Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
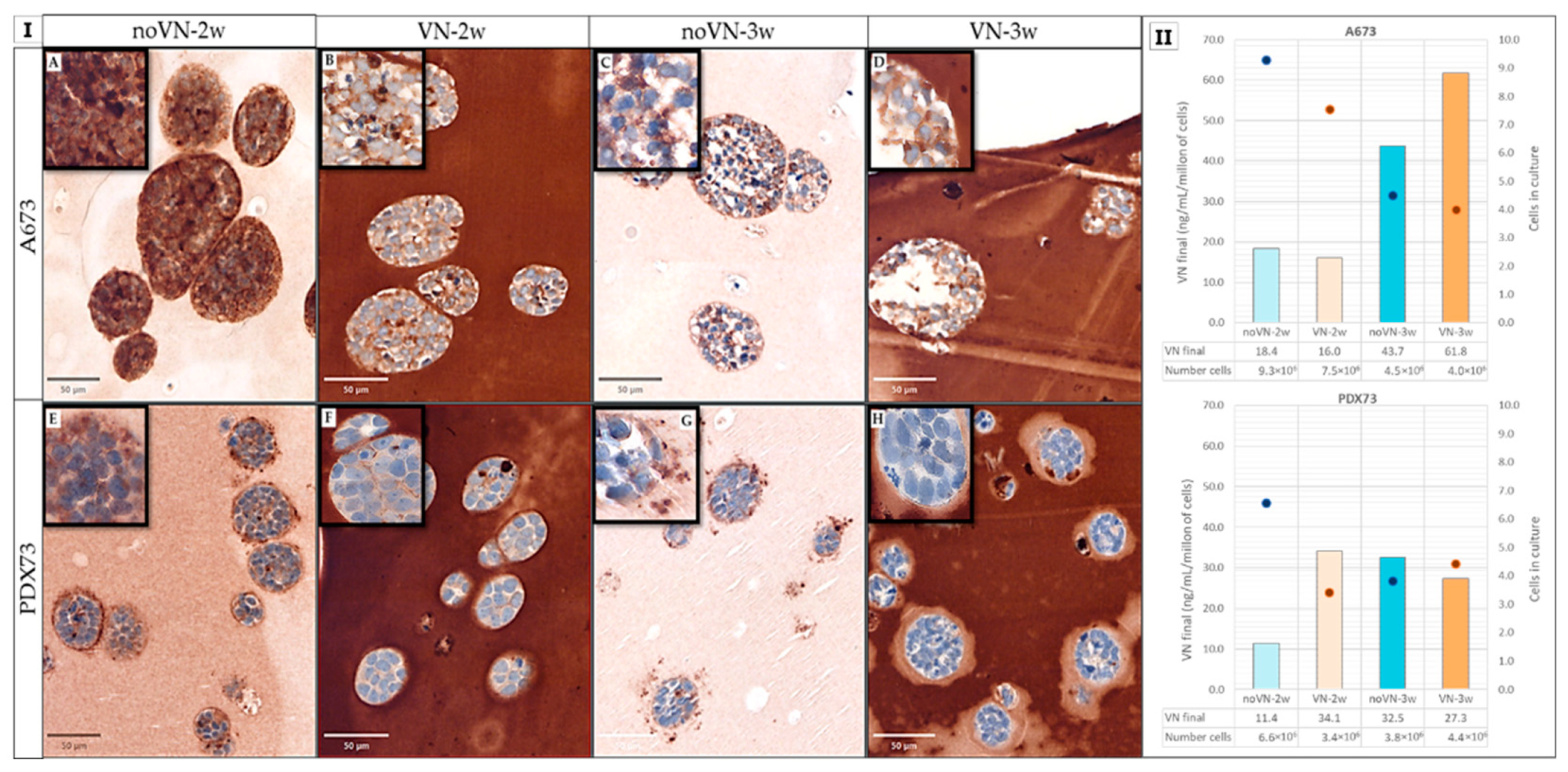
2.1. VN cell Expression and Secretion to Culture Media in 2D Models
2.2. VN Cell Expression and Secretion to the ECM in 3D Models
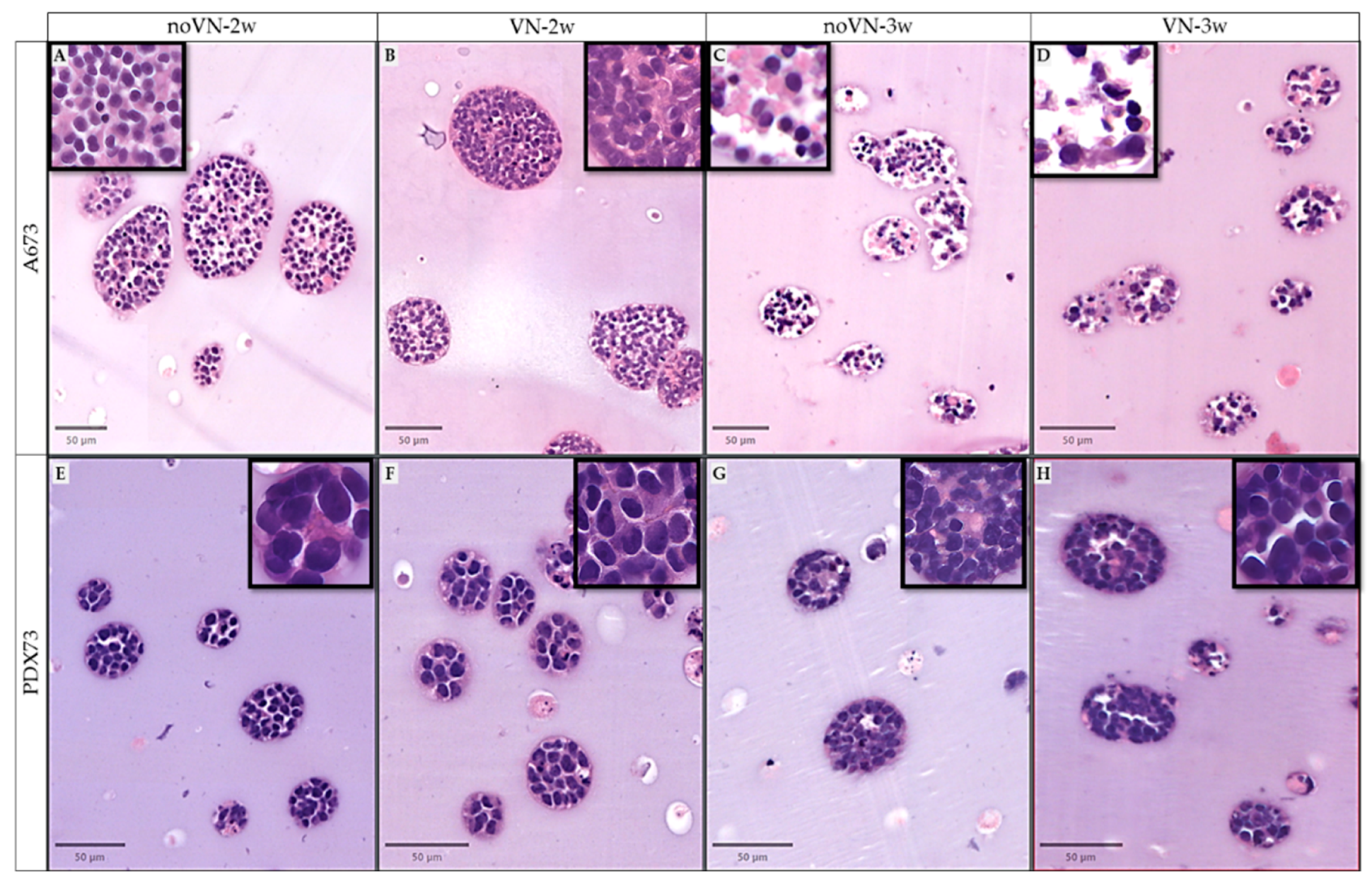
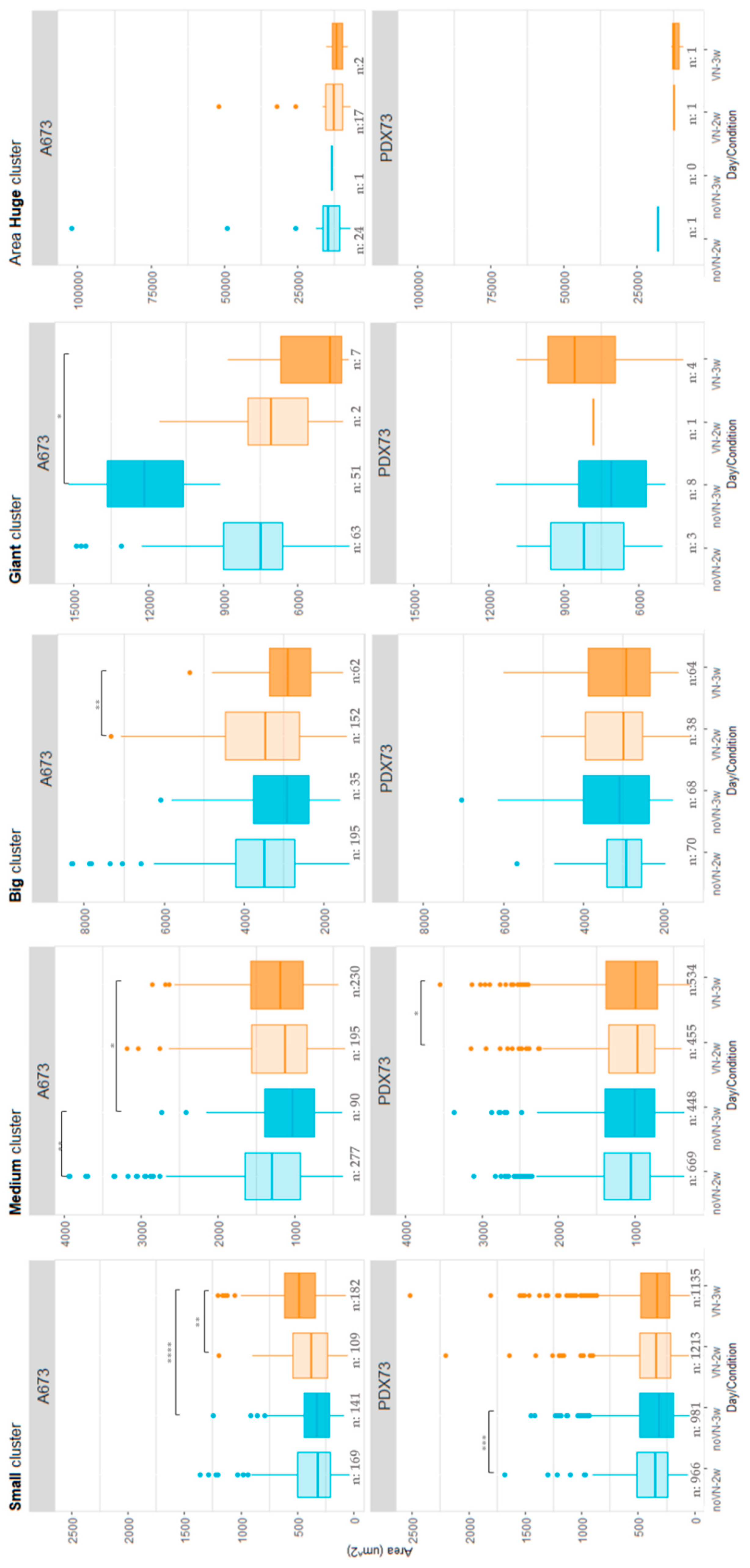
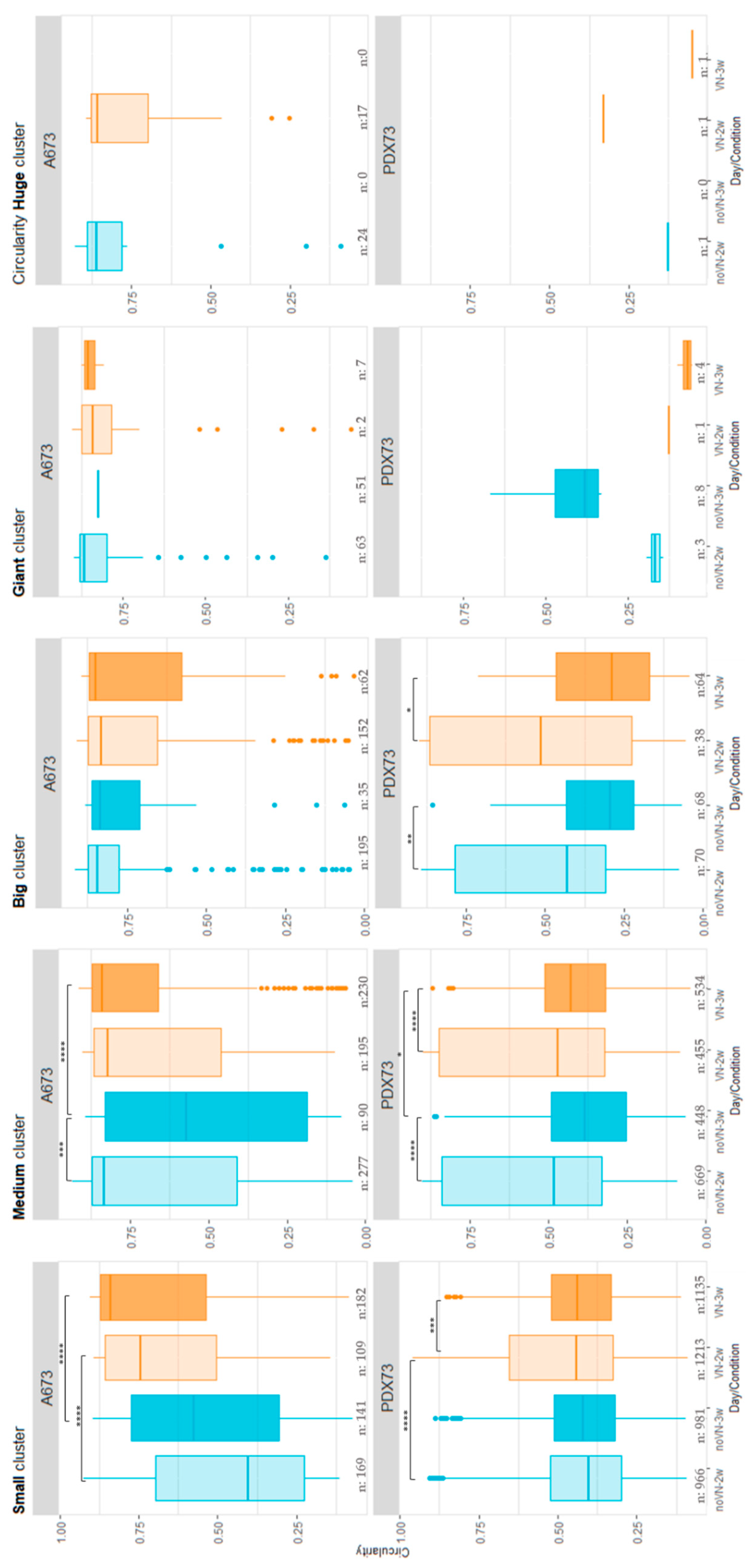
2.3. Digital Analisis and Morphometric Parameters of Clusters and Cells of 3D Models
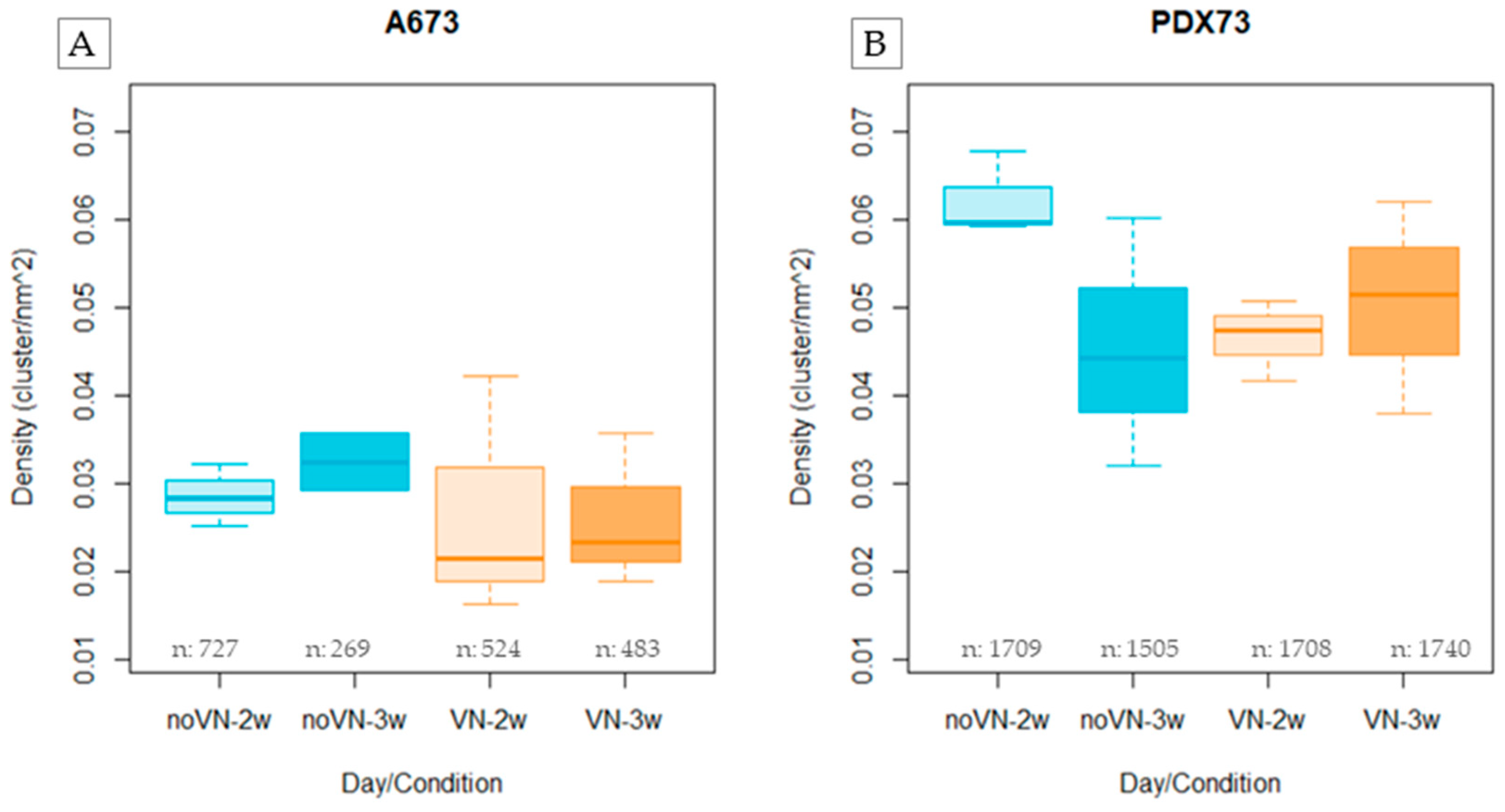
2.3.1. Cluster Density
2.3.1.1. Cell Density by Cluster Size
2.3.2. Cluster Areas
2.3.3. Circularity of Clusters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Monolayer Cultures (2D)
5.2. Hydrogel Cultures (3D)
5.3.2. D and 3D Sample Preparation for Digital Analysis
5.4. Digital Microscopic Analyses
5.5. Detection of VN in Culture Media
5.6. Statistical Analysis
Supplementary Materials
Author’s Note:
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Newman, E.A.; Abdessalam, S.; Aldrink, J.H.; Austin, M.; Heaton, T.E.; Bruny, J.; Ehrlich, P.; Dasgupta, R.; Baertschiger, R.M.; Lautz, T.B.; et al. Update on Neuroblastoma. J Pediatr Surg 2019, 54, 383–389. [CrossRef]
- Grünewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Álava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing Sarcoma. Nat Rev Dis Primers 2018, 4, 5. [CrossRef]
- Gargallo, P.; Yáñez, Y.; Juan, A.; Segura, V.; Balaguer, J.; Torres, B.; Oltra, S.; Castel, V.; Cañete, A. Review: Ewing Sarcoma Predisposition. Pathology & Oncology Research 2020, 26, 2057–2066. [CrossRef]
- Hameed, M. Small Round Cell Tumors of Bone. Arch Pathol Lab Med 2007, 131, 192-204. [CrossRef]
- Ozaki, T. Diagnosis and Treatment of Ewing Sarcoma of the Bone: A Review Article. Journal of Orthopaedic Science 2015, 20, 250–263. [CrossRef]
- Zöllner, S.K.; Amatruda, J.F.; Bauer, S.; Collaud, S.; de Álava, E.; DuBois, S.G.; Hardes, J.; Hartmann, W.; Kovar, H.; Metzler, M.; et al. Ewing Sarcoma—Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J Clin Med 2021, 10, 1685. [CrossRef]
- Eaton, B.R.; Claude, L.; Indelicato, D.J.; Vatner, R.; Yeh, B.; Schwarz, R.; Laack, N. Ewing Sarcoma. Pediatr Blood Cancer 2021, 68. [CrossRef]
- Setty, B.A.; Gikandi, A.; DuBois, S.G. Ewing Sarcoma Drug Therapy: Current Standard of Care and Emerging Agents. Pediatric Drugs 2023, 25, 389–397. [CrossRef]
- Sanegre, S.; Lucantoni, F.; Burgos-Panadero, R.; de La Cruz-Merino, L.; Noguera, R.; Álvaro Naranjo, T. Integrating the Tumor Microenvironment into Cancer Therapy. Cancers (Basel) 2020, 12, 1677. [CrossRef]
- Burgos-Panadero, R.; Lucantoni, F.; Gamero-Sandemetrio, E.; Cruz-Merino, L. de la; Álvaro, T.; Noguera, R. The Tumour Microenvironment as an Integrated Framework to Understand Cancer Biology. Cancer Lett 2019, 461, 112–122. [CrossRef]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New Horizons in Tumor Microenvironment Biology: Challenges and Opportunities. BMC Med 2015, 13, 45. [CrossRef]
- Noguera, R.; Nieto, O.A.; Tadeo, I.; Fariñas, F.; Álvaro, T. Extracellular Matrix, Biotensegrity and Tumor Microenvironment. An Update and Overview. Histol Histopathol 2012, 27, 693–705. [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep 2014, 15, 1243–1253. [CrossRef]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front Oncol 2020, 9. [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [CrossRef]
- López-Carrasco, A.; Martín-Vañó, S.; Burgos-Panadero, R.; Monferrer, E.; Berbegall, A.P.; Fernández-Blanco, B.; Navarro, S.; Noguera, R. Impact of Extracellular Matrix Stiffness on Genomic Heterogeneity in MYCN-Amplified Neuroblastoma Cell Line. Journal of Experimental & Clinical Cancer Research 2020, 39, 226. [CrossRef]
- Ben-David, U.; Ha, G.; Tseng, Y.-Y.; Greenwald, N.F.; Oh, C.; Shih, J.; McFarland, J.M.; Wong, B.; Boehm, J.S.; Beroukhim, R.; et al. Patient-Derived Xenografts Undergo Mouse-Specific Tumor Evolution. Nat Genet 2017, 49, 1567–1575. [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol Cancer 2023, 22, 48. [CrossRef]
- Hawkins, A.G.; Julian, C.M.; Konzen, S.; Treichel, S.; Lawlor, E.R.; Bailey, K.M. Microenvironmental Factors Drive Tenascin C and Src Cooperation to Promote Invadopodia Formation in Ewing Sarcoma. Neoplasia 2019, 21, 1063–1072. [CrossRef]
- Rodríguez-Núñez, P.; Romero-Pérez, L.; Amaral, A.T.; Puerto-Camacho, P.; Jordán, C.; Marcilla, D.; Grünewald, T.G.; Alonso, J.; de Alava, E.; Díaz-Martín, J. Hippo Pathway Effectors YAP1/TAZ Induce an EWS–FLI1 -opposing Gene Signature and Associate with Disease Progression in Ewing Sarcoma. J Pathol 2020, 250, 374–386. [CrossRef]
- Bierbaumer, L.; Katschnig, A.M.; Radic-Sarikas, B.; Kauer, M.O.; Petro, J.A.; Högler, S.; Gurnhofer, E.; Pedot, G.; Schäfer, B.W.; Schwentner, R.; et al. YAP/TAZ Inhibition Reduces Metastatic Potential of Ewing Sarcoma Cells. Oncogenesis 2021, 10. [CrossRef]
- Vasileva, E.; Warren, M.; Triche, T.J.; Amatruda, J.F. Dysregulated Heparan Sulfate Proteoglycan Metabolism Promotes Ewing Sarcoma Tumor Growth. Elife 2022, 11. [CrossRef]
- Wullkopf, L.; West, A.-K. V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer Cells’ Ability to Mechanically Adjust to Extracellular Matrix Stiffness Correlates with Their Invasive Potential. Mol Biol Cell 2018, 29, 2378–2385. [CrossRef]
- Aveic, S.; Davtalab, R.; Vogt, M.; Weber, M.; Buttler, P.; Tonini, G.P.; Fischer, H. Calcium Phosphate Scaffolds with Defined Interconnecting Channel Structure Provide a Mimetic 3D Niche for Bone Marrow Metastasized Tumor Cell Growth. Acta Biomater 2019, 88, 527–539. [CrossRef]
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Noel, A.; et al. Proteases, Extracellular Matrix, and Cancer. Am J Pathol 2004, 164, 1131–1139. [CrossRef]
- Shi, K.; Lan, R.-L.; Tao, X.; Wu, C.-Y.; Hong, H.-F.; Lin, J.-H. Vitronectin Significantly Influences Prognosis in Osteosarcoma. Int J Clin Exp Pathol 2015, 8, 11364–11371.
- Tas, F.; Karabulut, S.; Bilgin, E.; Tastekin, D.; Duranyildiz, D. Clinical Significance of Serum Fibronectin and Vitronectin Levels in Melanoma Patients. Melanoma Res 2014, 24, 475–479. [CrossRef]
- Zhu, W.; Li, W.; Yang, G.; Fu, C.; Jiang, G.; Hu, Q. Vitronectin Silencing Inhibits Hepatocellular Carcinoma in Vitro and in Vivo. Future Oncology 2015, 11, 251–258. [CrossRef]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M.; Radhakrishnan, S.; Gana, N.; Rothwell, S.; Pollard, H.; Hu, H.; Shriver, C.D.; et al. Functional Role of Vitronectin in Breast Cancer. PLoS One 2020, 15, e0242141. [CrossRef]
- Ciereszko, A.; Dietrich, M.A.; Słowińska, M.; Nynca, J.; Ciborowski, M.; Kisluk, J.; Michalska-Falkowska, A.; Reszec, J.; Sierko, E.; Nikliński, J. Identification of Protein Changes in the Blood Plasma of Lung Cancer Patients Subjected to Chemotherapy Using a 2D-DIGE Approach. PLoS One 2019, 14, e0223840. [CrossRef]
- Schneider, G.; Suszynska, M.; Kakar, S.; Ratajczak, M.Z. Vitronectin in the Ascites of Human Ovarian Carcinoma Acts as a Potent Chemoattractant for Ovarian Carcinoma: Implication for Metastasis by Cancer Stem Cells. J Cancer Stem Cell Res 2016, 4, 1. [CrossRef]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a Molecular Player of the Tumor Microenvironment in Neuroblastoma. BMC Cancer 2019, 19, 479. [CrossRef]
- Lopez-Carrasco, A.; Diaz-Martin, J.; Machado, I.; Navarro, S.; Alava, E.; Noguera, R. The Morphology of the Extracellular Matrix as a Classifier of Types and Subtypes of Ewing and Ewing-like Sarcomas: A Morpho-Molecular Study. Histology and Histopathology 2021, 37, supp. 1, p. 109.
- Guerrieri, A.N.; Bellotti, C.; Penzo, M.; Columbaro, M.; Pannella, M.; De Vita, A.; Gambarotti, M.; Mercatali, L.; Laranga, R.; Dozza, B.; et al. A Novel Patient-Derived Immortalised Cell Line of Myxofibrosarcoma: A Tool for Preclinical Drugs Testing and the Generation of near-Patient Models. BMC Cancer 2023, 23, 1194. [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An Organoid Platform for Ovarian Cancer Captures Intra- and Interpatient Heterogeneity. Nat Med 2019, 25, 838–849. [CrossRef]
- Braekeveldt, N.; Wigerup, C.; Gisselsson, D.; Mohlin, S.; Merselius, M.; Beckman, S.; Jonson, T.; Börjesson, A.; Backman, T.; Tadeo, I.; et al. Neuroblastoma Patient-derived Orthotopic Xenografts Retain Metastatic Patterns and Geno- and Phenotypes of Patient Tumours. Int J Cancer 2015, 136. [CrossRef]
- Rae, C.; Amato, F.; Braconi, C. Patient-Derived Organoids as a Model for Cancer Drug Discovery. Int J Mol Sci 2021, 22, 3483. [CrossRef]
- Munoz-Garcia, J.; Jubelin, C.; Loussouarn, A.; Goumard, M.; Griscom, L.; Renodon-Cornière, A.; Heymann, M.-F.; Heymann, D. In Vitro Three-Dimensional Cell Cultures for Bone Sarcomas. J Bone Oncol 2021, 30, 100379. [CrossRef]
- Santoro, M.; Lamhamedi-Cherradi, S.-E.; Menegaz, B.A.; Ludwig, J.A.; Mikos, A.G. Flow Perfusion Effects on Three-Dimensional Culture and Drug Sensitivity of Ewing Sarcoma. Proceedings of the National Academy of Sciences 2015, 112, 10304–10309. [CrossRef]
- Monferrer, E.; Sanegre, S.; Martín-Vañó, S.; García-Lizarribar, A.; Burgos-Panadero, R.; López-Carrasco, A.; Navarro, S.; Samitier, J.; Noguera, R. Digital Image Analysis Applied to Tumor Cell Proliferation, Aggressiveness, and Migration-Related Protein Synthesis in Neuroblastoma 3D Models. Int J Mol Sci 2020, 21, 8676. [CrossRef]
- Vieco-Martí, I.; Monferrer, E.; López-Carrasco, A.; Granados-Aparici, S.; Navarro, S.; Noguera, R. Building Silk-Fibroin 3D Hydrogels with Enzymatically Cross-Linked Vitronectin to Study Neuroblastoma Aggressiveness. Histology and Histopathology 2021, 37, supp. 1, p. 152.
- Choi, E.Y.K.; Gardner, J.M.; Lucas, D.R.; McHugh, J.B.; Patel, R.M. Ewing Sarcoma. Semin Diagn Pathol 2014, 31, 39–47. [CrossRef]
- Lamhamedi-Cherradi, S.-E.; Santoro, M.; Ramammoorthy, V.; Menegaz, B.A.; Bartholomeusz, G.; Iles, L.R.; Amin, H.M.; Livingston, J.A.; Mikos, A.G.; Ludwig, J.A. 3D Tissue-Engineered Model of Ewing’s Sarcoma. Adv Drug Deliv Rev 2014, 79–80, 155–171. [CrossRef]
- Cacho-Díaz, B.; García-Botello, D.R.; Wegman-Ostrosky, T.; Reyes-Soto, G.; Ortiz-Sánchez, E.; Herrera-Montalvo, L.A. Tumor Microenvironment Differences between Primary Tumor and Brain Metastases. J Transl Med 2020, 18, 1. [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Baradaran, B.; de la Guardia, M.; Mokhtarzadeh, A. Advanced Mechanotherapy: Biotensegrity for Governing Metastatic Tumor Cell Fate via Modulating the Extracellular Matrix. Journal of Controlled Release 2021, 335, 596–618. [CrossRef]
- Tadeo, I.; Berbegall, A.P.; Escudero, L.M.; Ãlvaro, T.; Noguera, R. Biotensegrity of the Extracellular Matrix: Physiology, Dynamic Mechanical Balance, and Implications in Oncology and Mechanotherapy. Front Oncol 2014, 4. [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Y. Artificial Intelligence Applications in Pediatric Oncology Diagnosis. Explor Target Antitumor Ther 2023, 157–169. [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Modern Pathology 2022, 35, 23–32. [CrossRef]
- Hanna, M.G.; Ardon, O.; Reuter, V.E.; Sirintrapun, S.J.; England, C.; Klimstra, D.S.; Hameed, M.R. Integrating Digital Pathology into Clinical Practice. Modern Pathology 2022, 35, 152–164. [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered Glycosylation in Cancer: A Promising Target for Biomarkers and Therapeutics. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2021, 1875, 188464. [CrossRef]
- Schneider, G.; Bryndza, E.; Poniewierska-Baran, A.; Serwin, K.; Suszynska, M.; Sellers, Z.P.; Merchant, M.L.; Kaliappan, A.; Ratajczak, J.; Kucia, M.; et al. Evidence That Vitronectin Is a Potent Migration-Enhancing Factor for Cancer Cells Chaperoned by Fibrinogen: A Novel View of the Metastasis of Cancer Cells to Low-Fibrinogen Lymphatics and Body Cavities. Oncotarget 2016, 7, 69829–69843. [CrossRef]
- Chen, M.H.; Lu, C.; Sun, J.; Chen, X.D.; Dai, J.X.; Cai, J.Y.; Chen, X.L. Diagnostic and Prognostic Value of Serum Vitronectin Levels in Human Glioma. J Neurol Sci 2016, 371, 54–59. [CrossRef]
- Kadowaki, M.; Sangai, T.; Nagashima, T.; Sakakibara, M.; Yoshitomi, H.; Takano, S.; Sogawa, K.; Umemura, H.; Fushimi, K.; Nakatani, Y.; et al. Identification of Vitronectin as a Novel Serum Marker for Early Breast Cancer Detection Using a New Proteomic Approach. J Cancer Res Clin Oncol 2011, 137, 1105–1115. [CrossRef]
- Turan, T.; Torun, M.; Atalay, F.; Gönenç, A. Assessment of Vitronectin, Soluble Epithelial-Cadherin and TGF-Β1 as a Serum Biomarker with Predictive Value for Endometrial and Ovarian Cancers. Turk J Pharm Sci 2017, 14, 141–147. [CrossRef]
- Repetto, O.; Caggiari, L.; De Zorzi, M.; Elia, C.; Mussolin, L.; Buffardi, S.; Pillon, M.; Muggeo, P.; Casini, T.; Steffan, A.; et al. Quantitative Plasma Proteomics to Identify Candidate Biomarkers of Relapse in Pediatric/Adolescent Hodgkin Lymphoma. Int J Mol Sci 2022, 23, 1–15. [CrossRef]
- Braoudaki, M.; Lambrou, G.I.; Vougas, K.; Karamolegou, K.; Tsangaris, G.T.; Tzortzatou-Stathopoulou, F. Protein Biomarkers Distinguish between High- and Low-Risk Pediatric Acute Lymphoblastic Leukemia in a Tissue Specific Manner. J Hematol Oncol 2013, 6. [CrossRef]
- López-Carrasco, A.; Vieco-Martí, I.; Granados-Aparici, S.; Acevedo-León, D.; Estañ-Capell, N.; Portugal, R.; Huerta-Aragonés, J.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin Levels in Plasma of Neuroblastoma Patients and Culture Media of 3D Models: A Prognostic Circulating Biomarker?. Preprints 2024, 2024070957. [CrossRef]
- Stepanek, O.; Brdicka, T.; Angelisova, P.; Horvath, O.; Spicka, J.; Stockbauer, P.; Man, P.; Horejsi, V. Interaction of Late Apoptotic and Necrotic Cells with Vitronectin. PLoS One 2011, 6, e19243. [CrossRef]
- Goyal, U.; Ta, M. A Novel Role of Vitronectin in Promoting Survival of Mesenchymal Stem Cells under Serum Deprivation Stress. Stem Cell Res Ther 2020, 11, 181. [CrossRef]
- Leavesley, D.I.; Kashyap, A.S.; Croll, T.; Sivaramakrishnan, M.; Shokoohmand, A.; Hollier, B.G.; Upton, Z. Vitronectin—Master Controller or Micromanager? IUBMB Life 2013, 65, 807–818. [CrossRef]
- Heyman, L.; Leroy-Dudal, J.; Fernandes, J.; Seyer, D.; Dutoit, S.; Carreiras, F. Mesothelial Vitronectin Stimulates Migration of Ovarian Cancer Cells. Cell Biol Int 2010, 34, 493–502. [CrossRef]
- Kurozumi K.; Ichikawa T.; Onishi M.; Fujii K.; Date I. Cilengitide treatment for malignant glioma: current status and future direction. Neurol Med Chir (Tokyo) 2012, 52, 539-47. [CrossRef] [PubMed]
- Stoks, M.; Vieco-Martí, I.; Noguera, I.; Sánchez-Sánchez, M.; Burgos-Panadero, R.; Navarro, S.; Noguera, R. Digital Image Analysis Workflows for Evaluation of Cell Behavior and Tumor Microenvironment to Aid Therapeutic Assessment in High-Risk Neuroblastoma. Comput Biol Med 2023, 164. [CrossRef]
- Burgos-Panadero, R.; El Moukhtari, S.H.; Noguera, I.; Rodríguez-Nogales, C.; Martín-Vañó, S.; Vicente-Munuera, P.; Cañete, A.; Navarro, S.; Blanco-Prieto, M.J.; Noguera, R. Unraveling the Extracellular Matrix-Tumor Cell Interactions to Aid Better Targeted Therapies for Neuroblastoma. Int J Pharm 2021, 608. [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. 2019. [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci Rep 2017, 7, 16878. [CrossRef]

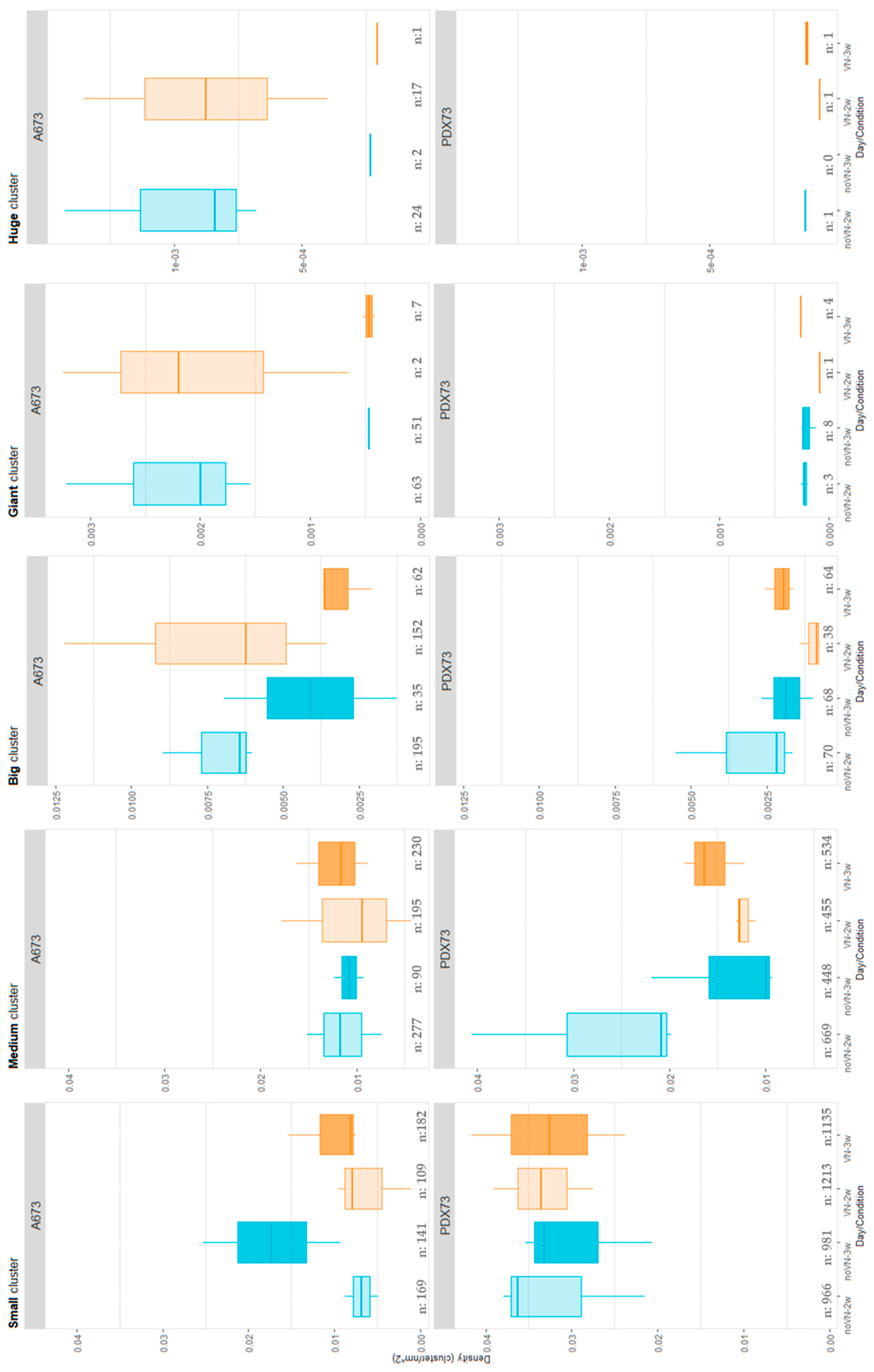
| Number of Cells | Classification | Color Code |
|---|---|---|
| <9 >10 and 38< |
Small Medium |
Cyan Green |
| >39 and 95< >96 and 169< >170 |
Big Giant Huge |
Yellow Magenta Blue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





