1. Introduction
The pharmaceutical industry places significant value on crisis management, particularly in relation to supply chain management, as it guarantees the constant and continuous provision of vital pharmaceuticals to patients, particularly in times of emergency or unforeseen circumstances. Manufacturers, distributors, suppliers, and healthcare professionals are merely a few of the many parties involved in the intricate pharmaceutical supply chain. A pharmaceutical manufacturer must have a strong crisis management department, particularly when it comes to supply chain management. For the pharmaceutical sector to guarantee that patients receive their necessary medications and medical supplies on time, a smooth supply chain is critical. Yet, a number of crisis scenarios, including pandemics, natural catastrophes, product recalls, transportation problems, or geopolitical conflicts, might cause the supply chain to break down. It takes effective crisis management techniques to lessen the effects of these interruptions and preserve corporate continuity. Because Indian pharmaceuticals are widely available, reasonably priced, and of high quality, they have made a substantial contribution to global healthcare. Supply chain management (SCM) has proven difficult, nevertheless, because of the industry's convergence, the ever-changing ground realities, and the demands for short product lifecycles.
For public health emergencies, quantity forecasting of commodities is crucial, notwithstanding their unpredictability and associated problems. Determine which distribution flaws occurred again and which ones still exist at various phases of the epidemic. Ignoring logistical gaps and anticipated shortages has ramifications that could make the tragedy worse. Lastly, prudent communication lowers the possibility of problems or shortages at every stage of the supply chain. Preserve and carry on teaching about the significance of communication in times of crisis. It is clear that during a pandemic, flaws seem straightforward to address but actually call for a sophisticated, integrated solution. Natural disasters are a category of dangerous events that do not have human causes but endanger human life all around the planet. Most of the time, these events are unpredictable. Although the majority of natural catastrophes seem to be beyond human control, there is a great deal of control over the harm they do. This immediately relates to human pre-emptive action, specifically planning and overseeing a continuous, effective schedule to supply the necessary medicinal supplies. The three goal functions of crisis management in pharmaceutical supply chains are to reduce overall network cost, decrease unmet demand, and maximise social responsibility satisfaction.
2. Literature Review
The purpose of this study (Wahab et al., 2023) is to list, evaluate, and rank the advantages, disadvantages, and opportunities of India's pharmaceutical supply chain management environment. Using a strengths, weaknesses, opportunity, and threat (SWOT) analysis, this study identifies tactics to take advantage of the opportunities and strengths, address the weaknesses, and eliminate threats. Planning, implementing, and overseeing the supply chain's activities with the goal of meeting customer demands as effectively as possible is known as supply chain management in pharmaceutical companies. For the past few years, despite being the third-largest pharmaceutical market in the world by volume, the Indian pharmaceutical industry has faced numerous challenges that have impacted its evolution (Jain et al., 2021; Jain, Phoghat, Ajmera, & Sirvi, 2022). SCM has the potential to revolutionize the pharmaceutical industry by enabling companies to more effectively utilize resources and services, optimize revenue, enhance shareholder value, and aggressively address the needs of customers (Desingh & R, 2021; Hosseini-Motlagh et al., 2020).
By reducing the amount of information needed to improve decision-making, SWOT analysis reduces the complexity of strategic challenges and solves them effectively (Lohrke et al., 2022). Earlier researchers have demonstrated that SWOT analysis provides a platform for comprehending the intended future role and marking issues (Ab Talib & Wahab, 2021; Jain, Ajmera, & Davim, 2021; Mbazima, Mbonane, & Masekameni, 2022). Along the supply chain, raw materials, work-in-process inventory, and all completed goods are transported and stored from the point of origin to the site of consumption. Business functions are actively and purposefully managed, integrated, and controlled. According to Moniruzzaman (2015), in order to improve performance, costs, flexibility, etc., supply chain management modifies and expands the company's supply chain, all of which eventually helps customers or end users. The supply chain function encompasses a wide range of sub-domains, including inventory management, transportation, warehousing, distribution, purchasing and procurement, operations, logistics, and customer support. Yet, finding a common supply chain management strategy utilised by businesses is difficult, particularly in the pharmaceutical sector. The purpose of this article (Ahlqvist et al., 2022) is to investigate how supply chain risk management (SCRM) can help avoid shortages of generic medications.
It examined how supply chain risk management (SCRM) was applied in the supply chains for paracetamol in seven different nations, both before and after the COVID-19 pandemic. According to the study (Bygballe et al., 2023), conventional SCRM tactics can be applied in many contexts and for various objectives. Before the pandemic, policymakers had put in place a large number of SCRMS, but few of them were designed to reduce risks. They were ill-prepared, but they managed to avert shortages by adjusting and utilizing last-resort tactics. Researchers in supply chain management (SCM) have consistently called for studies of crises (Remko, 2020; Al-Omoush et al., 2022) as well as an understanding of the strategic requirements and multifaceted complexities of SCM for crises (Siebert et al., 2020; Moretto & Caniato, 2021). According to Remko (2020) and Raj, Mukherjee, Jabbour, & and Srivastava (2022), supply chains often face the standard supply, demand, control, and logistical concerns that come with routine flow during such times. The general scheme of the supply chain network is shown in
Figure 1. As opposed to regular business disruptions, however, a crisis results in acute supply and demand shocks, including panic buying, alterations in consumer behaviour, and acute shortages of labour and necessary resources (Dubey et al., 2021; Ozdemir et al., 2022; Raj et al., 2022).
Measures related to public health also helped achieve this goal. supply chains beyond the apparent danger of shifting consumer demand. When the epidemic arrived, they were generally ill-prepared, but they managed to avoid shortages by continuing with business as usual, changing as needed, and being willing to use last-resort tactics that weren't in line with earlier plans (Roshan et.al., 2019).
Since there were fewer cases of other infectious diseases, including the common cold or flu, as a result of social distancing, enhanced hand hygiene, and other measures, the public health measures utilised to stop the spread of COVID-19 also favorably helped to this end (Blix & Høye, 2021). According to Okeagu et al. (2020), regular training exercises for preparation programs should be implemented to maintain the existing state of preparedness response surveillance. Bolstering emergency procedures in municipal, state, and federal healthcare systems. This research (Hopp, Brown, & Shore, 2022) focuses on these three functions. It also takes demand unpredictability into account when evaluating substitutability and perishability. Because it involves human life-saving concerns and necessitates the involvement of numerous stakeholders, including pharmaceutical manufacturers, wholesalers, distributors, customers, information service providers, and regulatory bodies, the management of pharmaceutical supply chains has grown increasingly complex. There is a dearth of studies on pharmaceutical supply chains.
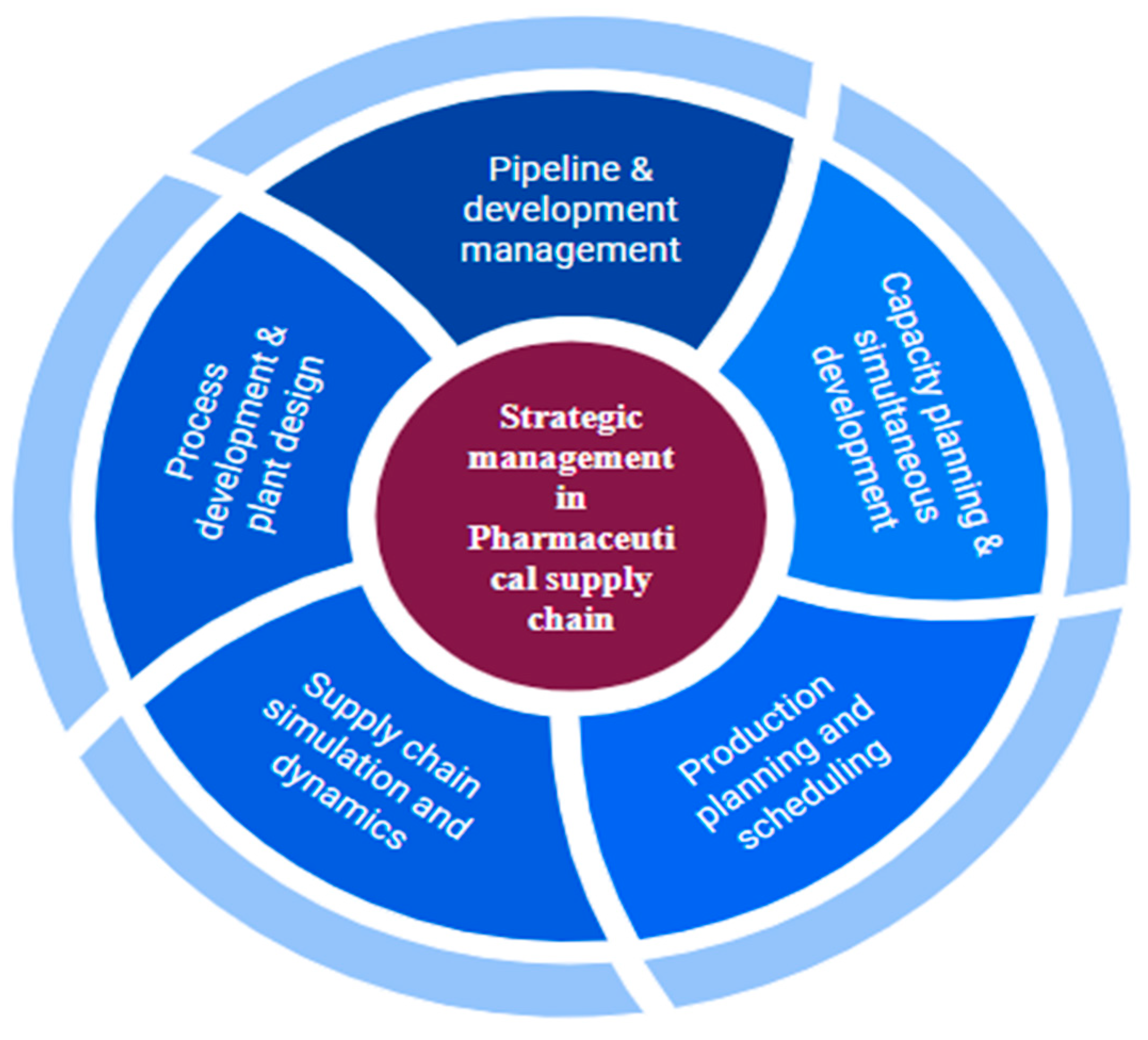
By examining research publications on several strategic concerns of supply chain management in the pharmaceutical industry, the author (Singh et al., 2016) hopes to identify gaps in the literature. They discovered that, because of the numerous intricacies in this supply chain, the pharmaceutical industry is not well studied in developing nations. However, pharmaceutical companies' proportion of the worldwide market is not particularly large. A thorough analysis of pharmaceutical supply chains has revealed research gaps in a number of areas, including reverse logistics, lean manufacturing, green supply chain management, inventory management, new product and process development, capacity planning, network and plant design, pipeline and development management, and the adoption of e-business and performance management systems. The three main categories of resources, processes, and performance are used to further categorise these strategic challenges.
3. Pharmaceutical Supply Chain
The pharmaceutical sector consists of businesses that create, produce, store, and distribute medications to wholesalers through production and importation. Usually, they collaborate with a single pharmaceutical wholesaler. The conceptual model (Whewell, 2016) used in this study shows how four aggregate notions are related to one another. (1) Crisis dynamics and related issues guided group formation and meeting frequency; (2) government agencies formed new issue-specific groups in accordance with legal mandates; and (3) government agencies and advocacy organisations played a bridging role. Pre-existing cross-sectoral structures, collaboration channels, and relationships were utilised in crisis management. Businesses need to invest in technology, create clear communication lines, monitor and manage supply chain risk, regularly evaluate suppliers, and have a strong risk management strategy in order to minimise interruptions in the pharmaceutical supply chain. Pharmaceutical firms can reduce risks and keep a dependable supply chain by adhering to the best practises and solutions.
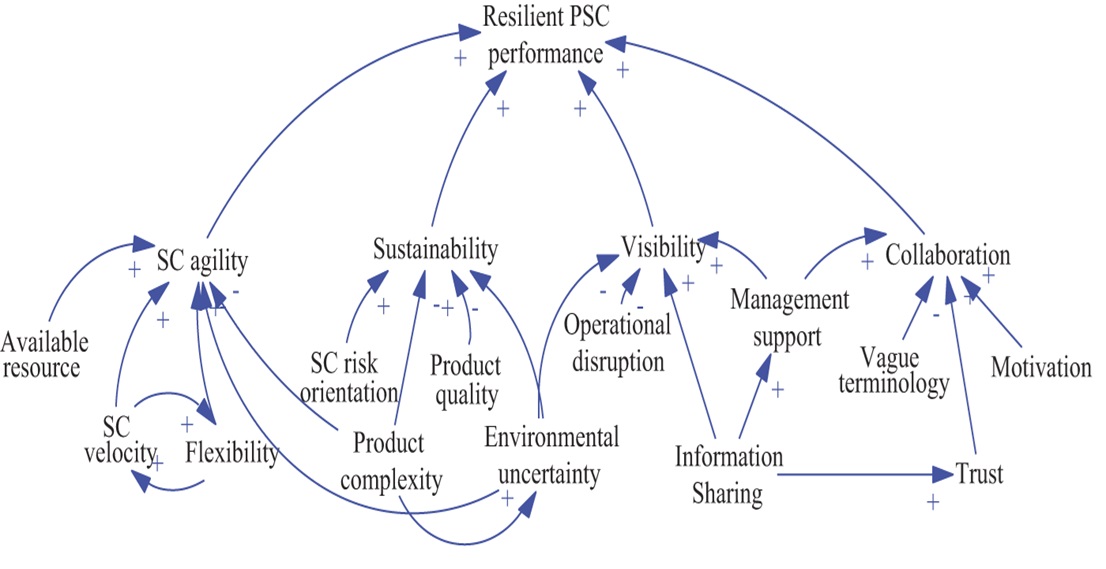
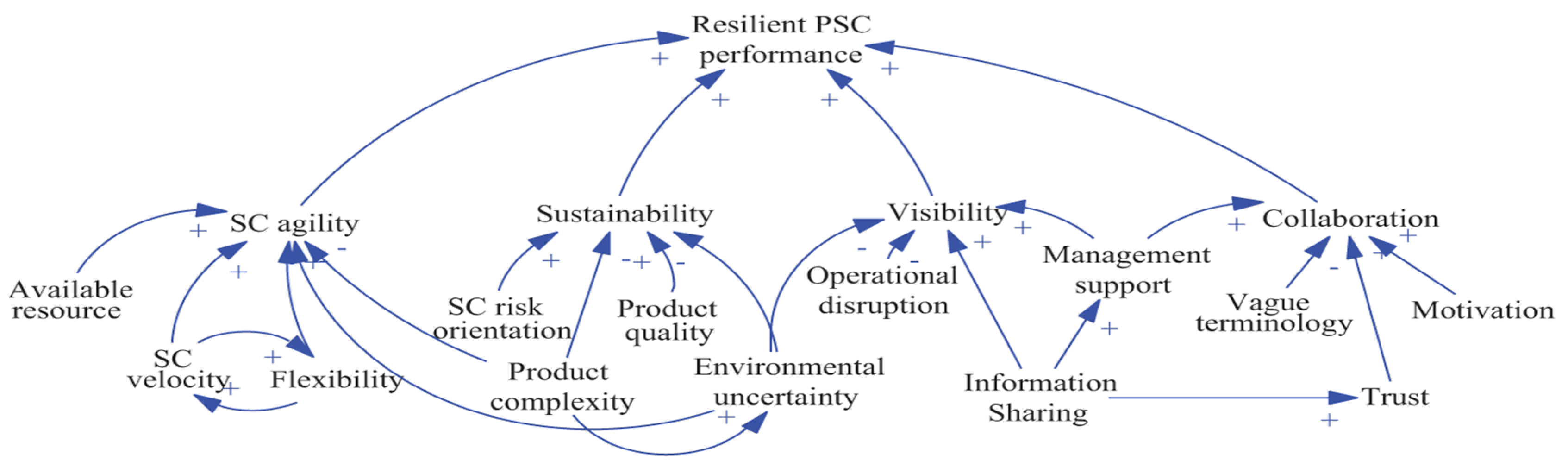
The supply chain plays a critical role in the pharmaceutical industry, ensuring the availability of drugs, medical devices, and healthcare products to consumers. Effective crisis management in the supply chain can help the pharmaceutical industry minimise the impact of unexpected events and maintain business continuity. Employees involved in supply chain management should attend regular training sessions with a focus on crisis management processes and standards (Hayes, 2023; Ergun, Hopp, & Keskinocak, 2022). Assess the preparedness and effectiveness of crisis management plans by conducting regular drills and role-plays. It's critical to diversify product sources, boost transparency, advance technology, create a proactive budgeting plan, and choose a capable emergency supply chain leader in order to reduce shortages during the pandemic. A resilient pharmaceutical supply chain is characterised by five key performance indicators: collaboration, supply chain agility, visibility, flexibility, and supply chain risk orientation (Graves et al., 2009).
Figure 1.
Loop diagram for resilient Pharmaceutical Supply Chain (PSC) performance (Chitra Lekha Karmaker, 2020).
Figure 1.
Loop diagram for resilient Pharmaceutical Supply Chain (PSC) performance (Chitra Lekha Karmaker, 2020).
IT capability, flexibility, supply chain network design, resource availability, supply chain risk orientation, and velocity were found to be in the cause category of the cause and effect connection. These factors are critical for building resilient supply chains. The supply chain's degree of resilience can be predicted using a model created by the system dynamics technique. Resilient Pharmaceutical Supply Chain (PSC) performance loop diagram as discussed by Chitra Lekha Karmaker (2020) in their research presented in
Figure 1.
Enhancing emergency protocols and providing regular training are also crucial. In an emergency involving public health, quantity forecasting is essential. It's critical to fill in logistical gaps and anticipate any shortfalls. At every stage of the supply chain, judgmental communication can lower the number of events and shortage probabilities. Deficits require a sophisticated integrative approach to be addressed (Okeagu et al., 2020). The health care sector is facing a significant issue in managing pharmaceutical inventories as they strive to improve performance and cut costs in a highly competitive economic climate. It has a lengthy history of being successfully applied in numerous industrial sectors to improve decision-making (e.g., airline, manufacturing industries, and telecommunication).
In order to establish a sustained competitive advantage, a pharmaceutical supply chain (PSC) is defined as "the integration of all operations related to the flow and transformation of medications from raw materials through to the end-user, as well as the accompanying information flows." The majority of the modern economy runs without formal contracts or data digitalization, as over 60% of employment worldwide is informal (Kaltenbach & Fath, 2022; Pinedo, 2009). For all healthcare sectors, providing excellent medical supply services and having efficient inventory management systems are critical goals.
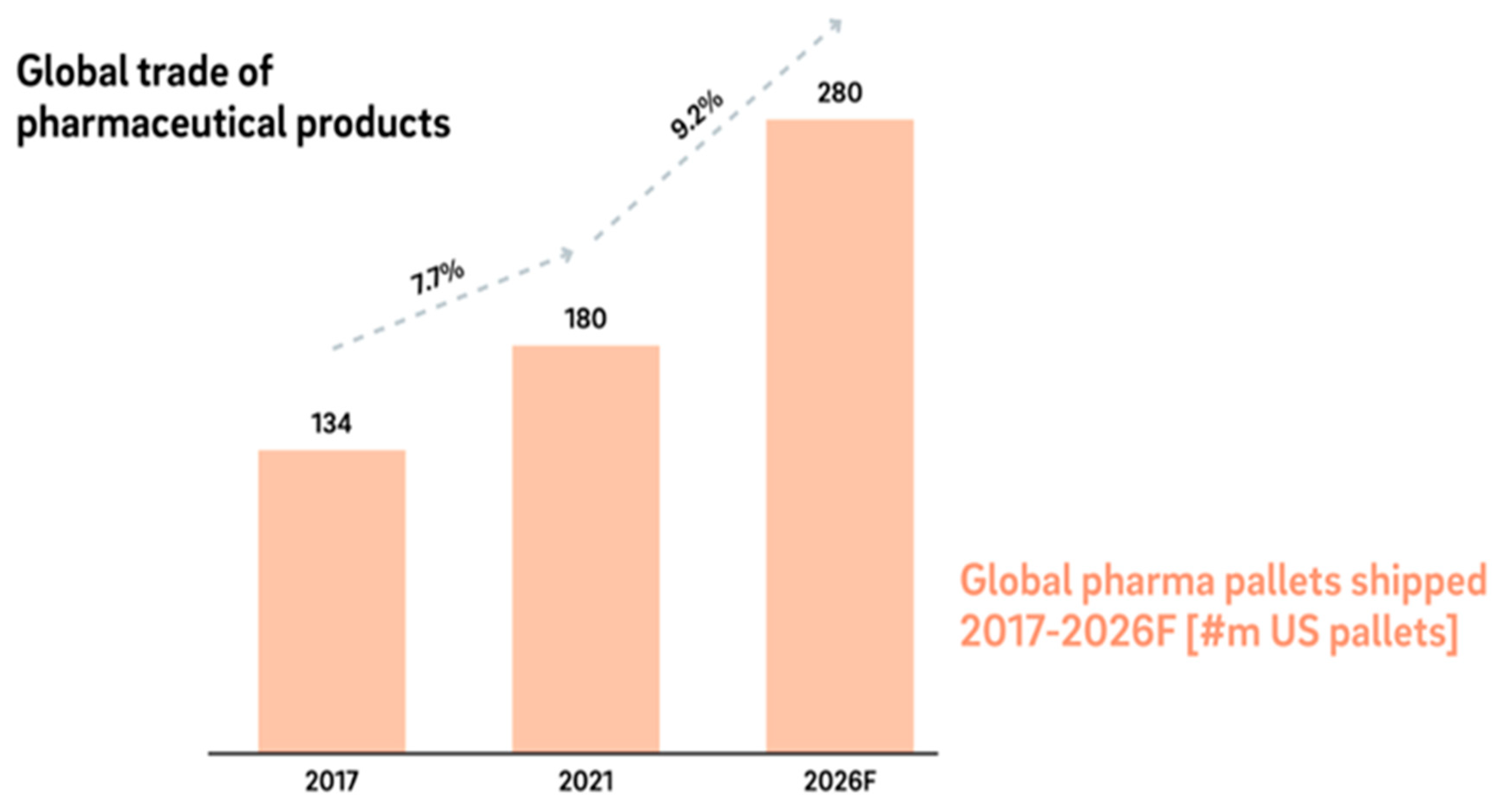
Figure 2.
Global trade of pharmaceutical products (Kaltenbach & Fath, 2022).
Figure 2.
Global trade of pharmaceutical products (Kaltenbach & Fath, 2022).
Lack of medications and inappropriate drug use can have a serious negative influence on patients in addition to causing financial losses. The management, supply, and use of medicines to promote health and save lives have not been addressed, which makes it difficult for many health systems and hospitals to meet these goals. Uthayakumar and Priyan (2013) state in their studies that it is necessary to comprehend the workings of the healthcare sector and to provide instruments for decision support that enhance patient safety, public health, health policy, and strategic decision-making in the pharmaceutical supply chain. Production facilities for pharmaceuticals are currently dispersed throughout the US, Europe, India, and China, with heavy traffic along international trade routes. Up until 2026, there will be a 9% annual growth in the global industry of pharmaceutical products shown in figure. More and more businesses are contracting out their production to other companies, frequently sourcing the "intermediates" or essential components needed to make APIs (active pharmaceutical ingredients) from a single region, most frequently China.
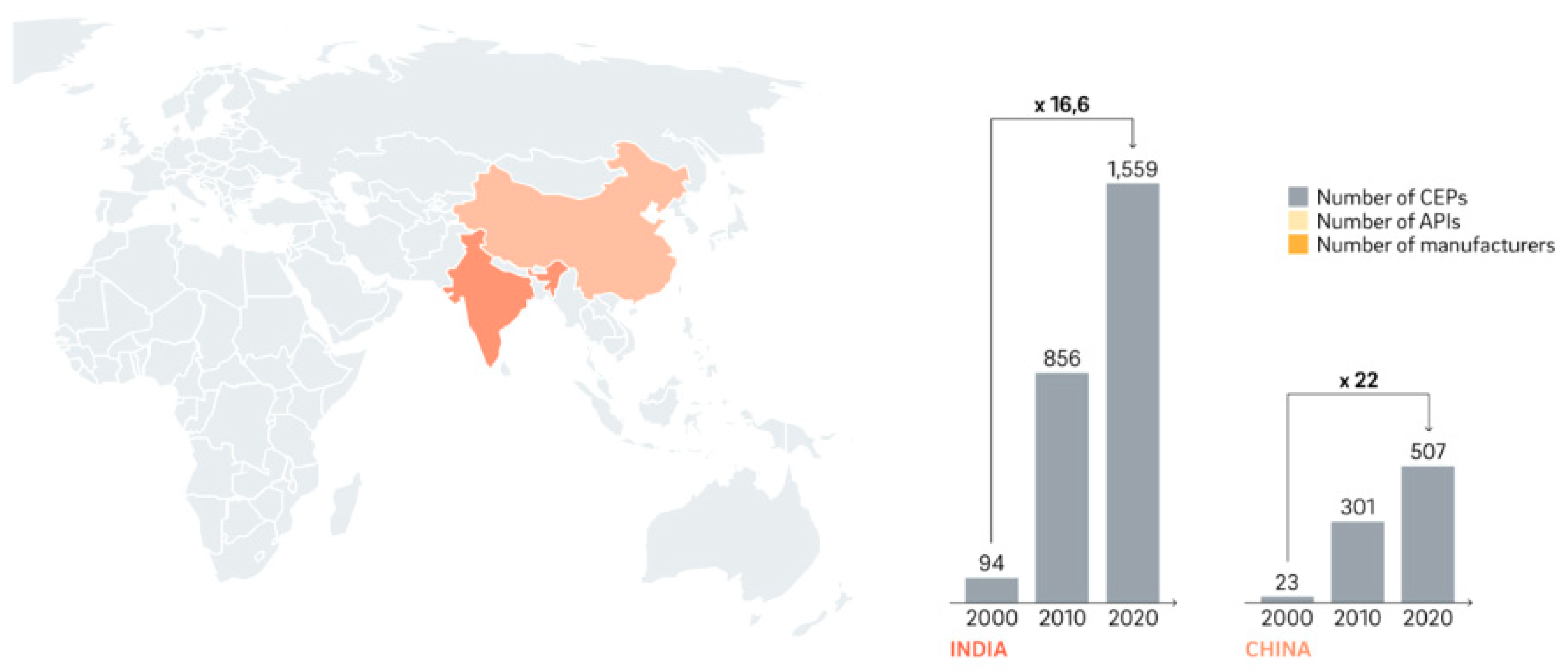
There are now excellence clusters emerging in some areas as a result of the globalisation process. Thus, during the past 20 years, China and India have significantly expanded their use of certificates of appropriateness (CEPs) shown in
Figure 3. They provided an inventory model for a supply chain including a pharmaceutical company and a hospital supply chain that combines continuous evaluation with manufacturing and distribution. The model takes into account a number of pharmaceutical goods, varying lead times, acceptable payment delays, space availability restrictions, and customer service levels.
Figure 3.
CEP's, API's and manufacturers of India and China (2000-2020) (Kaltenbach & Fath, 2022).
Figure 3.
CEP's, API's and manufacturers of India and China (2000-2020) (Kaltenbach & Fath, 2022).
4. Crisis Management in the Pharmaceutical Supply Chain
The pharmaceutical industry needs crisis management plans in order to reduce risks and prevent supply chain disruptions. These days, process operations and management research are heavily focused on supply chain optimization. Facility placement and design, inventory and distribution planning, capacity and production planning, and precise scheduling have all been the subject of extensive investigation. This industry is extremely well prepared and in need of advanced supply chain optimization methods. This business faces a unique challenge when designing the supply chain balancing projected demand and future capacity in light of the substantial uncertainty resulting from competitor action and clinical trials. As regulatory constraints rise and margins narrow, effective capacity utilization strategies and sound infrastructure investment decisions will become increasingly crucial. The capacity to identify supply chain nodes in tax havens and optimize trading and transfer price structures leads to intriguing degrees of freedom in the challenge of supply chain design. The issue of pipeline and testing planning, which involves choosing which products to create and when to complete them, comes before capacity planning. This involves carefully managing risk and possible rewards. Ensuring responsiveness is often difficult at the operation stage.
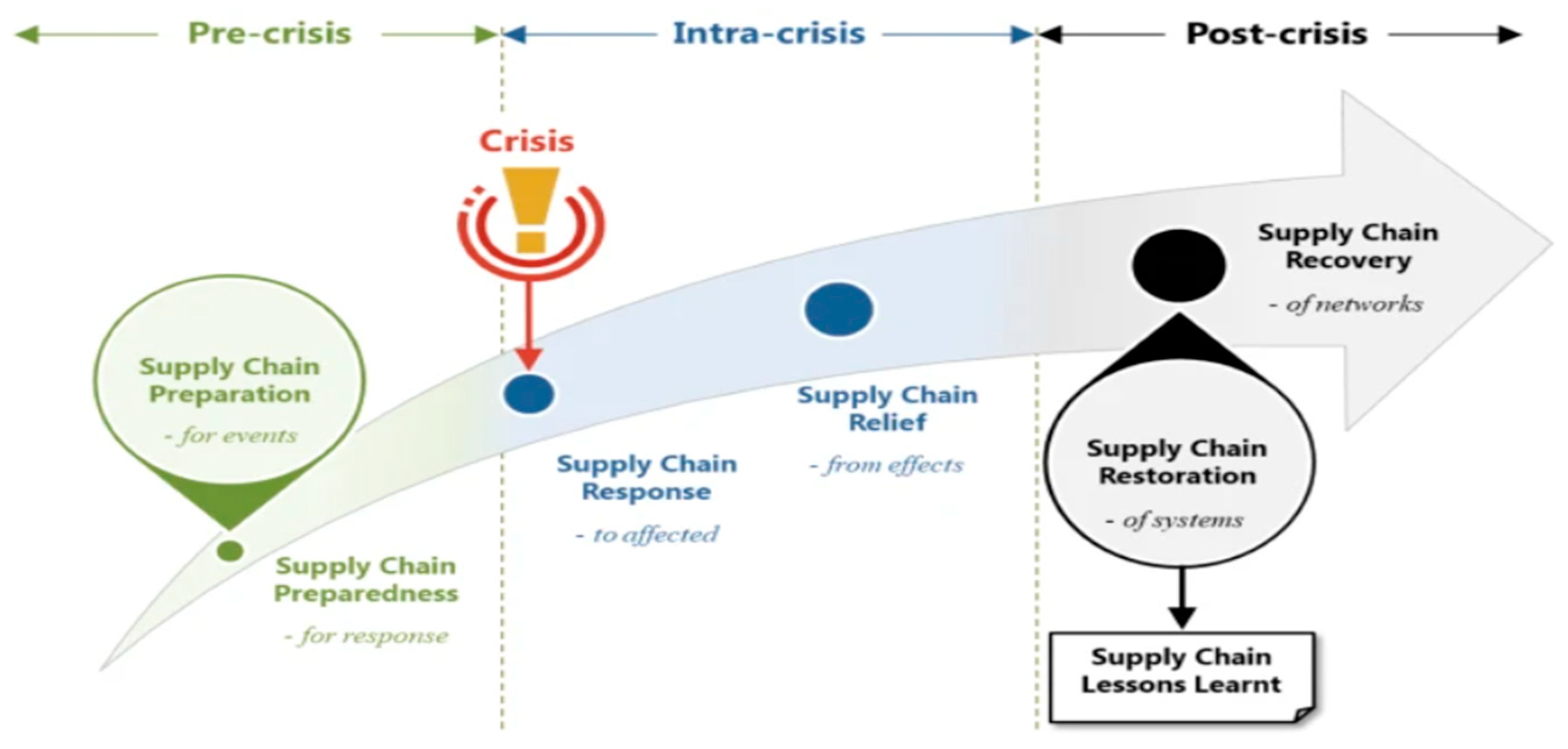
Coordinated inventory management, supply chain debottlenecking, and decoupling approaches are critical to responding swiftly to changing market trends. It's also essential to have a firm understanding of the basic ideas that underpin supply chain dynamics. Unpredictable dynamics can be prevented by rethinking internal business processes or supplier/customer relationships, as they are usually the result of company processes rather than external demand. This study will examine important issues related to supply chain design and operations, drawing from existing research as well as our collaborative efforts. A novel conceptual framework was created by Latonen et.al, (2023) to explain how collaborative crisis management is organised. In the absence of a predetermined crisis management organisation, cross-sector cooperation was arranged using past channels, institutions, and relationships, as well as through government agencies creating issue-specific committees in accordance with legal mandates. A synopsis of the phases involved in supply chain crisis response is shown in
Figure 4 (Durugbo & Al-Balushi, 2022).
The group's creation and meeting frequency were guided by crisis dynamics and related challenges. Governmental bodies and advocacy groups served as mediators between the various parties. Sharing information amongst stakeholders in the pharmaceutical supply chain promoted anticipatory and crisis-preparedness; pooling resources encouraged the upkeep of essential operations; and collaborative problem-solving permitted cross-sectoral solutions. According to Shah (2004), initially the focus of the pharmaceutical supply chain was on supply security as a means of efficiently bringing pharmaceuticals to market. Late modifications to the operational climate mean businesses are reviewing their supply chains' elements and figuring out how to get further advantages out of them. In this industry, the supply chain of interest is not just the material distribution and conversion activities carried out physically. The "value-chain" is equally significant in managing innovation and development from a perspective of procedures all the way to capacity and production scheduling.
There are still many fascinating research questions in this value chain, with a large number of the process engineering and systems engineering fields ideally positioned to address them. By conducting a thorough risk assessment to identify potential risks in the supply chain, one can find out the elements of the manufacturing and distribution supply chain shown in
Figure 5.
5. Developing a Crisis Management Plan
To efficiently handle emergencies, assemble a team of people with responsibilities in several areas, such as supply chains, operations, regulatory, and legal. Create a crisis management plan that specifies roles and actions in an emergency. Make sure the plan has techniques for allocating resources, communication procedures, and backup plans for different contingencies. Divide the process of creating a crisis management strategy into smaller, more manageable parts in order to do so quickly and effectively. This can assist you in identifying probable hazards without allowing the possibility of a crisis to overwhelm you (Asana, 2021). Use a crisis management template that includes the following five steps to structure your plan:
Define your crisis management team: A crisis management plan requires assembling a team of leaders, including public relations, security, and legal counsel, to promptly participate in the planning process and familiarise themselves with the plan's specifics.
Evaluate risk: To start preparing, have a brainstorming session to find any risks in your company. To determine the possibility of risks occurring, prepare for future setbacks, and see possible threats for an efficient response, use a risk register.
Assess the effect on business: Analyse high-probability threats with crisis leadership team to assess business impact, including client attrition, reputational damage, delayed sales, financial loss, or government fines.
Prepare your reply: Determine the steps your team should take to address each risk, such as contacting consumers, securing the network, and assessing damage in a cyberattack scenario.
Strengthen the plan: A crisis management plan should include an activation protocol, emergency contacts, and collaboration with key parties to discuss potential dangers, implications, and appropriate action, as well as a written or spoken approach.
6. Establishing Supply Chain Resilience
In order to improve supply chain flexibility and guarantee regulatory compliance, one should search for alternative suppliers or manufacturers. Plans will therefore be easier to prepare in the event that the primary provider does not live up to expectations. Another strategy to lessen supply disruptions is to ensure that there are strategic safety reserves. This suggests that in the case of a disruption in the supply chain, one can have a certain level of inventory on hand to support business operations (Tunisini et al., 2023). In conclusion, one can strengthen relationships with dependable vendors and establish more direct channels of communication. In addition to ensuring that suppliers can meet needs, this will establish solid relationships with them. Notwithstanding its diligence, any business may, for whatever reason, from a clinical trial death to a product safety recall, find itself unexpectedly thrown into a crisis. Industry sectors are simultaneously affected by cyber threats, data security and privacy, quality problems, and market economics. There could be urgent problems to deal with on any given day. Some business studies (Corbae, 2023) argue that a crisis will not affect them, but that is before it does, in which case it becomes even more crucial to be ready and assured with a crisis communications strategy. The pharmaceutical industry crisis management strategy checklist is shown in
Figure 6 (Asana, 2021). Planning messages and protocols ahead of time for any probable scenario is the answer. To get things going, follow these simple steps:
-
1.
Risk assessment and planning: The first step in crisis management is to conduct a comprehensive risk assessment within the supply chain. To ensure the smooth running of a supply chain, it is important to identify and address potential hazards and weak points. This includes assessing risks from natural disasters, disease outbreaks, geopolitical instability, supplier defaults, and other disruptions. By taking steps to mitigate these risks, businesses can protect their supply chains and keep their customers supplied with the products they need. Develop a crisis management team that is responsible for handling emergencies and implementing a robust crisis management plan.
-
2.
Business Continuity Planning: Create a thorough business continuity plan (BCP) that details what should be done in case of an emergency. Contingency plans, backup suppliers, emergency procurement procedures, and channels for keeping in touch with all parties involved should all be part of this strategy. Effective crisis management in the pharmaceutical industry requires proactive planning, collaboration, and the ability to adapt to changing circumstances. By integrating crisis management strategies into supply chain management practices, pharmaceutical companies can minimize the impact of disruptions and ensure the availability of medicines and healthcare products to patients in times of crisis.
-
3.
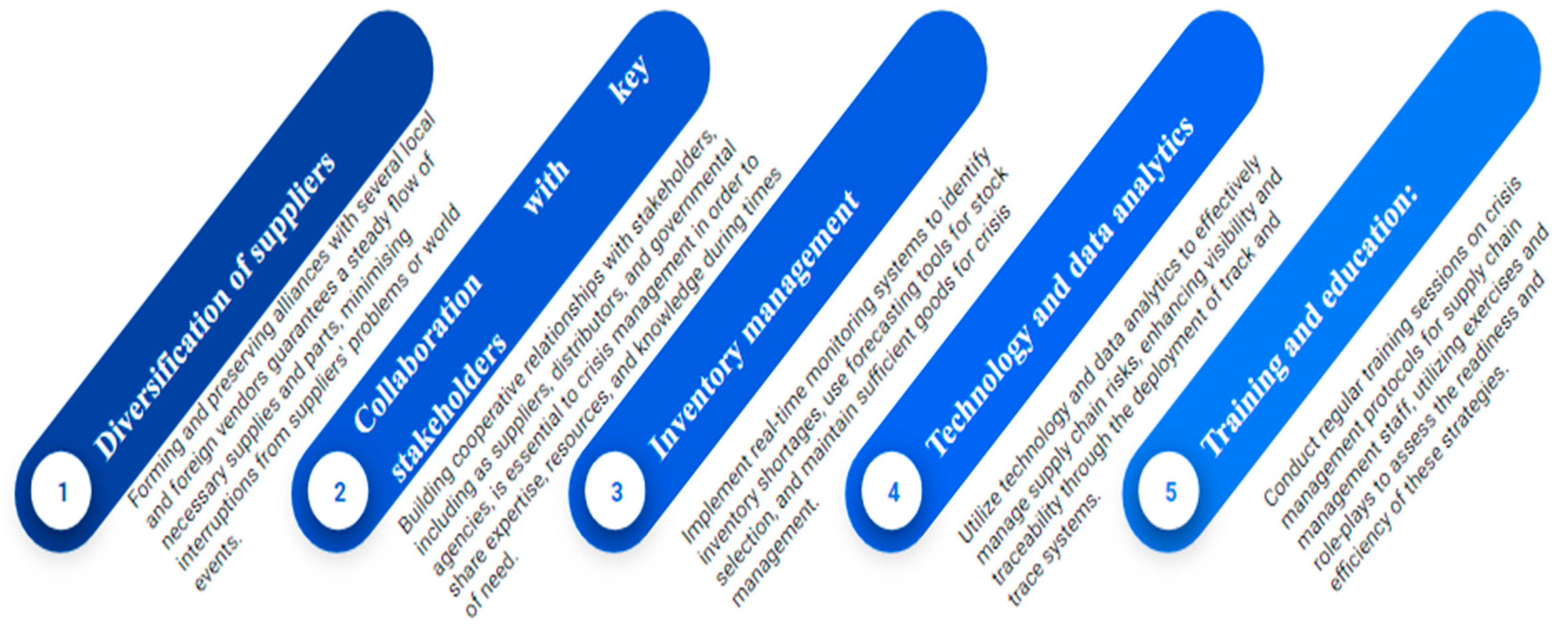
Supplier Relationship Management: Establish dependable relationships with suppliers and maintain open channels of communication. Regularly assess suppliers' operational, logistical, and financial soundness to ensure they can weather future challenges. Expand your pool of suppliers to lessen your need for one. Pharmaceutical businesses should diversify their procurement strategies in order to lessen their reliance on a single source. To assure a consistent supply of essential materials and components, establish and maintain partnerships with a number of domestic and international suppliers. By doing this, the effects of interruptions brought on by supplier problems or world events will be lessened.
-
4.
Inventory Management: Maintain the appropriate quantities of inventory to decrease the impact of any unforeseen disruptions to the supply chain. In order to identify potential inventory shortages and take relevant action, implement a real-time monitoring system. When deciding which prescriptions to stock, use forecasting tools to help you make selections. Keeping enough goods on hand is essential for crisis management. Provide a solid method for managing inventories that takes lead times, supply chain risks, and fluctuating demand into account. In the case of disruptions, such as unanticipated spikes in demand or delays in shipping, inventory should be proactively monitored and replenished to guarantee business continuity.
-
5.
Collaboration and Communication: Collaborate with trade associations, authorities, and other pharmaceutical companies to share information, resources, and expertise during times of crisis. Establish effective channels of communication with all stakeholders to provide transparency and timely updates regarding any disruptions or mitigating measures. In crisis management, fostering cooperative connections with important stakeholders, including distributors, suppliers, and governmental organisations, is crucial. To share knowledge, resources, and skills during emergencies, keep lines of communication open and set up mutual assistance systems. Provide regular updates to important stakeholders regarding the crisis management plan, including steps for handling product recalls and supply interruptions.
-
6.
Technology and Data-Driven Solutions: Use technology and data analytics to monitor and manage supply chain risks. Deploy track and trace systems to increase the visibility and traceability of the supply chain, enabling the quick identification and resolution of issues. To improve crisis management skills, make use of technological solutions like data analytics and supply chain management software. These technologies make it possible to monitor the performance of the supply chain in real time, identify possible disruptions early on, and prepare responses quickly. To avert or lessen possible crisis scenarios, use data analytics to spot trends, project demand, and streamline supply chain processes.
-
7.
Contingency Planning: Ensure that you have backup plans in case of unanticipated spikes in demand, production facility closures, or transportation disruptions. To cut down on downtime and guarantee drug supply, find and create backup manufacturing facilities or delivery networks.
-
8.
Regulatory Compliance: Keep yourself informed about supply chain management-related regulations and compliance obligations. Have Good Manufacturing Practises (GMP) in place and make sure all suppliers fulfil quality requirements, which include process validation and appropriate documentation.
-
9.
Training and Preparedness: Organise frequent training sessions with an emphasis on crisis management protocols and procedures for staff members who work in supply chain management. Conduct frequent exercises and role-plays to evaluate the readiness and efficiency of crisis management strategies. Conduct regular training sessions and educational programmes for employees involved in supply chain management. Provide them with the necessary skills and knowledge to handle crisis situations effectively. Ensure that employees are familiar with the crisis management plan, emergency response protocols, and relevant regulations.
-
10.
Continuous Improvement: Evaluate crisis management plans' efficacy and carry out post-crisis evaluations. Determine what needs to be improved, then apply the knowledge gained to future risk assessments and planning. The pharmaceutical industry is continuously evolving, and so are supply chain risks. Regularly review and update crisis management strategies based on lessons learned from past experiences, industry best practices, and emerging trends. Maintain a culture of continuous improvement by actively seeking feedback from stakeholders, conducting post-crisis evaluations, and implementing necessary changes.
7. Conclusions
In summary, proactive risk assessment, backup plans, teamwork, and efficient communication are necessary for crisis management in the pharmaceutical business, especially when it comes to supply chain management. Pharmaceutical businesses can enhance their crisis management capabilities and guarantee the uninterrupted provision of vital pharmaceuticals to patients, even in difficult circumstances, by employing these tactics. Conduct reviews after a crisis to determine the effectiveness of crisis management plans. Through these reviews, we summarise the conclusion shown in Fig. 7. Next, use the information obtained to inform future risk assessments and planning. Identify areas that need improvement. To sum up, collaboration, effective communication, proactive risk assessment, and backup plans are essential. The inadequate transportation infrastructure in India causes pharmaceutical businesses to spend one-third of their income on supply chain management (SCM) efforts. For numerous businesses, supply chain management (SCM) plays a crucial role since it helps reduce costs and boost revenue. At 13% of India's GDP, supply chain management expenses are higher in India than they are globally. This study set out to investigate SCM tactics shown in figure 7. Pharmaceutical sector executives from India have historically lowered the expensive SCM overhead. A solitary case study was employed in this investigation (Bolineni, 2016).
Figure 7.
Continuous improvement, learning, and Always Advancing (“Pharmaceutical Supply Chain Management,” 2024).
Figure 7.
Continuous improvement, learning, and Always Advancing (“Pharmaceutical Supply Chain Management,” 2024).
Reduced access to healthcare and insurance coverage are just two of the negative effects that can arise from a number of factors, such as an increase in supply chain waste, hurried decision-making, unmet expectations, callous managers, potential resource abuse, offending pharmaceutical distributors, and inefficient use of resources. Production-related goals are used to identify the best supply chain recovery strategies for multi-echelon closed-loop green supply chain models. These strategies aim to minimise total costs, which include costs related to the final products as well as costs resulting from green criteria in the event of a disruption (Hasan, Ali, Paul, & Kabir, 2023). Presumably, preserving the dimensions of the current model will bolster the pharmaceutical and consumable medical equipment supply chain in the event of a crisis.
This study (Bastani et al., 2021) builds on grounded theory and provides healthcare systems with a framework for dealing with uncertainties and unforeseen events in the future. Stated differently, the resilient model developed by the research provides healthcare systems with recommendations on how to minimise the risk of resource waste and efficiently manage their limited resources. To improve the resilience of pharmaceutical and consumable medical equipment in the event of a crisis, it is imperative to strengthen the different aspects of the resilience model and raise the supply chain's response rate. In particular, this work adds to our growing and deeper understanding of how to reduce the likelihood that the pharmaceutical supply chain will have unfavourable effects during emergencies or disasters. To improve supply chain resilience, policymakers should take into account the integration of modern technologies with the pharmaceutical supply chain and pay more attention to business complexity, economic development, intense competition, and quickly changing customer needs. One should also consider the appropriate relationship between manufacturers, distributors, prescribers, and insurance organisations as purchasers (Bastani, Dehghan, et al., 2021).
Funding
This research received no funding.
Institutional Review Board Statement
Not applicable in that there was no involvement of humans or animals in the study.
Informed Consent Statement
Not applicable in that there was no involvement of humans or animals in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interests
The authors declare no conflict of interest.
References
- Ab Talib, M. S., & Wahab, S. N. (2021). Halal logistics in a rentier state: an observation. Modern Supply Chain Research and Applications, ahead-of-print(ahead-of-print). [CrossRef]
- Ahlqvist, V., Dube, N., Jahre, M., Lee, J. S., Melaku, T., Moe, A. F., … Aardal, C. (2022). Supply chain risk management strategies in normal and abnormal times: policymakers’ role in reducing generic medicine shortages. International Journal of Physical Distribution & Logistics Management, 52(11). [CrossRef]
- Al-Omoush, K. S., Palacios-Marqués, D., & Ulrich, K. (2022). The impact of intellectual capital on supply chain agility and collaborative knowledge creation in responding to unprecedented pandemic crises. Technological Forecasting and Social Change, 178, 121603. [CrossRef]
- Asana. (2021, August 16). What Is a Crisis Management Plan? (6 Steps to Create One) • Asana. Retrieved from Asana website: https://asana.com/resources/crisis-management-plan.
- Bastani, P., Dehghan, Z., Kashfi, S. M., Dorosti, H., Mohammadpour, M., Mehralian, G., & Ravangard, R. (2021). Strategies to improve pharmaceutical supply chain resilience under politico-economic sanctions: the case of Iran. Journal of Pharmaceutical Policy and Practice, 14(1). [CrossRef]
- Bastani, P., Sadeghkhani, O., Ravangard, R., Rezaei, R., Bikine, P., & Mehralian, G. (2021). Designing a resilience model for pharmaceutical supply chain during crises: a grounded theory approach. Journal of Pharmaceutical Policy and Practice, 14(1). [CrossRef]
- Blix, H. S., & Høye, S. (2021). Bruk av antibiotika under covid-19-pandemien. In Tidsskrift for Den norske legeforening. [CrossRef]
- Bolineni, P. (2016). ScholarWorks The Indian Pharmaceutical Industry’ s Supply Chain Management Strategies. Retrieved from https://scholarworks.waldenu.edu/cgi/viewcontent.cgi?article=3497&context=dissertations&httpsredir=1&referer=.
- Bygballe, L. E., Dubois, A., & Jahre, M. (2023). The importance of resource interaction in strategies for managing supply chain disruptions. Journal of Business Research, 154, 113333. [CrossRef]
- Chitra Lekha Karmaker. (2020). Modeling performance indicators of resilient pharmaceutical supply chain. Modern Supply Chain Research and Applications, 2(3), 179–205. [CrossRef]
- Corbae, M. (2023, August 9). Crisis Communication Plans in Pharma. Retrieved from PharmExec website: https://www.pharmexec.com/view/crisis-communication-plans-in-pharma.
- Desingh, V., & R, B. (2021). Internet of Things adoption barriers in the Indian healthcare supply chain: An ISM-fuzzy MICMAC approach. The International Journal of Health Planning and Management, 37(1). [CrossRef]
- Dubey, R., Bryde , D. J., Blome, C., Roubaud , D., & Giannakis, M. (2021). Facilitating artificial intelligence powered supply chain analytics through alliance management during the pandemic crises in the B2B context. Industrial Marketing Management, 96, 135–146. [CrossRef]
- Durugbo, C. M., & Al-Balushi, Z. (2022). Supply chain management in times of crisis: a systematic review. Management Review Quarterly, 73. [CrossRef]
- Ergun, O., Hopp, W. J., & Keskinocak, P. (2022). A structured overview of insights and opportunities for enhancing supply chain resilience. IISE Transactions, 154, 1–18. [CrossRef]
- Graves, S., Lei, L., Melamed, B., Pinedo, M., Qi, L., Shen, Z., & Xu, X. (2009). (Position Paper for the 2009 DHS Workshop on Incident Management, Resource Management, and Supply Chain Management) New Challenges to Emergency Management of Pharmaceutical/Healthcare Supply Chain Disruptions. Retrieved from https://ics.uci.edu/~projects/cert/EMWS09/presentations/Position%20Papers/emws09_submission_2.pdf.
- Hasan, K. W., Ali, S. M., Paul, S. K., & Kabir, G. (2023). Multi-objective closed-loop green supply chain model with disruption risk. Applied Soft Computing, 136, 110074. [CrossRef]
- Hayes, A. (2023, March 28). The Supply Chain: From Raw Materials to Order Fulfillment. Retrieved from Investopedia website: https://www.investopedia.com/terms/s/supplychain.asp#:~:text=A%20supply%20chain%20is%20a%20network%20of%20companies%20and%20people.
- Hopp, W. J., Brown, L., & Shore, C. (Eds.). (2022). Building Resilience into the Nation’s Medical Product Supply Chains. Washington, D.C.: National Academies Press. [CrossRef]
- Hosseini-Motlagh, S.-M., Jazinaninejad, M., & Nami, N. (2020). Recall management in pharmaceutical industry through supply chain coordination. Annals of Operations Research, 324. [CrossRef]
- Jain, V., Ajmera, P., & Davim, J. P. (2021). SWOT analysis of Industry 4.0 variables using AHP methodology and structural equation modelling. Benchmarking: An International Journal, ahead-of-print(ahead-of-print). [CrossRef]
- Jain, V., Phoghat, S., Ajmera, P., & Sirvi, A. (2022). Modeling the barriers of Indian healthcare supply chain management using ISM. International Journal of Supply and Operations Management, 9(3), 321–337. [CrossRef]
- Kaltenbach, T., & Fath, S. (2022, July 11). How to master supply chain crises in the pharmaceutical industry. Retrieved from Roland Berger website: https://www.rolandberger.com/en/Insights/Publications/Mastering-supply-chain-crises-in-the-pharmaceutical-industry.html.
- Latonen, S. H., Suominen, R. M., Juppo, A. M., Airaksinen, M., & Seeck, H. (2023). Organisation of cross-sector collaboration and its influence on crisis management effectiveness among pharmaceutical supply chain stakeholders during the COVID-19 pandemic. Public Health, 222, 196–204. [CrossRef]
- Lohrke, F. T., Mazzei, M. J., & Frownfelter-Lohrke, C. (2021). Should It Stay or Should It Go? Developing an Enhanced SWOT Framework for Teaching Strategy Formulation. Journal of Management Education, 46(2), 105256292110211. [CrossRef]
- Mbazima, S. J., Mbonane, T. P., & Masekameni, M. D. (2021). A SWOT analysis of contemporary gaps and a possible diagnostic tool for environmental health in an upper-middle income country: a case study of South Africa. International Journal of Environmental Health Research, 32, 1–23. [CrossRef]
- Moniruzzaman, D. M. (2015). Supply chain management in pharmaceutical industries: a study on Eskayef Bangladesh Ltd. Retrieved from https://core.ac.uk/reader/74352570.
- Moretto, A., & Caniato, F. (2021). Can Supply Chain Finance help mitigate the financial disruption brought by Covid-19? Journal of Purchasing and Supply Management, 27, 100713. [CrossRef]
- Okeagu, C. N., Reed, D. S., Sun, L., Colontonio, M. M., Rezayev, A., Ghaffar, Y. A., … Kaye, A. D. (2020). Principles of supply chain management in the time of crisis. Best Practice & Research Clinical Anaesthesiology, 35(3). [CrossRef]
- Ozdemir, D., Sharma, M., Dhir, A., & Daim, T. (2022). Supply chain resilience during COVID 19 pandemic. Technology in Society, 68, 101847. [CrossRef]
- Pharmaceutical Supply Chain Management. (2024). Retrieved from TraceLink website: https://www.tracelink.com/agile-supply-chain/pharmaceutical-supply-chain-management#:~:text=%22Pharmaceutical%20supply%20chain%20management%22%20refers.
- Pinedo, M. (2009). New Challenges to Emergency Management of Pharmaceutical/Healthcare Supply Chain Disruptions. Www.academia.edu. Retrieved from https://www.academia.edu/25482784/New_Challenges_to_Emergency_Management_of_Pharmaceutical_Healthcare_Supply_Chain_Disruptions.
- Raj, A., Mukherjee, A. A., Jabbour, A. B. L. de S., & Srivastava, S. K. (2022). Supply Chain Management during and post-COVID-19 pandemic: Mitigation Strategies and Practical Lessons Learned. Journal of Business Research, 142(1), 1125–1139. NCBI. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8776498/.
- Remko, van H. (2020). Research opportunities for a more resilient post-COVID-19 supply chain – closing the gap between research findings and industry practice. International Journal of Operations & Production Management, 40(4), 341–355. [CrossRef]
- Roshan, M., Tavakkoli-Moghaddam, R., & Rahimi, Y. (2019). A two-stage approach to agile pharmaceutical supply chain management with product substitutability in crises. Computers & Chemical Engineering, 127, 200–217. [CrossRef]
- Shah, N. (2004). Pharmaceutical supply chains: key issues and strategies for optimisation. Computers & Chemical Engineering, 28(6-7), 929–941. [CrossRef]
- Siebert, J. U., Brandenburg, M., & Siebert, J. (2020). Defining and aligning supply chain objectives before, during and after the COVID-19 pandemic. IEEE Engineering Management Review, 48, 1–1. [CrossRef]
- Singh, R. Kr., Kumar, R., & Kumar, P. (2016). Strategic issues in pharmaceutical supply chains: a review. International Journal of Pharmaceutical and Healthcare Marketing, 10(3), 234–257. [CrossRef]
- Tunisini, A., Harrison, D., & Bocconcelli, R. (2023). Handling resource deficiencies through resource interaction in business networks. Industrial Marketing Management, 109, 154–163. [CrossRef]
- Uthayakumar, R., & Priyan, S. (2013). Pharmaceutical supply chain and inventory management strategies: Optimization for a pharmaceutical company and a hospital. Operations Research for Health Care, 2(3), 52–64. [CrossRef]
- Wahab, S. N., Ahmed, N., & Syazwan, M. (2023). An overview of the SWOT analysis in India’s pharmaceutical supply chain. Arab Gulf Journal of Scientific Research,. [CrossRef]
- Whewell, R. (2016). Supply Chain in the Pharmaceutical Industry: Strategic Influences and Supply Chain Responses. In Google Books. CRC Press. Retrieved from https://books.google.co.in/books?hl=en&lr=&id=tmHeCwAAQBAJ&oi=fnd&pg=PP1&dq=crisis+management+strategies+pharmaceutical+industries+supply+chain%2Bpdf&ots=pJ9M5Xi2wS&sig=8gE28O-9AkcvsmJwkYHG6yqH3s8#v=onepage&q&f=false.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).