1. Introduction
Sudden sensorineural hearing loss (SSNHL) is an acquired idiopathic hearing loss that develops within 72 hours [
1]. Recent studies report an incidence rate of 27-61cases per 100,000 individuals. These studies also detail age specific differences in incidence. A peak incidence rate of 77 per 100,000 was reported in the American population aged 65 years and older with an overall slight male predominance[
2]. While, Nakashima et al. found a peak incidence of 94 per 100,000 in 60-69 year old patients with the incidence declining thereafter and with an overall female predominance [
3]
MicroRNAs (miRNAs) are short RNA chains, typically consisting of 21-23 nucleotides that act as posttranscriptional gene expression regulators. According to current estimates, less than 2000 human miRNAs regulate more than one-third of the cellular transcriptome [
4]. MiRNAs play important regulatory roles in most cellular and developmental processes and have been linked to a wide range of human diseases [
5,
6]. MiRNAs have been shown to modulate up to 60% of protein-encoding genes, influencing the cellular cycle, differentiation, proliferation, and apoptosis [
7]. MiRNAs have been found in a variety of body fluids such as serum, plasma, urine, and cerebrospinal fluid and have been shown to be reliable markers of a number of diseases [
8]. Many studies have found evidence of miRNA involvement in cancer, coronary heart disease, and neurological disease [
9,
10,
11,
12]. Normal inner ear cells express miRNAs, which are important for their development, differentiation, and survival [
13,
14,
15]. Li
et al. and Nunez
et al. have recently found evidence of differentially expressed miRNAs (DEMs) in the blood of SSNHL patients recruited in China and Canada respectively compared to local controls [
16,
17].
The DEMs identified in plasma by comparing 9 SSNHL patients with 3 controls in Li et al.’s study were miR-296/ -3667/ -15a, miR-1180/ -18b/ -451a/ -24-1/ -210/ -99b/ -190a/ -660/ -3940/ -34a/ -1-1/ -1-2/ -548ay/ -95/ -1255a/ -143/ -23a/ -548n/ -3679/ -3074, and miR-4742. While, Nunez et al. identified hsa-miR-590-5p/ -186-5p/ -195-5p/ -140-3p/ -128-3p/ -132-3p/ -375-3p, and -30a-3p as DEMs in serum by comparing 36 SSNHL patients with 12 controls.
A number of the above miRNAs have been shown to affect cell metabolism and or survival in different organs. miR-186-5p/ -195-5p/ and --590-5p/ exert control over apoptosis by targeting different genes. The targeting of genes XIAP and CEP55 by miR -186-5p in cardiomyocytes [
18] and -195-5p in non-small cell lung cancer cells [
19] respectively triggers apoptosis. MiR-590-5p has also been linked to apoptosis, and inflammation through its targeting of FGF18 in osteoarthritis-related chondrocytes [
20].
Cellular metabolic effects have been documented for miR-140-3p through its activation of the phosphatase and tensin homolog (PTEN)/ phosphatidyl inositol 3 kinase/protein Kinase B (PI3K/AKT) signaling pathway in bone [
21]. In the nervous system MiR-30a-3p targets SNAP23, which has been linked to the control of synaptic vesicle trafficking and neurotransmitter release, suggesting that it is involved in synaptic plasticity and neuronal function [
22].
PI3K/AKT and Rat Sarcoma (RAS) gene signaling pathways are enriched in the target genes of the DEMs identified in SSNHL [
17,
34]. Additionally, the RAF1 (proto-oncogene, serine/threonine kinase) gene which initiates the Mitogen-Activated Protein Kinase (MAPK) pathway is targeted by miR-15a [
16] and -132/ -195 [
17]. Therefore, SSNHL DEMs likely exert similar metabolic effects to those of miRNAs identified in other organ diseases.
The packaging of miRNAs in various protein complexes or membrane-bound particles, such as exosomes or microvesicles, maintains their stability and shields them from RNase degradation [
11,
23,
24,
25]. Scientists have focused on the profile of plasma and serum miRNAs in recent years due to their remarkably high stability across a wide pH range, for extended storage periods including multiple freeze-thaw cycles and resistance to endogenous RNase activity [
7]. They are also, simple to sample via relatively non-invasive methods, easily detected, and highly disease specific [
26].
Studies of miRNA expression levels in the plasma and serum of healthy individuals arrive at conflicting conclusions with respect to which type of blood sample is ideal. Wang
et al. found that the scope and scale of miRNA detection was greater in serum compared to paired plasma samples using Exiqon PCR panels [
27]. The same samples when assessed with Taqman cards using 40-Cycle threshold values did not show a statistically significant difference in the concentration of miRNAs between serum and plasma samples. However, when the samples were tested with miRNA specific qPCR Taqman primers, the serum samples demonstrated significantly higher miRNA concentrations than the plasma samples. The pre-amplification step in the Taqman cards process that was not utilized with Exiqon panels or the specific qPCR assays may have obscured the differences in serum and plasma samples. Foye
et al. reported the reverse: the scope and scale of miRNA detection was greater in plasma than serum using Nanostring nCounter technology [
28]. These contrasting findings support Wang
et al’s conclusion that miRNA expression levels varied with the measurement technology adopted [
27]. Inadvertent hemolysis or lysis of other blood cell types during collection can affect both plasma [
4] and serum [
29] leading similarly to the release of intracellular miRNAs. These released miRNAs can be erroneously interpreted as disease specific markers. This study investigates if in SSNHL patients there are differences between the serum and plasma expression levels of miRNAs previously identified to be differentially expressed in SSNHL patients’ serum.
2. Results
Paired serum and plasma samples from 8 female and 9 male SSNHL patients with a mean age of 51.9 years (Std. deviation 13.9 years) were analyzed (
Table 1). One patient had symptoms of dizziness. The mean of the initial pure tone audiometric (PTA) averaged thresholds across four low or three high frequencies in the affected ears of the patients was 60.7±22.9 dB. This is consistent with moderately severe hearing loss on average in the patients studied [
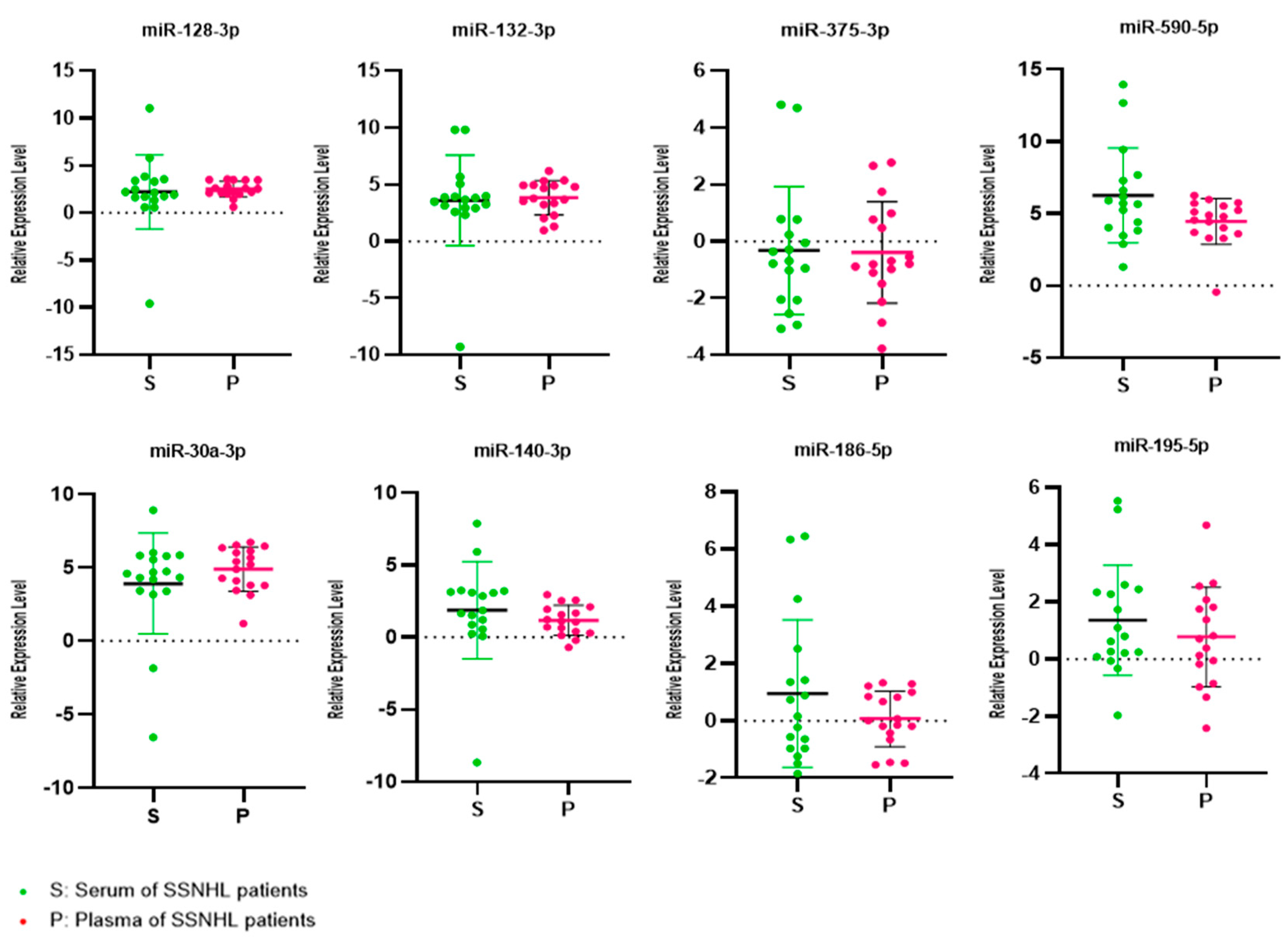
30] . The mean expression levels of miR-132-3p, -375-3p, -590-5p, -140-3p, -186-5p, -195-5p, 128-3p and -30a-3p were 3.59, -0.32, 6.24, 1.85, 0.95, 1.35, 2.22 and 3.89 in serum and 3.84, -0.39, 4.44, 1.16, 0.06, 0.77, 2.52 and 4.88 in plasma respectively (
Table 2). There was no statistically significant inter-group difference in the mean expression levels of the eight target miRNAs (
Figure 1).
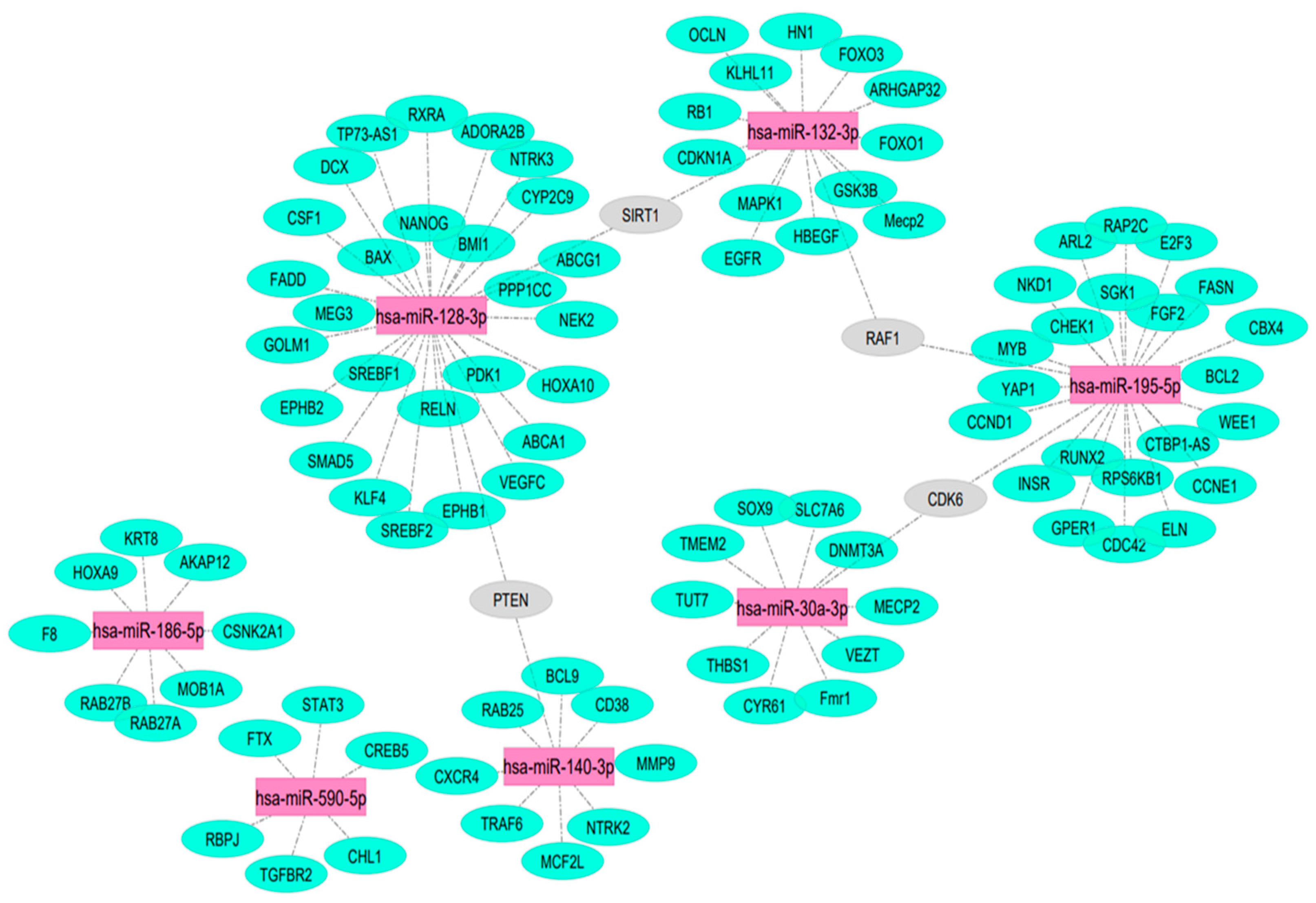
102 target mRNAs of miR-132-3p, -375-3p, -590-5p, -140-3p, -186-5p, -195-5p, 128-3p and -30a-3p validated by three strong types of experimental evidence were generated using miRTarBase [
31] and are illustrated in
Figure 2. PTEN, SIRT1, RAF1, and CDK8 mRNAs were targeted by more than one of these seven test miRNAs. No miR-375-5p mRNA targets were identified using the same robust methodology.
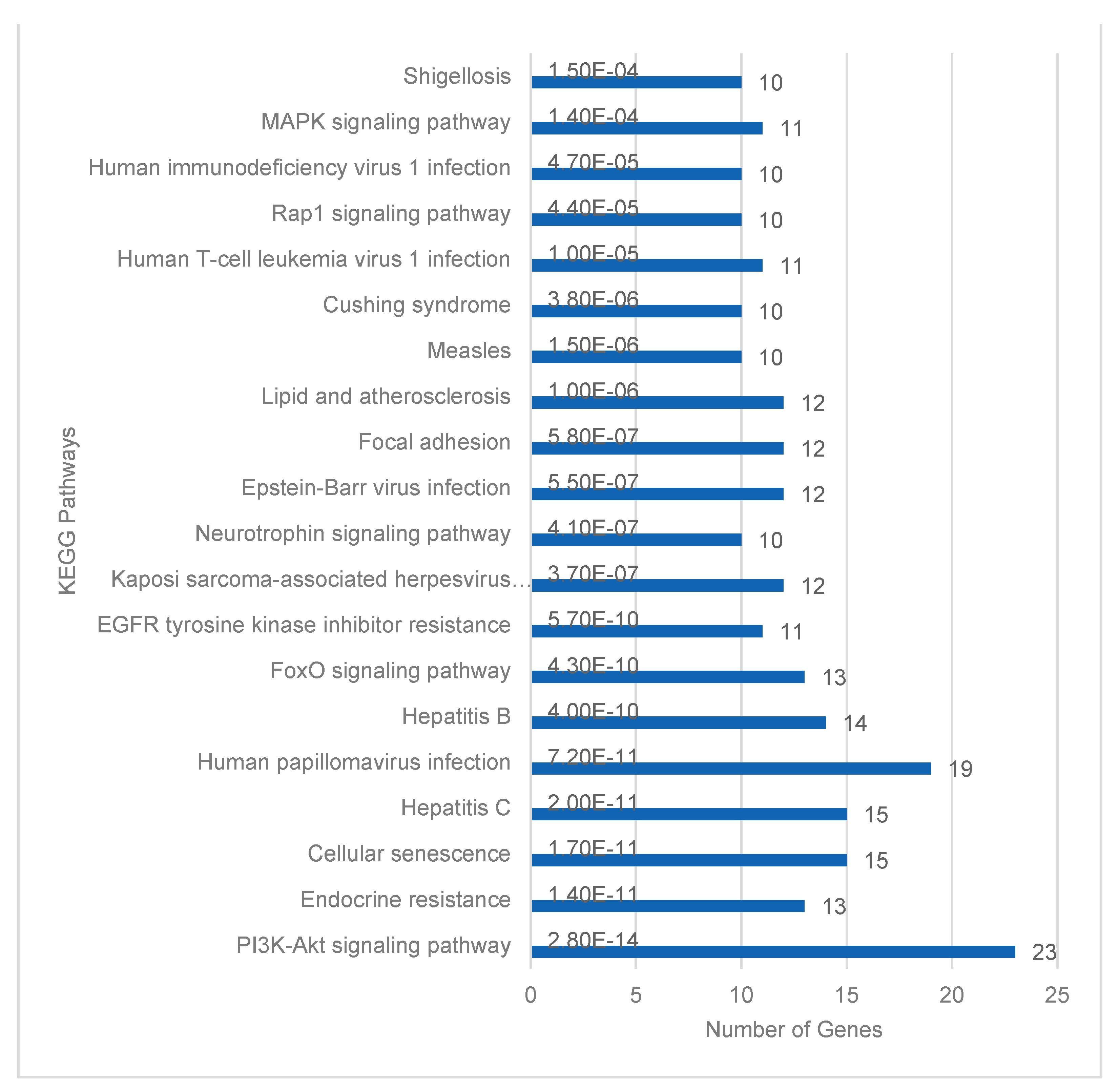
Database for Annotation, Visualization and Integrated Discovery (DAVID) for functional enrichment pathway analysis of the 205 target mRNAs identified 184 Homo sapiens genes [
32]. Twenty-three of these genes were identified in the Kyoto Encyclopedia of Genes and Genomes (KEGG) PI3K-Akt signaling pathway. This was the highest number of enriched genes in a single KEGG pathway (
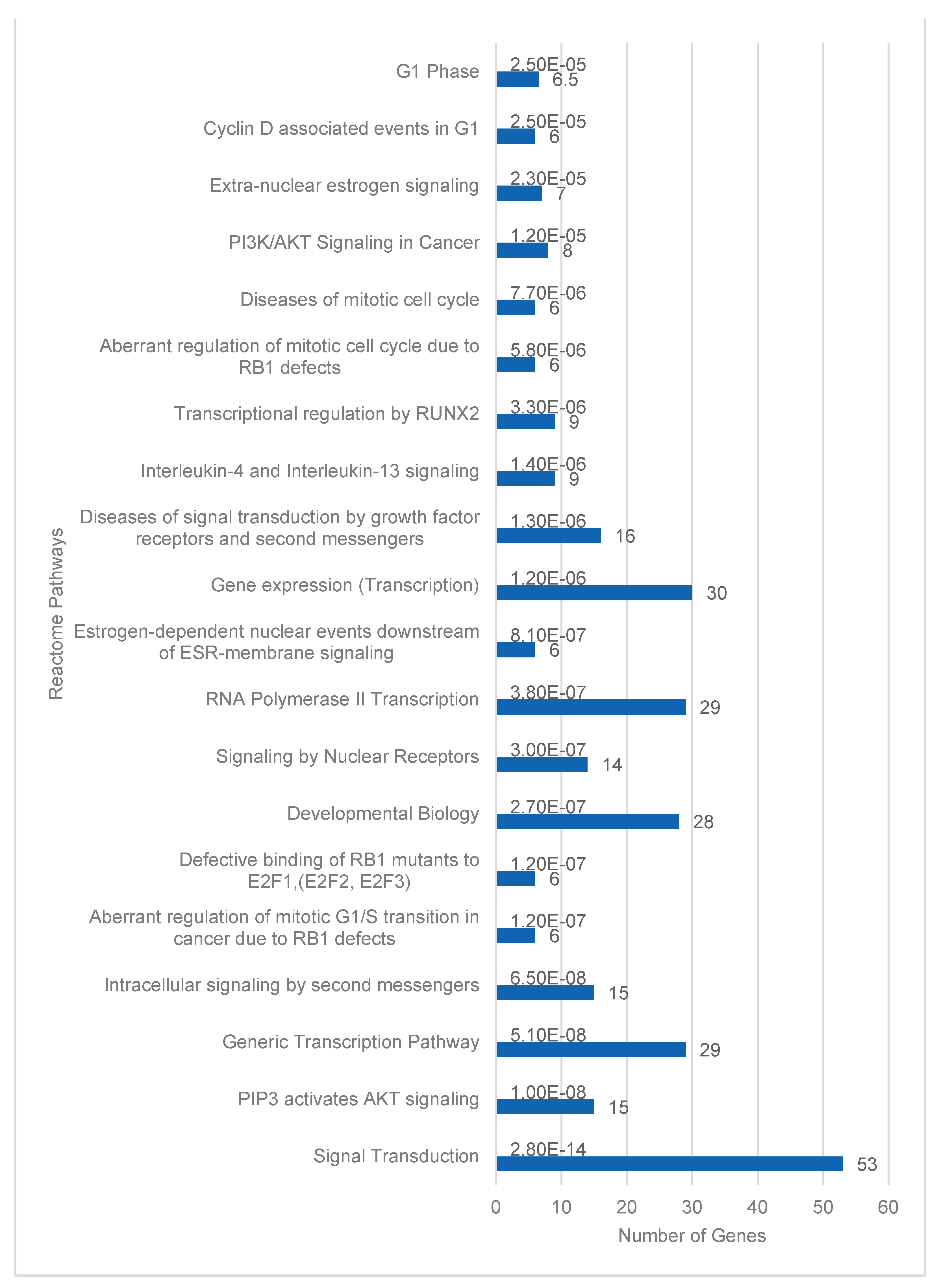
Figure 3). Signal transduction pathways with 53 target genes were similarly the most enriched Reactome pathways (
Figure 4). PI3-Akt, MAPK, RAF and FOXO genes were common to both KEGG and Reactome enriched pathways.
The top 20 pathways based on significant p-value and gene count are illustrated. The gene count in each pathway is enumerated at the end of each blue pathway bar on the graph.
The top 20 pathways based on significant p-values are illustrated. The pathways are listed by significance from p=2.8x10-14 to 2.5x10-5. Value labels in the bar chart indicate the number of target genes in each pathway.
3. Discussion
Previous investigations into miRNA expression levels in SSNHL studied either plasma [
16,
33] or serum [
17,
34] samples exclusively. miR-18b/-23a/-143/-210 were identified as significant DEMs in SSNHL patients' plasma compared to controls across two studies, albeit with some discrepancy in findings regarding the direction of expression changes [
16,
33]. Specifically Ha et al. reported downregulation of miR-18b and miR-23a, Li et al. found upregulation of miR-18b and downregulation of miR-23a [
16,
33]. This disparity might stem from differences in data normalization methods, with Li et al. employing a negative binomial distribution model and Ha et al. using miR-103 or miR-16 as reference miRNAs[
16,
33]. The serum studies differed from each other in the miRNA populations studied and normalization methods adopted. Zhang et al. investigated exosomal miRNAs using U6 as the reference gene, while Nunez et al. studied circulating miRNAs using the global mean value of 768 target genes[
17,
34] . These differences likely account for the different range of relevant DEMs quoted in the two serum studies. However, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis in both studies identified that the target genes of the DEMs were enriched in the phosphatidyl inositol 3 kinase/protein Kinase B (PI3K/Akt) and Ras signaling pathways. Furthermore, KEGG analyses in plasma and serum studies were congruent in postulating MAPK signaling pathway genes as targets of DEMs. Specifically, RAF1 (proto-oncogene, serine/threonine kinase) which initiates the MAPK pathway is targeted by miR-15a [
16] and -132/ -195 [
17] . The expression levels of 8 mature DEMs previously identified in SSNHL patients serum by Nunez et al. were studied here [
17].
Wang
et al. reported higher miRNA expression values in serum compared to paired plasma samples using miRNA-specific qPCR Taqman primers [
27]. Conversely, Foye et al. observed an increased scope and scale of miRNA expression in plasma using Nanostring nCounter technology. Additionally, Foye
et al. [
28] demonstrated that normalizing serum miRNA values against the median of all serum miRNA values effectively reduced the coefficient of variation, while for plasma samples, normalization against the most stable miRNAs proved optimal. In this study, we normalized both serum and plasma values against the value of hsa-miR-191-5p as this miRNA demonstrated consistent expression levels, in both sample types. The expression levels of the 8 miRNAs investigated in the current study were not statistically different in paired serum and plasma samples.
Differences in the time elapsed between sample collection and analysis in different studies can introduce variation in total RNA findings. Tsui
et al. found that serum and plasma concentrations of mRNA were initially similar when assessed within 4 hours of venipuncture [
35]. However, over 24 hours while the level in unfiltered plasma stored at 4˚C remained stable, that in serum increased. Serum RNA levels did not as anticipated decrease in response to RNase activity and supports the theory that RNAs anneal with DNA making them resistant to both RNase and DNase activity [
36] or the RNA is protected in extracellular vesicles or through other protein complexes. This finding also suggests that a greater than 4-hour delay in serum RNA analysis will likely result in erroneous miRNA expression level measurements unless some mitigating action possibly ultra-low temperature storage is taken. Kim
et al. corroborated Tsui et al’s findings that whole blood holding time even at 4
˚C before processing can have dramatic effects on analytical reliability and reproducibility [
37]. In the current study, RNA was extracted within one hour of venipuncture in all samples assessed.
Studies of haematological, vascular and cardiac diseases can be anticipated because of their direct relationship to circulating blood to illustrate if serum or plasma samples are superior for the identification of miRNA changes in blood. Mompeón
et al. reported that the expression levels of 6 miRNAs associated with myocardial infarction (MI) were similar in paired plasma and serum samples drawn from control participants without cardiovascular disease CVD [
38]. However, significant differences in miRNA expression patterns were observed between serum and plasma samples of CVD patients, with greater variation noted in plasma samples. Both serum and plasma samples of non-ST-elevation myocardial infarction (NSTEMI) patients showed an increase in miR-1 and miR-208b expression, although plasma samples showed greater variation (higher standard deviation) in expression levels. miR-499a expression was significantly increased only in NSTEMI patients’ plasma samples, and the expression of miR-133a and miR-26a was significantly increased and decreased respectively only in NSTEMI patients’ serum samples. Interestingly/ -21 displayed a different direction of expression level change in different samples, increasing in serum and decreasing in plasma. This sample dependant variation in miRNA findings suggests that standardisation of the blood samples plasma or serum utilised for miRNA analysis in CVD is required.
Little is known about the mechanisms that generate the miRNAs found in the circulation of individuals with different diseases especially diseases of tissues remote from the major blood vessels, or the biological impact of these miRNAs in other parts of the body distant from the primary disease. David functional enrichment analysis utilizing Reactome and KEGG pathways suggests that PI3-Akt, MAPK, RAF and FOXO genes are likely important targets of the DEMs studied. This agrees with previous KEGG pathway analysis findings on an earlier study of SSNHL patients and controls [
17]. The relevance of these circulating miRNAs and their putative targeted genes to the inner ear is supported by functional enrichment pathway analysis of DEMs in differentiated compared to undifferentiated House Ear Institute organ of Corti cells (HEI-OC1). Specifically, the PI3-Akt and MAPK signaling pathways contained the highest number of target genes of the DEMs in differentiated HEI-OC1 cells [
15]. In conclusion in SSNHL patients it appears that either serum or plasma samples can be studied to identify miRNA changes provided RNA is extracted within sixty minutes of venipuncture.
4. Materials and Methods
17 adult patients, 18 years and older, presenting with SSNHL as defined by the AAOHNS criteria [
39] were recruited at the Department of Otolaryngology, Vancouver General Hospital between 2017 and 2022. All participants’ pure tone audiometric responses were recorded by provincially registered audiologists and/or hearing instrument practitioners. The PTA averaged thresholds across 4 low (0.5, 1, 2, 3 or 4 kHz) or 3 high (3, or 4, 6 and 8 kHz) as previously described [
40] were calculated based on the frequencies demonstrating the greatest degree of hearing loss at presentation. The pure tone audiometric results were utilized to confirm the diagnosis of SSNHL based on the criteria described by Stachler
et al. [
39] and ascertain that control participants had normal hearing. Specifically, SSNHL participants required audiometrically documented evidence of sensorineural hearing loss of at least 30 dB across three contiguous frequencies.
Patients were recruited whether or not they had undergone some form of treatment. Otolaryngologist Head and Neck Surgeons or trainees under their supervision registered with the College of Physicians and Surgeons of British Columbia conducted a comprehensive clinical examination of the participants' ears. Otoscopic examinations of participants ear canals and tympanic membranes was performed to exclude possible causes of hearing loss arising from otitis externa, otitis media, foreign materials, trauma, or other evidence of ear disease. Patients with an identified cause of hearing loss, major medical illness, or coexisting ear pathology were excluded. The study was approved by the University of British Columbia’s Clinical Research Ethics Board and performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants.
Blood samples were collected in separate tubes designated for serum and plasma separation at the time of the patient’s initial clinical presentation or during their subsequent clinical follow-up visits. Serum and plasma aliquots were stored at -80˚C for RNA extraction. miRNA analysis was undertaken as previously described [
41]. In brief, total RNA was extracted from 200 microliters of serum and plasma using miRNeasy Mini Kit (Qiagen, Toronto, ON, Canada) according to the manufacturer’s instructions. Purified RNA was resuspended in 50 microliters of nuclease-free water and stored at -80˚ C before Reverse transcription (RT) was performed using a TaqMan cDNA synthesis kit (Applied Biosystems, Thermo Fisher Scientific, Waltham, USA), with a preamplification step. The reaction mixture consisted of 2 μL of microRNAs, 3 μL of poly(A) reaction mix, 10 μl of ligation reaction mix, and 15 μL of reverse transcriptase. This was reverse transcribed (RT) at 42˚C for 15 min and deactivated at 85˚C for 5 min. 5 μL of the RT reaction product was incubated with 45 μL of the miR-Amp Reaction Mix before extracting and diluting cDNA 1:10 with nuclease-free water for miRNA PCR analysis. Real-time PCR was then performed using Taqman® Advanced miRNA primers and Taqman Fast Advanced master mix on a QuantStudio 3 Real-time PCR System (Applied Biosystems,Thermo Fisher Scientific, Waltham, USA).
Individual qPCR assays were performed three times with a total reaction volume of 20 μL using hsa-miR-30a-3p/-128-3p/-132-3p/-140-3p/-186-5p/-195-5p/-375-3p/-590-5p/ primers (
Table 3) and test reference primers hsa-miR-16-5p/-103a-3p/-191-5p according to the manufacturer’s instructions. The PCR thermocycler was programmed for denaturation at 95˚C for 20 s, followed by 40 annealing cycles of 95˚C for 1 s and 60˚C for 20 s. Test miRNA expression levels were calculated for all samples with the delta Ct method using each of the assessed reference primers independently [
42]. The reference miRNA that displayed the least variation in delta Ct expression values across the samples was selected as the reference miRNA for all further analyses. The expression levels of the miRNAs of interest in SSNHL patients’ serum and plasma were then compared with paired t-tests using SPSS version 26 (IBM, Armonk, USA). Scatter plots with means and standard deviations were generated with GraphPad Prism version 9.0.
Putative target mRNAs and genes of the test miRNAs were identified by interrogating miRTarBase for miRNA- target mRNA interactions supported by at least three strong experimental validations such as reporter assay, western blot, and qPCR [
31]. Cytoscape version 3.10.1 [
43] was then used to visualize the validated test miRNA- target mRNA interactions. Functional enrichment pathway analysis of the identified target genes was undertaken with KEGG and Reactome pathway tools in DAVID [
32]. The first twenty most statistically significant (p < .05) pathways after Benjamini and Hochberg correction were selected.
Author Contributions
DAN generated the idea; DAN, PW, and CG designed the study; RA performed the experiments and analyzed the data; RA and DAN drafted the paper; PW performed the bioinformatic analyses; PW and CG critically revised the paper.
Funding
This research was funded by Vancouver Coastal Health Research Institute, grant number RD47.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of British Columbia (protocol code H15-03024 initial date of approval 17 February 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We kindly thank clinical and administrative staff of the Vancouver General Hospital Division of Otolaryngology Head and Neck Surgery and the Neurotology Service. We thank Hakha Behmanesh for improvements to the wording of the manuscript. We thank the Rotary Club of Vancouver Hearing Foundation, the Pacific Otolaryngology Foundation, and individual donors for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.H.; Harris, J.P. Incidence of Sudden Sensorineural Hearing Loss. Otol. Neurotol. 2013, 34, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Sato, H.; Gyo, K.; Hato, N.; Yoshida, T.; Shimono, M.; Teranishi, M.; Sone, M.; Fukunaga, Y.; Kobashi, G.; et al. Idiopathic sudden sensorineural hearing loss in Japan. Acta Oto-Laryngologica 2014, 134, 1158–1163. [Google Scholar] [CrossRef]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development 2005, 132, 4653–4662. [Google Scholar] [CrossRef] [PubMed]
- Sumathipala, M.; Weiss, S.T. Predicting miRNA-Based Disease-Disease Relationships through Network Diffusion on Multi-Omics Biological Data. Sci. Rep. 2020, 10, 8705. [Google Scholar] [CrossRef] [PubMed]
- Szelenberger, R.; Kacprzak, M.; Saluk-Bijak, J.; Zielinska, M.; Bijak, M. Plasma MicroRNA as a novel diagnostic. Clin. Chim. Acta 2019, 499, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, X.; Ma, K.; Li, G.; Liu, Z.; Rong, W.; Miao, H.; Zhu, F.; Cui, Q.; Wu, S.; et al. Circulating miRNAs Related to Long-term Adverse Cardiovascular Events in STEMI Patients: A Nested Case-Control Study. Can. J. Cardiol. 2020, 37, 77–85. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, P.; Xi, J.; Cui, J.; Campbell, M.; Pham, W.; A Matsubara, J. MicroRNAs in tear fluids predict underlying molecular changes associated with Alzheimer’s disease. Life Sci. Alliance 2023, 6, e202201757. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.M.; Dror, A.A.; Mor, E.; Tenne, T.; Toren, G.; Satoh, T.; Biesemeier, D.J.; Shomron, N.; Fekete, D.M.; Hornstein, E.; et al. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc. Natl. Acad. Sci. 2009, 106, 7915–7920. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, K.; Rudnicki, A.; Avraham, K.B. MicroRNAs in sensorineural diseases of the ear. Front. Mol. Neurosci. 2013, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, P.; Nunez, D.A.; Garnis, C. MicroRNA Signature and Cellular Characterization of Undifferentiated and Differentiated House Ear Institute-Organ of Corti 1 (HEI-OC1) Cells. J. Assoc. Res. Otolaryngol. 2022, 23, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, X.; Huang, H.; Li, J.; Wang, F.; Wang, J. RNA sequencing uncovers the key microRNAs potentially contributing to sudden sensorineural hearing loss. Medicine 2017, 96, e8837. [Google Scholar] [CrossRef] [PubMed]
- Nunez, D.A.; Wijesinghe, P.; Nabi, S.; Yeh, D.; Garnis, C. microRNAs in sudden hearing loss. Laryngoscope 2019, 130, E416–E422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, B. MicroRNA-186-5p is expressed highly in ethanol-induced cardiomyocytes and regulates apoptosis via the target gene XIAP. Mol. Med. Rep. 2019, 19, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Pan, J.; Jin, Y.; Li, M.; Chen, M. MiR-195-5p Inhibits Proliferation and Induces Apoptosis of Non-Small Cell Lung Cancer Cells by Targeting CEP55. OncoTargets Ther. 2019, 12, 11465–11474. [Google Scholar] [CrossRef]
- Jiang, P.; Dou, X.; Li, S.; Jia, Q.; Ling, P.; Liu, H.; Han, Q.; Sun, S. miR-590-5p affects chondrocyte proliferation, apoptosis, and inflammation by targeting FGF18 in osteoarthritis. Am. J. Transl. Res. 2021, 13, 8728–8741. [Google Scholar]
- Yin, R.; Jiang, J.; Deng, H.; Wang, Z.; Gu, R.; Wang, F. miR-140-3p aggregates osteoporosis by targeting PTEN and activating PTEN/PI3K/AKT signaling pathway. Hum. Cell 2020, 33, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, X.; Long, Q.; Zeng, H.; Sun, Q.; Chen, Y.; Wu, D.; Liu, L. Small extracellular vesicles containing miR-30a-3p attenuate the migration and invasion of hepatocellular carcinoma by targeting SNAP23 gene. Oncogene 2020, 40, 233–245. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Collino, F.; Deregibus, M.C.; Bruno, S.; Sterpone, L.; Aghemo, G.; Viltono, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of miRNAs. PLOS ONE 2010, 5, e11803. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Chiam, K.; Mayne, G.C.; Wang, T.; I Watson, D.; Irvine, T.S.; Bright, T.; Smith, L.T.; A Ball, I.; Bowen, J.M.; Keefe, D.M.; et al. Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus. World J. Gastroenterol. 2020, 26, 2570–2583. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.-H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLOS ONE 2012, 7, e41561. [Google Scholar] [CrossRef] [PubMed]
- Foye, C.; Yan, I.K.; David, W.; Shukla, N.; Habboush, Y.; Chase, L.; Ryland, K.; Kesari, V.; Patel, T. Comparison of miRNA quantitation by Nanostring in serum and plasma samples. PLOS ONE 2017, 12, e0189165. [Google Scholar] [CrossRef] [PubMed]
- A MacLellan, S.; MacAulay, C.; Lam, S.; Garnis, C. Pre-profiling factors influencing serum microRNA levels. BMC Clin. Pathol. 2014, 14, 27. [Google Scholar] [CrossRef]
- Clark, J.G. Uses and abuses of hearing loss classification. Asha 1981, 23, 493–500. [Google Scholar]
- Huang, H.-Y.; Lin, Y.-C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: an informative resource for experimentally validated miRNA–target interactions. Nucleic Acids Res. 2021, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.M.; Hwang, K.R.; Park, I.H.; Park, S.; Choi, J.S.; Park, D.J.; Park, J.-E.; Lee, S.H.; Lee, H.Y.; Seo, Y.J. Circulating microRNAs as potentially new diagnostic biomarkers of idiopathic sudden sensorineural hearing loss. Acta Oto-Laryngologica 2020, 140, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Yang, G.; Ke, J.; Sun, W.; Yang, L.; Kuang, S.; Li, H.; Yuan, W. Differentially expressed miRNA profiles of serum-derived exosomes in patients with sudden sensorineural hearing loss. Front. Neurol. 2023, 14, 1177988. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.B.; Ng, E.K.; Lo, Y.D. Stability of Endogenous and Added RNA in Blood Specimens, Serum, and Plasma. Clin. Chem. 2002, 48, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Sisco, K.L. Is RNA in Serum Bound to Nucleoprotein Complexes? Clin. Chem. 2001, 47, 1744–1745. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacIntyre, D.A.; Sykes, L.; Arianoglou, M.; Bennett, P.R.; Terzidou, V. Whole Blood Holding Time Prior to Plasma Processing Alters microRNA Expression Profile. Front. Genet. 2022, 12, 818334. [Google Scholar] [CrossRef] [PubMed]
- Mompeón, A.; Ortega-Paz, L.; Vidal-Gómez, X.; Costa, T.J.; Pérez-Cremades, D.; Garcia-Blas, S.; Brugaletta, S.; Sanchis, J.; Sabate, M.; Novella, S.; et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci. Rep. 2020, 10, 5373. [Google Scholar] [CrossRef] [PubMed]
- Stachler, R.J.; Chandrasekhar, S.S.; Archer, S.M.; Rosenfeld, R.M.; Schwartz, S.R.; Barrs, D.M.; Brown, S.R.; Fife, T.D.; Ford, P.; Ganiats, T.G.; et al. Clinical Practice Guideline: Sudden Hearing Loss. Otolaryngol. Neck Surg. 2012, 146, S1–S35. [Google Scholar] [CrossRef]
- Joshua, T.G.; Ayub, A.; Wijesinghe, P.; Nunez, D.A. Hyperbaric Oxygen Therapy for Patients with Sudden Sensorineural Hearing Loss: A Systematic Review and Meta-Analysis. JAMA Otolaryngol. Neck Surg. 2022. [CrossRef]
- Abgoon, R.; Wijesinghe, P.; Garnis, C.; Nunez, D.A. The Expression Levels of MicroRNAs Differentially Expressed in Sudden Sensorineural Hearing Loss Patients’ Serum Are Unchanged for up to 12 Months after Hearing Loss Onset. Int. J. Mol. Sci. 2023, 24, 7307. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).