Submitted:
04 August 2024
Posted:
05 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Conclusions and Future Direction
Scientific Support:
Funding Support:
Conflict of Interest/Competing Interest:
Availability of Data and Material/Data Transparency:
Author Contributions
References
- Driessen G, van der Burg M. Educational paper: primary antibody deficiencies. Eur J Pediatr 2011,170,693-702. [CrossRef]
- Eroglu FK, Kaya FA, Cagdas D, Ozgur TT, Yilmaz T, Tezcan I, et al. B lymphocyte subsets and outcomes in patients with an initial diagnosis of transient hypogammaglobulinemia of infancy. Scand J Immunol 2018,88,12709. [CrossRef] [PubMed]
- Szczawinska-Poplonyk A, Tapolska-Jozwiak K, Samara H. The B-cell compartment in antibody-deficient infants and young children – developing common variable immunodeficiency or transient immune maturation? Ital J Pediatr 2016, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Odnoletkova I, Kindle G, Quinti I, Grimbacher B, Knerr V, Gathmann B, et al. The burden of common variable immunodeficiency disorders: a retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J Rare Dis 2018,13,201. [CrossRef]
- Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract 2016,4,38-59. [CrossRef]
- Seidel M, Kindle G, Gathmann B, Quinti I, Buckland M, van Monfrans J, et al. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract 2019,7,1763-1770. [CrossRef]
- Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol 2019,10,33. [CrossRef]
- Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cell (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 2002, 99,1544-1551. [CrossRef]
- Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 2008,111,77-85. [CrossRef]
- Ramirez NJ, Posadas-Cantera S, Caballero-Oteyza A, Camacho-Ordonez N, Grimbacher B. There is no gene for CVID – novel monogenetic causes for primary antibody deficiency. Curr Opin Immunol 2021,72,176-185. [CrossRef]
- Bogaert DJA, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet 2016, 53, 575-–590. [CrossRef] [PubMed]
- Abolhassani H, Hammarstrom L, Cunningham-Rundles C. Current genetic landscape in common variable immunodeficiency. Blood 2020,135,:656-667. [CrossRef]
- Aggarval V, Banday AZ, Jindal AK, Das J, Rawat A. Recent advances in elucidating the genetics of common variable immunodeficiency. Genes Dis 2020, 7,26–37. [CrossRef] [PubMed]
- De Valles-Ibanez G, Esteve-Sole A, Piquer M, Gonzalez-Navarro EA, Hernandez-Rodriguez J, Laayouni H, et al. Evaluating the genetics of common variable immunodeficiency: monogenetic model and beyond. Front Immunol 2018,9,636. [CrossRef]
- Edwards ESJ, Bosco JJ, Ojaimi S, O’Heir RE, van Zelm MC. Beyond monogenic rare variants: tackling the low rate of genetic diagnoses in predominantly antibody deficiency. Cell Mol Immunol 2021,18,588-603. [CrossRef]
- Kienzler AK, Hargreaves CE, Patel SY. The role of genomics in common variable immunodeficiency disorders. J Clin Immunol 2017, 188, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Sanchez LA, Maggadottir SM, Pantell MS, Lugar P, Rundles CC, Sullivan KE, et al. Two sides of the same coin: pediatric-onset and adult-onset common variable immunodeficiency. J Clin Immunol 2017,37,592-602. [CrossRef]
- Carrabba M, Salvi M, Baselli LA, Serafino S, Zarantonello M, Trombetta E, et al. Long-term follow-up in common variable immunodeficiency: the pediatric onset and adult-onset landscape. Front Pediatr 2023,11,112994. [CrossRef]
- Esmailzadeh H, Jokar-Derisi A, Hassani AH, Yazdani R, Delavari S, Abolhassani H, et al. Assessment of the first presentations of common variable immunodeficiency in a large cohort of patients. BMC Immunol 2023,24,9. [CrossRef] [PubMed]
- Szczawińska-Popłonyk A, Tąpolska-Jóźwiak K, Schwartzmann E, Popłonyk N. Immune dysregulation in pediatric common variable immunodeficiency: implications for the diagnostic approach. Front Pediatr 2022,10,855200. [CrossRef]
- Costagliola G, Peroni DG, Consolini R. Beyond infections: new warning signs for inborn errors of immunity in children. Front Pediatr 2022,10,855445. [CrossRef]
- Ameratunga R, Lehnert K, Woon ST. All patients with common variable immunodeficiency disorders (CVID) should be routinely offered diagnostic genetic testing. Front Immunol 2019,10,2678. [CrossRef]
- Christiansen M, Offersen R, Jensen JMB, Petersen MS, Larsen CS, Mogensen TH. Identification of novel genetic variants in CVID patients with autoimmunity, autoinflammation, or malignancy. Front Immunol 2020,10,3022. [CrossRef]
- Peng XP, Caballero-Oteyza A, Grimbacher B. Common variable immunodeficiency: more pathways than roads to Rome. Annu Rev Pathol Mech Dis 2023,18,283-310. [CrossRef]
- Giardino G, Gallo V, Prencipe R, Gaudino G, Romano R, De Cataldis M, et al. Unbalanced immune system: immunodeficiencies and autoimmunity. Front Pediatr 2016,4,107. [CrossRef]
- Tessarin G, Baronio M, Lougaris V. Monogenic forms of common variable immunodeficiency and implications on target therapeutic approaches. Curr Opin Allergy Clin Immunol 2023, 23, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Vanselow S, Wahn V, Schuetz C. Activated PI3Kδ syndrome – reviewing challenges in diagnosis and treatment. Front Immunol 2023,14,1208567. [CrossRef]
- Fischer A, Provot J, Jais JP, Alcais A, Mahlaoui N, and CEREDITH PID study group. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol 2017,140,1388-1393. [CrossRef]
- Grimbacher B, Warnatz K, Yong PFK, Korganow AS, Peter HH. The crossroads of autoimmunity and immunodeficiency: lessons from polygenic traits and monogenic defects. Clin Rev Allergy Immunol 2016,137, 3-17. [CrossRef]
- Ochiai K, Igarashi K. Exploring novel functions of BACH2 in the acquisition of antigen-specific antibodies. Int Immunol 2023,35,257-265. [CrossRef]
- Vogel TP, Leiding JW., Cooper MA, Forbes Satter LR. STAT3 gain-of-function syndrome. Front Pediatr 2022,10,770077. [CrossRef]
- Rodriguez-Ubreva J, Calvillo CL, Forbes Satter LR, Ballestar E. Interplay between epigenetic and genetic alterations in inborn errors of immunity. Trends Immunol 2023,44,902-916. [CrossRef] [PubMed]
- Tuijnenburg P, Lango Allen H, Burns SO, Green D, Jansen MH, Staples E, et al. Loss-of-function nuclear factor κB subunit 1 (NFKB1) variants are the most common monogenic cause of common variable immunodeficiency in Europeans. J Allergy Clin Immunol 2018,142,1285-1296. [CrossRef]
- Zhang L, Xiao X, Arnold PR, Li XC. Transcriptional and epigenetic regulation of immune tolerance: roles of the NF-κB family members. Cell Mol Immunol 2019,16,315-323. [CrossRef]
- Kerner G, Bouaziz M, Cobat A, Bigio B, Timberlake AT, Bustamante J, Lifton RP, et al. A genome-wide case-only test for the detection of digenic inheritance in human genome. Proc Natl Acad Sci USA 2020,117,19367-19375. [CrossRef]
- Ameratunga R, Woon ST, Bryant VL, Steele R, Slade C, Leung EY,; et al Clinical implications of digenic inheritance and epistasis in primary immunodeficiency disorders. Front Immunol 2018,8,1965. [CrossRef] [PubMed]
- Ameratunga R, Edwards ESJ, Lehnert K, Leung E, Woon ST, Lea E, et al. The rapidly expanding genetic spectrum of common variable immunodeficiency-like disorders. J Allergy Clin Immunol Pract 2023,11,1646-1664. [CrossRef]
- Ameratunga R, Koopmans W, Woon ST, Leung E, Lehnert K, Slade CA, et al. Epistatic interactions between mutations in TACI (TNFRSF13B) and TCF3 result in a severe primary immunodeficiency disorder and systemic lupus erythematosus. Clin Transl Immunol 2017,6,159. [CrossRef]
- Dieli-Crimi R, Martinez-Gallo M, Franco-Jarava C, Antolin M, Blasco L, Paramonov I, et al. Th1-skewed profile and excessive production of proinflammatory cytokines in a NFKB1-deficient patient with CVID and severe gastrointestinal manifestations. Clin Immunol 2018,195,49-58. [CrossRef]
- Massaad MJ, Zhou J, Tsuchimoto D, Chou J, Jabara H, Janssen E,; et al Deficiency of base excision repair enzyme NEIL3 drives increased predisposition to autoimmunity. J Clin Invest 2016,126, 4219–4236. [CrossRef] [PubMed]
- Sic H, Speletas M, Cornacchione V, Seidel M, Beibel M, Linghu B, et al. An activating Janus Kinase 3 mutation is associated with Cytotoxic T Lymphocyte Antigen-4-dependent immune dysregulation syndrome. Front Immunol 2017,8,1824. [CrossRef]
- Li J, Wei Z, Li RY, Magadottir SM, Chang X, Desai A, et al. Understanding the genetic and epigenetic basis of common variable immunodeficiency disorder through omics approaches. Biochim Biophys Acta 2016;1860(11):2656-2663. [CrossRef]
- Fang M, Abolhassani H, Lim CK, Zhang J, Hammarstrom L. Next generation sequencing data analysis in primary immunodeficiency disorders – future directions. J Clin Immunol 2016,36,68-75. [CrossRef]
- Jorgensen SF, Fevang B, Aukrust P. Autoimmunity and inflammation in CVID: a possible crosstalk between immune activation, gut microbiota, and epigenetic modification. J Clin Immunol 2019,39,30-36. [CrossRef]
- Silva SL, Fonseca M, Pereira MLM, Silva SP, Barbosa RR, Serra-Caetano A, et al. Monozygotic twins concordant for common variable immunodeficiency: strikingly similar clinical and immune profile associated with a polygenic burden. Front Immunol 2019,10,2503. [CrossRef]
- Weifenbach N, Schneckenburger AAC, Lotters S. Global distribution of common variable immunodeficiency (CVID) in the light of the UNDP Human Development Index (HDI): a preliminary perspective of a rare disese. J Immunol Res 2020,2020,8416124. [CrossRef]
- Selenius JS, Martelius T, Pikkarainen S, Siitonen S, Mattila E, Pietikainen R,; et al. Unexpectedly high prevalence of common variable immunodeficiency in Finland. Front Immunol 2017, 8, 1190. [CrossRef] [PubMed]
- Ogawa H, Horitani K, Izumiya Y, Sano S. Somatic mosaicism in biology and disease. Annu Rev Physol 2022, 84, 113-–133. [CrossRef] [PubMed]
- Mensa-Vilaro A, Garcia-Morato MB, de la Calle-Martin O, Franco-Jarava C, Martinez-Saavedra MT, Gonzalez-Granado LI,; et al. Unexpected relevant role of gene mosaicism in patients with primary immunodeficiency diseases. J Allergy Clin Immunol 2019, 143, 359-–368. [CrossRef] [PubMed]
- Aluri J, Cooper MA. Genetic mosaicism as a cause of inborn errors of immunity. J Clin Immunol 2021, 41, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Aluri J, Cooper MA. Somatic mosaicism in inborn errors of immunity: current knowledge, challenges, and future perspectives. Semin Immunol 2023,67,101761. [CrossRef]
- Materna-Kiryluk A, Pollak A, Gawalski K, Szczawińska-Popłonyk A, Rydzyńska Z, Sosnowska A,; et al. Mosaic IL6ST variant inducing constitutive GP130 cytokine receptor signaling as a cause of neonatal onset immunodeficiency with autoinflammation and dysmorphy. Hum Mol Genet 2021,30, 226–233. [CrossRef] [PubMed]
- Savola P, Martelius T, Kankainen M, Huuhtanen J, Lundgren S, Koski Y, et al. Somatic mutations and T cell clonality in patients with immunodeficiency. Haematologica 2020, 105, 2557–2768. [CrossRef] [PubMed]
- Kwon SS, Cho YK, Hahn S, Oh J, Won D, Shin D,; et al. Genetic diagnosis of inborn errors of immunity using clinical exome sequencing. Front Immunol 2023, 14, 1178582. [CrossRef] [PubMed]
- Guevara-Hoyer K, Fuentes-Antras J, de la Fuente-Munoz E, Fernandez-Arquero M, Solano F, Perez-Segura P, et al. Genomic crossroads between non-Hodgkin lymphoma and common variable immunodeficiency. Front Immunol 2022,13,937872. [CrossRef]
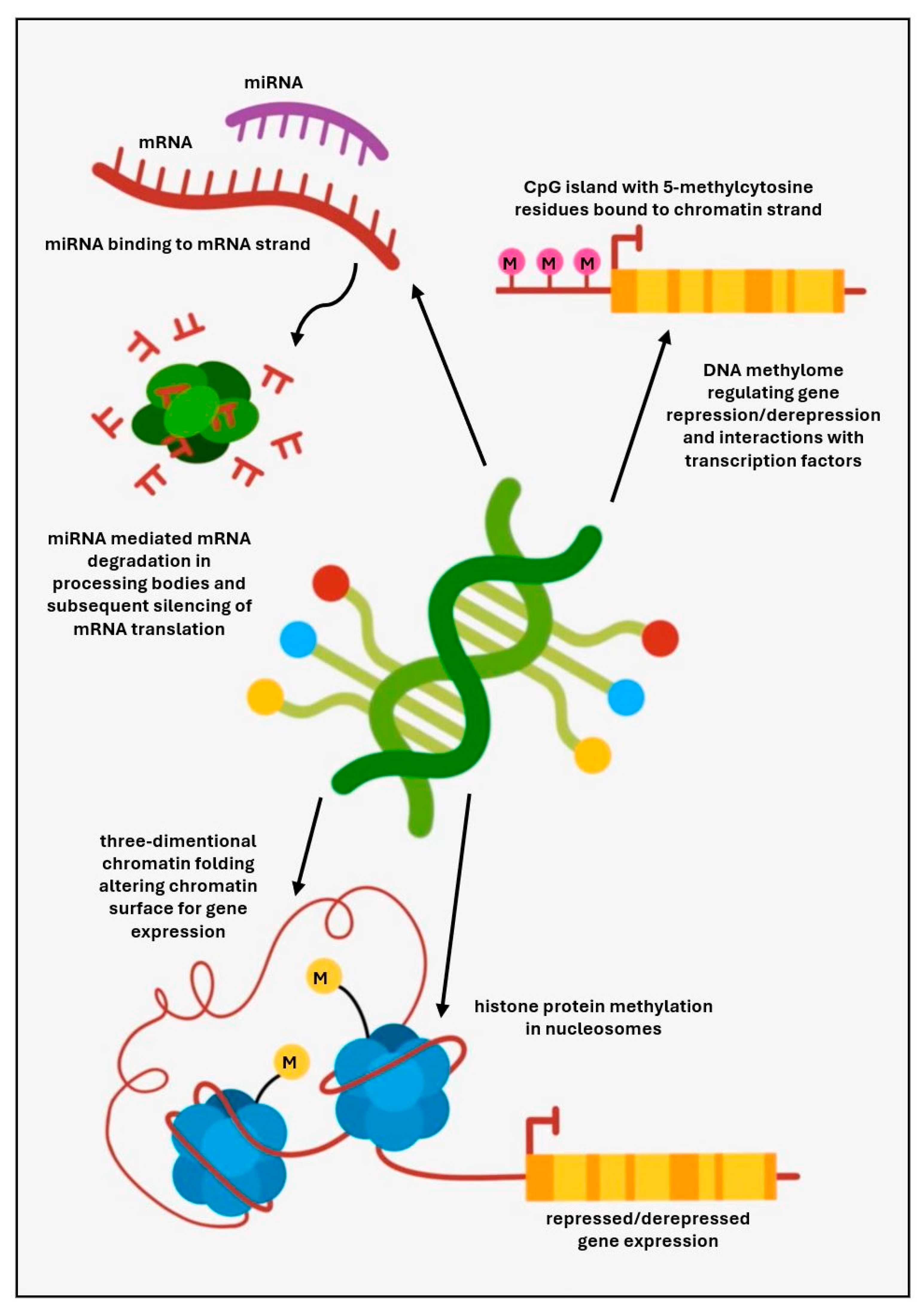
- Rae, W. Indications to epigenetic dysfunction in the pathogenesis of common variable immunodeficiency. Arch Immunol Ther Exp 2017, 65, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cano J, Campos-Sanchez E, Cobaleda C. Epigenetic priming in immunodeficiencies. Front Cell Dev Biol 2019, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Campos-Sanchez E, Martinez-Cano J, del Pino Molina L, Lopez-Granados E, Cobaleda C. Epigenetic deregulation in human primary immunodeficiencies. Trends Immunol 2019, 40, 49–65. [CrossRef]
- Camacho-Ordonez N, Ballestar E, Timmers HTM, Grimbacher B. What can clinical immunology learn from inborn errors of epigenetic regulators? J Allergy Clin Immunol 2021, 147, 1602–1618. [CrossRef] [PubMed]
- Ghafouri-Fard S, Niazi V, Taher M. Role of miRNAs and lncRNAs in hematopoietic stem cell differentiation. Noncoding RNA Res 2021, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chen CZ, Li L, Lodish HF, Bartel DP. Micro RNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [CrossRef] [PubMed]
- Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 2007,131,145-159. [CrossRef]
- Salunkhe S, Vaidya T. CD40-miRNA axis controls prospective cell fate determinants during B cell differentiation. Mol Immunol 2020,126,46-55.
- Bao Y, Cao X. Epigenetic control of B cell development and B cell related immune disorders. Clin Rev Allergy Immunol 2016, 51, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Rao DS, O’Connell, Chaudhuri AA, Garcia-Flores Y, Geiger TI, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity 2010,33,48-59. [CrossRef]
- O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles of micro RNAs in the immune system. Nat Rev Immunol 2010, 10, 111–122. [CrossRef] [PubMed]
- Babaha F, Yazdani R, Shahkarami S, Hamidi Esfahani Z, Abolhassani H, Sadr M, et al. Evaluation of miR-210 expression in common variable immunodeficiency: patients with unsolved genetic defect. Allergol Immunopathol 2021,49,84-93. [CrossRef]
- Haralambieva IH, Kennedy RB, Simon WL, Goergen KM, Grill DE, Ovsyannikova IG, et al. Differential miRNA expression in B cells is associated with inter-individual differences in humoral immune response to measles vaccination. PloS ONE 2018,13,e0191812. [CrossRef]
- Rosales, C. Fc gamma receptor heterogeneity in leukocyte functional responses. Front Immunol 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Surace AEA, Hedrich CM. The role of epigenetics in autoimmune/inflammatory disease. Front Immunol 2019,10,1525. [CrossRef]
- Gutierrez M, Gomez JL, Perez GF, Pancham K, Val S, Pillai DK, et al. Airway secretory microRNAome changes during rhinovirus infection in early childhood. PLoS ONE 2016,11,:e0162244. [CrossRef]
- De Felice B, Nigro E, Polito R, Rossi FW, Pecoraro A, Spadaro G, et al. Differently expressed microRNA in response to the first Ig therapy in common variable immunodeficiency patients. Sci Rep 2020, 10, 21482. [CrossRef] [PubMed]
- Baloh C, Reddy A, Henson M, Prince K, Buckley R, Lugar P. 30-year review of pediatric- and adult-onset CVID: clinical correlates and prognostic factors. J Clin Immunol 2019,39,678-687. [CrossRef]
- Sanchez LA, Magadottir MS, Pantell MS, Lugar P, Cunningham Rundles C, Sullivan K. Two sides of the same coin: pediatric-onset and adult-onset common variable immune deficiency. J Clin Immunol 2017,37,592-602. [CrossRef]
- Szczawinska-Poplonyk A, Schwartzmann E, Bukowska-Olech E, Biernat M, Gattner S, Korobacz T, et al. The pediatric common variable immunodeficiency – from genetics to therapy: a review. Eur J Pediatr 2022,181,1371-1383. [CrossRef]
- Janssen LMA, van der Flier M, de Vries E. Lessons learned from the clinical presentation of common variable immunodeficiency disorders: a systematic review and meta-analysis. Front Immunol 2021:620709. [CrossRef]
- Ammato G, Vita F, Quattrocchi P, Minciullo PL, Pioggia G, Gangemi S. Involvement of miR-142 and miR-155 in non-infectious complications in CVID. Molecules 2020,25,4760. [CrossRef]
- Zhu H, Wang G, Qian J. Transcription factors as readers and effectors of DNA methylation. Nat Rev Genet 2016, 17, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Kulis M, Merkel A, Heath S, Queiros AC, Schuyler RP, Castellano G, et al. Whole-genome fingerprint of the DNA methylome during human B-cell differentiation. Nat Genet 2017,47,746-756. [CrossRef]
- Alvarez-Errico D, Vento-Tormo R, Siewke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat Rev Immunol 2015,15,7-17. [CrossRef]
- Rodriguez-Cortez VC, de Pino-Molina L, Rodriguez-Ubreva J, Ciudad L, Gomez-Cabrero D, Company C, et al. Monozygotic twins discordant for common variable immunodeficiency reveal impaired DNA demethylation during naiv-to-memory B-cell transition. Nat Commun 2015,6,7335. [CrossRef]
- Rodriguez-Ubreva J, Arutyunyan A, Bonder JM, Del Pino-Molina L, Clark SJ, de la Calle-Fabregat C, et al. Single-cell atlas of common variable immunodeficiency shows germinal center-associated epigenetic dysregulation in B-cell responses. Nat Commun 2022,13,1779. [CrossRef]
- Del Pino-Molina L, Rodriguez-Ubreva J, Torres Canizales J, Coronel-Diaz M, Kulis M, Martin-Subero JI, et al. Impaired CpG demethylation in common variable immunodeficiency associates with B cell phenotype and proliferation rate. Front Immunol 2019, 10, 879. [CrossRef] [PubMed]
- Aird A, Lagos M, Vargas-Hernandez A, Posey JE, Coban-Akdemir Z, Jhangiani S, et al. Novel heterozygous mutation in NFKB2 is associated with early onset CVID and a functional defect in NK cells complicated by disseminated CMV infection and severe nephrotic syndrome. Front Pediatr 2019,7,303. [CrossRef]
- Klemann C, Camacho-Ordonez N, Yang L, Eskandarian Z, Rojas-Restrepo JL, Frede N,; et al Clinical and immunological phenotype of patients with primary immunodeficiency due to damaging mutations in NFKB2. Front Immunol 2019, 10, 297. [CrossRef] [PubMed]
- Lougaris V, Baronio M, Gazzurelli L, Lorenzini T, Fuoti M, Moratto D, et al. A de novo monoallelic CTLA-4 deletion causing pediatric onset CVID with recurrent autoimmune cytopenias and severe enteropathy. Clin Immunol 2018,197,186-188. [CrossRef]
- Rush-Kittle J, Gamez-Diaz L, Grimbacher B. Inborn errors of immunity associated with defects of self-tolerance checkpoints: the CD28 family. Pediatr Allergy Immunol 2022,33,13886. [CrossRef]
- Ballow M, Leiding JW. Precision medicine in the treatment of primary immune deficiency patients with disorders of immune dysregulation. Clin Rev Allergy Immunol 2022,63,1-8. [CrossRef]
- Majchrzak-Celińska A, Baer-Dubowska W. Pharmacoepigenetics: an element of personalized pharmacotherapy? Expert Opin Drug Metab Toxicol 2017, 13, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Lee TK, Gereige JD, Maglione PJ. State-of-the-art diagnostic evaluation of common variable immunodeficiency. Ann Allergy Asthma Immunol 2021,127,19-27. [CrossRef]
- Leiding, JW., Forbes LR. Mechanism-based precision therapy for the treatment of primary immunodeficiency and primary immunodysregulatory diseases. J Clin Immunol Pract, 2019; 7, 761–773. [Google Scholar] [CrossRef]
- Pinto MV, Neves JF. Precision medicine: the use of tailored therapy in primary immunodeficiencies. Front Immunol 2022,13,1029560. [CrossRef]
- Boz V, Zanchi C, Levantino L, Riccio G, Tommasini A. Druggable monogenic immune defects hidden in diverse medical specialties: focus on overlap syndromes. World J Clin Pediatr 2022,11,136-150.
- Fekrvand S, Khanmohammadi S, Abolhassani H, Yazdani R. B- and T-cell subset abnormalities in monogenic common variable immunodeficiency. Front Immunol 2022,13,912826. [CrossRef]
- Heimall JR, Hagin D, Hajjar J, Henrickson SF, Hernandez-Trujillo HS, Itan Y, et al. Use of genetic testing for primary immunodeficiency patients. J Clin Immunol 2018, 38, 320-–329. [CrossRef] [PubMed]
- Schmitt EG, Cooper MA. Genetics of pediatric immune-mediated diseases and human immunity. Annu Rev Immunol 2021,39,227-249. [CrossRef]
- Caballero-Oteyza A, Crisponi L, Peng XP, Yauy K, Volpi S, Giardino S, et al. GenIA, the Genetic Immunology Advisor database for inborn errors of immunity. J Allergy Clin Immunol 2024,153,831-843. [CrossRef]
- Beers BJ, Similuk MN, Ghosh R, Seifert BA, Jamal L, Kamen M, et al. Chromosomal microarray analysis supplements exome sequencing to diagnose children with suspected inborn errors of immunity. Front Immunol 2023, 14, 1172004. [CrossRef] [PubMed]
- Tadros S, Prevot J, Meyts I, Sanchez-Ramon S, Erwa NH, Fischer A, et al. The PID Odyssey 2030: outlooks, unmet needs, hurdles, and opportunities – proceedings from the IPOPI global multi-stakeholdrers’ summit. Front Immunol 2023,14,1245718.
| Predominantly antibody deficiencies associated with CVID phenotypes | |||||
|---|---|---|---|---|---|
| BCR costimulatory B cell surface proteins | TNF superfamily receptors and ligands | Lipid signaling molecules | Actin cytoskeleton regulators | Transcription factors mediating differentiation and crosstalk | Metabolic mitochondrial and glycosylation pathways |
|
CD19 MS4A1/ CD20 CR2/ CD21 CD81 |
TNFSF13B/ BAFF/ BLYS/ TALL1 TNFSF13/ APRIL TNFSF12/ TWEAK TNFRSF13C/ BAFF-R TNFRSF13B/ TACI TNFRSF17/ BCMA |
PIK3CD PIK3R1 PTEN PIK3CG TTC7A |
CXCR4 RAC2 ARHGEF1 TTC7A PSTPIP1 DOCK8 WAS |
NFKB1 NFKB2 IKZF1/ IKAROS |
MAGT1 ATP6AP1 PGM3 TNRT1 FNIP1 SBDS TAFAZZIN |
| Hypomorphic variants in other genes associated with predominantly antibody deficiencies | |||||
|
BTK TCF3 FNIP1 ICOS CTNNBL1 ZRSR2 | |||||
| Genes associated with immune dysregulation disorders | |||||
| Transcriptional regulators of central and peripheral tolerance | Membrane- bond organelle dynamics | Genes related to lymphoproliferative conditions | |||
|
AIRE FOXP3 STAT3 SOCS1 BACH2 |
CTLA4 LRBA SEC61A1 SH3KBP1 DEF6 SAMD9 |
CD27 CD70 MAGT1 SH2D1A PRKCD STXBP2 UNC13D FASLG |
|||
| Genes in combined cellular and humoral immunodeficiencies | |||||
| Genes associated with T cell signaling regulators | Genes associated with epigenetic regulation | ||||
|
ICOS CTLA4 FOXP3 GATA2 RFXANK LCK IL21R |
DNMT3B ZBTB24 IGH KMT2D/ MLL2 KDM6A/ UTX |
||||
| Genetic underpinnings of autoinflammatory disorders | |||||
|
DCLRE1C/ Artemis ADA2 RNF31 TNFA1P3 PLCG2 NLRC3, NLRC4, NLRP2, NLRP3, NLRP12 | |||||
| Digenic variants | Product functions | Clinical phenotype | Immunodeficiency | Authors (references) | ||
|---|---|---|---|---|---|---|
| Genes | Variants | Digenic proband | Monogenic variant | |||
|
TNFSFR13B /TACI (17p11.2) |
rs34557412 C104R |
T-cell independent CSR, MyD88 pathway | CVID Systemic lupus erythematosus |
Mild cytopenia Antibody deficiency | Defective T cell dependent and T cell independent B cell differentiation and activation | Ameratunga et al. [38] |
|
TCF3 (19p13.3) |
T168fsX191 | T-cell dependent and independent CSR, AID pathway | Antibody deficiency Arthritis Diabetes mellitus |
|||
|
NFKB1 (4q24) |
(c.1149delT/ p.Gly384Glu*48) | Key cellular driver of inflammation and immunity | CVID Inflammatory bowel disease Thrombocytopenia |
Asymptomatic | Th1-polarized T cell population B cell lymphopenia and B cell naïvete |
Dieli-Crimi et al. [39] |
|
NOD2 (16q12.1) |
rs 5743272 (p.His352Arg) |
Inflammatory response to pathogens NF-κB pathway |
Crohn disease | |||
|
LRBA (4q31.3) |
C7885delA (p.R2629fs) |
Peripheral B cell tolerance, stimulation of T regulatory cell development and functions, CTLA 4 pathway | Antibody deficiency Recurrent airway infections Sepsis Inflammatory bowel disease Throbocytopenia Autoimmune hemolytic anemia |
Immunodeficiency with autoimmunity | Decreased ability of T regulatory cells to control T effector cells Defective peripheral B cell tolerance Immunodeficiency |
Massaad et al. [40] |
|
NEIL3 (4q34.3) |
rs200055050 (p.D132V) |
Regulation of lymphoid cell proliferation, peripheral B cell tolerance | Asymptomatic High levels of autoantibodies |
|||
|
CTLA4 (2q33.2) |
rs1581573923 (p.Y139C) |
Negative regulator of T cell responses, expressed in activated T cells and T regulatory cells | CVID Recurrent airway infections Lymphoid hyperplasia Gastroenteritis Hypothyroidism |
Asymptomatic | Impaired memory B cell and plasma cell development T cell hyperactivity |
Sic et al. [41] |
|
JAK3 (19p13.11) |
rs200077579 (p.R840C) |
Signal transduction from JAK3-associated cytokine receptor common γ chain | Hashimoto thyroiditis | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





