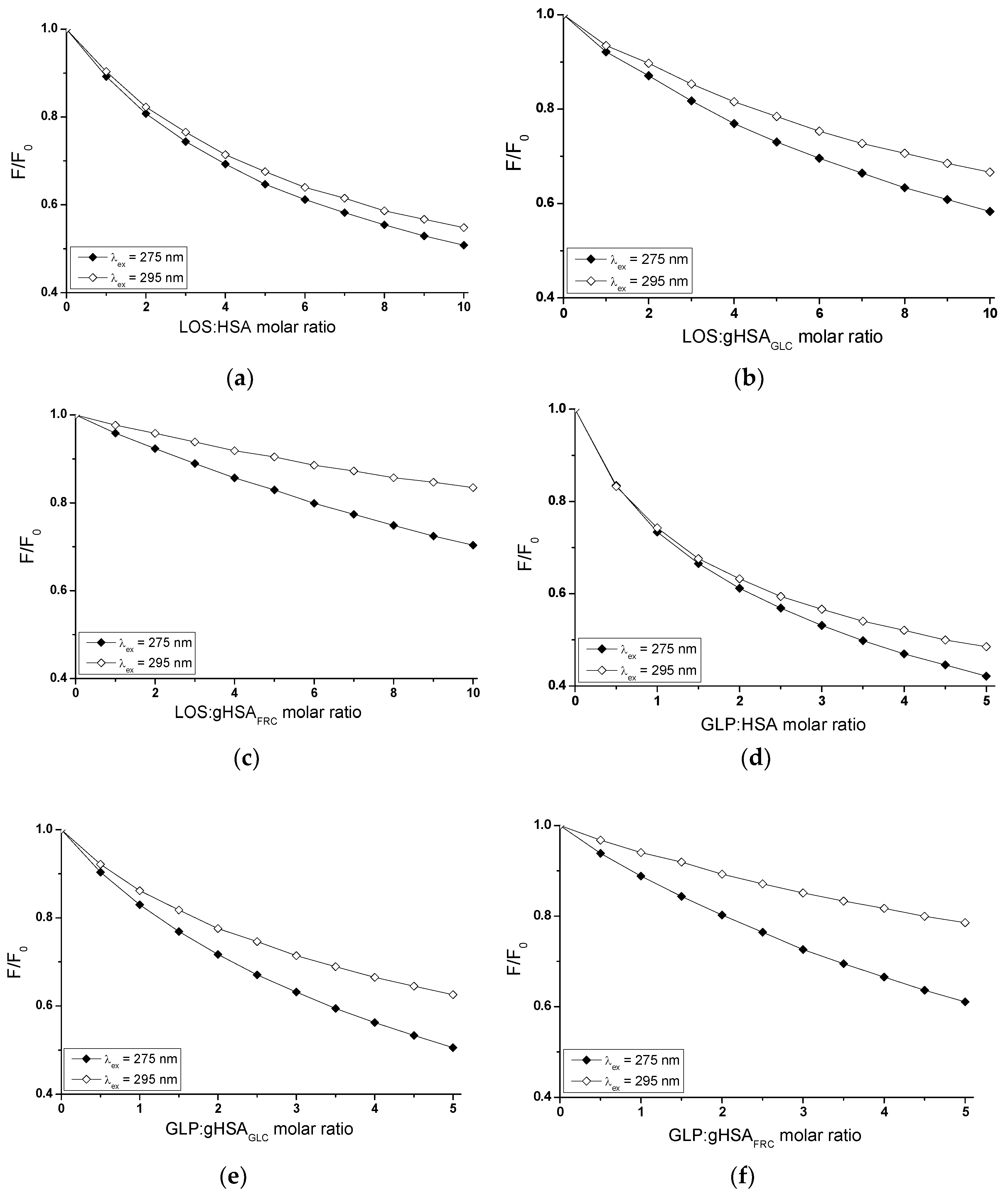
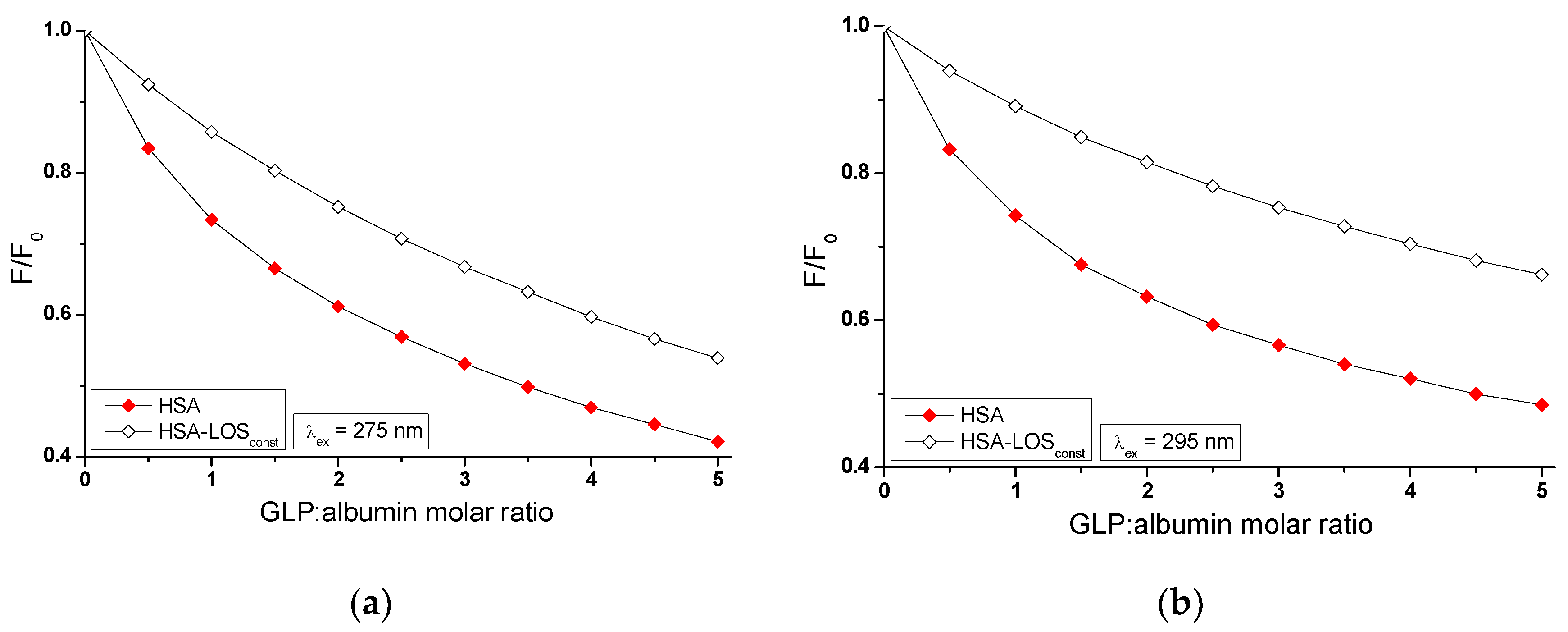
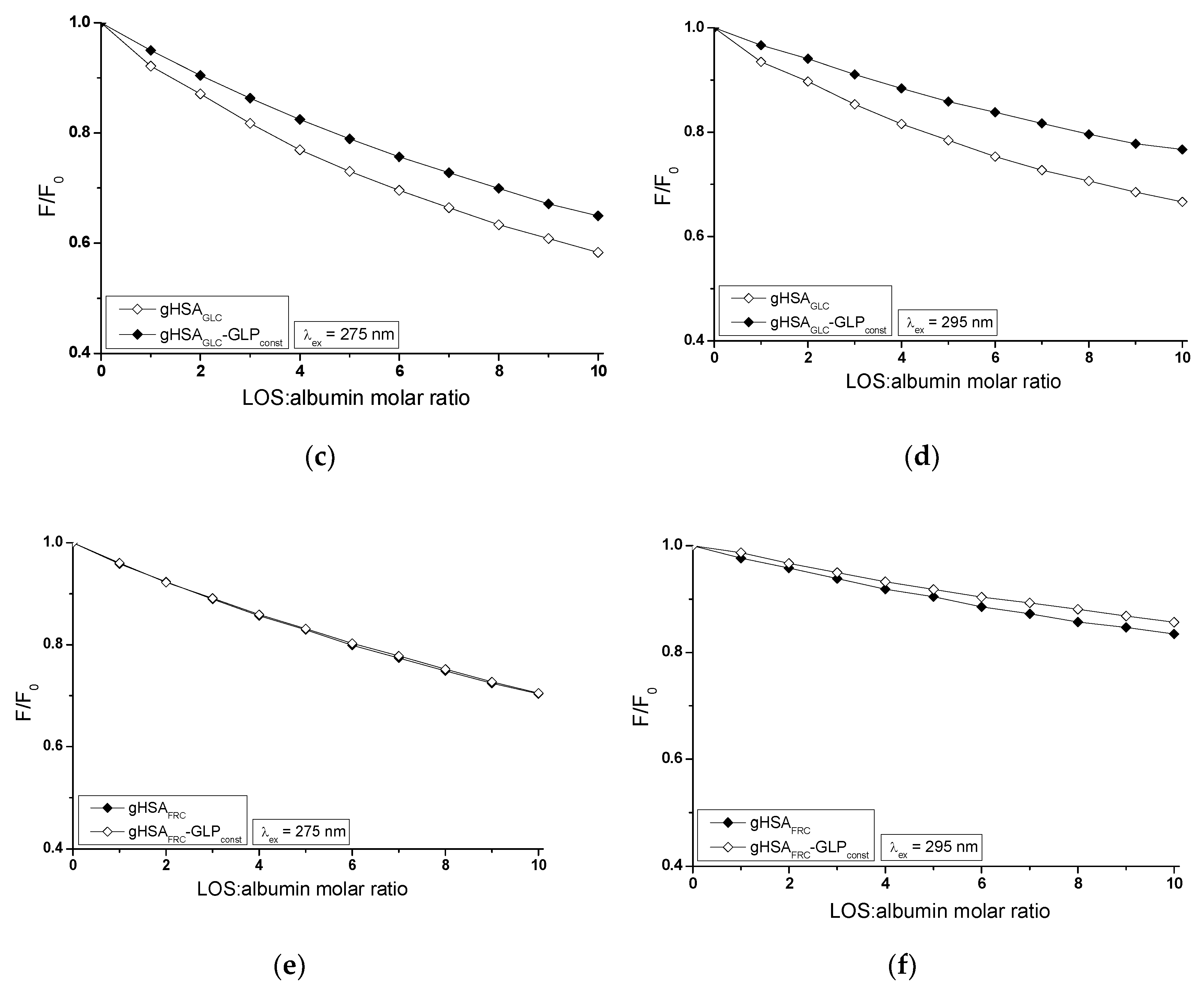2.2. Investigating the Interaction of Losartan and Glipizide with Non-Glycated and Glycated Human Serum Albumin in Binary and Ternary Complexes
Assessing changes in the fluorescence emission intensities of aromatic amino acid residues within proteins can effectively reveal intermolecular interactions [
19]. Human serum albumin (HSA) contains 18 fluorescent tyrosine residues (Tyr-9, Tyr-39, Tyr-84, Tyr-138, Tyr-140, Tyr-148, Tyr-150, Tyr-161, Tyr-263, Tyr-319, Tyr-332, Tyr-334, Tyr-340, Tyr-341, Tyr-372, Tyr-375, Tyr-401, Tyr-411), along with a single, strongly fluorescent tryptophan residue (Trp-214) buried within a hydrophobic pocket [
20]. The fluorescence quenching technique is commonly employed to determine the strength and mechanism of interactions between ligands (drugs) and albumin, especially involving the Trp-214 and Tyr residues located within albumin's subdomains IIA and/or IB, IIB, and IIIA.
Fluorescence quenching of non-glycated (HSA) and glycated (gHSA
GLC, gHSA
FRC) albumin, excited at λ
ex = 275 nm and λ
ex = 295 nm, was performed to determine the interaction of losartan (LOS) and glipizide (GLP) at high-affinity binding sites of albumins in the binary systems: LOS-HSA (
Figure 3a), LOS-gHSA
GLC (
Figure 3b), LOS-gHSA
FRC (
Figure 3c), GLP-HSA (
Figure 3d), GLP-gHSA
GLC (
Figure 3e), GLP-gHSA
FRC (
Figure 3f).
In fluorescence studies, attention is directed towards specific protein regions containing fluorophores. Using an excitation wavelength of 275 nm enables the observation of both tryptophan (Trp-214) and tyrosine (Tyr) residues, whereas a 295 nm wavelength selectively excites Trp-214 due to its unique spectral characteristics. The primary drug-binding sites are located within the IIA and IIIA subdomains of HSA. Both hydrophobic pockets contain at least one type of the mentioned amino acids, which can transfer energy to a ligand if it is close to the fluorophore [
21].
In Sudlow's site I, located in the IIA subdomain, one tryptophan (Trp-214) and one tyrosine (Tyr-263) residue can participate in drug binding. Although Trp-214 is the sole tryptophan in the HSA structure, it plays a vital role in ligand interactions. The IIIA subdomain includes three tyrosine residues (Tyr-401, Tyr-411, and Tyr-497), which can transfer energy to the acceptor [
22]. Additionally, Tyr-401 and Tyr-411 have been identified as amino acids that stabilize the binding of numerous ligands. To verify subdomain IIIA of human serum albumin as the specific binding site for LOS and GLP, quenching curves of HSA, gHSA
GLC and gHSA
FRC excited at λ
ex = 275 nm were compared with those excited at λ
ex = 295 nm with the addition of LOS (
Figure 3a–c) and GLP (
Figure 3d–f) at increasing concentrations.
As mentioned earlier, protein fluorescence quenching occurs when the distance between the chromophores in the aromatic rings of the ligand's chemical structure and the fluorophores of albumin is less than 10 nm according to Stryer [
23] and less than 7 nm according to Valeur [
21]. This proximity enables fluorescence resonance energy transfer (FRET) from a donor (fluorophore) to an acceptor (chromophore), leading to non-radiative, direct energy transfer to the drug molecule. The quenching curves of HSA (
Figure 3a,d), gHSA
GLC (
Figure 3b,e) and gHSA
FRC (
Figure 3c,f) in the presence of both losartan and glipizide at increasing concentrations (with molar ratios of ligand to albumin from 1:1 to 10:1 for LOS and 1:1 to 5:1 for GLP) indicate a decrease in fluorescence for non-glycated and glycated albumin at excitation wavelengths of 275 nm and 295 nm. This indicates effective energy transfer between albumin fluorophores and the ligands. After applying corrections for the inner filter effect, the observed fluorescence quenching of HSA, gHSA
GLC, gHSA
FRC can be attributed to the formation of LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC and GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC complexes.
Based on the data collected in
Table S1 (Supplementary Materials), the percentage of non-glycated HSA fluorescence quenching, used as a control albumin, is nearly similar, reaching approximately 49.20% and 45.17% for LOS and 57.86% and 51.46% for GLP at λ
ex = 275 nm and λ
ex = 295 nm, respectively. For glycated albumin, weaker fluorescence quenching was observed in the presence of increasing ligand concentrations compared to the control sample, with the weakest quenching seen for albumin glycated by fructose (gHSA
FRC). LOS quenches the fluorescence of gHSA
GLC by 41.67% at λ
ex = 275 nm and by 33.36% at λ
ex = 295 nm. The fluorescence of gHSA
FRC decreases by 29.62% at 275 nm and by 16.54% at 295 nm for the same molar ratio of ligand to albumin (10:1). GLP quenches the fluorescence of gHSA
GLC by 49.42% at λ
ex = 275 nm and by 37.41% at λ
ex = 295 nm, at molar ratio GLP:albumin 5:1. In contrast, the fluorescence of gHSA
FRC at λ
ex = 275 nm and λ
ex = 295 nm decreases by 38.93% and 21.46%, respectively. Moreover, the data presented in
Table S1 indicate that both LOS and GLP have a higher affinity towards non-glycated macromolecule than towards glycated proteins (gHSA
GLC and gHSA
FRC).
The quenching curves of albumins excited at λ
ex = 275 nm and λ
ex = 295 nm in the presence of LOS (
Figure 3a–c) and GLP (
Figure 3d–f) at increasing drug concentration do not overlap above the molar ratio LOS:HSA 2:1, LOS:gHSA
GLC 1:1, LOS:gHSA
FRC 1:1 and GLP:HSA 1.5:1, GLP:gHSA
GLC 0.5:1. The exact course of fluorescence quenchning curves indicates that there is no difference in energy transfer between the tyrosyl residues and the ligand, suggesting that initially only Trp-214 residue is likely involved in the interaction with LOS and GLP. This may allow the identification of subdomain IIA as a high-affinity binding site in the albumin structure. In contrast, a different trajectory of fluorescence quenching suggests that both tryptophanyl residue in subdomain IIA and tyrosyl residues located in hydrophobic subdomains IB, IIB, IIIA, and IIIB are involved in the interaction with LOS and GLP in the binding site environment. As shown in
Figure 3, the fluorescence quenching of HSA (
Figure 3a,d), gHSA
GLC (
Figure 3b,e), and gHSA
FRC (
Figure 3c,f) by LOS and GLP is more pronounced when excited at λ
ex = 275 nm compared to λ
ex = 295 nm. This likely indicates a significant involvement not only of Trp-214 but also of Tyr residues in the interaction between the ligands and albumins.
The quenching curves of non-glycated and glycated albumin in the presence of LOS (
Figure S1ab, Supplementary Materials) and GLP (
Figure S1cd, Supplementary Materials) exhibit significant differences in their profiles. Specifically, these differences result from 7.53% and 11.81%, 19.58% and 28.63% lower quenching of gHSA
GLC and gHSA
FRC by LOS relative to the LOS-HSA system at λ
ex = 275 nm and λ
ex = 295 nm, respectively. For the GLP-gHSA
GLC and GLP-gHSA
FRC systems, the differences in the quenching curves profiles amount to 8.44% and 14.05% for gHSA
GLC, and 18.93% and 30% for gHSA
FRC relative to the GLP-HSA system at λ
ex = 275 nm and λ
ex = 295 nm, respectively (
Table S1). Several factors may explain these observed differences. Glycation induces conformational changes in the albumin structure, modifying the binding sites and overall protein flexibility and affecting the interaction with quenchers. Additionally, glycation alters the microenvironment around crucial amino acid residues, such as Trp-214 and Tyr, influencing their accessibility to quenchers. The binding affinity of glycated albumin for LOS and GLP may also differ due to sugar moieties, which can either hinder or facilitate quencher binding [
24]. Furthermore, steric hindrance from added sugar groups can reduce quenching efficiency, while new chemical interactions and potential protein aggregation further impact the quenching dynamics. These combined factors contribute to the distinct quenching behaviours observed for non-glycated and glycated albumin at different excitation wavelengths.
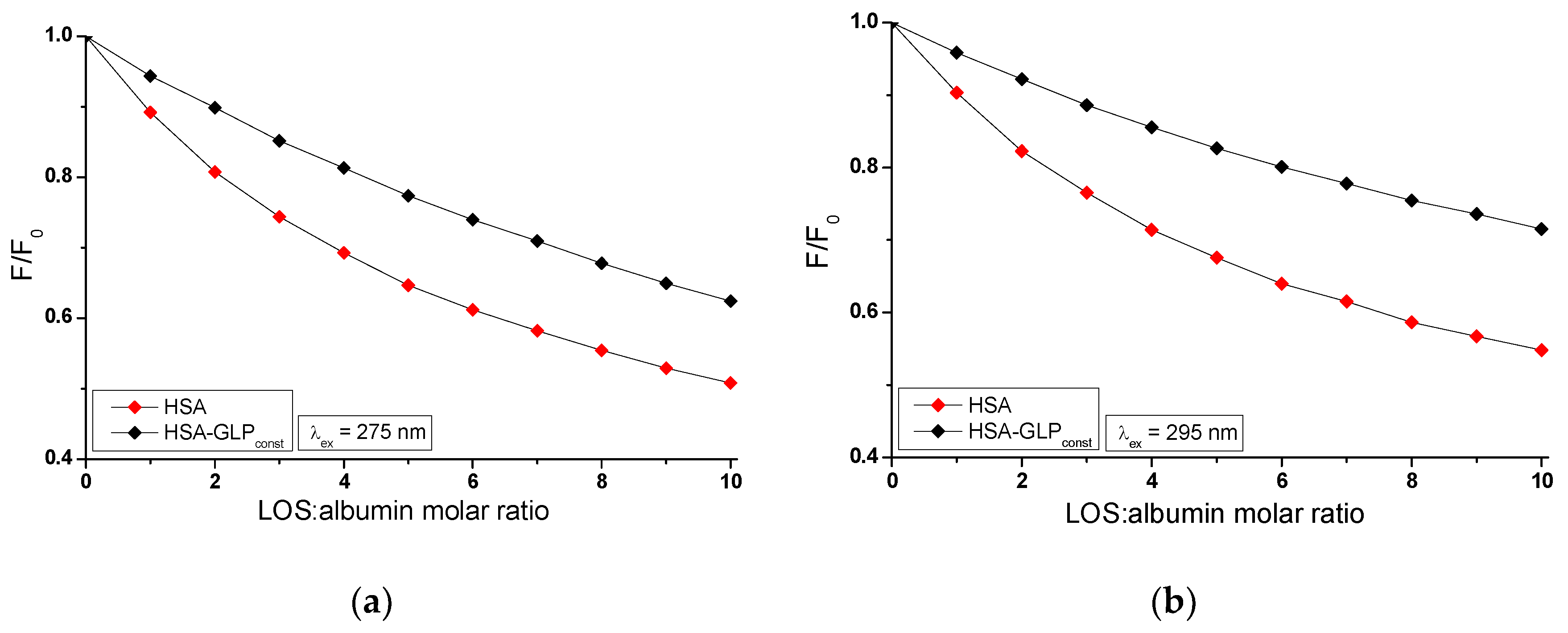
The influence of GLP on the LOS and LOS on the GLP affinity towards HSA, gHSA
GLC and gHSA
FRC was studied by comparing the quenching curves of albumins in the presence of LOS in the binary LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC and ternary complexes LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const (
Figure 4), and in the presence of GLP in the binary GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC and ternary complexes GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const (
Figure 5).
The fluorescence quenching observed in the LOS-HSA-GLP
const (
Figure 4a,b), LOS-gHSA
GLC-GLP
const (
Figure 4c,d) and GLP-HSA-LOS
const (
Figure 5a,b) systems differs from the quenching observed in the binary systems LOS-HSA (
Figure 4a,b), LOS-gHSA
GLC (
Figure 4c,d) and GLP-HSA (
Figure 5a,b), respectively. The quenching of HSA and gHSA
GLC by LOS and HSA by GLP at maximum concentration is more pronounced by 11.64% (
Figure 4a), 6.64% (
Figure 4c), 16.69% (
Figure 4b), 10.04% (
Figure 4d), 11.76% (
Figure 5a), and 17.7%% (
Figure 5b) compared to the systems with an additional ligand added to the binary system. An additional pharmaceutical in the system likely complicates the interaction between LOS-HSA and LOS-gHSA
GLC (or GLP-HSA) or interferes with forming these complexes. GLP (or LOS) may cause the displacement of LOS (or GLP) from its complex with non- and glycated HSA. This effect may arise from competitive binding sites on albumin, steric hindrance, or conformational alterations of the macromolecule induced by the binding of the second ligand. Furthermore, the differing affinities and binding dynamics of GLP and LOS for albumin could result in preferential binding of one ligand over the other, thereby influencing the observed quenching effect. In contrast, the absence of differences in the quenching of intrinsic fluorescence of glycated albumin by LOS or GLP in the binary systems LOS-gHSA
FRC (
Figure 4e,f), GLP-gHSA
GLC (
Figure 5c,d), and GLP-gHSA
FRC (
Figure 5e,f) compared to the ternary systems LOS-gHSA
FRC-GLP
const (
Figure 4e,f), GLP-gHSA
GLC-LOS
const (
Figure 5c,d), and GLP-gHSA
FRC-LOS
const (
Figure 5e,f) suggests that glycation, particularly glycation of albumin by fructose, alters the macromolecule's structure and/or binding characteristics. This modification prevents the additional drug – GLP in the LOS-gHSA
FRC complex or LOS in the GLP-gHSA
GLC and GLP-gHSA
FRC complexes – from competing for the binding site with LOS and GLP and prevents the displacement of drugs already bound in the gHSA
GLC and gHSA
FRC molecules.
Additionally, the study on the LOS-HSA, LOS-gHSAGLC, GLP-HSA, and LOS-HSA-GLPconst system revealed that an increase in drug concentration leads to a hypsochromic shift (∆λmax) of the fluorescence emission band relative to the maximum emission of unbound albumin. The hypsochromic shift indicates an increase in the hydrophobic nature of the fluorophore environment due to drug interaction with albumin. It also suggests the possibility of hydrophobic interactions between the aromatic rings of LOS and GLP molecules and the aromatic rings of amino acid residues. The more pronounced hypsochromic shift upon excitation of albumin fluorescence at λex = 275 nm than at λex = 295 nm indicates a less polar environment not only around Trp-214 but also around tyrosyl residues. The more substantial ∆λmax shift towards the blue in the double systems compared to the triple systems, i.e., in the LOS-HSA system compared to LOS-HSA-GLPconst by 5 nm, LOS-gHSAGLC compared to LOS-gHSAGLC-GLPconst by 4 nm, and GLP-HSA compared to GLP-HSA-GLPconst by 6 nm, and for non-modified compared to glycated albumin, may indicate a decrease in the hydrophobic nature of the environment of tryptophan or/and tyrosyl residues of albumin after glycation and in the presence of an additional drug in the drug-albumin system.
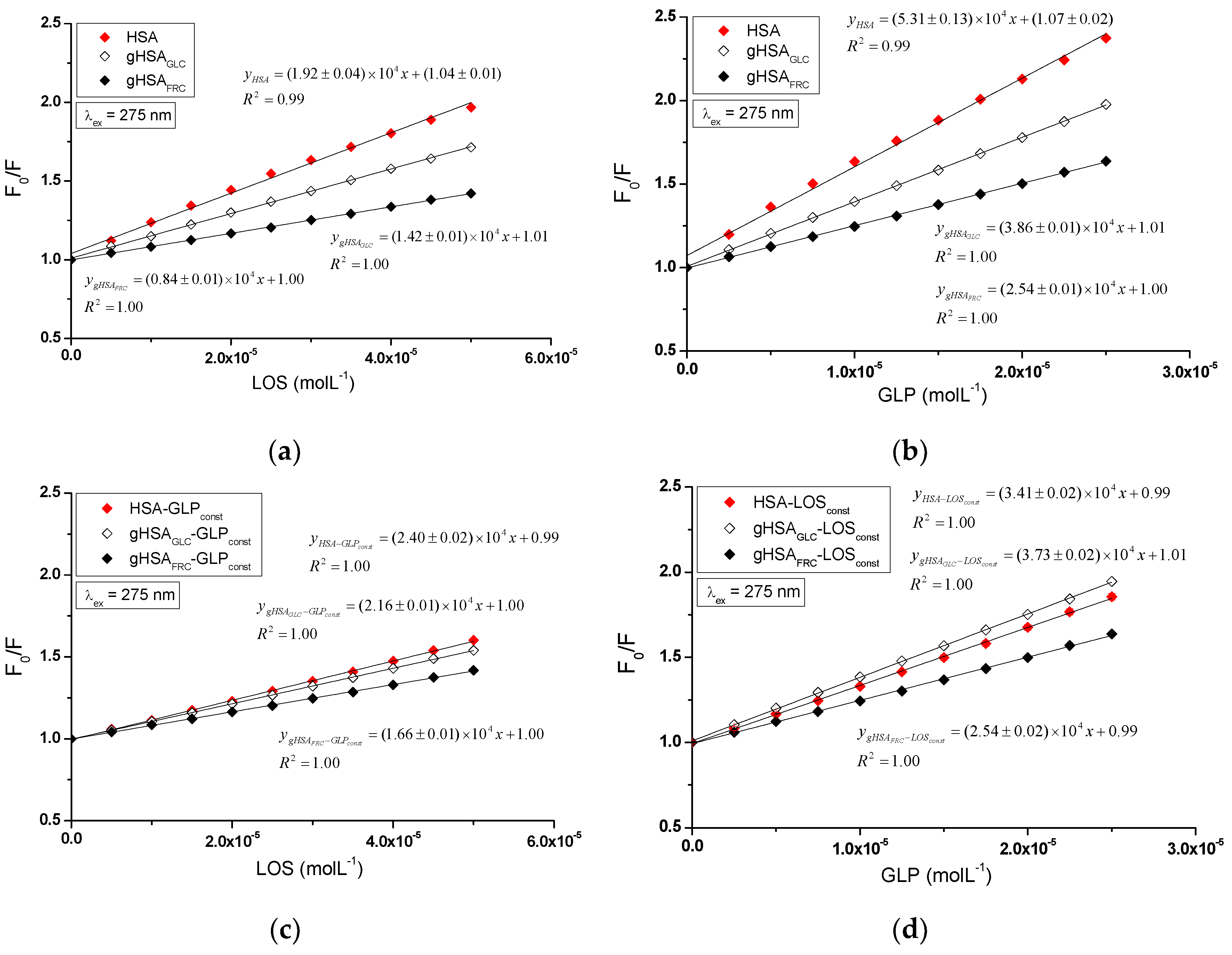
The quenching mechanism of losartan and glipizide interaction with both non-glycated and glycated serum albumin was determined using the Stern-Volmer plots. The analysis encompassed the binary systems LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC (
Figure 6a), GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC (
Figure 6b), as well as the ternary systems LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const (
Figure 6c), GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const (
Figure 6d) at λ
ex = 275 nm (
Figure 6) and λ
ex = 295 nm (
Figure S2,
Supplementary Materials).
The dependence of
on LOS or GLP concentration in the binary and ternary systems at λ
ex = 275 nm (
Figure 6) and λ
ex = 295 nm (
Figure S2, Supplementary Materials) demonstrated a linear correlation for ligand-albumin complexes. The linear Stern-Volmer plots for the ligand-albumin and ligand-albumin-ligand
const system may indicate a dynamic (collisional) or static quenching mechanism of fluorescence for both non-modified (HSA) and glycated albumins (gHSA
GLC, gHSA
FRC). According to the literature, the ligand penetrates the macromolecule's environment in dynamic quenching, and fluorescence quenching is caused by the collision between the quencher molecule and the albumin fluorophore(s). In contrast, static quenching leads to a decrease in the intensity of emitted fluorescence when the ligand binds to the fluorophore molecule in its ground (non-excited) state, thereby reducing the population of fluorophores capable of being excited [
25].
Table 1 and
Table 2 present the Stern-Volmer constants
, the bimolecular quenching rate constant
, and the fractional accessible protein fluorescence
calculated for binary (ligand-albumin) and ternary (ligand-albumin-ligand
const) systems at λ
ex = 275 nm and λ
ex = 295 nm.
The magnitude of the determined fluorescence quenching rate constants
(10¹²) for the investigated systems indicates a static fluorescence quenching mechanism in the LOS-albumin, LOS-albumin-GLP
const (
Table 1) and GLP-albumin, GLP-albumin-LOS
const systems (
Table 2). According to Lakowicz, for collisional fluorescence quenching, the maximum value of the constant
in an aqueous solution is 2 × 10¹⁰ (L∙mol
−1∙s
−1) [
27].
The Stern-Volmer constant serves as a means to assess the accessibility of the quencher to the excited fluorophore. A higher
value indicates a greater availability of ligand molecules in the macromolecule, which leads to the formation of a complex in the excited state [
28]. Glycation of HSA by glucose and fructose results in a decrease in the Stern-Volmer constant in both binary (LOS-albumin, GLP-albumin) and ternary systems (LOS-albumin-GLP
const) at excitation wavelengths of 275 nm and 295 nm. This indicates a lower quenching efficiency of glycated albumin fluorophores compared to non-modified albumin by losartan (
Table 1). In the ternary system (GLP-albumin-LOS
const), fructose-induced glycation decreased
, whereas glucose-induced glycation increased
(
Table 2). The
values determined for the system in the presence of an additional drug (LOS-albumin-GLP
const) are higher than those for the LOS-albumin system, both for unmodified and glycated albumin at λ
ex = 275 nm. At λ
ex = 295 nm, the same effect of increasing
is observed only for the system with glycated albumin, while the presence of GLP in the LOS-HSA system caused a slight decrease in
(
Table 1). Conversely, the
values determined for the system in the presence of an additional drug (GLP-albumin-LOS
const complex) are lower for HSA and gHSA
GLC at λ
ex = 275 nm and λ
ex = 295 nm. In contrast, for gHSA
FRC, they remain unchanged at λ
ex = 275 nm or are higher at λ
ex = 295 nm (
Table 2). At both excitation wavelengths (λ
ex = 275 nm and λ
ex = 295 nm), GLP complexes exhibit higher
values than to LOS complexes, indicating greater quenching efficiency. GLP molecules are closer to fluorophores of non-modified and glycated albumin than LOS molecules in binary and ternary complexes. It is also observed that
values are generally higher at λ
ex = 275 nm compared to λ
ex = 295 nm. The bimolecular quenching rate constants (
) align with the trends observed in
. Most complexes display fractional accessibility (
) close to or equal to 1, suggesting near-complete accessibility of the fluorophore to the quencher, with GLP-HSA showing the lowest accessibility (
Table 2 ).
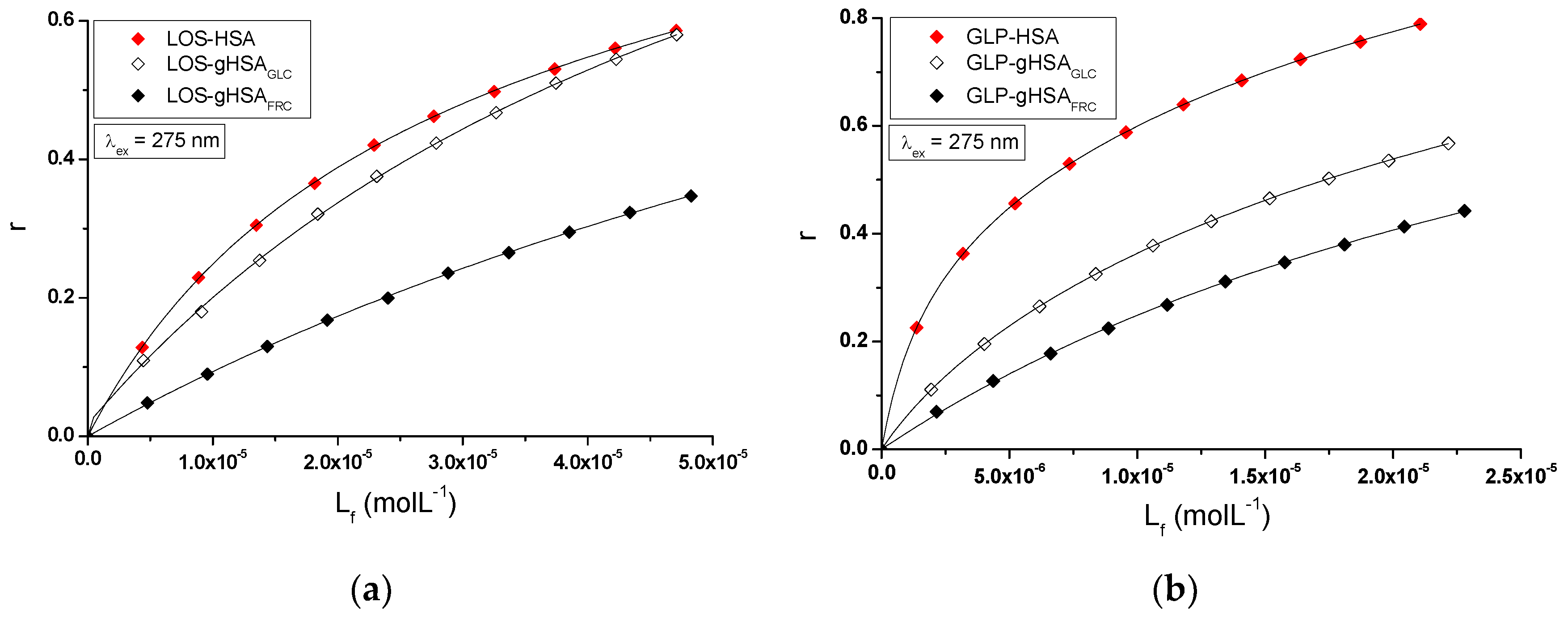
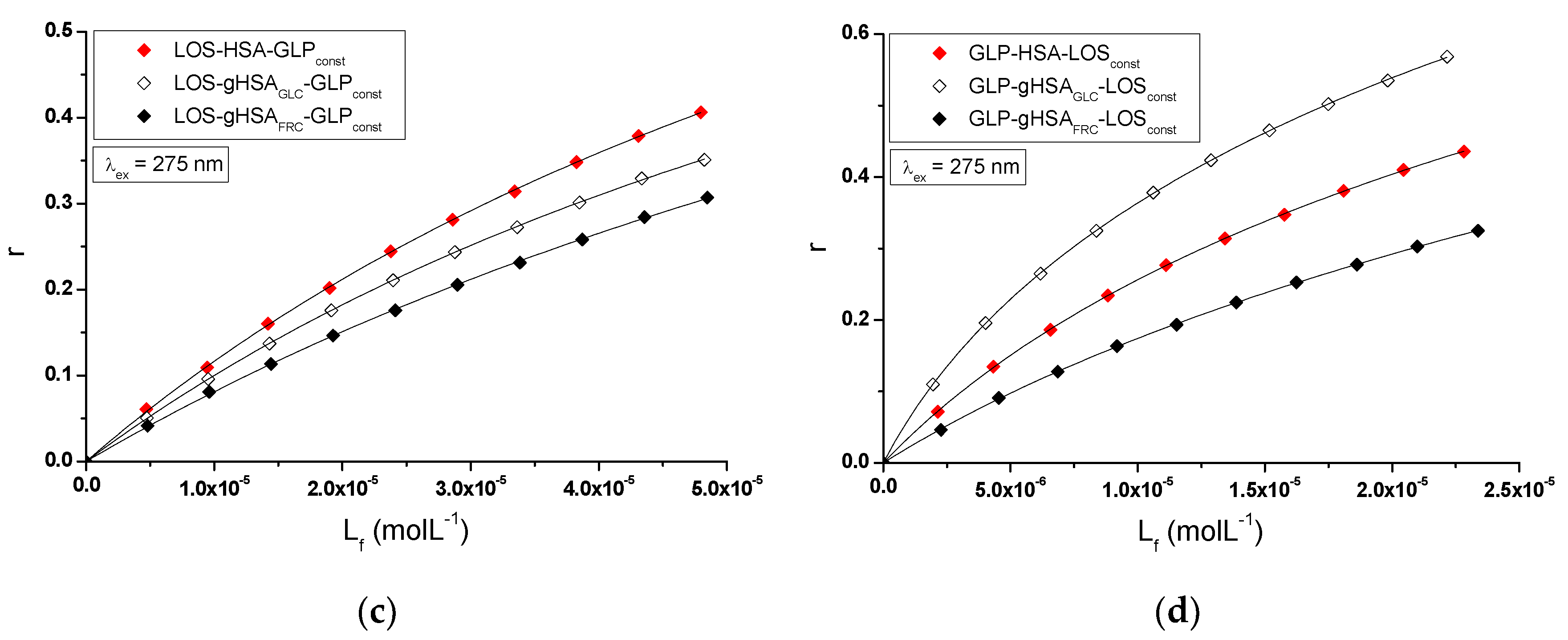
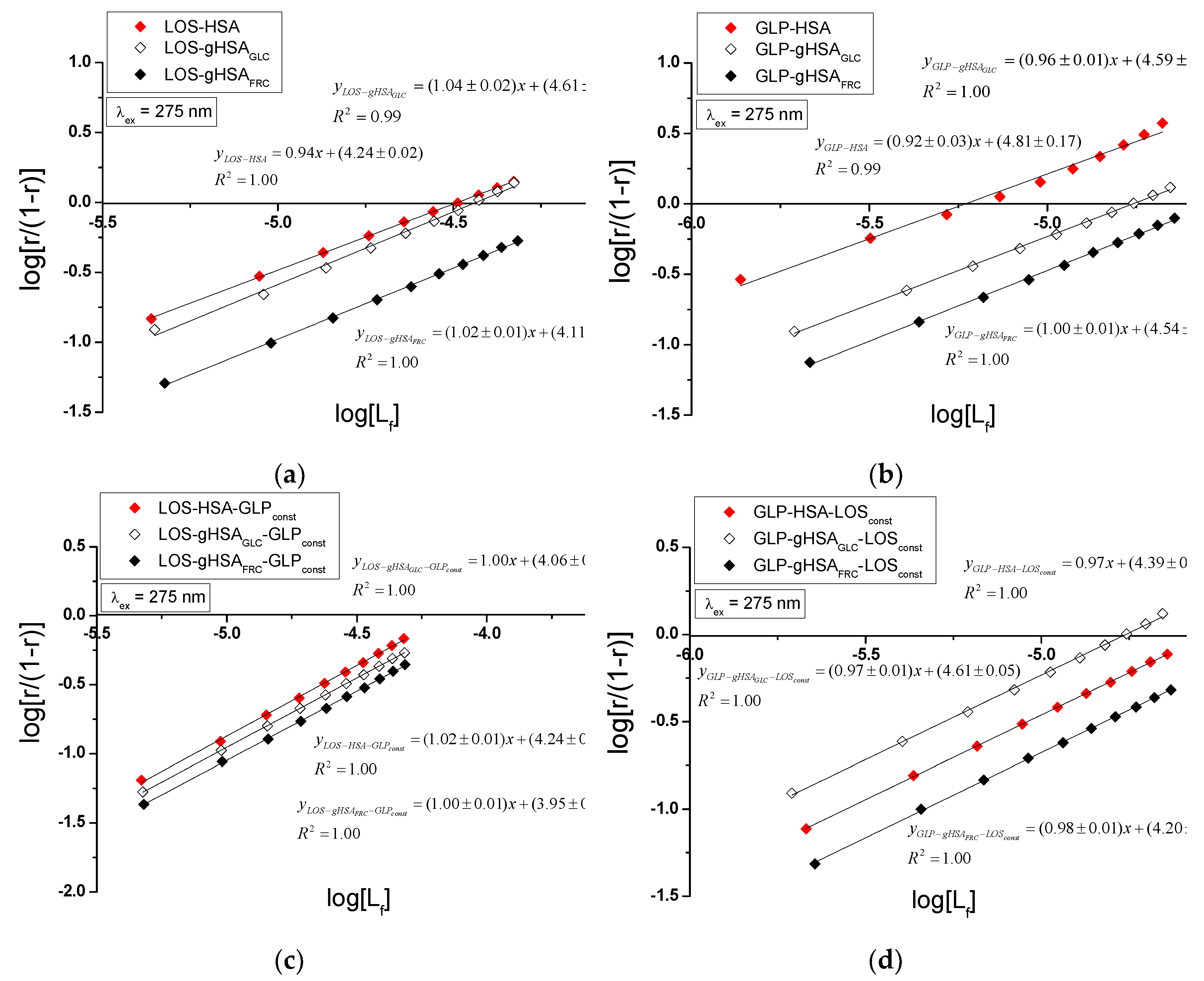
The nature of ligand binding to albumin (specificity of binding sites within the various classes of binding sites) was determined based on the binding isotherms (saturation curves) of LOS and GLP to non-glycated and glycated HSA in binary (
Figure 7 ab) and ternary systems (
Figure 7cd).
For LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC (
Figure 7a), GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC (
Figure 7b), LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const (
Figure 7c) and GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const (
Figure 7d) complexes at λ
ex = 275 nm (
Figure 7) and at λ
ex = 295 nm (
Figure S3,
Supplementary Materials), the course of the saturation curves is not linear across the entire range of ligand concentrations, as each of the binding isotherms exhibits an exponentially increasing course and does not reach a "plateau". Therefore, based on the analyzed plots, it can be inferred that LOS and GLP nonspecifically interact with the hydrophobic fragments of the surface of non-glycated and glycated albumin in both binary and ternary systems and specifically saturate the binding sites within the protein molecule, as confirmed by the literature [
29]. Specific binding is characterized by high affinity and low binding capacity, whereas nonspecific binding is characterized by low affinity and unlimited ligand binding capacity of the ligand [
29]. The physicochemical compatibility of both molecules determines the binding of ligands to serum albumin. Small structural changes in the protein molecule can influence the mutual interaction of the drug with albumin, which in turn affects the binding parameters.
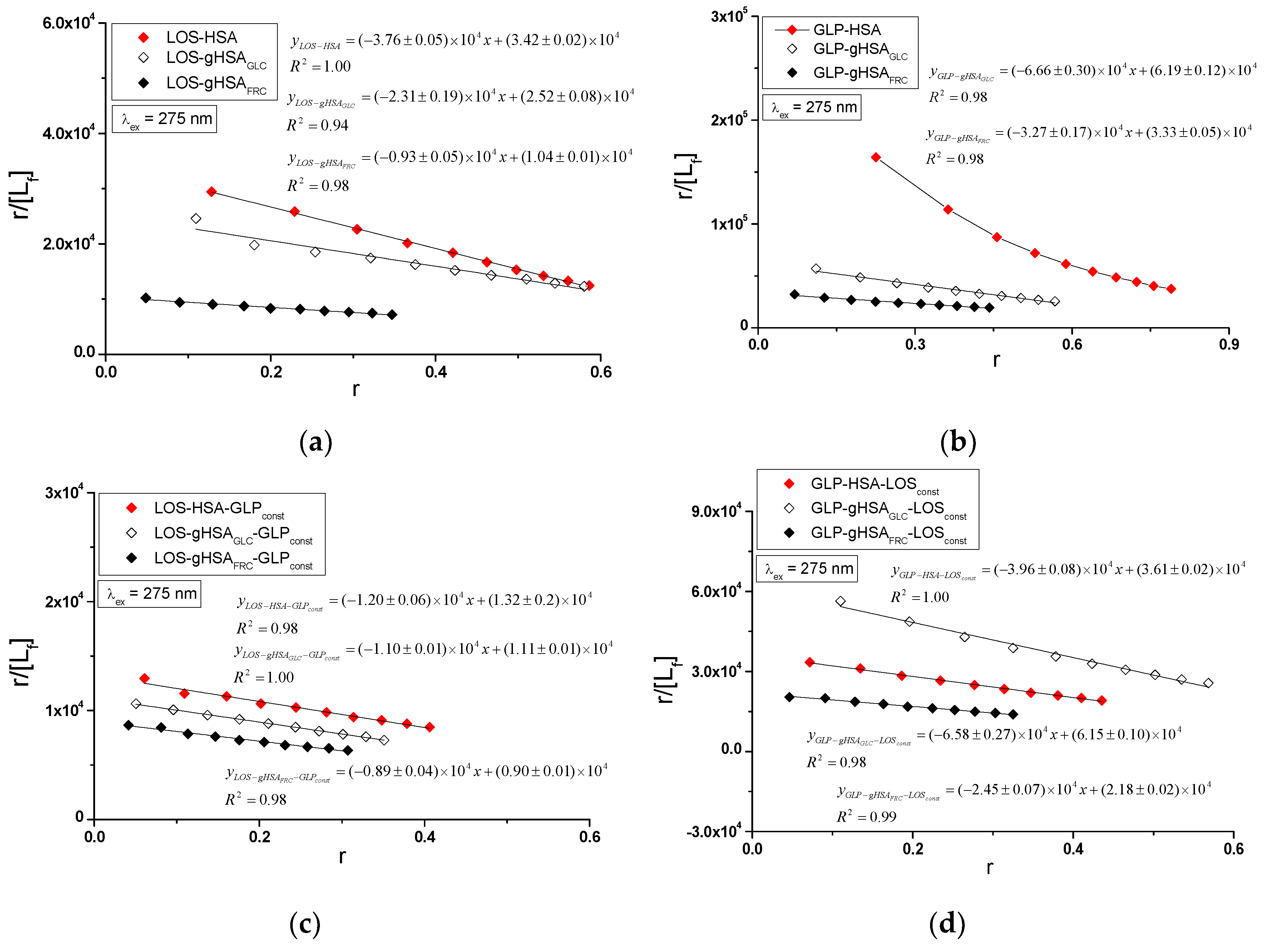
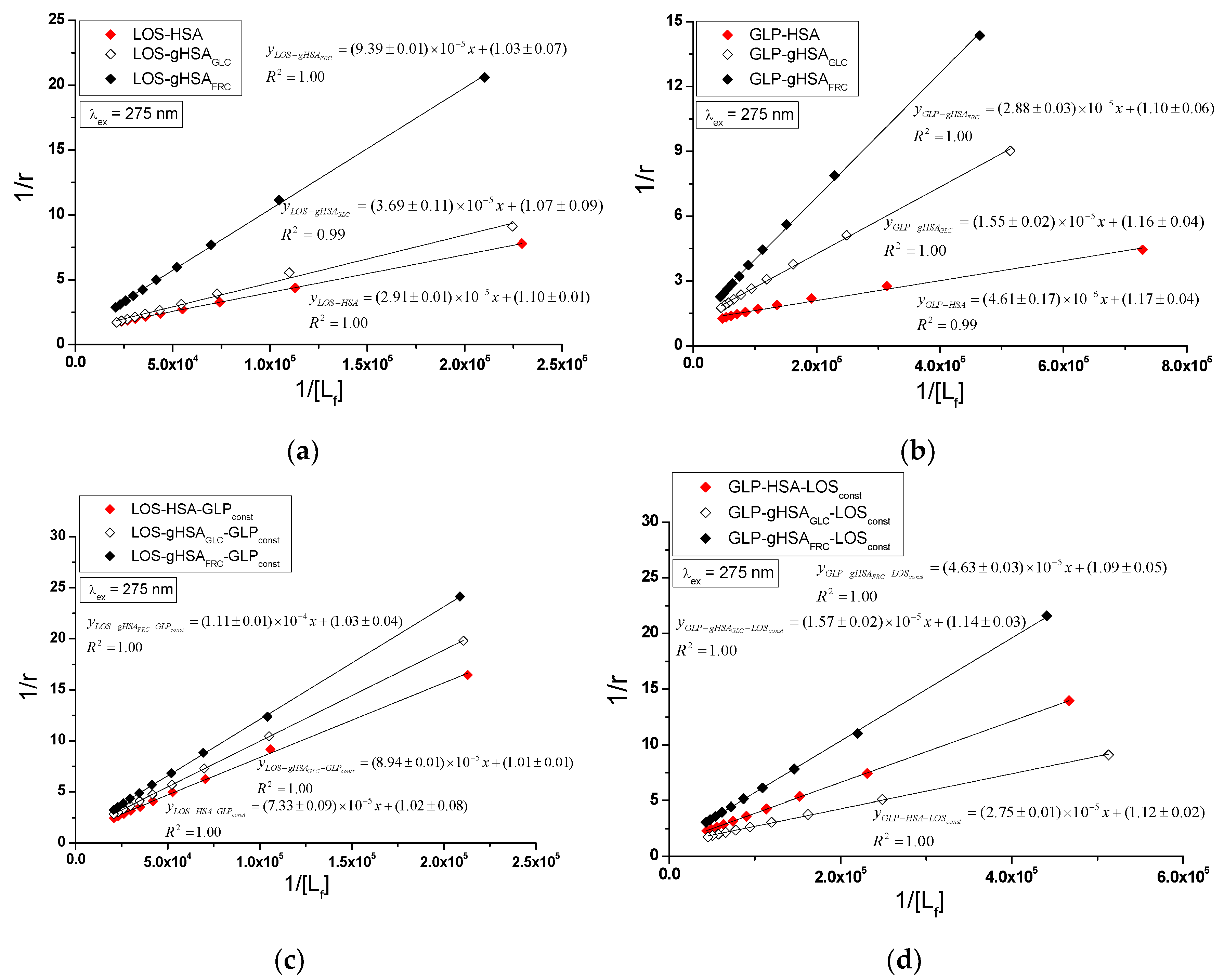
Specific binding of losartan and glipizide to non-modified and glycated albumin in the complexes LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC, GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC, LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const and GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const were quantitatively analyzed by calculating the association constant
using the Scatchard equation (with ligand-bound fraction concentration as the independent variable) (
Figure 8), the Klotz equation (with the inverse of the free ligand fraction concentration as the independent variable) (
Figure 9), and through non-linear regression based on the Levenberg-Marquardt algorithm, i.e., binding isotherms (
Figure 7). Additionally, Hill's coefficients
, representing cooperativity, were determined using the linear Hill plot (with the logarithm of the free ligand fraction concentration as the independent variable) (
Figure 10). Changes in the high-affinity binding of LOS and GLP to non-glycated and glycated albumin in binary and ternary systems, based on
, the number of LOS and GLP molecules bound to one mole of the macromolecule at a specific binding site (
), as well as Hill's coefficient of cooperativity, are summarized in
Table 3 and
Table 4, respectively (λ
ex = 275 nm, λ
ex = 295 nm).
The Scatchard model of ligand-protein interactions postulates that the protein molecule possesses a finite number of specific binding sites for ligands. In this case, the Scatchard dependence
is linear and intersects the x-axis of the coordinate system (the r-axis). The linear Scatchard plots for the complexes LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC (
Figure 8a), GLP-gHSA
GLC, GLP-gHSA
FRC (
Figure 8b), LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const (
Figure 8c) and GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const (
Figure 8d) at λ
ex = 275 nm (
Figure 8) and λ
ex = 295 nm (
Figure S4,
Supplementary Materials) indicate the existence of one class of equivalent, independent binding sites for LOS and GLP in both non-modified and glycated albumin structures (or a single binding site), characterized by the same association constant
. A non-linear Scatchard dependence resembling a hyperbola was observed for the GLP-HSA complex excited at λ
ex = 275 nm (
Figure 8b) and λ
ex = 295 nm (
Figure S4b, Supplementary Materials). This phenomenon may result from the presence of more than one class of ligand-binding sites within the albumin structure (heterogeneous binding), the non-specific nature of GLP binding to HSA, and/or negative cooperativity, where the binding of the drug at one site reduces its affinity for the remaining binding sites on the macromolecule. In contrast, for the LOS-gHSA
FRC-GLP
const complex excited at λ
ex = 295 nm (
Figure S4c,
Supplementary Materials), a "cone-shaped" Scatchard plot was obtained, which may indicate positive cooperativity or instability of losartan. Assuming the existence of two classes of binding sites, the binding parameters for GLP-HSA and LOS-gHSA
FRC-GLP
const were determined by non-linear regression using the Levenberg-Marquardt algorithm (
Table 3 and
Table 4).
Figure 9 illustrates the linear course of the Klotz dependence
for the binary (
Figure 9a and b) and ternary systems (
Figure 9c and d) at λ
ex = 275 nm, indicating the binding of ligands to albumins within a single class of binding sites. Notably, for the system LOS-gHSA
FRC-GLP
const at λ
ex = 295 nm, a non-linear course of the Klotz dependence was observed (
Figure S5,
Supplementary Materials).
In the first class of binding sites, the association constants
determined from the linear Scatchard and Klotz dependencies for the LOS-gHSA
GLC and LOS-gHSA
FRC complexes when excited at λ
ex = 275 nm and λ
ex = 295 nm are lower than those for the LOS-HSA complex (
Table 3). A comparable trend was noted in the ternary system, where the
are lower for the complexes with glycated (LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const) compared to non-modified albumin (LOS-HSA-GLP
const). This suggests that albumin glycation reduces the stability of the formed complex for both the excited Trp-214 and the Tyr residues. Losartan has the lowest affinity for fructose-glycated protein (gHSA
FRC). Moreover, the presence of an additional drug, i.e., GLP, in the LOS-albumin system weakens the binding of LOS to the macromolecules, as evidenced by a decrease in
(
Table 3). For the LOS-gHSA
FRC-GLP
const complex excited at λ
ex = 295 nm, the non-linear course of the Klotz plot made it impossible to determine the binding parameters (
Figure S5).
The
constants, determined by linear regression from the dependencies on the Klotz and the Scatchard equations, as well as based on binding isotherms, are significantly higher for the GLP-HSA compared to the GLP-gHSA
GLC and GLP-gHSA
FRC complex (
Table 4). This suggests that glipizide has a greater affinity for non-modified than glycated albumin, forming a more stable complex. These findings align with previous studies by Koyama et al. [
30], which used fluorescence quenching techniques to demonstrate that the binding capacity of hypoglycemic drugs to glycated albumin (G-HSA) was significantly lower than to non-modified HSA. Moreover, Wiglusz et al. [
31] demonstrated that gliclazide, a popular hypoglycemic drug, binds more weakly to glycated albumin than its native form. These results confirm that glycation alters the protein structure and drug-binding capacity. Furthermore, Chume et al. [
32] confirmed that glycation of albumin decreases its binding capacity for hypoglycemic drugs, which has profound implications for the pharmacokinetics and pharmacodynamics of these drugs in diabetic patients. Conversely, for the ternary system, a higher
was determined for GLP-gHSA
GLC-LOS
const compared to GLP-HSA-LOS
const and GLP-gHSA
FRC-LOS
const, indicating that glucose glycation increases the stability of the formed GLP-albumin-LOS
const complex. Furthermore, the presence of LOS in the GLP-albumin complex generally weakens the binding of GLP to HSA and gHSA
FRC, as reflected in a decrease in
(
Table 4). However, the presence of LOS does not significantly affect the
value in the complex with albumin glycated by glucose at λ
ex = 275 nm (
(GLP-gHSA
GLC) ≈
(GLP-gHSA
GLC-LOS
const)).
In addition, glipizide at a 5:1 GLP:albumin molar ratio has a higher affinity for non-glycated and glycated protein than losartan at a 10:1 LOS:albumin molar ratio. This effect indicates that the transfer of energy from albumin fluorophores (Trp-214 and Tyr residues) to GLP is more efficient than to LOS in both binary (ligand-albumin) and ternary (in the presence of an additional drug at a 1:1 molar ratio, ligand-albumin-ligand
const) complexes. The number of binding sites
close to one indicates the existence of a single specific binding site for LOS and GLP in non-modified and glycated molecules (
Table 3 and
Table 4).
To determine whether the binding of LOS and GLP to albumins affects the affinity of the ligand for other binding sites within the macromolecule, Hill’s coefficient
for cooperativity was calculated based on the linear Hill plot.
Figure 10 shows the linear Hill dependence
in the binary LOS-HSA, LOS-gHSA
GLC, LOS-gHSA
FRC (
Figure 10a), GLP-HSA, GLP-gHSA
GLC, GLP-gHSA
FRC (
Figure 10b), as well as the ternary systems LOS-HSA-GLP
const, LOS-gHSA
GLC-GLP
const, LOS-gHSA
FRC-GLP
const (
Figure 10c) and GLP-HSA-LOS
const, GLP-gHSA
GLC-LOS
const, GLP-gHSA
FRC-LOS
const (
Figure 10d) at λ
ex = 275 nm.
For the LOS-HSA (
Table 3) and the GLP-HSA complex (
Table 4), the Hill coefficient values were found to be less than one (
) at both excitation wavelengths, indicating negative cooperativity. This implies that binding one LOS or GLP molecule at a binding site decreases the affinity for subsequent ligand binding at other sites on non-glycated albumin [
33]. Conversely, for the LOS-gHSA
FRC-GLP
const complex (
Table 3), the Hill coefficient greater than one was observed (
), suggesting positive cooperativity. Here, the binding of one LOS molecule facilitates the binding affinity for additional molecules within the LOS-gHSA
FRC-GLP
const complex when excited at 295 nm. For the remaining systems, the Hill coefficient was approximately one (
≈ 1), indicating non-cooperative binding of LOS and GLP to the macromolecules, where the binding of one ligand molecule does not affect the binding affinity of subsequent molecules.