1. Introduction
Bloody stools in newborns may be a sign of several clinical entities of different severity. It may range from a transient haematochezia to food protein induced allergic proctocolitis (FPIAP) to more severe disease, such as necrotizing enterocolitis (NEC).
To distinguish among them at an early stage is challenging, but crucial, as the treatment and prognosis are radically different. NEC is a life-threatening condition that must be treated with a period of fasting and antibiotic therapy and in the most severe cases with intestinal resection surgery. Otherwise, food protein induced allergic procotoclitis is usually a benign condition that does not require pharmacological treatment, but only the avoidance of cow’s milk protein.
FPIAP is part of the larger group of gastrointestinal disorders associated with non-IgE-mediated food allergies (non-IgE-GI-FA). It is caused by an inflammation of the distal portion of the sigma and rectum characterised by oedema and erosion of the mucosa with eosinophilic infiltration of the epithelium and/or lamina propria. Onset most frequently occurs in the first three months of life, and it is manifested by blood in the faeces (haematochezia or occult blood), less frequently associated with half-formed alvo and mucus. Typically, infants with FPIAP appear healthy, with normal weight growth and have no other associated symptoms, except for mild anaemia in the case of chronic bleeding [
1].
The prevalence is not well known and has been estimated at between 0.16% and 64% of healthy infants with haematochezia. Cow’s milk is the allergen most frequently responsible for FPIAP.
More than 50% of infants with FPIAP are exclusively breastfed and up to 25% of cases have a positive family history of atopy. In breastfed infants, symptoms often occur later than in formula-fed infants/infants [
2]. It is possible that symptoms begin as early as the first week after birth, suggesting that in utero sensitisation may occur, although the exact mechanism is not yet fully understood [
3].
The main treatment of FPIAP is to avoid the allergen responsible, in most cases cow’s milk protein. In breastfed, symptomatic infants, a cow’s milk protein exclusion diet may be recommended for mothers, especially in cases of moderate/severe bleeding. In infants who continue to be symptomatic despite the mother’s strict cow’s milk protein exclusion diet, the elimination of other allergens, such as soy and eggs, from the mother’s diet should be considered (rarely other foods) [
2].
Visible bright red rectal bleeding, also associated with eosinophilic infiltration in the rectosigmoid mucosa, may disappear spontaneously in infants who have been excluded from surgical, infection, and other pathologies [
4,
5].
Hence, a “watch and wait” approach of 1 month before an elimination diet has been proposed to monitor whether spontaneous resolution occurs in some patients [
6].
The onset of FPIAIP symptoms is usually in the first months of life; the majority of patients develop symptoms during the second month of life [
7,
8].
However, the large cohort study by Senocak et al found that symptoms began in the neonatal period in one third of patients, finding an association between the onset of symptoms in the neonatal period and a history of premature birth [
8], although the available data inpreterm newborns is scarce and heterogeneous.
Cases of CMA reported in preterm infants are mostly non-IgE mediated [
3];Morita et al. found an incidence of CMPA with systemic symptoms of 1.1%, although to date the prevalence is not well established [
9].
Most preterm infants with CMPA were exposed to bovine milk protein before the development of symptoms ad the most common clinical manifestations were bloody stools, vomiting and abdominal distension [
10]. CMPA in preterms may present with a more acute illness that may mimic NEC, sepsis or shock [
11,
12], or they may have non-specific symptoms, including abdominal discomfort and fever [
13].
The aim of the present study was to describe the features of preterm newborns with bloody stools in a Neonatal Intensive Care Unit.
2. Materials and Methods
We conducted a monocentric retrospective study in a neonatal intensive care unit (NICU). The study population consisted of all preterm newborns admitted to the NICU of the Vittore Buzzi Children’s Hospital in Milan from December 2022 to May 2024 with blody stools.
At the onset of the symptoms all subjects were fasted for 48 hours and they underwent a diagnostic evaluation including blood examination, abdominal X-rays (single or serial, as appropriate), abdominal ultrasound and faecal infectious tests.
The diagnosis of NEC has been made on the basis of Bell’s modified criteria (Walsh & Kliegman, 1986), which include clinical and radiological criteria.
Patients for whom the diagnosis of NEC was ruled out were re-feed with amino acid formula and then a challenge was carried out to confirm or rule out a diagnosis of CMPA.
Newborns whose symptoms relapsed (e.g presence of bloody stools after cow’s milk reintroduction) were diagnosed as having cow’s milk allergy and they continued to be fed with the aminoacidic formula.
The following clinical characteristic sand laboratory data were collected from the medical records: date of birth, sex, gestational age, twin pregnancy (specifying whether monochorionic or biconchoric), type of delivery, birth weight, age at onset of haematochezia, presence of other symptoms (abdominal distension, vomiting and any other), type of feeding undertaken in the first few days of life, type of feeding in the days preceding the episode, blood exams (blood count, IgE, sIgE, blood culture, faecal antigens of adenovirus and rotavirus, faecal norovirus and enterovirus, X-rays of the abdomen, ultrasound of the abdomen, allergy counselling), duration and type of antibiotic therapy carried out, duration of withdrawal of enteral feeding, type of feeding undertaken when the episode was resolved.
Data analysis was conducted using R software. Quantitative and categorical variables available as metadata were compared among patients diagnosed with APLV, transient hematochezia, and NEC. Quantitative variables were compared between groups using the Kruskal-Wallis test, followed by post-hoc pairwise comparisons using the Mann-Whitney U test. The results were visualized with box plots. Categorical variables were compared between groups using the Chi-squared test, with the results visualized using bar plots. Statistical significance was defined as p-values < 0.05.
3. Results
A total of 43 prematures showed haematochezia during the study period.
In total, 40% (18/43) of infants with haematochezia were finally diagnosed as NEC, 37.2% (16/43) with FPIAP and 20% (9/43) with transient haematochezia.
11,6% (5/43) were extremely pretermnewborns (< 28 sg) and none among them were diagnosed with CMPA, while 2/5 presented with transient haematochezia. 32% (14/43) were very preterm (28+0-31+6) and 50% (7/14) had a diagnosis of FPIAP and 2/14 (14.2%) presented with transient haematochezia. 53% (23/43) were moderate to late preterm infants (32+0 - 36+6) and 40% (9/23) received a diagnosis of FPIAP while 21% (5/23) had transient haematochezia.
Two moderate preterm infants with an initial diagnosis of NEC were later diagnosed with CMPA.
The descriptive characteristics of the three study groups are summarized below (
Table 1).
The majority of preterm newborns with FPIAP were exposed to bovine-based milk proteins before the development of symptoms. At the time of the onset of symptoms 5/16 (31.3%) with FPIAP infants were fed with breast milk, 2/16 (12.5%) with formula milk and 9/16 (56.3%) with mixed feeding. 5/16 infants were receiving fortified breast milk.
In 93.7 % (15/16) of the cases, patients with FPIAP presented sIgE negative and were diagnosed with allergic proctocolitis. Only one patient (35.4 GA) had total IgE 14.5 KU/L, alpha lactalbumin <01 kU/L, beta lactoglobulin 2.31 kU/L, casein 0.41 kU/L and was the only one to present with vomiting, abdominal distension and gastric stagnation. Of the remaining 15 patients, only one (28+3 GA) presented with symptoms of gastroesophageal reflux and dermatitis in addition to rectal bleeding.
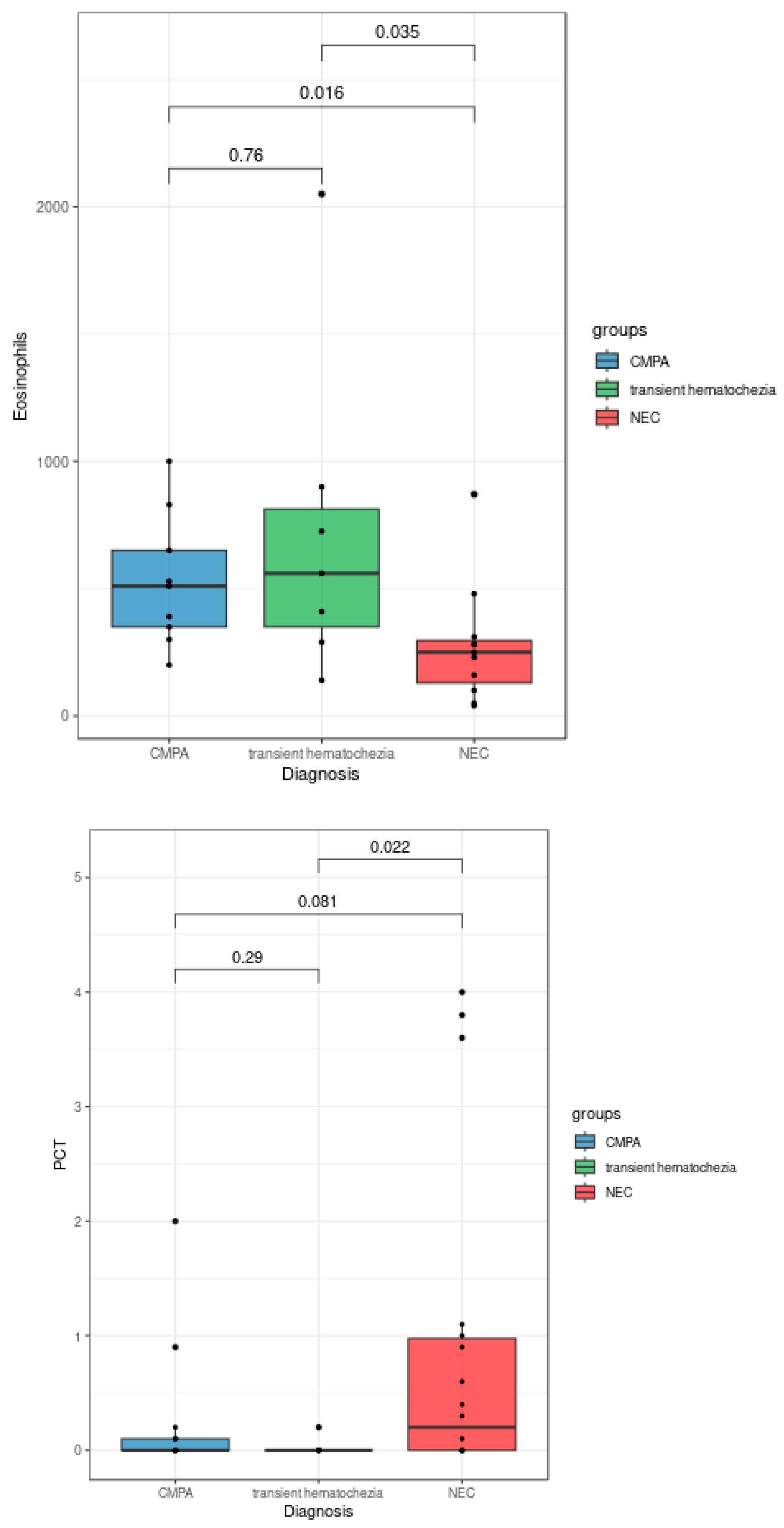
The statistical analyses of the clinical data revealed some features associated with CMPA, transient hematochezia, and NEC diagnoses. As shown in
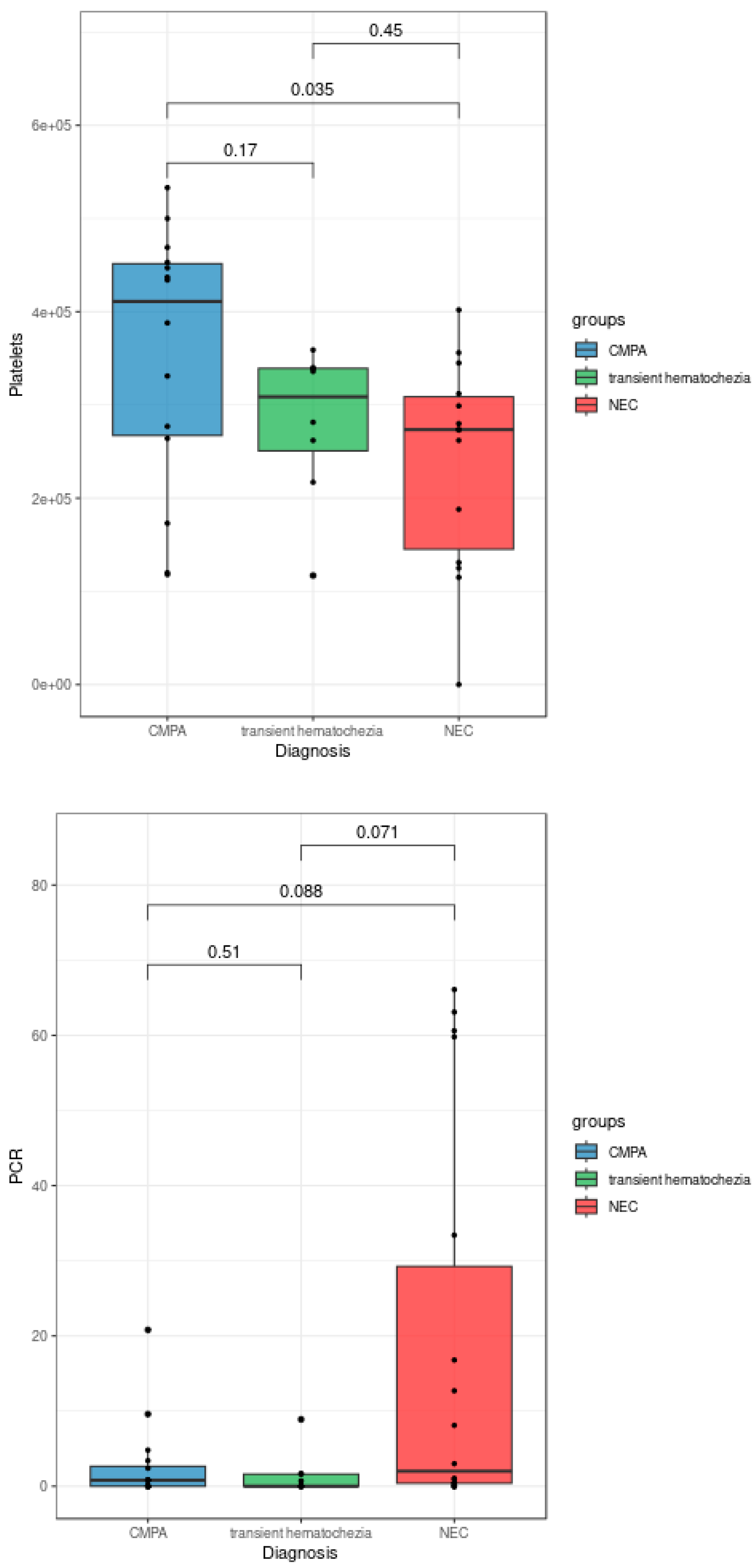
Figure 1, patients diagnosed with NEC exhibited a significantly lower eosinophil count compared to both patients with CMPA (p = 0.016) and patients with transient hematochezia (Kruskal-Wallis test, p = 0.035). Additionally, procalcitonin levels were significantly higher in patients with NEC compared to those with transient hematochezia (Kruskal-Wallis test, p = 0.022). Although not reaching full statistical significance in the Kruskal-Wallis test, blood platelet counts and C-reactive protein levels showed trends of decrease and increase, respectively, in patients diagnosed with NEC (
Figure 2).
Moreover, NEC patients demonstrated a statistically significant increase in pathological outcomes for ultrasounds (Chi-squared test, p = 0.046) and abdominal X-rays (Chi-squared test, p = 0.000096).
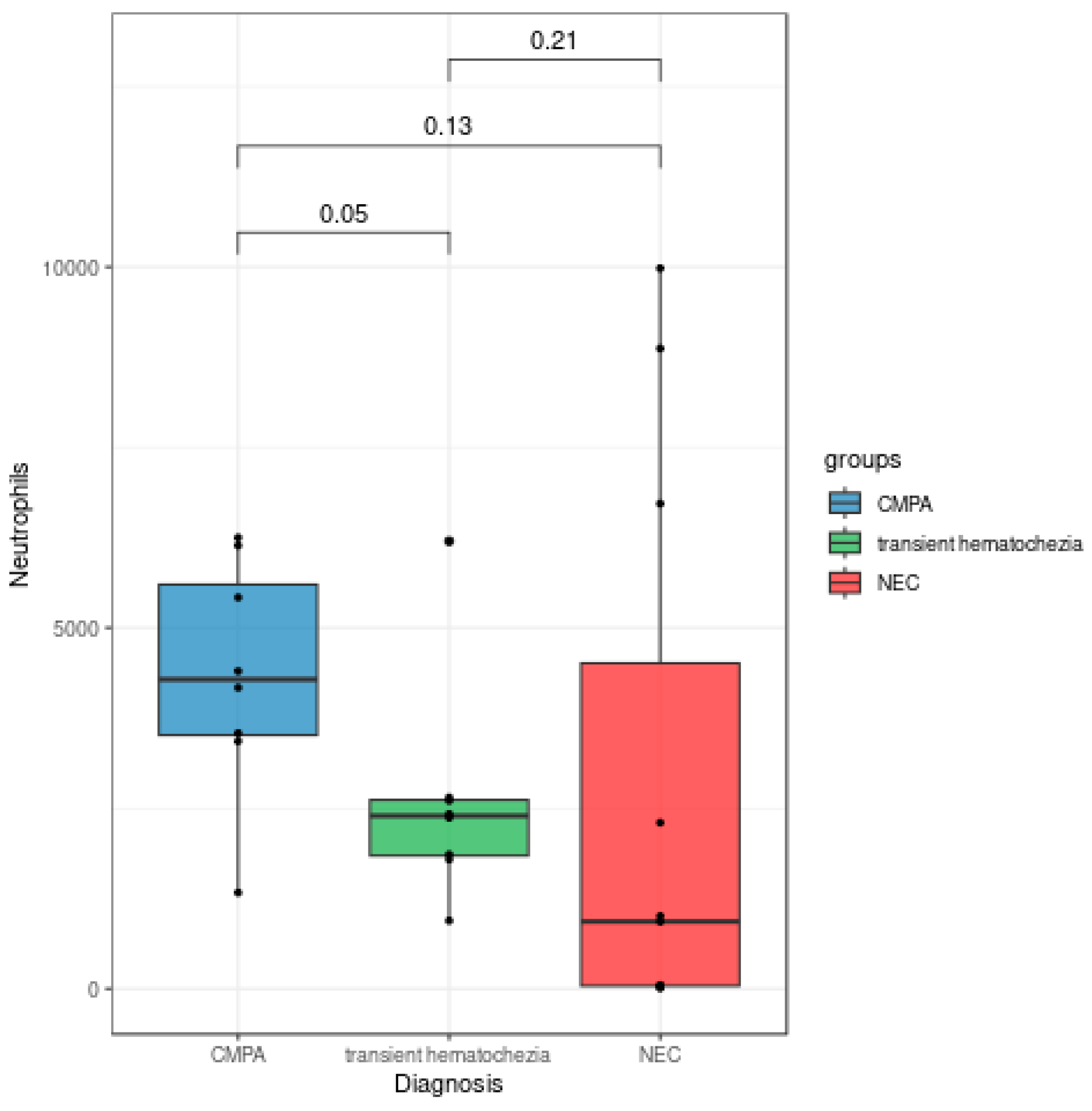
Despite the Kruskal-Wallis p-value (p = 0.097) not fully supporting the claim, the Mann-Whitney test p-value (p = 0.05) and the visual observation of
Figure 3 revealed an interesting trend of increase in neutrophil levels in patients with APLV compared to those with transient hematochezia.
4. Discussion
The main finding of our study is that no useful Lab markers or imaging may aid in distinguishing between transient hematochezia and FPIAIP, athough they may be useful,if combined to the clinical features to exclude the diagnosis of NEC, as well as for FPIES in prematures newborns [
11].
However, we should not overlook the 37% of patients diagnosed with CMPA who presented with pathological radiography. Intestinal pneumatosis can also be observedon X-ray of the abdomen, that may lead to a misdiagnosis of NEC, resulting in unnecessary antibiotic therapy.
In agreement with literature data, PCT showed superiority over PCR in neonatal age as a marker to distinguish NECfrom allergic conditions [
14].
Multiple food allergies were not described in our case series because all patients diagnosed with FPIAP had a resolution of their symptoms with the elimination of milk proteins (by mother feeding or formula).
Morita et al. showed no diagnosis of CMPA< 32 weeks GA; otherwise, in our study 7 patients were diagnosed with CMPA <32 sg, whilethere were no diagnoses < 28 sg GA. There is still limited understanding on the development of the immune system in preterms; in some studies, an association between prematurity and reduced functionality of Tregs has been shown, compared to term infants of adequate weight [
15].Human Tregs are a heterogeneous cellular subgroup in which functionally and phenotypically different subpopulations can be distinguished. Recent studies in a neonatal animal model have reported a delay in migration and ontogeny of Tregs in the intestinal tract and a reduced proportion of Foxp3+ Tregs in the intestinal mucosa, which may correlate with gestational age [
16,
17].Reduction in regulatory T cells in preterm newborns is associated with necrotizing enterocolitis [
18].Moreover since SGA neonates show reduced suppressive activity of Tregs [
19], this could be an additionl factor in promoting CMPA in our population.
In our cohort all patients underwent an oral food challenge (OFC), which allowed us to diagnose a “transient haematochezia” in thegroup of prematures whose symptoms did not relapse after reintroduction of cows milk proteines into the diet.
Making a correct diagnosis, distinguishing between these two entities, is important to avoid over treatment, with a prolonged and unnecessary diet in case of transient hematochezia.
On the other hand, a missing diagnosis of CMPA in early life can lead to potential complications, such as mild anemia associated with persistent bleeding and also to a prolonged colonic inflammation which in turn may predispose to the development of functional gastrointestinal disordersin childhood [
20]. All preterm newborn with haematochezia must be considered at risk of serious diseases (NEC or other surgical condition like malrotation, volvulus, Hirschsprung’s disease with enterocolitis, upper gastrointestinal haemorrhage, vascular malformations, gastrointestinal duplication) and investigations must be performed to first excludethese diagnoses. Once NEC and surgical conditions have been excluded, rectal bleeding is often labelled as CMPA in routine clinical practice. However, it must be borne in mind that within this group hides another with transient haematochezia, which if undiagnosed is at risk of receiving an unnecessary and prolonged diet. As clinical presentation, Lab markers and/or imaging are not useful to distinguish between these two conditions, an early oral provocation test remains the only way to exclude the diagnosis of FPIAIP, and therefore, the need for an avoidance diet.In our opinion, once NEC has been excluded, the only way to confirm the FPIAP is the OFC, which can be performed even in premature newborns presenting blood stools, otherwise healthy, in NICU.
In a retrospective study of 348 infants in a NICU who required parental nutrition, 5% received a final diagnosis of CMPA, manifested by feeding intolerance or ‘late-onset or recurrent NEC-like illness’ [
21]. In our case series, we also had two infants who were first diagnosed with NEC and later turned out to be CMPA due to the disappearance of symptoms when placed on a CMP-free diet. Thiscan be explained in two ways. The first diagnosis of NEC was wrong: the symptoms were actually caused by CMPA, but the first episode was severe, mimicking NEC, even with imaging compatible with this diagnosis. The second is that NEC itself may play a role in the development of CMPA. NEC causes damage to the intestinal barrier, which facilitates the onset of allergies due to contact between food antigens and the immune system. Chuang et al. demonstrated an up-regulation of proinflammatory cytokines after peripheral blood mononuclear cells from infants with NEC were stimulated by CMP (betalactoglobulin and casein), while controls showed no or negligible response. These results provide evidence of the immune system’s involvement with CMP and suggest the importance of CMPA in NEC [
22].
In regard to the mode of feeding, it should be pointed out that premature and very low-birth-weight infants have additional caloric requirements to human milk that are met through the use of human milk fortificants that are traditionally CMP-based. Recently, fortificants based on human milk protein have been proposed in the US [
23]. In our case series, 5 patients with CMPA had CMP-based fortificants added to their diet. If the use of these supplements in the first days of life may play a role in the development of CMPA is still unknown and need to be further investigated in the future.
This study has some limitations which consist of the retrsopective study design and the relatively small number of patients. The strength of the study consists of having perfomed an oral provocation test in preterm newborns, after a short period of elemental formula.
Further prospective studies are warranted to better elucidate FPIAIP in preterms and its management.
5. Conclusions
Our study highlights the importance of considering CMPA when taking careof preterm newbornswith blood stools, otherwise healthy. Both serial patological X-ray and ultrasound findings of the abdomen associated to high serum PCT levels and low eosinophils count help physicians in making a diagnosis of NEC. In this particular, fragile population an early oral food challenge could be considered to differentiating between transient hematochezia and FPIAIP. Further studies on larger populations are needed to confirm these findings.
Author Contributions
Conceptualization, E.D’A., M.A. and F.C.; methodology, L.S.; validation, G.V.Z and G.L.; formal analysis, L.S.; data curation, MA., P.A.L.V., C.P.; writing—original draft preparation, E.D’A., F.C., M.A.; writing—review and editing, E.D’A., M.A., A.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bahceci, S.; Kuyum Töz, P.; Celebi Celik, F.; Can, D. A Different Starting Line for Allergic March: Food Protein-Induced Allergic Proctocolitis. Allergol. Immunopathol. (Madr.) 2023, 51, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and Management of Non-IgE Gastrointestinal Allergies in Breastfed Infants—An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Burris, A.D.; Burris, J.; Järvinen, K.M. Cow’s Milk Protein Allergy in Term and Preterm Infants: Clinical Manifestations, Immunologic Pathophysiology, and Management Strategies. NeoReviews 2020, 21, e795–e808. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Shimizu, T.; Shoji, H.; Kudo, T.; Fujii, T.; Wada, M.; Sato, H.; Aoyagi, Y.; Haruna, H.; Nagata, S.; et al. Neonatal Transient Eosinophilic Colitis Causes Lower Gastrointestinal Bleeding in Early Infancy. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J.; Kim, A.S.; Hwang, J.-B. The Etiology of Small and Fresh Rectal Bleeding in Not-Sick Neonates: Should We Initially Suspect Food Protein-Induced Proctocolitis? Eur. J. Pediatr. 2012, 171, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Miceli Sopo, S.; Monaco, S.; Bersani, G.; Romano, A.; Fantacci, C. Proposal for Management of the Infant with Suspected Food Protein-induced Allergic Proctocolitis. Pediatr. Allergy Immunol. 2018, 29, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Koksal, B.T.; Barıs, Z.; Ozcay, F.; Yilmaz Ozbek, O. Single and Multiple Food Allergies in Infants with Proctocolitis. Allergol. Immunopathol. (Madr.) 2018, 46, 3–8. [Google Scholar] [CrossRef]
- Senocak, N.; Ertugrul, A.; Ozmen, S.; Bostanci, I. Clinical Features and Clinical Course of Food Protein—Induced Allergic Proctocolitis: 10-Year Experience of a Tertiary Medical Center. J. Allergy Clin. Immunol. Pract. 2022, 10, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Iwakura, H.; Ohtsuka, H.; Kohno, Y.; Shimojo, N. Milk Allergy in the Neonatal Intensive Care Unit: Comparison between Premature and Full-Term Neonates. Asia Pac. Allergy 2013, 3, 35–41. [Google Scholar] [CrossRef]
- Florquin, M.; Eerdekens, A. What Is Known About Cow’s Milk Protein Allergy in Preterm Infants? Breastfeed. Med. 2023, 18, 767–778. [Google Scholar] [CrossRef]
- D’Auria, E.; Cocchi, I.; Monti, G.; Sartorio, M.U.A.; Daniele, I.; Lista, G.; Zuccotti, G.V. Food Protein–Induced Enterocolitis Syndrome in Preterm Newborns. Pediatr. Allergy Immunol. 2022, 33, e13676. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.K. Enterocolitis in Low-Birth-Weight Infants Associated with Milk and Soy Protein Intolerance. J. Pediatr. 1976, 88, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Rodriquez, D.C.; Cecchi, S.; Tataranno, M.L.; Farmeschi, L.; Mori, A.; Buonocore, G. Different Clinical Manifestation of Cow’s Milk Allergy in Two Preterm Twins Newborns. J. Matern. Fetal Neonatal Med. 2012, 25, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Liao, W.; Chen, Z.; Tao, M.; Chen, S. The Mean Platelet Volume Combined with Procalcitonin as an Early Accessible Marker Helps to Predict the Severity of Necrotizing Enterocolitis in Preterm Infants. Int. J. Gen. Med. 2022, 15, 3789–3795. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Weaver, L.; Tobin, R.; Henderson, S.; Beeram, M.; Newell-Rogers, M.K.; Perger, L. Intrauterine Growth Restriction and Prematurity Influence Regulatory T Cell Development in Newborns. J. Pediatr. Surg. 2014, 49, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Lahl, K.; Loddenkemper, C.; Drouin, C.; Freyer, J.; Arnason, J.; Eberl, G.; Hamann, A.; Wagner, H.; Huehn, J.; Sparwasser, T. Selective Depletion of Foxp3+ Regulatory T Cells Induces a Scurfy-like Disease. J. Exp. Med. 2007, 204, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dingle, B.M.; Liu, Y.; Fatheree, N.Y.; Min, J.; Rhoads, J.M.; Tran, D.Q. FoxP3+ Regulatory T Cells Attenuate Experimental Necrotizing Enterocolitis. PLoS ONE 2013, 8, e82963. [Google Scholar] [CrossRef]
- Pacella, I.; Di Chiara, M.; Prota, R.; De Luca, C.; Cardillo, A.; Potenza, E.; Grimaldos, A.P.; Pinna, V.; Piconese, S.; Terrin, G. Reduction in Regulatory T Cells in Preterm Newborns Is Associated with Necrotizing Enterocolitis. Pediatr. Res. 2023, 94, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Engst, M.; Haensch, G.M.; Mahnke, K.; Schmitt, E.; Meuer, S.; Sohn, C. Small for Gestational Age (SGA) Neonates Show Reduced Suppressive Activity of Their Regulatory T Cells. Clin. Immunol. 2010, 134, 188–197. [Google Scholar] [CrossRef]
- Salvatore, S.; Folegatti, A.; Ferrigno, C.; Pensabene, L.; Agosti, M.; D’Auria, E. To Diet or Not to Diet This Is the Question in Food-Protein-Induced Allergic Proctocolitis (FPIAP)—A Comprehensive Review of Current Recommendations. Nutrients 2024, 16, 589. [Google Scholar] [CrossRef]
- Cordova, J.; Sriram, S.; Patton, T.; Jericho, H.; Gokhale, R.; Weinstein, D.; Sentongo, T. Manifestations of Cow’s-Milk Protein Intolerance in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S. -L.; Hayes, P.J.; Ogundipe, E.; Haddad, M.; MacDonald, T.T.; Fell, J.M. Cow’s Milk Protein-specific T-helper Type I/II Cytokine Responses in Infants with Necrotizing Enterocolitis. Pediatr. Allergy Immunol. 2009, 20, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, A.; Sharma, A.; Agarwal, R.; Thukral, A.; Deorari, A.; Sankar, M.J. Fortification of Breast Milk With Preterm Formula Powder vs Human Milk Fortifier in Preterm Neonates: A Randomized Noninferiority Trial. JAMA Pediatr. 2021, 175, 790. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).