1. Introduction
As a leading meat producer and exporter, the Australian industry maintains a proactive approach to assure food safety and product integrity from “paddock-to-plate” to protect public health and safeguard export market access [
1]. Accordingly, a review of organoleptic post-mortem inspection and carcase disposition criteria (PMID) in the
Australian Standard for Hygienic Production and Transportation of Meat and Meat Products for Human Consumption (the standard) [
2] capitalised on the opportunity and principles provided by the Codex Alimentarius Commission
Code of Hygienic Practice for Meat [
3].
While traditional organoleptic post-mortem inspection was developed in the late 19th and early 20th centuries to control important zoonotic diseases such as tuberculosis, anthrax and taeniasis in Europe and North America when these diseases were relatively prevalent [
4,
5], these inspection techniques have remained largely unamended in standards over the 20
th century. However, the 21
st century has seen substantial reform via application of risk-based approaches to modernise post-mortem inspection and disposition judgment [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17].
In addition to the international acceptance of risk-based reform, opportunity for substantial modernisation in Australia has also been enabled by improvements in animal health and reduced incidence and eradication of zoonoses over the past 40 years [
18,
19,
20,
21]. Consequently, this review of post-mortem inspection and disposition criteria (PMID) was initiated by industry, not the Competent Authority as a response to an unmanaged meat safety risk in domestic or export markets attributable to Australian meat products.
This report provides an overview of conducting a risk-based review of the standard from both a technical perspective of types of evidence required for determination of equivalence for a range of diseases and conditions, and lessons learned from applying the methodology at an industry level to modernise a national standard.
2. Materials and Methods
2.1. Terms of Reference
The formal review of the Australian standard 4696:2007 [
2] commenced in 2016.
The terms of reference (TOR) set by industry included:
removing techniques that are no longer necessary due to the improved animal health status of Australian herds and flocks
altering or removing techniques where new knowledge of animal or foodborne disease indicates current risk management techniques are not effective
assessing the effect of contamination of edible tissues arising from current organoleptic post-mortem inspection (inspection) techniques
reviewing disposition judgment criteria for total carcase condemnation where appropriate and
identifying techniques that are principally related to product quality rather than food safety that might be transferred to companies’ Quality Assurance systems.
2.2. Evidence-Based Approach
The scope of this review was restricted to organoleptic post-mortem inspection and carcase disposition criteria of the standard had not been subjected to a risk-based assessment when last reviewed [
2]. Carcase is defined as the entire body after bleeding that includes carcase parts i.e., head and viscera (the carcase). In Australia the Competent Authority determining the Australian Standard is comprised of state, territory, and federal meat safety jurisdictions. While the review utilised Australian data to amend the Australian standard, it applies and adapts methodologies reported internationally.
Taking a risk-based approach brought into play the following considerations:
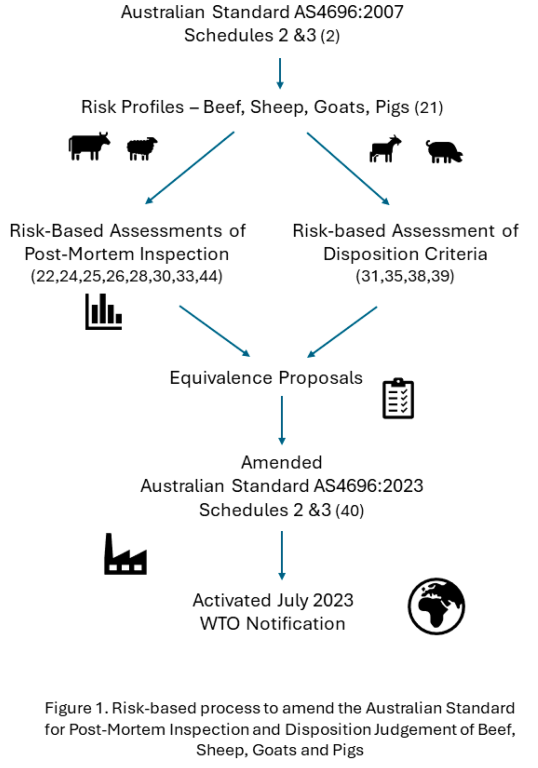
The review commenced with two risk profiles for cattle/sheep/goats and pigs (Figure 1) to identify hazards and rate the risk associated with hazard:gross abnormality combinations [
21]. These reflected assessments reported for the United Kingdom [
11,
12]. The first step involved examination of foodborne illness databases to identify contemporary meat-borne hazards. The process then assembled data from research reports on the occurrence and prevalence of identified hazards across the meat supply chain into an exposure assessment framework. Fourteen specific priorities were identified; seven to quantify and compare the effectiveness of alternative inspection techniques with the standard and seven assessments of criteria used for carcase disposition judgements (Figure 1).
For comparing the effectiveness of alternative inspection techniques, non-detection rates of gross abnormalities were extensively used as an indicator of consumer risk i.e. as a surrogate Acceptable Level of Protection (ALOP). This quantitative comparison was applied to gross abnormalities that only affected wholesomeness as well as those affecting food safety [
21,
24,
25]. For Cysticercus
bovis a full quantitative risk assessment was conducted to estimate the effect of amended inspection on consumer risk [
26]. Comparisons of microbial contamination rates between inspection techniques were presented as evidence [
22,
24].
2.3. Approaches to Compare the Performance of Alternative with Existing Post-Mortem Inspection Techniques
To compare the effectiveness of detection of gross abnormalities by alternative techniques, knowledge of the Se of detection of gross abnormalities by inspection was an important starting point. However, it became immediately apparent that there was a paucity of Sensitivity (Se) data for current inspection techniques for cattle, sheep and goats determined by studies undertaken in Australian abattoirs. Consequently, quantification of the performance of current inspection techniques was included in project methodology to enable comparison with alternative techniques.
This
Se data gap, however, did not apply to inspection of pigs where the assessment of equivalence of routine visual-only inspection utilised field data from Denmark and Australia [
24,
27,
28]. These
Se data were used in conjunction with more recent data on prevalence of gross abnormalities in Australia to compare the non-detection rates of gross abnormalities associated with routine visual-only inspection with the standard.
In this Australian review several approaches to address these Se data gaps were employed. These included:
quantitative risk assessment for C.
bovis where unconfirmed gross abnormalities were conservatively modelled as positive for C.
bovis using published
Se data [
26]
additional veterinary specialist inspection to provide a gold standard prevalence of gross abnormalities i.e., including those not detected by current and alternative inspection [
24,
29]
use of Delphi expert opinion [
9,
25]
only comparing non-detection rates when the initial prevalence of gross abnormalities affecting food safety was estimated as negligible i.e., the vast majority detectable abnormalities only affect wholesomeness [
21,
30,
31,
32]
To enable comparison of the non-detection rates, the prevalence of grossly detectable abnormalities at slaughter had to be determined in most assessments. The sampling framework for prevalence surveys applied exposure assessment principles, representing the spectrum of production systems, breeds, and regional production and processing numbers across Australia on a proportional basis, and including seasonality and animal age as relevant [
21,
25,
33,
34].
To acquire nationally representative data to meet the requirements of a national standard, data recording was undertaken by especially trained Australian Government Authorised Officers. Data acquisition and recording methods are detailed in each of the reports. The projects comparing inspection techniques for pigs were in most part implemented and managed in abattoirs by veterinarians that are authors of this report. For the historic major zoonoses of cattle, tuberculosis, and C.
bovis, suspect samples were submitted for laboratory testing [
19,
20].
2.4. Contamination and Net Effect of Post-Mortem Inspection
Application of the CAC risk assessment guidelines [
3] bring into scope an examination of the likely negative effect of traditional inspection where the net effect may be a poorer food safety outcome. A negative net effect is where the detection and removal of foodborne hazard:gross abnormality is outweighed by contamination of edible tissue with hazards resulting from the actual inspection techniques utilised [
7]. Australian data was used to model net effects [
28,
35].
Additionally, a prevalence survey in two abattoirs was undertaken to determine the contamination rate of inspectors’ hands by
Salmonella spp. during routine carcase inspection [
21].
2.5. Techniques Transferable to Companies’ Quality Assurance Systems
One of the TOR was to identify procedures that were principally related to product quality rather than food safety, that might be transferred to companies’ Quality Assurance systems.
To examine this proposition, a study was conducted to establish the
Se of detection of pleurisy in pigs by routine slaughter-line personnel when removing thoracic organs [
36]. Pleurisy of pigs was chosen for this assessment as it is not associated with foodborne hazards and is a leading reason for partial condemnation of pigs in Australia [
37]. This leads to processing inefficiency due to excessive numbers of carcases detained for trimming.
An assessment compared detection of pleurisy on the same carcases by experienced slaughter-line personnel at routine evisceration with official PMID.
2.6. Rationale for Assessment of Criteria Used for Carcase Disposition Judgement
During the conduct of the initial risk profiles the project team was advised that carcase condemnation rates for some species at some abattoirs were substantially greater than the national rates. These scenarios enabled opportunistic case studies of affected carcases at these abattoirs, to evaluate the effectiveness of carcase disposition criteria (Figure 1) that included:
discernment of acute from chronic carcase abnormalities
disposition outcomes for carcases with multiple, chronic abnormalities (prior septicaemia) and
interpretation of terms such as “systemic involvement”.
For many gross abnormalities that result from systemic infections, the aim of inspection is to determine the actual stage of infection the carcase lesions represent in the continuum from acute (current systemic), organising (infection localising to affected sites), resolving (localising lesions) to chronic (lesions localised and then diminishing in extent until no residual tissue damage remains), with or without sequelae (
Table 1). Each subsequent stage is associated with a decreasing probability of the causative organism still being present. The duration of each stage will vary considerably from animal to animal and will be further influenced by the effectiveness of treatments.
Knowledge of gross abnormalities associated with foodborne hazards is also paramount in determining PMID techniques. Consequently, the opportunity to provide information to assist inspectors was undertaken with the aim of reducing uncertainty in determining carcase disposition; a need heightened by the short time-frame available for routine inspection.
Accordingly, microbiological examination of gross abnormalities, lymph nodes and edible tissues were undertaken to provide additional hazard-based information to supplement pathology criteria in determining carcase disposition [
28,
31,
38,
39], replicating that developed for pig carcases affected by embolic pneumonia in Denmark [
13]. The approach taken not only considered detection of the infectious agents that may be causing the primary abnormalities and septicaemia (i.e., in non-draining lymph nodes), but also whether foodborne hazards (e.g.,
Salmonella spp.) occur in gross abnormalities and edible tissues as a sequel to the primary illness.
Priorities for the assessment of disposition criteria included pneumonia/pleurisy complex and polyarthritis of cattle and pig carcases. Cattle and pig carcases fitting the case definition leading to total condemnation due to multiple gross abnormalities and not showing signs of cachexia, were subjected to the following assessment and testing regime:
Description of gross abnormalities and carcase sites affected
Assessment of the acute versus chronic nature of gross abnormalities
Microbiological testing:
◦
of the primary lesion for causative infectious organisms
◦
of lymph nodes not directly draining the lesion/abnormality and edible tissue to determine if totally condemned carcases were septicaemic (i.e., acute systemic infection) and
◦
to assess of the presence or absence of food safety hazards in edible tissue.
2.7. Expert Panels
Expert panels with extensive experience provided practical guidance on survey methodologies to estimate prevalence to fill data gaps, assisted in interpretation of key findings, and provided information on practical design of validation projects conducted during routine slaughter operations [
21,
25]. The capabilities of the expert panels spanned:
experience with regulatory reform using Codex Risk Assessment guidelines
practical and long-standing field experience with meat inspection at the operational and plant management level
experience in Competent Authority roles (including domestic standards management and market access considerations)
veterinary experience in the field as an abattoir veterinarian; respected authorities in their field
experience in using published risk rating methods and publishing outcomes of related studies
awareness of the level of evidence required by Competent Authorities to assess equivalence
statistical and epidemiological skills to underpin data rigor.
3. Results and Discussion
3.1. Hazard Identification
The Hazard Identification (HI) demonstrated
Salmonella Typhimurium as the most likely hazard to occur in association with fresh meat products in Australia for these species. However,
Salmonella spp. was found to be a primary agent or secondary contaminant of a minority of gross abnormalities. Conversely, this Australian HI confirmed that most gross abnormalities found at slaughter, in both type and prevalence, are not associated with identified foodborne hazards and only affect wholesomeness that result from animal health and welfare issues [
21].
Staphylococcus aureus was identified as being a common cause of meat-borne illness in Australia with outbreaks attributed to human strains contaminating post-cooked product with subsequent temperature abuse enabling toxin build-up [
21], as reported by EFSA [
6,
7,
8]. However,
Staphylococcus aureus is commonly associated with gross abnormalities of carcases affecting wholesomeness and were considered accordingly in the review. A similar rationale was also found to apply to
Clostridium perfringens.
3.2. Findings and Priorities from Risk Profiles
The initial risk profiles conducted for the review found few reports on
Se of post-mortem inspection conducted in Australia [
21], though some were available from EU reports [
6,
7,
8,
26,
27]. Similarly, there is a paucity of prevalence surveys based on regional production/slaughter volumes, or for the range and prevalence of gross abnormalities occurring at specific carcase sites (e.g., liver, lymph nodes, etc.). needed for comparison of alternative inspection techniques. A review of published data indicated that most gross abnormalities detectable in beef, sheep, goat, and pig carcases in Australia only affect wholesomeness [
21].
The risk profiles defined fourteen priorities for a risk-based assessments of PMID activities for cattle, sheep, goats, and pigs Priorities identified included:
removing post-mortem inspection procedures that are no longer necessary due to the improving animal health status of Australian animals include e.g., bovine tuberculosis, Cysticercus bovis and caseous lymphadenitis of sheep and goats
altering or removing procedures where new knowledge of animal or foodborne disease indicates current risk management procedures are not effective e.gs., inspection of spleens and unenucleated kidneys of sheep and goats
assessing the effect of inspection on microbial contamination of edible product of pigs e.g., visual inspection of offal of sheep and goats
reviewing the criteria used to determine disposition e.gs., melanoma of pigs, pneumonia of cattle and pigs, polyarthritis of cattle and pigs
identifying procedures that are principally related to detecting gross abnormalities that affect product wholesomeness rather than food safety and might, therefore, be managed within quality assurance arrangements e.g., pleurisy of pigs.
3.3. Equivalent Alternative Post-Mortem Inspection Techniques
Criteria for assessing the equivalence of alternative inspection is set by the Competent Authority (Australian Meat Regulators Group). These include assessment of any adverse effects on food safety, wholesomeness, animal health (including zoonoses) and animal welfare. Key quantitative metrics applied for determination of equivalence of inspection techniques included comparisons of estimated public health risk, non-detection rates and microbial contamination, as appropriate.
In summary, the validation studies of alternative techniques based on national data demonstrated negligible:
differences in non-detection rates between observation and palpation i.e. undetected abnormality increase commonly in the order of 1/1,000 to 1/10,000 carcases
occurrence of gross abnormalities of foodborne significance
adverse effect on information available for carcase disposition judgement resulting from visual-only inspection; and
adverse effect on animal health and welfare surveillance.
Amended equivalent inspection techniques in AS4696:2023 Schedule 2 [
40] from applying an equivalence assessment include:
reduced incision of lymph nodes for bovine tuberculosis [
19,
41]
cessation of routine incision of masseters for Cysticercus bovis of cattle unless flagged as high-risk, wherein full carcase inspection is required; otherwise. Routine incision of hearts is retained [
20,
26,
40,
42,
43].
reduced palpation of lymph nodes for detection of caseous lymphadenitis of sheep and goats [
22,
25]
visual inspection of sheep and goat spleens instead of palpation, irrespective of being kept for human consumption [
44]
routine visual-only inspection of commercially reared pigs including cull breeding stock, irrespective of whether they are reared in intensive and extensive production systems [
24,
28,
33]
observing unenucleated kidneys of sheep, goat, and pig kidneys when not for human consumption and observation of enucleated kidneys when for human consumption [
30,
34].
3.4. Retention of Palpation and Incision
While routine incision and palpation is much reduced for cattle, sheep and goats and removed for pigs, provision is added for use of palpation and incision “where appropriate” for all conditions detected or suspected (Table 4, Schedule 2 AS4696:2023; [
40]) as provided by the European Commission [
45]. For example, in moving to routine visual-only inspection of pigs, such circumstances would be suspect carcases identified through visual detection of relevant abnormalities or herd health history particularly evidenced by recent PMID data [
46]. This approach is especially relevant to modern pig production in Australia, whereby sale pigs are shipped direct to the same abattoir from the same farm a weekly basis. It also applies to lot fed cattle where site related conditions may emerge, with carcases showing similar gross abnormalities at the same abattoir [
38,
39]. The provision of “where appropriate” in the amended standard allows varying PMID techniques in real-time .
In keeping with the risk-based approach, when palpation and incision are used, these additional techniques must be followed by effective decontamination of hands and associated equipment to minimise subsequent contamination of edible tissue [
40]. These assessments of equivalence and amended procedures mirror the risk-based modernisation of PMID occurring internationally in recent years [
6,
7,
8,
14,
15,
45].
However, routine palpation and/or incision is retained in some circumstances for cattle, sheep and goats. Routine palpation is retained for a lungs, liver, kidney, and tongue to detect deep-seated gross abnormalities e.gs. abscesses, granulomas etc. Palpation and incision also remain unchanged if specified diseases are detected or suspected, notably tuberculosis and parasitic zoonoses (Table 4, Schedule 2 of AS4696:2023; [
40]). For cattle and buffalo the incision of masseters is only required under specified circumstances [
40,
42]. For sheep and goats, while palpation is much reduced due to reduced palpation for caseous lymphadenitis, hearts are routinely palpated and bile ducts incised (Supplementary Material 1, Tables A7.1–7.4; [
21]).
3.5. Use of Food Chain Information
In terms of food chain information (FCI) being available from primary production to tailor meat hygiene requirements [
3,
16,
17], three forms of this is practiced or are developing in Australia. The first applies to all consignments of cattle, sheep, goats and pigs slaughtered for human consumption in Australia. These are accompanied by a mandated statutory vendor declaration of relevant food safety and traceability information [
47] that cover suspect bovines for C.
bovis, animals known to be carrying physical hazards (e.g. broken needles), and any that do not meet chemical residue withholding periods [
48,
49].
The second example of FCI practiced routinely is where pre-slaughter information is used to guide PMID inspection for C.
bovis [
40]; a principle advocated by Jacobs et al [
17]. Low risk cattle now require routine inspection of observation of masseters and only routine incision of heart muscle for C.
bovis; noting that while hearts are the most common site for CB, the retention of incision is primarily for detection of hydatids. For high-risk groups of cattle, defined as the animal has grazed properties where exposure to
Taenia saginata eggs may have occurred, full carcase inspection applies. This requires incision of masseter muscles, heart, and other sites (AS4696:2023, Schedule 2, Table 4: [
40]).
An accompanying revised CB risk management framework of FCI information provides the detail required by regulators, processors, producers, and other stakeholders along the supply chain about the actions and requirements to ensure risk-based PMID is applied to cattle classified as low or high-risk for C.
bovis [
42,
43].
This amended PMID for CB also applies to cattle exposed to adequately recycled human sewage water ensuring risk is identified and managed appropriately. Cattle grazed on lands treated with recycled water that has been treated with a helminth egg Log Reduction Value (LRV) of ≥4.0 or LRV 3.0 if an alternate clean water source is available for cattle to drink (i.e., no access to recycled water for livestock to drink) are now managed as low-risk cattle for PMID [
42,
43,
50,
51,
52].
The third form of FCI being implemented in parallel to the PMID review is the eventual adoption of reporting of information of gross abnormalities encountered at routine slaughter back to producers for pigs [
37,
46] and sheep and goats [
53]. This approach is especially suited the extensive rangeland production systems practiced in Australia for cattle, sheep and goats, while reflecting the intent of Codex-facilitated reform [
3].
3.6. Contamination and Net Effect of Post-Mortem Inspection
In the 2011 EFSA review of post-mortem inspection of pigs [
7], it is noted that hygiene, which is a pre-requisite for production of safe meat, is also hampered by manual meat inspection procedures. Making further incisions in tissues/organs following incision of even “normal appearance” lymph nodes is a pathway for spreading pathogens such as
Salmonella spp. and
Yersinia enterocolitica over the carcass, and between carcasses [
28,
54].
In view that the bulk of observable abnormalities in Australian slaughtered stock not being of food safety significance [
21], an assessment was undertaken to model whether inspection may be microbiologically counter-productive. Using previous Australian data to model net effect, Pointon
et al., [
28], (Appendix 2 [
35]) estimated visual-only inspection of lymph nodes of pigs’ heads outweighs incision by around 214 to 1 i.e., for each
Salmonella contaminated head lymph node abscess detected, 214 carcases (subsequent sites) handled are potentially contaminated. The ultimate effect on consumer risk was not estimated. Since publication of the previous standard [
2] there has been provision to observe, or excise and discard these lymph nodes, and that provision has been retained [
40].
To further examine potential contamination pathways, a prevalence survey in two large pig abattoirs found 14% of inspectors’ hands contaminated by
Salmonella spp. during routine carcase inspection under the previous standard [
21]. This is consistent with evidence of faecal contamination of these inspectors’ hands in the survey, with
E coli at 50% of samplings having a mean log count of 2.23 cfu/swab. By comparison, 9.3% of meat handlers tested positive for
Salmonella spp. in Portuguese abattoirs [
55].
However, inspection is only one of many processes involved in carcase processing that may contaminate edible tissues. While reports indicate the increase in bacterial counts due to inspection is generally low, increases in prevalence of hygiene indicator organisms and foodborne hazards from inspection may be substantial [
22,
23].
3.7. Techniques Transferable to Companies’ Quality Assurance Systems
The assessment of the ability of experienced slaughter-line personnel to detect pleuritic carcases in pigs at routine evisceration, found a high detection rate (
Se 0.9). This supports implementation of preliminary carcase trimming as part of routine carcase dressing (i.e., partial stripping of affected pleura before official PMID) to achieve carcase wholesomeness. This approach minimises carcases having to be detained for final trimming. In addition, there is no loss of information to inform carcase disposition as partially stripped pleura (i.e., dangling
in situ) are obvious to official inspectors when subsequently making the final carcase disposition judgement [
36].
These findings offer feasible improvement in efficiency for both processing and official carcase inspection and have been incorporated in Approved Arrangements without changes to the standard.
3.8. Amended Carcase Disposition Judgement Criteria
During the review, awareness of relatively high total carcase condemnation rates for beef became apparent at some abattoirs. This provided the opportunity for intensive carcase evaluations at these abattoirs to investigate the reason for high rates of carcase condemnation. Similar investigations were conducted for pig carcases, while desk-top studies were employed for sheep and goats as official meat safety risk managers required data for each species.
The consistent finding was that carcases with multiple gross abnormalities mostly reflected historic infections presenting as chronic phase lesions that are no more than a historical infection (i.e., prior septicaemia), which could be trimmed to achieve wholesomeness of meat for human consumption as proposed by Murray in 1986 [
56] and reported in beef by Petersen
et al. in 2022 [
57].
These assessments of disposition criteria included pneumonia/pleurisy complex, and polyarthritis of cattle and pig carcases found those with multiple chronic lesions mostly harboured primary infectious agents in the primary site, but not peripheral lymph nodes or edible tissues.
Salmonella spp. was not recovered from any of these sites in the beef and pig carcases investigated [
31,
38,
39].
Consequently, to minimise uncertainty in judging final carcase disposition use of acute or chronic classification is now consistently applied across these gross abnormalities in the standard. Amended equivalent disposition criteria now published as AS4696:2023 Schedule 2 [
40] for arthritis and pneumonia/pleurisy now includes:
replacement of “Systemic effects” with specific descriptions and disposition, for Acute (disease manifest as septicaemia; indicated by petechial haemorrhages and/or polyserositis) or Chronic (may show multiple localise abnormalities of lungs/joints, no signs of septicaemia) abnormalities
multiple chronic abnormalities not being interpreted as “systemic effect” and thereby a reason for total carcase condemnation, and
these carcases may now be trimmed to achieve wholesomeness instead of being totally condemned, when not showing generalised signs of septicaemia and/or cachexia.
Where uncertainty remains, particularly for high value beef carcases, additional testing to determine safety and wholesomeness may be undertaken to minimise unnecessary wastage [
40].
It is noted that total carcase condemnation rates varied unexpectedly across abattoirs processing comparable cohorts of beef carcases (e.gs. lot fed cattle). The results suggest that at some abattoirs judging final carcase disposition may have taken on a site interpretation and inspection culture that could benefit from objective data from carcase investigations [
38,
39], as noted by Petersen
et al. [
57] in Denmark.
Training for meat inspectors needs to include, for example, the continuum of clinical signs, gross abnormalities, and microbiology of diseases and conditions (e.g. bovine respiratory disease) from acute infection through to localisation of the pathology leading towards resolution, with or without sequelae (
Table 1, [
38]). Similar results were obtained for intensive testing of beef and pig carcases with arthritis [
31,
39]. It is noted that carcases investigated in these studies were predominantly at the resolving (localising) and chronic stage, even though the case definition for the carcase selection was total carcase condemnation determined at routine inspection. The information in
Table 1 is intended as a guideline to better define and minimise the number of carcases where uncertainty remains for final disposition judgment.
For melanoma of pigs, 1.53% of 130,000 pigs surveyed had visible melanoma lesions on the skin. Of these 1989 carcases with skin melanoma, 2.86% had related lesions in the immediate draining lymph nodes. None showed progression beyond these immediate lymph nodes ([
35];Appendix 4). For the specific condition of melanoma of pigs, the amended disposition is, “depending on extent, lesion trimmed and condemned or affected carcase part condemned” [
40]. This is now consistent with disposition judgements in other countries for this inherited gross abnormality.
4. Conclusions
By acquiring risk-based national data, the review has validated that the bulk of gross abnormalities detected by post-mortem inspection in Australia affect wholesomeness, not food safety. This is largely attributed to improved animal health and elimination of meat-borne zoonoses over the past 40 years. Consequently, it is unsurprising that the review was initiated by industry, not as a jurisdictional response to an unmanaged food safety risk. These findings for Australian production systems substantiate other evidence that post-mortem meat inspection serves to detect gross abnormalities that are almost entirely related to food quality and on-farm (health and welfare) management issues [
6,
7,
8,
9,
10,
11,
12,
14,
17,
58] . Consequently, a substantial number of amendments to PMID were long due, notably for C.
bovis [
26,
42] and caseous lymphadenitis of sheep and goats [
25].
There were many lessons learned from performing this risk-based review. In most part, a quantitative assessment of the performance of current PMID was found to be a frequent data gap, that had to be quantified to compare performance of alternative PMID. To address this fundamental gap, projects established prevalence of gross abnormalities across large data sets that were based on regional production and proportional abattoir volumes, the range of gross abnormalities occurring across the continent, age and seasonality where relevant. These also determined the proportional occurrence of gross abnormalities on an organ/carcase site as baseline data for determining the effectiveness of detection by post-mortem inspection. These data sets reported gross abnormalities affecting food safety, as well as those affecting wholesomeness alone.
The acquisition of these data on a national industry scale is well beyond the capacity any research team. While the core research team designed the assessments and monitored data quality, collection of the data was dependent on a combination of engagement with, and access granted by industry, together with official inspectors working in abattoirs nationally.
The expert panels provided essential industry guidance on how national data prevalence and technique comparison data could be recorded by competent industry personnel on a standardised basis during routine operations. This fostered engagement and contributions by industry to meet sampling framework requirements (i.e., exposure assessment data) that included proportional regional slaughter numbers and capturing regional disease and condition variation data for national assessments. The panels also assisted in estimating Se, interpretation of the data and recommending validated amendments for formal risk management consideration.
The equivalence of alternative techniques was based on estimating adverse effects on food safety, wholesomeness, and surveillance of animal health (including zoonoses) and welfare [
3,
10]. Quantitative metrics considered by the Competent Authority for determination of equivalence of alternative post-mortem inspection techniques included comparisons of estimated public health risk, non-detection rates for gross abnormalities, and microbial contamination resulting from inspection activities, as appropriate.
Selection of methodologies to compare inspection procedures also followed a risk-based approach. For the classic meat-borne zoonosis Cysticercus
bovis, a full quantitative risk assessment was conducted to predict the effect of different post-mortem inspection options on consumer risk. Whereas for gross abnormalities affecting wholesomeness alone, non-detection rates were compared. Meat hygiene assessments were used to identify any beneficial microbiological effects on meat safety in assessments of sheep and pigs [
22,
24].
The publication of peer-reviewed papers from the review that accompanied proposals (Figure 1) provided additional assurance to the Competent Authority when assessing equivalence for the amended standard. These publications also informed notification and acceptance of equivalence to maintain export market access.
Alternative PMID procedures were accepted as equivalent with the standard by Competent Authority risk managers resulting in the amended AS4696:2023 [
40] being activated throughout domestic and export-licenced abattoirs from 1 July 2023. While assuring equivalent meat safety and wholesomeness, the amended standard facilitates reduced product wastage, optimisation of carcase cuts, and a basis for allocation of official PMID resources commensurate with risk.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors wish to thank the members of expert panels that contributed to the design of abattoir projects and interpretation of results. Reflecting extensive industry collaboration, meat inspectors and quality assurance managers of the many participating beef, sheep, goat, and pig abattoirs nationally are gratefully acknowledged for their contribution in data recording. Jo Slade is gratefully thanked for the conduct of literature searches and editing of reports and papers. Meat & Livestock Australia, Australian Meat Processors Corporation, and Australian Pork Limited are acknowledged for funding these projects. Participating meat processors are thanked for their in-kind funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Safe Meat [Internet]. [cited 2024 Jul 22]. SAFEMEAT - a partnership between Government and Industry. Available from: https://www.safemeat.com.au/about/.
- Australian Standard AS 4696:2007 Hygienic Production and Transportation of Meat and Meat Products for Human Consumption. Technical Support Series 3 [Internet]. 2007 [cited 2024 Jul 21]. Available from: https://www.primesafe.vic.gov.au/wp-content/uploads/2024/03/AS-4696-2007.pdf.
- CAC/RCP 58-2005. The Codex Code of Hygienic Practice for Meat [Internet]. 2005 [cited 2024 Jul 21]. Available from: https://www.fao.org/fao-who-codexalimentarius/codex-texts/codes-of-practice/en/.
- von Ostertag R. Handbuch der Fleischbeschau für Tierärzte, Ärzte und Richter [Internet]. F. Enke; 1892. Available from: https://books.google.com.au/books?id=fu1UAAAAYAAJ.
- Meat Inspection Act of 1906 | History, Summary, & Facts | Britannica [Internet]. [cited 2024 Jul 21]. Available from:https://www.britannica.com/topic/Meat-Inspection-Act.
- Hazards (BIOHAZ) EP on B. Scientific Opinion on the public health hazards to be covered by inspection of meat (bovine animals). EFSA Journal. 2013;11(6):3266. [CrossRef]
- Hazards (BIOHAZ) EP on B. Scientific Opinion on the public health hazards to be covered by inspection of meat (swine). EFSA Journal. 2011;9(10):2351. [CrossRef]
- Hazards (BIOHAZ) EP on B. Scientific Opinion on the public health hazards to be covered by inspection of meat from sheep and goats. EFSA Journal. 2013;11(6):3265. [CrossRef]
- Hardstaff J, Nigsch A, Dadios N, Stärk K, Alonso S, Lindberg A. Contribution of meat inspection to animal health surveillance in Sheep and Goats. EFSA Supporting Publications. 2012;9(10):320E.
- Stärk KDC, Alonso S, Dadios N, Dupuy C, Ellerbroek L, Georgiev M, et al. Strengths and weaknesses of meat inspection as a contribution to animal health and welfare surveillance. Food Control. 2014 May 1;39:154–62.
- Hill AA, Horigan V, Clarke KA, Dewé TCM, Stärk KDC, O’Brien S, et al. A qualitative risk assessment for visual-only post-mortem meat inspection of cattle, sheep, goats and farmed/wild deer. Food Control. 2014 Apr 1;38:96–103.
- Hill A, Brouwer A, Donaldson N, Lambton S, Buncic S, Griffiths I. A risk and benefit assessment for visual-only meat inspection of indoor and outdoor pigs in the United Kingdom. Food Control. 2013 Mar 1;30(1):255–64.
- Kruse AB, Larsen MH, Skou PB, Alban L. Assessment of human health risk associated with pyaemia in Danish finisher pigs when conducting visual-only inspection of the lungs. International Journal of Food Microbiology. 2015 Mar 2;196:32–9.
- Alban L, Albuquerque ER, Cordeiro de Sá CVG, Buholzer P, Vieira Pinto M, Langkabel N, et al. Modernization of meat inspection of pigs. The world is on the move towards a more evidence-based type of inspection. Fleischwirtschaft international: journal for meat production and meat processing. 2018;(2):8–15.
- Food Safety and Inspection Service (FSIS). Federal Register. 2019 [cited 2024 Jul 21]. Modernization of Swine Slaughter Inspection. Available from: https://www.federalregister.gov/documents/2019/10/01/2019-20245/modernization-of-swine-slaughter-inspection.
- FAO. Technical guidance principles of risk-based meat inspection and their application. Food and Agriculture Organization of the United Nations; 2019.
- Jacobs P, Berends B, Lipman L. The Value of Current Ante Mortem Meat Inspection and Food Chain Information of Dairy Cows in Relation to Post Mortem Findings and the Protection of Public Health: A Case for a More Risk-Based Meat Inspection. Foods. 2023 Jan;12(3):616.
- Paton M, Walker S, Rose I, Watt G. Prevalence of caseous lymphadenitis and usage of caseous lymphadenitis vaccines in sheep flocks. Australian Veterinary Journal. 2003 Jan 1;81(1–2):91–5.
- Pearse B, Langbridge J, Cobbold R, Glanville R. Current activities add little to food safety. Fleischwirtschaft International. 2009;24(1):46–50.
- Pearse B, Traub R, Davis A, Cobbold R, Vanderlinde P. Prevalence of Cysticercus bovis in Australian cattle. Australian Veterinary Journal. 2010 Jul 1;88(7):260–2.
- Pointon A, Hamilton D, Kiermeier A. Assessment of the post-mortem inspection of beef, sheep, goats and pigs in Australia: Approach and qualitative risk-based results. Food Control. 2018 Aug;90:222–32.
- Jordan D, Sentance CB, Spooncer WF, Balan JA, Morris SM. Inspection of lymph nodes for caseous lymphadenitis and its effect on the density of microbes on sheep carcasses. Meat Science. 2012 Dec 1;92(4):837–40.
- de Freitas Costa E, Corbellini LG, da Silva APSP, Nauta M. A Stochastic Model to Assess the Effect of Meat Inspection Practices on the Contamination of the Pig Carcasses. Risk Anal. 2017 Oct;37(10):1849–64.
- Hamilton, D, Gallas, P, Lyall, L, Lester, S, McOrist, S, Hathaway, S.C., et al. Risk-based evaluation of postmortem inspection procedures for pigs in Australia. The Veterinary record [Internet]. 2002 Jul 27 [cited 2024 Jul 21];151(4). Available from: https://pubmed.ncbi.nlm.nih.gov/12180659/.
- Pointon A, Hamilton D, Kiermeier A. Comparison of postmortem inspection procedures for detecting caseous lymphadenitis of Australian sheep and goats. Veterinary Record. 2019 Jul 1;185(2):54–54.
- Kiermeier A, Hamilton D, Pointon A. Quantitative risk assessment for human Taenia saginata infection from consumption of Australian beef. Microbial Risk Analysis. 2019 Aug 1;12:1–10.
- Willeberg P, Wedam JM, Gardner IA, Holmes JC, Mousing J, Kyrval J, et al. A comparative study of visual and traditional post-mortem inspection of slaughter pigs: estimation of sensitivity, specificity and differences in non-detection rates. Épidémiologie et Santé Animale. 1997;(31):04.20.1-04.20.3.
- Pointon AM, Hamilton D, Kolega V, Hathaway S. Risk assessment of organoleptic postmortem inspection procedures for pigs. Vet Rec. 2000 Jan 29;146(5):124–31.
- Hathaway SC, Richards MS. Determination of the performance attributes of post-mortem meat inspection procedures. Preventive Veterinary Medicine. 1993 Jun 1;16(2):119–31.
- Pointon A, Hamilton D, Kiermeier A. Project V.RBP.0022 Report. Risk-based Review of Post-mortem Inspection of Unenucleated Kidneys of Sheep and Goats. Meat and Livestock Australia; 2018. Report No.: Project V.RBP.0022.
- Pointon A, Hamilton D, Kiermeier A. [Risk-Based Assessment of Criteria used for Disposition Judgement for Polyarthritis of Pigs]. Advances in Animal Biosciences. 2019;10(Suppl 1):s74.
- Australian Meat Regulators Group Guideline 2021.1. Fact Sheet 2.1 Post-Mortem Meat Inspection – Alternative techniques to Schedule 2 and 3 of AS 4696:2007 [Internet]. 2020. Available from: https://mintrac.com.au/docs/pages/175/Sched%202_1.%20Fact%20Sheet_Sheep%20and%20goat%20spleen%20inspection.pdf.
- Pointon A, Hamilton D, Kiermeier A. [Validation of Routine Visual Only Post-Mortem Inspection of Pigs in Australia]. Advances in Animal Biosciences. 2019;10(Suppl 1):s47.
- Pointon A, Hamilton D, Kiermeier A. [Risk-based Review of Post-mortem Inspection of Kidneys of Pigs in Australia]. Advances in Animal Biosciences. 2019;10(Suppl 1):s48.
- Pointon A, Hamilton D, Kiermeier A. Pork.Project 2015/023 report. Review of the Post-mortem Inspection and Disposition Schedules of the Australian Standard. Australian Pork Limited, Canberra ACT; 2017.
- Pointon A, Hamilton D, Kiermeier A. [Validation of alternative post-mortem inspection procedures with reference to pleurisy of pigs in Australia]. Advances in Animal Biosciences. 2019;10(Suppl 1):s75.
- Hamilton D, Jolley J. Health4Wealth- pilot trials for the pork industry and producer engagement and case studies. Australia Pork Limited; 2021.
- Pointon A, Nixon J, Ayton M, Seetanna S. Project P.PIP.0527 Report. Pilot Risk-based evaluation of disposition judgement criteria for peri-acute pneumonia complex of lot-fed cattle. [Internet]. Meat and Livestock Australia; 2017 [cited 2024 Jul 22]. Report No.: P.PIP.0527. Available from: https://www.mla.com.au/contentassets/3438361831ff4e39aeb4f686e9ba2531/p.pip.0527_final_report.pdf.
- Pointon A, Bennett M, Ayton M, Johnston M. Project P. PIP.0555 Report. Pilot Risk-Based Evaluation of Disposition Judgement Criteria used for Lot Fed Cattle Totally Condemned for Polyarthritis [Internet]. Meat and Livestock Australia; 2017 [cited 2024 Jul 22]. Report No.: P.PIP.0555. Available from: https://www.mla.com.au/contentassets/30e80f6187174b10aa08bbeac2ab5bbf/p.pip.0555_final_report.pdf.
- Australian Food Regulation Standing Committee. Australian Standard AS 4696:2023 Hygienic Production and Transportation of Meat and Meat Products for Human Consumption. [Internet]. 2023 [cited 2024 Jul 21]. Available from: https://i2.saiglobal.com/mpc2v/preview/1257463558681.pdf?sku=121579_SAIG_AS_AS_3234005.
- Sergeant E, Happold J, Langstaff I. Evaluation of Australian surveillance for freedom from bovine tuberculosis. Australian Veterinary Journal. 2017 Dec 1;95(12):474–9.
- Integrity Systems Company [Internet]. [cited 2024 Jul 21]. Recycled water use: New risk management framework - Integrity Systems. Available from: https://www.integritysystems.com.au/about/news--events/news/2023/new-risk-management-framework-for-recycled-water-use/.
- Stevens D, McDonald A, Pointon A. A risk management framework for Taenia saginata eggs in recycled water to minimise the risk of Cysticercus bovis in cattle. Water e-Journal. 2024;10(1):1–13.
- Pointon. A.M., Kiermeier, A., Hamilton, D. Project V.RBP.0022. Risk-based review of post-mortem inspection of spleens of sheep and goats (Supplementary Report). Meat and Livestock Australia; 2017.
- Commission Regulation (EU) No 219/2014 of 7 March 2014 amending Annex I to Regulation (EC) No 854/2004 of the European Parliament and of the Council as regards the specific requirements for post-mortem inspection of domestic swine Text with EEA relevance [Internet]. OJ L Mar 7, 2014. Available from: http://data.europa.eu/eli/reg/2014/219/oj/eng.
- Australian Pork Limited. Enhancing supply chain profitability through reporting and utilization of peri-mortem information by livestock producers. Final Report [Internet]. Rural R&D for Profit Program; 2022 [cited 2024 Jul 21]. Available from: https://www.ampc.com.au/getmedia/4227e57e-1280-4fa6-9e0d-25de57f215d6/2017-1099_RnD4Profit_Final-Report.pdf?ext=.pdf.
- Integrity Systems Company [Internet]. [cited 2024 Jul 21]. National Vendor Declaration (NVD) - Integrity Systems. Available from: https://www.integritysystems.com.au/on-farm-assurance/national-vendor-declaration-nvd/.
- Horchner PM, Brett D, Gormley B, Jenson I, Pointon AM. HACCP-based approach to the derivation of an on-farm food safety program for the Australian red meat industry. Food Control. 2006 Jul 1;17(7):497–510.
- Horchner PM, Pointon AM. HACCP-based program for on-farm food safety for pig production in Australia. Food Control. 2011 Oct 1;22(10):1674–88.
- Stevens DP, Surapaneni A, Thodupunuri R, O’Connor NA, Smith D. Helminth log reduction values for recycling water from sewage for the protection of human and stock health. Water Res. 2017 Nov 15;125:501–11.
- Stevens DP, Daniel V, Shahsavari E, Aburto-Medina A, Soni SK, Khudur LS, et al. Improvement of Log Reduction Values Design Equations for Helminth Egg Management in Recycled Water. Water. 2021 Nov 9;13(22):3149.
- Stevens D, Surapaneni A, Deere D, O’Connor N, Crosbie N, Keegan A, et al. The probability of cysticercus bovis detection in livestock from exposure to recycled water in non-endemic countries. Microbial Risk Analysis. 2021 Aug 1;18:100164.
- Meat and Livestock Australia. National Sheep Health Monitoring Project [Internet]. 2019 [cited 2024 Jul 21]. Available from: https://www.mla.com.au/contentassets/d2c48c5abdf2422d8094a353c9785fde/p.psh.0907_final_report_.pdf.
- Nesbakken T, Eckner K, Høidal HK, Røtterud OJ. Occurrence of Yersinia enterocolitica and Campylobacter spp. in slaughter pigs and consequences for meat inspection, slaughtering, and dressing procedures. Int J Food Microbiol. 2003 Feb 15;80(3):231–40.
- Gomes-Neves E, Antunes P, Tavares A, Themudo P, Cardoso MF, Gärtner F, et al. Salmonella cross-contamination in swine abattoirs in Portugal: Carcasses, meat and meat handlers. Int J Food Microbiol. 2012 Jun 15;157(1):82–7.
- Murray G. Ante-mortem and post-mortem meat inspection: an Australian Inspection Service perspective. Australian Veterinary Journal. 1986 Jul 1;63(7):211–5.
- Petersen JV, Abildgaard KS, Poulsen MK, Alban L. Investigating ways of detecting and handling findings indicating prior septicaemia in bovines. Food Control. 2022 Jul 1;137:108901.
- Mousing J, Pointon A. Liability of meat inspection, should post mortem inspection be abandoned. In: Proceedings World Congress on Food Hygiene The Hague. 1997.
Table 1.
Disposition guidance for carcasses totally condemned with Bovine Respiratory Disease complex [
38].
Table 1.
Disposition guidance for carcasses totally condemned with Bovine Respiratory Disease complex [
38].
| Criteria |
Acute stage |
Organising stage |
Resolving stage |
Chronic stage |
| Disease stage |
Bacteraemia/viraemia |
Primary bacteraemia/viraemia, secondary pathogens localising to susceptible organs |
Lesions localising |
Lesions localised and then diminishing in extent until no residual tissue damage remains |
| Indicative period post-infection1
|
0 – 14 days |
7 – 14 days |
10 – 28 days |
> 20 days |
| Ante-mortem |
May be showing signs of fever e.g., Lethargy, reluctance to move, increased rate of breathing, elevated temperature. |
May be showing signs of fever
e.gs. Lethargy, increased rate of breathing, elevated temperature. Possible muco-purulent nasal discharge. Cough, signs of discomfort on coughing |
No fever, but possible muco-purulent nasal discharge. Possible cough. |
Normal |
| Gross post-mortem abnormalities |
Carcase showing signs of fever/septicaemia (e.g., erythema and/or petechial haemorrhages and/or poly-serositis with straw coloured exudate with fibrin clots in cavities). |
Bronchopneumonia. Early fibrous adhesions between visceral (i.e., organ) and parietal (i.e., rib) serosal surfaces. Erythema of serosal surfaces. Possible purulent exudate. |
Adhesions well developed. Serosal erythema mild to moderate. Exudates resolving, abscess formation. Possible arthritis. |
Probable hyperaemia of serosa. Diffuse/localised pleurisy and peritonitis. Possible chronic abscess. Possible (poly) arthritis. |
| BRD agent present in primary lesion |
Yes |
Probably |
Possibly |
Unlikely |
| Septicaemic with primary BRD agent |
Probably |
Possibly |
No |
No |
| Muscle contaminated with primary BRD agent |
Unlikely |
No |
No |
No |
| Muscle contaminated with foodborne hazard |
Possibly |
Unlikely |
No |
No |
| Wholesomeness after stripping membranes |
No |
No |
Possibly |
Most likely |
| Disposition judgment |
Total carcase condemnation |
Total carcase condemnation |
If uncertain - Hold and Test |
Pass – trim affected parts |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





