Introduction
The cerebrospinal fluid-contacting raphe nucleus (CsfR) has recently been identified in the brain, initially reconstructed and located by Song et al. in both rats and non-human primates [
1,
2]. The mechanisms of signal transmission between nervous tissue and body fluid have remained elusive due to the brain’s barriers. However, the presence of CsfR offers a significant structure that facilitates the brain-CSF circuit. Given its consistent location, formation as an independent cluster, and unique neuron type distinct from any other known brain nuclei, this cluster has been designated the “cerebrospinal fluid-contacting raphe nucleus” or “CSF-contacting raphe nucleus” (CsfR).
Mice, which have a genome similar to that of human beings, serve as an ideal model for studying physiological and pathological mechanisms, particularly in neuroscience. However, the existence and detailed morphology of the CsfR in mice have not yet been demonstrated.
In this study, we chose C57BL/6J strain mice as our subjects to investigate the existence, location, and distributional regularity of the CsfR in detail. Our findings will also offer essential methodological support for researchers interested in studying this nucleus.
Materials and methods
1. Experimental animals and Tracer administration to label CsfR
All experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine. Male and female C57BL/6J mice, weighing 26-30g, were used in this study. The mice were anesthetized with 2% isoflurane, and their heads were secured in a stereotaxic instrument. A 0.2 μl injection of 1% CB-Alexa Fluor 594 (Invitrogen, Thermo Fisher, USA), a specific tracer for CsfR via the ventricular system, was administered into the lateral ventricle according to the stereotaxic coordinates provided by Paxinos and Franklin [
3]. After a survival period of 48 hours, the animals were perfused and sacrificed.
2. CsfR Visualization and Positive Neuron Counting
The sections were sequentially mounted on slides and coverslipped. Fluorescence microscopy was used to capture images of the sections at various positions under uniform standards. The number of positive neurons was then counted using Image-Pro Plus 7.0 software.
3. Statistics
SPSS 13.0 software was used for data analysis in the present study. Data were presented as mean±SD.
Results:
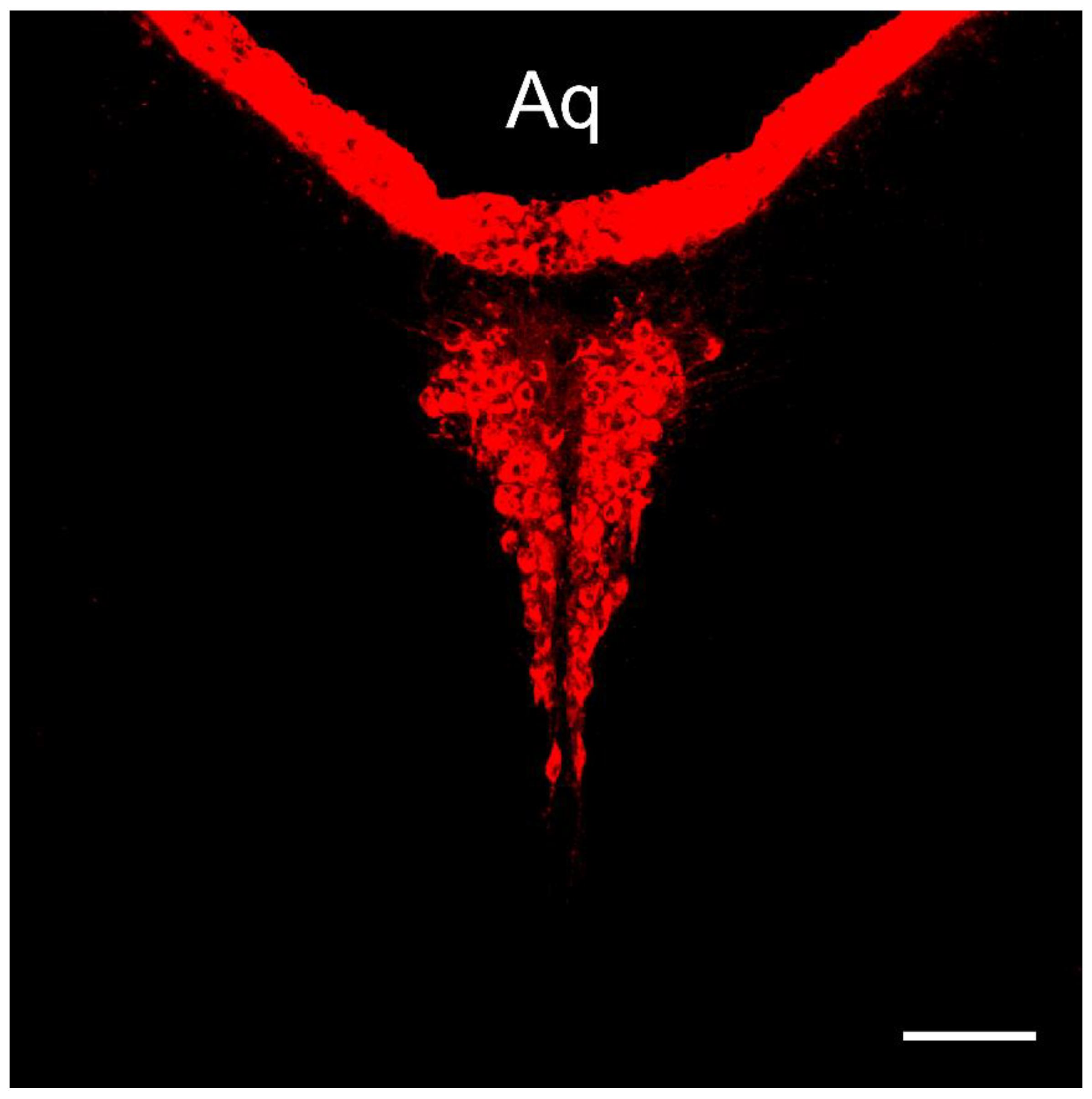
1. Label of CSF-contacting Raphe Nucleus in Mice
Similar to findings in rats and non-human primates, numerous CB-labeled neurons were observed in the dorsal part of the dorsal raphe (DR) nucleus and throughout its entire caudal region. These neurons exist independently, forming a distinct cluster that we refer to as the cerebrospinal fluid-contacting raphe nucleus (CsfR) (
Figure 1).
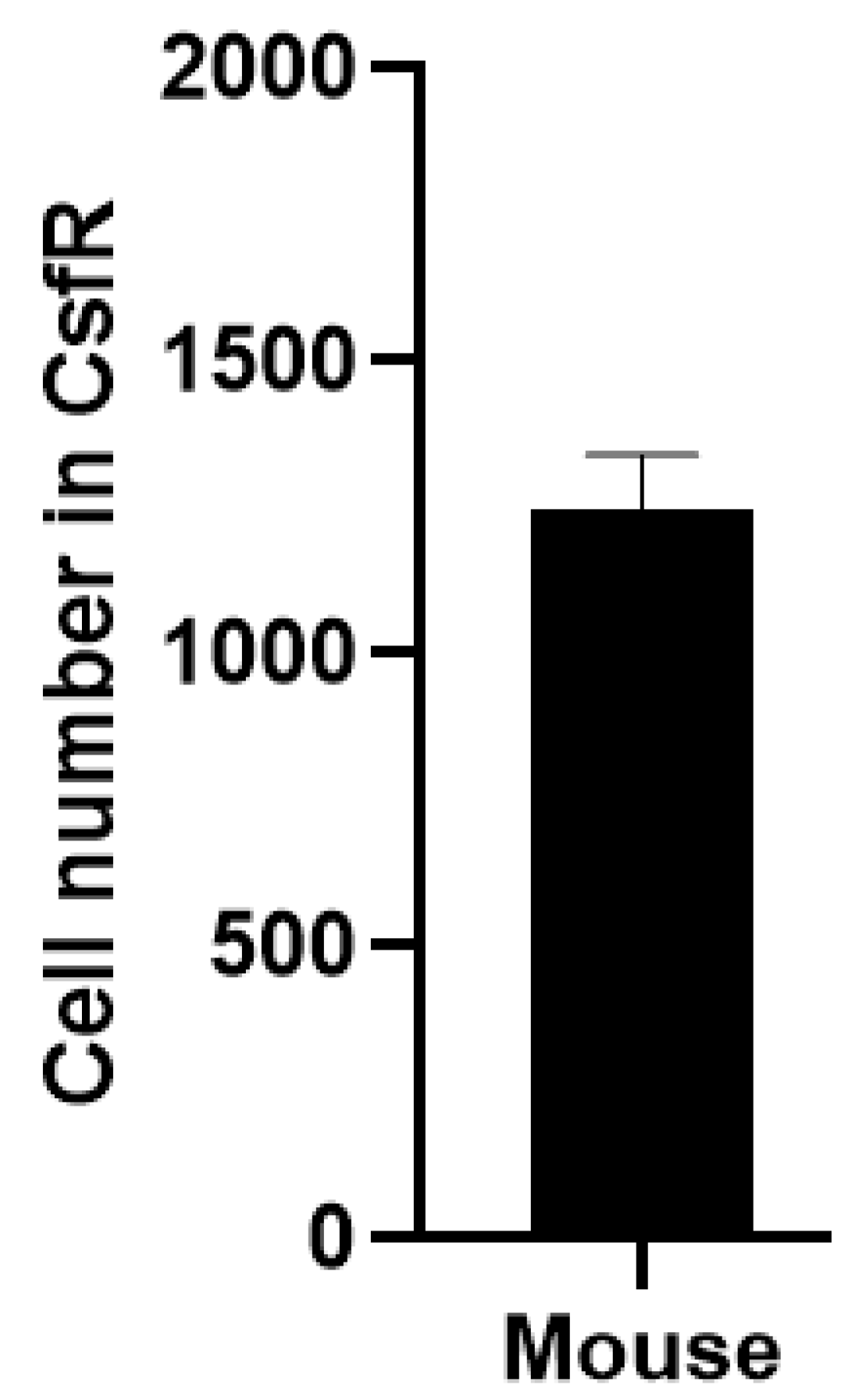
2. The Comparison of the CSF-contacting Raphe Nucleus Morphology in Mice and Rats on Coronal Sections
In both rats and non-human primates, the CsfR typically exhibits a Y-shaped structure. In mice, we observed a denser, heart-shaped structure with bilateral symmetry at corresponding stages. Although there are morphological differences, the overall structure remains consistent. The CsfR in rats contains approximately 1,400 neurons, whereas in mice, there are about 1,100 neurons. Despite the species differences, the neuron count varies only slightly (
Figure 2).
Discussion
When the tracer CB or its fluorescent conjugate was injected into the lateral ventricle of rats, it formed a clear outline along the cerebral ventricular system. The CSF-contacting raphe nucleus (CsfR), with its processes stretching into the ventricular system and directly contacting the cerebrospinal fluid, was specifically labeled [
1]. Our study shows that this phenomenon is also applicable to mice. The tracer CB forms a clear outline along the lateral ventricle, the third ventricle, the aqueduct, the fourth ventricle, the central canal of the spinal cord, and the surface of the brain and spinal cord. The CsfR, whose processes stretch into the ventricular system and directly contact the cerebrospinal fluid, have their somas consistently located, forming a distinct cluster separate from nearby non-CSF-contacting structures in the parenchyma. Given that this location has been previously identified as the raphe nucleus, but considering its capability to extend into the cerebrospinal fluid, we propose naming this special neuron cluster the “cerebrospinal fluid-contacting raphe nucleus” (CsfR).
In mice, the number of these neurons is approximately 1,100, similar to the ~1,400 neurons found in rats. In both rats and non-human primates, these neurons exhibit a rivet-like appearance. Future research should focus on the 3D reconstruction of the mouse CsfR and precise stereotactic coordinates to further investigate its unique functions. This paper provides a brief confirmation of the presence of CsfR in mice, and many questions remain to be explored.
Funding
The author declares no competing interests.
Data Access Statement
The author had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgement
I am grateful to the scholars for their support and attention to this research.
Availability of Data and Materials
Please contact the author for data requests.
References
- Song, S.Y.; Li, Y.H.; Bao, C.Y.; Li, Y.; Yin, P.C.; Hong, J.; Li, W.L.; Shi, Y.; Zhang, L.C. Stereotaxic Coordinates and Morphological Characterization of a Unique Nucleus (CSF-Contacting Nucleus) in Rat. Front. Neuroanat. 2019, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Zhai, X.M.; Shan, C.J.; Lu, L.L.; Hong, J.; Cao, J.L.; Zhang, L.C. A Special Cranial Nucleus (CSF-Contacting Nucleus) in Primates. Front. Neuroanat. 2020, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The mouse brain in stereotaxic coordinates. Compact 2004, 1. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).