1. Introduction
The adverse health effects of cigarette smoking are well recognised. Most smoking-related diseases are not related to nicotine [
1] but are associated with other toxic chemicals generated by the combustion of tobacco leaves. A heated tobacco product (HTP), also referred to as a heat-not-burn tobacco product, is an electrically operated device that delivers nicotine to the body by generating mainstream aerosols during the heating of processed tobacco-leaf materials [
2]. Recent comprehensive chemical analyses have shown that HTPs also generate numerous types of chemicals during heating, but the amount of generated chemicals is much less than that of conventional combustible cigarettes [
3,
4,
5,
6], mainly because of the lower heating temperature. Thus, HTPs are promoted as less hazardous or harmless products. Although there is little evidence that the short-to medium-term use of HTPs causes major harm to users, the effects of long-term use are still uncertain. Therefore, it is necessary to carefully investigate the aerosol components, including unknown and unidentified substances.
Formate (IUPAC name: methanoate) is a conjugate base of formic acid—the simplest carboxylic acid—with a potentially toxic effect on human health. Formic acid inhalation causes eye and nose irritation, sore throat, cough, chest tightness, headache, and confusion [
7]. It is also an intermediate in methanol poisoning. Methanol has low toxicity, and in the human body, it is first metabolised to formaldehyde by alcohol dehydrogenase, and then converted to formate. Formate accumulates in the body and causes visual damage, optic nerve injury, abdominal problems, nausea, and headaches [
8]. Tobacco leaves contain several organic acids, including formic acid, which contribute to leaf quality wherein the taste and aroma of tobacco products are closely linked to their organic acids [
9,
10]. Therefore, these organic acids are found in mainstream aerosols of combustible cigarette [
11]. However, despite their potential significance, formate generated by HTPs has received little attention.
The mainstream aerosol generated by the HTPs consists primarily of “water droplets”, which contain glycerine and/or propylene glycol that aid in aerosol forming [
12,
13]. Because formic acid is a water-soluble weak acid, it is presumed to be easily soluble in water droplets to form formate with counter cations such as protonated nicotine and ammonium ions. At present, Japan is the largest HTP-consumer country [
14] with a variety of devices which employ different heating temperatures ranging from 40 to 350 ℃. Here, we aimed to elucidate the actual emission levels of formate in mainstream aerosols produced by commercially available HTP devices in Japan and compare them with those of acetate, one of the most abundant chemicals found in mainstream HTP aerosols [
6].
2. Materials and Methods
2.1. Heated Tobacco Products
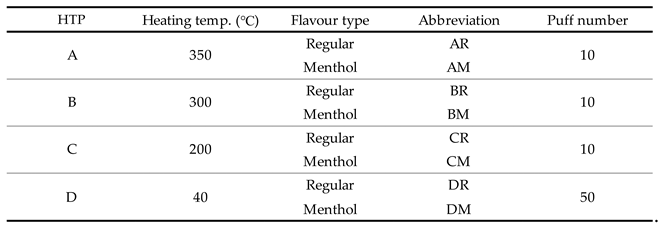
Mainstream aerosols were collected from four HTP types: A–D. All materials were purchased from a retail tobacco store in Tokyo, Japan.
Table 1 summarises the devices, their heating temperatures, flavour types, and the corresponding abbreviations used here. The heating temperature was obtained from a commercial catalogue or technical information provided by each manufacturer. The devices A-C were categorised as a “high-temperature type” and device D as a “low-temperature type”.
Device A consisted of a charger, holder, and consumable tobacco stick. After charging, the tobacco stick is inserted into the holder which has a flat heating blade. The blade-heated tobacco leaves are impregnated with aerosol formers in the tobacco stick. The aerosol is generated during the direct heating of the tobacco leaves with the blade at ~350 °C. The configuration of Device B was the same as that of Device A; however, the heating method is different. The tobacco stick has a metal heating element placed in its core. The aerosol is generated by direct heating of the tobacco leaves from inside to premiere by the inductively heated metal element at temperatures up to 350 °C. Device C comprises of a rechargeable battery, a heating furnace in the battery body, and a tobacco stick. Direct heating of tobacco leaves inserted in the furnace produces the mainstream aerosol at ~200 °C. Device D consists of a rechargeable battery, cartridge, and tobacco capsule. The aerosol is generated by heating liquid in the cartridge containing aerosol formers and passing this through the tailor-made tobacco capsule at ~40 °C. Each device has a variety of flavour sticks or capsules with regular and menthol (IUPAC name: 2-isopropyl-5-methylcyclohexanol) used here.
2.2. Measurement of Formate and Acetate
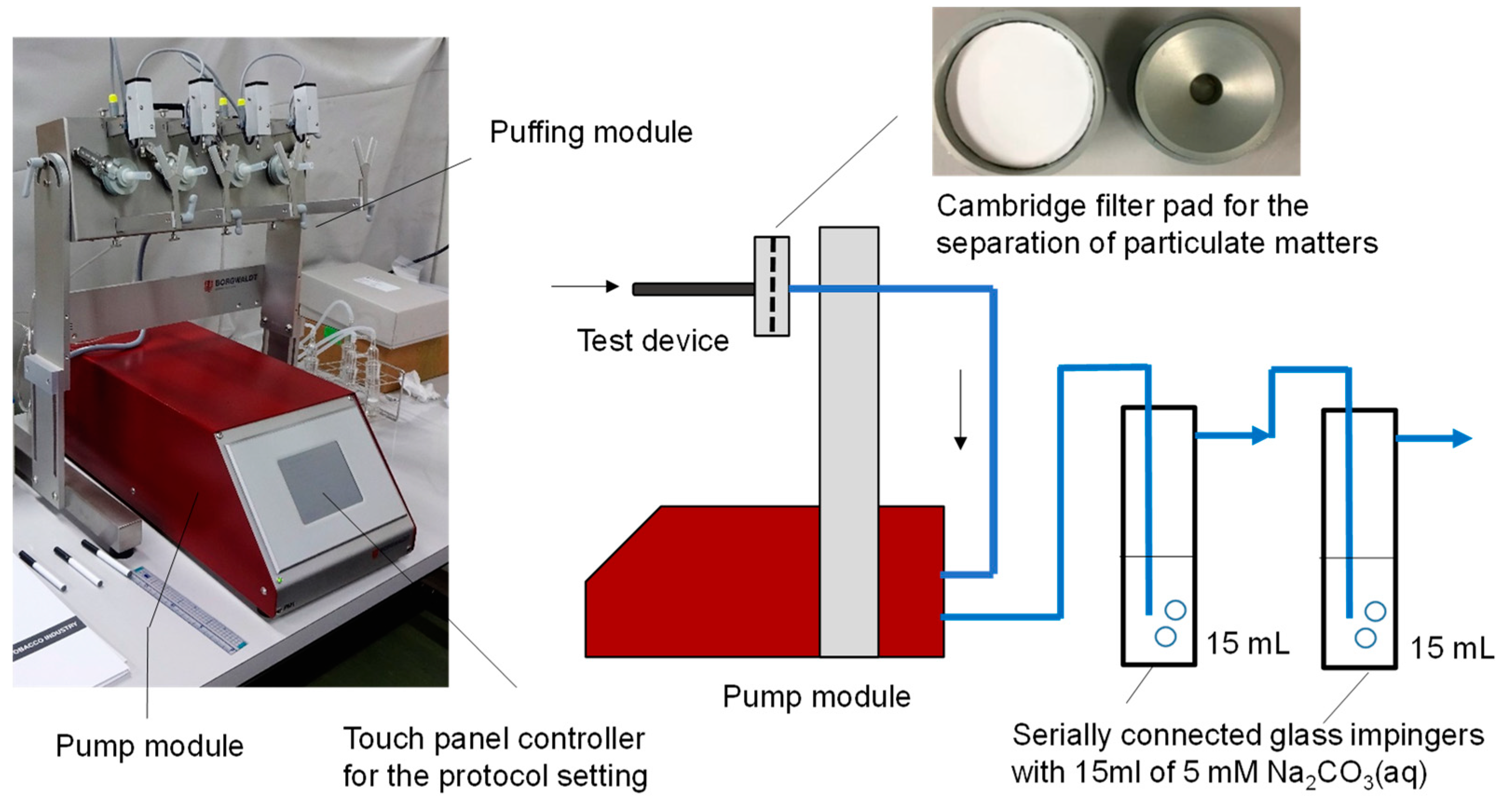
As shown in
Figure 1, The mainstream aerosols were generated from all devices using an LM4E Linear Vaping Machine for E-cigarettes (Borgwaldt KC, GmbH, Germany), following the puffing regime specified in the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA) Recommended Method 81 (CRM81) [
15]—55 mL puff volume, 3 s puff duration, 30 s puff interval, and a “Rectangle” type puffing profile. The vaping machine was operated in a separated booth with a local exhaust system at room temperature (~24 ℃) and 34 % relative humidity.
For the formate and acetate measurements in the aerosols, two glass impingers filled with trapping solution (15 mL) were connected in series to the outlet of the vaping machine with a silicon tube, with or without a Cambridge filter pad (44 mm diameter, Whatman, Buckinghamshire, UK), which was used for the separation of the gas and particle phases. As a trapping solution, 5 mM sodium carbonate (Na2CO3)—the ion chromatography effluent—was used to reduce the water dip in the chromatograms. Ten puffs were each collected from devices A, B, and C. Due to a lower emission of analytes from “low-temperature” type device D, the amount was set at 50 puffs for this device only. The formate and acetate contents were subsequently determined using ion chromatography after filling up to 15 mL with 5 mM Na2CO3. Ion chromatography was performed using a Thermo Fisher Scientific Dionex Aquion IC system with a chemical suppressor. The following condition was used: separation column, 4.0 mmφ × 250 mm, Dionex AS-9-HC Analytical (Thermo Fisher Scientific, Massachusetts, USA); guard column, Dionex AS-9-HC Guard (Thermo Fisher Scientific, Massachusetts, USA); eluent, 5 mM Na2CO3 at 1.0 mL min−1 (isocratic); and regenerant, 1.5 mM sulfuric acid (H2SO4). Dilution series of formate and acetate ranging from 0.0 to 1.0 mg L−1 in Milli-Q water were prepared from their sodium salts and used for calibration (r > 0.99 for each concentration versus peak area). All the reagents were purchased from Kanto Chemicals (Tokyo, Japan).
Three repeated measurements were conducted for all the runs. Blank samples were collected simultaneously without connecting HTPs. After subtracting the mean blank reading of the storage from sample readings, collection amounts of formate and acetate (mg) were converted to emission amounts in a puff volume
E (mg L
−1) by the following:
where
W is the collection amount of formate or acetate (µg) and
V is a total puff volume (0.55 L at 10 puffs and 2.75 L at 50 puffs). The limit of determination was defined as 10-fold the standard deviation of the blank reading and resulted in 3.9 and 0.78 µg L
-1 at 10 and 50 puffs, respectively for formate, and 5.1 and 1.0 µg L
-1 at 10 and 50 puffs, respectively for acetate.
3. Results
3.1. Emission Amounts of Formate and Acetate
Acidic substances from the mainstream aerosols of HTPs were collected in two serially connected impingers without installing a Cambridge filter pad. Significant peaks of acetate and formate—with retention times of 4.9 and 5.4 min, respectively—were observed in all HTP samples, whereas the peaks of chloride, nitrate, and sulfate ions were almost absent (blank levels). Acetate and formate were also found in the second impinger, resulting in collection efficiencies of 84 ± 1.9 and 81 ± 4.7 % for acetate and formate, respectively in all runs (n = 12). The emission amounts of both organic acid ions were then determined for all samples.
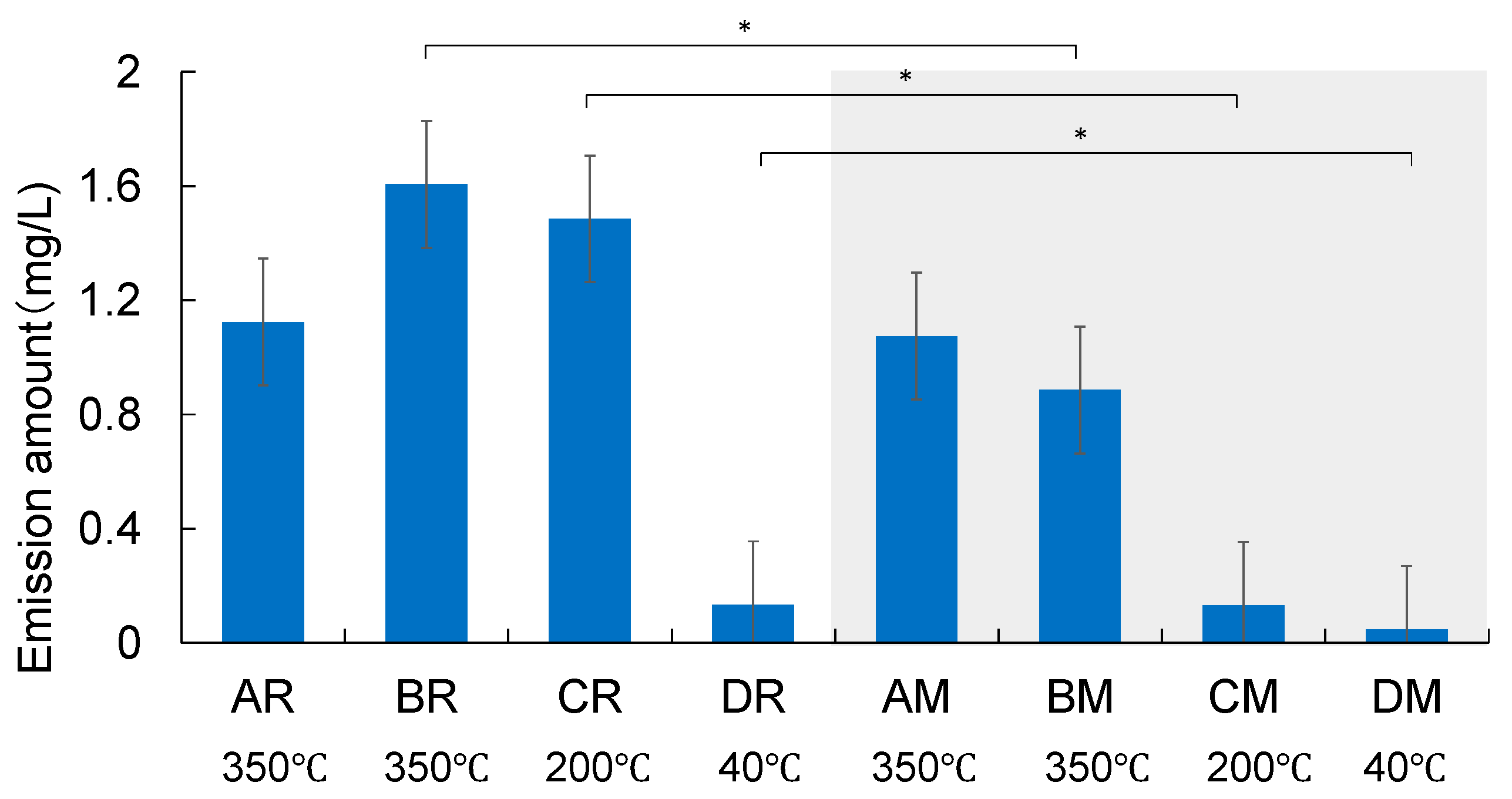
The arithmetic means of the emission amounts of acetate collected from all devices are shown in
Figure 2. Error bars indicate the standard deviation of triplicate runs. The amount of acetate emitted varies with the device and flavour type. Bentley et al. [
6] conducted a comprehensive chemical analysis of aerosols from the Tobacco Heating System 2.2 (commercial name: IQOS) and reported the emission amount of acetic acid was 944 µg item
-1 which corresponds to 1.5 mg L
-1. Even though the devices may not be exactly the same and storage conditions during distribution are not controlled, the “high-temperature type” devices showed equivalent levels of acetate emission—AR: 1.1 ± 0.20 mg L
-1, BR: 1.6 ± 0.11 mg L
-1, and CR: 1.5 ± 0.084 mg L
-1. The acetate emission from the “low-temperature type” device, DR, was much less. Meanwhile, except for AR and AM, significant differences in acetate emissions were found between regular and menthol flavour types (
t-test), probably because of the differences in the ingredients of the tobacco leaf materials.
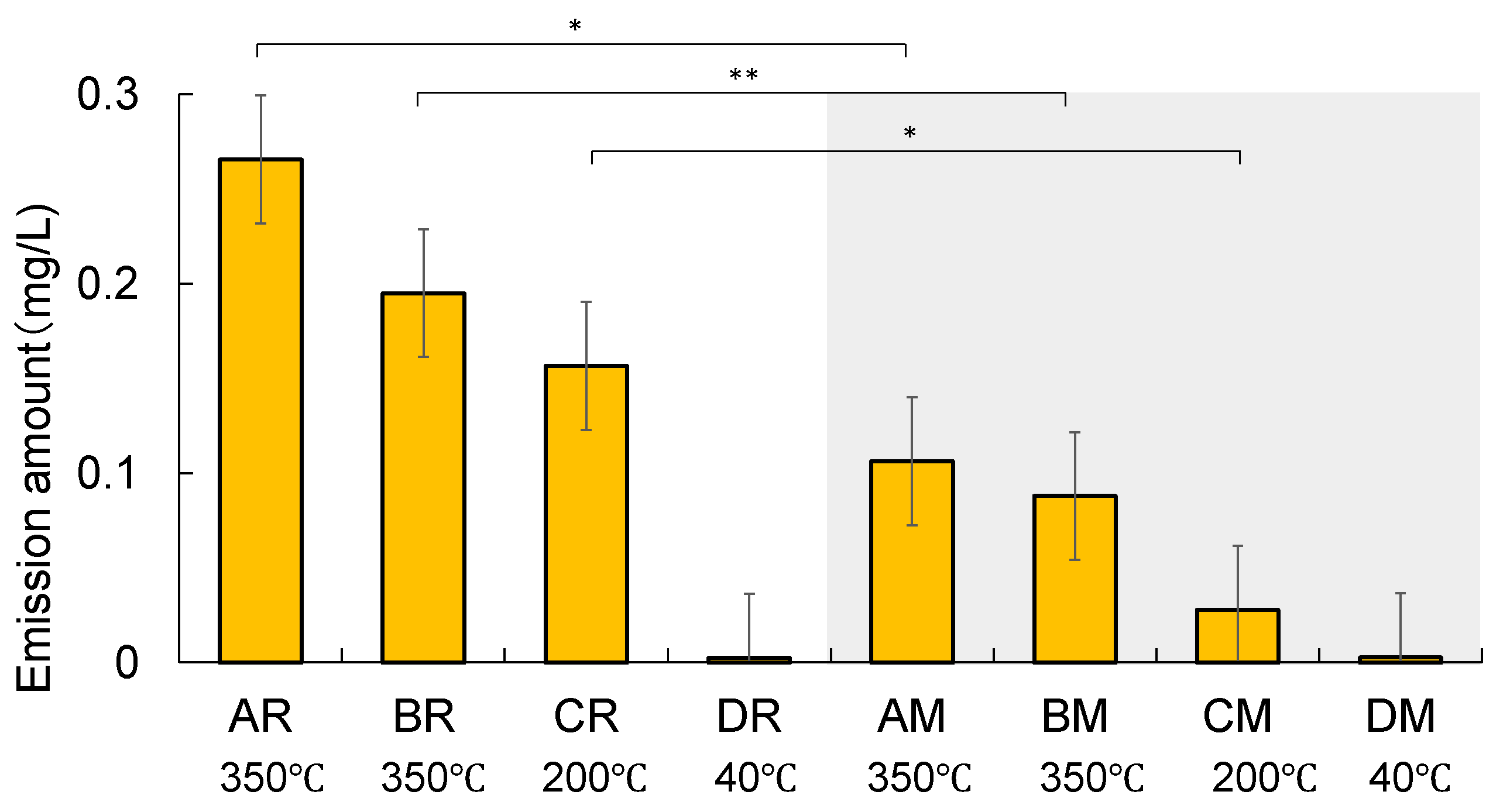
The arithmetic means of the emission amounts of formate collected from all devices are shown in
Figure 3. Error bars indicate the standard deviation of triplicate runs. The emission amount of formate increased with an increase in the heating temperature—AR: 0.27 ± 0.055 mg L
-1, BR: 0.19± 0.071 mg L
-1, CR: 0.048 ± 0.20 mg L
-1, and DR: 0.0027 ± 0.0031 mg L
-1. The levels were less than those of acetate. The emission levels of the regular type were significantly greater than those of the menthol type (
t-test) for devices A, B, and C. No significant difference was observed between DR and DM, mainly because of their lower emission levels. This means that the use of HTPs with a higher heating temperature and regular flavour results in the inhalation of more formate into the body. Here we need to pay attention to the validity of formate emission levels shown in
Figure 3, because Bentley et al. [
6] reported the emission amount of formic acid as 0.233 µg item
-1, corresponding to 0.00035 mg L
-1, which is much less than the values obtained here. The difference in trapping and/or analytical methodologies is a possible explanation. Thus, we also quantified the acetate and formate in the trapping solution using a reverse-phase HPLC method with UV detection (detection wavelength: 220 nm). The results of the HPLC method showed equivalent levels of both compounds: 1.0 ± 0.13 and 0.23 ± 0.13 mg L
-1 for acetate and formate, respectively (
n = 3). Therefore, the notable differences in results between the previous study and this work may lie in the analyte trapping methodology.
3.2. Gas to Particle Distribution of Formate and Acetate
Owing to the acid-base equilibrium of nicotine, the basic environment of aerosols is favourable for nicotine absorption into the body [
16,
17]. Actually, the “water droplets” generated from HTPs were weak-basic [
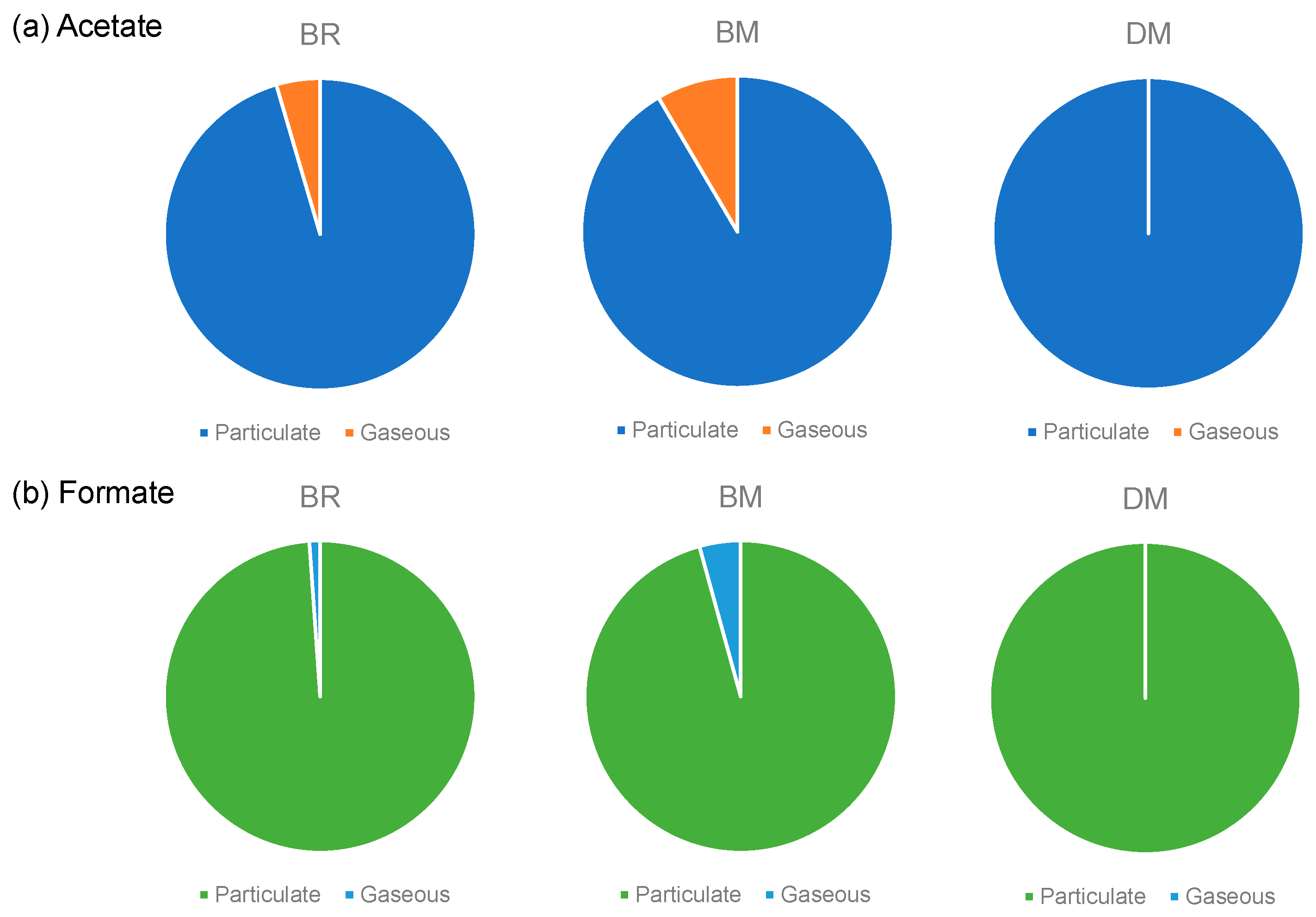
18], so that acetate and formate are likely to form salts and exist as particulate matter. The acetate and formate emissions from the selected devices BR, BM, and DM were measured using a Cambridge filter pad which trapped the particulate species emitted from them.
Figure 4 shows the percentages of the particulate and gaseous forms of acetate and formate. Gaseous acetate was found in BR and BM, but not in DR; thus, the percentage of particulate acetate was 95, 92, and 100 % for BR, BM, and DR, respectively. Similarly, the percentage of particulate formate was 99, 96, and 100 % for BR, BM, and DR, respectively. These results indicate that the formic acid in the mainstream aerosol of HTPs was distributed mainly in the particle phase.
4. Discussion
Here, we detected formate in the mainstream aerosols of HTPs distributed in Japan and found that their emission amounts depended on the heating temperature and varied depending on the type of flavour. The inhalation of formic acid vapour is known to severely irritate the mucous membranes of the nose and mouth, leading to inflammation. The inhalation of aerosolised formic acid also causes inhalation injuries [
19]. Even though the formate mostly existed as a particulate form in HTP aerosols, the emission amounts ranging from 0.0027 ± 0.0031 mg L
-1 (DR) to 0.27 ± 0.055 mg L
-1 (AR) were much greater than the workplace exposure limit of 9 mg cm
-3 (or 0.009 mg L
-1) [
7]. Thus, inhalation of formate from HTPs is a health concern, especially with long-term use. Similarly, we should note that the formate emitted from the exhaled breath of a user can be a source of air pollution. To assess this potential health risk, it is necessary to elucidate formate emission mechanisms.
Formate and other organic acids are originally present in tobacco leaves [
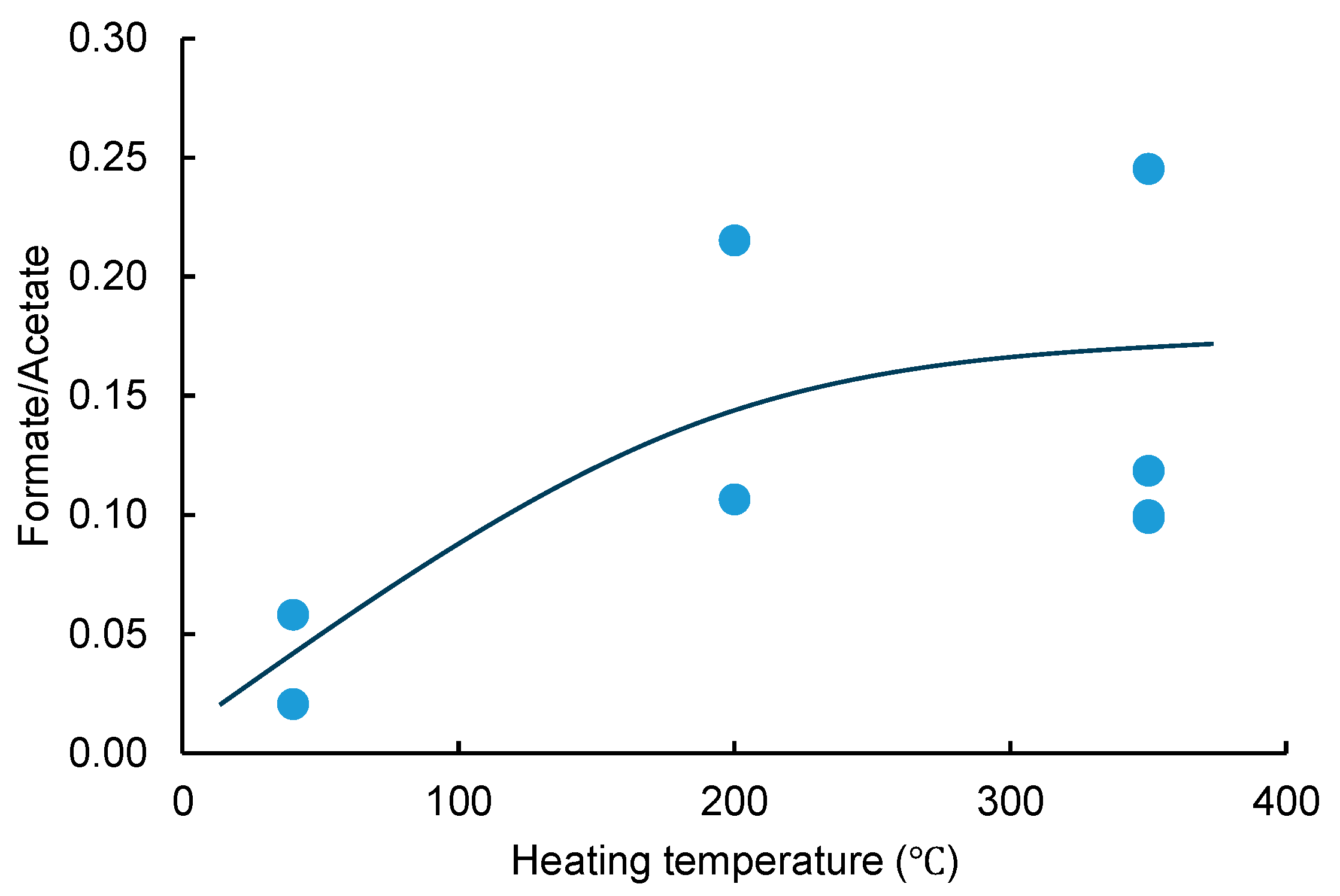
9]. Therefore, the transition from tobacco leaf material to water droplets during heating could be an emission route for formate from HTP products. However, despite the fact that formate and acetate have similar chemical properties, the ratio of formate and acetate emissions was not constant between the HTP devices, and the ratio tended to increase with the heating temperature, as shown in
Figure 5. This suggests the existence of another formate emission route.
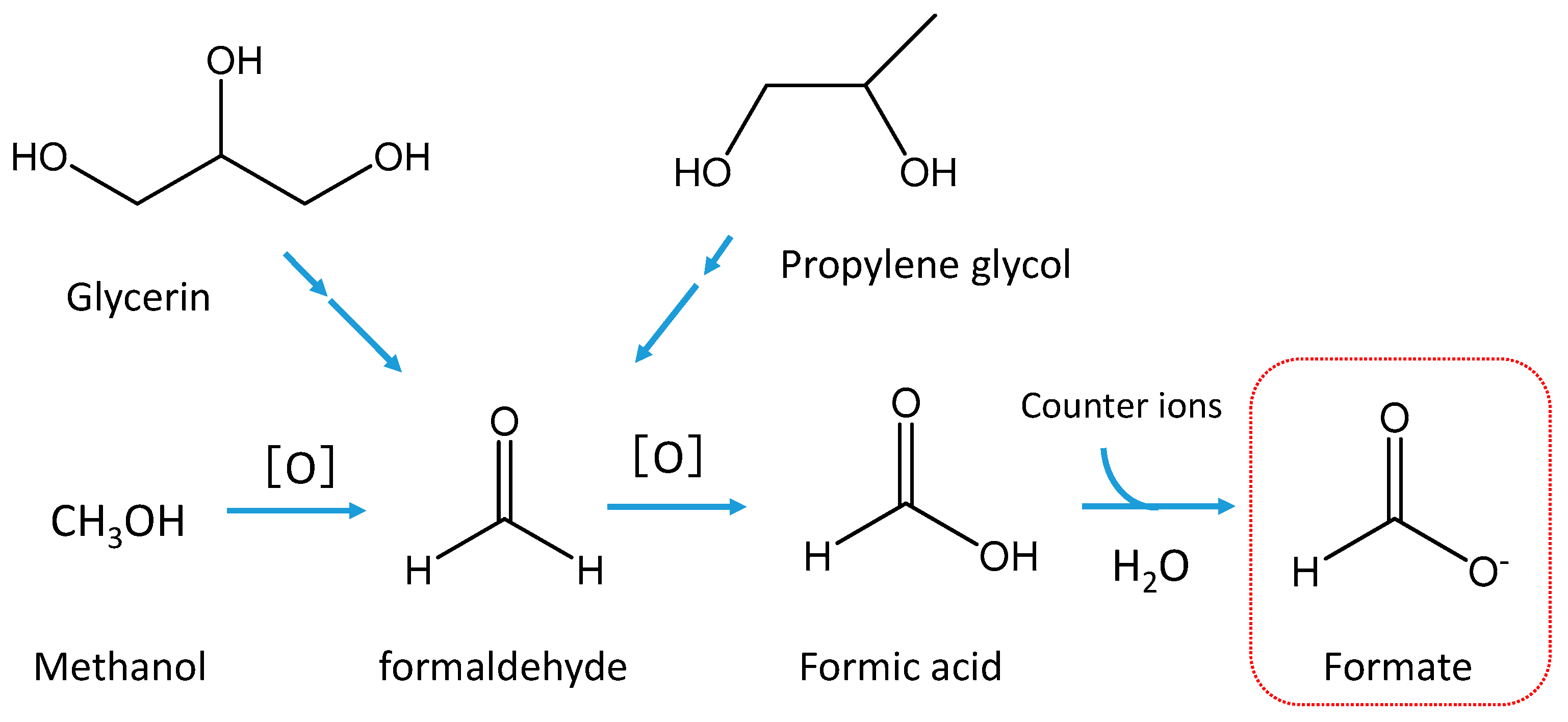
Glycerin and/or propylene glycol are used as HTP aerosol formers [
12,
13]. However, they are not necessarily stable, and the thermal decomposition of these ingredients is known to initiate the formation of formaldehyde and other aldehydes [
20] (
Figure 6). Formaldehyde is labile for further oxidation and thus produces formic acid. Formic acid can subsequently dissolve in water and is emitted mainly as formate in the particulate phase. Methanol can also be a precursor of formaldehyde in aerosols because certain amount of methanol (0.32 mg L
-1) was found in the aerosol of the IQOS [
6].
A limitation of this study is that the HTPs used were purchased from distribution, and the storage conditions from manufacturing and transportation to purchase were not controlled. Particularly, for imported products, the transportation period may be long, and it is necessary to investigate the influences of temperature and humidity on emission levels in association with the condition of tobacco leaf materials during storage. In addition, formate was deduced to be a by-product of aerosol formers and other ingredients. However, because the composition of the raw materials was not disclosed, it was not possible to fully consider the formate precursors. Formate is a highly hazardous chemical that can be considered a health concern, considering the emission amounts found in this study. In the future, we aim to ask tobacco manufacturers to cooperate and conduct tests that consider storage conditions and ingredients in tobacco leaf materials.
5. Conclusions
We aimed to determine the actual formate emissions from mainstream aerosols produced by commercially available HTPs in Japan. The results showed that the total amount of formate emitted increased with increasing heating temperature and varied with flavour type. The majority of formate exists in particulate form owing to the weak basic properties of the aerosol. It should be noted that formate emission levels were much greater than the workplace exposure limit when mainstream aerosols were directly inhaled. Therefore, formate in mainstream aerosols is a concern for human health when using the “high-temperature type” HTPs over a long period. Further studies are required to elucidate formate emission mechanisms for the safe use of HTPs.
Author Contributions
Conceptualization, Y.S.; methodology, M.K.; investigation, M.K.; data curation, Y.S. and M.K.; writing—original draft preparation, Y.S..; writing—review and editing, M.K.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Smoking Research Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in his study are available within the article.
Acknowledgement
Authors awfully thank emeritus Prof. Yukio Yanagisawa, The University of Tokyo, emeritus Prof. Hideaki Matsuki, Toki University, Prof. Satoshi Nakai, Yokohama National University, Mr. Daisuke Oikawa, AIREX inc. for their sincere advices.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Benowitz, N.L. Nicotine addiction. N Engl J Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tobacco Control. 2019, 28, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Dusautoir, R.; Zarcone, G.; Verriele, M.; Garçon, G.; Fronval, I.; Beauval, N.; Allorge, D.; Riffault, V.; Locoge, N.; Guidice, J.M.L.; Anthérieu, S. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J. Hazard Mat. 2021, 401, 123417. [Google Scholar] [CrossRef] [PubMed]
- Cancelada, L.; Sleiman, M.; Tang, X.; Russell, M.L.; Montesinos, V.N.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Heated tobacco products: Volatile emissions and their predicted impact on indoor air quality. Environ. Sci. Technol. 2019, 53, 7866–7876. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Noguchi, M.; Takagi, N.; Hayashida, H.; Inaba, Y.; Ogura, H.; Kunugita, N. Simple determination of gaseous and particulate compounds generated from heated tobacco products. Chem. Res. Toxicol. 2018, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.C.; Almstetter, M.; Arndt, D.; Knorr, A.; Martin, E.; Pospisil, P.; Maeder, S. Comprehensive chemical characterization of the aerosol generated by a heated tobacco product by untargeted screening. Anal. Bioanal. Chem. 2020, 412, 2675. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Occupational Safety and Health (NIOSH). Formic acid. https://www.cdc.gov/niosh/npg/npgd0296.html.
- Ghorbani, H.; Nezami, A.; Sheikholeslami, B.; Hedjazi, A.; Ahmadimanesh, M. Simultaneous measurement of formic acid, methanol and ethanol in vitreous and blood samples of postmortem by headspace GC-FID. J Occup Med Toxicol. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Sádecká, J.; Polonský, J. Determination of organic acids in tobacco by capillary isotachophoresis, J. Chromatogr. A, 2003, 988, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kalianos, A.G. Phenolics and acids in leaf and their relationship to smoking quality and aroma. Rec. Adv. Tob. Sci. 1976, 2, 61–79. [Google Scholar]
- Lu, X.; Zhang, H.; Cao, Y.; Pang, Y.; Zhou, G.; Huang, H.; Li, J.; Jiang, J.; Yang, Q. A Comprehensive Study on the Acidic Compounds in Gas and Particle Phases of Mainstream Cigarette Smoke. Processes 2023, 11, 1694. [Google Scholar] [CrossRef]
- Stevenson, T.; Proctor, R.N. The secret and soul of Marlboro: Phillip Morris and the origins, spread, and denial of nicotine freebasing. Am. J. Public Health 2008, 98, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Wayne, G.F.; Conolly, G.N.; Henningfield, J. Brand differences of free-base nicotine delivery in cigarette smoke: the view of the tobacco industry documents. Tobacco Control 2006, 15, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Yamamoto, T. Physical and chemical property of mainstream aerosol generated from heated tobacco products. Indoor Environ. 2021, 24, 135–144. (In Japanese) [Google Scholar] [CrossRef]
- Cooperation Centre for Scientific Research Relative to Tobacco: CORESTA Recommended Method No.81, Routine analytical machine for e-cigarette aerosol generation and collection-definitions and standard conditions. 2015.
- Pankow, J.; Mader, B.T.; Lorne, M.I.; Luo, W.; Pavlick, A.; Liang, C. Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ. Sci. Technol. 1997, 31, 2428–2433. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Sleijffers, A.; van Spiegel, P.; Blom, R.; Witte, M.; van de Kassteele, J.; Blokland, M.; Steerenberg, P.; Opperhuizen, A. Effect of ammonia in cigarette tobacco on nicotine absorption in human smokers. Food and Chem. Toxicol. 2011, 49, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sekine, Y.; Sohara, K.; Nakai, S.; Yanagisawa, Y. Effect of Heating Temperature on Ammonia Emission in the Mainstream Aerosols from Heated Tobacco Products. Toxics 2022, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Yelon, J.A.; Simpson, R.L.; Gudjonsson, O. Formic Acid Inhalation Injury: A Case Report. The Journal of Burn Care & Rehabilitation 1996, 17, 241–242. [Google Scholar] [CrossRef]
- Saliba, N.A.; Hellani, A.E.; Honein, E.; Salman, R.; Talih, S.; Zeaiter, J.; Shihadeh, A. Surface chemistry of electronic cigarette electrical heating coils: Effects of metal type on propylene glycol thermal decomposition, J. Anal. Appl. Pyro. 2018, 134, 520–525. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).