1. Introduction
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) is often used as a form of rescue therapy for patients with acute respiratory distress syndrome (ARDS) despite maximal mechanical ventilation. VV-ECMO has gained popularity over the past 15 years as a successful treatment modality for ARDS due to the 2009 influenza (H1N1) pandemic and the ongoing COVID-19 pandemic.[
1] Immunocompromise status had been traditionally regarded as a contraindication for VV-ECMO in ARDS given poor outcomes in this subset of patients.[
2,
3] Currently, evidence remains limited to case reports and small case series regarding VV-ECMO use in patients with ARDS secondary to human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) that failed to improve with optimal mechanical ventilation. Respiratory failure remains the most common cause of ICU admission in HIV/AIDS patients with bacterial pneumonia and Pneumocystis jirovecii pneumonia (PJP) being the most common etiologies.[
4] Since the use of invasive ventilation is an independent predictor for poor outcomes in HIV/AIDS-related ARDS patients, especially those with PJP[
5], VV-ECMO has been emerging as an alternative avenue of treatment. Majority of the available literature describe the successful use of ECMO in HIV/AIDS patients with PJP-associated ARDS.[
6] Our case report demonstrates successful use of VV-ECMO in a previously untreated HIV/AIDS patient with ARDS due to PJP and cytomegalovirus (CMV) pneumonia who achieved a full clinical recovery after adequately improving immune system and treating PJP and CMV.
2. Case Report
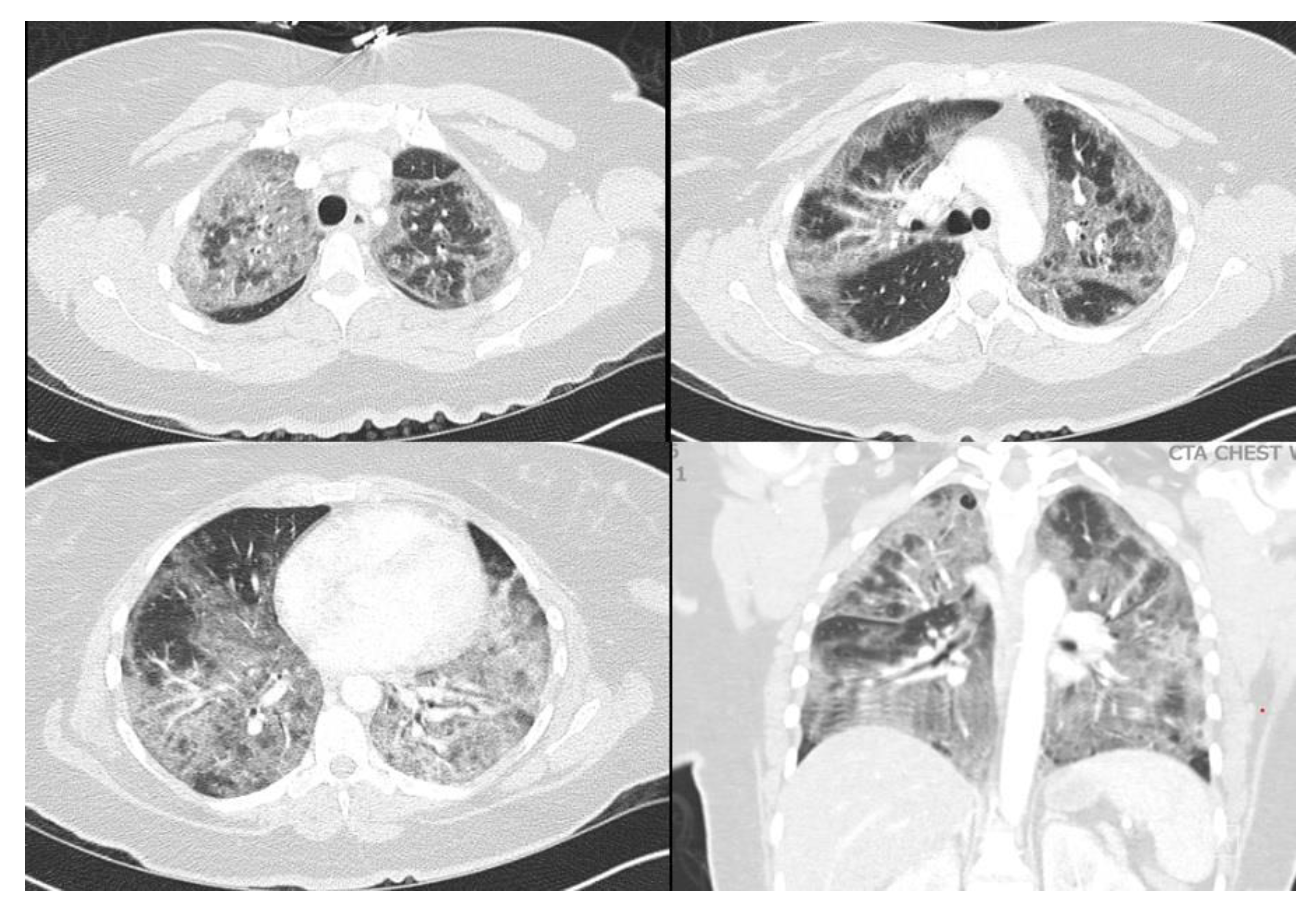
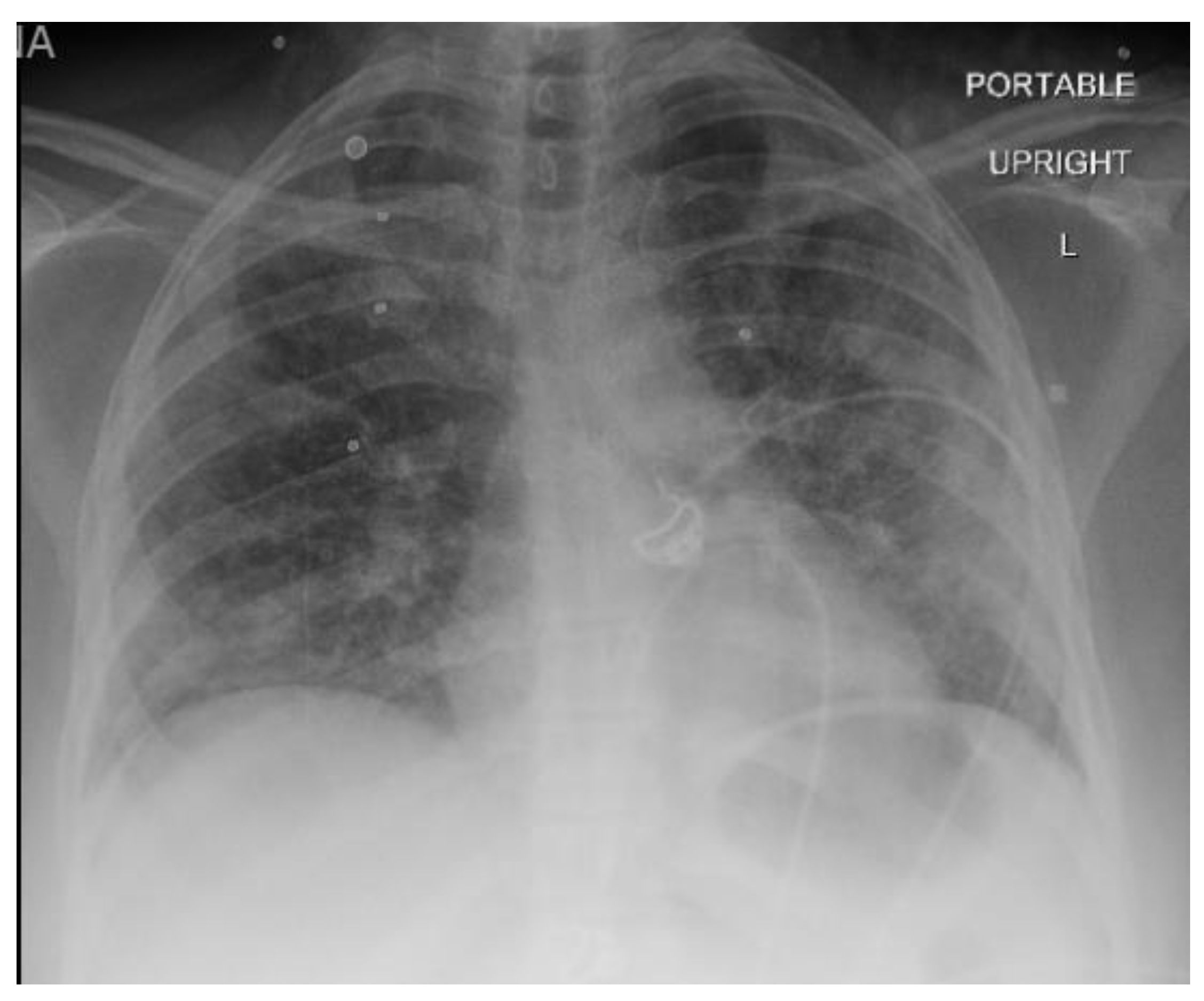
A 40-year-old female with a past medical history of asthma who presented to an outside hospital with a 3-week history of coughing, wheezing, and dyspnea, was found to have extensive bilateral ground glass opacities on CT scan and X-ray of the chest (
Figure 1 and
Figure 2). Admission testing revealed she was HIV positive with a CD4 count of 9 cells/ml and a viral load of 20,800. Additionally, she tested positive for P. jirovecii via direct immunofluorescence on admission for which she was treated with trimethoprim/sulfamethoxazole (TMP/SMX) and prednisone. Despite ongoing treatment, her clinical status continued to decline necessitating mechanical ventilation 10 days after admission. At this time, she underwent bronchoalveolar lavage which was positive for cytomegalovirus by polymerase chain reaction testing in addition to serum positive CMV (viral load of 175,000), for which ganciclovir was initiated and maintained for 11 days. PCR test of BAL was also positive for CMV, suggesting CMV pneumonia. Unfortunately, she progressed into ARDS refractory to lung-protective ventilation, prone positioning, and inhaled nitric oxide. She was transferred to our facility for evaluation of ECMO therapy at hospital day 18 (8 days after intubation). At the time of transfer, arterial blood gas analysis showed hypoxia (PaO2 of 79 mm Hg) on 100% fraction of inspired oxygen (FiO2), on Bi-level with P high/P low (30/1 cmH2O) and T high/T low (3/0.6 sec). Of note, patient was not able to promptly receive antiretroviral therapy (ART) upon diagnosis of HIV in the setting of ileus due to poor enteral absorption and acute kidney injury. ART with abacavir, lamivudine, and dolutegravir was immediately started on ECMO day 1 when her symptoms of ileus began to resolve.
Due to the failure to improve her respiratory condition, VV-ECMO was initiated via 28-French dual-lumen catheter placed into the right internal jugular vein under transesophageal echocardiography. The initial circuit settings were a flow of 4.3 L/min, sweep gas 5.5 L/min, on an FdO2 (fraction of delivered oxygen) of 100%. Her mechanical ventilator settings were adjusted to pressure control with a pressure difference of 10, positive end expiratory pressure (PEEP) of 10, respiratory rate (RR) of 10, and FiO2 of 40%.
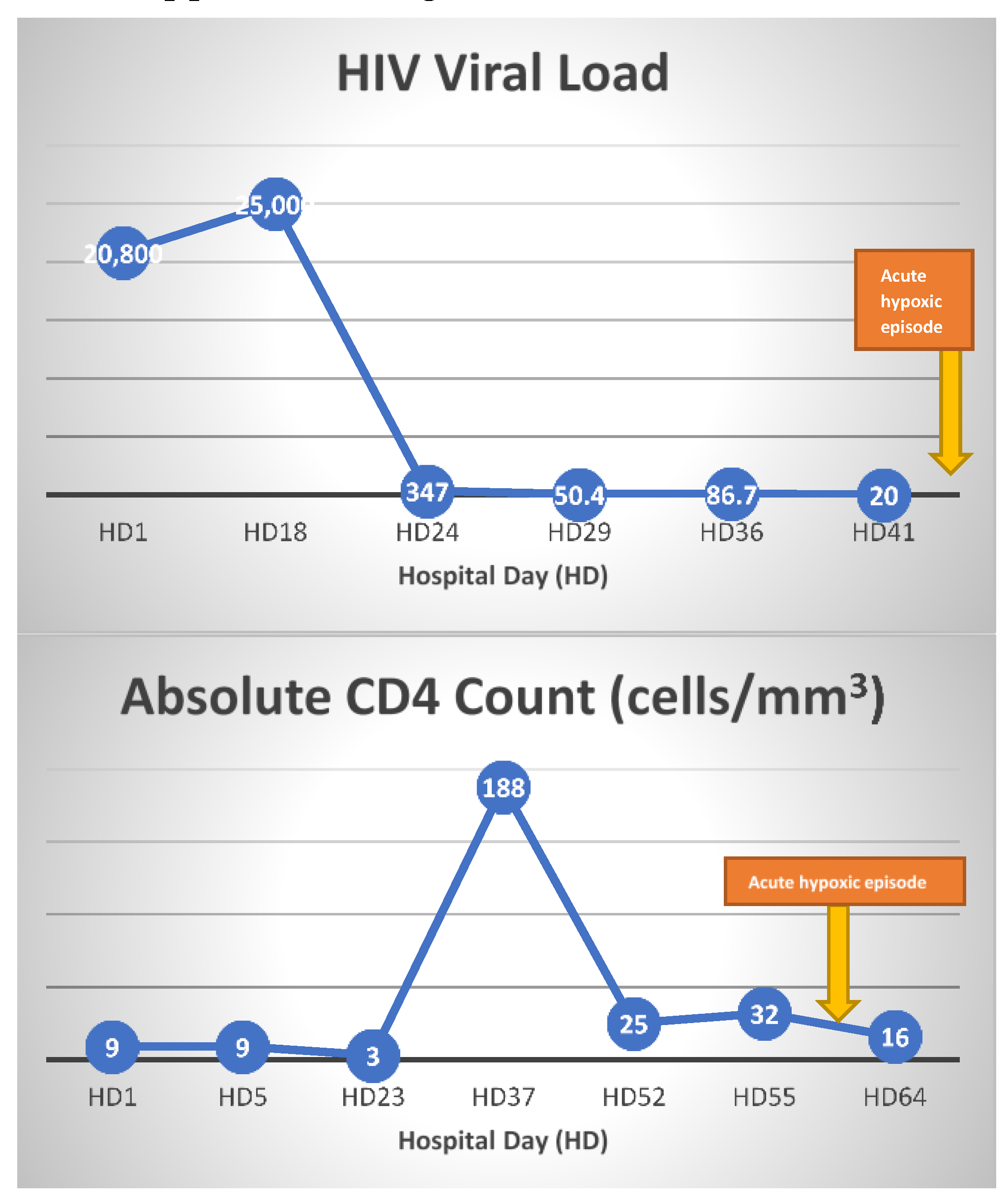
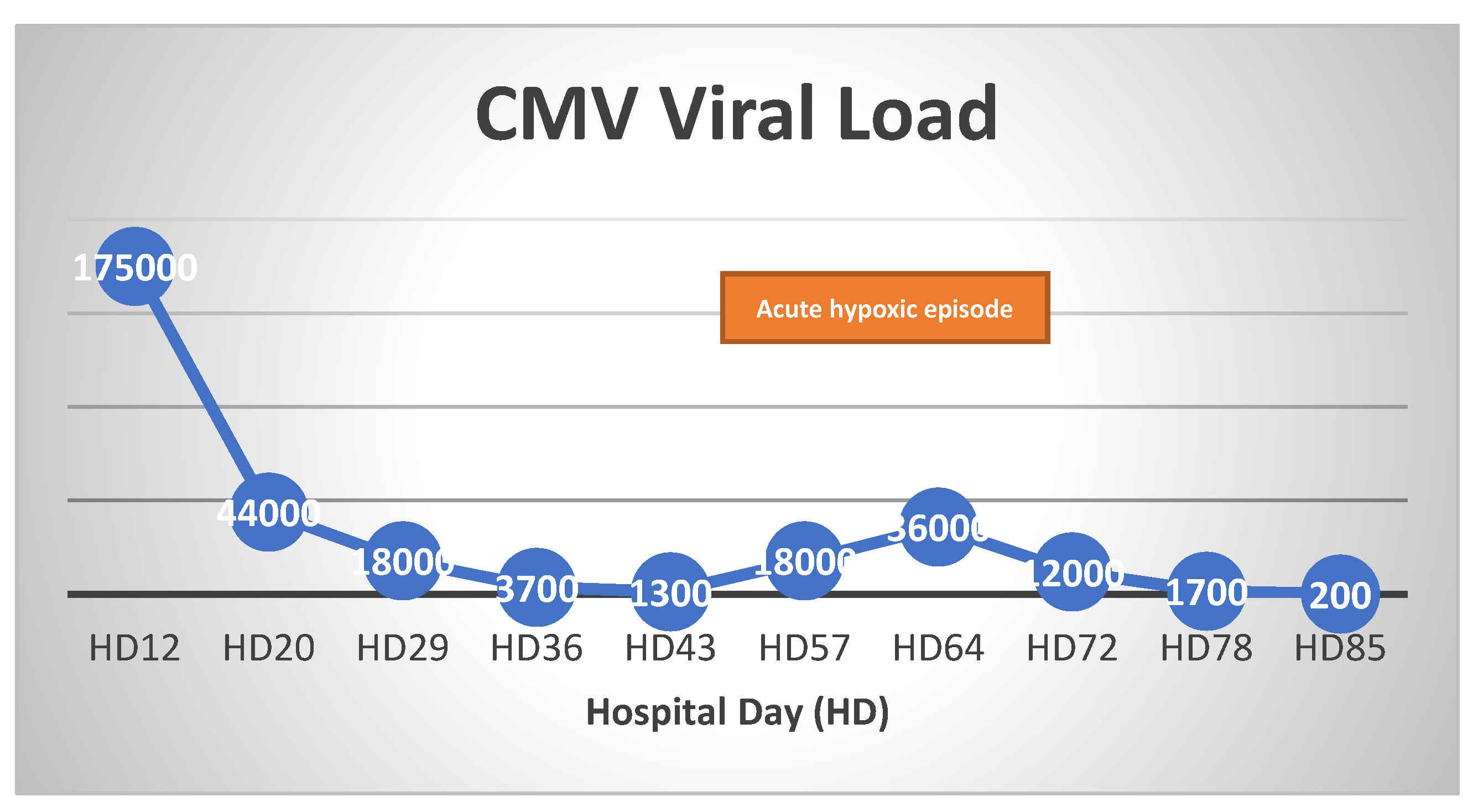
VV-ECMO settings were titrated to maintain flows between 4-6 L/min and a PaO2 >50 mmHg, until the patient began to have significant respiratory contribution with tidal volumes >200 ml on day 30 of ECMO support. During ECMO day 33 to 36, ECMO flow and sweep were weaned from 4.5 to 3 LPM and 5 to 0.5, respectively. However, patient progressively required more ECMO support from ECMO day 37 to 41. Repeat PCR testing for CMV showed increase in viral load to 18,000 from 1,290. IV ganciclovir was re-initiated on ECMO day 41. Her respiratory function markedly improved thereafter, allowing successful weaning of ECMO in the next 5 days. She was eventually decannulated from VV-ECMO on ECMO day 48 and maintained adequate ventilation on pressure support via tracheostomy. She continued to make clinical progress following VV-ECMO decannulation, and was discharged to acute inpatient rehabilitation facility at hospital day 82. ART was maintained throughout her hospitalization and her CD 4 count and HIV viral load improved from 9 to 38and 20,800 to <20 at discharge, respectively.
Of note, the patient’s clinical course was complicated by the development of recurrent bilateral pneumothoraces requiring chest tube placements, recurrent VRE bacteremia (treated with linezolid and daptomycin), subarachnoid hemorrhage (SAH), and appendicitis managed non-operatively. Heparin was held following SAH diagnosis and later resumed after repeat head CT demonstrated stability in the SAH.
3. Discussion
Exponential increase in the use of VV-ECMO, starting with the H1N1 pandemic in 2009, became more evident during the COVID-19 pandemic. VV-ECMO can alleviate the burden of mechanical power from mechanical ventilation, thereby reducing the incidence of ventilator induced lung injury, which is frequently seen in patients with ARDS. However, VV-ECMO is a resource-intensive therapy with high costs and is associated with life-threatening complications such as thrombosis, coagulopathy, infection, and brain injury. There are no clear guidelines established for the use of VV-ECMO in HIV/AIDS patients with severe ARDS. Current available ECMO guidelines states that immunocompromised status is considered as a relative contraindication because of high mortality—overall 6-month survival was only 30% and immunosuppression was an independent risk factor for mortality in prognosis scoring system used for respiratory failure in ECMO.[
7,
8] To this date, only case reports and small case series reported successful use of VV-ECMO to treat refractory ARDS in HIV/AIDS patients. It is important to note that the majority of the case reports (11 out of 14) describe the use of VV-ECMO in HIV/AIDS related ARDS due to PJP.[
6] Our patient had untreated HIV with PJP and concomitant CMV pneumonia whose respiratory function gained full recovery after treating CMV pneumonia. Her respiratory function, previously not improving despite prolonged VV-ECMO therapy and adequate medical treatment of PJP, finally turned the corner after treating the CMV pneumonia.
A retrospective study of 147 patients with HIV infection suggests that infectious etiology accounts for 78% of the causes of acute respiratory failure. Bacterial pneumonia and PJP were the two most common infectious etiologies with 50% and 35%, respectively.[
4] 3% of the patients experienced acute respiratory failure due to opportunistic pneumonia secondary to cytomegalovirus.[
4] Incidence of full-blown respiratory failure due to CMV is very rare and limited to case reports.[
9] To our knowledge, successful use of VV-ECMO in such a patient is limited to only one case report.[
10]
The incidence of CMV pneumonia in HIV patients is rare and reported to be 5-8%.[
11] The mainstay of treatment of CMV infection in an HIV patient is the restoration of immune system with ART.[
12] Even though our patient received ganciclovir treatment upon diagnosis of CMV pneumonia in the beginning of the hospital course, her immune function was not adequately restored to fight against CMV as ART was delayed due to ileus and poor enteral absorption. Although her diagnosis of PJP was quickly made and immediately treated with high dose TMP/SMX for 21 days and steroids, her respiratory function failed to improve resulting in continuous support on VV-ECMO. CMV viral load, which initially responded appropriately after initial ganciclovir treatment, was shown to be elevated again when the patient had an acute hypoxic episode requiring higher ECMO support around hospital day 55 (ECMO day 40). The CD4 count was less than 50 cells/mm3 at this time. Although HIV viral load during this time was lower than 20, her CD4 count was less than 30, suggesting incomplete restoration of immune system (
Figure 3). As a result, patient had an opportunistic infection with CMV pneumonia, as supported by the increase in CMV viral load (
Figure 4). As the 2-week course of ganciclovir treatment was re-initiated, patient began to pull better tidal volumes and required less VV-ECMO support—leading to successful decannulation after the next 7 days.
We believe that her ARDS was caused by both PJP and CMV pneumonia rather than immune reconstitution inflammatory syndrome (IRIS). IRIS is unlikely in this patient because the timing of acute respiratory deterioration episode did not correspond with the immune recovery. Also, IRIS associated Pneumocystis jirovecii infection usually presents after 1-3 weeks of ART[
13] when our patient experienced this episode 6 weeks after ART. Treatment of CMV pneumonia resulted in obvious improvement in the pulmonary function, which previously failed to achieve full recovery despite complete course of PJP treatment.
Despite the absence of well-established guidelines, our case report clearly suggests that VV-ECMO should be considered even in treatment naïve HIV/AIDS patients with persistent ARDS despite mechanical ventilation. Thorough work-up and diagnosis of the etiologies of ARDS should be completed to ensure prompt initiation of appropriate treatment. Also, rare causes of opportunistic infections causing ARDS should be explored when patient’s respiratory function is not progressing despite conventional management.
4. Conclusions
Management of ARDS in HIV/AIDS patients is challenging. Immediate ART should be initiated in addition to promptly Identifying the causative organisms and appropriate targeted treatment is the key step. As our case and recent case reports suggest, VV-ECMO can be a viable treatment modality for ARDS related to HIV/AIDS complications. Clinicians should consider it as an alternative option in carefully selected patients with refractory ARDS. Further studies are required to validate our outcome and adjust the indication for ECMO therapy in patients with severe immunosuppression.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, J.K.K., M.A., B.S.K.; methodology, J.K.K., M.A., B.S.K.; investigation, J.K.K.; resources, J.K.K., M.A., B.S.K.; data curation, J.K.K., M.A., B.S.K.; writing—original draft preparation, J.K.K., M.A.; writing—review and editing, J.K.K., B.S.K.; visualization, J.K.K.; supervision, B.S.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Informed consent was obtained.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; McAuley, D.F.; Firmin, R.K.; et al. Referral to an Extracorporeal Membrane Oxygenation Center and Mortality among Patients with Severe 2009 Influenza A(H1N1). JAMA 2011, 306, 1659–1668. [CrossRef]
- Collett, L.W.; Simpson, T.; Camporota, L.; Meadows, C.I.; Ioannou, N.; Glover, G.; Kulasegaram, R.; Barrett, N.A. The Use of Extracorporeal Membrane Oxygenation in HIV-Positive Patients with Severe Respiratory Failure: A Retrospective Observational Case Series. Int J STD AIDS 2019, 30, 316–322. [CrossRef]
- Barbas, C.S.V.; de Mato, G.F.J. Is It Worth to Apply Extra-Corporeal Membrane Oxygenation in the Immunocompromised Patients with Severe Acute Respiratory Distress Syndrome? J. Thorac. Dis. 2019, 11, S425–S427. [CrossRef]
- Barbier, F.; Coquet, I.; Legriel, S.; Pavie, J.; Darmon, M.; Mayaux, J.; Molina, J.-M.; Schlemmer, B.; Azoulay, E. Etiologies and Outcome of Acute Respiratory Failure in HIV-Infected Patients. Intensive Care Med 2009, 35, 1678–1686. [CrossRef]
- Akgün, K.; Miller, R. Critical Care in Human Immunodeficiency Virus–Infected Patients. Semin Respir Crit Care Med 2016, 37, 303–317. [CrossRef]
- Obata, R.; Azuma, K.; Nakamura, I.; Oda, J. Severe Acute Respiratory Distress Syndrome in a Patient with AIDS Successfully Treated with Veno-Venous Extracorporeal Membrane Oxygenation: A Case Report and Literature Review. Acute Med Surg 2018, 5, 384–389. [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting Survival after Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am J Respir Crit Care Med 2014, 189, 1374–1382. [CrossRef]
- Schmidt, M.; Schellongowski, P.; Patroniti, N.; Taccone, F.S.; Reis Miranda, D.; Reuter, J.; Prodanovic, H.; Pierrot, M.; Dorget, A.; Park, S.; et al. Six-Month Outcome of Immunocompromised Patients with Severe Acute Respiratory Distress Syndrome Rescued by Extracorporeal Membrane Oxygenation. An International Multicenter Retrospective Study. Am J Respir Crit Care Med 2018, 197, 1297–1307. [CrossRef]
- Poh, K.C.; Zheng, S. A Rare Case of CMV Pneumonia in HIV-Infection. Respir Med Case Rep 2019, 28, 100945. [CrossRef]
- Morley, D.; Lynam, A.; Carton, E.; Martin-Loeches, I.; Sheehan, G.; Lynn, N.; O’Brien, S.; Mulcahy, F. Extracorporeal Membrane Oxygenation in an HIV-Positive Man with Severe Acute Respiratory Distress Syndrome Secondary to Pneumocystis and Cytomegalovirus Pneumonia. Int J STD AIDS 2018, 29, 198–202. [CrossRef]
- Millar, A.B.; Patou, G.; Miller, R.F.; Grundy, J.E.; Katz, D.R.; Weller, I.V.; Semple, S.J. Cytomegalovirus in the Lungs of Patients with AIDS. Respiratory Pathogen or Passenger? Am Rev Respir Dis 1990, 141, 1474–1477. [CrossRef]
- O’Sullivan, C.E.; Drew, W.L.; McMullen, D.J.; Miner, R.; Lee, J.Y.; Kaslow, R.A.; Lazar, J.G.; Saag, M.S. Decrease of Cytomegalovirus Replication in Human Immunodeficiency Virus Infected-Patients after Treatment with Highly Active Antiretroviral Therapy. J Infect Dis 1999, 180, 847–849. [CrossRef]
- Wislez, M.; Bergot, E.; Antoine, M.; Parrot, A.; Carette, M.F.; Mayaud, C.; Cadranel, J. Acute Respiratory Failure Following HAART Introduction in Patients Treated for Pneumocystis Carinii Pneumonia. Am J Respir Crit Care Med 2001, 164, 847–851. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).