Submitted:
06 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. S-sulfenylation
2.1. S-Sulfenylation is Promoted by Oxidative Conditions and is a Stepping-Stone Towards Other Cys Redox PTMs
2.2. S-Sulfenylation is a Reversible Primary Cys Oxidation
2.3. S-sulfenylation as a Redox-Control Mechanism in Plant Primary Metabolism
2.4. Involvement of Protein S-Sulfenylation in Stress Signal Transduction
3. S-Sulfinylation and S-Sulfonylation
4. Disulfide Bridge Formation
4.1. Disulfide Bridge Reduction is an Important Regulatory Mechanism that Links Light Harvesting and CO2 Fixation in the Chloroplast
4.2. Disulfide Bridge Formation in the Chloroplast under Dark Conditions
5. S-Glutathionylation
5.1. S-Glutathionylation as a Means of Protecting Metabolic Enzymes against Irreversible Oxidation
5.2. Metabolic Enzymes Targeted by Regulatory S-Glutathionylation under Oxidative Conditions
5.3. Involvement of S-Glutathionylation in Signaling
6. Protein Persulfidation
6.1. Addition of Sulfide on Cys Results from Direct Persulfidation or Transpersulfidation
6.2. Metabolic Targets of Cys Persulfidation
6.3. Cys Persulfidation Involvement in ABA-Mediated Stomatal Movement
7. S-Cyanylation
8. S-Nitrosation
8.1. Mechanisms Involved in Protein S-Nitrosation
8.2. Protein Denitrosation
8.3. Targets of Protein S-Nitrosation in Plant Metabolism
8.4. Involvement of S-Nitrosation in Signal Transduction Pathways
9. S-Carbonylation by Reactive Carbonyl Species
9.1. Interrelations between RCS and ROS Signaling
9.2. Protein Thiols Modification by RCSs and S-OPDAylation
10. S-Acylation
10.1. S-Acylation Appears to be Critical for Target Proteins Membrane Localization
10.2. De-S-Acylation Players and Evidence of Their Involvement in Plant Signal Transduction
11. Prenylation
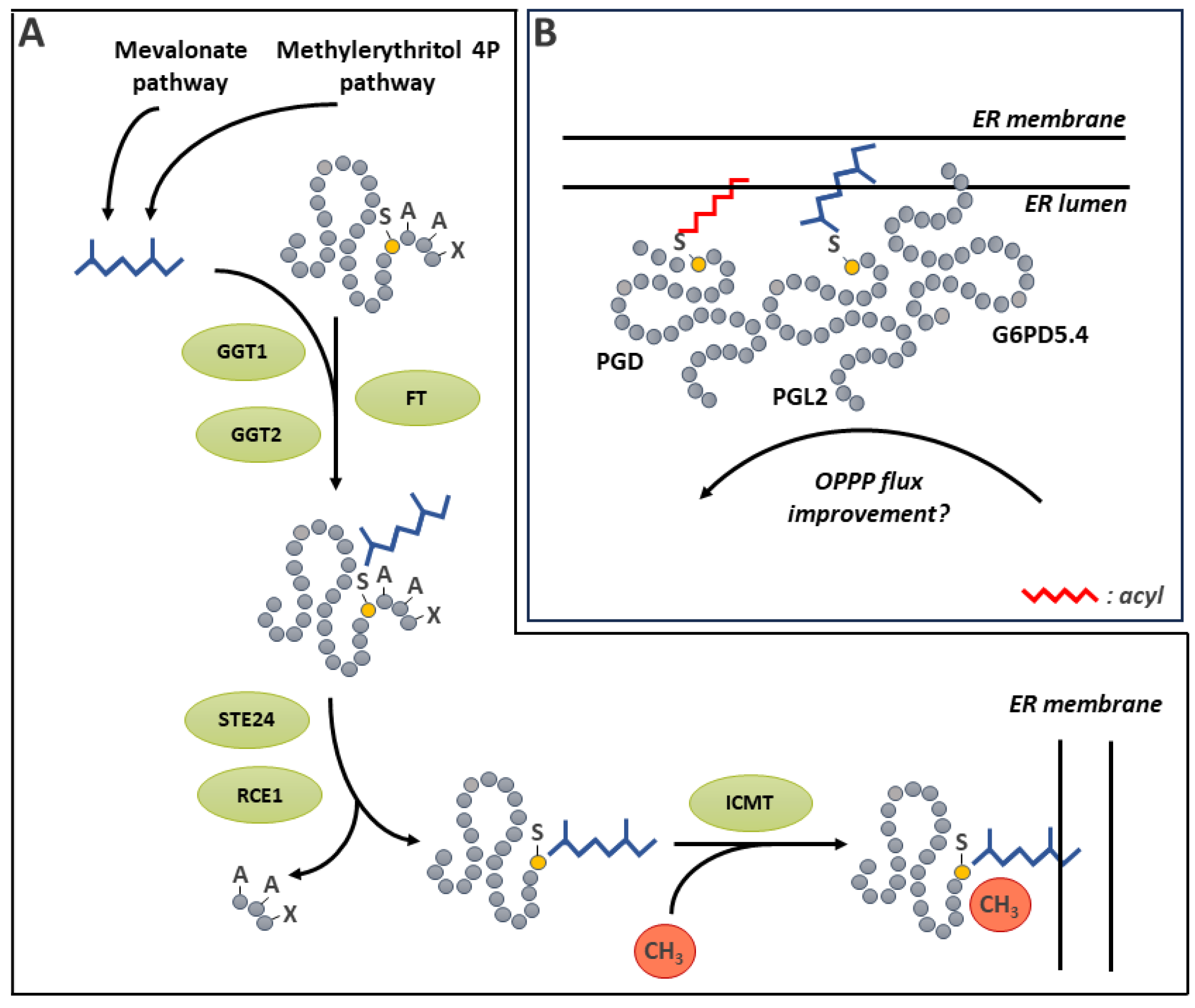
12. CoAlation
13. Thiohemiacetal Formation
14. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beltrao, P.; Bork, P.; Krogan, N.J.; van Noort, V. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013, 9, 714. [Google Scholar] [CrossRef]
- Spoel, S.H. Orchestrating the proteome with post-translational modifications. J. Exp. Bot. 2018, 69, 4499–4503. [Google Scholar] [CrossRef]
- Stührwohldt, N.; Schaller, A. Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol. 2019, 21, 49–63. [Google Scholar] [CrossRef]
- Willems, P.; Horne, A.; Van Parys, T.; Goormachtig, S.; De Smet, I.; Botzki, A.; Van Breusegem, F.; Gevaert, K. The Plant PTM Viewer, a central resource for exploring plant protein modifications. The Plant Journal 2019, 99, 752–762. [Google Scholar] [CrossRef]
- O’Leary, B.; Plaxton, W.C. Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr. Opin. Plant Biol. 2020, 55, 28–37. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M. ; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiology and Biochemistry 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Mathe, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling-Facts and Hypotheses. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10. [Google Scholar] [CrossRef]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox Signaling in Plant Heat Stress Response. Antioxidants 2023, 12. [Google Scholar] [CrossRef]
- Chaput, V.; Martin, A.; Lejay, L. Redox metabolism: the hidden player in carbon and nitrogen signaling? J. Exp. Bot. 2020, 71, 3816–3826. [Google Scholar] [CrossRef]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.-P. Redox-mediated responses to high temperature in plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- Leichert, L.I.; Dick, T.P. Incidence and physiological relevance of protein thiol switches. Biol. Chem. 2015, 396, 389–399. [Google Scholar] [CrossRef]
- Trifonov, E.N. The triplet code from first principles. J. Biomol. Struct. Dyn. 2004, 22, 1–11. [Google Scholar] [CrossRef]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 293–322. [Google Scholar] [CrossRef]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radical Biology and Medicine 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Miseta, A.; Csutora, P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Molecular Biology and Evolution 2000, 17, 1232–1239. [Google Scholar] [CrossRef]
- Wiedemann, C.; Kumar, A.; Lang, A.; Ohlenschläger, O. Cysteines and Disulfide Bonds as Structure-Forming Units: Insights From Different Domains of Life and the Potential for Characterization by NMR. Frontiers in Chemistry 2020, 8. [Google Scholar] [CrossRef]
- Willems, P.; Huang, J.J.; Messens, J.; Van Breusegem, F. Functionally annotating cysteine disulfides and metal binding sites in the plant kingdom using AlphaFold2 predicted structures. Free Radical Biology and Medicine 2023, 194, 220–229. [Google Scholar] [CrossRef]
- Marino, S.M.; Gladyshev, V.N. Cysteine Function Governs Its Conservation and Degeneration and Restricts Its Utilization on Protein Surfaces. J. Mol. Biol. 2010, 404, 902–916. [Google Scholar] [CrossRef]
- Clement, G.E.; Hartz, T.P. Determination of the microscopic ionization constants. J. Chem. Educ. 1971, 48, 395. [Google Scholar] [CrossRef]
- Trost, P.; Fermani, S.; Calvaresi, M.; Zaffagnini, M. Biochemical basis of sulphenomics: how protein sulphenic acids may be stabilized by the protein microenvironment. Plant Cell and Environment 2017, 40, 483–490. [Google Scholar] [CrossRef]
- Habjanič, J.; Chesnov, S.; Zerbe, O.; Freisinger, E. Impact of naturally occurring serine/cysteine variations on the structure and function of Pseudomonas metallothioneins†. Metallomics 2019, 12, 23–33. [Google Scholar] [CrossRef]
- Hol, W.G.J.; van Duijnen, P.T.; Berendsen, H.J.C. The α-helix dipole and the properties of proteins. Nature 1978, 273, 443–446. [Google Scholar] [CrossRef]
- Kortemme, T.; Creighton, T.E. Ionisation of Cysteine Residues at the Termini of Model α-Helical Peptides. Relevance to Unusual Thiol pKaValues in Proteins of the Thioredoxin Family. J. Mol. Biol. 1995, 253, 799–812. [Google Scholar] [CrossRef]
- Miranda, J.J.L. Position-dependent interactions between cysteine residues and the helix dipole. Protein Sci. 2003, 12, 73–81. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Ramos, M.J. Theoretical insights into the mechanism for thiol/disulfide exchange. Chemistry-a European Journal 2004, 10, 257–266. [Google Scholar] [CrossRef]
- Netto, L.E.S.; de Oliveira, M.A.; Tairum, C.A.; Neto, J.F.D. Conferring specificity in redox pathways by enzymatic thiol/disulfide exchange reactions. Free Radic. Res. 2016, 50, 206–245. [Google Scholar] [CrossRef]
- Radzinski, M.; Oppenheim, T.; Metanis, N.; Reichmann, D. The Cys Sense: Thiol Redox Switches Mediate Life Cycles of Cellular Proteins. Biomolecules 2021, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Reddie, K.G.; Carroll, K.S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochimica Et Biophysica Acta-General Subjects 2014, 1840, 847–875. [Google Scholar] [CrossRef]
- Huang, J.J.; Willems, P.; Van Breusegem, F.; Messens, J. Pathways crossing mammalian and plant sulfenomic landscapes. Free Radical Biology and Medicine 2018, 122, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Kaushik, P. Free Radicals Mediated Redox Signaling in Plant Stress Tolerance. Life-Basel 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Yu, K.; Wu, G.D.; Zhang, Q.F.; Wang, P.Q.; Zheng, J.; Liu, Z.X.; Wang, J.C.; Gao, X.J.; Cheng, H. pCysMod: Prediction of Multiple Cysteine Modifications Based on Deep Learning Framework. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gonzalez-Gordo, S.; Palma, J.M. Protein nitration: A connecting bridge between nitric oxide (NO) and plant stress. Plant Stress 2021, 2. [Google Scholar] [CrossRef]
- Mukherjee, S.; Corpas, F.J. H2O2, NO, and H2S networks during root development and signalling under physiological and challenging environments: Beneficial or toxic? Plant Cell and Environment 2023, 46, 688–717. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering hydrogen peroxide-induced signalling towards stress tolerance in plants. 3 Biotech 2019, 9, 395. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The ascorbate–glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Cejudo, F.J.; Sandalio, L.M.; Van Breusegem, F. Understanding plant responses to stress conditions: redox-based strategies. J. Exp. Bot. 2021, 72, 5785–5788. [Google Scholar] [CrossRef]
- Vogelsang, L.; Dietz, K.J. Regulatory thiol oxidation in chloroplast metabolism, oxidative stress response and environmental signaling in plants. Biochem. J. 2020, 477, 1865–1878. [Google Scholar] [CrossRef]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; de Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J.Exp.Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Liebthal, M.; Maynard, D.; Dietz, K.J. Peroxiredoxins and Redox Signaling in Plants. Antioxidants & Redox Signaling 2018, 28, 609–624. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Yang, J.; Liebler, D.C.; Carroll, K.S. Diverse Redoxome Reactivity Profiles of Carbon Nucleophiles. J. Am. Chem. Soc. 2017, 139, 5588–5595. [Google Scholar] [CrossRef]
- Huang, J.; Willems, P.; Wei, B.; Tian, C.; Ferreira, R.B.; Bodra, N.; Martínez Gache, S.A.; Wahni, K.; Liu, K.; Vertommen, D. Mining for protein S-sulfenylation in Arabidopsis uncovers redox-sensitive sites. Proceedings of the National Academy of Sciences 2019, 116, 21256–21261. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Carroll, K.S. Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation. Acc. Chem. Res. 2020, 53, 20–31. [Google Scholar] [CrossRef]
- Monteiro, G.; Horta, B.B.; Pimenta, D.C.; Augusto, O.; Netto, L.E.S. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 4886–4891. [Google Scholar] [CrossRef]
- Pulido, P.; Cazalis, R.; Cejudo, F.J. An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. The Plant Journal 2009, 57, 132–145. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zito, E.; Hansen, Henning G. ; Yeo, Giles S.H.; Fujii, J.; Ron, D. Endoplasmic Reticulum Thiol Oxidase Deficiency Leads to Ascorbic Acid Depletion and Noncanonical Scurvy in Mice. Mol. Cell 2012, 48, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.; Le Marechal, P.; Rouhier, N.; Lemaire, S.D.; Rey, P. Regeneration mechanisms of Arabidopsis thaliana methionine sulfoxide reductases B by glutaredoxins and thioredoxins. J. Biol. Chem. 2009, 284, 18963–18971. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Laugier, E.; Zaffagnini, M.; Marchand, C.H.; Le Maréchal, P.; Lemaire, S.D.; Rey, P. Plant Thioredoxin CDSP32 Regenerates 1-Cys Methionine Sulfoxide Reductase B Activity through the Direct Reduction of Sulfenic Acid *<sup></sup>. J. Biol. Chem. 2010, 285, 14964–14972. [Google Scholar] [CrossRef] [PubMed]
- Toriu, M.; Horie, M.; Kumaki, Y.; Yoneyama, T.; Kore-eda, S.; Mitsuyama, S.; Yoshida, K.; Hisabori, T.; Nishiyama, Y. Chloroplast translation factor EF-Tu of Arabidopsis thaliana can be inactivated via oxidation of a specific cysteine residue. Biochem. J. 2023, 480, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Bedhomme, M.; Adamo, M.; Marchand, C.H.; Couturier, J.; Rouhier, N.; Lemaire, S.D.; Zaffagnini, M.; Trost, P. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 2012, 445, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, F.; Wei, F.; Fanella, B.; Allen, D.K.; Wang, X. Cytosolic Phosphorylating Glyceraldehyde-3-Phosphate Dehydrogenases Affect <em>Arabidopsis</em> Cellular Metabolism and Promote Seed Oil Accumulation. Plant Cell 2014, 26, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Miller, C.G.; Grossmann, J.; Szyrwiel, L.; Schwecke, T.; Demichev, V.; Mikecin Drazic, A.-M.; Mayakonda, A.; Lutsik, P.; Veith, C.; et al. The GAPDH redox switch safeguards reductive capacity and enables survival of stressed tumour cells. Nature Metabolism 2023, 5, 660–676. [Google Scholar] [CrossRef]
- Fu, Z.-W.; Feng, Y.-R.; Gao, X.; Ding, F.; Li, J.-H.; Yuan, T.-T.; Lu, Y.-T. Salt stress-induced chloroplastic hydrogen peroxide stimulates pdTPI sulfenylation and methylglyoxal accumulation. Plant Cell 2023, 35, 1593–1616. [Google Scholar] [CrossRef]
- Hoque, T.S.; Uraji, M.; Ye, W.; Hossain, M.A.; Nakamura, Y.; Murata, Y. Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J. Plant Physiol. 2012, 169, 979–986. [Google Scholar] [CrossRef]
- Cosse, M.; Rehders, T.; Eirich, J.; Finkemeier, I.; Selinski, J. Cysteine oxidation as a regulatory mechanism of Arabidopsis plastidial NAD-dependent malate dehydrogenase. Physiol. Plant. 2024, 176, e14340. [Google Scholar] [CrossRef]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K. Sulfenome mining in Arabidopsis thaliana. Proceedings of the National Academy of Sciences 2014, 111, 11545–11550. [Google Scholar] [CrossRef]
- Yu, L.Q.; Dai, Z.Z.; Zhang, Y.T.; Iqbal, S.; Lu, S.P.; Guo, L.; Yao, X. Proteome-wide identification of S-sulfenylated cysteines reveals metabolic response to freezing stress after cold acclimation in <i>Brassica napus</i>. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Hino, Y.; Inada, T.; Yoshioka, M.; Yoshioka, H. NADPH oxidase-mediated sulfenylation of cysteine derivatives regulates plant immunity. J. Exp. Bot. 2024. [Google Scholar] [CrossRef]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biology 2017, 11, 192–204. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kadota, Y.; Sklenar, J.; Derbyshire, P.; Stransfeld, L.; Asai, S.; Ntoukakis, V.; Jones, Jonathan D. ; Shirasu, K.; Menke, F.; Jones, A.; et al. Direct Regulation of the NADPH Oxidase RBOHD by the PRR-Associated Kinase BIK1 during Plant Immunity. Mol. Cell 2014, 54, 43–55. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-Dependent Protein Kinases Regulate the Production of Reactive Oxygen Species by Potato NADPH Oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nature Reviews Immunology 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Chaouch, S.; Queval, G.; Noctor, G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. The Plant Journal 2012, 69, 613–627. [Google Scholar] [CrossRef]
- Siodmak, A.; Shahul Hameed, U.F.; Rayapuram, N.; Völz, R.; Boudsocq, M.; Alharbi, S.; Alhoraibi, H.; Lee, Y.-H.; Blilou, I.; Arold, S.T.; et al. Essential role of the CD docking motif of MPK4 in plant immunity, growth, and development. New Phytol. 2023, 239, 1112–1126. [Google Scholar] [CrossRef]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Doczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol.Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Kosetsu, K.; Matsunaga, S.; Nakagami, H.; Colcombet, J.; Sasabe, M.; Soyano, T.; Takahashi, Y.; Hirt, H.; Machida, Y. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell 2010, 22, 3778–3790. [Google Scholar] [CrossRef]
- Puerto-Galán, L.; Pérez-Ruiz, J.M.; Guinea, M.; Cejudo, F.J. The contribution of NADPH thioredoxin reductase C (NTRC) and sulfiredoxin to 2-Cys peroxiredoxin overoxidation in Arabidopsis thaliana chloroplasts. J. Exp. Bot. 2015, 66, 2957–2966. [Google Scholar] [CrossRef]
- Lee, E.S.; Kang, C.H.; Park, J.H.; Lee, S.Y. Physiological Significance of Plant Peroxiredoxins and the Structure-Related and Multifunctional Biochemistry of Peroxiredoxin 1. Antioxidants & Redox Signaling 2018, 28, 625–639. [Google Scholar] [CrossRef]
- Liu, X.P.; Liu, X.Y.; Zhang, J.; Xia, Z.L.; Liu, X.; Qin, H.J.; Wang, D.W. Molecular and functional characterization of sulfiredoxin homologs from higher plants. Cell Res. 2006, 16, 287–296. [Google Scholar] [CrossRef]
- Iglesias-Baena, I.; Barranco-Medina, S.; Lazaro-Payo, A.; Lopez-Jaramillo, F.J.; Sevilla, F.; Lazaro, J.J. Characterization of plant sulfiredoxin and role of sulphinic form of 2-Cys peroxiredoxin. J. Exp. Bot. 2010, 61, 1509–1521. [Google Scholar] [CrossRef]
- Barranco-Medina, S.; Lazaro, J.J.; Dietz, K.J. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009, 583, 1809–1816. [Google Scholar] [CrossRef]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two Enzymes in One: Two Yeast Peroxiredoxins Display Oxidative Stress-Dependent Switching from a Peroxidase to a Molecular Chaperone Function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jang, H.H.; Lee, J.R.; Sung, N.R.; Lee, H.B.; Lee, D.H.; Park, D.-J.; Kang, C.H.; Chung, W.S.; Lim, C.O.; et al. Oligomerization and chaperone activity of a plant 2-Cys peroxiredoxin in response to oxidative stress. Plant Sci. 2009, 177, 227–232. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; Seidel, T.; Laxa, M.; Nunes de Miranda, S.M.; Gärtner, F.; Ströher, E.; Kandlbinder, A.; Dietz, K.-J. Multiple Redox and Non-Redox Interactions Define 2-Cys Peroxiredoxin as a Regulatory Hub in the Chloroplast. Mol. Plant 2009, 2, 1273–1288. [Google Scholar] [CrossRef]
- Hassan, H.M. Exacerbation of superoxide radical formation by Paraquat. In Methods Enzymol; Academic Press, 1984; Volume 105, pp. 523–532. [Google Scholar]
- Cerveau, D.; Ouahrani, D.; Marok, M.A.; Blanchard, L.; Rey, P. Physiological relevance of plant 2-Cys peroxiredoxin overoxidation level and oligomerization status. Plant, Cell & Environment 2016, 39, 103–119. [Google Scholar] [CrossRef]
- Aran, M.; Caporaletti, D.; Senn, A.M.; Tellez de Iñon, M.T.; Girotti, M.R.; Llera, A.S.; Wolosiuk, R.A. ATP-dependent modulation and autophosphorylation of rapeseed 2-Cys peroxiredoxin. The FEBS Journal 2008, 275, 1450–1463. [Google Scholar] [CrossRef]
- Weits, D.A.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Hubberten, H.M.; Riegler, H.; Hoefgen, R.; Perata, P.; van Dongen, J.T.; Licausi, F. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nature Communications 2014, 5. [Google Scholar] [CrossRef]
- Weits, D.A.; Zhou, L.; Giuntoli, B.; Carbonare, L.D.; Iacopino, S.; Piccinini, L.; Lombardi, L.; Shukla, V.; Bui, L.T.; Novi, G.; et al. Acquisition of hypoxia inducibility by oxygen sensing N-terminal cysteine oxidase in spermatophytes. Plant, Cell & Environment 2023, 46, 322–338. [Google Scholar] [CrossRef]
- White, M.D.; Carbonare, L.D.; Puerta, M.L.; Lacopino, S.; Edwards, M.; Dunne, K.; Pires, E.; Levy, C.; McDonough, M.A.; Licausi, F.; et al. Structures of Arabidopsis thaliana oxygen-sensing plant cysteine oxidases 4 and 5 enable targeted manipulation of their activity. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 23140–23147. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Q.; Wu, G.; Wen, J.; Liao, S.; Xu, C. Molecular basis for cysteine oxidation by plant cysteine oxidases from Arabidopsis thaliana. J. Struct. Biol. 2021, 213, 107663. [Google Scholar] [CrossRef]
- Sriram, S.M.; Kim, B.Y.; Kwon, Y.T. The N-end rule pathway: emerging functions and molecular principles of substrate recognition. Nature Reviews Molecular Cell Biology 2011, 12, 735–747. [Google Scholar] [CrossRef]
- Hu, R.-G.; Sheng, J.; Qi, X.; Xu, Z.; Takahashi, T.T.; Varshavsky, A. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature 2005, 437, 981–986. [Google Scholar] [CrossRef]
- Dissmeyer, N. Conditional Protein Function via N-Degron Pathway-Mediated Proteostasis in Stress Physiology. In Annual Review of Plant Biology, Vol 70; Merchant, S.S., Ed.; Annual Review of Plant Biology; 2019; Volume 70, pp. 83–117. [Google Scholar]
- Giuntoli, B.; Perata, P. Group VII Ethylene Response Factors in Arabidopsis: Regulation and Physiological Roles. Plant Physiol. 2017, 176, 1143–1155. [Google Scholar] [CrossRef]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.; Perata, P.; van Dongen, J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 2011, 479, 419–U177. [Google Scholar] [CrossRef]
- White, M.D.; Kamps, J.J.A.G.; East, S.; Taylor Kearney, L.J.; Flashman, E. The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. J. Biol. Chem. 2018, 293, 11786–11795. [Google Scholar] [CrossRef]
- White, M.D.; Klecker, M.; Hopkinson, R.J.; Weits, D.A.; Mueller, C.; Naumann, C.; O’Neill, R.; Wickens, J.; Yang, J.; Brooks-Bartlett, J.C.; et al. Plant cysteine oxidases are dioxygenases that directly enable arginyl transferase-catalysed arginylation of N-end rule targets. Nature Communications 2017, 8, 14690. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The Many Facets of Hypoxia in Plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef]
- Kunkowska, A.B.; Fontana, F.; Betti, F.; Soeur, R.; Beckers, G.J.M.; Meyer, C.; De Jaeger, G.; Weits, D.A.; Loreti, E.; Perata, P. Target of rapamycin signaling couples energy to oxygen sensing to modulate hypoxic gene expression in <i>Arabidopsis</i>. Proceedings of the National Academy of Sciences 2023, 120, e2212474120. [Google Scholar] [CrossRef]
- Weits, D.A.; Kunkowska, A.B.; Kamps, N.C.W.; Portz, K.M.S.; Packbier, N.K.; Nemec Venza, Z.; Gaillochet, C.; Lohmann, J.U.; Pedersen, O.; van Dongen, J.T.; et al. An apical hypoxic niche sets the pace of shoot meristem activity. Nature 2019, 569, 714–717. [Google Scholar] [CrossRef]
- Weits, D.A.; van Dongen, J.T.; Licausi, F. Molecular oxygen as a signaling component in plant development. New Phytologist 2021, 229, 24–35. [Google Scholar] [CrossRef]
- Gibbs, D.J.; Tedds, H.M.; Labandera, A.M.; Bailey, M.; White, M.D.; Hartman, S.; Sprigg, C.; Mogg, S.L.; Osborne, R.; Dambire, C.; et al. Oxygen-dependent proteolysis regulates the stability of angiosperm polycomb repressive complex 2 subunit VERNALIZATION 2. Nature Communications 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Labandera, A.-M.; Tedds, H.M.; Bailey, M.; Sprigg, C.; Etherington, R.D.; Akintewe, O.; Kalleechurn, G.; Holdsworth, M.J.; Gibbs, D.J. The PRT6 N-degron pathway restricts VERNALIZATION 2 to endogenous hypoxic niches to modulate plant development. New Phytol. 2021, 229, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation-mediated pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641. [Google Scholar] [CrossRef]
- Kolšek, K.; Aponte-Santamaría, C.; Gräter, F. Accessibility explains preferred thiol-disulfide isomerization in a protein domain. Sci. Rep. 2017, 7, 9858. [Google Scholar] [CrossRef]
- Sun, M.A.; Wang, Y.; Zhang, Q.; Xia, Y.; Ge, W.; Guo, D. Prediction of reversible disulfide based on features from local structural signatures. BMC Genomics 2017, 18, 279. [Google Scholar] [CrossRef]
- Barinova, K.V.; Serebryakova, M.V.; Eldarov, M.A.; Kulikova, A.A.; Mitkevich, V.A.; Muronetz, V.I.; Schmalhausen, E.V. S-glutathionylation of human glyceraldehyde-3-phosphate dehydrogenase and possible role of Cys152-Cys156 disulfide bridge in the active site of the protein. Biochimica et Biophysica Acta (BBA) - General Subjects 2020, 1864, 129560. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.-c. Oxidative protein folding fidelity and redoxtasis in the endoplasmic reticulum. Trends Biochem. Sci. 2023, 48, 40–52. [Google Scholar] [CrossRef]
- Meyer, A.J.; Riemer, J.; Rouhier, N. Oxidative protein folding: state-of-the-art and current avenues of research in plants. New Phytol. 2019, 221, 1230–1246. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, Q.; Zhang, Y.; Huang, G.; Liang, X.; Wang, C.-c.; Wang, L.; Lu, D. Two protein disulfide isomerase subgroups work synergistically in catalyzing oxidative protein folding. Plant Physiol. 2021, 188, 241–254. [Google Scholar] [CrossRef]
- Wilkinson, B.; Gilbert, H.F. Protein disulfide isomerase. Biochim. Biophys. Acta 2004, 1699, 35–44. [Google Scholar] [CrossRef]
- Couturier, J.; Chibani, K.; Jacquot, J.P.; Rouhier, N. Cysteine-based redox regulation and signaling in plants. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Wolosiuk, R.A.; Schürmann, P. Thioredoxin and enzyme regulation. Trends Biochem. Sci. 1979, 4, 93–96. [Google Scholar] [CrossRef]
- Chibani, K.; Pucker, B.; Dietz, K.-J.; Cavanagh, A. Genome-wide analysis and transcriptional regulation of the typical and atypical thioredoxins in Arabidopsis thaliana. FEBS Lett. 2021, 595, 2715–2730. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Spoel, S.H. Thioredoxin-mediated redox signalling in plant immunity. Plant Sci. 2019, 279, 27–33. [Google Scholar] [CrossRef]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radical Biology and Medicine 2019, 140, 28–35. [Google Scholar] [CrossRef]
- Cejudo, F.J.; González, M.-C.; Pérez-Ruiz, J.M. Redox regulation of chloroplast metabolism. Plant Physiol. 2020, 186, 9–21. [Google Scholar] [CrossRef]
- da Fonseca-Pereira, P.; Souza, P.V.L.; Fernie, A.R.; Timm, S.; Daloso, D.M.; Araújo, W.L. Thioredoxin-mediated regulation of (photo)respiration and central metabolism. J. Exp. Bot. 2021, 72, 5987–6002. [Google Scholar] [CrossRef]
- Geissel, F.; Lang, L.; Husemann, B.; Morgan, B.; Deponte, M. Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction. Nature Communications 2024, 15, 1733. [Google Scholar] [CrossRef]
- Lemaire, S.D.; Michelet, L.; Zaffagnini, M.; Massot, V.; Issakidis-Bourguet, E. Thioredoxins in chloroplasts. Curr. Genet. 2007, 51, 343–365. [Google Scholar] [CrossRef]
- Dziubek, D.; Poeker, L.; Siemitkowska, B.; Graf, A.; Marino, G.; Alseekh, S.; Arrivault, S.; Fernie, A.R.; Armbruster, U.; Geigenberger, P. NTRC and thioredoxins m1/m2 underpin the light acclimation of plants on proteome and metabolome levels. Plant Physiol. 2023, 194, 982–1005. [Google Scholar] [CrossRef]
- Wolosiuk, R.A.; Buchanan, B.B. Regulation of chloroplast phosphoribulokinase by the ferredoxin/thioredoxin system. Arch. Biochem. Biophys. 1978, 189, 97–101. [Google Scholar] [CrossRef]
- Brandes, H.K.; Larimer, F.W.; Hartman, F.C. The molecular pathway for the regulation of phosphoribulokinase by thioredoxin f. J. Biol. Chem. 1996, 271, 3333–3335. [Google Scholar] [CrossRef]
- Sparla, F.; Pupillo, P.; Trost, P. The C-terminal Extension of Glyceraldehyde-3-phosphate Dehydrogenase Subunit B Acts as an Autoinhibitory Domain Regulated by Thioredoxins and Nicotinamide Adenine Dinucleotide*. J. Biol. Chem. 2002, 277, 44946–44952. [Google Scholar] [CrossRef]
- Chiadmi, M.; Navaza, A.; Miginiac-Maslow, M.; Jacquot, J.P.; Cherfils, J. Redox signalling in the chloroplast: structure of oxidized pea fructose-1,6-bisphosphate phosphatase. The EMBO Journal 1999, 18, 6809–6815. [Google Scholar] [CrossRef]
- Dunford, R.P.; Durrant, M.C.; Catley, M.A.; Dyer, T.A. Location of the redox-active cysteines in chloroplast sedoheptulose-1,7-bisphosphatase indicates that its allosteric regulation is similar but not identical to that of fructose-1,6-bisphosphatase. Photosynthesis Res. 1998, 58, 221–230. [Google Scholar] [CrossRef]
- Wedel, N.; Soll, J.; Paap, B.K. CP12 provides a new mode of light regulation of Calvin cycle activity in higher plants. Proc.Natl.Acad.Sci.U.S.A 1997, 94, 10479–10484. [Google Scholar] [CrossRef]
- Marri, L.; Trost, P.; Pupillo, P.; Sparla, F. Reconstitution and Properties of the Recombinant Glyceraldehyde-3-Phosphate Dehydrogenase/CP12/Phosphoribulokinase Supramolecular Complex of Arabidopsis. Plant Physiol. 2005, 139, 1433–1443. [Google Scholar] [CrossRef]
- Wolosiuk, R.A.; Buchanan, B.B. Thioredoxin and glutathione regulate photosynthesis in chloroplasts. Nature 1977, 266, 565–567. [Google Scholar] [CrossRef]
- Yoshida, K.; Hisabori, T. Determining the Rate-Limiting Step for Light-Responsive Redox Regulation in Chloroplasts. Antioxidants 2018, 7, 153. [Google Scholar] [CrossRef]
- Née, G.; Aumont-Nicaise, M.; Zaffagnini, M.; Nessler, S.; Valerio-Lepiniec, M.; Issakidis-Bourguet, E. Redox regulation of chloroplastic G6PDH activity by thioredoxin occurs through structural changes modifying substrate accessibility and cofactor binding. Biochem. J. 2013, 457, 117–125. [Google Scholar] [CrossRef]
- Moon, J.C.; Jang, H.H.; Chae, H.B.; Lee, J.R.; Lee, S.Y.; Jung, Y.J.; Shin, M.R.; Lim, H.S.; Chung, W.S.; Yun, D.-J.; et al. The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochemical and Biophysical Research Communications 2006, 348, 478–484. [Google Scholar] [CrossRef]
- Pérez-Ruiz, J.M.; Naranjo, B.; Ojeda, V.; Guinea, M.; Cejudo, F.J. NTRC-dependent redox balance of 2-Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proceedings of the National Academy of Sciences 2017, 114, 12069–12074. [Google Scholar] [CrossRef]
- Lampl, N.; Lev, R.; Nissan, I.; Gilad, G.; Hipsch, M.; Rosenwasser, S. Systematic monitoring of 2-Cys peroxiredoxin-derived redox signals unveiled its role in attenuating carbon assimilation rate. Proceedings of the National Academy of Sciences 2022, 119, e2119719119. [Google Scholar] [CrossRef]
- Ojeda, V.; Pérez-Ruiz, J.M.; Cejudo, F.J. 2-Cys Peroxiredoxins Participate in the Oxidation of Chloroplast Enzymes in the Dark. Mol. Plant 2018, 11, 1377–1388. [Google Scholar] [CrossRef]
- Gromes, R.; Hothorn, M.; Lenherr, E.D.; Rybin, V.; Scheffzek, K.; Rausch, T. The redox switch of gamma-glutamylcysteine ligase via a reversible monomer-dimer transition is a mechanism unique to plants. Plant J. 2008, 54, 1063–1075. [Google Scholar] [CrossRef]
- Marty, L.; Siala, W.; Schwarzländer, M.; Fricker, M.D.; Wirtz, M.; Sweetlove, L.J.; Meyer, Y.; Meyer, A.J.; Reichheld, J.-P.; Hell, R. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 9109–9114. [Google Scholar] [CrossRef]
- Marty, L.; Bausewein, D.; Muller, C.; Bangash, S.A.K.; Moseler, A.; Schwarzlander, M.; Muller-Schussele, S.J.; Zechmann, B.; Riondet, C.; Balk, J.; et al. Arabidopsis glutathione reductase 2 is indispensable in plastids, while mitochondrial glutathione is safeguarded by additional reduction and transport systems. New Phytol. 2019, 224, 1569–1584. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem.Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Aldini, G.; Carini, M.; Rossi, R.; Dalle-Donne, I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxidants & redox signaling 2005, 7, 930–939. [Google Scholar]
- Ercolani, L.; Scirè, A.; Galeazzi, R.; Massaccesi, L.; Cianfruglia, L.; Amici, A.; Piva, F.; Urbanelli, L.; Emiliani, C.; Principato, G.; et al. A possible S-glutathionylation of specific proteins by glyoxalase II: An in vitro and in silico study. Cell Biochemistry and Function 2016, 34, 620–627. [Google Scholar] [CrossRef]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel role for glutathione S-transferase Pi regulator of protein s-glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef]
- Mo, Z.; Huang, Y.; Pu, T.; Duan, L.; Pi, K.; Luo, J.; Long, B.; Lu, A.; Liu, R. Genome-wide identification and characterization of Glutathione S-Transferases (GSTs) and their expression profile under abiotic stresses in tobacco (Nicotiana tabacum L.). BMC Genomics 2023, 24, 341. [Google Scholar] [CrossRef]
- Bender, Kyle W. ; Wang, X.; Cheng, George B.; Kim, Hyoung S.; Zielinski, Raymond E.; Huber, Steven C. Glutaredoxin AtGRXC2 catalyses inhibitory glutathionylation of Arabidopsis BRI1-associated receptor-like kinase 1 (BAK1) in vitro. Biochem. J. 2015, 467, 399–413. [Google Scholar] [CrossRef]
- Mao, J.; Li, J. Regulation of Three Key Kinases of Brassinosteroid Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 4340. [Google Scholar] [CrossRef]
- van der Linde, K.; Gutsche, N.; Leffers, H.-M.; Lindermayr, C.; Müller, B.; Holtgrefe, S.; Scheibe, R. Regulation of plant cytosolic aldolase functions by redox modifications. Plant Physiology and Biochemistry 2011, 49, 946–957. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Couturier, J.; Gao, X.H.; Rouhier, N.; Trost, P.; Lemaire, S.D. Glutaredoxin S12: Unique Properties for Redox Signaling. Antioxidants & Redox Signaling 2012, 16, 17–32. [Google Scholar] [CrossRef]
- Peltoniemi, M.J.; Karala, A.-R.; Jurvansuu, J.K.; Kinnula, V.L.; Ruddock, L.W. Insights into deglutathionylation reactions: Different intermediates in the glutaredoxin and protein disulfide isomerase catalyzed reactions are defined by the γ-linkage present in glutathione. J. Biol. Chem. 2006, 281, 33107–33114. [Google Scholar] [CrossRef]
- Randi, E.B.; Zuhra, K.; Pecze, L.; Panagaki, T.; Szabo, C. Physiological concentrations of cyanide stimulate mitochondrial Complex IV and enhance cellular bioenergetics. Proc. Natl. Acad. Sci. U. S. A. 2021, 118. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Morisse, S.; Trost, P.; Lemaire, S.D. Redox Regulation in Photosynthetic Organisms: Focus on Glutathionylation. Antioxidants & Redox Signaling 2012, 16, 567–586. [Google Scholar] [CrossRef]
- Gurrieri, L.; Distefano, L.; Pirone, C.; Horrer, D.; Seung, D.; Zaffagnini, M.; Rouhier, N.; Trost, P.; Santelia, D.; Sparla, F. The thioredoxin-regulated α-amylase 3 of Arabidopsis thaliana is a target of S-glutathionylation. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Rouhier, N.; Couturier, J.; Jacquot, J.P. Genome-wide analysis of plant glutaredoxin systems. J. Exp. Bot. 2006, 57, 1685–1696. [Google Scholar] [CrossRef]
- He, X.Y.; Chen, W.Y.; Sun, X.C.; Gao, Y.; He, Y.R.; Xu, X.T.; Su, C.J.; Lv, Y.F.; Ren, B.Y.; Yin, H.Y.; et al. Genome-Wide Identification and Characterization of Glutaredoxin Family Genes in Common Wheat. Agronomy-Basel 2023, 13. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, S.P. New insights on thioredoxins (Trxs) and glutaredoxins (Grxs) by in silico amino acid sequence, phylogenetic and comparative structural analyses in organisms of three domains of life. Heliyon 2022, 8, e10776. [Google Scholar] [CrossRef]
- Bodnar, Y.; Gellert, M.; Hossain, F.M.; Lillig, C.H. Breakdown of Arabidopsis thaliana thioredoxins and glutaredoxins based on electrostatic similarity-Leads to common and unique interaction partners and functions. PLoS One 2023, 18. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Colombo, R.; Dalle-Donne, I. S-Glutathionylation: from redox regulation of protein functions to human diseases. Journal of Cellular and Molecular Medicine 2004, 8, 201–212. [Google Scholar] [CrossRef]
- Dixon, D.P.; Skipsey, M.; Grundy, N.M.; Edwards, R. Stress-Induced Protein S-glutathionylation in Arabidopsis. Plant Physiol. 2005, 138, 2233–2244. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Vanacker, H.; Le Marechal, P.; Marchand, C.; Schroda, M.; Lemaire, S.D.; Decottignies, P. In vivo targets of S-thiolation in Chlamydomonas reinhardtii. J. Biol. Chem. 2008, 283, 21571–21578. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Bedhomme, M.; Groni, H.; Marchand, C.H.; Puppo, C.; Gontero, B.; Cassier-Chauvat, C.; Decottignies, P.; Lemaire, S.D. Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: A proteomic survey. Mol. Cell. Proteomics 2012, 11. [Google Scholar] [CrossRef]
- Gietler, M.; Nykiel, M.; Orzechowski, S.; Fettke, J.; Zagdańska, B. Proteomic analysis of S-nitrosylated and S-glutathionylated proteins in wheat seedlings with different dehydration tolerances. Plant Physiology and Biochemistry 2016, 108, 507–518. [Google Scholar] [CrossRef]
- Müller-Schüssele, S.J.; Bohle, F.; Rossi, J.; Trost, P.; Meyer, A.J.; Zaffagnini, M. Plasticity in plastid redox networks: evolution of glutathione-dependent redox cascades and glutathionylation sites. BMC Plant Biol. 2021, 21, 322. [Google Scholar] [CrossRef]
- Kitajima, S.; Kurioka, M.; Yoshimoto, T.; Shindo, M.; Kanaori, K.; Tajima, K.; Oda, K. A cysteine residue near the propionate side chain of heme is the radical site in ascorbate peroxidase. FEBS J. 2008, 275, 470–480. [Google Scholar] [CrossRef]
- Seung, D.; Thalmann, M.; Sparla, F.; Abou Hachem, M.; Lee, S.K.; Issakidis-Bourguet, E.; Svensson, B.; Zeeman, S.C.; Santelia, D. Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic α-amylase. J. Biol. Chem. 2013, 288, 33620–33633. [Google Scholar] [CrossRef] [PubMed]
- Niazi, A.K.; Bariat, L.; Riondet, C.; Carapito, C.; Mhamdi, A.; Noctor, G.; Reichheld, J.-P. Cytosolic Isocitrate Dehydrogenase from Arabidopsis thaliana Is Regulated by Glutathionylation. Antioxidants (Basel) 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J. Exp. Bot. 2002, 53, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, J.; Hanson, A.D. Choline-O-sulfate biosynthesis in plants: Identification and partial characterization of a salinity-inducible choline sulfotransferase from species of Limonium (Plumbaginaceae). Plant-Physiol. 1994, 106, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Varin, L.; Marsolais, F.; Richard, M.; Rouleau, M. Sulfation and sulfotransferases 6: Biochemistry and molecular biology of plant sulfotransferases. FASEB J. 1997, 11, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.C.; Grootwassink, J.W.D.; Kolenovsky, A.D.; Underhill, E.W. Purification and properties of 3'-phosphoadenosine-5'-phosphosulfate desulfoglucosinolate sulfotransferase from Brassica juncea cell cultures. Phytochemistry 1990, 29, 1425–1428. [Google Scholar] [CrossRef]
- Yang, L.M.; Fernandez, M.D.; Lamppa, G.K. ACYL CARRIER PROTEIN (ACP) IMPORT INTO CHLOROPLASTS - COVALENT MODIFICATION BY A STROMAL HOLOACP SYNTHASE IS STIMULATED BY EXOGENOUSLY ADDED COA AND INHIBITED BY ADENOSINE 3',5'-BISPHOSPHATE. Eur. J. Biochem. 1994, 224, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Crisp, P.; McQuinn, R.; Pogson, B.J. Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond. Annu. Rev. Plant Biol. 2016, 67, 25–53. [Google Scholar] [CrossRef]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef]
- Chan, K.X.; Phua, S.Y.; Van Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef]
- Chan, K.X.; Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N.; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M.; et al. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E4567–E4576. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef]
- Lisjak, M.; Teklic, T.; Wilson, I.D.; Whiteman, M.; Hancock, J.T. Hydrogen sulfide: environmental factor or signalling molecule? Plant, Cell & Environment 2013, 36, 1607–1616. [Google Scholar] [CrossRef]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on Plant Functioning. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- González-Gordo, S.; Palma, J.M.; Corpas, F.J. Appraisal of H2S metabolism in Arabidopsis thaliana: In silico analysis at the subcellular level. Plant Physiology and Biochemistry 2020, 155, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, Y. Hydrogen Sulfide Signaling in Plants. Antioxidants & Redox Signaling 2023, 39, 40–58. [Google Scholar] [CrossRef]
- Moseler, A.; Dhalleine, T.; Rouhier, N.; Couturier, J. Arabidopsis thaliana 3-mercaptopyruvate sulfurtransferases interact with and are protected by reducing systems. J. Biol. Chem. 2021, 296, 100429. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Scuffi, D.; Blatt, M.R.; García-Mata, C. Hydrogen Sulfide Regulates Inward-Rectifying K+ Channels in Conjunction with Stomatal Closure. Plant Physiol. 2015, 168, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-Sulfhydration: A Cysteine Posttranslational Modification in Plant Systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, J.; Zhou, H.; Zhao, D.; Duan, T.; Wang, S.; Yuan, X.; Xie, Y. Hydrogen Sulfide-Linked Persulfidation Maintains Protein Stability of ABSCISIC ACID-INSENSITIVE 4 and Delays Seed Germination. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Yruela, I.; Gotor, C.; Bassham, D.C. Persulfidation of ATG18a regulates autophagy under ER stress in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Dóka, É.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Science Advances 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Macinkovic, I.; Devarie-Baez, N.O.; Pan, J.; Park, C.-M.; Carroll, K.S.; Filipovic, M.R.; Xian, M. Detection of Protein S-Sulfhydration by a Tag-Switch Technique. Angewandte Chemie International Edition 2014, 53, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Flores, A.; Romero, L.C.; Gotor, C. Label-Free Quantitative Proteomic Analysis of Nitrogen Starvation in Arabidopsis Root Reveals New Aspects of H2S Signaling by Protein Persulfidation. Antioxidants 2021, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Jurado-Flores, A.; Filipovic, M.R.; Gotor, C.; Romero, L.C. Detection of protein persulfidation in plants by the dimedone switch method. In Methods Enzymol.; Jez, J., Ed.; Academic Press, 2022; Volume 676, pp. 385–402. [Google Scholar]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2020, 72, 830–847. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W.; et al. Ethylene-Induced Hydrogen Sulfide Negatively Regulates Ethylene Biosynthesis by Persulfidation of ACO in Tomato Under Osmotic Stress. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Hu, Y.; Ma, Y.; Yi, Y.; Jia, H.; Li, F.; Sun, H.; Li, T.; Wang, X.; et al. Persulfidation maintains cytosolic G6PDs activity through changing tetrameric structure and competing cysteine sulfur oxidation under salt stress in Arabidopsis and tomato. New Phytol. 2023, 240, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Schneider, M.; Scheibe, R.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Regulates the Cytosolic/Nuclear Partitioning of Glyceraldehyde-3-Phosphate Dehydrogenase by Enhancing its Nuclear Localization. Plant Cell Physiol. 2017, 58, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.; Knuesting, J.; Berndt, C.; Morgan, B.; Scheibe, R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 2015, 396, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Feng, J.; Zhu, S. Biological Functions of Hydrogen Sulfide in Plants. Int. J. Mol. Sci. 2022, 23, 15107. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen Sulfide Positively Regulates Abscisic Acid Signaling through Persulfidation of SnRK2.6 in Guard Cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based Modification of Cysteine Desulfhydrase and the NADPH Oxidase RBOHD Controls Guard Cell Abscisic Acid Signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Flores, A.; Aroca, A.; Romero, L.C.; Gotor, C. Sulfide promotes tolerance to drought through protein persulfidation in Arabidopsis. J. Exp. Bot. 2023, 74, 4654–4669. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.-K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.-M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. The EMBO Journal 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Fukao, Y.; Fujiwara, M.; Umezawa, T.; Shinozaki, K.; Hibi, T.; Taniguchi, M.; Miyake, H.; Goto, Derek B. ; et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009, 424, 439–448. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Hou, Y.-J.; Zhao, Y.; Hsu, C.-C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.-P.; Zhu, J.-K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proceedings of the National Academy of Sciences 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: an indispensable combination for plant functioning. Trends Plant Sci. 2021, 26, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak, E.; Polkowska, Ż.; Narkowicz, S.; Namieśnik, J. Cyanides in the environment-analysis-problems and challenges. Environ. Sci. Pollut. Res. Int. 2017, 24, 15929–15948. [Google Scholar] [CrossRef] [PubMed]
- Yulvianti, M.; Zidorn, C. Chemical Diversity of Plant Cyanogenic Glycosides: An Overview of Reported Natural Products. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Zuhra, K.; Szabo, C. The two faces of cyanide: an environmental toxin and a potential novel mammalian gasotransmitter. FEBS J. 2022, 289, 2481–2515. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.M.; Moller, B.L. Cyanogenic Glycosides: Synthesis, Physiology, and Phenotypic Plasticity. In Annual Review of Plant Biology, Vol 65; Merchant, S.S., Ed.; Annual Review of Plant Biology; 2014; Volume 65, pp. 155–185. [Google Scholar]
- Dong, J.G.; Fernández-Maculet, J.C.; Yang, S.F. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proceedings of the National Academy of Sciences 1992, 89, 9789–9793. [Google Scholar] [CrossRef] [PubMed]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Westphal, L.; Schmotz, C.; Prade, E.; Scheel, D.; Glawischnig, E. The Multifunctional Enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) Converts Cysteine-Indole-3-Acetonitrile to Camalexin in the Indole-3-Acetonitrile Metabolic Network of <i>Arabidopsis thaliana</i>. Plant Cell 2009, 21, 1830–1845. [Google Scholar] [CrossRef]
- Hucklesby, D.P.; Dowling, M.J.; Hewitt, E.J. Cyanide formation from glyoxylate and hydroxylamine catalysed by extracts of higher-plant leaves. Planta 1982, 156, 487–491. [Google Scholar] [CrossRef]
- Álvarez, C.; García, I.; Romero, L.C.; Gotor, C. Mitochondrial Sulfide Detoxification Requires a Functional Isoform O-Acetylserine(thiol)lyase C in Arabidopsis thaliana. Mol. Plant 2012, 5, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Zidenga, T.; Siritunga, D.; Sayre, R.T. Cyanogen Metabolism in Cassava Roots: Impact on Protein Synthesis and Root Development. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E. Impact of Hydrogen Sulfide on Mitochondrial and Bacterial Bioenergetics. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Fasco, M.J.; Hauer, C.R.; Stack, R.F.; O'Hehir, C.; Barr, J.R.; Eadon, G.A. Cyanide Adducts with Human Plasma Proteins: Albumin as a Potential Exposure Surrogate. Chem. Res. Toxicol. 2007, 20, 677–684. [Google Scholar] [CrossRef] [PubMed]
- García, I.; Arenas-Alfonseca, L.; Moreno, I.; Gotor, C.; Romero, L.C. HCN Regulates Cellular Processes through Posttranslational Modification of Proteins by S-cyanylation. Plant Physiol. 2018, 179, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.J.; Kharasch, N. Derivatives of Sulfenic Acids. XXXVI. The Ionic Scission of the Sulfur-Sulfur Bond. 1 Part 1. J. Am. Chem. Soc. 1960, 82, 3071–3075. [Google Scholar] [CrossRef]
- Gawron, O.; Fernando, J. Kinetics of the cyanide-cystine reaction. J. Am. Chem. Soc. 1961, 83, 2906–2908. [Google Scholar] [CrossRef]
- Catsimpoolas, N.; Wood, J.L. Specific Cleavage of Cystine Peptides by Cyanide. J. Biol. Chem. 1966, 241, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yu, G.; Leeuwon, S.Z.; Liu, W.R. Site-Specific Conversion of Cysteine in a Protein to Dehydroalanine Using 2-Nitro-5-thiocyanatobenzoic Acid. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Arenas-Alfonseca, L.; Yamada, M.; Romero, L.C.; García, I. New Insights on the Role of ß-Cyanoalanine Synthase CAS-C1 in Root Hair Elongation through Single-Cell Proteomics. Plants 2023, 12, 4055. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: an update. J. Exp. Bot. 2017, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Hasinoff, B.B.; Igamberdiev, A.U.; Manac'h, N.; Rivoal, J.; Hill, R.D. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C.; Hasinoff, B.B.; Rivoal, J.; Hill, R.D. Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 2004, 219, 66–72. [Google Scholar] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide: A radical molecule with potential biotechnological applications in fruit ripening. J. Biotechnol. 2020, 324, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, I.; Lindermayr, C. Nitric oxide-based protein modification: formation and site-specificity of protein <i>S</i>-nitrosylation. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Dent, M.R.; DeMartino, A.W. Nitric oxide and thiols: Chemical biology, signalling paradigms and vascular therapeutic potential. Br. J. Pharmacol. n/a. [CrossRef]
- Suarez, S.A.; Muñoz, M.; Alvarez, L.; Venâncio, M.F.; Rocha, W.R.; Bikiel, D.E.; Marti, M.A.; Doctorovich, F. HNO Is Produced by the Reaction of NO with Thiols. J. Am. Chem. Soc. 2017, 139, 14483–14487. [Google Scholar] [CrossRef]
- Sánchez-Vicente, I.; Albertos, P.; Sanz, C.; Wybouw, B.; De Rybel, B.; Begara-Morales, J.C.; Chaki, M.; Mata-Pérez, C.; Barroso, J.B.; Lorenzo, O. Reversible S-nitrosylation of bZIP67 by peroxiredoxin IIE activity and nitro-fatty acids regulates the plant lipid profile. Cell Rep. 2024, 43, 114091. [Google Scholar] [CrossRef]
- Frungillo, L.; Skelly, M.J.; Loake, G.J.; Spoel, S.H.; Salgado, I. <i>S</i>-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nature Communications 2014, 5. [Google Scholar] [CrossRef]
- Jahnová, J.; Luhová, L.; Petrivalsky, M. S-Nitrosoglutathione Reductase-The Master Regulator of Protein S-Nitrosation in Plant NO Signaling. Plants-Basel 2019, 8. [Google Scholar] [CrossRef]
- Kalinina, E.; Novichkova, M. Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Sakamoto, A.; Ueda, M.; Morikawa, H. Arabidopsis glutathione-dependent formaldehyde dehydrogenase is an S-nitrosoglutathione reductase. FEBS Lett. 2002, 515, 20–24. [Google Scholar] [CrossRef]
- Xu, S.; Guerra, D.; Lee, U.; Vierling, E. S-nitrosoglutathione reductases are low-copy number, cysteine-rich proteins in plants that control multiple developmental and defense responses in Arabidopsis. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Cutrona, M.C.; Wienkoop, S.; Begara-Morales, J.C.; Sandal, N.; Orera, I.; Barroso, J.B.; Stougaard, J.; Becana, M. Altered Plant and Nodule Development and Protein <i>S</i>-Nitrosylation in <i>Lotus japonicus</i> Mutants Deficient in <i>S</i>-Nitrosoglutathione Reductases. Plant Cell Physiol. 2020, 61, 105–117. [Google Scholar] [CrossRef]
- Kovacs, I.; Holzmeister, C.; Wirtz, M.; Geerlof, A.; Frohlich, T.; Romling, G.; Kuruthukulangarakoola, G.T.; Linster, E.; Hell, R.; Arnold, G.J.; et al. ROS-mediated inhibition of S-nitrosoglutathione reductase contributes to the activation of anti-oxidative mechanisms. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Guerra, D.; Ballard, K.; Truebridge, I.; Vierling, E. S-Nitrosation of Conserved Cysteines Modulates Activity and Stability of <i>S</i>-Nitrosoglutathione Reductase (GSNOR). Biochemistry 2016, 55, 2452–2464. [Google Scholar] [CrossRef]
- Tichá, T.; Lochman, J.; Činčalová, L.; Luhová, L.; Petřivalský, M. Redox regulation of plant S-nitrosoglutathione reductase activity through post-translational modifications of cysteine residues. Biochemical and Biophysical Research Communications 2017, 494, 27–33. [Google Scholar] [CrossRef]
- Stomberski, C.T.; Anand, P.; Venetos, N.M.; Hausladen, A.; Zhou, H.L.; Premont, R.T.; Stamler, J.S. AKR1A1 is a novel mammalian <i>S</i>-nitroso-glutathione reductase. J. Biol. Chem. 2019, 294, 18285–18293. [Google Scholar] [CrossRef]
- Treffon, P.; Rossi, J.; Gabellini, G.; Trost, P.; Zaffagnini, M.; Vierling, E. Quantitative Proteome Profiling of a S-Nitrosoglutathione Reductase (GSNOR) Null Mutant Reveals a New Class of Enzymes Involved in Nitric Oxide Homeostasis in Plants. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Treffon, P.; Vierling, E. Focus on Nitric Oxide Homeostasis: Direct and Indirect Enzymatic Regulation of Protein Denitrosation Reactions in Plants. Antioxidants 2022, 11, 1411. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Gelineau, S.; Tada, Y.; Loake, G.J.; Spoel, S.H. Selective Protein Denitrosylation Activity of Thioredoxin-<i>h</i>5 Modulates Plant Immunity. Mol. Cell 2014, 56, 153–162. [Google Scholar] [CrossRef]
- Sunico, C.R.; Sultan, A.; Nakamura, T.; Dolatabadi, N.; Parker, J.; Shan, B.; Han, X.M.; Yates, J.R.; Masliah, E.; Ambasudhan, R.; et al. Role of sulfiredoxin as a peroxiredoxin-2 denitrosylase in human iPSC-derived dopaminergic neurons. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E7564–E7571. [Google Scholar] [CrossRef]
- Ren, X.; Sengupta, R.; Lu, J.; Lundberg, J.O.; Holmgren, A. Characterization of mammalian glutaredoxin isoforms as S-denitrosylases. FEBS Lett. 2019, 593, 1799–1806. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Morisse, S.; Bedhomme, M.; Marchand, C.H.; Festa, M.; Rouhier, N.; Lemaire, S.D.; Trost, P. Mechanisms of Nitrosylation and Denitrosylation of Cytoplasmic Glyceraldehyde-3-phosphate Dehydrogenase from Arabidopsis thaliana*. J. Biol. Chem. 2013, 288, 22777–22789. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, M.; Chen, T.; Zhang, L.; Fan, L.; Zhang, W.; Wei, B.; Li, S.; Xuan, W.; Noctor, G.; et al. Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant, Cell & Environment 2020, 43, 1175–1191. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef]
- Lindermayr, C.; Saalbach, G.; Durner, J.r. Proteomic Identification of S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, X.; Chen, L.; Sun, X.; Lu, C.; Zhang, L.; Wang, Y.; Zuo, J. Site-Specific Nitrosoproteomic Identification of Endogenously S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.S.; Xu, G.; Stancevic, B.; Jaffrey, S.R. Nitrosothiol Reactivity Profiling Identifies S-Nitrosylated Proteins with Unexpected Stability. Chem. Biol. 2008, 15, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, R.; Stuehr, D.J. Thioredoxin-1 Regulates Cellular Heme Insertion by Controlling S-Nitrosation of Glyceraldehyde-3-phosphate Dehydrogenase*. J. Biol. Chem. 2012, 287, 16179–16186. [Google Scholar] [CrossRef] [PubMed]
- Serrato, A.J.; Romero-Puertas, M.C.; Lázaro-Payo, A.; Sahrawy, M. Regulation by S-nitrosylation of the Calvin-Benson cycle fructose-1,6-bisphosphatase in Pisum sativum. Redox Biology 2018, 14, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Kolbert, Z.; Durner, J.; Lindermayr, C.; Corpas, F.J.; Brouquisse, R.; Barroso, J.B.; Umbreen, S.; Palma, J.M.; Hancock, J.T.; et al. Regulating the regulator: nitric oxide control of post-translational modifications. New Phytol. 2020, 227, 1319–1325. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Rahim, W.; Khan, M.; Lee, D.S.; Lee, G.M.; Al Azzawi, T.N.I.; Hussain, A.; Kim, C.K.; Yun, B.W. Phytohormonal Regulation Through Protein S-Nitrosylation Under Stress. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Dua, Y.Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.J.; Zhu, X.H.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Bowling, S.A.; Gordon, A.S.; Dong, X. Characterization of an Arabidopsis Mutant That Is Nonresponsive to Inducers of Systemic Acquired Resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.; apos; Amick, M. ; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Despres, C. The Arabidopsis NPR1 Protein Is a Receptor for the Plant Defense Hormone Salicylic Acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.H.; Dong, X.N. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science (New York, N.Y.) 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiology and Biochemistry 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Izbiańska, K.; Deckert, J. Products of lipid, protein and RNA oxidation as signals and regulators of gene expression in plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Alché, J.d.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biology 2019, 23, 101136. [Google Scholar] [CrossRef]
- Knieper, M.; Viehhauser, A.; Dietz, K.J. Oxylipins and Reactive Carbonyls as Regulators of the Plant Redox and Reactive Oxygen Species Network under Stress. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiology and Biochemistry 2012, 59, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J.i. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environmental and Experimental Botany 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.S.; Sugimoto, K. Reactive Carbonyl Species: A Missing Link in ROS Signaling. Plants (Basel) 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Vick, B.A.; Farmer, E.E. Dinor-oxo-phytodienoic acid: a new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 10473–10478. [Google Scholar] [CrossRef]
- Gosset, V.; Harmel, N.; Göbel, C.; Francis, F.; Haubruge, E.; Wathelet, J.P.; du Jardin, P.; Feussner, I.; Fauconnier, M.L. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009, 60, 1231–1240. [Google Scholar] [CrossRef]
- Gorshkov, V.Y.; Toporkova, Y.Y.; Tsers, I.D.; Smirnova, E.O.; Ogorodnikova, A.V.; Gogoleva, N.E.; Parfirova, O.I.; Petrova, O.E.; Gogolev, Y.V. Differential modulation of the lipoxygenase cascade during typical and latent Pectobacterium atrosepticum infections. Ann. Bot. 2022, 129, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Mano, J. Lipid Peroxide-Derived Reactive Carbonyl Species as Mediators of Oxidative Stress and Signaling. Front. Plant Sci. 2021, 12, 720867. [Google Scholar] [CrossRef]
- Mano, J.i.; Kanameda, S.; Kuramitsu, R.; Matsuura, N.; Yamauchi, Y. Detoxification of Reactive Carbonyl Species by Glutathione Transferase Tau Isozymes. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J.i. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef]
- Yin, L.; Mano, J.i.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant. 2017, 161, 211–223. [Google Scholar] [CrossRef]
- Roach, T.; Stöggl, W.; Baur, T.; Kranner, I. Distress and eustress of reactive electrophiles and relevance to light stress acclimation via stimulation of thiol/disulphide-based redox defences. Free Radical Biology and Medicine 2018, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Mano, J. Lipid Peroxide-Derived Short-Chain Carbonyls Mediate Hydrogen Peroxide-Induced and Salt-Induced Programmed Cell Death in Plants. Plant Physiol. 2015, 168, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Ye, W.; Matsushima, D.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, S.; Mano, J.; Murata, Y. Reactive Carbonyl Species Mediate ABA Signaling in Guard Cells. Plant Cell Physiol 2016, 57, 2552–2563. [Google Scholar] [CrossRef]
- Islam, M.M.; Ye, W.; Matsushima, D.; Rhaman, M.S.; Munemasa, S.; Okuma, E.; Nakamura, Y.; Biswas, M.S.; Mano, J.; Murata, Y. Reactive Carbonyl Species Function as Signal Mediators Downstream of H2O2 Production and Regulate [Ca2+]cyt Elevation in ABA Signal Pathway in Arabidopsis Guard Cells. Plant Cell Physiol 2019, 60, 1146–1159. [Google Scholar] [CrossRef]
- Biswas, M.S.; Terada, R.; Mano, J. Inactivation of carbonyl-detoxifying enzymes by H2O2 is a trigger to increase carbonyl load for initiating programmed cell death in plants. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Mano, J.i.; Miyatake, F.; Hiraoka, E.; Tamoi, M. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 2009, 230, 639–648. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.J.; Berger, S. Reactive electrophilic oxylipins: Pattern recognition and signalling. Phytochemistry 2009, 70, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Tola, A.J.; Jaballi, A.; Missihoun, T.D. Protein Carbonylation: Emerging Roles in Plant Redox Biology and Future Prospects. Plants 2021, 10, 1451. [Google Scholar] [CrossRef]
- Carbone, D.L.; Doorn, J.A.; Kiebler, Z.; Petersen, D.R. Cysteine Modification by Lipid Peroxidation Products Inhibits Protein Disulfide Isomerase. Chem. Res. Toxicol. 2005, 18, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; Marcocci, L.; Das, D.; Wang, X.; Luo, H.; Zungu-Edmondson, M.; Suzuki, Y.J. Mechanism of protein decarbonylation. Free Radic. Biol. Med. 2013, 65, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Chételat, A.; Reymond, P.; Farmer, E.E. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. The Plant Journal 2004, 37, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Tola, A.J.; Missihoun, T.D. Iron Availability Influences Protein Carbonylation in Arabidopsis thaliana Plants. Int. J. Mol. Sci. 2023, 24, 9732. [Google Scholar] [CrossRef]
- Maynard, D.; Viehhauser, A.; Knieper, M.; Dreyer, A.; Manea, G.; Telman, W.; Butter, F.; Chibani, K.; Scheibe, R.; Dietz, K.-J. The In Vitro Interaction of 12-Oxophytodienoic Acid and Related Conjugated Carbonyl Compounds with Thiol Antioxidants. Biomolecules 2021, 11, 457. [Google Scholar] [CrossRef]
- Millar, A.H.; Leaver, C.J. The cytotoxic lipid peroxidation product, 4-hydroxy-2-nonenal, specifically inhibits decarboxylating dehydrogenases in the matrix of plant mitochondria. FEBS Lett. 2000, 481, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Oeste, C.L.; Pérez-Sala, D. Modification of cysteine residues by cyclopentenone prostaglandins: Interplay with redox regulation of protein function. Mass Spectrom. Rev. 2014, 33, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Knieper, M.; Vogelsang, L.; Guntelmann, T.; Sproß, J.; Gröger, H.; Viehhauser, A.; Dietz, K.-J. OPDAylation of Thiols of the Redox Regulatory Network In Vitro. Antioxidants 2022, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Schedl, A.; Lafleur, C.; Martinez Henao, J.; van Dam, N.M.; Rivoal, J.; Bede, J.C. Arabidopsis Transcriptomics Reveals the Role of Lipoxygenase2 (AtLOX2) in Wound-Induced Responses. Int. J. Mol. Sci. 2024, 25, 5898. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Molecular and Cellular Biology 2009, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, G.; Dörmann, P. Chloroplast Lipids and Their Biosynthesis. Annu. Rev. Plant Biol. 2019, 70, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, P.A. S-acylation in plants: an expanding field. Biochem. Soc. Trans. 2020, 48, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Smotrys, J.E.; Linder, M.E. Palmitoylation of Intracellular Signaling Proteins: Regulation and Function. Annu. Rev. Biochem. 2004, 73, 559–587. [Google Scholar] [CrossRef]
- Kumar, M.; Carr, P.; Turner, S.R. An atlas of Arabidopsis protein S-acylation reveals its widespread role in plant cell organization and function. Nat. Plants 2022, 8, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.F.; Feng, Y.; Chen, L.; Davis, N.G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 2002, 159, 23–28. [Google Scholar] [CrossRef]
- Hou, H.; John Peter, A.T.; Meiringer, C.; Subramanian, K.; Ungermann, C. Analysis of DHHC Acyltransferases Implies Overlapping Substrate Specificity and a Two-Step Reaction Mechanism. Traffic 2009, 10, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, D.A.; Meng, X.; Veit, M. S-Acylation of Proteins of Coronavirus and Influenza Virus: Conservation of Acylation Sites in Animal Viruses and DHHC Acyltransferases in Their Animal Reservoirs. Pathogens 2021, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, H.; Wu, J.; Huang, J.; Gao, Q.; Tang, D.; Cai, L.; Liao, Z.; Wang, Y.; Liu, X.; et al. Screening DHHCs of S-acylated proteins using an OsDHHC cDNA library and bimolecular fluorescence complementation in rice. The Plant Journal 2022, 110, 1763–1780. [Google Scholar] [CrossRef] [PubMed]
- Batistič, O. Genomics and Localization of the Arabidopsis DHHC-Cysteine-Rich Domain S-Acyltransferase Protein Family. Plant Physiol. 2012, 160, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, D.; Hemsley, P.A. Fats and function: protein lipid modifications in plant cell signalling. Curr. Opin. Plant Biol. 2017, 40, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, R.N.P.; Snijder, J.; van de Waterbeemd, M.; Schouten, A.; Granneman, J.; Heck, A.J.R.; Gros, P. Stochastic palmitoylation of accessible cysteines in membrane proteins revealed by native mass spectrometry. Nature Communications 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Li, E.; Feng, Q.N.; Zhao, X.Y.; Ge, F.R.; Zhang, Y.; Li, S. Protein palmitoylation is critical for the polar growth of root hairs in Arabidopsis. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef]
- Blaskovic, S.; Blanc, M.; van der Goot, F.G. What does S-palmitoylation do to membrane proteins? FEBS J. 2013, 280, 2766–2774. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.; Nagaraj, R. Interaction of Peptides Corresponding to Fatty Acylation Sites in Proteins with Model Membranes (∗). J. Biol. Chem. 1995, 270, 16749–16755. [Google Scholar] [CrossRef]
- Purushotham, P.; Ho, R.; Zimmer, J. Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science 2020, 369, 1089–1094. [Google Scholar] [CrossRef]
- Kumar, M.; Wightman, R.; Atanassov, I.; Gupta, A.; Hurst, C.H.; Hemsley, P.A.; Turner, S. S-Acylation of the cellulose synthase complex is essential for its plasma membrane localization. Science 2016, 353, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Beckmann, L.; Kudla, J.; Batistic, O. N-terminal S-acylation facilitates tonoplast targeting of the calcium sensor CBL6. FEBS Lett. 2017, 591, 3745–3756. [Google Scholar] [CrossRef]
- Saito, S.; Hamamoto, S.; Moriya, K.; Matsuura, A.; Sato, Y.; Muto, J.; Noguchi, H.; Yamauchi, S.; Tozawa, Y.; Ueda, M.; et al. N-myristoylation and S-acylation are common modifications of Ca2+-regulated Arabidopsis kinases and are required for activation of the SLAC1 anion channel. New Phytol. 2018, 218, 1504–1521. [Google Scholar] [CrossRef]
- Fan, R.; Zhao, F.; Gong, Z.; Chen, Y.; Yang, B.; Zhou, C.; Zhang, J.; Du, Z.; Wang, X.; Yin, P.; et al. Insights into the mechanism of phospholipid hydrolysis by plant non-specific phospholipase C. Nature Communications 2023, 14, 194. [Google Scholar] [CrossRef]
- Ali, U.; Lu, S.; Fadlalla, T.; Iqbal, S.; Yue, H.; Yang, B.; Hong, Y.; Wang, X.; Guo, L. The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog. Lipid Res. 2022, 86, 101158. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, K.; Jin, X.; Yan, J.; Lu, S.; Shen, Q.; Guo, L.; Hong, Y.; Wang, X.; Guo, L. Acylation of non-specific phospholipase C4 determines its function in plant response to phosphate deficiency. The Plant Journal 2021, 106, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Dekker, F.J.; Rocks, O.; Vartak, N.; Menninger, S.; Hedberg, C.; Balamurugan, R.; Wetzel, S.; Renner, S.; Gerauer, M.; Schölermann, B.; et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 2010, 6, 449–456. [Google Scholar] [CrossRef]
- Lin, D.T.S.; Conibear, E. ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife 2015, 4, e11306. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Li, Y.; Chen, Z.; Zhuge, C.; Ouyang, Y.; Zhao, Y.; Lin, Y.; Xie, Q.; Yang, C.; et al. An ABHD17-like hydrolase screening system to identify de-S-acylation enzymes of protein substrates in plant cells. Plant Cell 2021, 33, 3235–3249. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, R.; Zhu, F.; Zhang, Z.; Gou, L.; Wen, J.; Dong, J.; Wang, T. A Lipid-Anchored NAC Transcription Factor Is Translocated into the Nucleus and Activates Glyoxalase I Expression during Drought Stress. Plant Cell 2017, 29, 1748–1772. [Google Scholar] [CrossRef]
- Ji, T.; Zheng, L.H.; Wu, J.L.; Duan, M.; Liu, Q.W.; Liu, P.; Shen, C.; Liu, J.L.; Ye, Q.Y.; Wen, J.Q.; et al. The thioesterase APT1 is a bidirectional-adjustment redox sensor. Nature Communications 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses[OPEN]. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Chen, Z.; Huang, L.T.; Ouyang, Y.W.; Wang, Z.Y.; Wu, S.; Ye, W.X.; Yu, B.Y.; Zhang, Y.H.; Yang, C.W.; et al. Salicylic acid attenuates brassinosteroid signaling via protein de-S-acylation. EMBO J. 2023, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Casey, P.J. Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996, 65, 241–269. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat.Prod.Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef]
- Yalovsky, S. Protein prenylation CaaX processing in plants. In The Enzymes; Tamanoi, F., Hrycyna, C.A., Bergo, M.O., Eds.; Academic Press, 2011; Volume 29, pp. 163–182. [Google Scholar]
- Downes, B.P.; Saracco, S.A.; Lee, S.S.; Crowell, D.N.; Vierstra, R.D. MUBs, a Family of Ubiquitin-fold Proteins That Are Plasma Membrane-anchored by Prenylation*. J. Biol. Chem. 2006, 281, 27145–27157. [Google Scholar] [CrossRef]
- Bracha-Drori, K.; Shichrur, K.; Lubetzky, T.C.; Yalovsky, S. Functional analysis of Arabidopsis postprenylation CaaX processing enzymes and their function in subcellular protein targeting. Plant Physiol. 2008, 148, 119–131. [Google Scholar] [CrossRef]
- Hála, M.; Žárský, V. Protein prenylation in plant stress responses. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Running, M. The role of lipid post–translational modification in plant developmental processes. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Wilson, A.L.; Erdman, R.A.; Castellano, F.; Maltese, W.A. Prenylation of Rab8 GTPase by type I and type II geranylgeranyl transferases. Biochem. J. 1998, 333, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Stroh, S.; Eisenhaber, F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005, 6, R55. [Google Scholar] [CrossRef] [PubMed]
- Quinn, O.; Kumar, M.; Turner, S. The role of lipid-modified proteins in cell wall synthesis and signaling. Plant Physiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Pentose Phosphate Pathway Reactions in Photosynthesizing Cells. Cells 2021, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Hölscher, C.; Schwöppe, C.; von Schaewen, A. Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. The Plant Journal 2011, 66, 745–758. [Google Scholar] [CrossRef]
- Linnenbrügger, L.; Doering, L.; Lansing, H.; Fischer, K.; Eirich, J.; Finkemeier, I.; von Schaewen, A. Alternative splicing of Arabidopsis G6PD5 recruits NADPH-producing OPPP reactions to the endoplasmic reticulum. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Zhang, Y.-M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Kupke, T.; Hernández-Acosta, P.; Steinbacher, S.; Culiáñez-Macià, F.A. Arabidopsis thaliana Flavoprotein AtHAL3a Catalyzes the Decarboxylation of 4′-Phosphopantothenoylcysteine to 4′-Phosphopantetheine, a Key Step in Coenzyme A Biosynthesis*. J. Biol. Chem. 2001, 276, 19190–19196. [Google Scholar] [CrossRef]
- Kupke, T.; Hernández-Acosta, P.; Culiáñez-Macià, F.A. 4′-Phosphopantetheine and Coenzyme A Biosynthesis in Plants*. J. Biol. Chem. 2003, 278, 38229–38237. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Peak-Chew, S.Y.; Newell, C.; Miller-Aidoo, S.; Mangal, S.; Zhyvoloup, A.; Bakovic´, J.; Malanchuk, O.; Pereira, G.C.; Kotiadis, V.; et al. Protein CoAlation: a redox-regulated protein modification by coenzyme A in mammalian cells. Biochem. J. 2017, 474, 2489–2508. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Zhyvoloup, A.; Bakovic, J.; Thomas, N.; Yu, B.Y.K.; Das, S.; Orengo, C.; Newell, C.; Ward, J.; Saladino, G.; et al. Protein CoAlation and antioxidant function of coenzyme A in prokaryotic cells. Biochem. J. 2018, 475, 1909–1937. [Google Scholar] [CrossRef] [PubMed]
- Aloum, L.; Brimson, C.A.; Zhyvoloup, A.; Baines, R.; Baković, J.; Filonenko, V.; Thompson, C.R.L.; Gout, I. Coenzyme A and protein CoAlation levels are regulated in response to oxidative stress and during morphogenesis in Dictyostelium discoideum. Biochemical and Biophysical Research Communications 2019, 511, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Clares, R.A.; González-Segura, L.; Díaz-Sánchez, Á.G. Crystallographic evidence for active-site dynamics in the hydrolytic aldehyde dehydrogenases. Implications for the deacylation step of the catalyzed reaction. Chemico-Biological Interactions 2011, 191, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.N.; Haines, B.E.; Stauffacher, C.V.; Helquist, P.; Wiest, O. Computational Study of Base-Catalyzed Thiohemiacetal Decomposition in Pseudomonas mevalonii HMG-CoA Reductase. The Journal of Physical Chemistry B 2023, 127, 4931–4938. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Rathinasabapathi, B.; Rivoal, J.; Burnet, M.; Dillon, M.O.; Gage, D.A. Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc.Natl.Acad.Sci.U.S.A 1994, 91, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Zárate-Romero, A.; Murillo-Melo, Darío S. ; Mújica-Jiménez, C.; Montiel, C.; Muñoz-Clares, Rosario A. Reversible, partial inactivation of plant betaine aldehyde dehydrogenase by betaine aldehyde: mechanism and possible physiological implications. Biochem. J. 2016, 473, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, respiration and growth: A carbon and energy balancing act for alternative oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Umbach, A.L.; Siedow, J.N. The reaction of the soybean cotyledon mitochondrial cyanide-resistant oxidase with sulfhydryl reagents suggests that alpha-keto acid activation involves the formation of a thiohemiacetal. J. Biol. Chem. 1996, 271, 25019–25026. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Sweet, C.R.; Lennon, A.M.; Rauch, G.S.; Siedow, J.N. Regulation of the cyanide-resistant alternative oxidase of plant mitochondria -: Identification of the cysteine residue involved in α-keto acid stimulation and intersubunit disulfide bond formation. J. Biol. Chem. 1998, 273, 30750–30756. [Google Scholar] [CrossRef]
- Selinski, J.; Hartmann, A.; Kordes, A.; Deckers-Hebestreit, G.; Whelan, J.; Scheibe, R. Analysis of Posttranslational Activation of Alternative Oxidase Isoforms. Plant Physiol. 2017, 174, 2113–2127. [Google Scholar] [CrossRef]
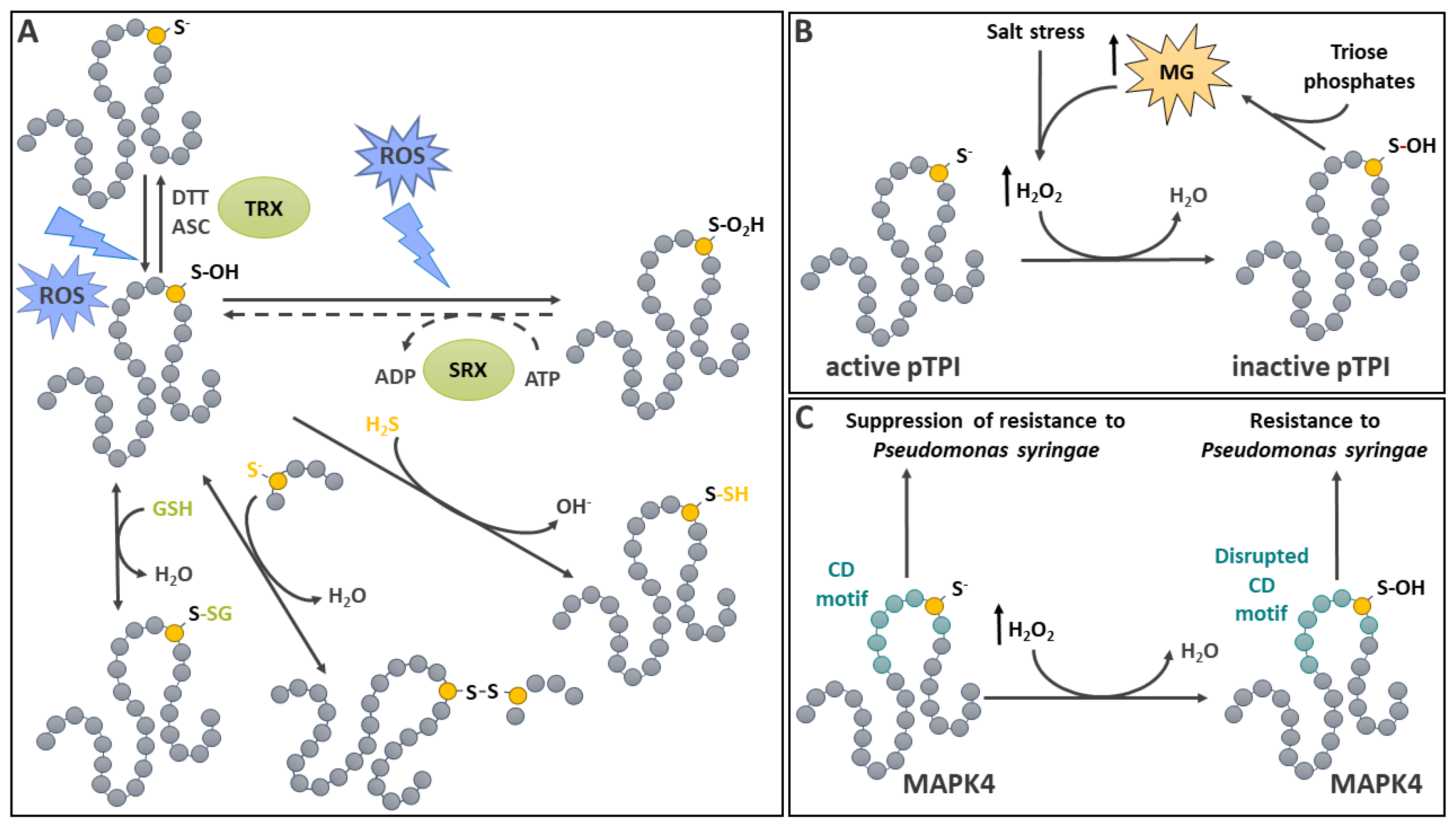

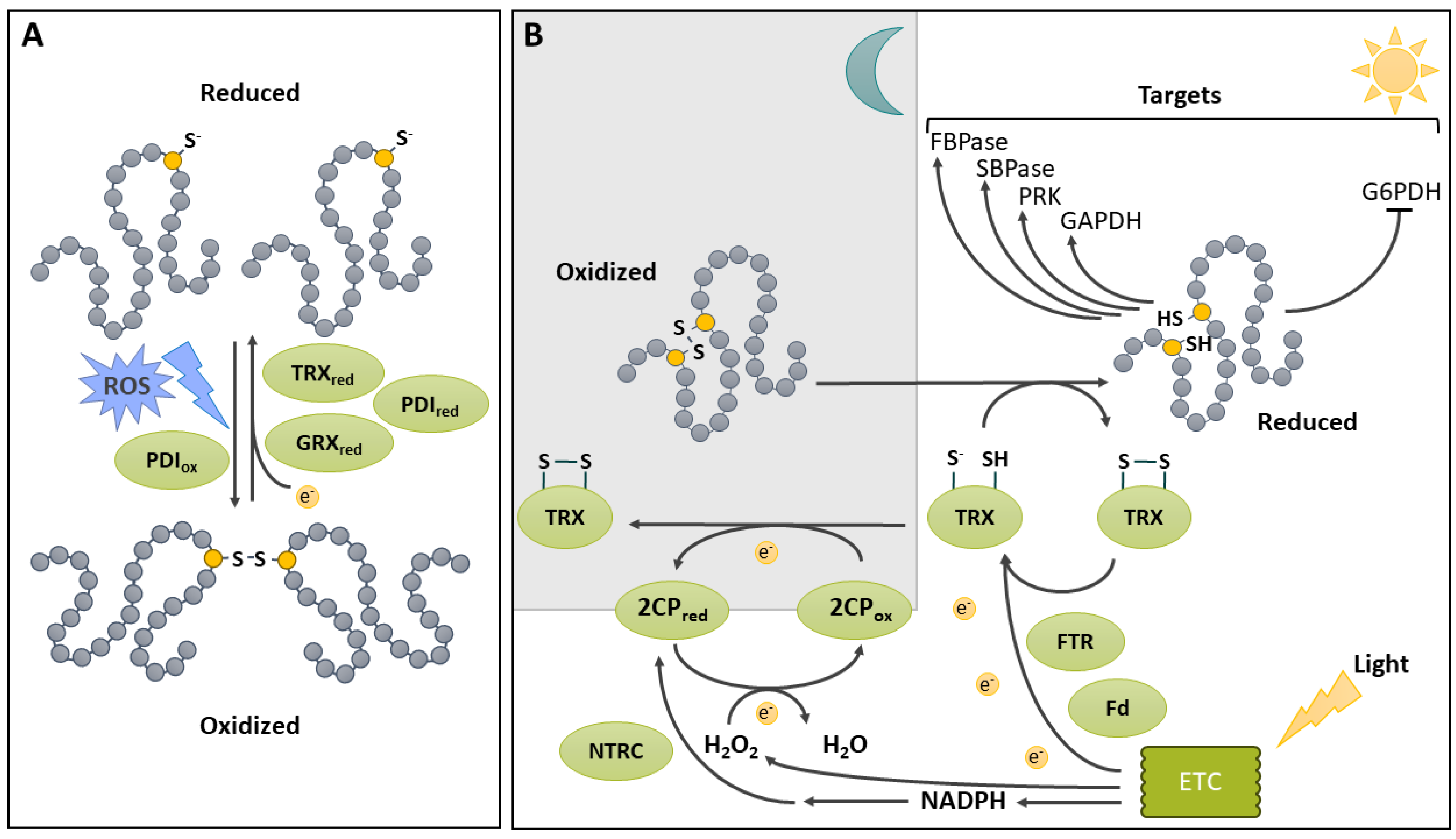
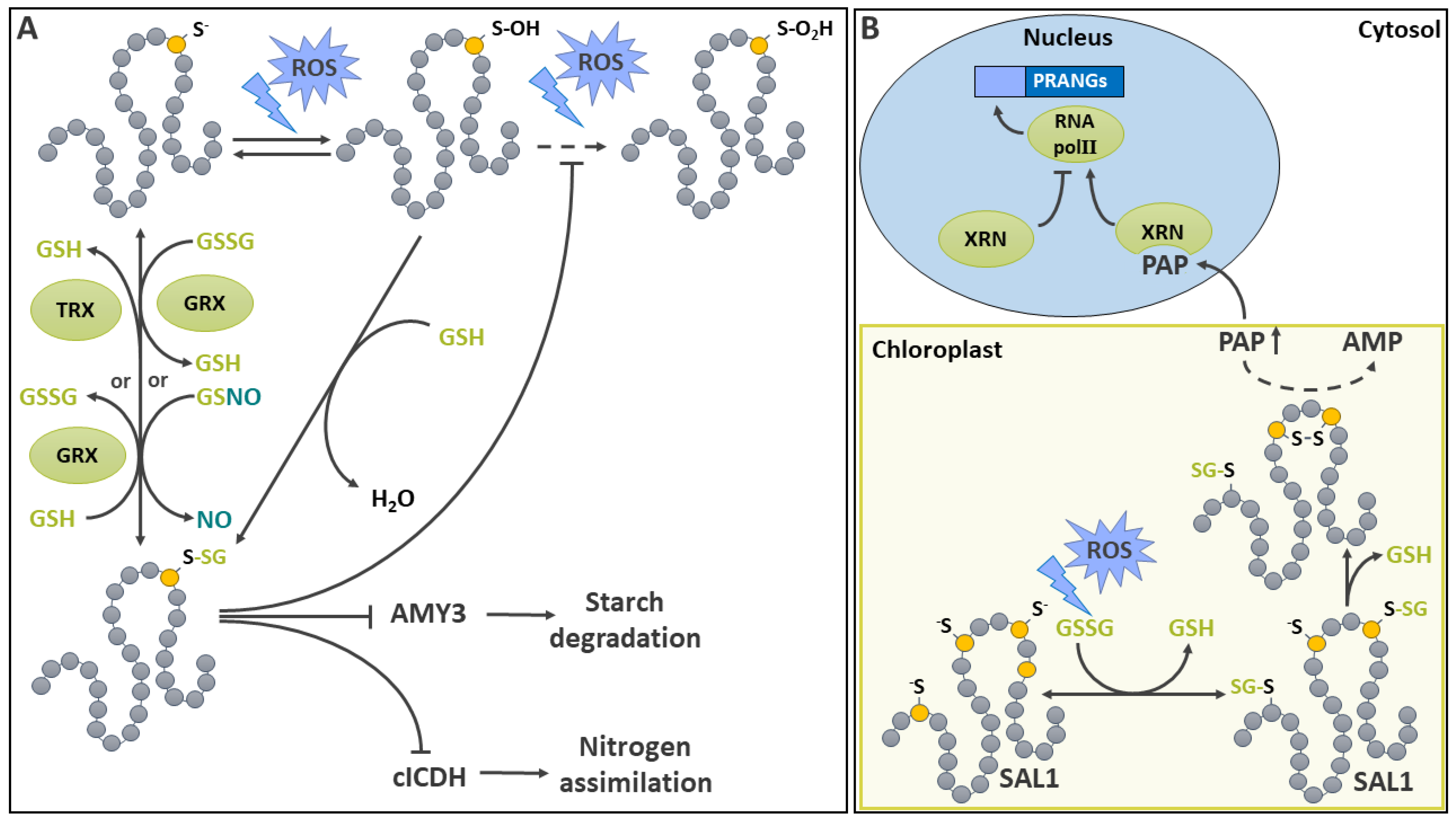
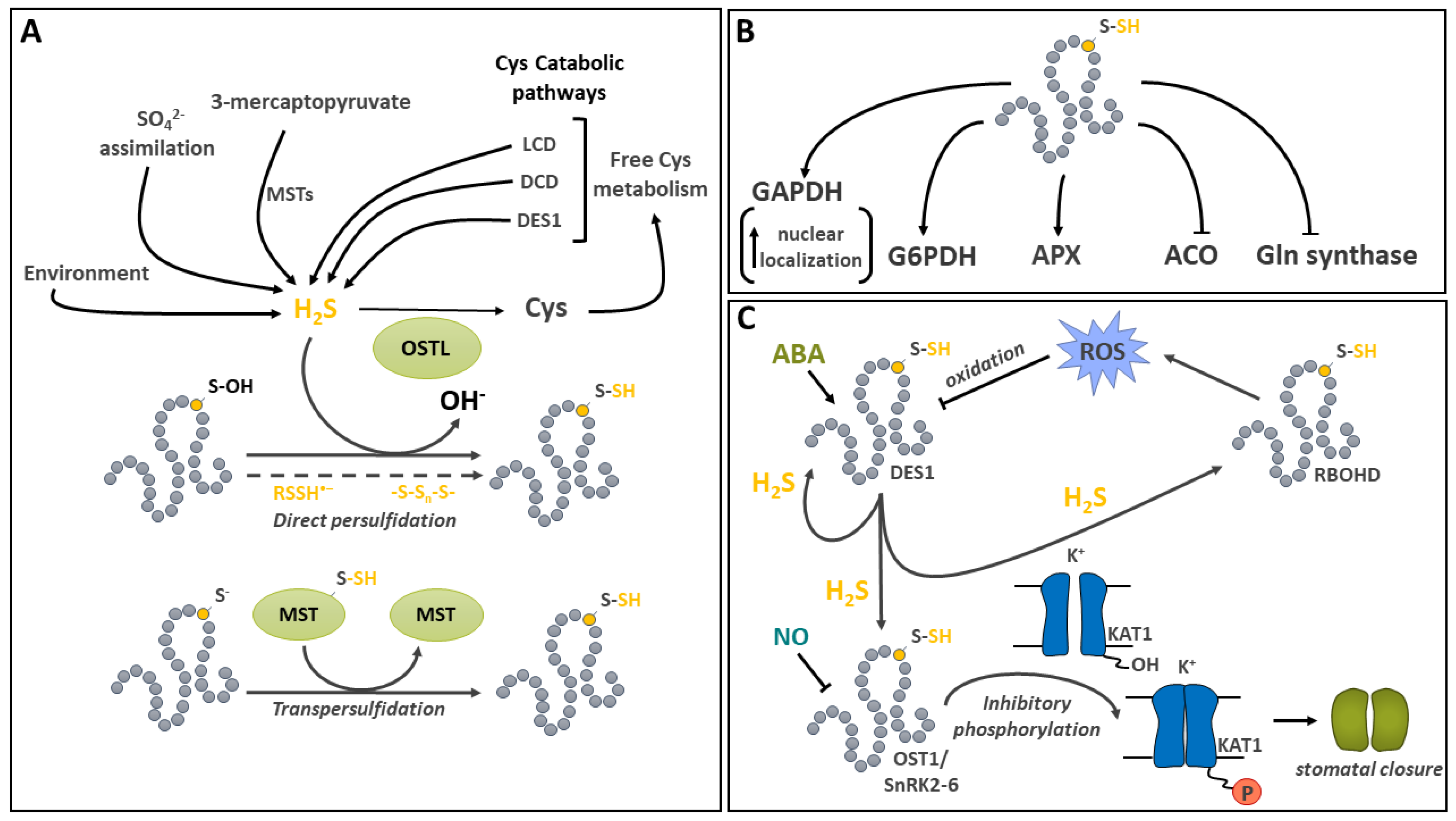
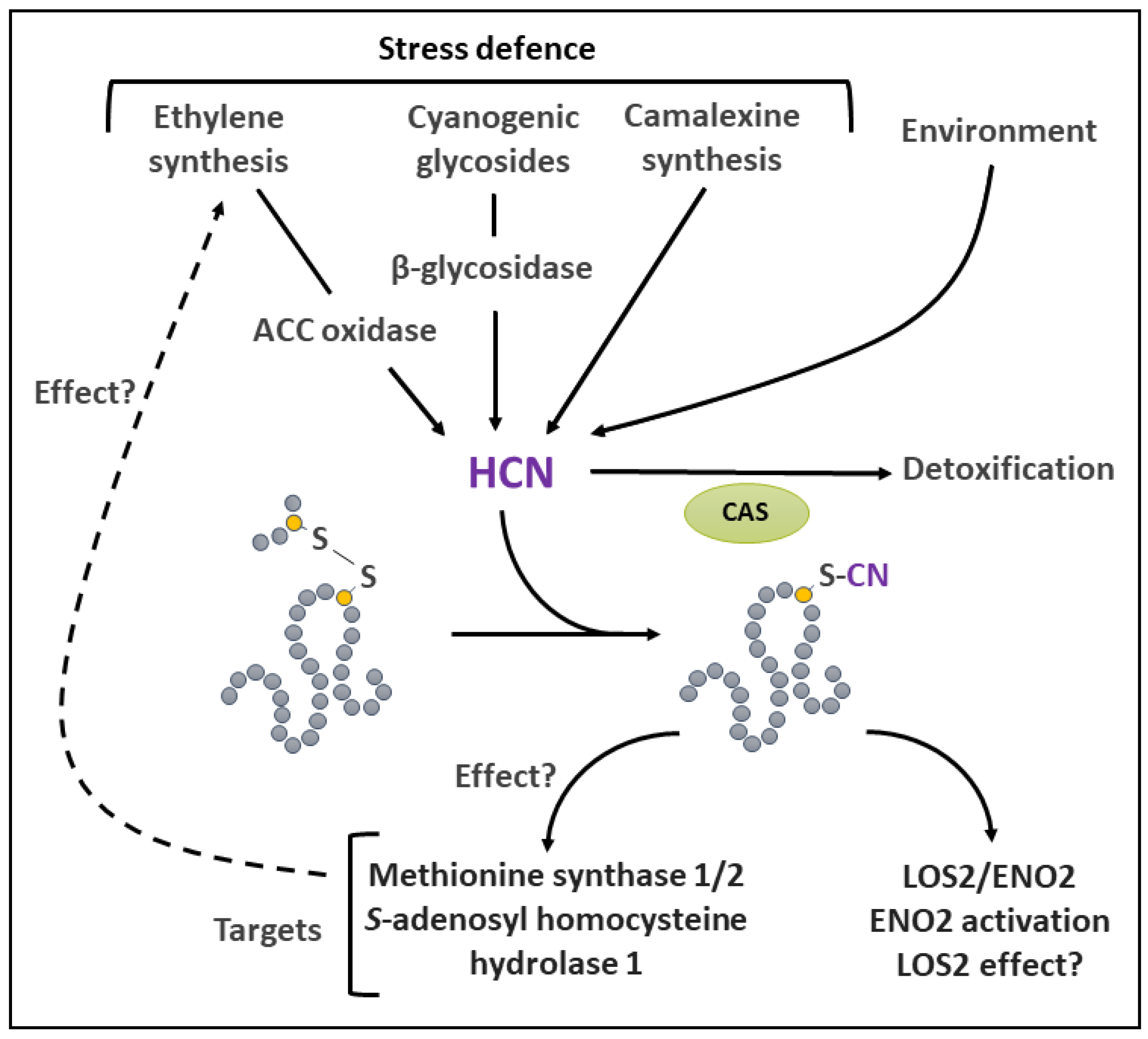

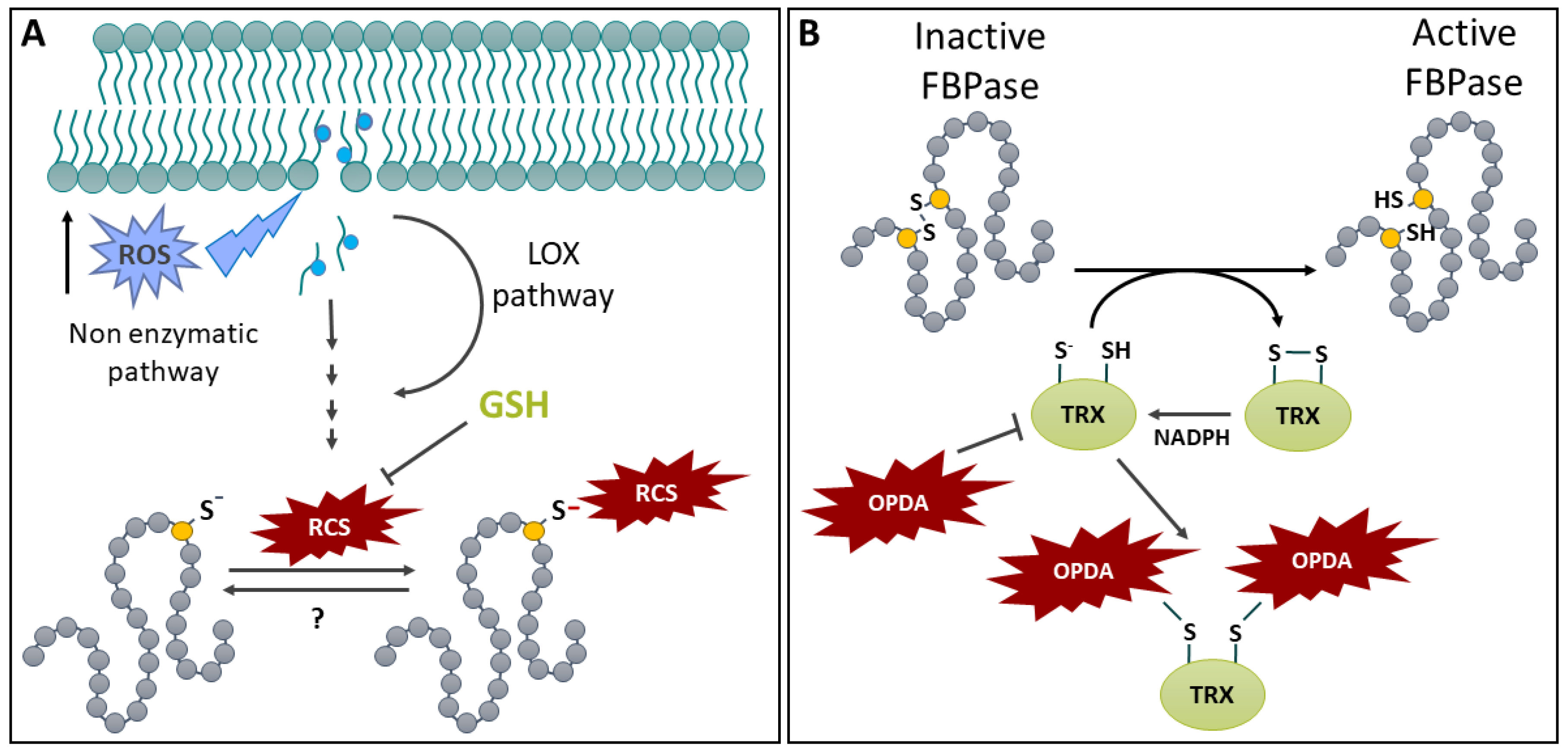
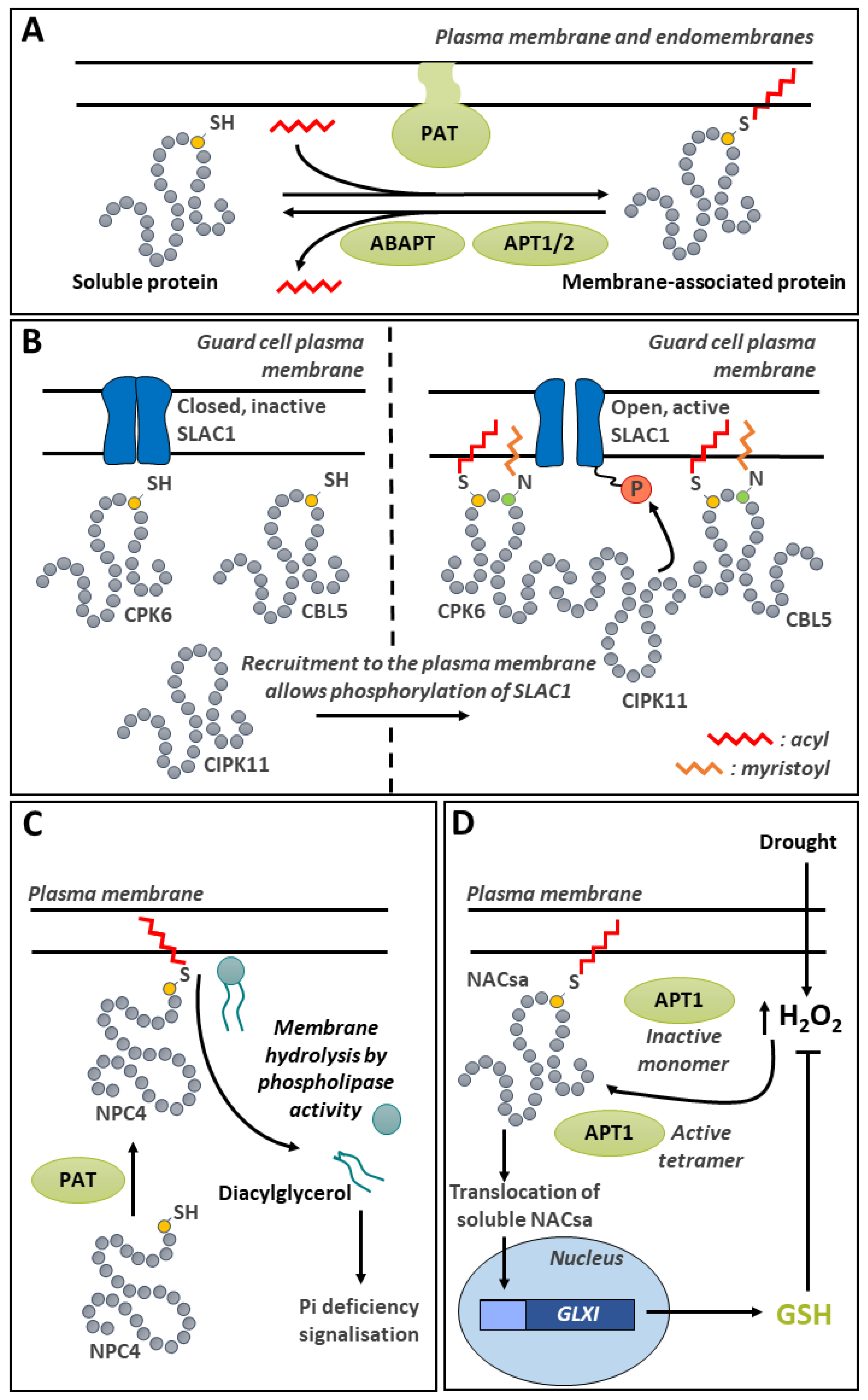
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




