1. Introduction
Infective endocarditis (IE) is a rare and potentially life-threatening infection that primarily affects the heart valves. This condition can be challenging to diagnose and has a high mortality rate owing to its elusive nature [
1,
2].
The incidence of infective endocarditis has been rising in recent years, largely due to the increasing use of vascular and cardiac devices, particularly in elderly individuals with comorbidities [
2].
Left-sided valves are most commonly affected by vegetation growth, which often leads to valve failure. In contrast, right-sided valves were more commonly affected by intravenous drug use [
2].
Over the years, the causative organisms have shifted, with a growing incidence of Staphylococcus species [
3].
IE is a complex medical condition that can present with a variety of symptoms including fever, heart failure, and stroke. Clinical suspicion is essential for proper diagnosis and treatment in individuals with risk factors. This condition can also lead to severe complications such as myocardial infarction. Accurate diagnosis of IE requires a comprehensive approach that integrates clinical, imaging, and laboratory analyses [
3,
4].
Laboratory diagnostic techniques include serological tests, molecular methods, and pathological examination. The pathological examination of valve tissue samples is considered the optimal method for diagnosing infective endocarditis when these specimens are accessible [
4].
The criteria for this diagnosis encompass histological demonstration of pathological lesions, such as vegetation and inflammatory infiltrates [
5].
Treatment of infective endocarditis usually involves long-term antimicrobial therapy and surgical intervention to remove the affected valve. Although antibiotics are the primary treatment option, surgery may be necessary in cases of extensive disease or complications. It is crucial to understand microbiological sensitivity in order to effec-tively treat infective endocarditis in light of the growing issue of antibiotic resistance [
5].
Pathological examination of prosthetic heart valves is considered to be one of the most accurate methods for diagnosing IE. However, identification of causative agents is challenging [
4,
5].
PVE is a severe condition that poses a significant threat to both individual and societal well-being as it is associated with extended hospitalization periods, substantial socioeconomic factors, and diminished quality of life.
Recently, there has been a notable increase in the incidence of infective endocarditis (IE) in adults. Several factors have been proposed to contribute to this increase, including an aging population, growing prevalence of cardiac surgery for prosthetic heart valves, and increase in the use of cardiac electronic devices [
5,
6].
Although some of these factors have been found to be unrelated to the increased incidence, there is a need for a comprehensive evaluation of this disease in patients with prosthetic heart valves [
6,
7].
The annual incidence of PVE around the globe is approximately 2%, and it is generally regarded as an uncommon complication that may occur following valve replacement surgery [
6,
7].
Individuals who have undergone valve replacement surgery or transcatheter valve replacement are at a higher risk of developing PVE because of the presence of a prosthetic valve, which can become infected and cause severe complications if not treated promptly. Therefore, it is crucial for healthcare providers to consider PVE a potential diagnostic tool when assessing patients with IE [
7,
8].
Furthermore, healthcare providers should be cognizant of the potential of PVE to mimic other medical conditions, which could result in misdiagnosis if not considered thoroughly. The mortality rate of patients with PVE remains significantly higher than that of patients with native valve endocarditis (NVE) [
8].
PVE is a serious and potentially life-threatening condition that has historically posed significant challenges to resource utilization and treatment improvement. The clinical outcomes associated with PVE have evolved with changes in underlying causes [
8,
9].
Owing to enhanced therapeutic management, there is a more comprehensive understanding of the prognosis of PVE when it is identified at an early stage. Therapeutic measures typically require the coordination of a multidisciplinary team of specialists to determine the most appropriate approach for each individual case. The timing of the surgical intervention is particularly critical and requires careful analysis to prevent irreversible tissue damage [
7,
8].
The importance of the endocarditis team in managing patients with PVE and associated systemic complications is well-recognized. This study sheds light on the pathological aspects of PVE in mechanical heart valves and provides valuable insights into the complexity of this condition [
8].
Pathological examination of resected valve tissues is the gold standard for diagnosing PVE. The Duke and von Reyn criteria, which include histological findings, are considered important for confirming diagnosis. To meet these criteria, it is necessary to demonstrate the presence of microorganisms in cardiac vegetation [
9,
10].
Histological examination may not always reveal the presence of microorganisms in cardiac valve tissue, and it is possible that vegetation may not be present. In these cases, PVE can be diagnosed based only on the presence of inflammatory infiltrates in the valve tissues. Nonetheless, degenerative valves that are not infected can exhibit inflammation upon histological examination, which can lead to diagnostic con(-)fusion and suggest the possibility of infective endocarditis [
10].
The interpretation of histological analyses is often subjective and may not be uniform among the pathologists. In addition, the term „active endocarditis” in the Duke criteria has not been clearly defined [
10,
11,
12].
Furthermore, the lack of a clear definition for „active endocarditis” in the Duke criteria hinders the ability to accurately diagnose PVE, and more research is needed to determine the specificity of the histological examination results for this condition.
The importance of obtaining data on the histological changes in patients with both early- and late-onset PVE cannot be overstated.
The objective of the present study was to evaluate the histological findings in patients with PVE who underwent surgery to replace the prosthetic cardiac valve by comparing early onset and late-onset PVE to gain a more comprehensive understanding of the factors that contribute to the high morbidity and mortality rates associated with this disease.
2. Materials and Methods
2.1. Study Design
This study was a population-based cohort analysis that utilized data from individuals who were admitted to the Infectious Disease Department of the “Dr. Carol Davila” Central Military Emergency University Hospital in Bucharest between January 1, 2017 and December 31, 2022. The investigation followed ethical guidelines and was authorized by the Ethics Committee of the “Dr. Carol Davila” Central Military Emergency University Hospital in Bucharest (Decision No. 562/20.12.2022). Informed consent was obtained from all the patients included in this study.
2.2. Setting
The collection of specimens was conducted in the Department of Cardiothoracic Surgery at "Dr. Carol Davila" Central Military Emergency University Hospital in Bucharest for patients with PVE. The specimens were then sent to the Pathology Department and fixed in 10% buffered formalin for 24-72 hours. Fixed samples were subsequently processed using a Leica Peloris 3 Histoprocessor, which involved the following steps: dehydration, clarification, paraffinization, and paraffin embedding. Tissue fragments were placed in successive ethanol baths (70%, 80%, 96%, and absolute) to eliminate water and replace it with a substance miscible with an organic solvent (ethanol). Tissue fragments were then placed in successive toluene or xylene baths to replace the ethanol with an organic solvent (toluene and xylene). Tissue fragments were placed in liquid paraffin baths at 56-58ºC. The clarifying liquid was replaced with paraffin, which provided tissue with the necessary consistency for microtome sectioning. Tissue fragments were embedded in paraffin at the Leica Arcadia H Leica Arcadia embedding station for microtome sectioning. Paraffin blocks were recorded according to the registration number accompanying necropsy fragments. Embedded paraffin blocks were sectioned at 3-5 micrometers thickness using a Leica RM 2265 microtome. Staining was performed using a Leica Spectra ST staining station. Immunohistochemical examination was performed using Leica Bond III immunostainer. Histological preparations were examined under an Olympus BX43 light microscope, and image acquisition was performed with an XC30 camera using Cell-Sense software.
2.3. Study Population
From January 2017 through December 2022, a total of 27 patients underwent surgical removal of a mechanical valve at "Dr. Carol Davila" Central Military Emergency Uni-versity Hospital in Bucharest. All patients had tissue harvested from the mechanical cardiac valve and were analyzed for the pathology of their infection. Patients were divided into two groups: early onset and late-onset. Patients were considered to have confirmed PVE with microorganisms detected by standard blood cultures, according to the Duke criteria.
2.4. Statistical Analysis
The Mann-Whitney U test was applied to assess the statistical significance of the results for each histological parameter of the valve tissue samples from patients in the early onset and late-onset groups. The Kruskal-Wallis test yielded p-values, which indicate the probability of obtaining differences as significant or greater than those observed in our data, assuming that the null hypothesis is valid. If the p-value was less than the predetermined significance level of 0.05, we rejected the null hypothesis and concluded that there were significant differences between at least two groups. Statistical analyses were performed using SPSS software version 26.
3. Results
Among the 10 patients in the early onset group, 80% were male and 20% were female. In contrast, the late-onset group, which included 17 patients, comprised 58.8% male patients and 41.2% female patients. The mean age of patients in the early-onset group was 63.46 years, with a standard deviation of +/- 19.12 years, while the mean age of patients in the late-onset group was 72.31 years, with a standard deviation of +/- 15.61 years. In the early onset group, the ratio of male to female patients was 1.93 in the early onset group and 0.62 late-onset group. The distribution of valve involvement differed between the two groups, with aortic and mitral valves being involved in 60% and 40% of patients, respectively, in the early onset group, and in 64.7% and 35.3% of patients, respectively, in the late-onset group.
3.1. Histological Findings
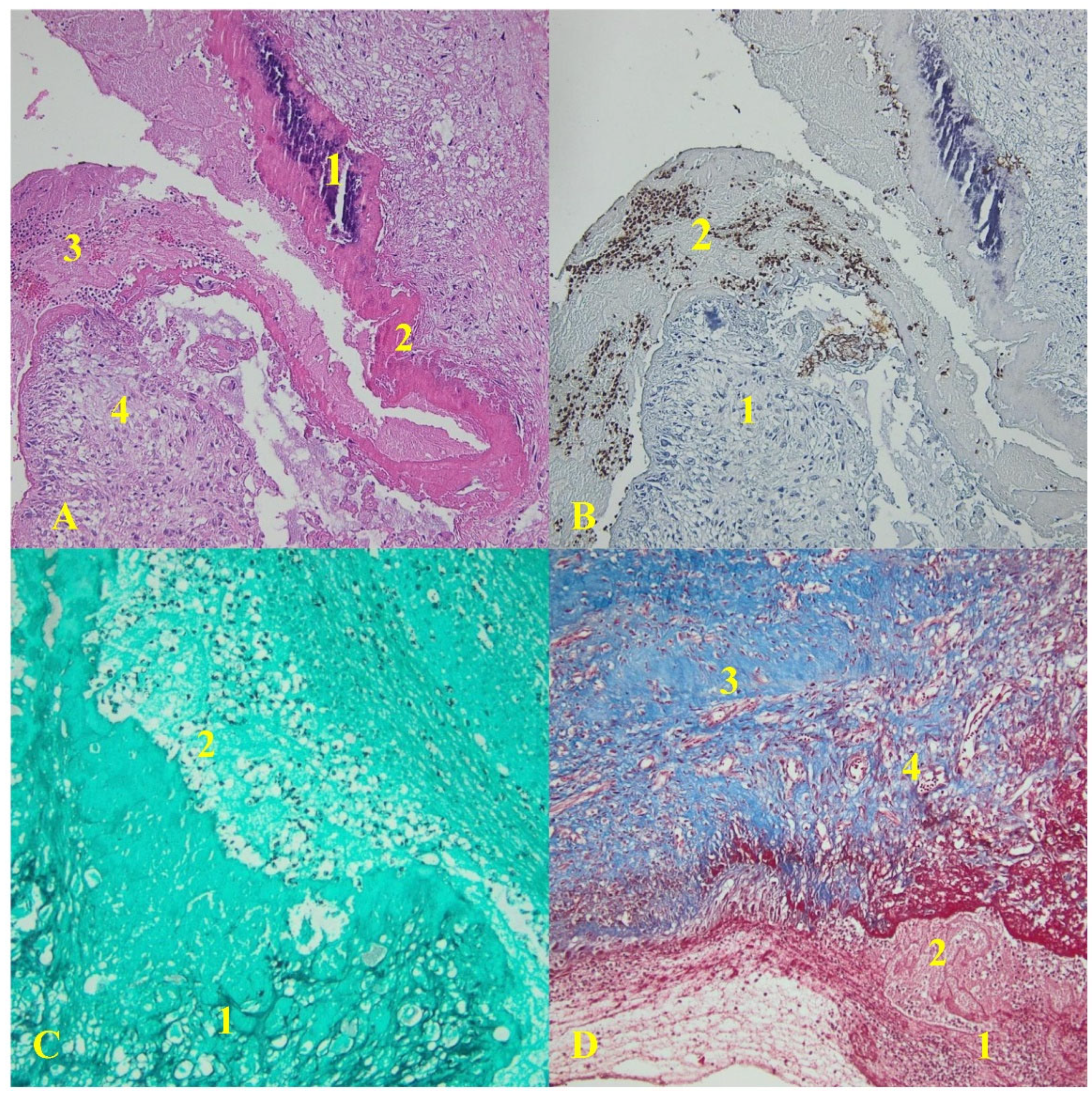
All the collected valve tissue specimens displayed evidence of vegetation. Ten mechanical heart valve specimens were analyzed in the early onset group, whereas 17 specimens were examined in the late-onset group. Histological analysis revealed that all the collected specimens exhibited signs of intense inflammation. In the early onset group, valve tissue specimens collected from each patient revealed extensive inflammatory infiltrates, composed primarily of neutrophils, and an organized fibrin network. The infection process was mainly evident in the vegetation, as illustrated in
Figure 1.
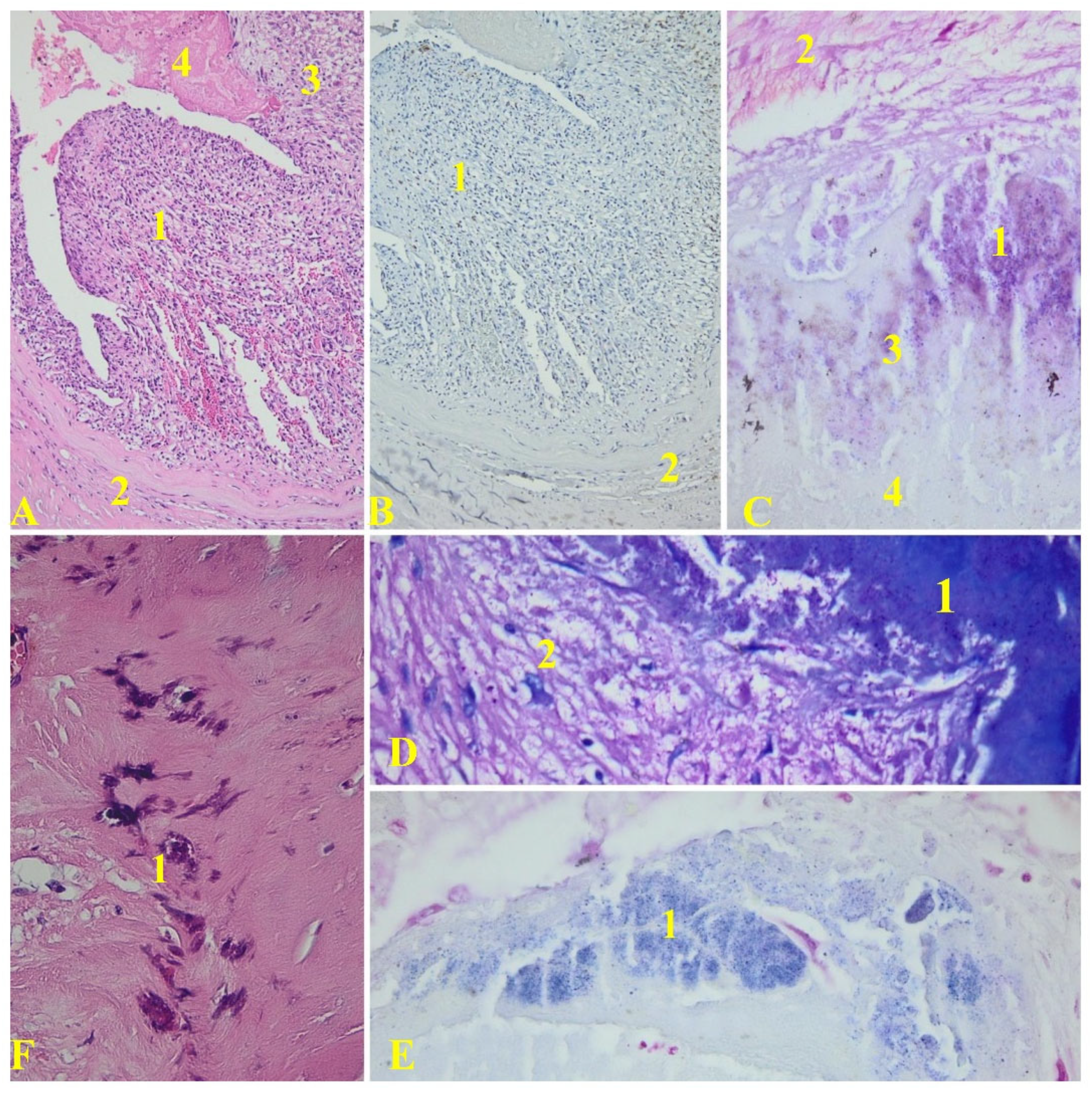
The late-onset group’s specimens exhibited a subendothelial inflammatory infiltrate, primarily composed of lymphocytes, monocytes, and fibrin in the subendothelial connective tissue, as depicted in
Figure 2.
In the late onset group, the inflammatory infiltrates were primarily composed of macrophages. Vegetation constituted a significant portion of the valve tissue area (approximately 52.38% of the surface was covered by vegetation in the examined samples).
Fibrin deposits and PMNs were found in 75% of the valve specimens. Histological analysis revealed fibrin deposits, neovascularization, lymphocytes, foamy histiocytes, and fibrosis. Acute inflammation (presence of PMNs) was detected in 67% of the cases, while 59% had chronic inflammation (neovascularization, lymphocytes, and histiocytes). Both acute and chronic inflammation were present in 42% of the cases.
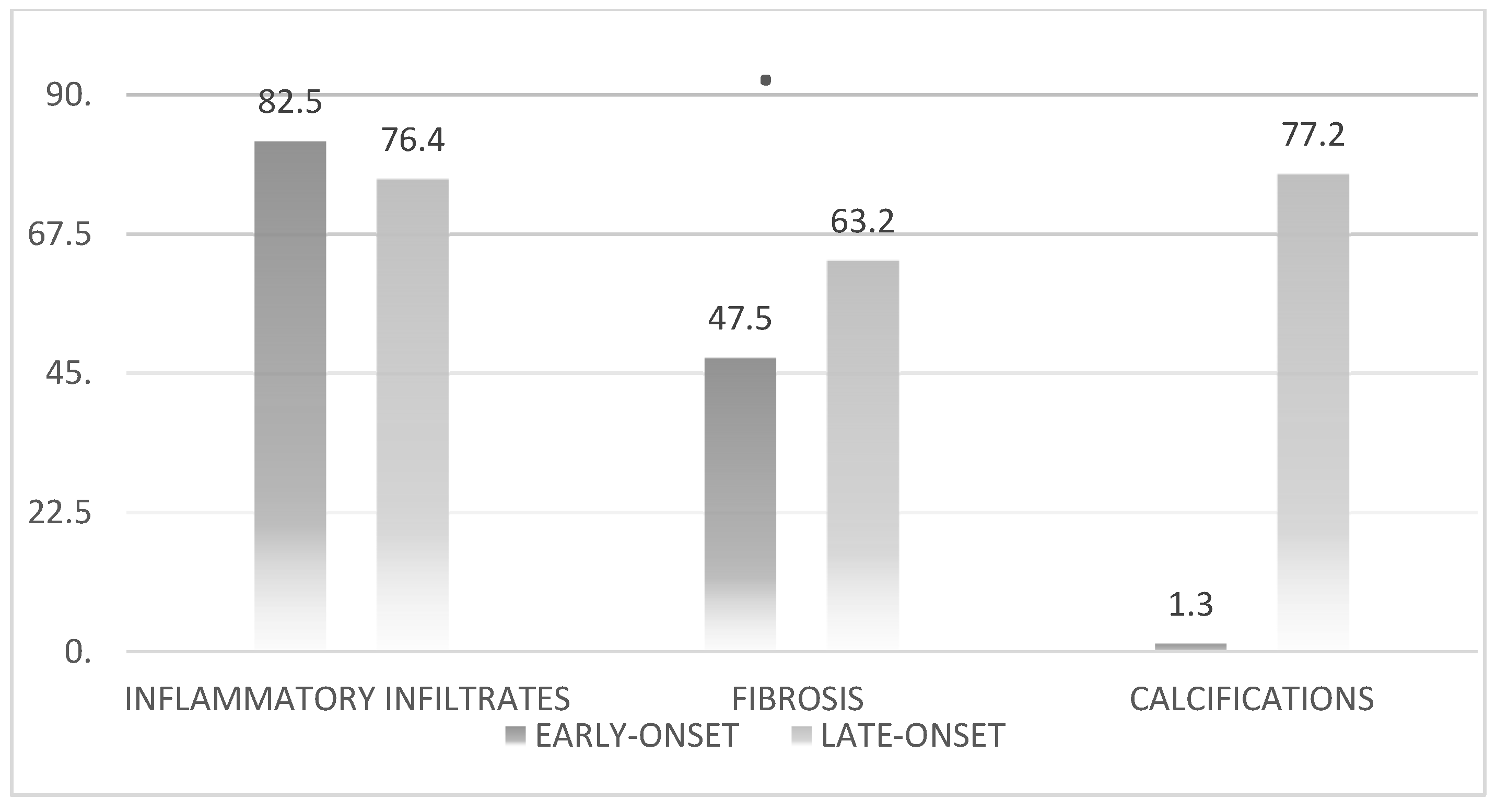
After conducting a comprehensive statistical analysis of the histological parameters, we compared the two groups to gain a clearer understanding and enhance the validity of our findings. The results are shown in
Figure 3.
Statistical analysis of inflammatory infiltrates revealed no significant differences between the early- and late-onset groups (p=0.06). However, significant differences were observed in the statistical analysis of calcifications (p=0.003).
3.2. Bacteriological Findings
The etiological agents found in the samples of mechanical heart valves collected during surgery were Staphylococcus aureus (seven cases), Enterococcus faecalis (four cases), and Streptococcus mitis (two cases). S. aureus was identified as the primary infectious agent with a higher prevalence observed in the early-onset group, followed by E. faecalis, which demonstrated a higher prevalence in the late-onset group.
The remaining samples examined in this study were sterile (n = 14). The distribution of etiological agents according to the disease onset is shown in
Table 1.
3.3. Follow-Up
The study’s follow-up period for participants concluded in February 2023. Following surgery, the patients underwent physical examination and echocardiography at three months, as well as on-demand additional examinations, including computed tomography. Postoperative morbidities included dialysis and stroke. Early 60-day mortality occurred in four patients. No reoperations were performed during the follow-up period. Histological examination of the prosthetic cardiac valve revealed tissue degeneration and inflammation, which could indicate IE. Removal and examination of the tissue valve specimen can be beneficial in determining the appropriate medication regimen for patients after surgery.
4. Discussions
IE of the mechanical cardiac valves continues to be a major complication. The mortality rate remains high in situations that involves etiological agents such as S. aureus [
7,
8].
According to the Duke criteria, a diagnosis of IE can be established through histological evidence of microorganisms or the presence of vegetation and active endocarditis, regardless of whether the affected valve is native or prosthetic [
8,
9].
Managing patients with IE calls for a multidisciplinary strategy that engages physicians, cardiologists, cardiac surgeons, electrophysiologists, microbiologists, histopathologists, infectious disease specialists, radiologists, and echocardiographers, owing to the heterogeneity of the condition [
10,
11].
Despite advances in detection and treatment, disappointing results continue to emerge, as evidenced by inpatient mortality rates of 18% and a 6-month mortality rate of 30%. Poor outcomes are heavily influenced by variables such as S. aureus infection, age progression, persistent positive blood cultures despite appropriate antibiotic treatment, and presence of significant concurrent health issues [
10,
11,
12].
It is crucial to promptly establish an accurate diagnosis and administer antimicrobial treatment to prevent complications and improve overall prognosis. In approximately 40-50% of cases, surgical intervention is necessary for infective endocarditis in order to avert progressive heart failure, mitigate irreversible structural damage caused by un-controlled infection, and preclude embolism [
11,
12].
The term "active infective endocarditis" has not yet been clearly defined, and the specific histological characteristics that distinguish IE have not been uniformly established [
11].
According to recent studies, acute and chronic inflammation are distinct. Acute inflammation is identified by the presence of polymorphonuclear leukocytes, specifically neutrophils, in the cardiac valvular inflammatory infiltrates. In contrast, chronic inflammation is characterized by the presence of inflammatory infiltrates consisting of mononuclear cells such as macrophages and lymphocytes [
12].
Macrophages and lymphocytes are frequently found in the inflammatory infiltrates of cardiac valves in cases of chronic inflammation. The use of statistical analysis in studies of IE histology in mechanical valves can help avoid subjective impressions that may lead to inaccurate reports. Statistical analysis can also aid in identifying patterns and trends in the data collected from IE studies on mechanical valves, which can provide a more objective and accurate understanding of the disease [
13].
Histological examination is a reliable method for differentiating IE from other non-infectious inflammatory processes. To accomplish this, the current study included patients with mechanical valves removed because of suspected PVE. These specimens were used to establish precise histological criteria that delineated the features of PVE in individuals who underwent valve removal because of malfunction.
The histological diagnosis of IE in mechanical cardiac valves requires a different approach from that for native valves. In individuals with mechanical heart valves, the primary location of infection is at the interface between the prosthesis and the host, and it concentrates on the annulus, resulting in abscesses in the annular region without causing any harm to the material or the presence of small or insignificant vegetations [
13].
A lack of vegetation may lead to underdiagnosis of IE in patients with mechanical valves. In our study, all the valve tissue samples showed evidence of vegetation. The presence of vegetation, extensive inflammatory infiltrates predominantly consisting of lymphocytes and neutrophils, and identification of microorganisms in valve tissue are well-known criteria for histological diagnosis of IE [
12,
13].
It is important to note that the last criterion may not always be applicable in the diagnosis of IE, as preoperative antibiotic treatment may eradicate infectious agents, rendering them undetectable by histological and biological methods. In our series, microorganisms were identified in valve tissues from only 13 patients, six from the early onset group, and seven from the late-onset group. In the present study, we showed that vegetation represented an important proportion of the valve tissue area (an average of 52.38% in our study). Mechanical cardiac valves typically exhibit avascular tissue, similar to native valves [
13].
The presence of an inflammatory infiltrate in the histological findings of valve tissue leads to neovascularization, allowing leukocytes to infiltrate the valve tissue [
14].
Neovascularization serves as a crucial histological criterion for accurate diagnosis of this pathology. In our study, we observed that degenerative processes, particularly fibrosis, were more commonly detected in the late onset group.
These two histological characteristics may aid in defining IE more accurately, based on the histopathological Duke criteria [
13,
14].
Features that characterize IE from a histological perspective have not yet been accurately standardized. This study aimed to analyze methalic cardiac valves that were removed after PVE using positive hemoculture and transesophageal echocardiography.
In the early onset group, we included patients who presented with IE symptoms less than 12 months after surgery. The histological features that characterized this group were slightly different from those of the late-onset group, with extensive inflammatory infiltrates composed primarily of neutrophils and lymphocytes in the first group and lymphocytes and macrophages in the second group. As noted in various studies, acute inflammation, which is defined by the presence of neutrophils in inflammatory infiltrates, is a highly indicative histological characteristic of infection, whereas chronic inflammation primarily consists of mononuclear cells, including macrophages and lymphocytes. Inflammatory infiltrates are frequently encountered in the histological features of metallic valves that are surgically removed from patients with PVE [
13,
14].
Valvular inflammatory infiltrates typically include neutrophils and macrophages. Nevertheless, qualitative estimation of various leukocyte subtypes through histological examination may result in inaccurate conclusions. A recent investigation of mechanical cardiac valves established that identifying the pattern of inflammation may be essential for diagnosing PVE [
15].
The diagnostic criteria for infectious endocarditis (IE) include the presence of vegetation and identification of the etiological agent in valve tissue samples. Vegetation can vary in size and covers only a small portion of the valve specimen. However, preoperative antibiotic treatment can destroy the incriminating agent, making it undetectable using histological methods [
13,
14,
15].
In our study, we observed 14 cases of prosthetic valve endocarditis (PVE) in which microorganisms could not be identified. It is possible that errors in the valve tissue sampling procedure contributed to these discrepancies. Nonetheless, the absence of visualization of vegetation and microorganisms does not preclude a histological diagnosis of IE. Furthermore, adherent thrombi can develop on the surfaces of the cardiac valves.
Although infective endocarditis of mechanical cardiac valves may involve fibrin and inflammation, our observations show that the infectious process primarily develops in the vegetation.
Adherent thrombi on the surface of mechanical heart valves may be a crucial initial event in the infection process, in which the etiological agent is encapsulated within fibrin and can survive and evade the host defense. In our study, we found that
S. aureus was the most commonly identified infectious agent, with a higher prevalence in the early onset group. These results are consistent with those of previous studies [
14,
15,
16].
Based on the histological analysis of mechanical cardiac valve tissues, the present study aimed to more accurately define the features of PVE. Specifically, the presence of vegetation and microorganisms in valve tissues in conjunction with an inflammatory infiltrate in a surgically removed mechanical heart valve may be sufficient for diagnosing PVE. The findings of this research suggest the need for adjustment to the pathological criteria for diagnosing IE, enabling differentiation between IE and non-infective valve processes.
5. Conclusions
All patients enrolled in this study exhibited indicators of inflammation, including both acute and chronic types, as determined by histological analysis.
The primary objective of this study was to evaluate the histological diagnosis of patients with PVE according to the disease onset.
This study presents a histological examination of excised tissue from mechanical cardiac valves, with the aim of gaining a deeper understanding of the features of PVE. The absence of microorganisms in valve tissues through histological analysis may indicate an inflammatory pattern that is crucial for an accurate diagnosis.
To confirm the diagnosis of PVE, surgical removal of all valves in these individuals should be subjected to histological examination, and the absence of such findings raises doubts regarding the accuracy of the diagnosis.
The literature and guidelines on the histological findings of IE developed on mechanical prosthetics heart valves are mainly based on observational studies. Owing to clinical scenarios, IE teams often find it difficult to decide the most appropriate strategy. The best clinical decisions are made by sharing the management approaches. Including a phatologist in the IE team is important for improving the clinical outcomes of this disease. This study aimed to demonstrate that PVE exhibits extensive inflammatory infiltrates.
Limitations
The current study had certain limitations that should be acknowledged. First, it was limited to only one hospital, which may have influenced the results. A further study with a larger patient sample should be conducted for gaining a more comprehenive understanding of the histological characteristics of PVE.
Author Contributions
Conceptualization: C.-I.A. and S.D.; methodology F.S.; software: A.T.Ș. and C.-I.A.; validation: A.S.-C. and I. Ș. ; formal analysis: C.-I.A.; investigation: C.-I.A.; resources: I.Ș.; data curation: C.-I.A.; writing, original draft preparation: C.-I.A.; writing, review and editing: C.-I.A.; visualization: C.A.B..; supervision: C.-I.A.; project administration: C.-I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Informed Consent Statement
All patients who have been admitted and examined within our institution are invited to read and sign a document authorizing the department to anonymously analyze their data for medical research purposes, preserving the confidentiality of their identity.
Data Availability Statement
The data presented in this study are available upon request. The data are not publicly available because of the confidentiality of health data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, A.; Gaca, J.G.; Chu, V.H. Management considerations in infective endocarditis: a review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Tinica, G.; Tarus, A.; Enache, M.; et al. Infective endocarditis after TAVI: a meta-analysis and systematic review of epidemiology, risk factors and clinical consequences. Rev Cardiovasc Med. 2020, 21, 263–274. [Google Scholar] [PubMed]
- Vogkou, C.T.; Vlachogiannis, N.I.; Palaiodimos, L.; Kousoulis, A.A. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2016, 35, 1227–1245. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.T.E.; Westaby, J.D.; Griffin, K.J.; Sheppard, M.N. The role of endocarditis in sudden cardiac death: highlighting the value of the autopsy, pathological features andcardiac complications. Cardiovasc Pathol. 2021, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Greub, G; Lepidi, H; Rovery, C; Casalta, J-P; Habib, G; Collard, F; Fournier, P-E; Raoult, D. Diagnosis of infectious endocarditis in patients undergoing valve surgery. AM J Med. 2005, 118, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Celik, M; Milojevic, M; Durko, AP; Oei, FBS; Bogers, AJJC; Mahtab, EAF. Comparative study of male and female patients undergoing surgical aortic valve replacement. Interdiscip CardioVasc Thor Surg. 2023, 36, 1–9. [Google Scholar]
- Witten, JC; Tan, CD; Rodriguez, ER; Shrestha, NK; Gordon, SM; Hussain, ST; Apte, SS; Unai, S; Blackstone, EH; Petterson, GB. Invasive aortic valve endocarditis: clinical and tissue findings from a prospective investigation. Ann Thorac Surg. 2022, 113, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Morris AJ, Drinkovic D, Pottumarthy S, et al. Gram stain, culture, and histopathological examination findings for heart valves removed because of infective endocarditis, Clin Infect Dis, 2017.
- Zhou, Y.; Wilkinson, S.R.; Cain, M.; Litovsky, S.H.; Reilly, S.D. A 12-year autopsy review of infective endocarditis. Am J Clin Pathol. 2012, 138, A206. [Google Scholar] [CrossRef]
- Guimaron, S; Kalavrouziotis, D; Maranda-Robitaille, M; Dumont, E; Joubert, P; Babaki, S; Rodes-Cabau, J; Mohammadi, S. Macroscopic and microscopic features of surgically explanted transcatheter aortic valve prosthesis. J Card Surg. 2022, 37, 3178–3187. [Google Scholar] [CrossRef] [PubMed]
- Ely, D; Tan, CD; Rodriguez, ER; Hussain, S; Pettersson, G; Gordon, S; Shrestha, N. Histological findings in infective endocarditis. Open Forum Infect Dis. 2016, 3, 1111. [Google Scholar] [CrossRef]
- Witten, JC; Tan, CD; Rodriguez, ER; Shrestha, NK; Gordon, SM; Hussain, ST; Apte, SS; Unai, S; Blackstone, EH; Petterson, GB. Invasive aortic valve endocarditis: clinical and tissue findings from a prospective investigation. Ann Thorac Surg. 2022, 113, 535–544. [Google Scholar] [CrossRef] [PubMed]
- van Kesteren, F; Wiegerinck, EMA; Rizzo, S; Baan, J, Jr; Planken, RN; von der Thusen, JH; Niessen, HWM; van Oosterhout, MFM; Pucci, A; Thiene, G; et al. Autopsy after transcatheter aortic valve implantation. Virchows Arch. 2017, 470, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Brandao, TJD; Januario-da-Silva, CA; Crreia, MG; Zappa, M; Abrantes, JA; Dantas, AMR; Golebiovski, W; Barbosa, GIF; Weksler, C; Lamas, CC. Histopathology of valves in infective endocarditis, diagnostic criteria and treatment considerations. Infection. 2017, 45, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Brandao, T.J.; Januario-da-Silva, C.A.; Correia, M.G.; Zappa, M.; Abrantes, J.A.; Dantas, A.M.; Golebiovski, W.; Barbosa, G.I.; Weksler, C.; Lamas, C.C. Histopathology of valves in infective endocarditis, diagnostic criteria and treatment considerations. Infection. 2017, 45, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur. Heart J. 2014, 35, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).