Submitted:
07 August 2024
Posted:
07 August 2024
You are already at the latest version
Abstract
Keywords:
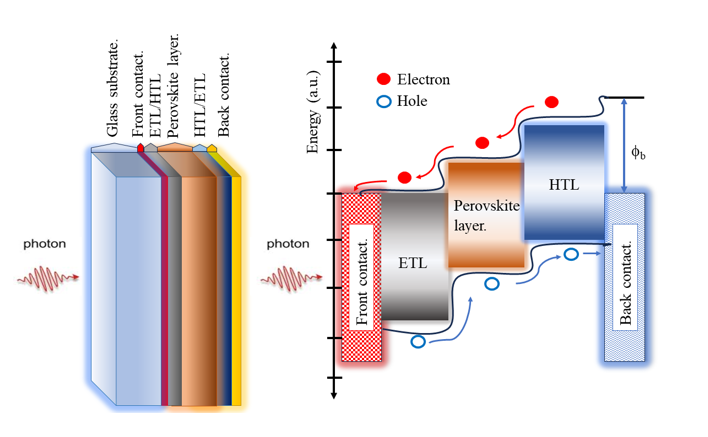
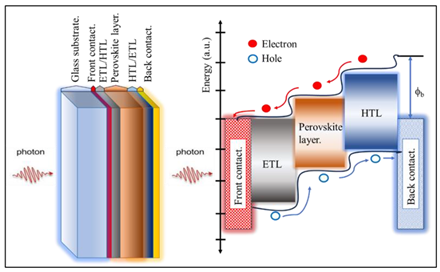 Graphical abstract.
Graphical abstract.
1. Introduction
2. Materials and Methods
2.1. Chemicals Used in Experimental Work
2.2. Substrates’ Cleaning
2.3. Preparation of CuO Thin Films
2.4. Characterizations
3. Results and Discussion
3.1. Thin Film Thickness Measurement
3.2. XRD
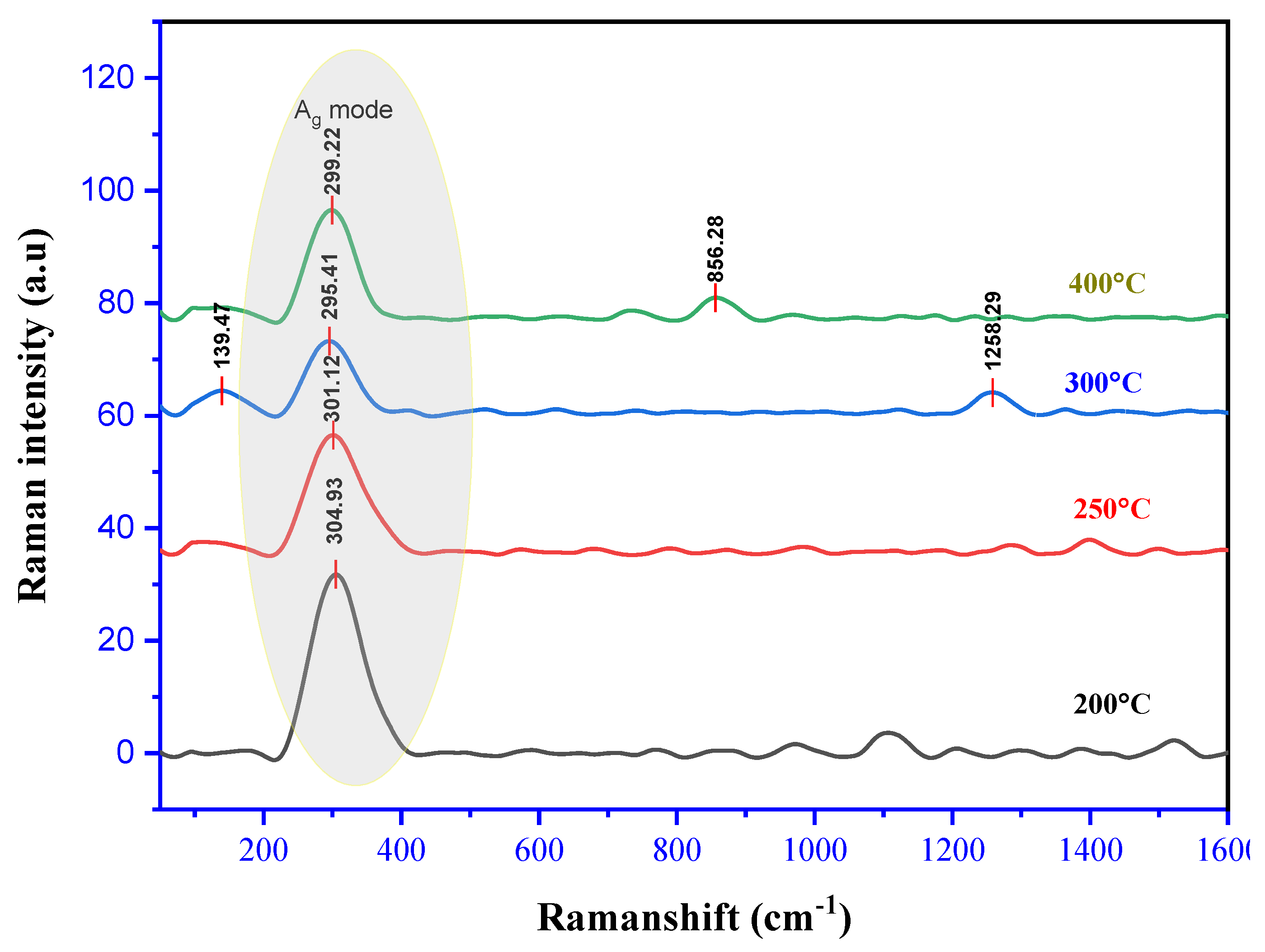
3.3. Raman Spectra
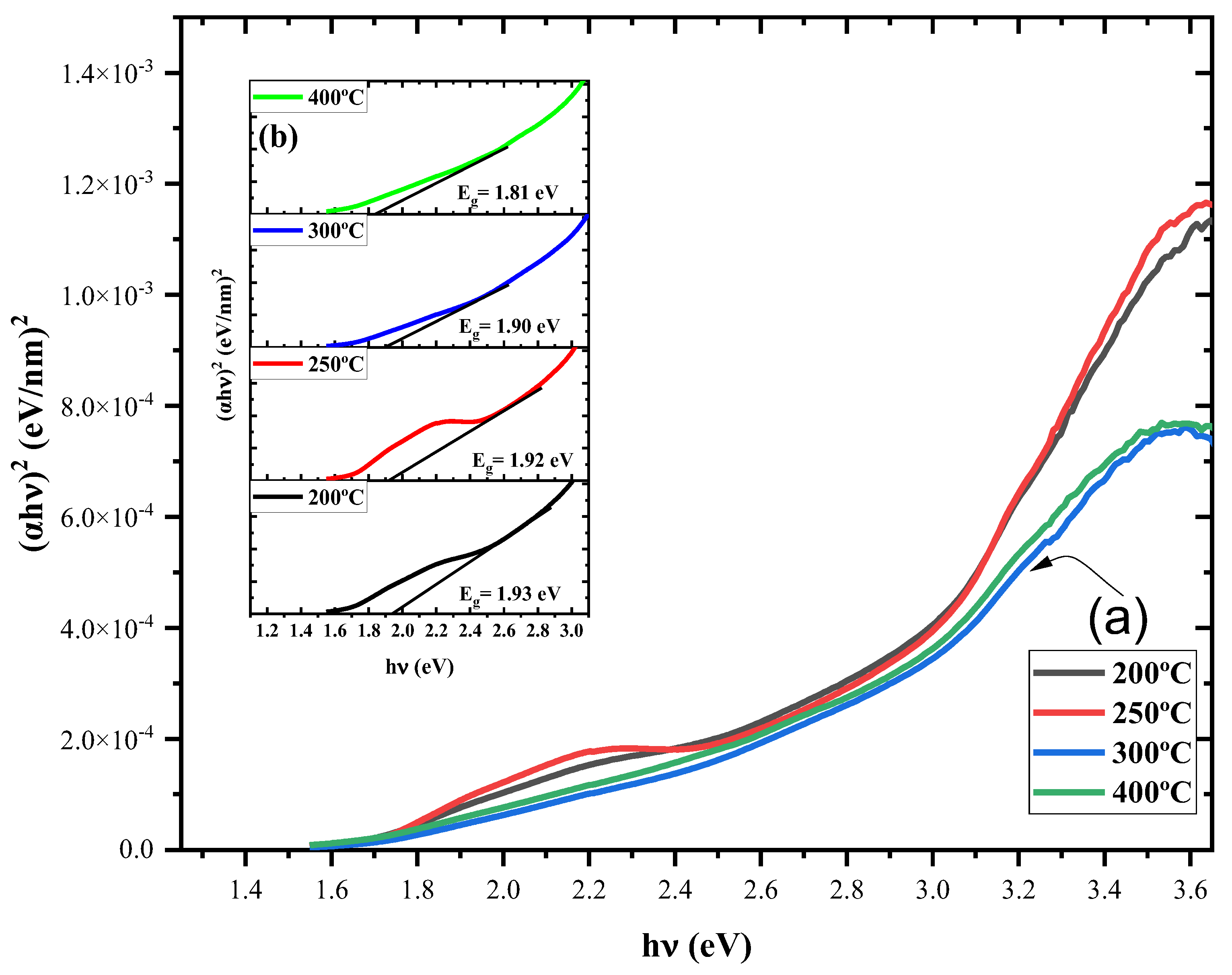
3.4. Ultra Violet – Visible Spectroscopy
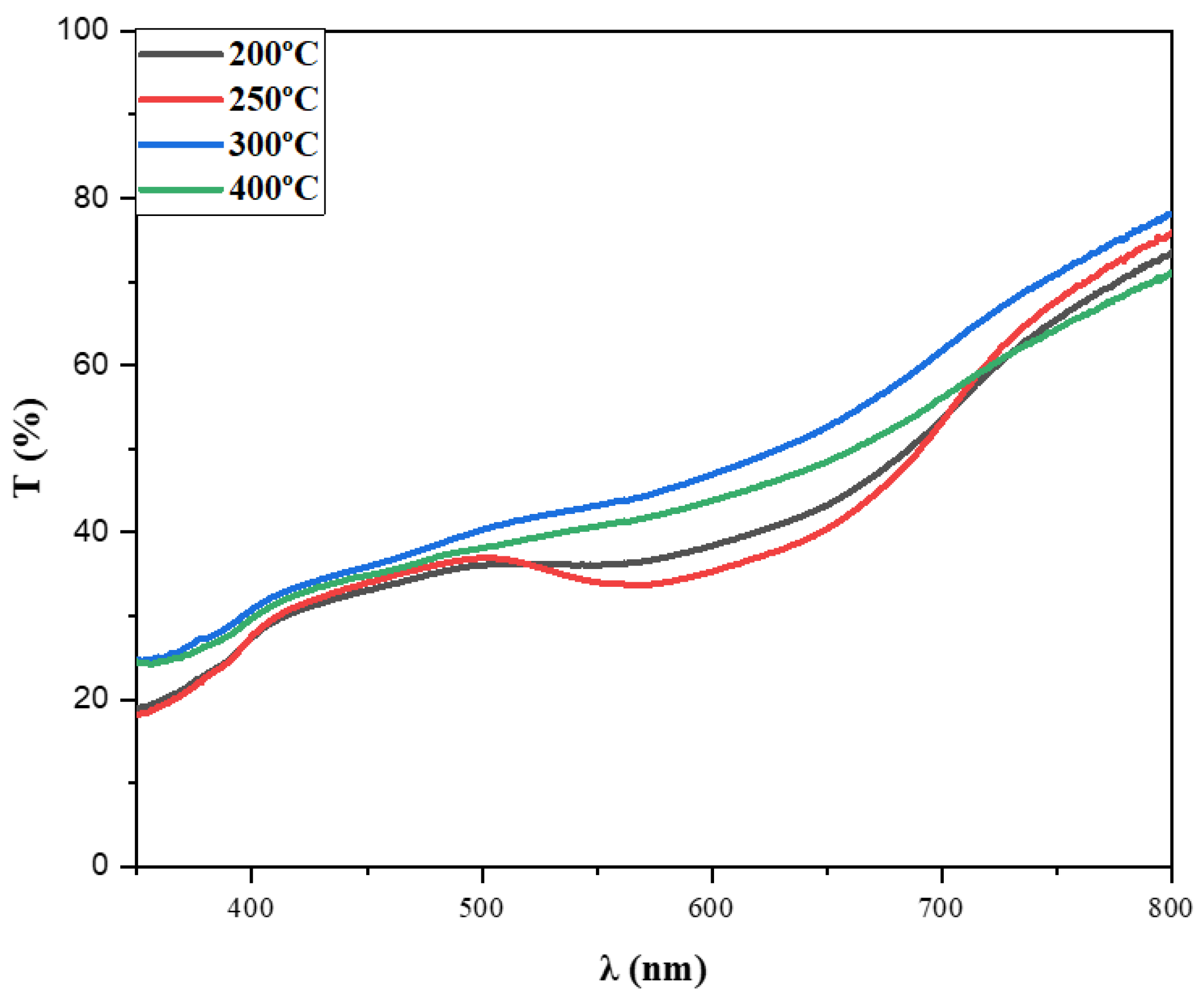
3.4.1. Transmission Spectra
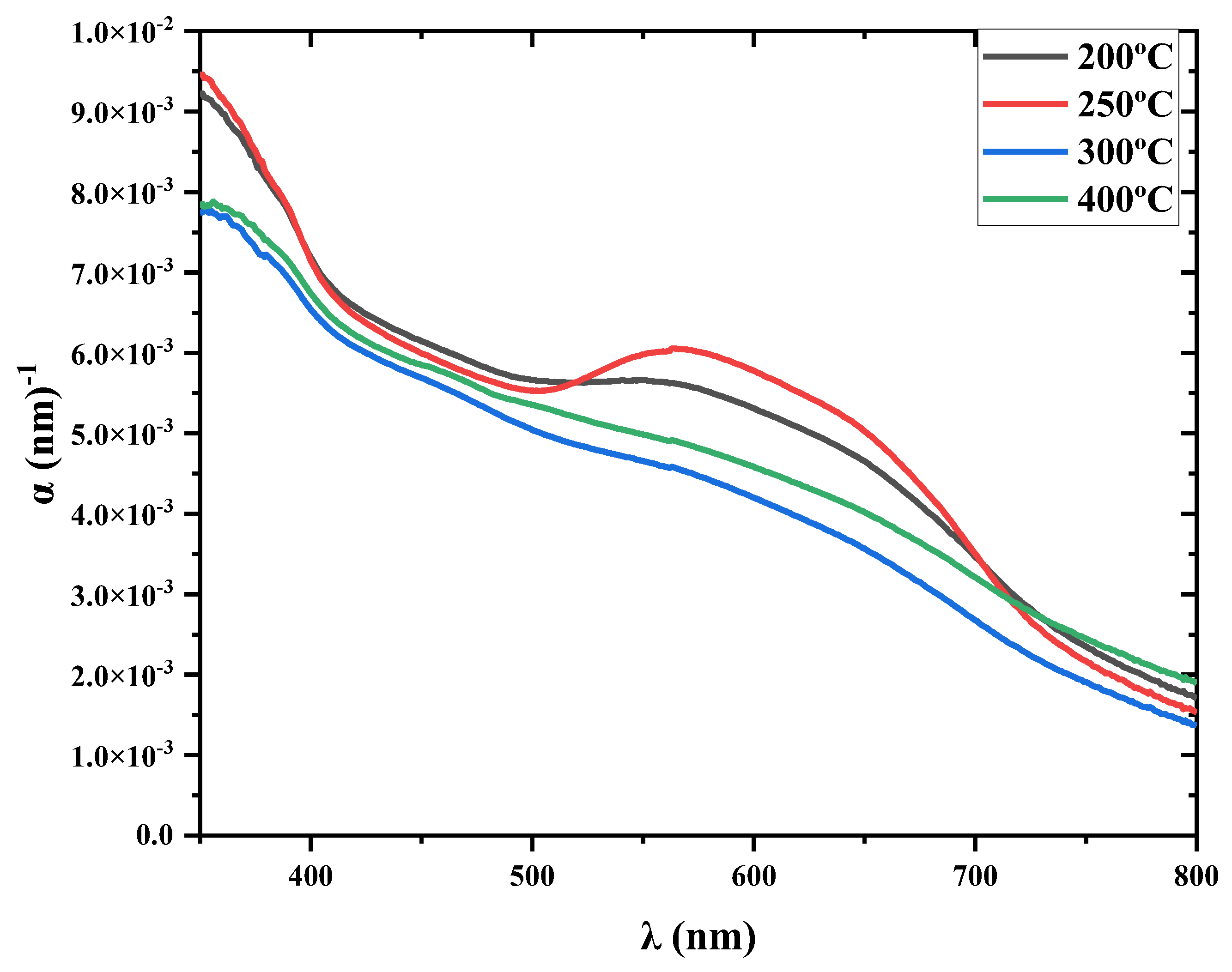
3.4.2. Absorption Coefficient
3.4.3. Bandgap
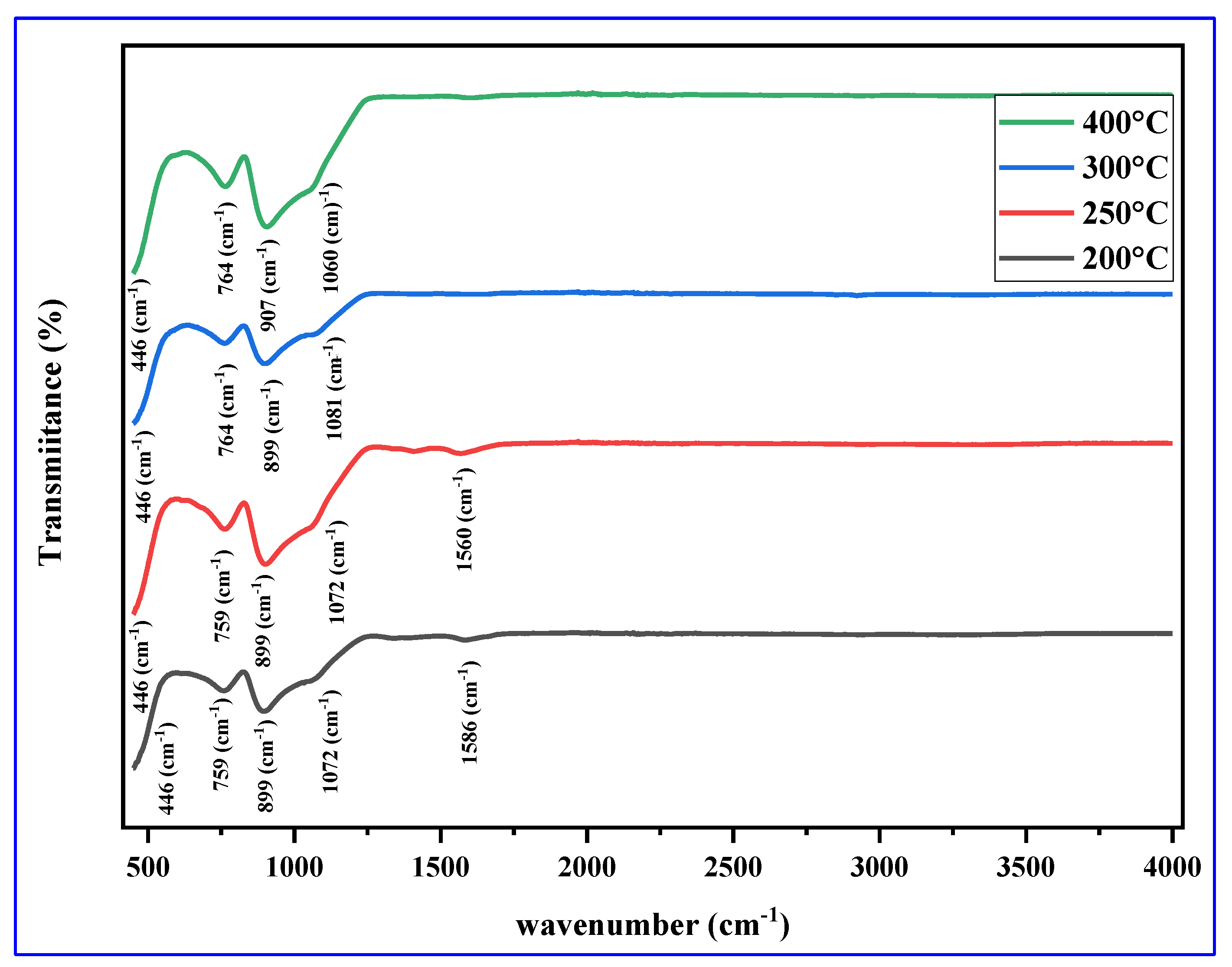
3.5. FTIR Spectra
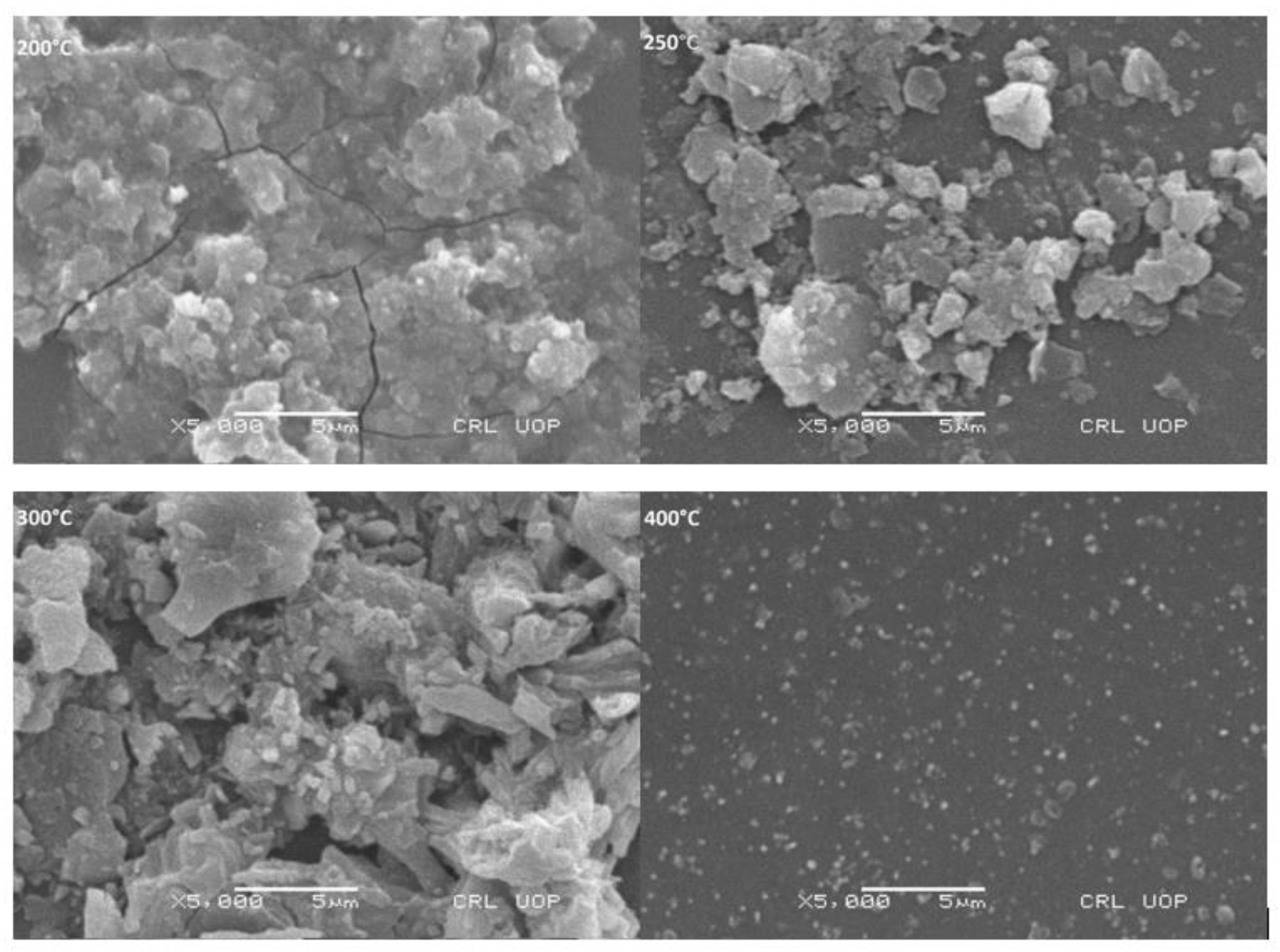
3.6. Surface Morphology
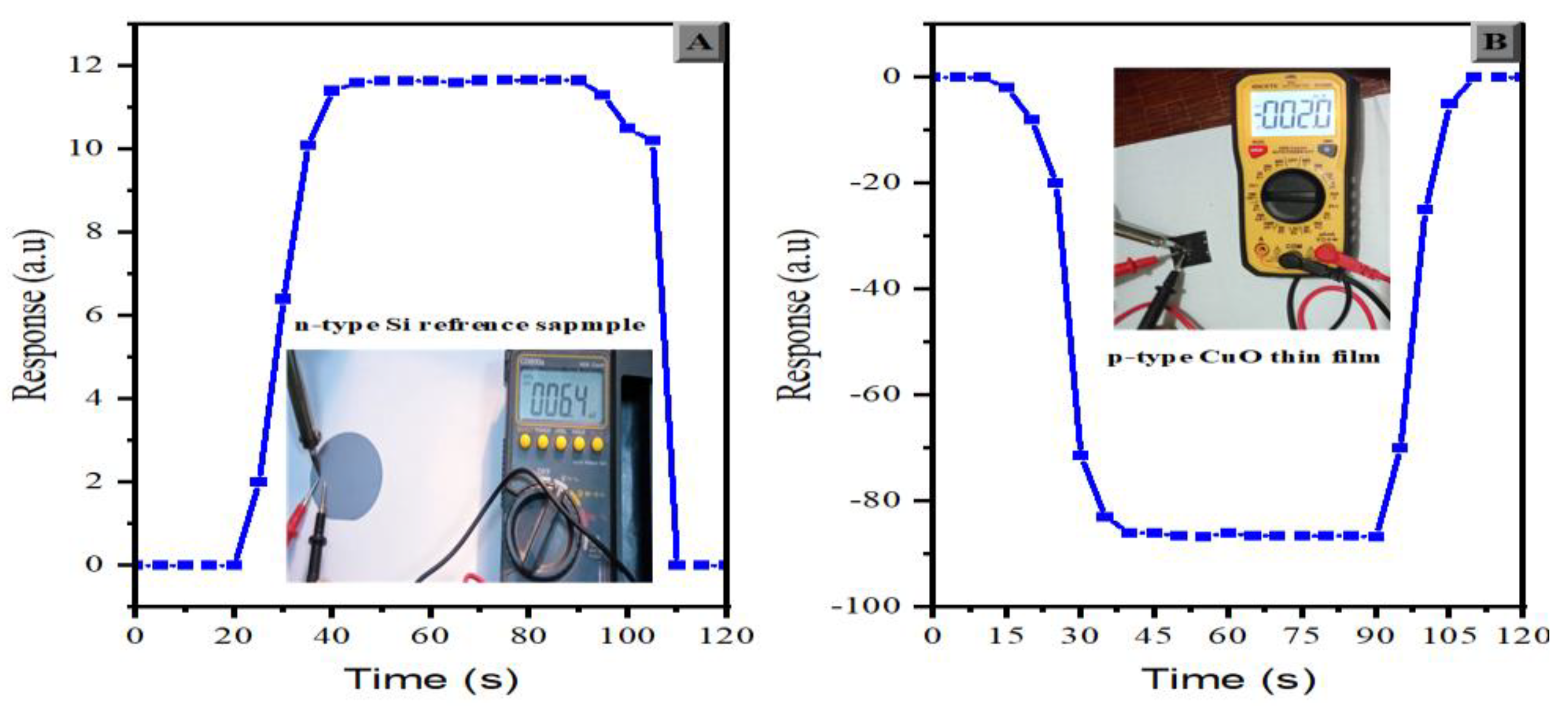
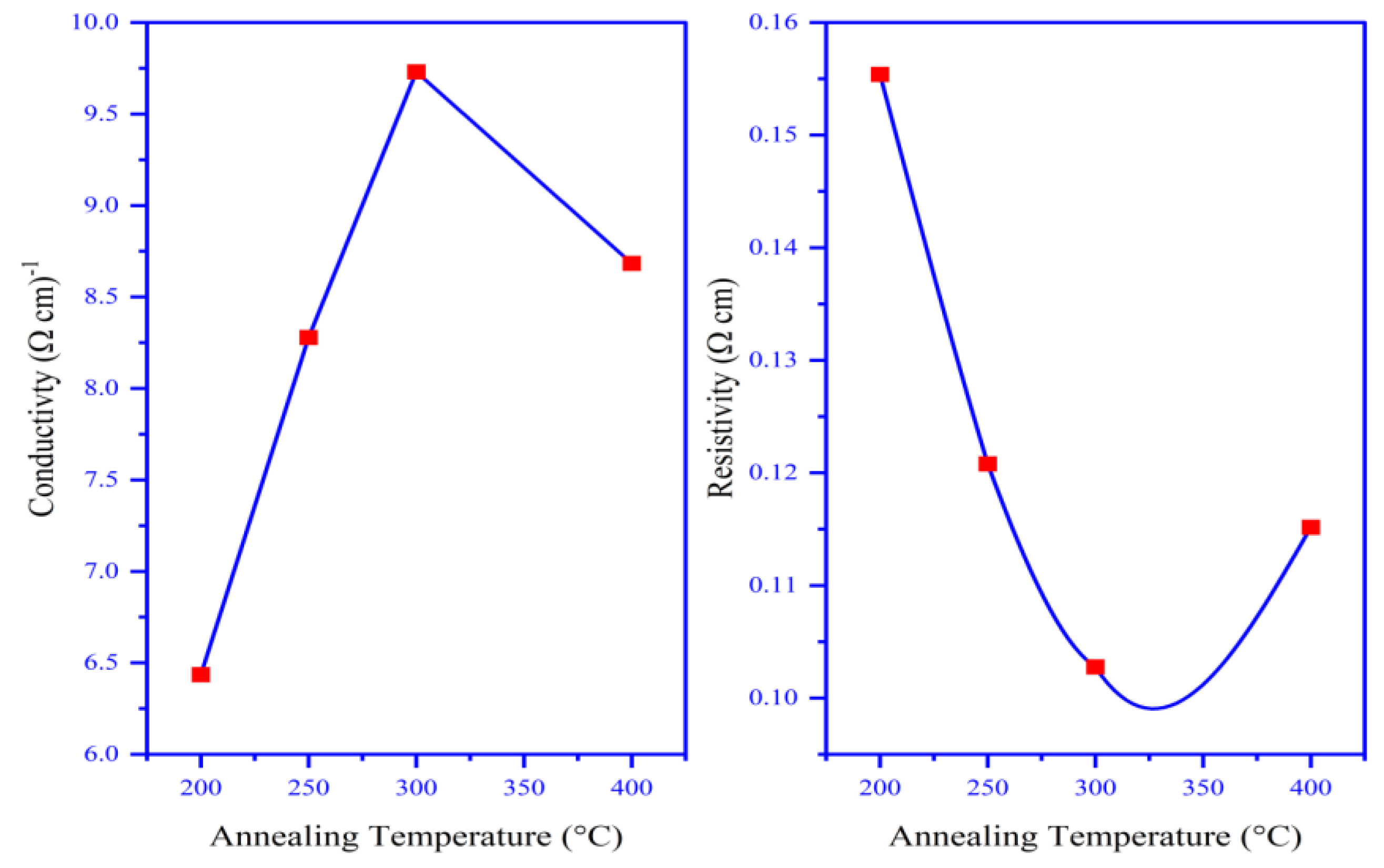
3.7. Electrical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Su, Z.; Canil, L.; Hughes, D.; Aldamasy, M.H.; Dagar, J.; Trofimov, S.; Wang, L.; Zuo, W.; Jerónimo-Rendon, J.J.; et al. Highly efficient p-i-n perovskite solar cells that endure temperature variations. Science 2023, 379, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yuan, Y.; Huang, J. Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells. Nature Energy 2016, 1, 1–6. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.M.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nature nanotechnology 2016, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.H.; Ibrahim, H.; Ayon, S.A.; Billah, M.M.; Neaz, S. Structural, nonlinear optical and antimicrobial properties of sol-gel derived, Fe-doped CuO thin films. Heliyon 2022, 8, e10609. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, T.; Kati, N.; Gül, B. Characterization of CuO doped CdO nanomaterials synthesized by sol gel spin coating and hydrothermal method. Materials Science and Engineering: B 2023, 290, 116306. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Prakash, J.; Viljoen, B.C.; Roos, W.; Swart, H. Band gap tailoring of cauliflower-shaped CuO nanostructures by Zn doping for antibacterial applications. Journal of Alloys and Compounds 2020, 832, 154968. [Google Scholar] [CrossRef]
- Bashir, A.; Naz, A.; Sultan, M.; Bashir, R.; Ali, N.Z.; Iqbal, A.; Akhter, Z. Influence of manganese and nickel doping on optical and electric properties of CuO nanostructures for optoelectronic applications. International Journal of Nanoparticles 2022, 14, 13–30. [Google Scholar] [CrossRef]
- Moumen, A.; Kumarage, G.C.; Comini, E. P-type metal oxide semiconductor thin films: Synthesis and chemical sensor applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef]
- Bougharouat, A.; Touka, N.; Talbi, D.; Baddari, K. Hydrophobic Properties of CuO Thin Films Obtained by Sol-Gel Spin Coating Technique-Annealing Temperature Effect. In Proceedings of the Annales de Chimie-Science des Matériaux; 2021; pp. 439–445. [Google Scholar]
- Benaissa, N.; Bentour, H.; Garmim, T.; El Jouad, Z.; Louardi, A.; Hartiti, B.; Monkade, M.; El Kenz, A.; Benyoussef, A. Experimental and DFT TB-mBJ calculations studies of structural, morphological, electronic, optical and electrical properties of copper oxide thin films. Optical Materials 2023, 136, 113433. [Google Scholar] [CrossRef]
- Güney, H.; İskenderoğlu, D.; Güldüren, M.E.; Demi̇r, K.Ç.; Karadeniz, S.M. An investigation on CuO thin films grown by ultrasonic spray pyrolysis at different substrate temperatures: Structural, optical and supercapacitor electrode characterizations. Optical Materials 2022, 132, 112869. [Google Scholar] [CrossRef]
- Kim, C.K.; Drozdov, I.K.; Fujita, K.; Davis, J.S.; Božović, I.; Valla, T. In-situ angle-resolved photoemission spectroscopy of copper-oxide thin films synthesized by molecular beam epitaxy. Journal of Electron Spectroscopy and Related Phenomena 2022, 257, 146775. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Wal, A.; Potera, P.; Bester, M.; Płoch, D.; Sibiński, M.; Cholewa, M.; Ruszała, M. TiO2: ZnO/CuO thin film solar cells prepared via reactive direct-current (DC) magnetron sputtering. Applied Materials Today 2022, 29, 101673. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lai, C.-W.; Jiang, W.-C.; Li, Y.-S.; Choi, C.; Yu, H.-C.; Chen, S.-J.; Choi, Y. Fabrication and characterization of P-type semiconducting copper oxide-based thin-film photoelectrodes for solar water splitting. Coatings 2022, 12, 1206. [Google Scholar] [CrossRef]
- AF Lahiji, F.; Bairagi, S.; Magnusson, R.; Sortica, M.A.; Primetzhofer, D.; Ekström, E.; Paul, B.; Le Febvrier, A.; Eklund, P. Growth and optical properties of NiO thin films deposited by pulsed dc reactive magnetron sputtering. Journal of Vacuum Science & Technology A 2023, 41, 063402. [Google Scholar] [CrossRef]
- Rahman, R.A.; Zulkefle, M.A.; binti Alip, R.I.; Herman, S.H. Determination of CuO Concentration for ZnO-NRs/P3HT/CuO as the Potential Thin Film in Solar Cell Application. Proceedings of 2022 IEEE International Conference on Semiconductor Electronics (ICSE); 2022; pp. 136–139. [Google Scholar] [CrossRef]
- Möllers, P.V.; Wei, J.; Salamon, S.; Bartsch, M.; Wende, H.; Waldeck, D.H.; Zacharias, H. Spin-Polarized Photoemission from Chiral CuO Catalyst Thin Films. ACS nano 2022, 16, 12145–12155. [Google Scholar] [CrossRef]
- Aftab, M.; Butt, M.; Ali, D.; Bashir, F.; Khan, T.M. Optical and electrical properties of NiO and Cu-doped NiO thin films synthesized by spray pyrolysis. Optical Materials 2021, 119, 111369. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-film coating methods: A successful marriage of high-quality and cost-effectiveness—A brief exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Al-Dujayli, S.; Ali, N. The effects of CuO doping on structural, electrical and optical properties of CdO thin films deposited by pulsed laser deposition technique. JOURNAL OF OVONIC RESEARCH 2022, 18, 579–590. [Google Scholar] [CrossRef]
- Yildirimcan, S. Effect of ageing on electrical properties of Fe-doped CuO thin films deposited by spin coating technique. Indian Journal of Physics 2023, 97, 1707–1716. [Google Scholar] [CrossRef]
- Absike, H.; Labrim, H.; Hartiti, B.; Tahri, M.; Ez-Zahraouy, H. Influence of spinning speed on the physical properties of sol-gel spin coated CuO films. Molecular Crystals and Liquid Crystals 2020, 711, 18–31. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, C.H.; Du, T.; Morbidoni, M.; Lin, C.-T.; Xu, S.; Durrant, J.R.; McLachlan, M.A. ZnO-PCBM bilayers as electron transport layers in low-temperature processed perovskite solar cells. Science Bulletin 2018, 63, 343–348. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Wang, L.; Wu, Y.; Tu, B.; Yu, B.; Liu, F.; Tam, H.W.; Wang, G.; Djurišić, A.B. Molecule-doped nickel oxide: Verified charge transfer and planar inverted mixed cation perovskite solar cell. Advanced Materials 2018, 30, 1800515. [Google Scholar] [CrossRef] [PubMed]
- Touka, N.; Tabli, D.; Badari, K. Effect of annealing temperature on structural and optical properties of copper oxide thin films deposited by sol-gel spin coating method. Journal of Optoelectronics and Advanced Materials 2019, 21, 698–701. [Google Scholar]
- Koshy, A.M.; Sudha, A.; Yadav, S.K.; Swaminathan, P. Effect of substrate temperature on the optical properties of DC magnetron sputtered copper oxide thin films. Physica B Condensed Matter 2023, 650, 414452. [Google Scholar] [CrossRef]
- Al Armouzi, N.; El Hallani, G.; Liba, A.; Zekraoui, M.; Hilal, H.S.; Kouider, N.; Mabrouki, M. Effect of annealing temperature on physical characteristics of CuO films deposited by sol-gel spin coating. Materials Research Express 2019, 6, 116405. [Google Scholar] [CrossRef]
- Kamarozaman, N.S.; Zulkefle, M.A.; Rahman, R.A.; Rosli, A.B.; Zainal, N.; Him, N.R.N.; Herman, S.H.; Zulkifli, Z. Annealing Effect on the EGFET Based pH Sensing Performance of Solgel Spin-coated CuO Thin Film. International Journal of Nanoelectronics & Materials 2022, 15. [Google Scholar]
- Akl, A.A.; Hassanien, A.S. Comparative microstructural studies using different methods: Effect of Cd-addition on crystallography, microstructural properties, and crystal imperfections of annealed nano-structural thin CdxZn1-xSe films. Physica B Condensed Matter 2021, 620, 413267. [Google Scholar] [CrossRef]
- Kul, M. Electrodeposited SnS film for photovoltaic applications. Vacuum 2014, 107, 213–218. [Google Scholar] [CrossRef]
- Faridi, A.W.; Imran, M.; Tariq, G.H.; Ullah, S.; Noor, S.F.; Ansar, S.; Sher, F. Synthesis and characterization of high-efficiency halide perovskite nanomaterials for light-absorbing applications. Industrial & Engineering Chemistry Research 2022, 62, 4494–4502. [Google Scholar]
- Chang, M.-H.; Liu, H.-S.; Tai, C.Y. Preparation of copper oxide nanoparticles and its application in nanofluid. Powder technology 2011, 207, 378–386. [Google Scholar] [CrossRef]
- Fath, F.N.; Ramadhanti, S.; Purwaningsih, S.Y.; Asrori, Z.; Cahyono, Y. Optical and structural properties of CuO thin film by spin coating method. AIP Conf. Proc. 2023, 2604, 020023. [Google Scholar] [CrossRef]
- Nesa, M.; Sharmin, M.; Bhuiyan, A. Role of Zn dopants on the surface morphology, chemical structure and DC electrical transport properties of nanostructured p-type CuO thin films. Materials Science in Semiconductor Processing 2021, 122, 105479. [Google Scholar] [CrossRef]
- Begum, A.; Hussain, A.; Rahman, A. Effect of deposition temperature on the structural and optical properties of chemically prepared nanocrystalline lead selenide thin films. Beilstein Journal of Nanotechnology 2012, 3, 438–443. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; Shaban, M.; Said, G. Characterization of Different Metal Oxides Thin Films Deposited by Spin Coating Technique. Labyrinth Fayoum Journal of Science and Interdisciplinary Studies 2023, 1, 59–66. [Google Scholar] [CrossRef]
- Tabli, D.; Touka, N.; Baddari, K.; Selmi, N. Pb-doped CuO thin films synthetized by sol-gel method. Advances in Materials Science 2022, 22, 5–13. [Google Scholar] [CrossRef]
- Jhansi, N.; Balasubramanian, D.; Raman, R.; Mohanraj, K. Influence of aluminum ion on the structural, optical, and electrical properties of CuO thin films for the PN-Junction diode application. Materials Science for Energy Technologies 2022, 5, 433–443. [Google Scholar] [CrossRef]
- Shehab, A.A.; Aabed, M.A.; Abd, A.N. Fabrication And Characterization Of P-Cuo/N-Si Heterojunction For Solar Cell Applications. Engg Sci Technol 2017, 4, 2458–9403. [Google Scholar]
- Saadaoui, F.; Rjeb, A.; Akharkhach, B.; Nfissi, A.; Sayouri, S. Effect of thickness and annealing temperature on the structural properties of CuO thin films prepared by sol-gel spin coating technique. Journal of Ceramic Processing Research 2019, 20, 139–142. [Google Scholar] [CrossRef]
- Sabli, N.; Talib, Z.A.; Wan Mahmood, M.Y.; Zainal, Z.; Hilal, H.S.; Husin, M.S. Effect of annealing on the properties of SnSe film prepared by thermal vacuum evaporation in the presence of argon gas. Advanced Materials Research 2014, 1024, 323–326. [Google Scholar] [CrossRef]
- Armouzi, N.A. Effect of annealing temperature on physical characteristics of CuO films deposited by sol-gel spin coating. Material Research Expesses 2019, 6, 116405. [Google Scholar] [CrossRef]
- Dhaouadi, M.; Jlassi, M.; Sta, I.; Miled, I.B.; Mousdis, G.; Kompitsas, M.; Dimassi, W. Physical properties of copper oxide thin films prepared by sol–gel spin–coating method. Am. J. Phys. Appl 2018, 6, 43–50. [Google Scholar] [CrossRef]
- Abouabassi, K.; Atourki, L.; Sala, A.; Ouafi, M.; Boulkaddat, L.; Ait Hssi, A.; Labchir, N.; Bouabid, K.; Almaggoussi, A.; Gilioli, E. Annealing effect on one step electrodeposited CuSbSe2 thin films. Coatings 2022, 12, 75. [Google Scholar] [CrossRef]
- Chand, P.; Gaur, A.; Kumar, A.; Gaur, U.K. Structural and optical study of Li doped CuO thin films on Si (1 0 0) substrate deposited by pulsed laser deposition. Applied surface science 2014, 307, 280–286. [Google Scholar] [CrossRef]
- Musa, A.; Farhad, S.; Gafur, M.; Jamil, A. Effects of withdrawal speed on the structural, morphological, electrical, and optical properties of CuO thin films synthesized by dip-coating for CO2 gas sensing. AIP Advances 2021, 11, 115004. [Google Scholar] [CrossRef]
- Bunea, R.; Saikumar, A.K.; Sundaram, K. A comparison of optical properties of CuO and Cu2O thin films for solar cell applications. Materials Sciences and Applications 2021, 12, 315–329. [Google Scholar] [CrossRef]
- Hossain, M.F.; Pervez, M.S.; Nahid, M. Influence of film thickness on optical and morphological properties of TiO2 thin films. Emerging Materials Research 2020, 9, 186–191. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Wisz, G.; Rybak-Wilusz, E.; Cholewa, M. Numerical analysis and optimization of Cu2O/TiO2, CuO/TiO2, heterojunction solar cells using SCAPS. Journal of Physics: Conference Series 2018, 1033, 012002. [Google Scholar] [CrossRef]
- Lee, Y.S.; Heo, J.; Winkler, M.T.; Siah, S.C.; Kim, S.B.; Gordon, R.G.; Buonassisi, T. Nitrogen-doped cuprous oxide as a p-type hole-transporting layer in thin-film solar cells. Journal of Materials Chemistry A 2013, 1, 15416–15422. [Google Scholar] [CrossRef]
- Diachenko, O.; Kováč Jr, J.; Dobrozhan, O.; Novák, P.; Kováč, J.; Skriniarova, J.; Opanasyuk, A. Structural and optical properties of CuO thin films synthesized using spray pyrolysis method. Coatings 2021, 11, 1392. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Hussain, A.N.; Hassoon, K.I.; Hassan, M.A. Effect of annealing on copper oxide thin films and its application in solar cells. J. Phys.: Conf. Ser. 2020; 1530, p. 012140. [Google Scholar] [CrossRef]
- Dahham, N.A. Annealing temperature effect on the Structure, Morphology and Optical properties of Copper Oxide CuO thin Films. Tikrit Journal of Pure Science 2018, 22, 115–124. [Google Scholar] [CrossRef]
- Jundale, D.; Joshi, P.; Sen, S.; Patil, V. Nanocrystalline CuO thin films: Synthesis, microstructural and optoelectronic properties. Journal of Materials Science Materials in Electronics 2012, 23, 1492–1499. [Google Scholar] [CrossRef]
- Kabir, M.H.; Ibrahim, H.; Billah, M.M. Effect of stabilizer on sol ageing for CuO thin films synthesized by sol-gel spin coating. AIP Conf. Proc. 2021, 2324, 030007. [Google Scholar] [CrossRef]
- Cahyono, Y.; Ramadhanti, S.; Fatimah, S.; Dewi, Y.; Asrori, M. Structure and optical properties study of annealed CuO films for development of perovskite-based solar cells. Proceedings of Journal of Physics: Conference Series; p. 012061.
- Sharma, M.S.D.a.S.K. Structural, morphological and optical studies of nanocrystalline CuO thin films by solution processed Spin Coating technique. Journal of Emerging Technologies and Innovative Research 2019, 6, 669–674. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. Journal of non-crystalline solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Keabadile, O.P.; Aremu, A.O.; Elugoke, S.E.; Fayemi, O.E. Green and traditional synthesis of copper oxide nanoparticles—Comparative study. Nanomaterials 2020, 10, 2502. [Google Scholar] [CrossRef]
- Prakash, V.; Diwan, R.; Niyogi, U. Characterization of synthesized copper oxide nanopowders and their use in nanofluids for enhancement of thermal conductivity. 2015.
- Varughese, A.; Kaur, R.; Singh, P. Green synthesis and characterization of copper oxide nanoparticles using Psidium guajava leaf extract. IOP Conf. Ser.: Mater. Sci. Eng. 2020, 961, 012011. [Google Scholar] [CrossRef]
- Shafiey Dehaj, M.; Zamani Mohiabadi, M. Experimental study of water-based CuO nanofluid flow in heat pipe solar collector. Journal of Thermal Analysis and Calorimetry 2019, 137, 2061–2072. [Google Scholar] [CrossRef]
- Sudha, V.; Murugadoss, G.; Thangamuthu, R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Scientific reports 2021, 11, 3413. [Google Scholar] [CrossRef]
- Sharma, K. Structural, morphological and optical studies of nanocrystalline CuO thin films by solution processed Spin Coating technique.
- Khmissi, H.; El Sayed, A.; Shaban, M. Structural, morphological, optical properties and wettability of spin-coated copper oxide; influences of film thickness, Ni, and (La, Ni) co-doping. Journal of Materials Science 2016, 51, 5924–5938. [Google Scholar] [CrossRef]
- Abdel-Karim, A.M.; Ahmed, Y.M.; El-Masry, M.M. Ag-CuO/epoxy hybrid nanocomposites as anti-corrosive coating and self-cleaning on copper substrate. Scientific Reports 2023, 13, 19248. [Google Scholar] [CrossRef] [PubMed]
- Bouachma, S.; Ayouz-Chebout, K.; Kechouane, M.; Manseri, A.; Yaddadene, C.; Menari, H.; Gabouze, N. Synthesis of PSi-n/CuO-p/Cu 2 On heterostructure for CO 2 gas sensing at room temperature. Applied Physics A 2022, 128, 1–13. [Google Scholar] [CrossRef]
- Datta, P.; Sharmin, M.; Podder, J.; Choudhury, S. Influence of substrate temperature on the morphological, structural, optical and electrical properties of nanostructured CuO thin films synthesized by spray pyrolysis technique. Journal of Optoelectronics and Advanced Materials 2021, 23, 35–42. [Google Scholar]
- Taherimakhsousi, N.; MacLeod, B.P.; Parlane, F.G.; Morrissey, T.D.; Booker, E.P.; Dettelbach, K.E.; Berlinguette, C.P. Quantifying defects in thin films using machine vision. npj Computational Materials 2020, 6, 111. [Google Scholar] [CrossRef]
- Wang, R.; Moos, R. Electrical conductivity determination of semiconductors by utilizing photography, finite element simulation and resistance measurement. Journal of Materials Science 2021, 56, 10449–10457. [Google Scholar] [CrossRef]
- Axelevitch, A. Hot-probe characterization of transparent conductive thin films. Materials 2021, 14, 1186. [Google Scholar] [CrossRef]
- Alsmadi, A.; Masmali, N.; Jia, H.; Guenther, J.; Abu Jeib, H.; Kerr, L.L.; Eid, K. Hot probe measurements of n-type conduction in Sb-doped ZnO microwires. Journal of Applied Physics 2015, 117, 155703. [Google Scholar] [CrossRef]
- Tariq, G.H.; Asghar, G.; Shifa, M.S.; Anis-Ur-Rehman, M.; Ullah, S.; Shah, Z.A.; Ziani, I.; Tawfeek, A.M.; Sher, F. Effect of copper doping on plasmonic nanofilms for high performance photovoltaic energy applications. Physical Chemistry Chemical Physics 2023, 25, 31726–31740. [Google Scholar] [CrossRef]
- Philips’Gloeilampenfabrieken, O. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep 1958, 13, 1–9. [Google Scholar]
- Djebian, R.; Boudjema, B.; Kabir, A.; Sedrati, C. Physical characterization of CuO thin films obtained by thermal oxidation of vacuum evaporated Cu. Solid State Sciences 2020, 101, 106147. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Subramaniam, V.; Mahalingam, T.; Helen, S.; Sumathi, P. Improved properties of spray pyrolysed CuO nanocrystalline thin films. Journal of Materials Science Materials in Electronics 2017, 28, 4211–4218. [Google Scholar] [CrossRef]
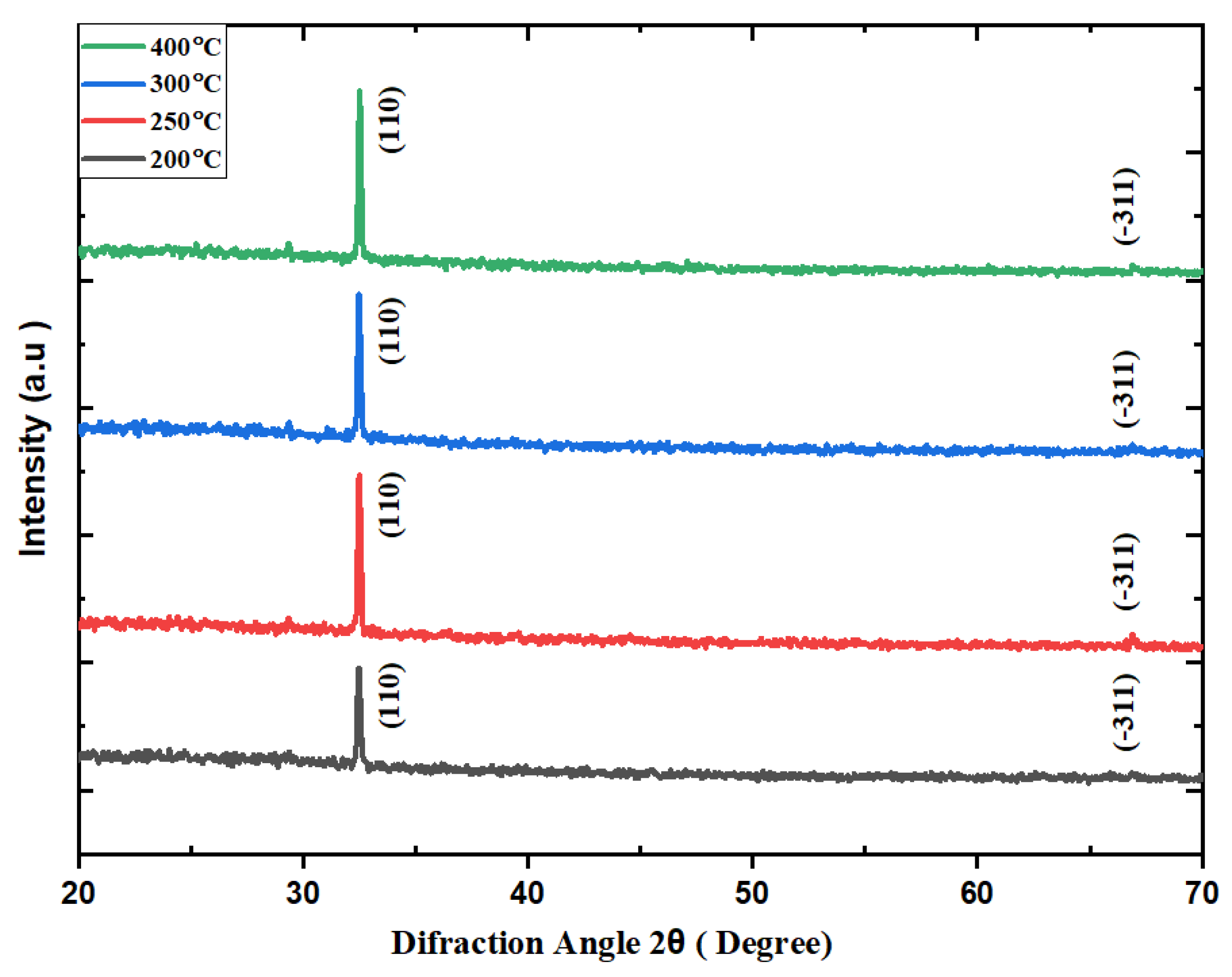
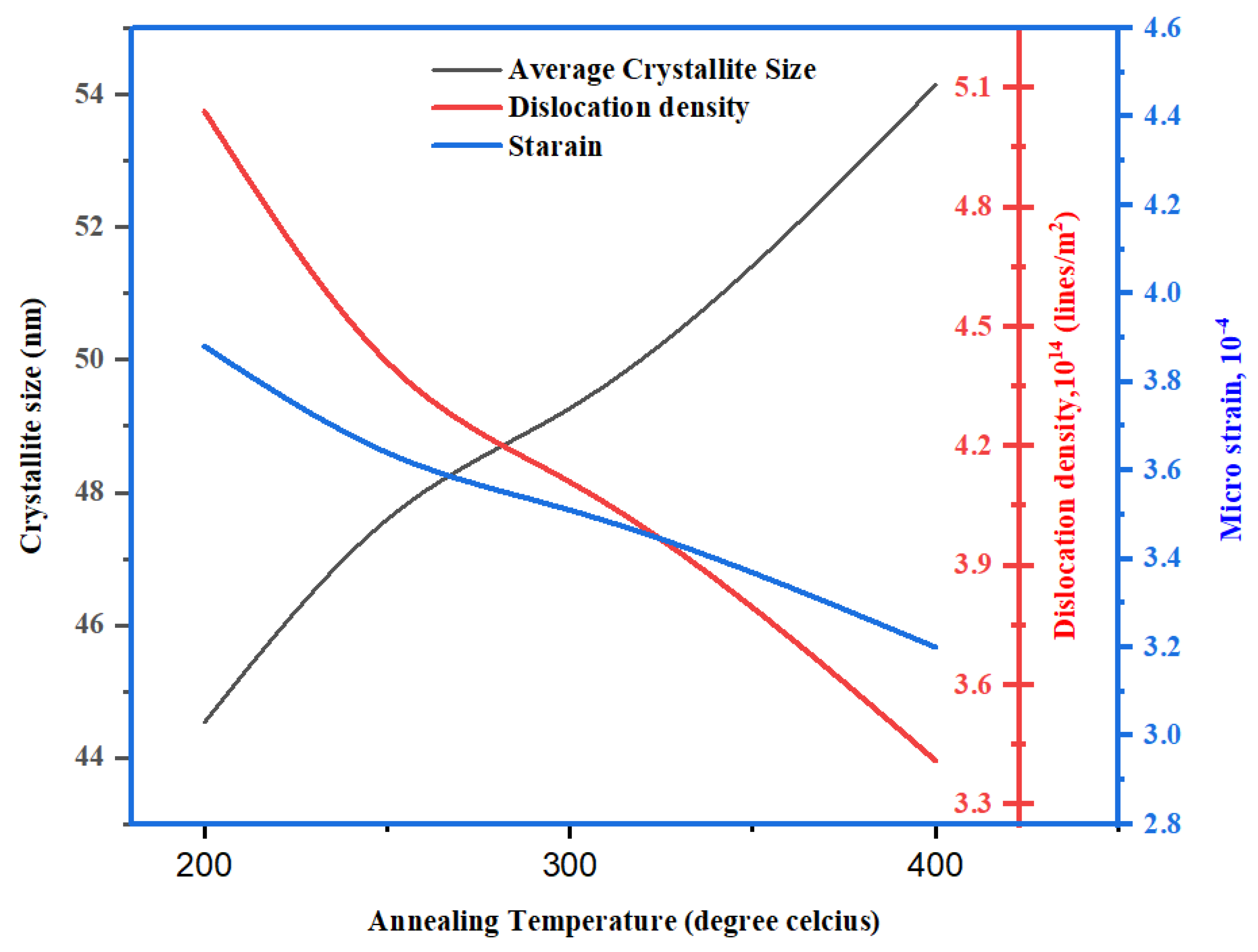
| Method | Temperature T(°C) | FWHM 2θ (Degree) | Crystallite size D(nm) | Micro strain ε(10-4) | Dislocation density δ(1014/m2) | Reference |
|---|---|---|---|---|---|---|
| Drop casting | 200 | 0.2160 | 40.70 | 8.64 | 6.22 | [39] |
| Plasma focus | RT | 0.1710 | 122.6 | 0.28 | 0.6 | [39] |
| Spin coating | 600 | 0.2334 | 37.68 | 9.61 | 7.04 | [40] |
| Spin coating | 200 | 0.1850 | 44.54 | 3.88 | 5.04 | Present work |
| Spin coating | 250 | 0.1738 | 47.60 | 3.64 | 4.41 | Present work |
| Spin coating | 300 | 0.1694 | 49.27 | 3.51 | 4.11 | Present work |
| Spin coating | 400 | 0.1529 | 54.14 | 3.20 | 3.41 | Present work |
| Annealing temperature (°C) | Raman shift (cm-1) | Phonon mode | Corresponding mode | Reference |
|---|---|---|---|---|
| 450 | 624.00 | CuO | [43] | |
| 250 | 89.00 | Ag | CuSbSe2 | [44] |
| 500 | 286.00 | Ag | CuO | [45] |
| 450 | 292.00 | Ag | CuO | [6] |
| 200 | 304.93 | Ag | CuO | Present work |
| 250 | 301.12 | Ag | CuO | Present work |
| 300 | 295.41 | Ag | CuO | Present work |
| 400 | 299.22 | Ag | CuO | Present work |
| Deposition Method | Annealing temperature (°C) | Band gap energy (eV) | Reference |
|---|---|---|---|
| Chemical spray pyrolysis | 400 – 600 | 2.60 – 2.45 | [53] |
| Spin coating | 250 – 450 | 3.99 – 3.94 | [54] |
| Spin coating | 300 – 700 | 1.64 – 1.46 | [55] |
| Spin coating | 300 – 500 | 2.70 – 1.80 | [27] |
| Spin coating | 450 – 550 | 3.72 – 3.48 | [56] |
| Spin coating | RT – 600 | 1.61 – 1.44 | [57] |
| Spin coating | 150 – 500 | 1.62 – 1.43 | [58] |
| Spin coating | 250 – 400 | 1.93 – 1.81 | Present work |
| Band position (cm-1) | Assigned group or stretching/ bending | Reference |
|---|---|---|
| 646 | Cu-O | [65] |
| 455 | Cu-O | [66] |
| 443,535 | Cu-O | [67] |
| 784 | Cu-O | [62] |
| 420,503,615,740 | Cu-O | [68] |
| 446,759,764 | Cu-O | Present work |
| Annealing- temperature (°C) | 200 | 250 | 300 | 400 |
|---|---|---|---|---|
| Conductivity type | P | P | P | P |
| Carriers type | Holes | Holes | Holes | Holes |
| Sheet Resistance (Ω) | 8221.2 | 6711.2 | 5872.3 | 6543.4 |
| Resistivity (Ω.cm) | 0.1554 | 0.1208 | 0.1028 | 0.1152 |
| Conductivity (Ω.cm)-1 | 6.4358 | 8.27803 | 9.7039 | 8.68325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





