Introduction
Vaccines are administered to protect people fromcertain diseases, at the same time reducing their transmission. Since the inception of the concept of vaccination in 1796, there have been tremendous advancements in the field of vaccinology. From the early developed whole-pathogen-inactivated vaccines to the recent discovery of mRNA vaccines, the field has shown great progress with an inflection during this pandemic [
1]. Vaccines against SARS-CoV-2 have proved their capability of mitigating severe symptoms and reducing hospitalization, but little is known about the cellular memory responses they impart [
2]. Besides antibodies, the generation and maintenance of immune memory is an important element to tackle any pathogen during future encounters [
3]. Generation of immune memory is the change in the immune status of the host which imparts its footprint that may last for much longer duration [
4]. Both the T and B cells play critical roles in generating immune memory and shape each other’s functionality [
5,
6]. Cytotoxic CD8+ T cells are important in killing and eliminating the cells infected with an intracellular pathogen such as a virus, while CD4+ T cells generate a cytokine milieu conducive for the maturation of the B and CD8+ T cells, and concomitantly development of their memory phenotype [
7,
8]. Long-lasting memory B cells are generated in responseto natural infection or vaccination andadd a further layer of protection [
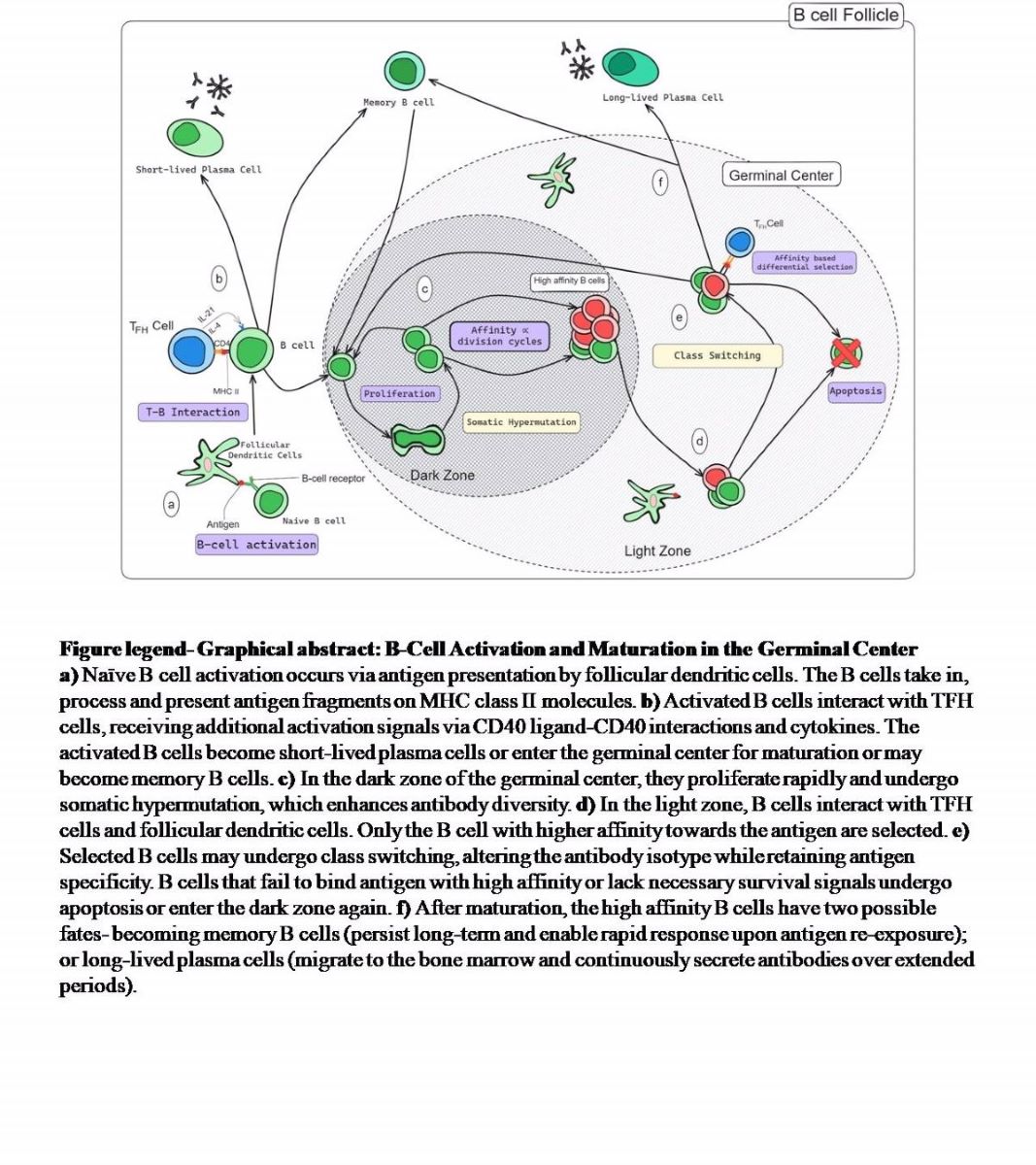
9]. B-cell maturation occurs in the transient but specialized structures known as the germinal centre. After a proliferative stage where a B cell clone undergoes somatic hypermutation and antigen-based selection, only those with the ability to effectively bind an antigen survive [
10,
11]. This process continues for several months after vaccination or the infection. This makes the newer generations of B cells produce antibodies with better avidity and superior ability of virus neutralization. A subset of these B cells- become memory B cells and dictates the immune response in case of any subsequent encounter with the antigen [
5]. The affinity maturation process expands the breadth of recognition with increased affinity of the antibodies and the generated pool of the MBCs are multi-pronged with anticipatory memory potential [
11]. Of note, besides the role of IgG in viral infections IgA and IgM have also been shown to be able to neutralize them [
12,
13].
A comparative assessment of memory B-cell response elicited by different vaccine platforms holds the potential to provide invaluable insights into their relative effectiveness in promoting enduring immune memory, hence facilitating the development of evidence-based vaccination strategies. It is also important to understand the level of memory B cells present in the peripheral circulation of the vaccinees over time and whether they have the ability to respond to therecurrent invasion by the pathogen [
14]. There are concerns regarding the potential decrease in the vaccine efficacy, as viruses carrying mutations in key neutralizing antibody epitopes propagate in the community, allowing them to partially/completely evade antibody recognition [
15,
16]. There are also reports that severe COVID-19 may compromise affinity maturation of the B cells [
17]. In India, three viral vector-based vaccines (Covishield, Sputnik Light and Sputnik V), an inactivated virus-based vaccine (Covaxin) [
18], and a protein subunit vaccine (Corbevax) were widely administered [
19]. Covaxin- is manufactured by Bharat Biotech. International Ltd. India uses the whole SARS-CoV-2 virus in an inactivated form. Corbevax, from Biological E. Limited India, utilizes a yeast (
Komagataellaphaffii)-produced version of the SARS-CoV-2 spike protein’s RBD along with adjuvants aluminium hydroxide gel and CpG1018 to trigger an effective immune response. It is based on the technology utilized for the well accepted for the recombinant hepatitis B vaccine production [
20]. Covishield is manufactured by Serum Institute of India Pvt. Ltd., a two-dose COVID-19 vaccine that uses a chimpanzee adenovirus vector called ChAdOx1 to deliver SARS-CoV-2 spike protein into the cell. The Sputnik Light vaccine manufactured by Gamaleya Research Institute of Epidemiology and Microbiology; Russia is a single-dose regimen that also utilizes a recombinant human adenovirus type 26 (rAd26) vector. It is the first dose of the Sputnik V (Gam-COVID-Vac) vaccine used as a standalone for vaccination [
21]. All these vaccines showed great potential during phase 3 trials with 77.8% efficacy for Covaxin (BBV152) [
22], 70.4% for Covishield (AZD1222/ChAdOx1) [
23], 91.6% for Sputnik V (Gam-COVID-Vac) [
24] and >90% for Corbevax (BECOV2D) (biologicale.com).
Despite the success shown by these vaccines during the clinical trials, the protection set forth by them had a waning effect. Although it is known that the absence of antibodies need not necessarily correspond to the absence of immune memory, a considerable amount of breakthrough cases were observed in many of the vaccinated cohorts, though with less disease severity [
25,
26,
27,
28]. This is partly linked to the immune evasion ability of the virus due to a high mutation rate observed in SARS-CoV-2 genome. However, the comprehensive data regarding the immunological aspects of this inadequate protection are limited. Moreover, limited in-depth studies have been conducted regarding the vaccines given in India despite having one of the most extensive vaccine drives in the world. The study of the long-term effects of immunological memory imparted by vaccines given in India is important to understand how immunological memory is established.
In this study, we chose the above-mentioned vaccines differing in either their development platform or dose regimen to study the different aspects of the antigen specific B cell immune response three months after vaccination. Memory B cells are made to quickly proliferate and differentiate into antibody secreting cells upon re-exposure to the pathogen [
29]. Our readouts were based on the Receptor Binding Domain (RBD) of the Spike protein of the SARS-CoV-2 Wuhan strain. A corollary is that immune response against RBD has been shown to be dominant and correlates well with the virus neutralization [
14,
30,
31,
32]. We compared the memory B cell response generated by the above-mentionedvaccines in the peripheral circulation of these individuals and the ability of these cells to proliferate and secrete anti-RBD antibodies. We observed a noticeable effect by Corbevax and Sputnik Light vaccines on the ASC response of the vaccinated participants in comparison to the unvaccinated individuals. Some of our findings were also corroborated by the studies on the T cell response by other groups [
20,
33].
Materials and Methods
Ethics Statement
This study was conducted at the BRIC-Translational Health Science and Technology Institute, Faridabad, India. Participant enrolment and sample collection were done at ESIC Medical College and Hospital, Faridabad. Written informed assent/consent was obtained from each of the study participants. This study was approved by the Institutional Ethics Committees from both institutes (ESIC Hospital and Medical College, Faridabad File no._134 X/11/13/2021- IEC/43 and BRIC-THSTI Faridabad Ref No: THS 1.8.1/ (130) dated 27th Oct 2021).
Participant Details
Samples were collected from a total of 180 participants and their age, sample collection date, and vaccination details were recorded. The participants were of the following categories- Unvaccinated - 30 participants and Vaccinated (Corbevax, Covaxin, Covishield, and Sputnik Light; 30 participants from each group except Sputnik Light where we collected samples from 27 participants) (
Table 1). Blood samples were collected in the Greiner Bio-One tubes (Cat. No. 22-040-134) for the isolation of PBMCs and plasma.
Indirect Anti-RBD IgG ELISA
96-well Maxisorp plates (Thermo Fisher Scientific- Cat No. 442404) were coated with 100 µl of RBD protein (2 µg/ml in PBS) per well and incubated overnight at 4°C. Plates were washed with PBST (PBS + 0.1% Tween 20) and blocked for two hours at 37°C with the block buffer (PBST + 3% skimmed milk). After incubation, the block buffer was discarded, and the diluted Plasma samples 100 µl per well were added in duplicates. Plasma samples were diluted in PBST + 3% skimmed milk at a ratio of 1:The plate was incubated for 30 minutes at 37°C. After that, the plate was washed with PBST and secondary antibodies were added 100 µl per well. Goat anti-human IgA HRP-conjugated (Southern Biotech 2050-05; 1:5000 dilution), Goat anti-human IgG HRP-conjugated (Jackson Immunoresearch 109-035-088; 1:10000 dilution) and Goat anti-human IgM HRP-conjugated (Jackson Immunoresearch 109-035-129; 1:10000 dilution)] were used as secondary antibodies. The plate was incubated for 30 minutes at room temperature and then washed with PBST. TMB substrate was added in the dark and incubated for 3 minutes. The reaction was stopped with 1N H
2SO
4 before measuring the optical densities at 450 nm using a microplate reader [
34].
Expression and Purification of the RBD Protein of SARS-CoV-2 (Wuhan Strain-Hu-1)
The following reagent was contributed by David Veesler for distribution through BEI Resources, NIAID, NIH: Vector pcDNA3.1(-) Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Spike Glycoprotein Receptor Binding Domain (RBD), NR-52422as mentioned previously [53,54]. The recombinant his-tagged SARS-CoV-2 ancestral Wu-RBD protein was expressed in transiently transfected Expi293F cells in suspension culture using Expifectamine Transfection kit (Thermo Fisher Scientific, Cat no. A14524) as per manufacturer protocol. Post-transfection and expression, cell culture supernatants were harvested after 5–6 days or until cells showed more than 60% cell death. The supernatant was passed through a Ni–NTA column for protein purification. Bound proteins were eluted with 500 mM imidazole and were concentrated with an Amicon 10 kDa filter (Millipore), protein fractions were aliquoted and stored at −80 °C.
Labeling of RBD Protein with the Alexa Fluor-488
Purified RBD was tested using SDS PAGE, and was taken for the fluorophore labeling at a concentration of 1 mg/ml. We labeled RBD protein using Alexa Fluor Microscale Protein Labeling kit (Cat No. #A30006; Thermo Fisher Scientific) as per the manufacturer’s instructions. Fluorophorelabeled RBDs were aliquoted and stored at -20 °C for further use.
Estimation of Memory B Cells in the Peripheral Circulation
To estimate the percentage of RBD-specific memory B cells in the peripheral circulation of the study participants, a flow-cytometry-based method was utilized [
14]. Alexa Fluor-488 labelled RBD was used as a probe for estimation of antigen specific memory B cells. Briefly, 1.5-2.5 x 10
6 cells were labelled with the antibodies against different human B cell markers which included markers for memory phenotype as well (
Table 2). Samples were acquired on Canto II Flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software (FlowJo LLC).
Estimation of Antibody Secreting Cells
Antigen specific antibody secreting cell estimation was performed as described by Crotty et al., 2004 with slight modifications. Peripheral blood mononuclear cells (PBMCs) and blood plasma were isolated from blood samples of the study participants and stored for later use [
29]. For culturing, fresh stimulant media was prepared by adding polyclonal stimulants to complete RPMI medium. Thawed PBMCs were washed with complete RPMI medium, counted and cultured at a density of 3 million cells per sample, and added at 0.5 million cells per well in a 24-well plate. An additional 0.5 million cells were cultured in a medium without stimulants as a control. Polyclonal stimulation reagents used were Protein A from Staphylococcus aureus (Sigma Aldrich P7155), Lectin from Phytolacca americana (pokeweed) (L9379), and ODN 2006 (TLR GRADE
®) (synthetic) (Enzo Life Sciences- ALX-746-056-M001). The culture plate was maintained in a CO
2 incubator for 5 days at 37°C.
An ELISpot plate (Sigma Aldrich-MSIPS4510) was activated with 35% Ethanol. After washing with PBST (PBS + 0.05% Tween 20) and PBS respectively, the plate was coated with polyvalent Goat anti-human Ig mix (Thermo Fisher Scientific - H17000) and SARS-CoV-2 spike RBD at a concentration of 10 μg/ml in PBS. The plate was incubated overnight in the dark at 4°C. Following coating, the plate was washed and blocked with 200 µl of blocking buffer per well for at least 2 hours at 37°C or overnight at 4°C. After culturing for 5 days, cells were washed and seeded onto the ELISpot plate (Figure 3). Subsequent steps included incubation with secondary antibodies [(Goat Anti-human IgA secondary antibody, Biotin (A18785), Goat Anti-human IgG Fc Bio Affinity (Thermo Fisher Scientific - A18821), Goat Anti-human IgM secondary antibody, Biotin (Thermo Fisher Scientific - PA1-86071)], Avidin-D-HRP (Thermo Fisher Scientific 18-4100-51), and substrate solution. The substrate was prepared by mixing 110 µl of AEC-DMF solution (3-Amino-9-Ethylcarbazole in Dimethyl Formamide, 60 mg/mL) with 10 mL of 0.1 M sodium acetate and adding 165 µl of H2O2 [Sigma Aldrich (323381)] after filtering the solution. The plate was incubated, rinsed gently with tap water, and dried overnight in the dark.
Spot counting was performed using the Auto-counter feature of the ImmunoSpot 7.0.36.0 device with CTL software, which visualized and recorded the number of spots in each well. Quality control was performed to check for any errors in the counting and corrections were made accordingly. The average of the spot-forming units (SFUs) from the unstimulated control wells was deducted from each of the SFU values.
Statistics
All the data visualization and statistical analyses were done using GraphPad Prism 9.5.Kruskal-Wallis’ test and Dunn’s multiple comparison test were used for statistical analysis. P value <0.05 was considered statistically significant.
Results
All Participants Including Unvaccinated Individuals Showed Marked Levels of Anti-RBD IgG Antibodies
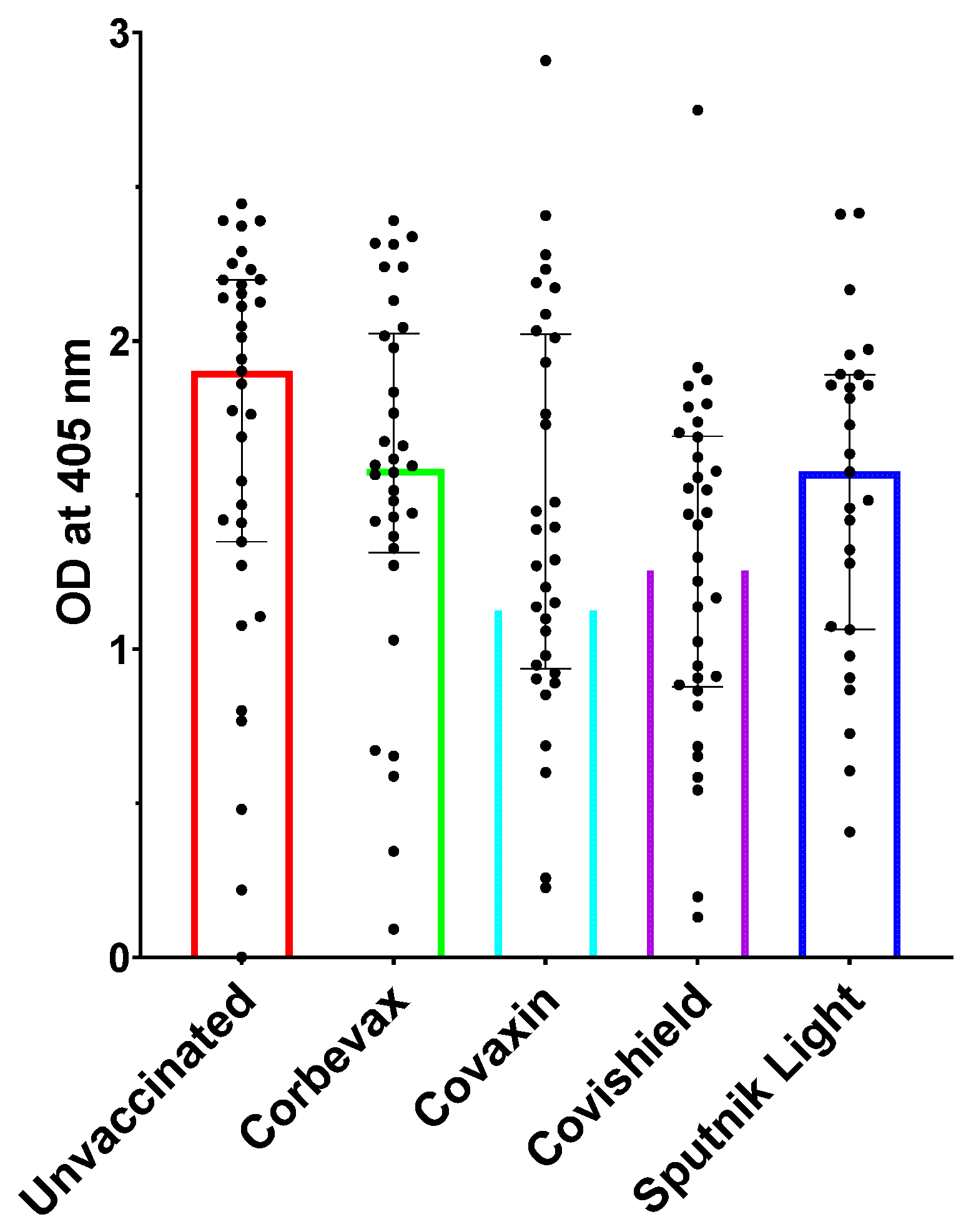
We tested the IgG antibody levels of the participants in our study using RBD-specific IgG ELISA [
34]. Vaccinated participants from all four vaccines showed a remarkable IgG response towards SARS-CoV-2 RBD. This indicated that the vaccinations led to the development of a distinct humoral response against the virus (
Figure 1). However, the unvaccinated participants also showed an elevated RBD-specific IgG response, which suggests that the unvaccinated participants have beenexposedto SARS-CoV-The blood samples for this study from unvaccinated participants were collected during Omicron surge in India; it is likely that the participants contracted the virus during this period. The presence of IgM antibodies post-recovery phase for a prolonged time among these participants highlights the importance of these multimeric antibodies in tackling the virus. Similar observations have also been made in certain other viral infections [
12,
35]. This is probably because of the high avidity of these antibodies towards the antigens.
Study Participants Exhibited RBD-Specific Memory B Cells in Their Peripheral Circulation
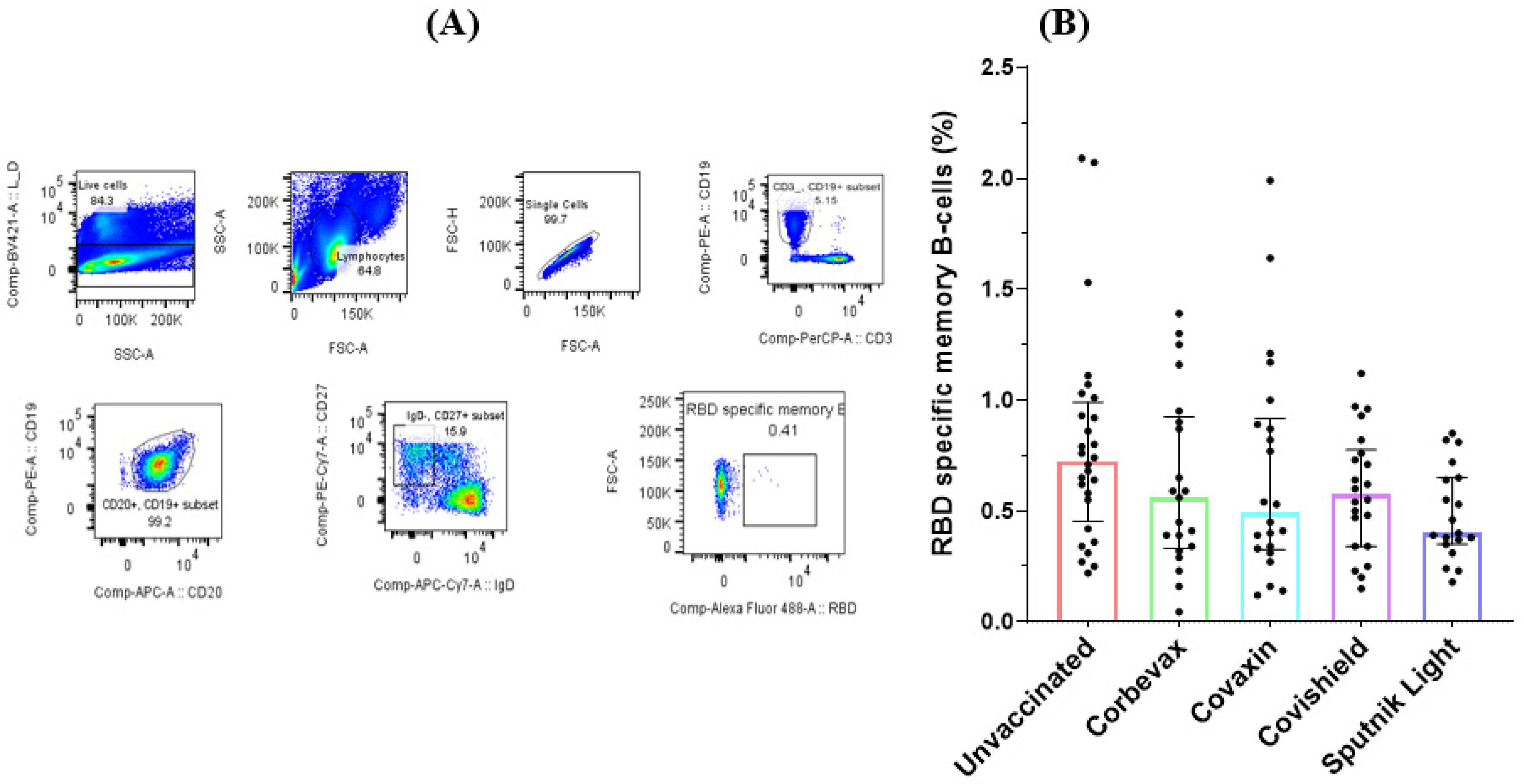
Our analysis of the memory B cell responses to the SARS-CoV-2 RBD in the peripheral blood of the participants yielded mixed results. Flow cytometry analysis on peripheral blood mononuclear cells (PBMCs) from 118 participants (90 vaccinated and 28 unvaccinated) revealed a higher percentage of RBD-specific memory B cells in the unvaccinated group compared to any of the vaccinated groups. This is probably because of the recent natural exposure of the unvaccinated participants to the pathogen at the time when the Omicron variant swept the country (
Figure 2). Although we recruited these unvaccinated participants based on theirasymptomatic profile during the last three months, they showed a good antibody response against SARS-CoV-2 RBD, suggesting a recent asymptomatic exposure. All the participants of vaccinated groups showed comparable percentages of RBD specific memory B cells. As observed for the RBD specific antibody levels, the group of unvaccinated participants had a slightly higher amount of RBD specific memory B cells as compared to all the vaccinated groups, possibly impelled by the recent exposure to the virus. However, there was no statistically significant difference seen among participants of vaccinated groups when compared with that of the unvaccinated participants.
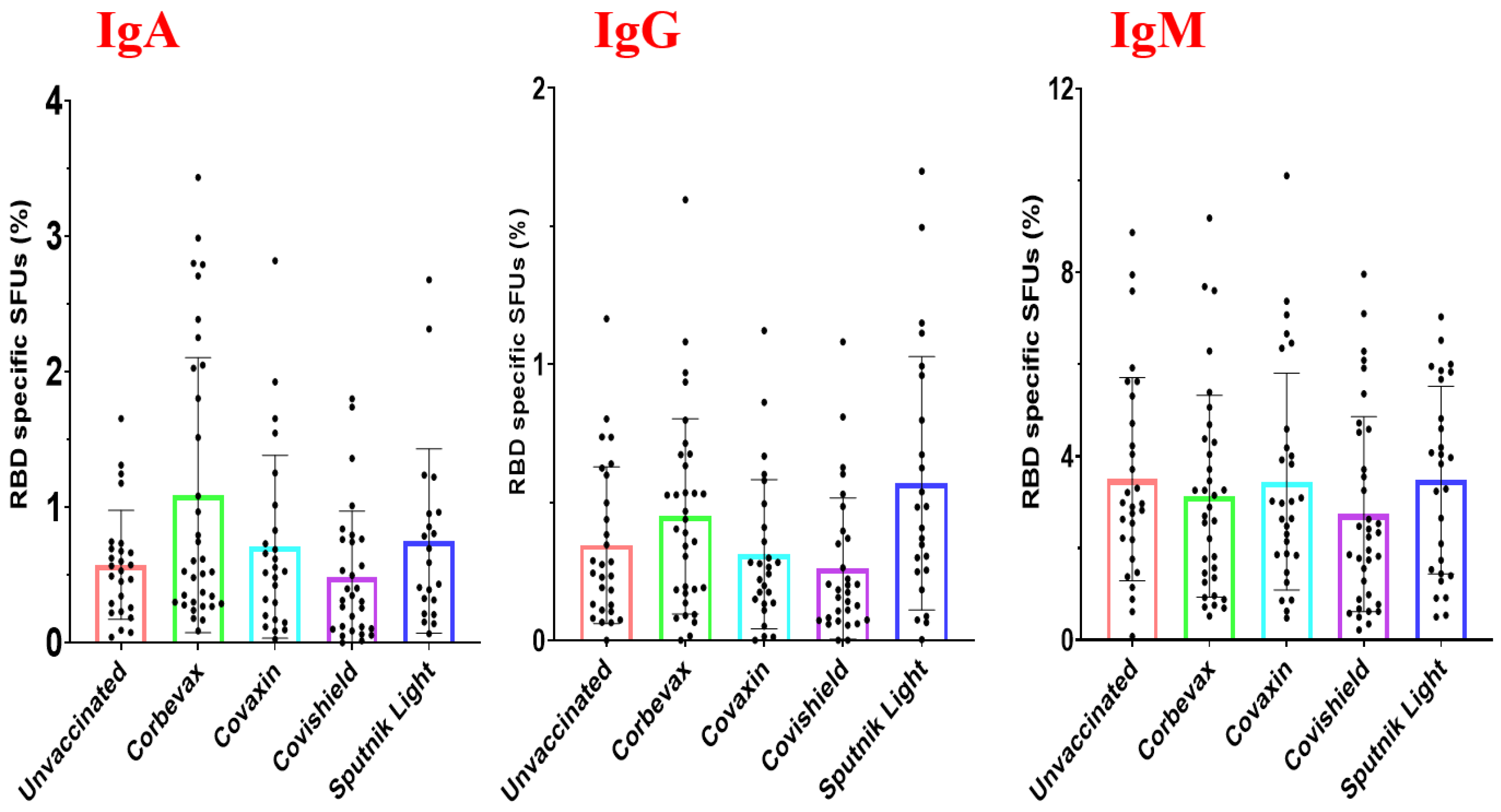
Magnitude of the RBD-Specific Antibody-Secreting B Cells among Vaccinees from Different Vaccine Groups
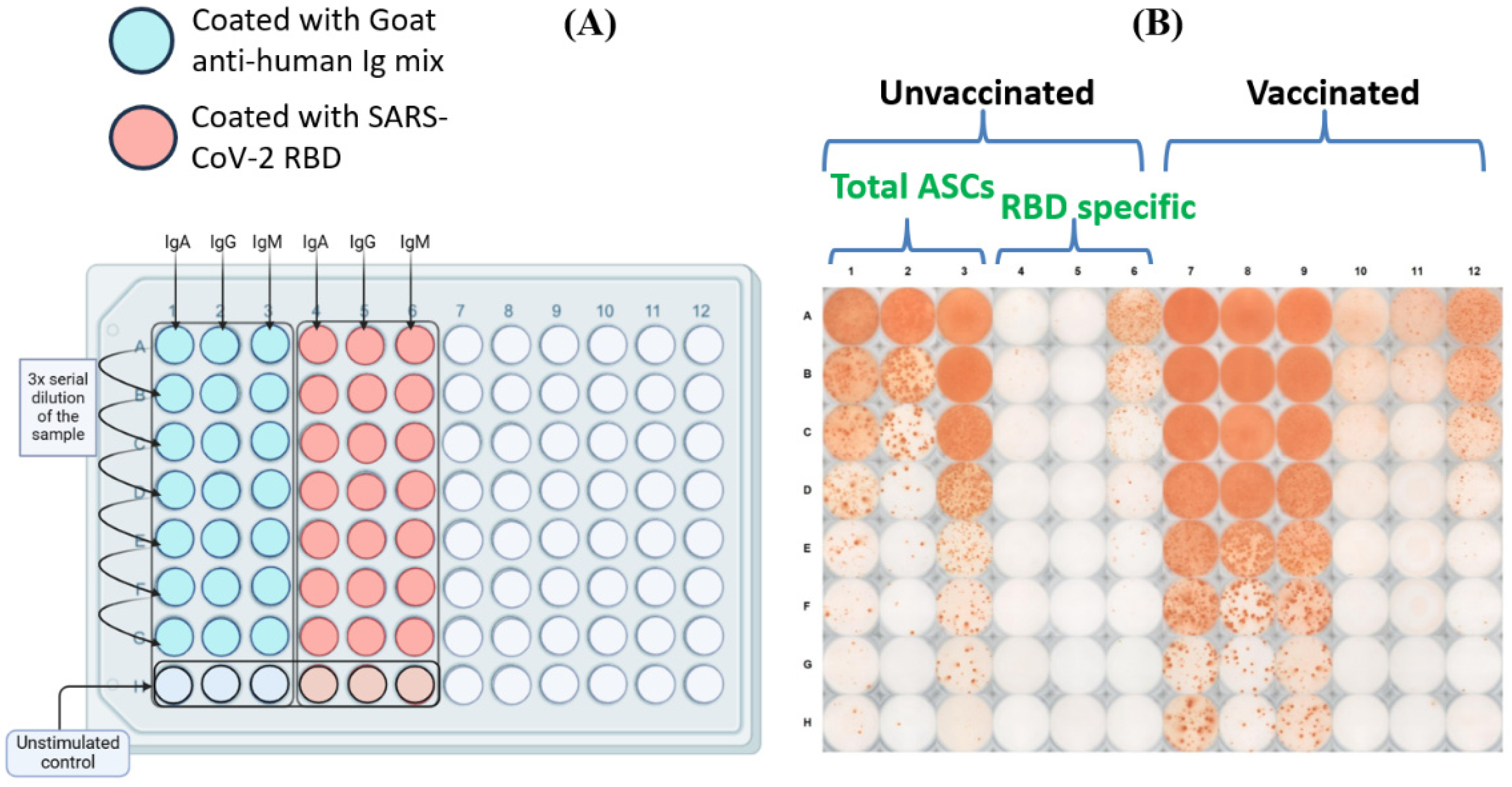
To test the magnitude of the RBD-Specific antibody-secreting B cells among vaccinees from different vaccine groups, we did polyclonal stimulation of the PBMCs obtained from these participants and tested the ability of the B cells to proliferate and secrete antigen specific antibodies [
29]. The schematic diagram of the plate map and a representative image of the B cell ELISPOT plate have been shown in
Figure 3A,B. Our data revealed a distinct distribution of RBD-specific IgM, IgA and IgG antibody secreting B cells among the participant samples. The percentage of RBD-specific IgM antibody secreting B cells was higher than that of IgA and IgG antibody secreting B cells across all the analyzed groups (
Figure 4). However, the percentage of RBD-specific IgM antibody secreting B cells wasmore or less similar across all the groups. The median percentage of IgA and IgG antibody secreting B cells were slightly higher among participants vaccinated with the Corbevax and Sputnik Light groups as compared to the other groups including the unvaccinated participants. There was no statistically significant difference between groups because of a considerable variability within the ASC as evident from
Figure 4. The increase in the median percentage of IgA and IgG antibody secreting B cells among the participants of Corbevax and Sputnik Light groups as compared to the unvaccinated participants is of interest which showed lower RBD specific IgG antibodies as compared to those of the unvaccinated participants in the plasma samples and RBD specific memory B cells in the peripheral circulation- a reversal in the pattern. Of note, a single dose of the Sputnik Light vaccine mounted a marked level of the RBD specific IgG antibody secreting B cells at par with the other vaccines.
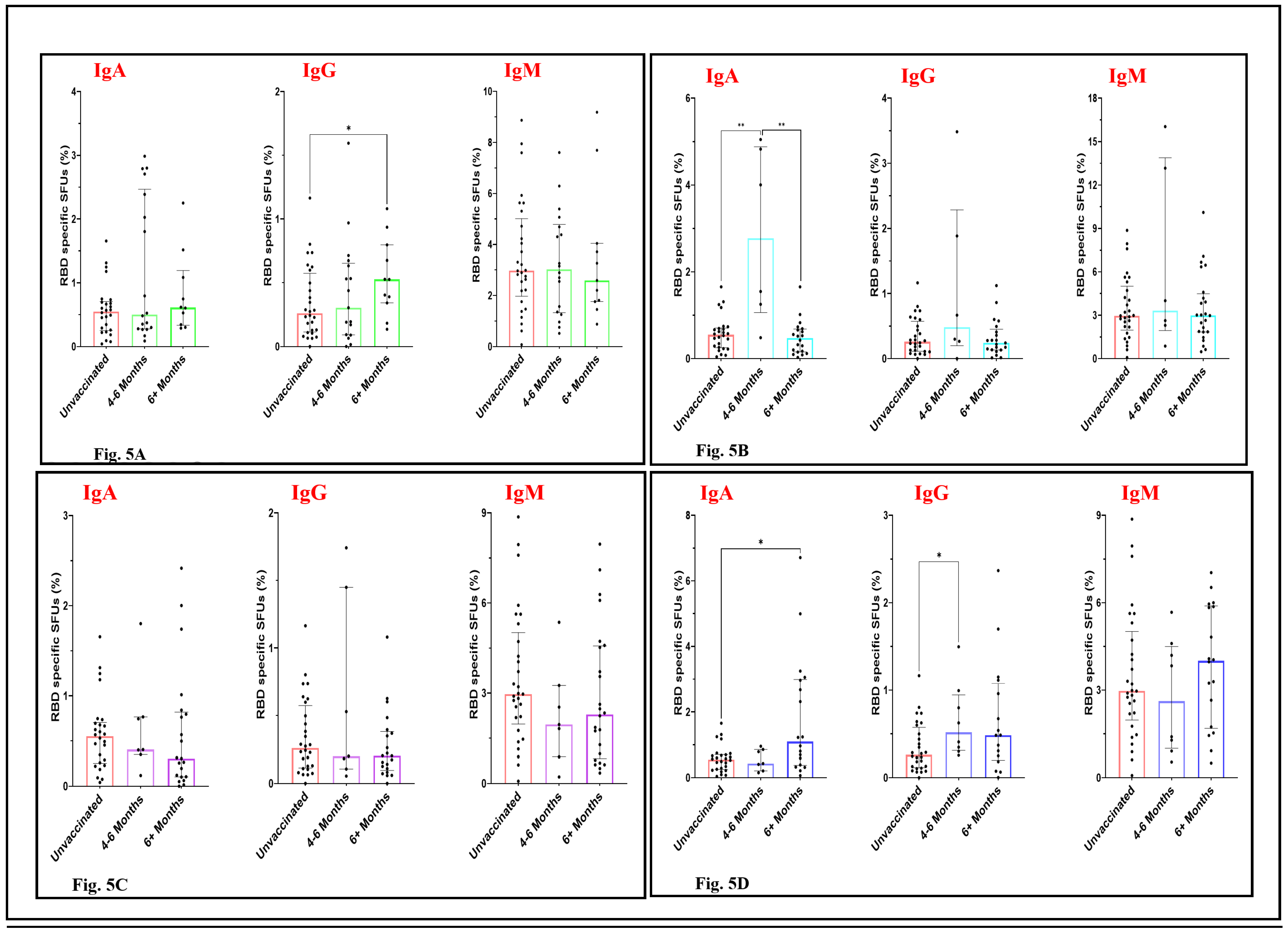
Temporal Patterns in the RBD-Specific Antibody-Secreting B Cells Post-Vaccination
To test the durability of the ASC response of the participants of different vaccine groups, we categorized the samples of each vaccination group by 4-6 months and beyond 6 months post vaccination. The levels of IgA and IgM-secreting B cells among the Corbevax-immunised participants, did not differ significantly from the participants in the unvaccinated group as well as between 4-6 months and beyond 6 months post vaccination groups (
Figure 5A). However, individuals who had been vaccinated with Corbevax for more than 6 months before sample collection showed a statistically significant increase in RBD specific IgG ASC response as compared to the unvaccinated participants suggesting a better affinity maturation of IgG antibodies among these participants over time (
Figure 5A). IgM ASC response after Covaxin administration remained more or less similar to that of the unvaccinated group. However, there is an increase in the median RBD specific IgA and IgG SFUs at an early time point with a huge inter-individual variation (
Figure 5B). Although we can’t make any conclusion based on the above result as the number of samples were less in the 4-6 months post vaccination group. Participants immunized with Covishield did not show a noticeable trend in the memory B cell immune response for any of the antibodies. The percentage of RBD-specific antibody-secreting B cells remained similar for 4-6 months and afterwards in these participants (
Figure 5C). Individuals who received the Sputnik Light vaccine displayed an interesting temporal pattern in the levels of RBD-specific antibody-secreting B cells (
Figure 5D). An enhancement of RBD-specific antibody-secreting B cells can be seen in the samples collected 6 months post-vaccination. IgG responses peaked early and remained consistent among the 6 months post vaccination group participants. However, IgA ASC response also enhanced over the time, no significant difference was observed for IgM specific ASC response in these participants although the median SFU percentage increased among the samples collected after the 6 months post vaccination. This observation suggests the development of an enduring and broader antibody response over time following Sputnik Light vaccination. A restraint on the precise conclusion over the durable impact of different vaccines on ASC responses is the smaller number of the samples in the 4-6 months time window among participants vaccinated with Covaxin, Covishield and Sputnik Light vaccines.
Discussion and Conclusions
Understanding the immune memory response helps us in getting insights into the longevity of the protection against a pathogen. The memory response of T and B cells and theirfunctionality are shaped not only by the antigens of a pathogen and their presentations but also by the interactions between these two cell types besides others [
36]. The B cell memory phenotype is generated as a result of multiple iterations of antigen-based selection of antibody-secreting B-cell populations [
37]. The instability of the viral genome and the resulting mutations in the antigens, mainly the Spike protein in case of the SARS-CoV-2, diminishes the impact of B-cell memory response during a reinfection or a breakthrough infection [
38]. The efficacy of various vaccination platforms against SARS-CoV-2 and natural infection in conferring immunity has been a subject of extensive research and debate [
19]. It has been shown that MBC responses were more pronounced when individuals experienced a natural infection followed by a single vaccine dose as opposed to vaccination alone [
39]. These studies show the impact of various vaccines on generating immune memory, or enhancing the one already produced as a result of a natural infection.
We observed that the unvaccinated individuals also exhibited antibody response as well as memory B cells in their peripheral circulation with an ability to proliferate. This is probably due to the asymptomatic infections they might have had as these samples were collected during the peak of the Omicron wave in the country. Studies regarding memory B cells in the peripheral circulation and the estimation of ASCs have been done in the BNT162b2 vaccinated and naturally infected individuals. The generation and viability of the MBCs have been linked to the severity of the disease, where moderate to severe infections have resulted in a better B cell recall response. It has been noted in several studies that the overall antibody titers decline rapidly a few months after infection and vaccination sub-sequential a peak in the initial days [
40,
41,
42]. Few studies have been performed to gain insight into the protection provided by memory B cells mostly for the mRNA-based vaccines [
43,
44,
45,
46,
47]. Some studies carried out with a cohort of BNT162b2 vaccinees found that there is indeed an increase in the amount of memory B cells 8 months after the second dose and an even better increase after a booster dose [
48,
49]. Another study by Nayak et al., 2021 observed that there was a positive correlation between RBD-specific memory B cells and RBD-specific IgG titers while also mentioning that the individuals with a low amount of neutralizing antibodies also had a low amount of memory B cells in their peripheral circulation [
14]. Various aspects of immunological memory produced by BNT162b2 vaccination against SARS-CoV-2 have been widely studied.
In this study, we evaluated the memory B cells induced by vaccines in India where the BNT162b2 vaccine was rarely administered. The blood samples particularly of the unvaccinated groups were collected during the Omicron surge in India and it is likely that these unvaccinated participants were exposed to the virus. Despite this limitation, the results give us a glimpse into the potential complexity of the B cell immune response as among the vaccinated groups, the amount of ASCs shows variation from vaccine to vaccine. BECOV2D and Gam-COVID-Vac showed promising results with slight increase in the number of ASCs six months after vaccination- similar to what was previously observed in BNT162bOn the other hand, though BBV152 and AZD1222 induced a considerable amount of ASCs after vaccination, they did not show any significant change in the B cell recall response over time. To summarize, vaccines induced a B-cell recall response similar to a mild infection but its temporal dynamics depended on the specific vaccine. These findings highlight the complex interplay between vaccination, prior infection and RBD-specific memory B cell generation.
Although the ASCestimation is tedious to perform and takes almost a week to the results, one needs to study this on a larger sample size for better understanding. Larger sample size would have also helped in categorizing the ASC response in different time intervals and making comparison among these vaccines. A limitation in our study is the exposure status of the unvaccinated participants. It would have been better to have PBMCs and blood plasma collected from the people before the pandemic to have an actual comparison between the vaccinated and unvaccinated groups. We observed a great deal of interindividual variation among these responses with heterogeneity at the level of RBD-specific memory B cells in the peripheral circulation and also secretion of antigen-specific antibodies; our study is one among the few done to understand the memory B cell response after vaccination against COVID-19 [
50,
51]. Particularly, this aspect of evidence-based understanding was lacking for the vaccines given to Indians. The study of the memory B cell response is essential in understanding the long-term protection offered by vaccinations. The memory response of the B cells mounted by the vaccines to respond to an infection, its durability, and effectiveness play a key role in preventing the recurrence of the disease. This knowledge could also help in making policy decisions by the Government bodies, use of a particular vaccine development platform or whether we need a booster vaccination for such infectious diseases.
Our study highlights the immune memory response, particularly the B cell memory response generated after vaccination against COVID-The levels of memory B cells generated by vaccination, or the natural infection serve better for the immune correlate of protection than the antibody levels in the blood circulation [
52]. Probably due to asymptomatic exposure by circulating Omicron variants in the Indian community during the time when participants’ samples were collected, even the unvaccinated individuals exhibited high levels of RBD-specific IgG antibodies. They also showed the presence of RBD-specific B cells in their peripheral circulation. However, the vaccinated people, especially those vaccinated with Corbevax and Sputnik Light showed better response in terms of secreting RBD-specific antibodies after polyclonal stimulation. This suggests an effective and durable B cell memory response generated by these vaccines. This study is unique in a way that can provide tailored and more equitable vaccination strategies based on the understanding of the durability of the memory response of the B cells, its ability to proliferate and secrete antigen specific antibodies.
Author Contributions
Atharv Athavale- performed the experiments, made tables and figures, wrote, reviewed and edited the different versions of the manuscript; Anmol Gaur and Nafees Ahmed- collected samples and performed the experiments; Adarsh Subramaniam, Jyotsana Dandotiya and Sneha Raj- performed the experiments; Santosh K. Upadhyay- edited and reviewed the different versions of the manuscript; Sweety Samal- provided RBD reagent, reviewed the manuscript; Anil Kumar Pandey- provided samples for the study, reviewed the different versions of the manuscript; Ramesh Chandra Rai- conceptualized the study, wrote the manuscript, reviewed and edited the different versions of the manuscript, tables and figures; Amit Awasthi- conceptualized the study, and reviewed the different versions of the manuscript; All authors read and approved the final version of the manuscript.
Data Availability Statement
Authors will provide data keeping the participants details confidential and as per the journal policy.
Acknowledgments
Authors wish to acknowledge BRIC-THSTI, Faridabad for intramural funding support to this work. We would like to acknowledge Dr. Sankalp and Dr. Rohit Dhaka from ESIC Medical College and Hospital, Faridabad for their help in participant enrollment. We also thank Amit Kumar Yadav, Aftab Hussain, Richa Kumari, Deepak Rathore, Akshay Binayake, Aymaan Jaheer, Manas Ranjan Tripathy and Jitender Chandilla, Immunobiology and Immunotherapy laboratory, BRIC-THSTI, Faridabad for the suggestions and help. We are thankful to the participants for donating their blood samples which made this study feasible. We are also thankful to the BEI resources for providing the RBD-Wuhan plasmid NR-The figure for the graphical abstract is created by using BioRender.com.
Conflicts of Interest
Authors declare no known competing financial interests that could have influenced this work.
References
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat Rev Immunol 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. The Success of SARS-CoV-2 Vaccines and Challenges Ahead. Cell Host & Microbe 2021, 29, 1111–1123. [Google Scholar] [CrossRef]
- Pušnik, J.; König, J.; Mai, K.; Richter, E.; Zorn, J.; Proksch, H.; Schulte, B.; Alter, G.; Streeck, H. Persistent Maintenance of Intermediate Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. J Virol 2022, 96, e0076022. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Tarlinton, D. B Cell Memory: Understanding COVID-Immunity2021, 54, 205–210. [CrossRef]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B Cells. Nat Rev Immunol 2015, 15, 149–159. [Google Scholar] [CrossRef]
- Inoue, T.; Moran, I.; Shinnakasu, R.; Phan, T.G.; Kurosaki, T. Generation of Memory B Cells and Their Reactivation. Immunological Reviews 2018, 283, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br J Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The Multifaceted Role of CD4(+) T Cells in CD8(+) T Cell Memory. Nat Rev Immunol 2016, 16, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.-K.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, M.; Obara, M.; Chiyyeadu, A.; Costa, B.; Salam, A.; Ziegler, A.; Waltl, I.; Pavlou, A.; Bonifacius, A.; Hoffmann, M.; et al. Memory B Cells Anticipate SARS-CoV-2 Variants through Somatic Hypermutation. J Infect 2024, 88, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.-L.; Sam, I.-C.; Chiam, C.-W.; Chan, Y.-F. The Neutralizing Role of IgM during Early Chikungunya Virus Infection. PLoS ONE 2017, 12, e0171989. [Google Scholar] [CrossRef] [PubMed]
- Lizeng, Q.; Nilsson, C.; Sourial, S.; Andersson, S.; Larsen, O.; Aaby, P.; Ehnlund, M.; Björling, E. Potent Neutralizing Serum Immunoglobulin A (IgA) in Human Immunodeficiency Virus Type 2-Exposed IgG-Seronegative Individuals. J Virol 2004, 78, 7016–7022. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Gottimukkala, K.; Kumar, S.; Reddy, E.S.; Edara, V.V.; Kauffman, R.; Floyd, K.; Mantus, G.; Savargaonkar, D.; Goel, P.K.; et al. Characterization of Neutralizing versus Binding Antibodies and Memory B Cells in COVID-19 Recovered Individuals from India. Virology 2021, 558, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A. Targeting Spike Protein: Modified Antibody for Broad-Spectrum Binding to Coronaviruses: An In Silico Study. VIJ 2023, 7, 1–15. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. A Cross-Reactive Human IgA Monoclonal Antibody Blocks SARS-CoV-2 Spike-ACE2 Interaction. Nat Commun 2020, 11, 4198. [Google Scholar] [CrossRef] [PubMed]
- Hajilooi, M.; Keramat, F.; Moazenian, A.; Rastegari-Pouyani, M.; Solgi, G. The Quantity and Quality of Anti-SARS-CoV-2 Antibodies Show Contrariwise Association with COVID-19 Severity: Lessons Learned from IgG Avidity. Med Microbiol Immunol 2023, 212, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Vadrevu, K.M.; Ganneru, B.; Reddy, S.; Jogdand, H.; Raju, D.; Sapkal, G.; Yadav, P.; Reddy, P.; Verma, S.; Singh, C.; et al. Persistence of Immunity and Impact of Third Dose of Inactivated COVID-19 Vaccine against Emerging Variants. Sci Rep 2022, 12, 12038. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Athavale, A.; Tripathi, A.H.; Subramaniam, A.; Upadhyay, S.K.; Pandey, A.K.; Rai, R.C.; Awasthi, A. To Be Remembered: B Cell Memory Response against SARS-CoV-2 and Its Variants in Vaccinated and Unvaccinated Individuals. Scandinavian Journal of Immunology 2024, 99, e13345. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.; Yerroju, V.; Mogulla, R.; Suneetha, P.V.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Adabala, S.; et al. Immunogenicity and Safety of Biological E’s CORBEVAXTM Vaccine Compared to COVISHIELDTM (ChAdOx1 nCoV-19) Vaccine Studied in a Phase-3, Single Blind, Multicentre, Randomized Clinical Trial. Hum Vaccin Immunother 2023, 19, 2203632. [Google Scholar] [CrossRef] [PubMed]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An Open, Non-Randomised, Phase 1/2 Trial on the Safety, Tolerability, and Immunogenicity of Single-Dose Vaccine “Sputnik Light” for Prevention of Coronavirus Infection in Healthy Adults. Lancet Reg Health Eur 2021, 11, 100241. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBV152: A Double-Blind, Randomised, Phase 1 Trial. The Lancet Infectious Diseases 2021, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. The Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and Immunogenicity of an rAd26 and rAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine in Two Formulations: Two Open, Non-Randomised Phase 1/2 Studies from Russia. The Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.; Gupta, A.; Meena, K.; Gaba, A.; Krishna, S.; Jyoti, R.; Aeron, N.; Prashanth, S.; Samriti; Ganapathy, U. Prevalence, Severity, and Risk Factor of Breakthrough Infection after Vaccination with Either the Covaxin or the Covishield among Healthcare Workers: A Nationwide Cross-Sectional Study. J Anaesthesiol Clin Pharmacol 2022, 38, S66–S78. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A. Curing the Pandemic of Misinformation on COVID-19 mRNA Vaccines through Real Evidence-Based Medicine - Part Journal of Insulin Resistance 2022, 5, 72. [CrossRef]
- Vallejo, A.; Vizcarra, P.; Martín-Hondarza, A.; Gómez-Maldonado, S.; Haemmerle, J.; Velasco, H.; Casado, J.L. Impact of SARS-CoV-2-Specific Memory B Cells on the Immune Response after mRNA-Based Comirnaty Vaccine in Seronegative Health Care Workers. Front Microbiol 2022, 13, 1002748. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Brokstad, K.A. Not Just Antibodies: B Cells and T Cells Mediate Immunity to COVID-Nat Rev Immunol2020, 20, 581–582. [CrossRef]
- Crotty, S.; Aubert, R.D.; Glidewell, J.; Ahmed, R. Tracking Human Antigen-Specific Memory B Cells: A Sensitive and Generalized ELISPOT System. Journal of Immunological Methods 2004, 286, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.-M.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 Neutralizing Antibody Structures Inform Therapeutic Strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, Q.; Guo, B.; Mu, D.; Lu, X.; Ma, Q.; Guo, Y.; Fang, L.; Zhang, B.; Zhang, G.; et al. A Method To Prevent SARS-CoV-2 IgM False Positives in Gold Immunochromatography and Enzyme-Linked Immunosorbent Assays. J Clin Microbiol 2020, 58, e00375-20. [Google Scholar] [CrossRef] [PubMed]
- Thuluva, S.; Paradkar, V.; Gunneri, S.R.; Yerroju, V.; Mogulla, R.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Medigeshi, G.; Singh, J.; et al. Evaluation of Safety and Immunogenicity of Receptor-Binding Domain-Based COVID-19 Vaccine (Corbevax) to Select the Optimum Formulation in Open-Label, Multicentre, and Randomised Phase-1/2 and Phase-2 Clinical Trials. eBioMedicine 2022, 83, 104217. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, F.; Chattopadhyay, S.; Thiruvengadam, R.; Yadav, S.; Kumar, M.; Sinha, S.K.; Goswami, S.; Kshetrapal, P.; Wadhwa, N.; Chandramouli Natchu, U.; et al. Development of a Fast SARS-CoV-2 IgG ELISA, Based on Receptor-Binding Domain, and Its Comparative Evaluation Using Temporally Segregated Samples From RT-PCR Positive Individuals. Front Microbiol 2020, 11, 618097. [Google Scholar] [CrossRef] [PubMed]
- Skountzou, I.; Satyabhama, L.; Stavropoulou, A.; Ashraf, Z.; Esser, E.S.; Vassilieva, E.; Koutsonanos, D.; Compans, R.; Jacob, J. Influenza Virus-Specific Neutralizing IgM Antibodies Persist for a Lifetime. Clin Vaccine Immunol 2014, 21, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Kaji, T.; Takahashi, Y.; Shimoda, M.; Rajewsky, K. Generation of Memory B Cells inside and Outside Germinal Centers. European Journal of Immunology 2014, 44, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, M.J.; Weisel, F. Germinal Center Selection and the Development of Memory B and Plasma Cells. Immunological Reviews 2012, 247, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.L.; Essigmann, H.T. Original Antigenic Sin: The Downside of Immunological Memory and Implications for COVID-19. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological Memory and Neutralizing Activity to a Single Dose of COVID-19 Vaccine in Previously Infected Individuals. International Journal of Infectious Diseases 2021, 108, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody Titers against SARS-CoV-2 Decline, but Do Not Disappear for Several Months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.-H.; Minn, D.; Lim, J.; Lee, K.-D.; Kang, Y.-M.; Choe, K.-W.; Kim, K.-N. Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines 2021, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, H.R.; Parai, D.; Chandra Dash, G.; Kshatri, J.S.; Mishra, N.; Choudhary, P.K.; Pattnaik, D.; Panigrahi, K.; Behera, S.; Ranjan Sahoo, N.; et al. Persistence of Antibodies Against Spike Glycoprotein of SARS-CoV-2 in Healthcare Workers Post Double Dose of BBV-152 and AZD1222 Vaccines. Front Med (Lausanne) 2021, 8, 778129. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Kaku, C.I.; Bergeron, A.J.; Ahlm, C.; Normark, J.; Sakharkar, M.; Forsell, M.N.E.; Walker, L.M. Recall of Preexisting Cross-Reactive B Cell Memory after Omicron BA.1 Breakthrough Infection. Sci Immunol 2022, 7, eabq3511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Raspe, R.; Johannsen, F.; Turroja, M.; Canis, M.; ElTanbouly, M.A.; Santos, G.S.S.; Johnson, B.; Baharani, V.A.; et al. Memory B Cell Development Elicited by mRNA Booster Vaccinations in the Elderly. J Exp Med 2023, 220, e20230668. [Google Scholar] [CrossRef] [PubMed]
- Morales-Núñez, J.J.; García-Chagollán, M.; Muñoz-Valle, J.F.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Rodríguez-Reyes, S.C.; Santoscoy-Ascencio, G.; Sierra García de Quevedo, J.J.; Hernández-Bello, J. Differences in B-Cell Immunophenotypes and Neutralizing Antibodies Against SARS-CoV-2 After Administration of BNT162b2 (Pfizer-BioNTech) Vaccine in Individuals with and without Prior COVID-19 - A Prospective Cohort Study. J Inflamm Res 2022, 15, 4449–4466. [Google Scholar] [CrossRef] [PubMed]
- Terreri, S.; Piano Mortari, E.; Vinci, M.R.; Russo, C.; Alteri, C.; Albano, C.; Colavita, F.; Gramigna, G.; Agrati, C.; Linardos, G.; et al. Persistent B Cell Memory after SARS-CoV-2 Vaccination Is Functional during Breakthrough Infections. Cell Host Microbe 2022, 30, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Busà, R.; Miele, M.; Sorrentino, M.C.; Amico, G.; Timoneri, F.; Miceli, V.; Di Bella, M.; Russelli, G.; Gallo, A.; Zito, G.; et al. Long-Term Effectiveness of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine on B Cell Compartment: Efficient Recall of SARS-CoV-2-Specific Memory B Cells. Int J Mol Sci 2022, 23, 15046. [Google Scholar] [CrossRef] [PubMed]
- Mise-Omata, S.; Ikeda, M.; Takeshita, M.; Uwamino, Y.; Wakui, M.; Arai, T.; Yoshifuji, A.; Murano, K.; Siomi, H.; Nakagawara, K.; et al. Memory B Cells and Memory T Cells Induced by SARS-CoV-2 Booster Vaccination or Infection Show Different Dynamics and Responsiveness to the Omicron Variant. The Journal of Immunology 2022, 209, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, E.; Del Rio Estrada, P.M.; DaSilva, J.; Boukadida, C.; Zhang, F.; Luna-Villalobos, Y.A.; Rodríguez-Rangel, X.; Pitén-Isidro, E.; Luna-García, E.; Díaz Rivera, D.; et al. Antibody and Memory B-Cell Immunity in a Heterogeneously SARS-CoV-2-Infected and -Vaccinated Population. mBio13, -22. [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451. [Google Scholar] [CrossRef] [PubMed]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.J.; O’Hehir, R.E.; van Zelm, M.C. Humoral Immunity and B-Cell Memory in Response to SARS-CoV-2 Infection and Vaccination. Biochem Soc Trans 2022, 50, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Parray, H.A.; Siddiqui, G.; Chiranjivi, A.K.; Raj, S.; Kaul, R.; Maithil, V.; Samal, S.; Ahmed, S. Biophysical and Biochemical Characterization of the Receptor Binding Domain of SARS-CoV-2 Variants. Protein J 2022, 41, 457–467. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).