1. Introduction
Recurrent pregnancy loss (RPL) is defined as experiencing 2 or more miscarriages before reaching 20 weeks of gestation [
1]. The prevalence of RPL among women of childbearing age around the world is around 2% [
1,
2,
3]. Among the causes include genetic, anatomical, endocrine, infectious factors, and immune abnormalities such as increased levels of antibodies and increased circulating NK cell cytotoxicity [
1,
2,
3,
4]. Compared to normal pregnancies, high Interleukin-17 (IL-17) circulating levels were found in RPL patients [
5,
6,
7]. The cytokine is involved in the endometrium's extravasation and accumulation of neutrophils. It worsens local inflammation and damage and thereby increases the risk of miscarriage [
5,
6,
7]. This suggests that IL-17 may play a crucial role in the occurrence and progression of RPL [
7]. The cell types usually involved in IL-17 production are CD3CD4 and Th17 cell subpopulations; however, CD8, NK cells, and T γδ cells are also known to produce IL-17 [
8,
9]. There are mixed reports on NKT cells, although this cell subpopulation could also be involved in RPL [
9].
Over the past decade, many studies have explored the connection between IL-17 gene polymorphism and RPL [
10,
11,
12,
13,
14,
15,
16,
17]. Variations in the IL-17A and IL-17F genes have been associated with the development of various human diseases [
18]. Specifically, polymorphic loci known as rs2275913 and rs763780 in the coding regions of IL-17A and IL-17F are closely linked to IL-17 secretion [
19]. However, these findings have been conflicting and have sparked controversy based on the possible impact of ethnic and population differences on genetic polymorphisms and backgrounds [
18,
19,
20].
There is no specific data on Venezuela concerning the risk of RPL; however, there is a general consensus that the rates may be similar to other populations. Part of the Venezuelan population is genetically admixed with the prevalence of Caucasian genes but mixed with African and Amerindian groups [
21,
22]. This admixed population differs from other Latin American countries, probably due to gene segregation among Eastern African and Amerindian populations [
21,
22,
23].
Most reports regarding RPL and IL-17 refer to the local production of the cytokine by stimulated cells [
12,
13], and only a few have dealt with the circulating number of Th17 cells [
24]. Moreover, no reports have addressed the relationship between genetic IL-17 SNP, circulating lymphocytes, and the response to cell activation.
The present report aims to analyze the relationship between genetic polymorphism, circulating IL-17 cells, the effect of stimulation on intracellular IL-17, and the circulating levels of IL-17A using two groups with identical genetically admix conditions: controls and patients with RPL.
2. Materials and Methods
2.1. Human Samples
The study is a case-control study involving 50 patients who met the criteria for RPL. The patients with RPL underwent screening for immunological and hormonal factors and showed no abnormalities in paraclinical analysis, indicating the absence of infectious or genetic diseases. Patients with cancer, severe endometriosis, or HPV infection were excluded. The study also enrolled 50 healthy women with normal pregnancies and no medical conditions such as viral diseases, hypertension, diabetes, metabolic syndrome, or hormonal imbalances. The samples were taken when patients were attending control medical checkups.
Written consent was obtained from all individuals interested in participating in the study. The genetic admixture of both the patients and controls was previously verified. [
21,
22]. The study received approval from the Ethical Committee of the Institute of Immunology, Faculty of Medicine, Caracas, Venezuela (approval number 20052308).
Twelve ml of blood was taken from each individual and divided into three parts. Two aliquots of 1 ml were used, one for assessing cell subpopulations and the other for cell stimulation, and the other 10 ml were centrifuged at 90xg. The plasma was stored to assess IL-17A, and the buffy coat was used to obtain the cDNA.
2.2. Antibodies and Reagents
Anti-human IL-17A/F monoclonal mouse IgG1 Clone # 41802 with PE (R&D systems). The following antibodies were from BioLegend: anti-CD3 (clone UCHT1), CD4 (clone OKT4), CD8 (clone Leu2), CD25 (clone M-A251), CD56 (QA17A16), CD45RA (clone HI100), CD45RO (clone UCHL1), HLA-DR (clone L243), and FoxP3 (clone 206D). Cytofix™ and Cytoperm™ kits from Becton Dickinson. Cell Activation Cocktail (with Brefeldin A) ™ from Biolegend.
2.3. Genetic Polymorphism Analysis
Venous blood samples of 10 mL were collected in EDTA tubes from all individuals, and the cDNA was extracted from the buffy coat on the same day. Genomic DNA isolation was performed using the AxyPrep Blood Genomic DNA Miniprep Kit from Axygen Biosciences, Union City, CA, USA. This kit can purify up to 12 µg of genomic DNA in 250 µL of non-coagulated blood. DNA isolation protocol was performed as described by the manufacturer with minor modifications described previously [
25].
The samples' pure DNA (260/280 ratio ≥1.8) was expressed in µg/mL for the assays. The forward and reverse primers for IL-17A (rs2275913) are 5’-TCTCCATCTCCATCACCTTTG-3’ and 5’-GTCCAAATCAGCAAGAGCATC-3’, respectively. For IL-17F (rs763780), the forward and reverse primers are 5’- CACTGGTGCTCTGATGAGGA -3’ and 5’- CATTGTGCTTTGGCTTGCT -3’, respectively.
The primers were obtained from the literature [
26,
27] along with the protocol, which was slightly modified. Briefly, DNA (20 ng) was mixed with a 40 µL reaction mixture containing 1.5 mM MgCl2, 100 ng of each primer, 500 µM deoxyribonucleoside triphosphate, and 0.6 IU Taq DNA polymerase (Promega®). The DNA was amplified via polymerase chain reaction (PCR) using a thermal cycler Minicycler™ (MJ Research, Waltham, MA, USA) consisting of an initial single cycle of initial single cycle of 10 min at 95 °C, followed by 35 cycles of 30 s at 94 °C, 45 s at 60 °C, and 30 s at 72 °C. Then, all PCR products were incubated with endonucleases
XmnI (for polymorphism rs2275913) and
NIaIII (for polymorphism rs763780). The PCR products were incubated at 37°C with the enzyme and visualized using ethidium bromide staining after separation by electrophoresis on a 3.0% agarose gel at 80 V for 100 minutes. The sequencing service at the Venezuelan Institute for Scientific Research (IVIC) sequenced the amplification products. All the results of the RFLP were confirmed.
2.4. Analysis of Intracellular IL-17 in Different Cell Subpopulations.
The blood was lysed using the standard protocol, and the lymphocytes were washed and adjusted to 250000 cells/tube. Anti-human IL-17A/F-PE was used for intracellular detection of IL-17. The detection of IL-17 was performed by flow cytometry using the kits from Becton Dickinson according to the protocol provided by the protocol suggested by the supplier. The cells were labeled extracellularly with anti-CD4, anti-CD8, or anti-CD56 from BioLegend. The analysis was performed using Beckman Coulter's EPICS XL apparatus.
Intracellular analysis of FoxP3 was also performed in non-stimulated cells. Then, the cells were labeled with CD4 and CD25 to determine the cell subpopulation.
To ascertain the effect of cell stimulation, the cells with 1 ml of blood were incubated with the Cell Activation Cocktail for 6 hr according to the manufacturer’s protocol. The cells were treated for 12 hr with the optimal concentration of the cocktail and then fixed with cytoplasm, permeabilized, and anti-IL17 PE was added to the permeabilized cells. The cells were then washed and labeled with CD4, CD8, or CD56 to analyze the different subpopulations.
2.5. Determination of Il-17A from Serum Samples and Supernatants.
The Quantikine HS ELISA Kit from R&D Systems was used to analyze IL-17A in the plasma of controls and RPL patients. The analysis was performed according to the manufacturer's instructions.
2.6. Statistical Analysis
The minimum sample size was set at 45. The calculation was performed using the online calculator (
www.calculator.net, accessed on 2 April 2024) based on the SNP data of 3% frequency in the normal population for rs1051740 (
https://opensnp.org, accessed on 2 April 2024).
The program GraphPad Prism 5 was used for calculations. The results were analyzed using chi-square with Yate’s correction. Both absolute and relative frequency have been utilized, and significance has been attributed to a p-value less than 0.05.
3. Results
Table 1 shows the population characteristics. The only significant (P<0.0001) differences were in the number of abortions registered.
Table 2 shows the analysis of the SNPs rs2275913, which corresponds to IL-17A, and re763780, which corresponds to IL-17F. There are no statistical differences between controls and RPL patients.
Table 3 illustrates the difference in total leukocytes, different lymphocyte populations, and subpopulations. Most of the analyzed populations were significantly different between the groups. Even though the percentage of lymphocytes was higher in the RPL group compared to the controls, the rate of T lymphocytes was significantly lower, and the amount of NK cells was considerably higher. No general effects were observed in NKT cells or CD3 naive or memory cells, but the total number of HLADR+ cells and CD3+/HLADR+ cells was significantly higher. The percentage of Treg between both groups was also significantly lower in the RPL group.
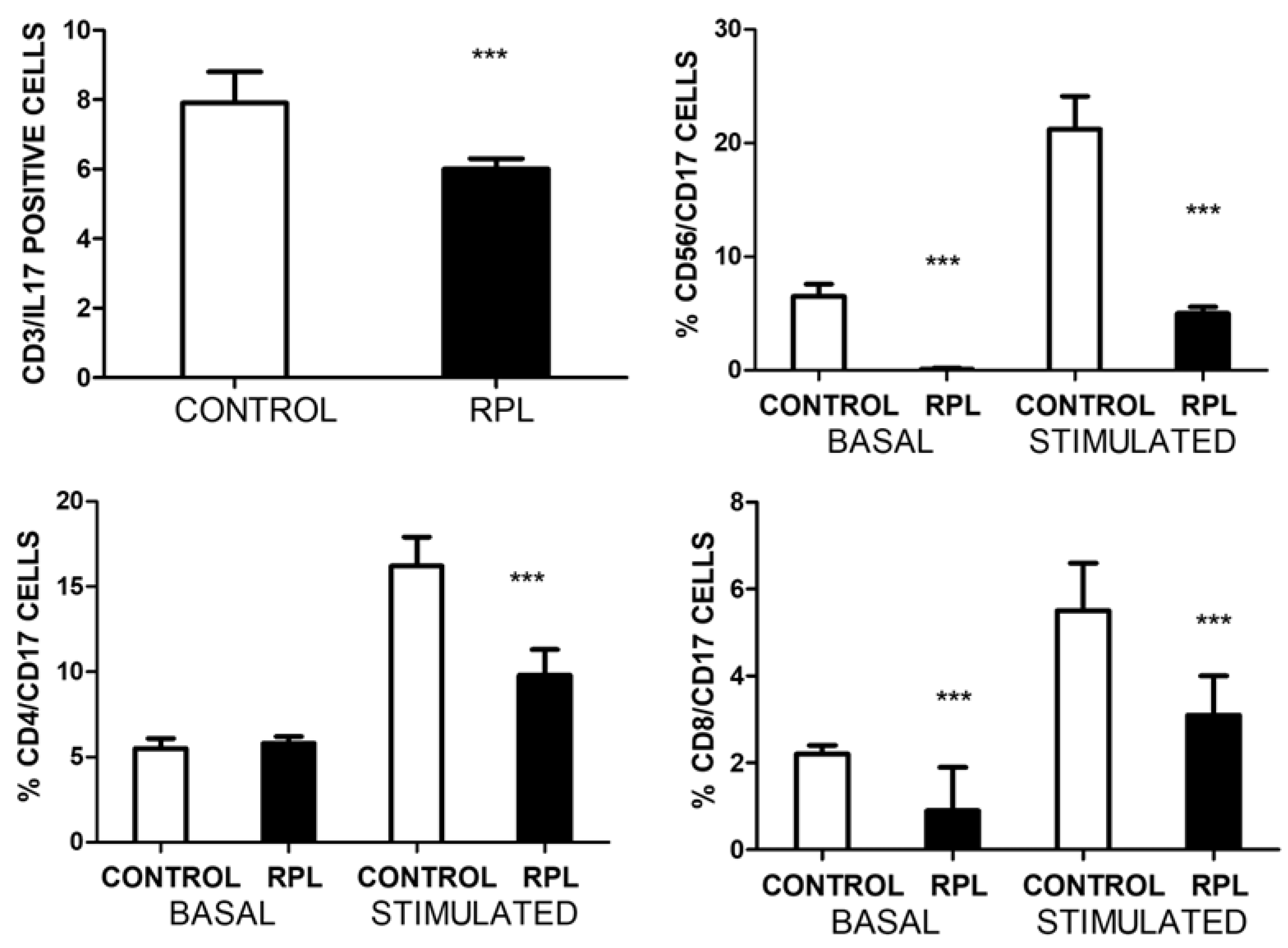
Figure 1 illustrates the total number of IL-17 positives and the different subpopulations before and after stimulation with PMA/ionomycin. Significant differences were recorded in the total T and NK cell populations in basal conditions. Interestingly, the number of CD4/CD17 cells in both groups was similar; however, upon stimulation, there was a clear difference between both groups in each cell population assessed. The number of NK cells that produce IL-17 was almost undetectable in all patients.
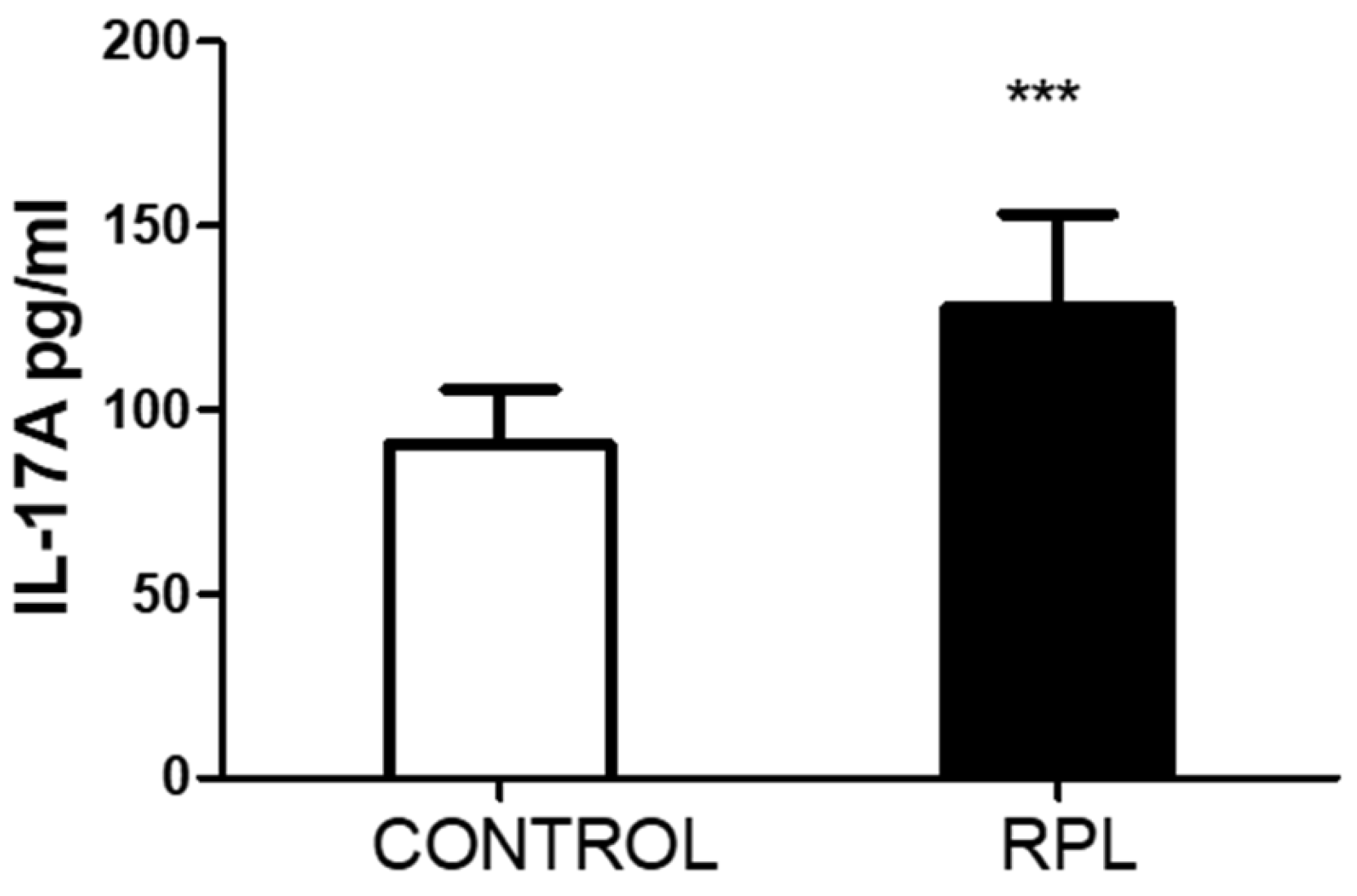
A significant increase in IL17A is observed in the plasma samples of patients as compared to controls. This increase does not correlate with how many cells in which IL-17A/F are detected. There is also no significant correlation with the amount of total activated HLADR+ cells; however, a mild significant correlation (r=0.3, p<0.05) was observed between the number of CD3/HLADR+ cells and IL-17A levels in RPL patients, but not in controls (r=0.05, p=0.6). No correlation was observed between the number of IL17 cells and Treg cells. No other significant correlations were observed with the other parameters.
3. Discussion
RPL is a complex medical entity, and it involves two types: primary (no successful pregnancy) and secondary, a successful pregnancy followed by recurrent miscarriage. There is no medical explanation in most cases; the karyotype of the fetus is normal, and there are no accountable medical alterations to explain the miscarriages.
In the endometrium, tolerogenic NK, macrophages, dendritic cells, and T cells are essential to maintain pregnancy [
1,
2,
3,
4,
5]. However, local exposition to pathogens, vaginal or endometrial bacterial, fungal, or viral infections induce the migration of cells, which compromise the tolerogenic milieu and consequently attack induce pregnancy termination [
1,
2,
3,
4]. IL-17 is a marker of the inflammatory process for cell migration of neutrophils and other subpopulations [
5,
6,
7]; Th17 cells are involved in the rejection of the fetus or affect the implantation of the zygote [
5,
6,
7,
8]. There are, however, questions referring to the role of circulating proinflammatory cell populations in the bloodstream of RPL patients. The migration of these cells in the endometrial compartment has not been well analyzed due to the ethical complexity involved. The only available information refers to pregnancy termination in normal conditions and compares them to pathological ones. A one-time point may not be sufficient for a critical assessment of events.
The present report shows that IL-17A levels are increased in patients with RPL, but this increase does not match the amount of circulating IL-17A/F-positive cells. No difference in genetic polymorphism could explain the phenomenon, nor was there a clear indication that the circulating CD4/IL-17A/F cells differed between both groups. The remarkable difference was among the CD8 and NK subpopulations. It is unclear why there is a difference between these subpopulations as compared to CD4/IL-17 cells. Moreover, the effect of cell stimulation is also complex to interpret. Since we used total blood for stimulation to recreate the physiological conditions and not in purified cell types, we cannot define if the secretion of other protein or non-protein intermediates secreted by different cell types are responsible for the decrease in IL-17 production. Based on the data recorded on HLA-DR expression, we propose that PMA/ionomycin may have affected the stimulated cell subpopulations, which were responsible for producing intermediates that suppress the production of IL-17A/F. The amount of IL-17A in the plasma is not accountable for modulating the number of IL-17-positive cells, as we could not reproduce the effects (results not shown).
Unfortunately, we could not quantify the number of CD4/CD25/FoxP3 cells in the stimulated samples, which could have been an important point for comparing with IL-17. The significant differences recorded in the basal levels suggest that upon higher circulating levels of IL-17, the number of T regulatory cells decreases. The other important issue is that some of these RPL patients did not continue to go to the fertility clinic for other procedures due to the costs, so we could not do a follow-up.
The association of the two genetic polymorphisms IL-17A rs2275913 and IL-17F rs763780 with RPL is debatable [
10,
11,
14,
15,
16,
17]. Essentially, the association reports are contradictory; some suggest that rs763780 is associated with a decreased risk of RPL in the Iranian population, while others suggest that rs2275913 is protective in the same population [
10,
11,
16,
20]. Also, contradictory reports have been observed in the Chinese population [
15,
16,
17,
18,
19]. However, in our admix population, there is no difference; similar frequencies are observed in both groups. New, well-defined genetic studies are required to determine if there is a relationship between these two polymorphisms and RPL. Moreover, based on the data reported in this manuscript, it is highly improbable that significant changes recorded in the cell populations are due to genetic polymorphisms. The conclusion is based on the dichotomy of the number of circulating cells positive for IL-17 and the values recorded in plasma. The CD4/CD17 subpopulation is similar in both groups, suggesting that the amount of Th17 cell subpopulation may be comparable. The decrease in IL-17 in stimulated cells requires attention and should be further studied.
In the present manuscript, our group has tried combining genetic analysis with cell subpopulation, cell activation, and cytokine detection in plasma patients with RPL. Indeed, several questions remain to be solved. However, the manuscript is one of the few related to the genetic analysis of IL-17 SNP polymorphisms with cell subpopulations and their effect on cell stimulation. This information is crucial for the admixed, well-defined genetic population that we studied. Even though we are unaware of the possible activation of cells in the endometrial cavity or even in the early stages of implantation or decidua, we can assume peripheral inflammation in RPL, cell activation markers, and IL-17A plasma levels. Eventually, peripheral inflammation affects local inflammation. It can be proposed that the assessment of inflammation parameters can be critical in RPL and may serve as an initial marker for future studies in evaluating the role of IL-17 in RPL and the response of those patients to therapy.
4. Conclusions
There is no association between the two polymorphisms analyzed, IL-17A rs2275913 and IL-17F rs763780, with RPL. The values of plasma RPL recorded were independent of the genetic polymorphisms.
The peripheral lymphocytes of RPL patients were activated based on the percentage of HLA-DR expression. In addition, the number of T regulatory cells decreased.
The number of IL-17-positive cells, CD3 and CD56, was significantly lower in RPL patients than in controls. However, the differences were in the CD3CD8 subpopulation compared to the CD3CD4 subpopulation, which was similar in both control and RPL patients. However, IL-17 was almost undetectable in NK cells of RPL patients.
In stimulated cells, the response of all different cell populations and subpopulations was lower in RPL patients than in the controls. The effect of PMA/ionomycin stimulation on whole blood may probably be responsible for this effect.
Author Contributions
Conceptualization, J.V.G. and I.B..; methodology, J.V.G., I.B., M.J.P., J.B.D.S..; validation, J.B.D.S.., C.V.D.S. and J.V.G.; formal analysis, C.V.D.S., J.B.D.S.; investigation, M.J.P., J.V.G. I.B..; resources, J.V.G..; data curation, J.B.D.S..; writing—original draft preparation, J.V.G., C.V.D.S.; writing—review and editing, J.B.D.S..; visualization, J.B.D.S,.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript,
Funding
This research was funded by the Universidad Central de Venezuela's Counsel for Scientific and Human Development (CDCH-UCV), grant number PG 09-6599-2006/1.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Institute of Immunology, Faculty of Medicine, Universidad Central de Venezuela (protocol code 20052308, date of approval 15/09/2005).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The crude data is available from the authors.
Acknowledgments
The authors thank the patients and controls involved in the study, bachelor students Dariana Parra and Irene Rodríguez, and the technician BSc Perla Chirinos for their valuable help in sample processing.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
The images of the sample electrophoresis for both SNP polymorphisms is shown.
References
- Pillarisetty, L.S.; Mahdy, H. Recurrent Pregnancy Loss. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554460/ assessed June 4, 2024.
- Stephenson, M.D. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996 Jul;66(1):24-9. [CrossRef]
- Ford, H.B.; Schust, D.J. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76-83.
- Cuadrado-Torroglosa, I.; García-Velasco, J. A.; Alecsandru, D. Maternal-Fetal Compatibility in Recurrent Pregnancy Loss. J Clin Med, 2024; 13(8), 2379. [CrossRef]
- Saito, S.; Nakashima, A.; Ito, M.; Shima, T. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert Rev Clin Immunol, 2011; 7(5), 649–657. [CrossRef]
- Fu, B.; Tian, Z.; Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol, 2014; 11(6), 564–570. [CrossRef]
- Dai, M.; Xu, Y.; Gong, G.; Zhang, Y. Roles of immune microenvironment in the female reproductive maintenance and regulation: novel insights into the crosstalk of immune cells. Front Immunol., 2023; 14, 1109122. [CrossRef]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: crosstalk between ancient "Yin-Yang" theory and modern immunology. Cell comm sig 2024; 22(1), 99. [CrossRef]
- Moura, G. A.; Rocha, Y. M.; Moura, F. L. D.; Freitas, J. O.; Rodrigues, J. P. V.; Gonçalves, V. P.; Nicolete, R. Immune system cells modulation in patients with reproductive issues: A systematic review approach. JBRA assis reprod, 2024; 28(1), 78–89. [CrossRef]
- Najafi, S., Hadinedoushan, H., Eslami, G., & Aflatoonian, A. Association of IL-17A and IL-17 F gene polymorphisms with recurrent pregnancy loss in Iranian women. Journal of assisted reproduction and genetics, 2014; 31(11), 1491–1496. [CrossRef]
- Zhang, M.; Xu, J.; Bao, X.; Niu, W.; Wang, L.; Du, L.; Zhang, N.; Sun, Y. Association between Genetic Polymorphisms in Interleukin Genes and Recurrent Pregnancy Loss - A Systematic Review and Meta-Analysis. PloS one, 2017; 12(1), e0169891. [CrossRef]
- Abdolmohammadi Vahid, S.; Ghaebi, M., Ahmadi, M.; Nouri, M.; Danaei, S.; et al. Altered T-cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. J Cell Physiol, 2019; 234(4), 4924–4933. [CrossRef]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; et al. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. Journal of clinical and translational research, 2022; 8(3), 256–265.
- Ali, S.; Majid, S.; Ali, M. N.; Banday, M. Z.; et al. Immunogenetic Role of IL17A Polymorphism in the Pathogenesis of Recurrent Miscarriage. J Clin Med, 2022; 11(24), 7448. [CrossRef]
- Ma, Y.; Ma, M.; Ye, S.; Liu, Y.; Zhao, X.; Wang, Y. Association of IL-17 and IL-27 polymorphisms with susceptibility to recurrent pregnancy loss and pre-eclampsia: A systematic review and meta-analysis. Immunity, inflammation and disease, 2023; 11(10), e1057. [CrossRef]
- Keshavarz Motamed, A.; Zarei, Z. H.; Mirfakhraee, H.; Shariatinia, F.; Akbari, M.; Ziagham, S.; Igder, S.; Zarei, N. Association of Interleukin-17A rs2275913 Polymorphism with Recurrent Miscarriage: A Systematic Review and Meta-Analysis Study. International journal of fertility & sterility, 2023; 18(1), 7–11.
- Li, D.; Uskenbayeva, N.; Fang, L.; Xu, Y.; Yan, H.; Zhang, K.; Wang, J. Genetic polymorphism of IL-17 influences susceptibility to recurrent pregnancy loss in a Chinese population. Medicine, 2024; 103(23), e38333. [CrossRef]
- Gao, J. F.; Zhang, H.; Lv, J.; Wang, L.; Fan, Y. Y. Associations of the IL-17A rs2275913 and IL-17F rs763780 polymorphisms with the risk of digestive system neoplasms: A meta-analysis. International immunopharmacology, 2019; 67, 248–259. [CrossRef]
- Li, J.; Tian, H.; Jiang, H. J.; Han, B. Interleukin-17 SNPs and serum levels increase ulcerative colitis risk: a meta-analysis. World J Gastroenterol, 2014; 20, 15899–15909. [CrossRef]
- Stavros, S.; Panagopoulos, P.; Machairiotis, N.; Potiris, A.; Mavrogianni, D.; et al. Association between cytokine polymorphisms and recurrent pregnancy loss: A review of current evidence. Intern J Gynecol Obst, 2024; 10.1002/ijgo.15575. [CrossRef]
- Conesa, A.; Fernández-Mestre, M.; Padrón, D.; Toro F.; Silva, N.; Tassinari, P.; Blanca, I.; Martin, M.P.; Carrington, M.; Layrisse, Z. Distribution of killer cell immunoglobulin-like receptor genes in the mestizo population from Venezuela. Tissue Antigens. 2010 ;75(6):724-9. [CrossRef]
- Del Pilar Fortes. M.; Gill, G.; Paredes, M.E.; Gamez, L.E.; Palacios, M.; Blanca, I.; Tassinari, P. Allele and haplotype frequencies at human leukocyte antigen class I and II genes in Venezuela's population. Ann Biol Clin (Paris). 2012; 70(2):175-81. [CrossRef]
- Bryc, K.; Velez, C.; Karafet, T.; Moreno-Estrada, A.; Reynolds, A.; et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. PNAS USA, 2010; 107 Suppl 2, 8954–8961.
- Niafar, M.; Samaie, V.;, Soltani-Zangbar, M. S.; Motavalli, R.; et al. The association of Treg and Th17 cells development factors and anti-TPO autoantibodies in patients with recurrent pregnancy loss. BMC research notes, 2023; 16(1), 302. [CrossRef]
- Peña MJ, De Sanctis CV, De Sanctis JB, Garmendia JV. Frequency of Gene Polymorphisms in Admixed Venezuelan Women with Recurrent Pregnancy Loss: Microsomal Epoxy Hydroxylase (rs1051740) and Enos (rs1799983). Curr Issues Mol Biol. 2024 Apr 17;46(4):3460-3469.
- Xie, Z.; Ding, X.; Wang, Y.; Zhang, M. The rs2275913 polymorphism of the interleukin-17A gene is associated with the risk of ovarian endometriosis. Journal of obstetrics and gynaecology, 2023; 43(1), 2199852. [CrossRef]
- Li, H.; Zhou, Z.; Tai, W.; Feng, W.; Zhang, D.; Gu, X.; Yang, R. Decreased Frequency of IL-17F rs763780 Site Allele G is Associated With Genetic Susceptibility to Immune Thrombocytopenia in a Chinese Population. Clinical and applied thrombosis/hemostasis, 2017; 23(5), 466–471. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).