Submitted:
06 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
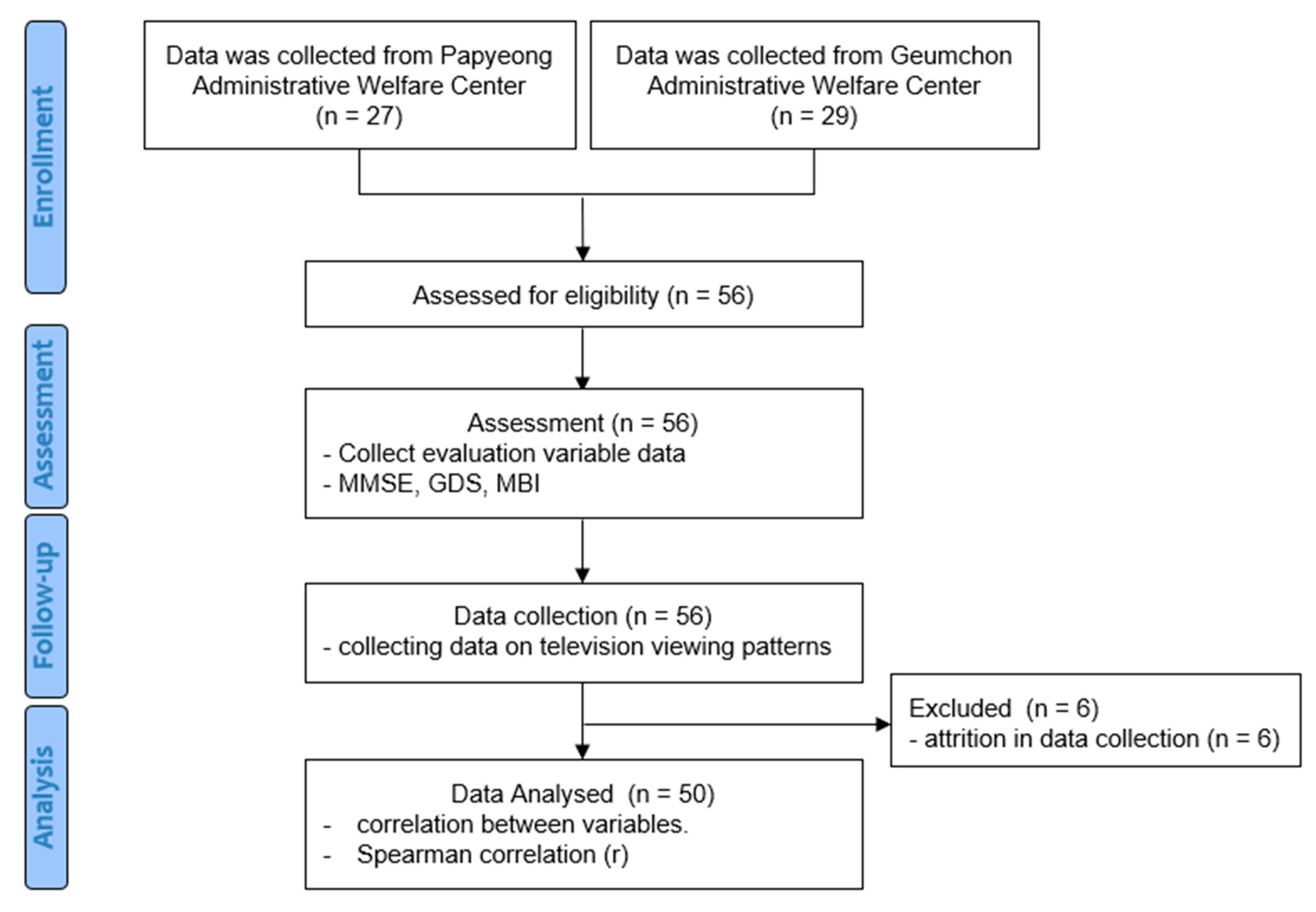
2.1. Subjects
2.2. Experimental Procedures
2.3. Data Collection
2.4. Measurements and Instrumentation
2.4.1. MMSE
2.4.2. GDS
2.4.3. MBI
2.5. Statistical Analysis
2.5.1. MMSE-K
- Severe cognitive impairment group (n = 6): score≤19
- Moderate cognitive impairment group (n = 19): 20≤score≤23
- No cognitive impairment group (n = 25): score≥24
2.5.2. KGDS
- Severe depression group (n = 6): score≥22
- Moderate depression group (n = 2): 19≤score≤21
- Mild depression group (n = 14): 14≤score≤18
- No depression group (n = 28): score≤13
2.5.3. KMBI
- Normal ADL performance group (n = 35): score≥95
- Grade 6 group (n = 10): 85≤score≤94
- Grade 5 (n = 1): 70≤score≤84
- Grade 3 (n = 1): 40≤score≤54
- Grade 1 group (n = 3): score≤24
3. Results
3.1. General Characteristics of Subjects
3.2. Analysis of Correlations between TV Viewing Data and Cognitive Function, Depression, and ADL Performance
3.3. Analysis of Peak Viewing Hours by MMSE-K, KGDS, and KMBI Score Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumacher, A.E.; Kyu, H.H.; Aali, A.; Abbafati, C.; Abbas, J.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; Abd ElHafeez, S.; Abdelmasseh, M. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. The Lancet 2024, 403, 1989–2056. [Google Scholar]
- Osareme, J.; Muonde, M.; Maduka, C.P.; Olorunsogo, T.O.; Omotayo, O. Demographic shifts and healthcare: A review of aging populations and systemic challenges. International Journal of Science and Research Archive 2024, 11, 383–395. [Google Scholar]
- Krellman, J.W.; Mercuri, G. Cognitive interventions for neurodegenerative disease. Current Neurology and Neuroscience Reports 2023, 23, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, W.; Zhou, J. Advances and challenges of non-invasive brain stimulation in age-related neurodegenerative diseases, volume II. Frontiers in Aging Neuroscience 2023, 15, 1275530. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, F.K.; Hoffmann, K.; Siersma, V.; Sobol, N.; Beyer, N.; Andersen, B.B.; Vogel, A.; Lolk, A.; Gottrup, H.; Høgh, P. The role of physical and cognitive function in performance of activities of daily living in patients with mild-to-moderate Alzheimer’s disease–A cross-sectional study. BMC Geriatrics 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, N.A.; Farghaly Abdelaliem, S.M.; Malki, A.; Gad, I.; Ewis, A.; Atlam, E. Advanced machine learning techniques for cardiovascular disease early detection and diagnosis. Journal of Big Data 2023, 10, 144. [Google Scholar] [CrossRef]
- Wilson, A.D. Developments of recent applications for early diagnosis of diseases using electronic-nose and other VOC-detection devices. Sensors 2023, 23, 7885. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Meng, Y.; Hu, Q.; Du, Q.; Wu, X.; Zou, W.; Zhu, M.; Chen, J.; Luo, L.; Cheng, Y. Obstacles to access to community care in urban senior-only households: A qualitative study. BMC Geriatrics 2022, 22, 122. [Google Scholar] [CrossRef]
- Fingerman, K.L.; Kim, Y.K.; Ng, Y.T.; Zhang, S.; Huo, M.; Birditt, K.S. Television viewing, physical activity, and loneliness in late life. Gerontologist 2022, 62, 1006–1017. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kawashima, R. Effects of television viewing on brain structures and risk of dementia in the elderly: Longitudinal analyses. Frontiers in Neuroscience 2023, 17, 984919. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Andrade, E.; Martínez-Gómez, D.; Caballero, F.F.; López-García, E.; Rodríguez-Artalejo, F. Television viewing time as a risk factor for frailty and functional limitations in older adults: Results from 2 European prospective cohorts. International Journal of Behavioral Nutrition and Physical Activity 2017, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Billis, A.S.; Batziakas, A.; Bratsas, C.; Tsatali, M.S.; Karagianni, M.; Bamidis, P.D. Enabling active and healthy ageing decision support systems with the smart collection of TV usage patterns. Healthcare Technology Letters 2016, 3, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.; Oliveira, R.; Garcia-Crespo, A.; Rodriguez-Goncalves, R. TV interaction as a non-invasive sensor for monitoring elderly well-being at home. Sensors 2021, 21, 6897. [Google Scholar] [CrossRef] [PubMed]
- Kyan, H.; Inoue, J. Agent-based modeling of zapping behavior of viewers, television commercial allocation, and advertisement markets. In Econophysics of Agent-Based Models; Springer: 2014; pp. 3–26.
- Shin, J.H. Diagnosis of dementia: Neuropsychological test. Korean Journal of Family Medicine 2010, 31, 253–266. [Google Scholar] [CrossRef]
- Kwon, Y.C. Korean version of Mini-Mental State Examination (MMSE-K) Part I: Development of the test for the elderly. Journal of the Korean Neuro-psychiatric Association 1989, 28, 125. [Google Scholar]
- Kim, D.; Kang, Y.; Yun, J.; Lee, K.; Han, K.; Chung, H. A correlation between the Mini Mental State Examination-Korean Version and the Neuro-behavioral Cognitive Status Examination in stroke patients. PNF and Movement 2012, 10, 45–52. [Google Scholar]
- Jamison, C.; Scogin, F. Development of an interview-based geriatric depression rating scale. The International Journal of Aging and Human Development 1992, 35, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Albiński, R.; Kleszczewska-Albińska, A.; Bedyńska, S. Geriatric Depression Scale (GDS). Validity and reliability of different versions of the scale--Review. Psychiatr. Pol. 2011, 45, 555–562. [Google Scholar] [PubMed]
- Montorio, I.; Izal, M. The Geriatric Depression Scale: A review of its development and utility. International Psychogeriatrics 1996, 8, 103–112. [Google Scholar] [CrossRef]
- Leung, S.O.; Chan, C.C.; Shah, S. Development of a Chinese version of the Modified Barthel Index—Validity and reliability. Clin. Rehabil. 2007, 21, 912–922. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, W.; Park, E.; Kim, E. The validity, reliability and discriminative index of the Korean version of Modified Barthel Index (K-MBI) in stroke patients. Journal of the Korea Academia-Industrial cooperation Society 2012, 13, 4119–4125. [Google Scholar] [CrossRef]
- Rovai, A.P.; Baker, J.D.; Ponton, M.K. Social Science Research Design and Statistics: A Practitioner's Guide to Research Methods and IBM SPSS; Watertree Press LLC: 2013.
- Maranhao Neto, G.A.; Pavlovska, I.; Polcrova, A.; Mechanick, J.I.; Infante-Garcia, M.M.; Medina-Inojosa, J.; Nieto-Martinez, R.; Lopez-Jimenez, F.; Gonzalez-Rivas, J.P. The combined effects of television viewing and physical activity on cardiometabolic risk factors: The Kardiovize Study. Journal of Clinical Medicine 2022, 11, 545. [Google Scholar] [CrossRef]
- McAnally, H.M.; Young, T.; Hancox, R.J. Childhood and adolescent television viewing and internalising disorders in adulthood. Preventive Medicine Reports 2019, 15, 100890. [Google Scholar] [CrossRef] [PubMed]
- Spinsante, S.; Gambi, E. Remote health monitoring for elderly through interactive television. Biomedical Engineering Online 2012, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Rehg, J.M. Watching the TV watchers. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 2018, 2, 1–27.
- Santos, J.; Ihle, A.; Peralta, M.; Domingos, C.; Gouveia, É.R.; Ferrari, G.; Werneck, A.; Rodrigues, F.; Marques, A. Associations of physical activity and television viewing with depressive symptoms of the European adults. Frontiers in Public Health 2022, 9, 799870. [Google Scholar] [CrossRef] [PubMed]
- Tolba, A.A.; Zoghaib, S.Z. Understanding the binge-watching phenomenon on Netflix and its association with depression and loneliness in Egyptian adults. Media Watch 2022, 13, 264–279. [Google Scholar] [CrossRef]
- Yu, B.; Gu, Y.; Bao, X.; Meng, G.; Wu, H.; Zhang, Q.; Liu, L.; Sun, S.; Wang, X.; Zhou, M. Distinct associations of computer/mobile devices use and TV watching with depressive symptoms in adults: A large population study in China. Depress. Anxiety 2019, 36, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Fingerman, K.L.; Ng, Y.T.; Huo, M.; Birditt, K.S.; Charles, S.T.; Zarit, S. Functional limitations, social integration, and daily activities in late life. The Journals of Gerontology: Series B 2021, 76, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Park, J.; Hsueh, M.; Sun, W.; Liao, Y. Prevalence of total physical activity, muscle-strengthening activities, and excessive TV viewing among older adults; and their association with sociodemographic factors. International Journal of Environmental Research and Public Health 2018, 15, 2499. [Google Scholar] [CrossRef] [PubMed]
- Shree, M.K.R. An examination on causes for anxiety and depression among elderly people. The Journal of Contemporary Issues in Business and Government 2020, 26, 823–828. [Google Scholar]
- Kim, S.; Jeon, S.; Lee, M.Y.; Shin, D.; Lim, W.; Shin, Y.; Oh, K. The association between physical activity and anxiety symptoms for general adult populations: An analysis of the dose-response relationship. Psychiatry Investigation 2020, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi-Koolaee, A.; Frouzan, F. Loneliness and death anxiety: Differences between active and bedridden older men. OMEGA-Journal of Death and Dying 2023, 00302228231153460. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Zhuang, S.; Shen, Y.; Tang, X.; Sun, H.; Fang, Q. Exploring the bidirectional associations between short or long sleep duration and lower cognitive function: A 7-year cohort study in China. Frontiers in Aging Neuroscience 2021, 13, 727763. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Yu, Y.; Tang, C.; Liu, Z.; Li, X.; Tan, Y.; Mai, X.; Li, R.; Xu, C.; Xie, G. Effect of early morning awakening in major depressive disorder. 2022.
| Participants (n=50) | |
|---|---|
| Age (years) | 82.12 ± 4.32 |
| Height (cm) | 156.94 ± 6.59 |
| Weight (kg) | 56.76 ± 9.99 |
| MMSE-K (score: 0–30) | 23.72 ± 4.24 |
| KGDS (score: 0–30) | 12.60 ± 6.94 |
| KMBI (score: 0–100) | 90.02 ± 21.28 |
| Daily average viewing time (min) | 639.83 ± 297.21 |
| Upper zapping threshold (number of days) | 34.82 ± 27.79 |
| Lower zapping threshold (number of days) | 0.62 ± 1.39 |
| Average zapping per hour | 3.09 ± 1.65 |
| AGE | DAV | PVH | UZT | LZT | AZH | MMSE-K | KGDS | KMBI | |
|---|---|---|---|---|---|---|---|---|---|
| AGE | 1.000 | 0.102 | 0.070 | -0.001 | -0.063 | -0.192 | -0.208 | 0.012* | -0.245 |
| DAV | 0.102 | 1.000 | 0.161 | 0.812** | 0.150 | 0.495** | 0.264 | 0.320* | -0.313* |
| PVH | 0.070 | 0.161 | 1.000 | 0.091 | 0.129 | 0.230 | -0.216 | 0.088 | -0.022 |
| UZT | -0.001 | 0.812** | 0.091 | 1.000 | 0.046 | 0.590** | 0.145 | 0.308* | -0.352* |
| LZT | -0.063 | 0.150 | 0.129 | 0.046 | 1.000 | 0.324 | 0.033 | 0.098 | -0.127 |
| AZH | -0.192 | 0.495** | 0.230 | 0.590** | 0.324 | 1.000 | 0.087 | 0.218 | 0.088 |
| MMSE-K | -0.208 | 0.264 | -0.216 | 0.145 | 0.033 | 0.087 | 1.000 | -0.249 | -0.091 |
| KGDS | 0.012 | 0.320* | 0.088 | 0.308* | 0.098 | 0.218 | -0.249 | 1.000 | -0.153 |
| KMBI | -0.245 | -0.313* | -0.022 | -0.352* | -0.127 | 0.088 | -0.091 | -0.153 | 1.000 |
| Hour of Day |
Mean ± SD | p | ||
| Severe (n=6) |
Moderate (n=19) |
None (n=25) |
||
| 0 | 2.83 ± 2.48 | 2.84 ± 1.95 | 2.92 ± 1.93 | 0.873 |
| 1 | 1.00 ± 1.55 | 2.26 ± 1.66 | 3.28 ± 2.36 | 0.226 |
| 2 | 0.50 ± 1.22 | 1.79 ± 1.44 | 1.92 ± 1.73 | 0.105 |
| 3 | 0.33 ± 0.82 | 1.51 ± 1.82 | 1.88 ± 1.83 | 0.048* |
| 4 | 0.33 ± 0.52 | 1.89 ± 1.76 | 2.00 ± 1.66 | 0.041* |
| 5 | 1.00 ± 2.00 | 2.63 ± 2.54 | 2.88 ± 1.64 | 0.049* |
| 6 | 2.17 ± 2.64 | 3.84 ± 2.67 | 3.37 ± 2.47 | 0.307 |
| 7 | 3.17 ± 2.79 | 4.84 ± 2.12 | 5.24 ± 1.29 | 0.152 |
| 8 | 5.17 ± 2.32 | 5.05 ± 2.59 | 5.48 ± 1.20 | 0.501 |
| 9 | 6.17 ± 2.64 | 6.05 ± 2.14 | 5.36 ± 1.25 | 0.786 |
| 10 | 5.83 ± 3.61 | 4.31 ± 2.06 | 4.08 ± 1.22 | 0.190 |
| 11 | 6.33 ± 4.36 | 4.60 ± 2.13 | 4.92 ± 1.15 | 0.268 |
| 12 | 6.50 ± 4.25 | 3.79 ± 2.27 | 3.84 ± 1.39 | 0.593 |
| 13 | 4.00 ± 2.45 | 3.58 ± 2.10 | 3.92 ± 1.33 | 0.291 |
| 14 | 5.33 ± 3.22 | 5.05 ± 2.47 | 4.08 ± 1.22 | 0.165 |
| 15 | 5.83 ± 3.61 | 5.89 ± 2.94 | 5.84 ± 1.20 | 0.645 |
| 16 | 4.00 ± 2.35 | 5.36 ± 2.74 | 5.92 ± 1.08 | 0.326 |
| 17 | 2.33 ± 2.74 | 4.37 ± 2.51 | 4.76 ± 1.23 | 0.623 |
| 18 | 3.50 ± 2.34 | 5.05 ± 2.04 | 5.44 ± 1.66 | 0.715 |
| 19 | 2.67 ± 3.25 | 4.37 ± 2.40 | 4.37 ± 1.74 | 0.657 |
| 20 | 4.00 ± 2.47 | 4.05 ± 2.19 | 3.76 ± 1.98 | 0.774 |
| 21 | 2.33 ± 2.94 | 4.77 ± 2.54 | 4.36 ± 1.35 | 0.540 |
| 22 | 3.67 ± 3.20 | 4.37 ± 2.44 | 4.72 ± 1.83 | 0.614 |
| 23 | 4.00 ± 2.37 | 3.74 ± 2.94 | 3.64 ± 1.99 | 0.913 |
| Hour of Day |
Mean ± SD | p | |||
| None (n=28) |
Mild (n=14) |
Moderate (n=2) |
Severe (n=6) |
||
| 0 | 2.71 ± 2.24 | 2.93 ± 1.54 | 3.00 ± 1.41 | 3.50 ± 1.87 | 0.921 |
| 1 | 1.93 ± 1.90 | 2.29 ± 1.54 | 1.50 ± 0.71 | 3.17 ± 1.72 | 0.710 |
| 2 | 1.57 ± 1.64 | 1.64 ± 1.34 | 3.00 ± 1.41 | 2.67 ± 1.86 | 0.618 |
| 3 | 1.39 ± 1.62 | 1.57 ± 1.34 | 0.50 ± 0.71 | 2.67 ± 1.75 | 0.322 |
| 4 | 1.61 ± 1.75 | 1.79 ± 1.58 | 1.00 ± 0.00 | 3.67 ± 1.75 | 0.544 |
| 5 | 2.50 ± 2.25 | 2.64 ± 1.54 | 3.00 ± 2.83 | 2.83 ± 1.72 | 0.814 |
| 6 | 3.82 ± 2.89 | 3.14 ± 2.35 | 3.50 ± 2.12 | 3.17 ± 1.72 | 0.936 |
| 7 | 5.46 ± 2.25 | 4.14 ± 1.79 | 3.50 ± 2.12 | 4.00 ± 0.83 | 0.068 |
| 8 | 6.57 ± 3.89 | 5.21 ± 2.35 | 5.00 ± 0.00 | 5.00 ± 1.10 | 0.017* |
| 9 | 5.07 ± 2.89 | 5.07 ± 2.35 | 5.00 ± 0.00 | 3.33 ± 0.52 | 0.279 |
| 10 | 3.36 ± 2.15 | 4.21 ± 2.02 | 4.00 ± 1.41 | 4.67 ± 1.37 | 0.672 |
| 11 | 3.93 ± 2.15 | 4.14 ± 2.02 | 4.00 ± 1.41 | 3.67 ± 1.21 | 0.395 |
| 12 | 5.93 ± 4.15 | 5.21 ± 1.64 | 4.00 ± 0.00 | 5.50 ± 2.23 | 0.524 |
| 13 | 5.07 ± 2.45 | 5.50 ± 1.51 | 6.00 ± 1.41 | 5.67 ± 1.86 | 0.572 |
| 14 | 5.93 ± 4.29 | 4.21 ± 1.64 | 5.00 ± 2.12 | 5.50 ± 1.87 | 0.175 |
| 15 | 5.57 ± 2.49 | 4.64 ± 2.35 | 5.00 ± 1.41 | 5.33 ± 2.04 | 0.299 |
| 16 | 5.96 ± 2.40 | 5.07 ± 1.79 | 6.00 ± 1.41 | 5.67 ± 2.68 | 0.800 |
| 17 | 5.93 ± 2.45 | 4.71 ± 1.64 | 5.00 ± 2.12 | 5.33 ± 1.37 | 0.977 |
| 18 | 5.93 ± 2.89 | 4.57 ± 1.79 | 5.50 ± 2.12 | 4.83 ± 1.87 | 0.796 |
| 19 | 3.57 ± 2.89 | 4.71 ± 1.64 | 5.00 ± 2.12 | 3.83 ± 2.04 | 0.576 |
| 20 | 5.00 ± 2.25 | 5.07 ± 1.52 | 5.00 ± 1.41 | 3.83 ± 1.99 | 0.143 |
| 21 | 4.93 ± 2.40 | 4.50 ± 1.51 | 4.00 ± 1.41 | 3.67 ± 1.94 | 0.254 |
| 22 | 3.36 ± 1.52 | 4.71 ± 1.76 | 4.67 ± 2.87 | 3.83 ± 2.23 | 0.439 |
| 23 | 3.57 ± 2.45 | 4.00 ± 1.52 | 4.50 ± 1.87 | 3.64 ± 1.99 | 0.840 |
| Hour of Day |
Mean ± SD | p | ||
| Grade 1 (n=3) |
Grade 6 (n=10) |
Normal (n=35) |
||
| 0 | 3.67 ± 1.53 | 4.20 ± 1.32 | 2.60 ± 1.96 | 0.036* |
| 1 | 2.67 ± 2.08 | 3.50 ± 1.18 | 1.86 ± 1.72 | 0.022* |
| 2 | 1.67 ± 2.08 | 2.80 ± 1.48 | 1.49 ± 1.52 | 0.029* |
| 3 | 1.67 ± 2.08 | 2.50 ± 1.51 | 1.37 ± 1.50 | 0.071 |
| 4 | 2.33 ± 2.52 | 2.80 ± 1.71 | 1.54 ± 1.58 | 0.118 |
| 5 | 3.67 ± 2.52 | 2.90 ± 1.66 | 2.31 ± 2.22 | 0.247 |
| 6 | 4.33 ± 2.08 | 3.60 ± 1.84 | 3.31 ± 2.00 | 0.614 |
| 7 | 5.33 ± 0.58 | 4.10 ± 1.66 | 4.89 ± 2.23 | 0.398 |
| 8 | 4.33 ± 1.15 | 5.00 ± 1.49 | 6.34 ± 3.52 | 0.083 |
| 9 | 4.33 ± 1.15 | 4.50 ± 1.49 | 5.14 ± 2.58 | 0.094 |
| 10 | 4.33 ± 2.08 | 4.50 ± 1.72 | 5.14 ± 2.58 | 0.332 |
| 11 | 4.33 ± 2.08 | 4.00 ± 1.97 | 4.23 ± 2.04 | 0.704 |
| 12 | 4.33 ± 2.08 | 3.70 ± 1.42 | 4.89 ± 4.17 | 0.608 |
| 13 | 4.67 ± 1.53 | 3.80 ± 1.14 | 4.40 ± 2.37 | 0.319 |
| 14 | 4.00 ± 2.65 | 3.20 ± 1.14 | 4.03 ± 2.27 | 0.416 |
| 15 | 3.33 ± 2.89 | 3.20 ± 1.23 | 4.20 ± 2.63 | 0.291 |
| 16 | 3.67 ± 2.31 | 4.00 ± 0.82 | 4.49 ± 1.95 | 0.058 |
| 17 | 4.67 ± 1.53 | 3.00 ± 0.85 | 5.29 ± 1.78 | 0.017 |
| 18 | 5.67 ± 0.58 | 5.50 ± 0.95 | 5.94 ± 2.46 | 0.214 |
| 19 | 5.67 ± 0.58 | 5.00 ± 1.25 | 5.86 ± 2.88 | 0.681 |
| 20 | 5.33 ± 0.58 | 4.20 ± 1.63 | 5.71 ± 2.30 | 0.985 |
| 21 | 5.67 ± 0.58 | 5.00 ± 1.10 | 5.31 ± 2.61 | 0.671 |
| 22 | 5.67 ± 0.58 | 5.70 ± 1.63 | 4.34 ± 2.31 | 0.356 |
| 23 | 5.00 ± 1.00 | 5.10 ± 1.10 | 3.49 ± 2.21 | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





