1. Introduction
Companies worldwide produce metallurgical-grade silicon, with key production regions including China, Russia, Brazil, the USA, Canada, and European countries. According to data from the US Geological Survey (Mineral Commodity Summaries), in 2023, around 4000 metric tons of metallurgical-grade silicon were produced globally, with 3000 metric tons produced in China.
Table 1 provides an overview of production capacities using various technologies and materials.
The table shows that traditional materials for smelting metallurgical-grade silicon are quartz and coke (reductant). Additionally, the charge includes additives such as wood chips (shavings).
Technological operations in silicon production involve the preparation of the charge, its smelting in a submerged arc furnace, casting of silicon, and grinding it to remove slag inclusions.
The raw materials for carbothermic silicon production are:
- -
For the ore part of the charge: quartz or quartzite containing at least 98.0% SiO₂;
- -
As reducing agents, traditionally used are charcoal and petroleum coke, the use of the latter is mainly due to its low cost and low impurity content. To loosen the charge and improve its gas permeability, wood chips are also introduced into the charge.
All raw materials must meet certain requirements for fractional composition. Quartz or quartzite is crushed into pieces no larger than 80 mm, with the use of fines below 20 mm not allowed, as their melting in the upper zone of the charge worsens the gas permeability and disrupts the furnace operation. Petroleum coke, having relatively high electrical conductivity, is crushed into pieces no larger than 15 mm. Charcoal is fed into the furnace crushed to 80 mm. Fines of the reducing agents with a size class of -2 mm are sieved out, as their direct combustion in the charge leads to a lack of reducing agent.
Due to the high purity requirements of silicon, there have been no instances of using alternative raw materials in practice. However, it is worth noting that global industrial enterprises are focusing on recycling production waste and creating comprehensive resource-saving technologies that involve the processing of waste and non-standard raw materials.
Production of metallurgical grade silicon is accompanied with a large amount of dust emissions, i.e. microsilica from 300 kg to 900 kg per tonne of melted silicon. It contains a substantial amount of silicon oxide [
1,
2].
A major problem of the technological production process of the technical-grade silicon is formation of a large amount of microsilica. However, this material can be a significant source of raw materials for its own production [
3].
Hence, this study is aimed to solve the problem of microsilica utilization, i.e. the technogenic dust-like waste generated during the melting of the technical-grade silicon.
Despite numerous attempts to solve this problem, microsilica is practically not recycled and constantly accumulates during the silicon production.
The issue to use the finely dispersed materials in the ore-thermal melting is very relevant. Developments are underway worldwide to improve the existing methods and to create the alternative ways to produce silicon from the small particle size materials. An important stage in producing of the high-quality silicon is preparation of such raw materials.
Microsilica is predominantly used to produce concrete with special properties, such as ultra-high strength, increased frost resistance, sulfate and corrosion resistance, and waterproofing [
4,
5]. In practice, its use in metallurgical processing as an additional source of silicon has not been recorded.
Research is underway to use the fine raw materials by the briquetting method. They concern the processing of industrial dust-like waste, i.e. the condensed microsilica [
6,
7,
8,
9]. These technologies have not been realized due to the scattering of briquettes on the top of an ore-thermal furnace during the thermal shock.
The problem to process microsilica is due to the fact that microsilica is a fine powder. Its direct processing (without preliminary preparation) into the technical-grade silicon is impossible in the ore-thermal furnaces. However, microsilica is a valuable raw material for silicon production. It has the ultra-low contents of the harmful components (e.g., the iron content is hundredths of a percent, 0.01-0.02%), and these components are removed during the formation of microsilica.
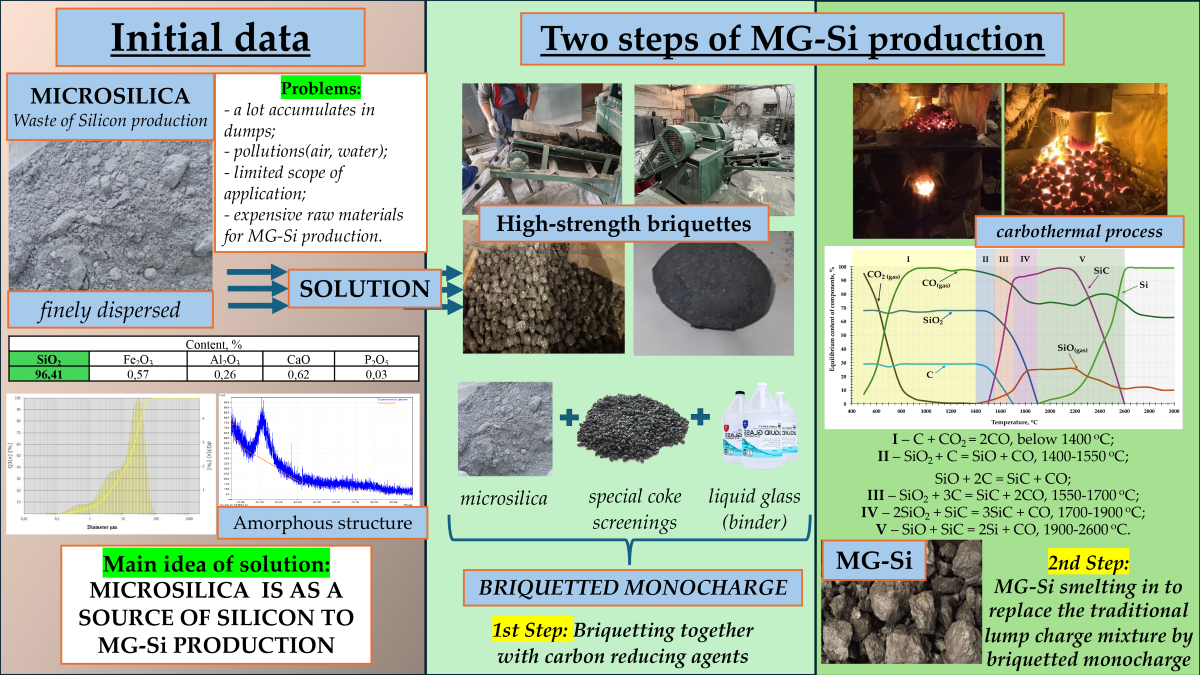
Within this study, the technology of the joint briquetting of microsilica was investigated with the carbonaceous reducing agents, i.e. by obtaining of mono-charge and by melting of the technical-grade silicon from the obtained mono-charge in the 200 kVA ore-thermal furnace.
Based on a close contact between silicon oxides of microsilica and solid carbon of carbonaceous reducing agents in the briquette, the most favourable conditions for silicon reduction and melting are achieved. Thus, deficient low ash reducing agents can be involved as the reducing agents for fine fractions.
For instance, a lump charcoal, imported mainly from the Russian Federation and obtained from whole pieces of wood, is currently used as a main reducing agent. The technology being developed can involve the charcoal fines which are not currently used. It can be obtained from the small wood and plant waste on an industrial scale in the conditions of the Republic of Kazakhstan.
Development and implementation of this technology will allow to solve the ecological problem of formation and storage of microsilica, and to increase the end-to-end silicon recovery up to 90-92%, i.e. currently the recovery is 65-75%. Therefore, the silicon obtained from microsilica will be of higher quality in terms of purity.
2. Materials and Methods
Microsilica is an ultra-dispersed material. It consists of the spherical particles. It is obtained in the fume cleaning process of furnaces during the production of the technical-grade silicon and ferrosilicon. The primary component of this material is amorphous silicon dioxide.
The chemical composition of microsilica is presented by, %: SiO2 – 96.41; Al2O3 – 0.26; CaO – 0.62; P2O5 – 0.03.
Briquettes containing screenings of the semi-coke and microsilica were produced on a large-scale laboratory roller briquetting press ZZXM-4 made by Henan Zhongzhou Heavy Industry Technology Co., Ltd (China). The carbonaceous reducing agent (semi-coke) was obtained from the long-flame gas coals of the Shubarkol deposit (Kazakhstan). It was characterized by the following technical composition (where: A - Ash Content; W – Coal moisture; V - Release of volatile substances; С – solid carbon content), %:
А – 2.81; W – 10.59; Va – 37.90; and Сsolid – 48.26.
The chemical composition of Shubarkol semi-coke ash, %:
SiO2 – 50.91; Fe2O3 – 7.53; Al2O3 – 21.07; CaO – 1.46; MnО – 1.45; MgO– 4.70; P – 0.133; and S – 0.06.
As a binding additive for briquetting, a liquid glass solution was used in the amount of 10-12% of the total mass of the charge mixture, consisting of 35% coke fines and 65% microsilica. This combination of components was chosen based on the fact that it contains enough solid carbon to intensify the reduction processes during the smelting of crystalline silicon.
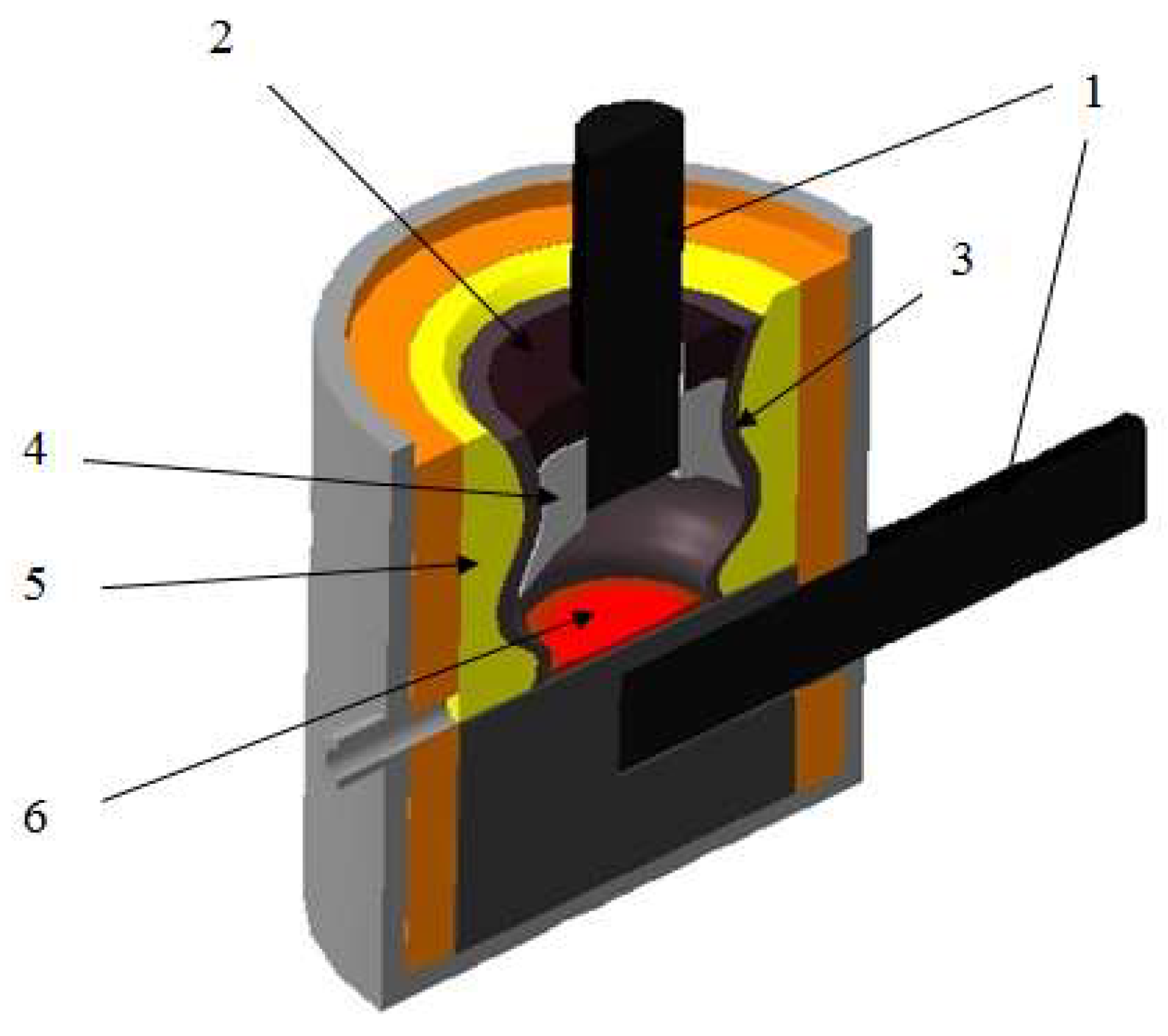
The melting tests of the technical-grade silicon using briquetted monocharge obtained from Shubarkol semi-coke (35%) and dust from gas purification system (microsilic - 65%) were conducted in a large laboratory single-phase arc furnace with a graphite electrode and a conductive hearth with a power of 200 kVA (
Figure 1). The electric furnace is two-electrode. One electrode is coked in the hearth by the hearth mass, i.e. the electric furnace has a structure similar to the Mige electric furnace. The transformer is powered by a voltage of 380 V [
10].
The electric furnace was powered by two OSU-100/0.5 transformers connected in parallel. The arc discharge temperature of 2500-4500°C is provided by a graphite electrode with a diameter of 150 mm. The furnace is lined with fireclay bricks. The furnace bath is formed as an ellipse with axes of 55-65 cm, elongated towards the tap hole. The distance from an electrode to a tap hole block is 21-22 cm, and to the back wall of the furnace is 29-30 cm. Bath depth is 35-40 cm. The hearth to a level of the furnace tap hole is sintered from an electrode mass which has been coked for 12 h under current with periodic shutdowns of the furnace. An electrode is moved mechanically by hand. The furnace is equipped with an electric meter connected via a 400/5 current transformer (with a factor of 80). Devices for measuring primary current and secondary voltage are also available. The limits of current variation on the high side are 0-500A, and voltage on the low side is 0-50 V.
The crystalline silicon is discharged through a tap hole. The tap hole is closed and opened with a wooden pole. The secondary voltage can be varied in steps. The furnace transformer has four voltage steps: 18.4V; 24.5V; 36.8V and 49.0V. The voltage drop in furnace operation is about 4-8V depending on a voltage step. A larger voltage drop is recorded at high steps.
The main electrical characteristics and technical parameters of the 200kVA ore-thermal electric furnace are listed in
Table 2 and
Table 3.
Prior to testing, work was conducted to prepare the ore-thermal furnace for the electric melting. The electric furnace was heated for 24 h on a coke bed used as a conductor of the electric current and to preserve the hearth. At the end of the heating period, the electric furnace was completely cleaned of coke bed residues. The electrical mode of the heating period: secondary voltage is 24.6 V; and high-side current is 150-200 A.
The hearth and assembly of tap hole were sintered with the crushed electrode mass. The electrical parameters of the melting process were maintained as follows.
The rated secondary voltage is 36V including voltage sag is 32V. The electric current intensity is 2500 A. The heavy first two furnace charges were loaded with a shortage of reducing agent to form the wall accretion.
3. Theoretical Mechanism of the Silicon Reduction Process
The process of silicon reduction in the electric furnace is continuous. As the charge melts, more charge is loaded into the furnace, and silicon is released from the furnace through a tap hole. The composition of the charge is calculated for 100 kg of quartzite or quartz with an excess of reducing agent by 10-15% above the theoretically required amount according to reaction 1:
The carbothermic reduction of silicon from silica is implemented in several stages and is accompanied by the formation and consumption of intermediate interaction products — gaseous silicon monoxide (SiO) and solid crystalline silicon carbide (SiC). Silicon carbide is formed as a result of the interaction of SiO with a solid carbonaceous reducing agent at the phase boundary "reducer - gas phase". It is known that the interaction of silica with carbon can only occur through the formation of a reactive gas phase that ensures contact between the solid oxide and the reducer.
To describe the mechanism of silicon reduction during its smelting in submerged arc furnaces, the HSC Chemistry 9 software package was used in this study. With the help of HSC Chemistry 9, a thermodynamic diagram analysis of the metallurgical silicon smelting process was conducted based on the Si-O-C system. Elements such as Al, Ca (in the form of oxides from the charge Al₂O₃ and CaO), and Fe also participate in the smelting process. However, their content is quite low, so they were not considered in these thermodynamic calculations.
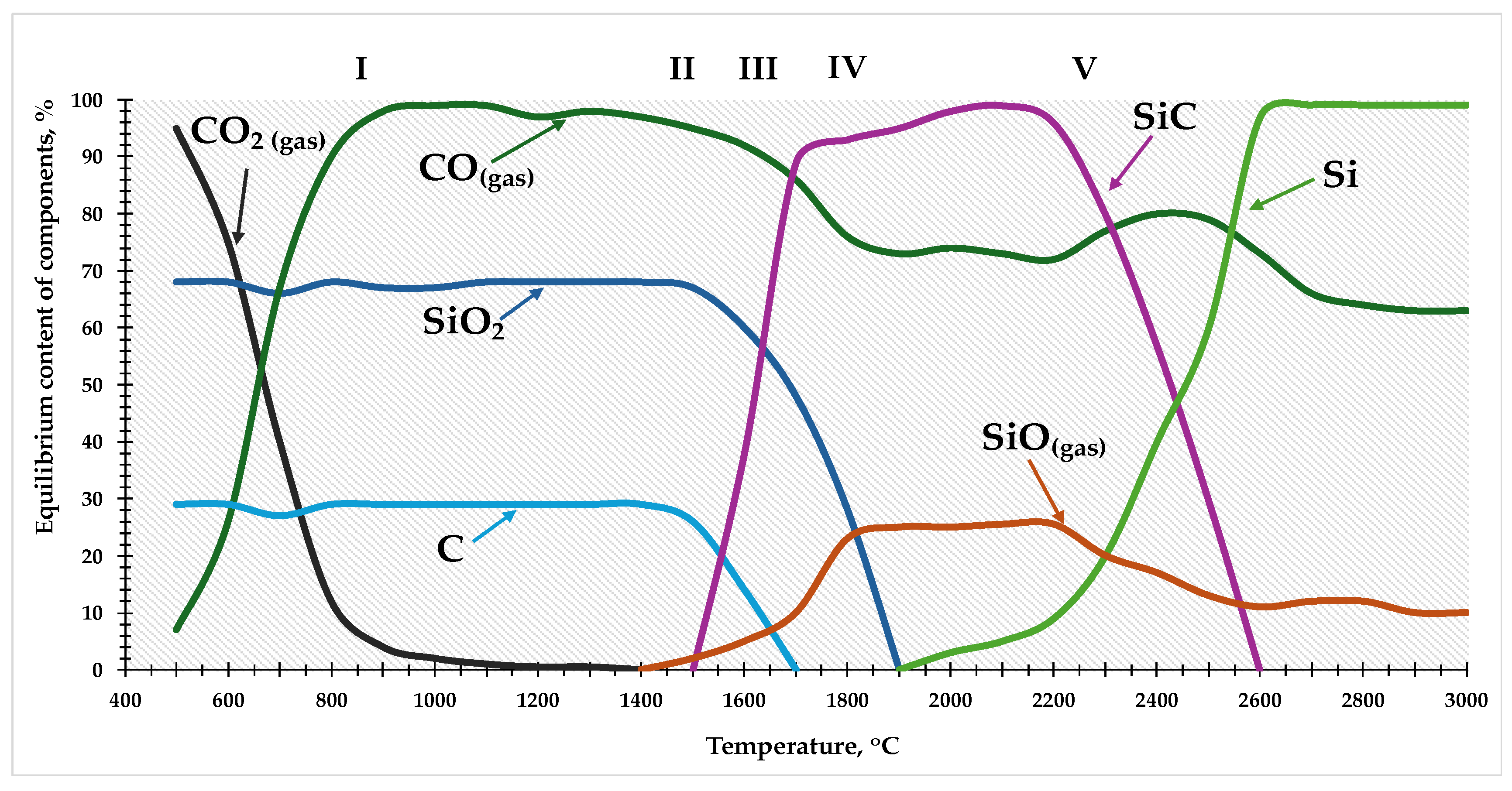
The results of the thermodynamic diagram analysis can be presented as the temperature dependence of the equilibrium composition of the Si-O-C system in the range of 500-3000
oC, as shown in
Figure 2.
The results of the thermodynamic analysis show that the carbothermic reduction of silicon can be implemented in several stages and accompanied by the formation and consumption of intermediate interaction products — gaseous silicon monoxide (SiO) and solid crystalline silicon carbide (SiC).
According to
Figure 2, theoretically the process of silicon reduction can be divided into 5 stages, which can be represented in the form of the following reactions 2-7:
In the system under consideration, the main components of the gas phase are silicon and carbon monoxides, while the condensed phases are SiO₂, SiC, C, and Si. All these phases are practically mutually insoluble. The real composition of the gas phase is undoubtedly much more complex, but due to the small amounts (less than the main substances by 1-2 orders of magnitude), such components as Si₂O₂, Si, SiC₂, Si₂C, and others are not taken into account and, for this reason, do not participate in the calculation procedures.
The primary condensed product of the interaction of silicon dioxide with carbon is silicon carbide, which is formed at the phase boundary "carbon reducer - gas phase," and the formation of SiC is an integral stage of the overall reduction process. According to [
11,
12,
13], the interaction of silica with carbon can only occur through the stage of forming a reactive gas phase, which ensures mass transfer between the oxide and the reducer, and the most significant influence on the reaction rate is exerted by the surface area of the reducer. Silicon carbide forms on the surface of the carbonaceous reducer, and the properties of the carbide phase are largely determined by the state of the carbonaceous reducer, its nature, and the conditions for carrying out the reduction process.
Analyzing the curves of the main component contents in
Figure 2, it is possible to trace the dynamics of the formation and consumption of intermediate components in the silicon reduction process. As noted above, the crucial role in the complex multi-stage mechanism of silicon reduction belongs to gaseous silicon monoxide and solid silicon carbide, with SiO in this scheme functioning as a reactive gas, ensuring diffusional mass transfer between silica and the reducer.
As the charge materials are heated and move deeper into the bath, conditions are created for reduction processes to occur. As seen in the figure, at 1550 ºC, there is a decrease in the concentration of silica and solid carbon, indicating the possible occurrence of reaction (5) with the formation of silicon carbide and SiO. This reaction is a summary, including the occurrence of two consecutive processes by reactions (3) and (4), evidenced by the noticeable increase in silicon carbide concentration from 1550 oC and higher.
As mentioned earlier, the reaction of silicon monoxide formation by reaction (3) precedes its consumption by reaction (4), which is one of the reasons for high silicon losses as SiO gas and products of its disproportionation and condensation with outgoing furnace gases.
The disappearance of free carbon at 1700 oC contributes to the enrichment of the gas phase with gaseous silicon monoxide, formed in the range of 1700-1900 oC by reaction (6). This phenomenon has a positive value as it is a necessary condition for the formation of elemental silicon by reaction (7), the occurrence of which, as seen in the figure, becomes possible at a temperature of 1900 oC and higher. Further temperature increases sharply intensify the reaction of silicon carbide and silicon monoxide consumption, resulting in the concentration of elemental silicon in the condensed phase reaching 100% at 2600 oC, while silicon carbide completely disappears, and the SiO content in the gas phase falls to 9.5%.
As seen from the provided data, a crucial condition for the complete reduction of silicon is a high reaction space temperature. The zone of the highest temperature and active silica reduction, where conditions for reaction (7) exist, is limited to the sphere in the lower part of the submerged arc furnace. The average temperature in the sphere is 2680 oC, with temperature fluctuations from 2000 to 3000 oC (according to some data, up to 3500 oC). Outside this area, even with a favorable ratio of reagents, the main reduction reaction will be hindered, as at lower temperatures (up to 1900 oC), reaction (7) does not develop, and silicon monoxide cannot participate in the formation of elemental silicon. This situation is exacerbated by the instability of the size and location of the high-temperature zone in the furnace, due to the uneven loading of the charge. As a result, a significant part of the SiO gas is carried out of the reaction space and irretrievably lost.
The use of charge materials in the form of briquettes, or briquetted monocharge, can significantly improve these conditions. Briquettes ensure a more uniform distribution of materials in the furnace and help maintain consistently high temperatures. This reduces the likelihood of silicon losses in the form of gaseous SiO and increases the yield of elemental silicon.
4. Results and Discussion
As a result of briquetting, a batch of high-strength briquettes (
Figure 3) was obtained, consisting of microsilica and a carbonaceous reducer.
After drying, the briquettes were tested as a raw material for the smelting of metallurgical silicon. Results of the study shows that the briquettes in the melting process of the technical-grade silicon can be used in the range from 0 to 50%. The using of briquettes may significantly improve the technological parameters. When briquetted charge is applied only, the technological parameters will deteriorate significantly. However, it should be stated that to work with this material and achieve high performance, it is necessary to adhere to some specifics of ore-thermal furnace operation. These features were found when we studied a standard charge (I period). In
Table 4 we presented the technological parameters obtained during the tests.
The material balance of silicon melting showed the recovery rates of the main elements (Si, Al, Ca and Fe). It recorded a decrease in content of aluminum from 1.9-2.1% to 0.5-0.7%, calcium from 0.2-0.3% to 0.07-0.1% and titanium from 0.2-0.23% to 0.11-0.13% during the transition to briquetted monocharge. Phosphorus content increased from 0.004-0.005 to 0.011-0.013%.
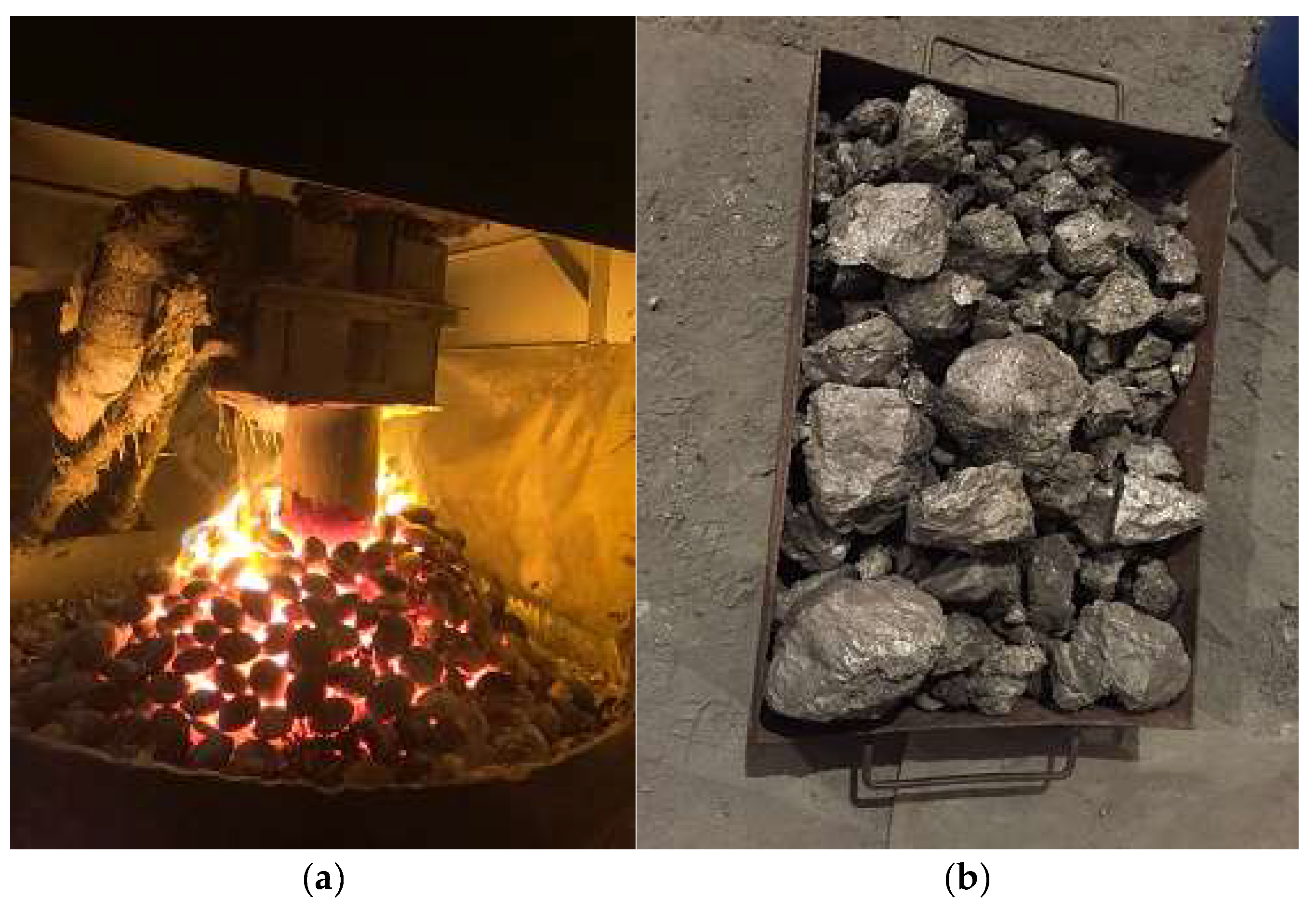
Figure 4 shows a general view of briquettes melting in a furnace, as well as metallurgical silicon ingots.
Practically the entire test period of 11 days, the operation of 200kVA ore-thermal furnace was characterized by the deep fitting of an electrode (at the level of the hearth) and the stable electrical mode.
Stoppages of the furnace due to burning out of inserts, overheating of contacts and current conductors, which are typical for unstable electrical operation of the furnace, were not found. The stable electrical mode and the deep electrode fitting allowed us to switch to the highest transformer stage - 49 volts (stage No. 4) and to operate on it for 6 days. The electrical modes of melting process at different variants of charge composition are given in
Table 5.
Our operation on a high top allowed the working crucible of the furnace top to be significantly enlarged. It helped to stabilize and increase the current load (part of the current went through the furnace top). For instance, when we operate on a low furnace top and constant fitting of the furnace top, the crucible was narrowed to a size of 30-35 cm (maximum).
During the operation on a high furnace top, the dimensions of the crucible reached 50-55 cm in diameter by the end of the campaign. The partial erosion, corrosion and recovery of the fireclay lining were found. The partial erosion and corrosion of the second outer layer of fireclay brick near the furnace shell were observed. Such erosion of fireclay lining during the melting of technical-grade silicon and complex ferroalloys has not been previously recorded by us and can be explained:
- -
by the stable and continuous operation on the 4th stage;
- -
by operation on the high furnace top - heat accumulation;
- -
by aggressive impact on the lining of alkali metal oxides (Na2O and K2O) contained in liquid glass;
- -
by long-term stable operation of the furnace with an excess of reducing agent.
Based on a result of melting tests of technical-grade silicon with using the briquetted mono-charge, the intensification of reduction processes and in general the melting process was recorded in comparison with the study on the standard charge.
The following conclusions can be drawn from our tests:
- -
we achieved an increase in furnace productivity from 2.1 kg (with standard charge) to 2.4-2.45 kg (with 30% share of briquettes in the charge) of technical-grade silicon per hour;
- -
during the entire test period, the maximum silicon recovery rate of 83-84% was achieved at 30% replacement of briquettes in the charge;
- -
total recovery of silicon including metal from the hearth was 92-93%, aluminum - 68-70% and calcium - 26-28%;
- -
negative influence of phase composition of microsilica on melting process of the technical-grade silicon was not found;
- -
shock destruction of briquettes under the influence of thermal and current load on the furnace top was not recorded.
Based on the research carried out, it is also possible to make a tentative conclusion about the economic efficiency of the monocharge used. Approximate calculations of the charge mixture using standard traditional technology and the developed technology using briquetted monocharge are given in
Table 6.
As can be seen from the calculations, the positive economic effect can amount to from 300 to 400 dollars per ton of technical silicon, which, with a production volume of 20 thousand tons, will provide additional income of 2-2.5 million dollars.
The economic effect was calculated without taking into account energy costs, since with the recommended 30% replacement share, the energy consumption was 42.38 kWh/kg, which, within the limits of error, coincides with the electricity consumption for a standard charge of 41.96 kWh/kg and the average for the entire company is 42.41 kWh/kg.
At the same time, the calculations carried out clearly demonstrate what economic effect can be achieved only by replacing charge materials and with a partial transition to monobriquettes.
5. Discussion and Conclusions
In general, the process of smelting metallurgical silicon in an ore-thermal furnace with a transformer power of 200 kVA was satisfactory and stable.
In all periods of furnace operation, the slag accumulation and output were not recorded at excess of reducing agent and deficiency. During the output, the tap hole always gasped. It indicated the formation of a working gas cavity with a high temperature (full flow of chemical reactions) and the absence of excessive slag which would hinder the output of metal. It was found that it is necessary to reduce the carbon excess ratio in a charge from 1.3-1.4 (on a standard charge in the 200kVA ore-thermal furnace at a heating stage) to 1.15-1.2 when using briquetted mono-charge. It was connected with:
- -
less solid carbon burn-up in briquettes where the carbonaceous reducing agent was isolated from exposure to air oxygen;
- -
partial mechanical removal of microsilica from surface of briquettes by hot flue gases;
- -
the need to take into account a content of solid carbon (3-4%) in the composition of microsilica.
The main disadvantage of the prolonged study on a high furnace top is the sintering of the furnace top and the problems during the fitting this furnace top. When we operated on the standard charge and charge with replacement to briquettes in the amount of 30%, we did not find problems practically, i.e. the sintered furnace top had a small thickness and was easily knocked down. During the transition to 50 and 100 per cent replacement, the thickness of the furnace top increased to 20-30 cm, i.e. the furnace top rose from the inside. This made it difficult to knock down the furnace top and resulted in the necessity to recovery segments of the furnace top in the form of sinters. Therefore, we believe that it is unacceptable to increase the share of briquettes in the charge more than 30%. During the first period of operation of furnaces using briquetted mono-charge in industrial conditions, the proportion of briquettes in the charge should not be more than 10-15%. A slight increase in dust content of flue gases was observed in the melting process of technical-grade silicon with using of briquetted mono-charge. We observed an increase in the dust content of flue gases using briquettes, i.e. in the melting of high-carbon ferrochrome using briquetted dust from gas purification system, and in the melting of ferrum silicon manganese using a mono-charge of fine manganese ore and coke [
9].
Organization of the production of briquetted mono-charge at existing productions of metallurgical silicon will require the accumulation and using of a large number of screenings of carbonaceous reducing agents, and the question of the need to organize fractionation of charge materials will arise. The using of fractionated charge materials will reduce the uncontrolled removal and degree of inefficient burn-up of small fractions of carbonaceous reducing agents. Thus, it will allow for a more accurate adjustment of the amount of a reducing agent.
Therefore, we believe that production of briquettes at existing productions of metallurgical silicon will harmoniously fit into the technological line. The increase in dust emissions with the use of briquettes will be not significant. We believe that this large-scale problem, which we have begun to explore in our research, cannot be resolved by one test campaign. This is due to the fact that it is necessary to solve a number of the technical problems such as development of the method of briquettes production, increasing the strength of ready briquettes, issues on transportation and feeding of briquettes into the furnace, stabilization of the furnace operation before the start of briquettes feeding, bringing the furnace to a stable mode using briquettes.
Thus, wherein some topical problems will be solved:
the ecological problem of utilization of production waste -microsilica;
the raw material problem for quartz and its quality;
intensification of the melting process due to closer contact of carbonaceous reducing agent and silicon oxide;
possibility to purchase cheaper carbonaceous reducing agents with smaller size (charcoal, special coke, etc.) [
14];
possibility of wider involvement in silicon production of such limitedly used carbonaceous reducing agents as screenings of charcoal and petroleum coke.
Author Contributions
Conceptualization, methodology, investigation, supervision. A.B. and Ye.Sh.; writing—original draft preparation, writing—review and editing N.V.; methodology, investigation, visualization Az.M., Am.M. and S.Sh. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP 14870218).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karlina, A.I. Technology of processing of dust from gas purification system of silicon production in modifying nanoadditives for cast irons. Dissertation for the degree of candidate of technical sciences, Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences, Ekaterinburg 2019. p. 134.
- Nemchinova, N.; Mineev, G.; Tyutrin, A.; Yakovleva, A. Utilization of Dust from Silicon Production. Steel in Translation 2017, 47, 763–767. [Google Scholar] [CrossRef]
- Galevsky, G.; Rudneva, V.; Galevsky, S. Microsilica in the production of silicon carbide: the results of testing and evaluation of technological challenges. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 20.10.2018.
- Ahmad, Sh.; Al-Amoudi, O.; Khan, S.; Maslehuddin, M. Effect of Silica Fume Inclusion on the Strength, Shrinkage and Durability Characteristics of Natural Pozzolan-Based Cement Concrete. Case Studies in Construction Materials 2022, 17, e01255. [Google Scholar] [CrossRef]
- Mermerdaş, K., İpek, S., Algın, Z., Ekmen, Ş., Güneş, İ. Combined effects of microsilica, steel fibre and artificial lightweight aggregate on the shrinkage and mechanical performance of high strength cementitious composite. Construction and Building Materials 2020, 262, 120048. [Google Scholar] [CrossRef]
- Briquetted mixture to produce the technical-grade silicon and method of its preparation, RF patent 2036144: IPC classes: C01B33/025 / Varyushenkov A.M., Okladnikov V.P., Issayeva Ye.P., Saltykov A.M., Khrennikova L.P.; Applicant and patentee Joint stock company of Open type “All-Russian aluminum and magnesium institute”, App. dated 09.03.1992; published on 27.05.1995.
- Kurbanov, M.Sh.; Abdurakhmanov, B.M.; Ashurov, Kh.B.; Kim, Ye.P. Return of fine wastes of production of technical-grade silicon and ferrosilicon into the technological process. In Proceedings of the XI conference on actual problems of physics, materials science, technology and diagnostics of silicon, nanometre structures and devices based on silicon, 2016, Novosibirsk, p.211.
- Nemchinova, N.V.; Leonova, M.S.; Tyutrin, A.A. Experimental works on the melting of pelletized charge in silicon production. Bulletin of Irkutsk state technical university 2017, 21, 209–217. [Google Scholar] [CrossRef]
- Zhunussov A.K.; Tolymbekova L.B .; Bykov P.O.; Zayakin O.V. Melting Ferrochrome Using Chrome-Ore Briquettes. Metallurgist 2024, 67, 606–613. [Google Scholar]
- N. Vorobkalo, A. Baisanov, Y. Makhambetov, Y. Mynzhasar, N. Nurgali. Technological Research of Process for Producing Titanium Rich Slag and Complex Titanium-Containing Ferroalloy. Heliyon 2023, 9, e18989. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.; Opila, E. Thermodynamics of Si-CO System. Metallurgical Transactions A 1993, 24, 1212–1214. [Google Scholar] [CrossRef]
- Haase, V. The Phase Diagram Si-C-O. In Si Silicon (Part of the book series: Gmelin Handbook of Inorganic and Organometallic Chemistry - 8th edition); Katscher, H., Sangster, R., Schröder, F., Eds.; Publisher: Springer, Berlin, Heidelberg, 1985. [Google Scholar] [CrossRef]
- Danes, F.; Saint-Aman, E.; Coudurier, L. The Si-C-O system. Journal of materials science 1993, 28, 489–495. [Google Scholar] [CrossRef]
- Isin, D.K.; Baisanov, S.O.; Mekhtiev, A.D.; Baisanov, A.S.; Isin, B.D. Technology for Producing Crystalline Silicon with the Use of Non-Traditional Reducing Agents. Metallurgist 2014, 57, (11–12). [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).