1. Introduction
Papillomaviruses (PVs) are small non-enveloped oncogenic viruses with an icosahedral capsid and a genome composed of double-stranded circular DNA that is approximately 8,000 bp in length [
1,
2]. PVs infect the skin and mucous membranes of various hosts including humans. These infections usually cause benign lesions that spontaneously regress [
3]. However, in some cases showing no occasional regression malignant progression may occur, in which benign papilloma transforms into squamous cell carcinoma [
4].
The papillomavirus genome contains open reading frames (ORFs) that encode the early (E) and late (L) proteins; E1 and E2 proteins promote viral replication, E4 participates in the production of virions, and the oncoproteins E5, E6, and E7 are linked to cellular transformation. The late proteins L1 and L2 are structural proteins involved in the formation of the capsid and the completion of the viral cycle [
1,
2].
Among the
Papillomaviridae family, 44 types of bovine papillomavirus (BPV) have been reported to infect cattle. These diverse viruses belong to the genera
Deltapapillomavirus (BPV1, 2, 13, and 14),
Dyoxipapillomavirus (BPV7, 19, and 21),
Epsilonpapillomavirus (BPV5 and 8),
Xipapillomavirus (BPV3, 4, 6, 9, 10, 11, 12, 15, 17, and 23), unclassified
Dyokappapapillomavirus (BPV16, 18, and 22), unclassified
Xipapillomavirus (BPV20, 24, 26, 28, and 29), unclassified
Epsilonpapillomavirus (BPV25), and unclassified types awaiting official classification (BPV 27, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, and 44) [
5,
6].
In cattle, BPVs can cause several clinical entities, such as cutaneous papillomatosis characterized by benign tumors at different anatomical sites on the skin, which can be histologically classified as fibropapillomas and true papillomas [
7]. Other BPV infection-associated clinical conditions in cattle include bovine enzootic hematuria (BEH), a chronic inflammatory and neoplastic disease of the urinary bladder [
4], and the development of tumors in the upper alimentary tract; both clinical alterations may be related to the chronic ingestion of bracken fern (
Pteridium aquilinum). In addition to its toxic, carcinogenic, and mutagenic effects,
P. aquilinum has an immunosuppressive effect, which contributes to papilloma persistence [
8,
9]. In tumors developed in the upper digestive tract, BPV4 has mainly been detected in squamous papillomas [
10], whereas, BPV2 is associated with fibropapillomas [
11].
The rolling circle amplification (RCA) followed by high-throughput sequencing (HTS) supported significant advances in recent research on papillomaviruses. This non-specific molecular strategy helped amplify and sequence the genomes of existing and potential new viral types of PVs with greater sensitivity [
12,
13,
14]. In this study, we aimed to investigate the BPV types associated with hyperplastic lesions located in the upper alimentary tract of an affected bull and to characterize the involved viral strains through complete genome sequencing using this molecular methodology.
2. Materials and Methods
2.1. Biological Samples and Histopathological Analysis
Through the post-slaughter inspection of the mucosa lining the whole upper alimentary tract, two proliferative lesions were identified and collected from the esophagus of a Girolando breed mature dairy bull provenient from a farm located in the North Central mesoregion of the Parana state, Southern Brazil. A portion of each esophageal lesion was fixed by immersing in 10% neutral-buffered formalin and stained using hematoxylin and eosin for routine histopathological evaluation. The proliferative mucosal lesions were histopathologically evaluated as previously described [
15]. The remaining fragment of each proliferative lesion was stored at −80 °C for further molecular evaluations.
The sample collection and all procedures performed in this study were based on ethical and animal welfare considerations. All the applicable international, national, and institutional guidelines for animal care and use were followed. This study was approved by the Ethics Committee for Animal Use at the Universidade Estadual de Londrina (Protocol No. 050.2021).
2.2. PCR Amplification of Partial BPV L1 and E1 Genes
Frozen fragments acquired from each esophageal lesion were disrupted and homogenized using TissueLyser LT (Qiagen, Hilden, Germany). Subsequently, total DNA was purified from these tissue homogenates using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) following the instructions provided by the manufacturer. Purified DNA was stored at −80 °C for further molecular analyses. Aliquots of ultrapure sterile water were used as negative controls in all DNA extraction procedures.
To investigate the presence of PV strains potentially associated with the hyperplastic lesions collected from the esophageal mucosa of the dairy bull, three degenerate primer pairs designed to detect the conserved regions of the L1 and E1 genes of a broad spectrum of cutaneous and mucosal PV strains were tested using DNA purified from both lesions. The sequences and features of the primers used are listed in
Table 1.
PCR mixtures (50 μl) contained 5 μl of the purified DNA, 0.4 to 1 μM of each primer, 200 μM of each deoxynucleoside triphosphate (dNTP) (Invitrogen, Carlsbad, CA, USA), 2.5 U of HotStar Taq DNA polymerase (Qiagen, Hilden, Germany), 1× PCR buffer, 1.5 to 2.5 mM MgCl
2, and ultrapure sterile water used to make up the final volume. Amplification was performed with the following cycling profile: an initial step of 15 min at 95 °C, followed by 45 cycles of 1 min at 94 °C, 1 min at an optimal temperature for primer annealing (
Table 1), and 1 min at 72 °C, with a final extension for 10 min at 72 °C. Aliquots of the PCR-amplified products were analyzed via electrophoresis using 2% agarose gels, stained with ethidium bromide (0.5 mg/ml), and examined under UV light.
2.3. Sequencing of Subgenomic Fragments
Amplified partial fragments of the BPV L1 and E1 genes were purified using a Wizard SV Gel and PCR Clean-Up System kit (Promega, Madison, WI, USA) following the protocol provided by the manufacturer. Next, direct sequencing was performed on a 3500 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA) using the BigDye Terminator v.3.1 cycle sequencing kit (Applied Biosystems, Carlsbad, CA, USA) and the corresponding forward and reverse primers, according to the instructions provided by the manufacturer. The generated sequences were examined using the Phred application for quality analysis of chromatogram readings. The sequences with a base quality ≥20 were considered acceptable. The consensus sequences were determined using CAP3 software, and their identities were analyzed based on the public database GenBank using the BLASTn program.
2.4. Rolling-Circle Amplification
The BPV genomes were inespecifically amplified using DNA purified from two esophageal lesions through the multiple-primed rolling-circle amplification (RCA) technique using the TempliPhi 100 Amplification kit (Cytiva, Marlborough, MA, USA), following a previously reported protocol optimized for the amplification of papillomaviral genomes [
19]. We verified the amplification of PV DNA through electrophoretic analysis of the RCA product (2 μl) using a 0.8% agarose gel generating a band corresponding to multiple tandem copies of the full-length PV DNA.
The RCA products were purified using a Wizard SV Gel and PCR Clean-Up System kit (Promega, Madison, WI, USA) and quantified through fluorometry using a Qubit 4.0 (Invitrogen).
2.5. High-Throughput Sequencing (HTS)
Libraries were prepared using 1 ng of purified DNA using an Illumina DNA Library Prep kit (Illumina, San Diego, CA, USA). The Agilent D1000 ScreenTape kit (Agilent Technologies, Santa Clara, CA, USA) was used for the quality control of the library on the Agilent TapeStation 4150 System (Agilent Technologies, Santa Clara, CA, USA) and determination of the library size. The library was quantified on a Qubit 4.0 Fluorometer using the Qubit dsDNA Quantification High Sensitivity Assay kit (Invitrogen, Waltham, MA, USA). The library was sequenced via the paired-end sequence strategy using the MiniSeq High Output kit (2 × 150 cycles) and MiniSeq system platform (Illumina, San Diego, CA, USA). The quality of the sequencing reads was evaluated using FastQC software version 0.11.3 (
www.bioinformatics.babraham.ac.uk/projects/fastqc). To minimize possible base-calling errors, reads were trimmed based on a Phred quality score cutoff of 30; sequence reads shorter than 150 nucleotides (nt) were discarded. Complete BPV genomes were assembled using a
de novo strategy using SPAdes software version 3.9.0 [
20].
Putative ORFs were identified using a combination of the ORF Finder program and BLASTp developed by the National Center for Biotechnology Information (NCBI) and compared with previously characterized BPV strains.
2.6. Phylogenetic Analysis
Pairwise and multiple sequence alignments at the nucleotide (nt) and amino acid (aa) levels and sequence similarities were estimated using ClustalW in the MEGA v11 software [
21]. Complete nt sequences of the L1 gene derived from PV types, previously characterized through complete genome sequencing and representing the
Deltapapillomavirus and
Xipapillomavirus genera, were included in the analyses. Two phylogenetic trees were reconstructed based on the alignment of complete L1 nt sequences using the Maximum Likelihood method with the general time-reversible model in MEGA v11 software.
3. Results
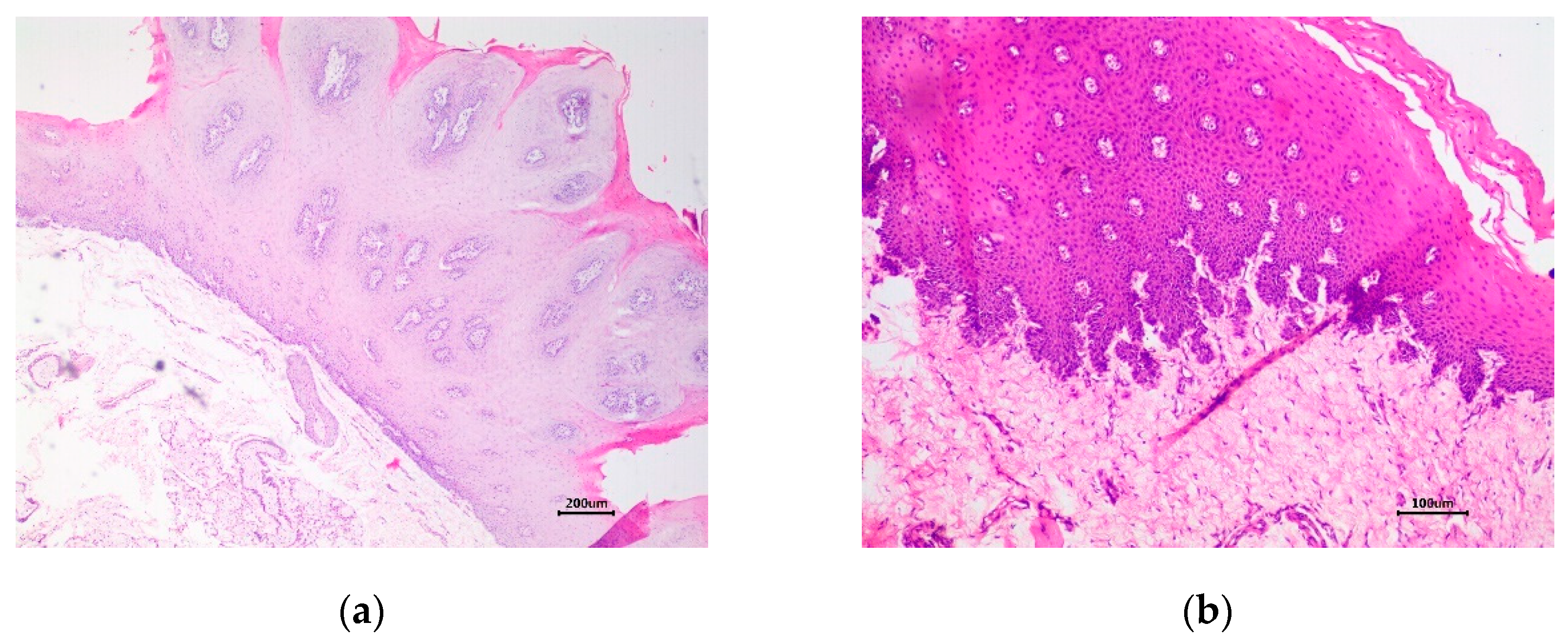
Histopathological analysis revealed marked epithelial hyperplasia, parakeratotic hyperkeratosis, and koilocytosis, resulting in exophytic folding in the first lesion, which is characteristic of a squamous papilloma (
Figure 1a). These cytopathic effects indicate BPV infection (
Figure 1a). In the second lesion, the proliferation of disorganized and anaplastic epithelial cells without invasion through the basement membrane into the submucosa was detected, which characterizes a carcinoma
in situ (CIS) and preneoplastic lesion (
Figure 1b).
In contrast to PCR with MY primer pair, the selected PCR using the primer pairs FAP59/FAP64 and AR-E1F2/AR-E1R4, whose amplicons represented partial fragments of the BPV L1 and E1 genes, successfully yielded PCR products with the expected lengths for DNA samples from both lesions evaluated. No amplicons were obtained from the negative controls included in the PCR assays. Direct sequencing of the FAP amplicon obtained from the squamous papilloma DNA sample generated a partial L1 nt sequence shown to belong to BPV4 by BLASTn evaluation (99.32% identity; accession number: OP682875). Moreover, the partial nt sequence of E1, which was obtained from the same lesion, showed the highest identity (98.96%) with a BPV4 strain available in GenBank (accession number MW436424). Contrastingly, direct sequencing of the amplicon representing a partial fragment of the L1 gene obtained from the CIS lesion revealed the highest similarity of this amplicon with BPV2, with an identity of 99.78% (accession number: KF284153). Similarly, for the esophageal CIS DNA sample, sequencing of the PCR product obtained using the primer pair targeting the E1 gene generated a partial nt sequence highly similar to the same viral type, exhibiting 99.51% identity (accession number: MF045490) through BLASTn-based analysis.
Sequencing analysis of the library using the RCA product of the esophageal squamous papilloma generated a total of 1,796,770 reads. After trimming, 1,127,632 high-quality reads were retained for the sequence analysis. These sequences were de novo assembled into contigs that were further compared with the GenBank database using BLASTn and BLASTx. For the squamous papilloma-derived DNA sample, only one contig was characterized as PV, representing the complete genome sequence of BPV4 (7,274 nt, GC content of 41.9%, a mean coverage of 4,081). Through BLASTn analysis, the highest identity obtained by comparison with other BPV complete genomic sequences deposited in GenBank was 99,11% with the BPV4 strain 4827RS16-BR node 9 recovered from a teat papilloma in cattle in Brazil in 2016 (Accession number: MW436424).
In contrast, the library obtained from the RCA product amplified from the CIS lesion generated a total of 1,867,724 reads, with 1,310,390 high-quality reads remaining after trimming. From this esophageal lesion,
de novo assembly generated one contig representing the whole-genome sequence of the BPV2 strain (7,947 nt, GC content of 45.8 %, and mean coverage of 2,177). BLASTn analysis of this sequence revealed that the BPV2 strains E170428-1 and E181025 (accession numbers: LC510376 and LC510384), identified from equine sarcoids in Japan in 2017 and 2018, respectively, exhibited the closest similarity to the BPV2 genome obtained (nt identity of 99.94%). Additionally, three other contigs, corresponding to BPV subgenomic sequences highly similar to BPV4, represented partial sequences of the L1, L2, and E7 genes (
Table 2).
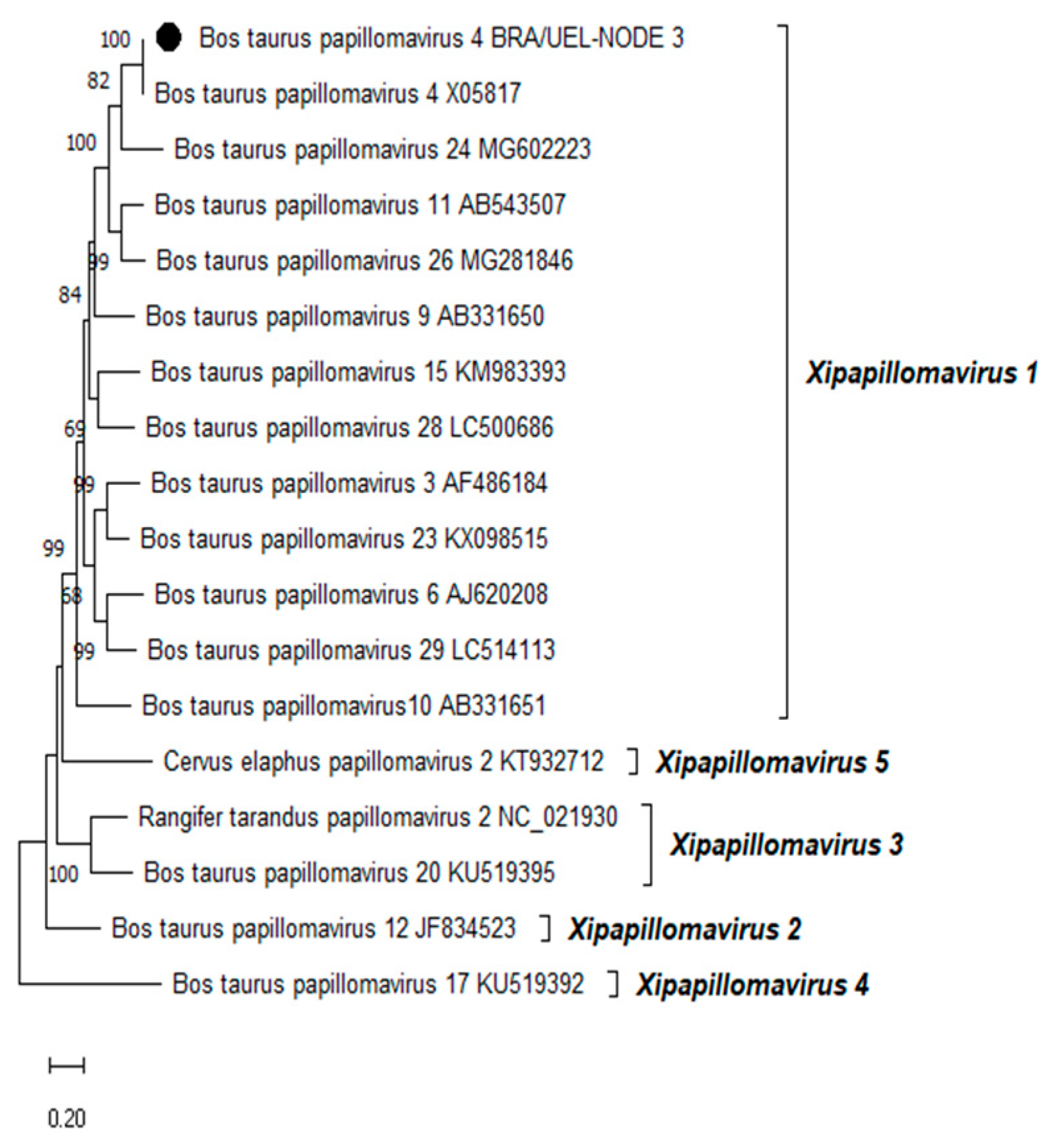
Phylogenetic reconstruction based on the complete L1 nt sequences of all PVs, already characterized through the complete genome sequencing and classified in the
Xipapillomavirus genus, confirmed that the BPV4 genome derived from esophageal squamous papilloma belongs to the
Xipapillomavirus 1 viral species (
Figure 2). Additionally, the phylogenetic tree of the complete L1 nt sequences of PVs fully sequenced and classified in the
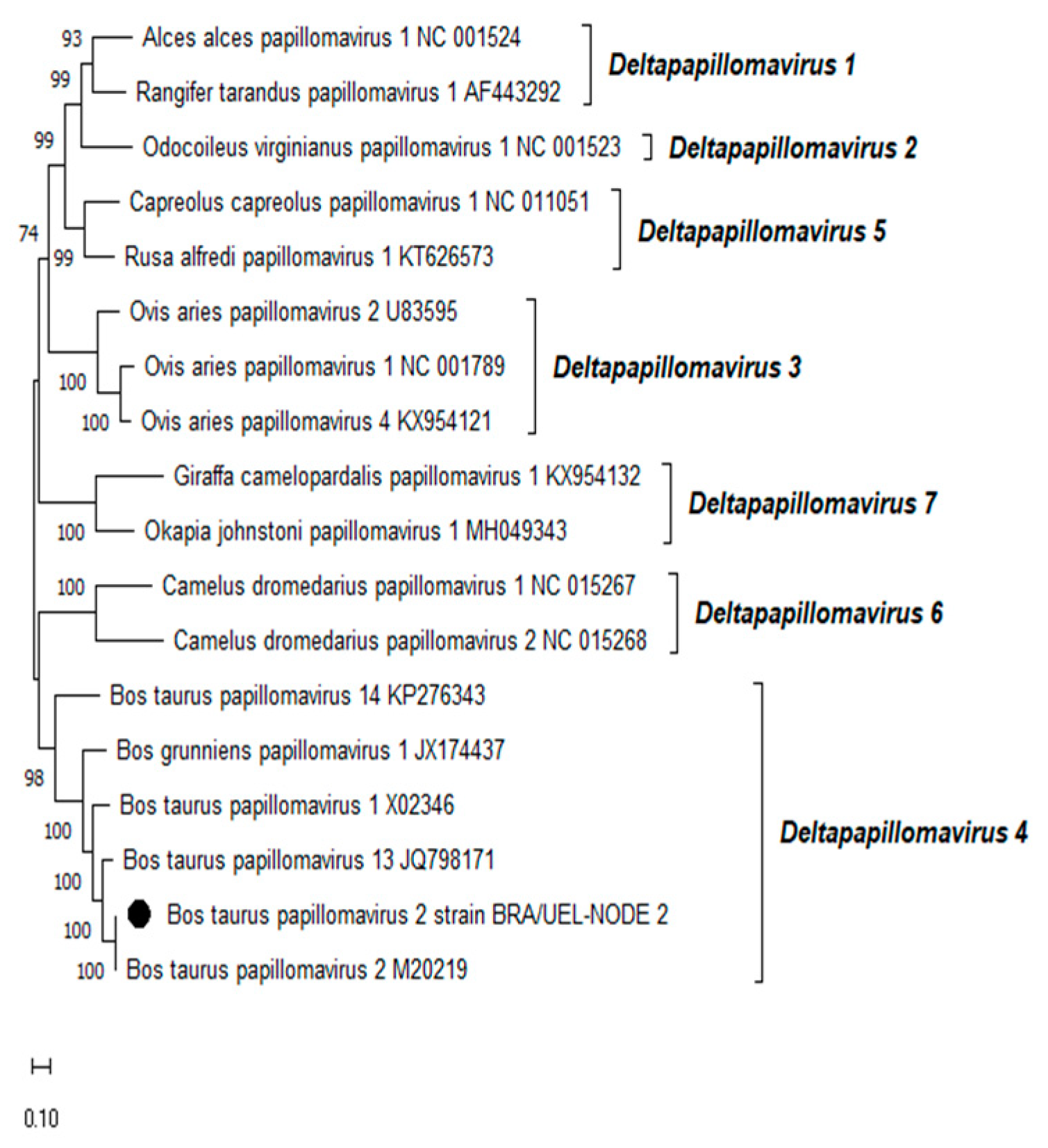
Deltapapillomavirus genus revealed that the Brazilian BPV2 strain present in the CIS lesion on the same organ belonged to the
Deltapapillomavirus 4 viral species, with high statistical support (
Figure 3).
4. Discussion
In this study, BPV2 and BPV4 were identified in lesions with different histological classifications developed in the esophagus of an asymptomatic dairy bull in Southern Brazil. The viral types were detected by PCR assays using degenerate primers that amplify a broad range of PV sequences and through a non-specific strategy involving the RCA of BPV genomes followed by sequencing via HTS. Additionally, deep sequencing of the RCA product derived from the esophageal CIS lesion revealed mixed infection by BPV2 and 4; the BPV2 complete genomic sequence and partial nucleotide sequences from the BPV4-specific genes E7, L2, and L1 were obtained.
Although BPV4 is the viral type mostly associated with tumor lesions that develop in the mucous membrane lining the upper digestive canal of cattle, both BPV4 belonging to the genus
Xipapillomavirus and BPV2 belonging to the genus
Deltapapillomavirus, serve as causal agents of hyperplastic lesions of the bovine upper alimentary tract, as previously reported [
10,
11,
22]. The BPV4 infection was associated with progression to malignancy [
23,
24], however, BPV2 infections seems to rarely lead to fibropapillomas at this anatomical site [
11]. As only BPV4 was identified in alimentary cancers of cattle, a link between BPV2 infection of the upper digestive tract and the development of malignancy has not been established in cattle [
11].
In this study, the BPV2 complete genomic sequence was amplified from an esophageal CIS DNA sample and sequenced. To the best of our knowledge, we, for the first time, have identified this viral type in a premalignant lesion of the bovine upper alimentary tract. Additionally, the results reflect that a mixed infection with BPV4 can be characterized in the CIS lesion through RCA amplification followed by HTS. A recent investigation carried out in the midwestern region of Brazil revealed the occurrence of a mixed infection caused by BPV4 and BPV2 in a squamous papilloma developed in the esophagus of a dairy cow; viral types were identified using conventional PCR employing the degenerate primer pairs FAP59/FAP64 and AR-E1F2/AR-E1R4, followed by cloning and sequencing of the amplified L1 and E1 gene fragments [
25]. Mixed infection by BPV2 and BPV4 has been previously described in a squamous papilloma of the esophagus of a dairy cow; however, this study, for the first time, presents its occurrence in a CIS in the same organ of cattle.
The RCA and HTS of the complete genome of BPV4 in the squamous papilloma of the esophagus of the dairy bull are comparable to the previous findings. Previously, BPV4 was reported in papillomatous lesions without malignant progression developed in cattle raised in Scotland and England. The conventional PCR-based molecular analyses of these biological samples confirmed the viral type, whereas, histopathological analyses revealed papillomatous changes throughout the upper alimentary tract [
26]. In Italy, through histopathological evaluation, esophageal papilloma (inverted and exophytic) was detected in 147 cattle among a total of 1,133 animals slaughtered between 2000 and 2001; BPV4 was present in > 60% of the examined samples [
27].
In addition to the viral types commonly detected in this clinical entity, studies carried out in India revealed BPV5 in lesions from the upper alimentary tract of cattle for the first time. This viral type was detected in six hyperproliferative lesions acquired from the rumen, reticulum, and esophagus of cattle and was histologically diagnosed to be fibropapillomas and papillomas [
28]. In the present study, we did not detect BPV5 in the lesions developed in the upper digestive tract of the dairy bull via broad-spectrum PCR assays using consensus primers or non-specific amplification by RCA, combined with deep sequencing.
Although BPV4 was associated with the emergence of papillomas in the upper alimentary tract of cattle, a retrospective survey was conducted using 47 papillomas in the mouth and esophagus of 30 animals with upper digestive canal carcinomas, collected from Southern Brazil between 2003 and 2014; PCR assays conducted using both the FAP primer pair and specific primers targeting the BPV4 L1 gene did not reveal BPV DNA in these lesions [
29]. In the present study, RCA of the BPV genomes followed by HTS successfully identified BPV2 and 4 sequences in the CIS lesion, as well as BPV4 in a squamous papilloma in the upper alimentary tract of a dairy bull, which indicates that BPV4 is not the only BPV type present in the CIS developed in the bovine esophageal mucous membrane. This molecular strategy potentially allows the identification of diverse BPV types, even in mixed infections, in different hyperproliferative lesions of cattle, deepening the research on BPV. In the northern region of Brazil, cutaneous papillomas collected from cattle were reported to contain three viral types previously characterized in one sample and BPV24, a novel type of BPV, in another sample; notably, both samples were collected from the skin lesions of a single animal [
30]. In an investigation carried out on cows slaughtered in another state of Southern Brazil, RCA and HTS-based assay using 23 teat warts revealed seven known viral types (BPV3, 4, 6, 8, 9, 12, and 27); moreover, 14 new types (BPV30–BPV43) were first characterized [
14].
In conclusion, the present study, for the first time, demonstrates the association of BPV2 with a CIS lesion in the bovine upper alimentary tract and reveals the occurrence of mixed infection by BPV2 and BPV4 in the tumor. Since mixed infection in esophageal CIS could not be detected by common molecular tools, such as PCR using degenerate primers followed by Sanger sequencing of the amplicons, the use of non-specific molecular techniques, such as RCA and HTS, is essential for advancement in PV research. This is the first study that revealed the association between BPV and proliferative lesions in the bovine upper digestive canal using this molecular strategy.
Author Contributions
Conceptualization, M.L., B.F.M. and A.A.A.; methodology, M.L., B.F.M., L.A.V. and A.A.A.; validation, M.L. B.F.M., and A.A.A.; formal analysis, M.L., B.F.M. and K.C.B.G.; investigation, M.L., B.F.M., A.P.F.B., L.F.C.C.F. and A.F.A.; resources, A.A.A., B.F.M., L.F.C.C.F., L.A.V. and A.P.F.B.; data curation, K.C.B.G. and L.A.V.; writing—original draft preparation, B.F.M. and M.L.; writing—review and editing, M.L., B.F.M., A.A.A. and A.P.F.B.; visualization, M.L., B.F.M., A.A.A., A.F.A., L.A.V. and L.F.C.C.F..; supervision, A.A.A.; project administration, M.L.; funding acquisition, A.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the following Brazilian Institutes for their financial support: the National Council of Technological and Scientific Development (CNPq); the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES); and the Financing of Studies and Projects (FINEP). This work was supported by the National Institute of Science and Technology of Dairy Production Chain (CNPq/INCT-Leite) [Grant No. 465725/2014-7]. AAA is a recipient of CNPq Fellowships.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee for Animal Use of the Universidade Estadual de Londrina (protocol number 050.2021).
Data Availability Statement
The original data presented in the study are openly available in GenBank database under the following accession numbers: PQ140660-PQ140664.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Daudt, C.; da-Silva, F.R.C.; Lunardi, M.; Alves, C.B.D.T.; Weber, M.N.; Cibulski, S.P.; Alfieri, A.F.; Alfieri, A.A.; Canal, C.W. Papillomaviruses in ruminants: An update. Transbound. Emerg. Dis. 2018, 00, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Freese, U. K.; Gissmann, L.; Mayer, W.; Roggenbuck, B.; Stremlau, A.; Hausen, H. Z. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985, 314, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S. Bovine papillomavirus and cancer. Vet. J. 1997, 154, 175–188. [Google Scholar] [CrossRef] [PubMed]
- ICTV International Committee on Taxonomy of Viruses. Available online: https://ictv.global (accessed on 15 March 2024).
- PaVE Papillomavirus Episteme. Available online: https://pave.niaid.nih.gov (accessed on 13 March 2024).
- Munday, J.S. Bovine and human papillomaviruses: A comparative review. Vet. Pat. 2014, 51, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Jarret, W.F.H.; McNeil, P.E.; Grimshaw, W.T.R.; Selman, I.E.; McIntyre, W.I.M. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature 1978, 274, 215–217. [Google Scholar] [CrossRef]
- Borzacchiello, G.; Roperto, F. Bovine papillomavirus, papillomas and cancer in cattle. Vet. Res. 2008, 39, 45. [Google Scholar] [CrossRef]
- Campo, M.S.; O’Neil, B.W.; Barron, R.J.; Jarret, W.F.H. Experimental reproduction of the papiloma-carcinoma complex of the alimentary canal in cattle. Carcinogenesis 1994, 15, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Jarret, W.F.H.; Campo, M.S.; Blaxter, M.L.; O’Neil, B.W.; Laird, H.M.; Moar, M.H.; Sartirana, M.L. Alimentary fibropapilloma in cattle: A spontaneous tumor, nonpermissive for papillomavirus replication. J. Natl. Cancer Inst. 1984, 73, 499–504. [Google Scholar] [CrossRef]
- Rector, A.; Bossart, G.D.; Ghim, S.; Sundberg, J.P.; Jenson, A.B.; Ranst, M.V. Characterization of a novel close-to-root papillomavirus from a Florida manatee by using multiply primed rolling-circle amplification: Trichechus manatus latirostris papillomavirus type 1. J. Virol. 2004, 78, 12698–12702. [Google Scholar] [CrossRef]
- Daudt, C.; da-Silva, F.R.C.; Streck, A.F.; Weber, M.N.; Mayer, F.Q.; Cibulski, S.P.; Canal, C.W. How many papillomavirus species can go undetected in papiloma lesios? Sci. Rep. 2016, 6, 36480. [Google Scholar] [CrossRef]
- Sauthier, J.T.; Daudt, C.; da-Silva, F.R.C.; Alves, C.D.B.T.; Mayer, F.Q.; Bianchi, R.M.; Driemeier, D.; Streit, R.S.A.; Staats, C.C.; Canal, C.W.; Weber, M.N. The genetic diversity of “papillomavirome” in bovine teat papiloma lesions. Anim. Microbiome 2021, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Melo, T.C.; Neves, A.C.; Spadacci-Morena, D.D.; Magnelli, D.G.; Modolo, D.G.; de-Sá-Júnior, P.L.; Mazucchelli-de-Souza, J.; Carvalho, R.F.; Beçak, W.; Stocco, R.C. Hyperproliferative action of bovine papillomavirus: Genetic and histopathological aspects. Genet. Mol. Res. 2015, 14, 12942–12954. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.M.; Ting, Y.; Wright, D.K.; Lewis, A.J.; Broker, T.R.; Wolinsky, S.M. The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cell 1989, 7, 209–214. [Google Scholar]
- Forslund, O.; Antonsson, A.; Nordin, P.; Stenquist, B.; Hansson, B.G. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J. Gen. Virol. 1999, 80, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; Van-Doorslaer, K.; Bertelsen, M.; Barker, I.K.; Olberg-Lemey, R.A.P.; Sundberg, J.P.; Van-Ranst, M. Isolation and cloning of the raccoon (Procyon lotor) papillomavirus type 1 by using degenerate papillomavirus-specific primers. J. Gen. Virol. 2005, 86, 2029–2033. [Google Scholar] [CrossRef]
- Rector, A.; Tachezy, R.; Van Ranst, M. A sequence-independent strategy for detection and cloning of circular DNA virus genomes by using multiply primed rolling-circle amplification. J. Virol. 2004, 78, 4993–4998. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: A quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Araldi, R.P.; Assaf, S.M.R.; de-Carvalho, R.F; de-Carvalho, M.A.C.R.; de-Souza, J.M.; Magnelli, R.F.; Módolo, D.G.; Roperto, F.P.; Stocco, R.C.; Beçak, W. Papillomaviruses: A systematic review. Genet. Mol. Bio. 2017, 40, 1–21. [Google Scholar] [CrossRef]
- Campo, M.S.; Moar, M.H.; Sartirana, M.L.; Kennedy, I.M.; Jarret, W.F.H. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. 1985, 4, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.E.; O’Brien, V.; Morgan, I.M.; Grindlay, G.J.; Campo, M.S. Bovine papillomavirus type 4: Neoplastic cell transformation and control of infection by vaccination (review). Int. J. Oncol. 1996, 9, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.P.; Alfieri, A.A.; Darold, G.M.; Boabaid, F.M.; Agnol, A.M.D.; Lunardi, M. Case report: Mixed infection of bovine papillomaviruses associated with squamous papiloma of the upper alimentary tract in a dairy cow. Front. Vet. Sci. 2022, 9, 1020166. [Google Scholar] [CrossRef] [PubMed]
- Tsirimonaki, E.; O’Neil, B.W.; Williams, R.; Campo, M.S. Extensive papillomatosis of the bovine upper gastrointestinal tract. J. Comp. Pathol. 2003, 129, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, G.; Ambrosio, V.; Roperto, S.; Poggiali, F.; Tsirimonakis, E.; Venuti, A.; Campo, M.S.; Roperto, F. 2003. Bovine papillomavirus type 4 in oesophageal papillomas of cattle from the south of Italy. J. Comp. Pathol. 2003, 128, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, N.; Saikumar, G.; Arya, R.S.; Somvanshi, R. Detection of bovine papilloma viruses in wart-like lesions of upper gastrointestinal tract of cattle and buffaloes. Transbound. Emerg. Dis. 2013, 62, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Faccin, T.C.; Cargnelutti, J.F.; Rodrigues, F.S.; de-Menezes, F.R.; Piazer, J.V.M.; de-Melo, S.M.P.; Lautert, B.F.; Flores, E.F.; Kommers, G.D. Bovine upper alimentary squamous cell carcinoma associated with bracken fern poisoning: Clinical-pathological aspects and etiopathogenesis of 100 cases. PLoS ONE 2018, 13, e0204656. [Google Scholar] [CrossRef]
- Daudt, C.; da-Silva, F.R.C.; Cibulski, S.P.; Streck, A.F.; Laurle, R.E.; Munday, J.S.; Canal, W.C. Bovine papillomavirus 24: A novel member of the genus Xipapillomavirus detected in the Amazon region. Arch. Virol. 2019, 164, 637–641. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).