1. Introduction
The global incidence of HIV-associated lymphoma (HIV-Ly) decreased consistently after the widespread use of combination antiretroviral therapy (cART). Nevertheless Hodgkin (HL) and non-Hodgkin lymphoma (NHL)
remain a major cause of morbidity and mortality in people living with HIV (PLWH) [
1,
2]
. Treatment outcomes of HIV-Ly have improved in recent decades, due to effective cART and better supportive care, making standard therapeutic regimens established for HIV-negative patients also feasible in the HIV setting. Outcomes are nowadays similar in HIV-positive and HIV-negative patients with lymphoma when treated with the same standard therapies [
3]. However, several population-based studies have highlighted a major health care disparity between the HIV-positive and negative population and studies of novel cancer therapeutics still usually exclude patients with HIV infection [
4,
5].
The use of high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) has been shown feasible and highly effective in HIV-Ly since several years [
6,
7,
8,
9,
10]. To date ASCT is offered to HIV-positive patients with lymphoma with the same indication as for HIV-negative patients, at least in developed countries, and concerns about potentially higher infections toxicity do not limit the use of this procedure [
11]. A surprisingly low relapse rate has been reported in some series of PLWH who received ASCT for HIV-Ly [
7,
12,
13,
14,
15]. Some of these studies have compared the results of monocentric or multicentric cohorts of HIV-positive subjects with historical series of HIV-negative patients undergoing ASCT or with controls identified among HIV-negative groups of patients according to various matching criteria. Although the HIV-positive patients had usually a higher clinical risk, a trend in favour of lower relapse rate and longer progression-free survival (PFS), has been reported for PLWH, although statistically not significant [
11,
13,
14,
15].
In this study, we performed a comparison between two series of consecutive HIV-positive and negative patients with lymphoma receiving HDT and ASCT at two Italian centers, based on a 1:1 propensity score-match (PSM) analysis, considering the main confounding variables. The aim was to directly compare, in a statistically reliable manner, the impact of ASCT, in terms of clinical outcome and toxicity, according to patient’s HIV status.
2. Material and Methods
2.1. Study Design and Population
We compared the clinical outcome of a case series of HIV-Ly that underwent ASCT and a cohort of comparable HIV-negative patients with lymphoma who received the same treatment, using a propensity score system to appropriately match the two groups.
The HIV-positive cohort comprised 66 consecutive patients receiving HDT and ASCT at the Hematology Department of Spedali Civili di Brescia, Italy, from January 2001 to December 2019. As comparison group we choose two consecutive series of HIV-negative patients who underwent ASCT at the Hematology Unit of Spedali Civili di Brescia and of Policlinico Borgo Roma, Verona, Italy, to increase the likelihood of finding adequately matched cases. Patients who underwent a first ASCT in a tandem program of autologous-autologous or autologous-allogeneic transplantation were excluded as patients who had a prior ASCT or allogeneic transplantation. Two hundred and eighty-five HIV-negative patients between 2010 and 2017 were identified.
All patient data were collected from an anonymized database previously gathered with patients’consent, for research and publication purposes. To adjust for confounders in baseline differences between the two groups, a balance was achieved using a 1:1 PSM analysis that led to a final population of 88 patients (44 HIV-positive and 44-HIV negative). Matching criteria included age, sex, histology, disease status at the time of transplant and number of previous therapy regimens. Disease status at ASCT was classified according to the following three categories: first complete remission (CR), chemotherapy-sensitive disease (chemo-s), that includes partial response (PR) after first-line therapy, or chemotherapy-sensitive disease (at least PR) after salvage therapy, and chemotherapy-resistant disease (chemo-r), when less than PR was achieved with the latest therapy received. Disease response was defined by the Revised Response Criteria for Malignant Lymphoma [
16]. All cases were diagnosed by expert Pathologists, and a histological review was deemed unnecessary.
The primary objective of this study was to compare the PFS of the two groups of HIV-positive and negative. Secondary objectives included evaluation of overall survival (OS), relapse rates, toxicity, pre- and post-engraftment infectious events, mortality rates, lymphoma specific-mortality (LSM) and other cause mortality (OCM) rates.
2.2. Statistics and Definition of Outcomes
Statistical analyses comprised a few steps. First, medians and interquartile ranges (IQR) or frequencies and proportions were reported for continuous or categorical variables, respectively. A Mann-Whitney U test and a Chi-square test were applied to determine the statistical significance of differences in medians and proportions, respectively. Second, propensity score match analysis was performed to achieve a comparable cohort of patients affected and not by HIV, respectively. Propensity scores were computed for each patients using the 1:1 nearest-neighbour approach with a caliper width of 0.1, by modeling logistic regression with the dependent variable as the presence of HIV and accounting for the following confounders: age, sex (male, female), histology, disease status at ASCT (1st CR, chemo-s, chemo-r) and number of prior lines of therapy (1, 2, ≥3). Third, survival analyses were performed by means of Kaplan–Meier curves Using a log-rank test to compare different groups. Fourth, Cox regression analysis was performed for univariable survival analyses. For univariate analysis of prognostic factors for survival, the following parameters were considered: age, sex, histology, disease status at transplant, and number of prior lines of therapy. Finally, cumulative incidence (CI) analyses were used to plot LSM and OCM. PFS was defined as the time from ASCT to relapse, progression or death for any cause. OS was measured from ASCT to death for any cause, or the last time the patient was known to be alive. LSM was defined as mortality due to active lymphoma, and OCM as mortality due to any cause other than disease relapse or progression. Neutrophil engraftment was defined as the first day with an absolute neutrophil count greater than 500/μL and platelet engraftment as self-supporting platelet count greater than 20,000/μL.
Post-ASCT toxicity was graded using the Common Terminology Criteria for Adverse Events, version 5.0 [
17]. Grade >2 toxicities were considered. Infectious events were classified by post-ASCT time frames: pre-engraftment period (PRE-EP) refers to the period between HDT and hematological engraftment and post engraftment period (POST-EP) refers to the time frame after the hematological engraftment and the first 2 years from ASCT All analyses were performed using the R software v.3.6.3. All tests were two-sided with significance level set at p <0.05.
3. Results
3.1. Patients’ Characteristics
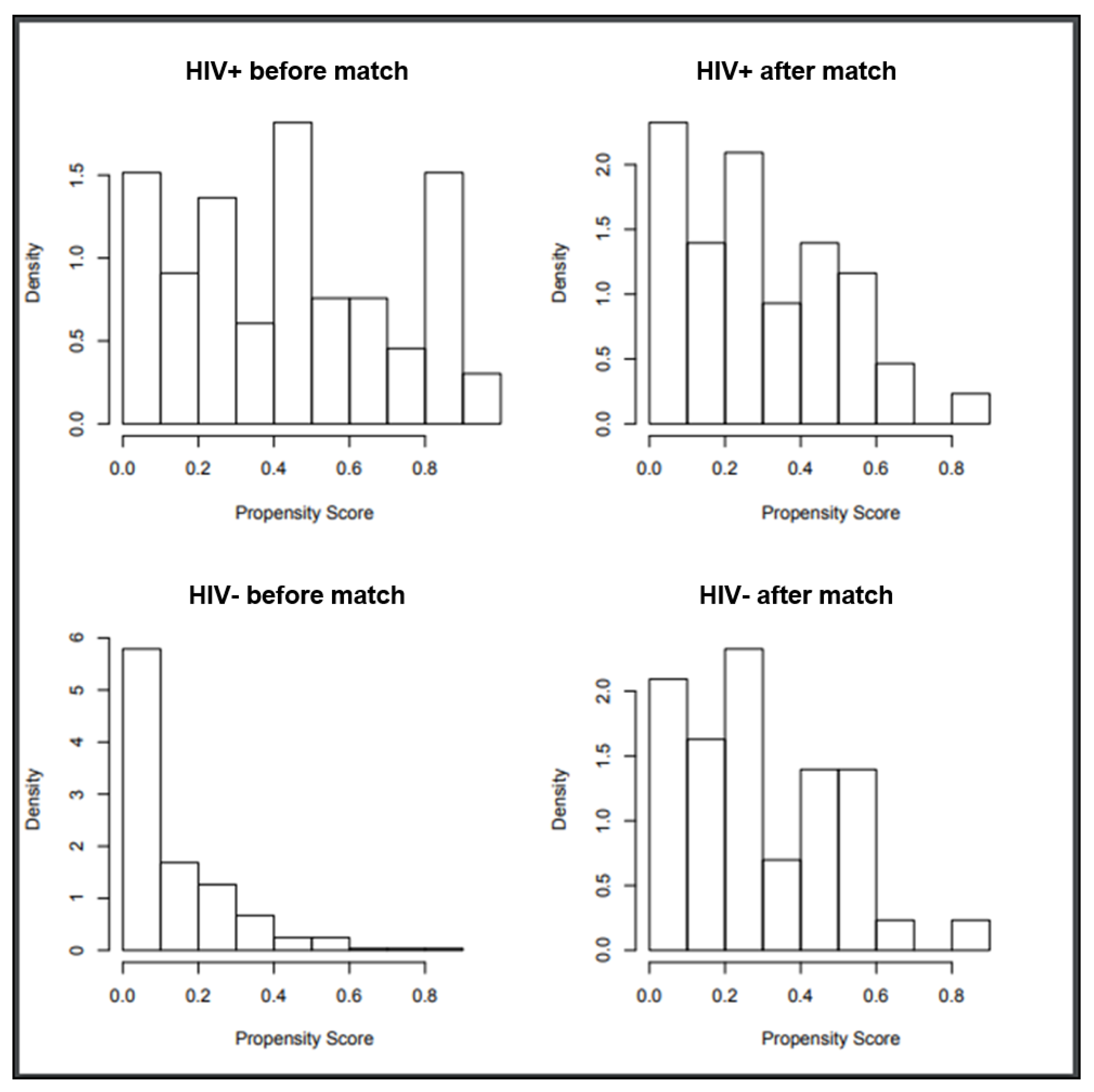
Following propensity score-based nearest neighbor 1:1 matching, we identified 44 HIV-positive patients (from the initial 66) and 44 HIV negative patients (from the initial 285) (
Figure 1). Baseline patients’ characteristics are shown in
Table 1. Half of the patients had a Diffuse Large B Cell Lymphoma, the great majority had a chemo-s disease and 50% had received more than 2 lines of previous therapy. Within the HIV-positive population, the median CD4+ T cells before ASCT was 219/mL (range 60-720) and only one patient (2%) had detectable HIV viremia. All patients were on cART at time of ASCT. Only 2 patients discontinued cART for at least 1 week after ASCT due to oral mucositis and hepatic toxicity, respectively. Co-infection with hepatitis B and hepatitis C virus was present in 1 (2%) and 12 (27%) HIV positive patients and in 1 (2%) and 5 (11%) HIV negative patients.
Table 1.
Descriptive characteristics of 88 patients with lymphoma (44 HIV-positive and 44 HIV-negative) receiving ASCT, after propensity score matching.
Table 1.
Descriptive characteristics of 88 patients with lymphoma (44 HIV-positive and 44 HIV-negative) receiving ASCT, after propensity score matching.
Variables
|
Overall n=88 |
HIV - (n=44) |
HIV + (n=44) |
p-value |
Age at ASCT, yrs
Median
IQR |
47
36-54 |
46
35-57 |
47
38-54 |
0.7 |
Sex, n (%)
M
F |
78 (89)
10 (11) |
38 (86)
6 (14) |
40 (91)
4 (9) |
0.8 |
| Histology, n (%) |
|
|
|
|
| HL |
24 (27) |
13 (30) |
11 (25) |
0.9 |
| DLBCL/PBL |
44 (50) |
20 (46) |
24 (54) |
|
| Burkitt/Burkitt-like Lymphoma |
4 (5) |
2 (4.5) |
2 (4.5) |
|
| PTCL |
11 (13) |
6 (14) |
5 (11) |
|
| FL |
3 (3.4) |
2 (4.5) |
1 (2.3) |
|
| PMBCL |
2 (2.3) |
1 (2.3) |
1 (2.3) |
|
| Status at ASCT, n (%) |
|
|
|
|
| 1st CR |
13 (15) |
3 (7) |
10 (23) |
0.1 |
| Chemo-s |
68 (77) |
37 (84) |
31 (71) |
|
| Chemo-r |
7 (8) |
4 (9) |
3 (7) |
|
| Number of prior therapies, n (%) |
|
|
|
|
| 1 |
22 (25) |
12 (27) |
10 (23) |
0.6 |
| 2 |
22 (25) |
9 (21) |
13 (30) |
|
| ≥3 |
44 (50) |
23 (52) |
21 (48) |
|
Table 2.
Toxicities and infections.
Table 2.
Toxicities and infections.
| |
HIV+ |
HIV- |
P value |
Grade 3-4 toxicity during PRE-EP
Oral mucositis
Gastrointestinal toxicities
Seizure
|
18/44 (41%)
10
9
1
|
15/44 (34%)
14
2
|
0.6 |
PRE-EP infections
FUO
Bacterial infection
Viral infection
Fungal infection
Pneumonia
|
29/44 (65%)
19
9
4
1
1 |
34/44 (77%)
26
7
1
1
2 |
0.9
0.9 |
POST-EP infections
FUO
Bacterial infection
Viral
Pneumonia |
23/43 (53%)
5
4
19
6 |
13/44 (29.5%)
2
2
10
3 |
0.02
0.13 |
Table 3.
Univariable and Cox regression analysis predicting relapse.
Table 3.
Univariable and Cox regression analysis predicting relapse.
| Predictor Factors |
Univariable analysis for PFS |
Univariable analysis for OS |
| HR (95% CI) |
p-value |
HR (95% CI) |
p-value |
| HIV |
0.43 (0.20-0.93) |
0.03 |
0.55 (0.24-1.25) |
0.14 |
| Age |
1.02 (0.98-1.05) |
0.2 |
1.04 (1.00-1.08) |
0.03 |
| Sex |
|
|
|
|
| M |
- |
- |
- |
|
| F |
0.8 (0.24-2.66) |
0.7 |
0.30 (0.04-2.20) |
0.15 |
| Histology |
|
|
|
|
| HD |
- |
- |
- |
|
| DLBCL/PBL |
0.7 (0.3-1.6) |
0.4 |
0.9 (0.33-2.5) |
0.9 |
| BL/B like |
0.7 (0.09-5.4) |
0.7 |
1.03 (0.12-8.6) |
0.9 |
| NHL/T |
1.7 (0.6-5.2) |
0.3 |
3.1 (1.1-9.8) |
0.05 |
| FL |
2.9 (0.8-10.8) |
0.1 |
2.2 (0.4-11.1) |
0.3 |
| PMBCL |
0.001 (NA) |
0.9 |
0.001 (NA) |
0.9 |
| MALT evol |
4.1 (0.5-33.6) |
0.2 |
0.001 (NA) |
0.9 |
| N. of prior therapies |
|
|
|
|
| 1 |
- |
- |
- |
- |
| 2 |
0.07 (0.01-0.53) |
0.01 |
0.48 (0.14-1.64) |
0.2 |
| ≥3 |
0.67 (0.32-1.44) |
0.31 |
0.85 (0.34-2.11) |
0.7 |
| Status at transplant |
|
|
|
|
| 1st CR |
- |
- |
- |
- |
| ChemoS disease |
5.4 (0.72-39) |
0.1 |
1.23 (0.36-4.2) |
0.7 |
| ChemoR disease |
32 (3.8-277) |
<0.01 |
6.6 (1.6-27) |
0.01 |
3.2. Clinical Outcomes
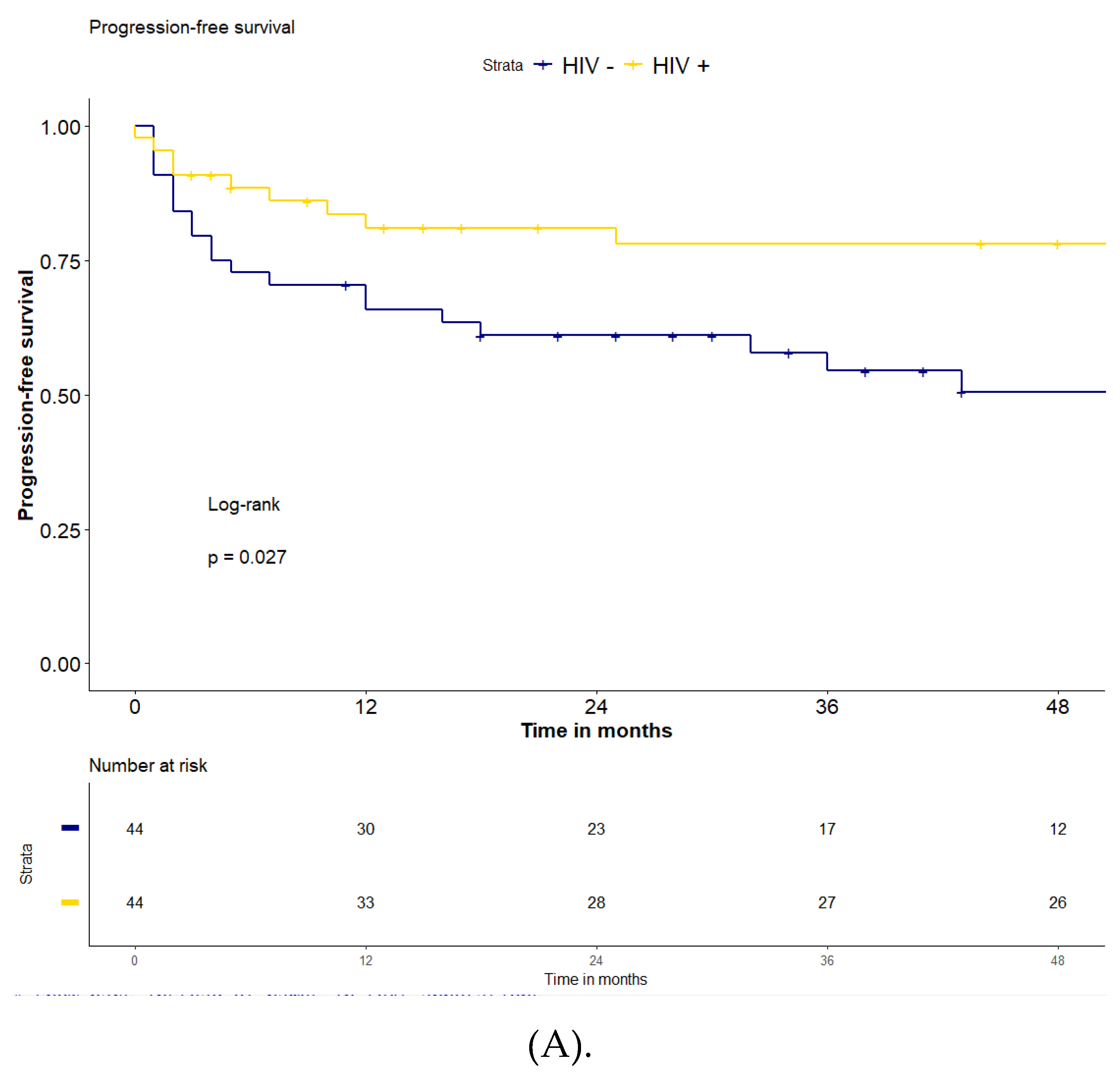
Overall, after a median follow-up of 51 months (IQR: 15-80), the 2-years and 4-years PFS were 71% and 65%, and the 2-years and 4-years OS were 78% and 74% for the entire series of 88 patients. The PFS in the HIV-positive group was significantly higher compared to the HIV-negative population (2-years and 4-years PFS 81% and 78% vs 61% and 51%, respectively, p=0.027)
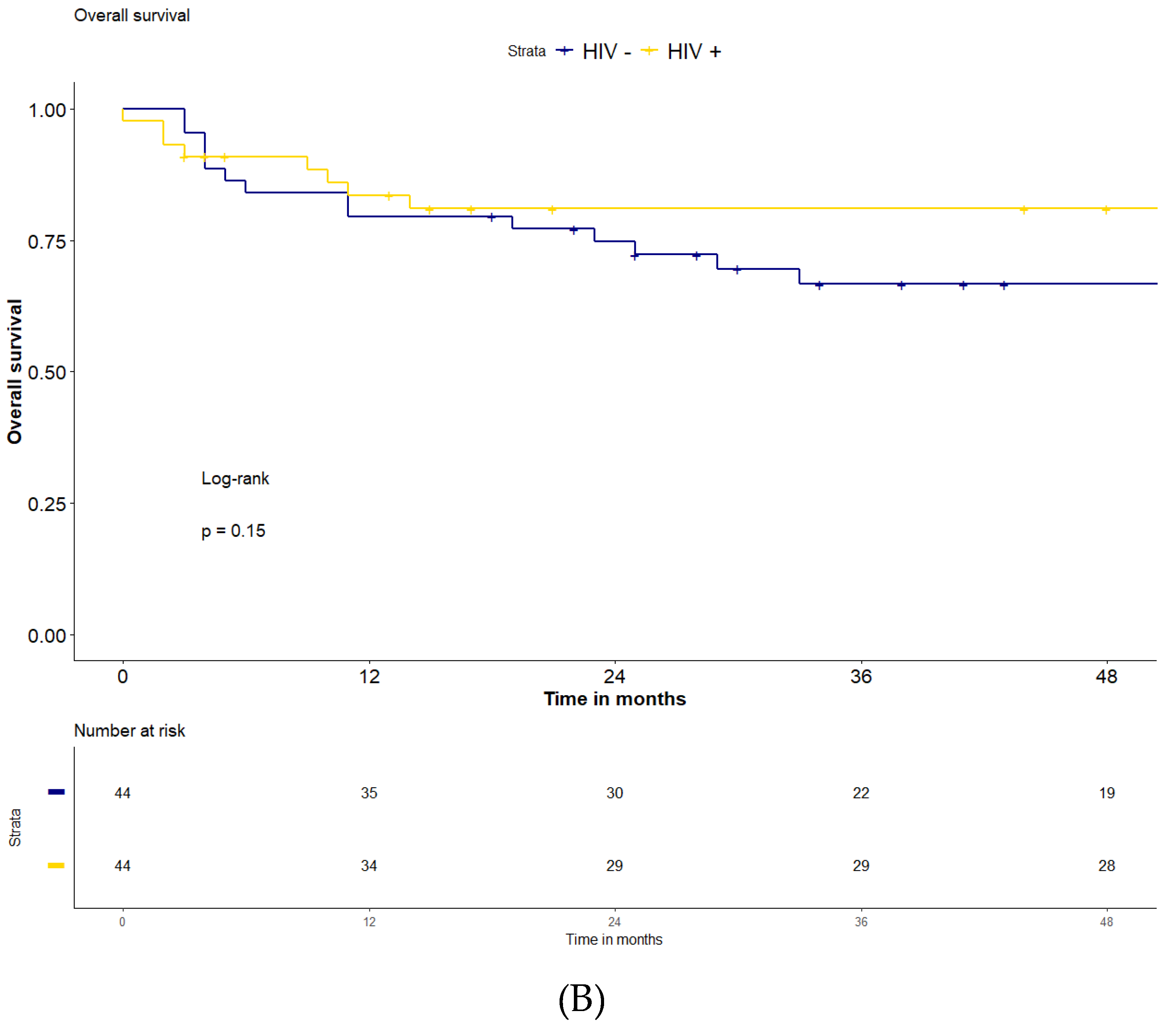
(Figure 2). The 2-years OS and 4-years OS were both 81% for the HIV-positive patients and 75% and 67% in the HIV-negative patients (p=0.15). The median follow-up for the two groups was 51 months (IQR: 8-87) for HIV-positive and 43 (IQR 24-73) for HIV-negative population, respectively.
Overall, 30 patients (34%) had lymphoma relapse after ASCT. Relapse rate was significantly higher in HIV-negative (20 patients, 45%) compared to HIV-positive patients (10 patients, 23%) (p=0.04). Median time to relapse was 57 months (IQR 12-89) for HIV-positive versus 27 months (IQR 5-53) for HIV-negative patients (p=0.03). Twenty-six cases of deaths were recorded. Ten patients (23%) in the HIV-positive group died: 7 from lymphoma, 2 from infection during CR (one had stopped cART by his own decision several months after ASCT), and one due to suicide. In the HIV-negative group 16 patients (36%) died: 11 from lymphoma, 4 from infection (one patient in CR due to pneumonia, 11 months after ASCT, and 3 while receiving further therapies for lymphoma relapse), and one from a second tumor (pancreatic adenocarcinoma).
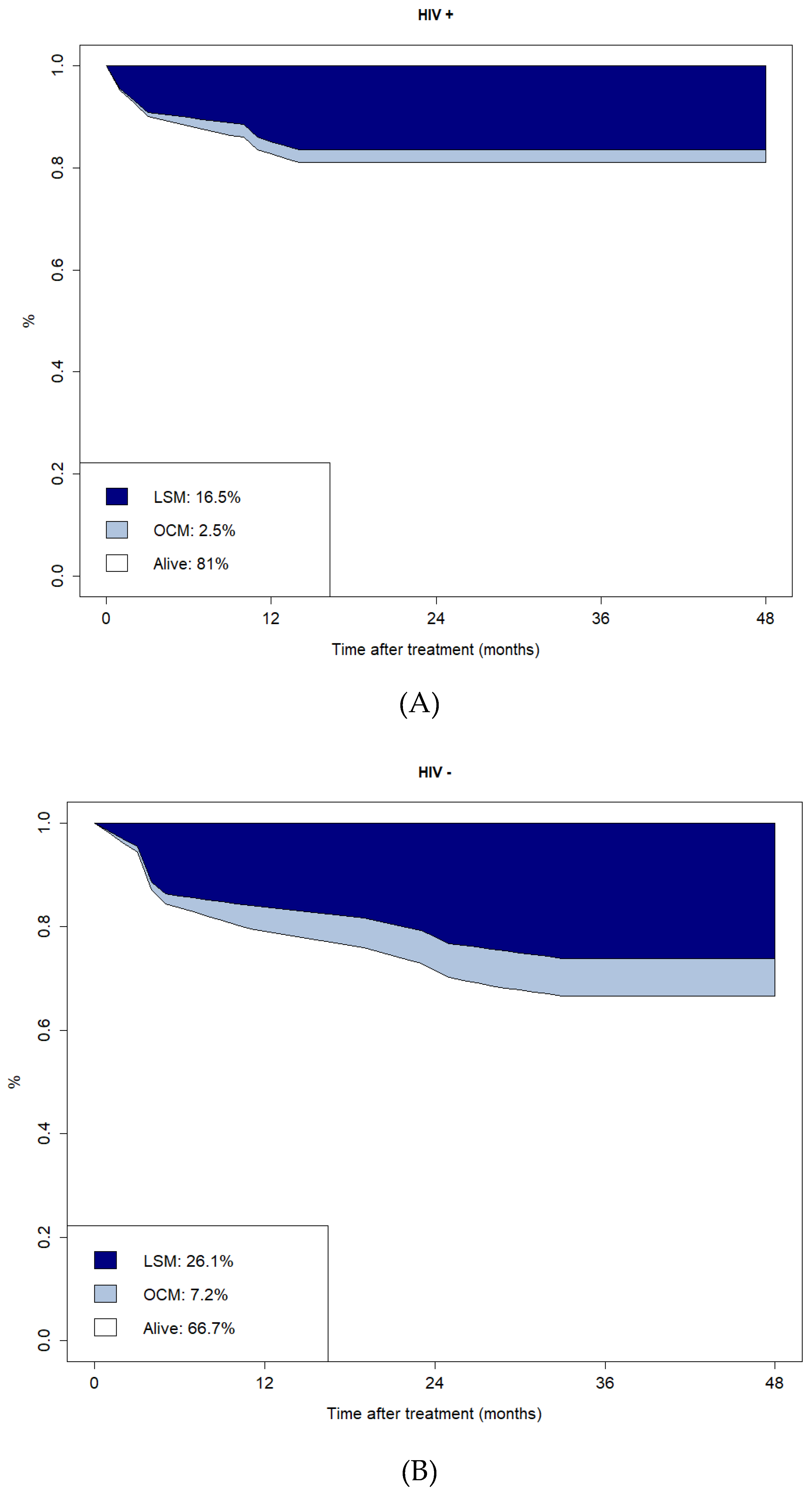
The CI plots of LSM and OCM are reported in
Figure 3. The CI of LSM and OCM at four years for HIV-positive versus HIV-negative patients were 16.5% vs 26.1% and 2.5% vs 7.2%, respectively.
3.3. Engraftment, Toxicity, and Infections
In all patients, peripheral hematopoietic stem cells were used for transplantation. The median number of CD34+ cells infused was 6.3 (IQR 5.0-7.3) ×10^6/kg in the HIV-positive and 5.75 (IQR 4.9-7.9) ×10^6/kg in the HIV-negative patients (p=0.72). Neutrophil and platelet engraftment was achieved in all patients, except for one HIV-positive patient with chemo-r disease who died 10 days after ASCT because of sepsis. Median time to neutrophil and platelet engraftment was 10 days and 13 days for both HIV-positive and negative patients.
As infection prophylaxis HIV-negative patients usually received acyclovir and trimethoprim sulfamethoxazole until 3 months after ASCT. HIV-positive patients received acyclovir and fluconazole for 3 months after ASCT, and trimethoprim sulfamethoxazole for at least 6 months (or until CD4 count > 200/mcl). Levofloxacin was used in both groups during the neutropenic period before engraftment.
Eighteen (41%) HIV-positive patients had a total of 20 grade 3-4 treatment-related toxicity events (10 oral mucositis, 6 gastrointestinal toxicity/diarrhea, 3 enteritis, 1 seizure), while in the HIV-negative group 16 grade 3-4 toxicity events were seen in 15 (34%) patients (14 oral mucositis, 2 enteritis) (p=0.6). Nineteen patients (43%) in the HIV-positive group had an episode of fever of unknown origin (FUO) versus 26 (59%) in the HIV-negative group (P=0.2). During the PRE-EP, there were a similar number of infectious events between the two groups (15 events in 14 patients and 11 events in 9 patients within the HIV-positive and negative group, respectively, p= 0.48). A documented bacterial infection was seen in 9 HIV-positive patients (20%) (including 3 gram-negative and 4 gram-positive episodes of sepsis), and in 7 HIV-negative patients (16%) (including 3 gram-negative and 1 gram-positive episodes of sepsis). A pneumonitis was recorded in one HIV-positive and two HIV-negative patients. Two cases (4%) of asymptomatic cytomegalovirus (CMV) reactivation, one (2%) herpes zoster (HZV) infection and one (2%) human herpes virus-6 (HHV-6) infection were seen in the HIV-positive group while only one (2%) HHV-6 infection was observed in the HIV-negative group. One C.parapsilosis sepsis was observed in one HIV-negative patient and one HIV-positive patient had a probable invasive pulmonary aspergillosis. During the POST-EP there were a significant majority of infections or febrile events in the HIV-positive population compared to the HIV-negative group (34 vs 17, respectively, p= 0.02). A total of 29 documented late infections occurred in 23 HIV-positive patients, including 5 bacterial infections, 20 viral infections (8 CMV, 11 HZV, 1 Rhinovirus pneumonia), and 4 cases of pneumonia. In the HIV negative-population, 12 patients had 15 documented infection events: two bacterial infections, 10 viral (7 CMV and 3 HZV) and 3 cases of pneumonia.
3.4. Predictive Factors for Relapse and Mortality
Considering the whole series of 88 patients, at univariable Cox regression analysis, two treatment lines prior to ASCT (HR: 0.07; p=0.01) and a chemo-refractory disease at ASCT (HR: 32; p<0.01) significantly correlated with lower PFS. HIV infection resulted to be a protective factor for PFS (HR: 0.43; p=0.03). Age (HR: 1.04; p=0.03), NHL T histology (HR: 3.1; p=0.05) and chemo-refractory disease at ASCT (HR: 6.6; 95% CI: 1.6-27; p=0.01) were significantly correlated with a higher risk of death.
4. Conclusions
Thanks to the widespread use of cART, and the improvement of supportive care, standard dose chemotherapy and HDT with ASCT are nowadays offered to HIV-positive patients with lymphoma with the same indications as for HIV-negative general population.
However, access to target therapies and modern immunotherapies is still limited to PLWH, due to socio-economic factors and inadequate research to support safety and efficacy in this population. In the present study, we compared the outcomes of ASCT among two series of lymphoma patients affected or not by HIV infection using a 1:1 propensity score matching
to reduce the bias of the retrospective comparison. Unpredictable satisfactory outcomes were previously reported for HIV-positive patients, comparable to HIV-negative subjects, with a surprising trend toward less relapses in the HIV-positive population [
11,
13,
14,
15].
The main result of our study was the significant lower relapse rate in the HIV-positive group, that results in a statistically significant higher PFS in PLWH compared to HIV-negative patients. This has also resulted in a not statistically significant trend toward improved OS in HIV-positive patients. PFS and OS of HIV-positive patients in the present study are in line with previous reports [
9,
18].
The fascinating finding of a better PFS in patients with HIV infection compared to the general population is difficult to interpret. A beneficial effect of ASCT on the underlying HIV infection and on the immune status of HIV-infected patients, may be hypothesized. Highly myelotoxic chemotherapy could deplete the reservoir that harbor HIV and facilitate immune recovery, and the restoration of an effective immune system might be relatively more beneficial in lymphomas developing in the context of failing immune response than in immunocompetent patients [
19]. In hematologic malignancies there are several examples of immunodeficiency-driven diseases that benefit from immune reconstitution, by simply reducing or modifying immune suppression, and several
cases of lymphoma regression with treatment of HIV alone have been reported [
20,
21,
22]
. Moreover, a favourable long-term effect of cART may also play a role in the reduction of the relapse risk in HIV-Ly.
Antiviral drugs included in cART regimens have been reported to exert a direct antineoplastic action [
23,
24,
25]
. Even if antiviral drugs have not been approved as antiproliferative agents, promising in vitro studies have demonstrated that nucleoside/nucleotide analogue, reverse transcriptase inhibitors or integrase inhibitors can modulate cell growth and differentiation across various cancer types [
26,
27,
28]
. Investigations are ongoing to identify agents with the ability to inhibit key enzymes that play a crucial role in DNA metabolism and their possible application as antiretroviral and antitumoral agents [
29]
.
In our study, we did not find significant differences both in acute toxicity and early infections after ASCT between the two groups of HIV-positive and negative patients. Instead, infections occurring later after engraftment were significantly higher in PLWH, and were mainly of viral origin. An increased rate of infections was also reported by the Spanish group [
11], without detrimental effect on survival. A previous study found a comparable immunologic recovery after ASCT between HIV-positive and negative patients, while other studies reported a delayed recovery in PLWH irrespective of efficient cART [
11,
19]. It is also known that chronic inflammation, immune cell metabolic dysregulation, and cellular dysfunction persist in PLWH even in the presence of effective cART and of suppressed HIV viremia [
30]. Our experience underlines the need of optimal supportive measures and careful follow up in HIV-positive patients. Anyway, in our study not only LSM but also OCM showed a trend in favor of PLWH.
In conclusion, our results increase the awareness of ASCT as an effective curative option for patients with HIV-Ly and chemo-sensitive disease. ASCT indeed appeared in our study highly effective, and we reported for the first time a significant lower relapse rate in HIV-positive patients compared to the general HIV-negative population.
We are aware that future clinical studies in the HIV setting should focus on novel molecules and immunological approaches, while considering the effects on the underlying HIV disease, and some preliminary experiences paved the way in this direction [
31,
32,
33]. While waiting that these new therapeutic approaches, rapidly developing in the HIV negative population [
34], are demonstrated to be safe and effective in the setting of HIV, ASCT remains a highly effective treatment strategy for HIV-Ly.
A collaboration between all the involved professional figures, particularly infectious disease specialists and hemato-oncologists, represents the key to the correct management of these patients to guarantee the best achievable oncological outcome.
Author Contributions
Conceptualization, Alessandro Re, Giuseppe Rossi and Mauro Krampera; Methodology, Alessandro Re, Margherita Oberti, Armando Stabile and Mauro Krampera; Software, Armando Stabile; Investigation, Chiara Cattaneo, Chiara Pagani, Salvatore Casari, Maria Antonia Forleo, Cristina Tecchio, Camillo Almici, Alessandra Tucci and Francesco Castelli; Data curation, Alessandro Re, Margherita Oberti, Angelo Andreini, Chiara Cattaneo and Chiara Pagani; Writing – original draft, Alessandro Re and Margherita Oberti; Writing – review & editing, Alessandro Re and Giuseppe Rossi; Supervision, Alessandro Re. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In this section, you should add the Institutional Review Board Statement and approval number, if relevant to your study. You might choose to exclude this statement if the study did not require ethical approval. Please note that the Editorial Office might ask you for further information. Please add “The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving humans. OR “The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving animals. OR “Ethical review and approval were waived for this study due to REASON (please provide a detailed justification).” OR “Not applicable” for studies not involving humans or animals.
Informed Consent Statement
Any research article describing a study involving humans should contain this statement. Please add “Informed consent was obtained from all subjects involved in the study.” OR “Patient consent was waived due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans. You might also choose to exclude this statement if the study did not involve humans. Written informed consent for publication must be obtained from participating patients who can be identified (including by the patients themselves). Please state “Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robbins HA; Pfeiffer RM; Shiels MS; Li J; Hall I; Engels EA. Excess cancers among HIV-infected people in the United States. J. Natl. Cancer. Inst. 2015, 107, 1–8. [Google Scholar]
- Kimani SM; Painschab MS; Horner MJ; Muchengeti M; Fedoriw J; Shiels MS; Gopal S. Epidemiology of haematological malignancies in people living with HIV. Lancet HIV 2020, 7, 1–21. [Google Scholar]
- Re A; Cattaneo C; Montoto S. Treatment management of haematological malignancies in people living with HIV. Lancet Haematol. 2020, 7, e679–e689. [Google Scholar] [CrossRef] [PubMed]
- Coghill A; Suneja G. Cancer care disparities in people with HIV in the United States. Curr. Opin. HIV AIDS 2017, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Uldrick TS; Ison G; Rudelk MA; Noy A; Schwartz K; Bruinooge S; Schenkel C; Miller B; Dunleavy K; Wang J; et al. Modernizing Clinical Trial Eligibility Criteria: Reccomendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. J. Clin. Oncol. 2017, 35, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Gabarre J; Azar N; Autran B; Katlama C; Leblond V. High-dose therapy and autologous haematopoietic stem-cell transplantation for HIV-1-associated lymphoma. Lancet 2000, 25, 1071–1072. [Google Scholar]
- Re A; Cattaneo C; Michieli M; Casari S; Spina M; Rupolo M; Allione B; Nosari A, Schiantarelli C; Vigano M, et al. High-dose therapy and autologous peripheral-blood stem-cell transplantation as salvage treatment for HIV-associated lymphoma in patients receiving highly active antiretroviral therapy. J. Clin. Oncol. 2003, 1, 4423–4427. [Google Scholar]
- Krishnan A; Molina A; Zaia J; Nademanee A; Kogut N, Rosenthal J, Woo D, Forman SJ. Autologous stem cell transplantation for HIV associated lymphoma. Blood 2001, 98, 3857–3859. [Google Scholar] [CrossRef] [PubMed]
- Re A; Michieli M; Casari S; Allione B; Cattaneo C; Rupolo M; Spina M; Manuele R; Vaccher E, Mazzucato M, et al. High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for AIDS-related lymphoma: Long-term results of the Italian Cooperative Group on AIDS and Tumors (GICAT) study with analysis of prognostic factors. Blood 2009, 114, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre P; Díez-Martín JL; Re A; Michieli M; Ribera JM; Canals C; Rosselet A; Conde E; Varela R; Cwynarski K; et al. Autologous stem-cell transplantation in patients with HIV-related lymphoma. J Clin Oncol. 2009, 27, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Oreiro M; Balsalobre P; Miralles P; Berenguer J; Dorado N; Bailen R; Obreoscoa G; Anguita J; Serrano D; Díez-Martín JL; et al. Autologous stem cell transplantation for lymphoma in HIV+ patients: higher rate of infections compared with non-HIV lymphoma. Bone Marrow Transplant. 2020, 55, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Re A; Gini G; Rupolo M; Levis A; Bandera A; Liberati AM; Tozzi P, Cattaneo C; Casari C; Skert C; et al. Early consolidation with high-dose therapy and autologous stem cell transplantation is a feasible and effective treatment option in HIV-associated non-Hodgkin lymphoma at high risk. Bone Marrow Transplant. 2018, 53, 28–230. [Google Scholar]
- Díez-Martín JL; Balsalobre P; Re A; Michieli M; Ribera JM; Canals C; Conde E; Rosselet A; Gabriel I; Varela R; et al. Comparable survival between HIV+ and HIV- non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation, Blood 2009, 113, 6011–6014. [Google Scholar] [CrossRef] [PubMed]
- Krishnan A; Palmer JM; Zaia JA, Tsai NC; Alvarnas J; Forman SJ. HIV status does not affect the outcome of autologous stem cell transplantation (ASCT) for non-hodgkin lymphoma (NHL), Biol. Blood Marrow Transplant., 2010, 16, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Alvarnas JC; Le Rademacher J; Wang Y; Little RF; Akpek G. Autologous hematopoietic cell transplantation for HIV-related lymphoma: results of the BMT CTN 0803/AMC 071 trial. Blood 2016, 128, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Cheson BD; Fisher RI; Barrington SF; Cavalli F; Schwartz LH; Zucca E; Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Cancer Institute.
- Hubel K; Re A; Boumendil A; Finel H; Hentrich M; Robinson S; Wyen C; Michieli M; Kanfer E; Diez-Martin JL; et al. Autologous stem cell transplantation for HIV-associated lymphoma in the antiretroviral and rituximab era: a retrospective study by the EBMT Lymphoma Working Party, Bone Marrow Transplant., 2019, 54, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Bertoli D; Re A; Chiarini M; Sottini A; Serana F; Giustini V; Roccaro AM; Cattaneo C; Caimi L; Rossi G; et al. B- and T-lymphocyte number and function in HIV+/HIV- lymphoma patients treated with high-dose chemotherapy and autologous bone marrow transplantation. Sci. Rep. 2016, 6, 1–11. [Google Scholar]
- Lee M; Abousaud, A; Harkins A; Marin E; Balasubramani D; Churnetski MC; Peker D; Singh A; Koff JL. Important Considerations in the Diagnosis and Management of Post-transplant Lymphoproliferative Disorder. Curr Oncol Rep, 2023, 25, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Mu W; Patankar V; Kitchen S; Zhen A. Review Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses, 2024; 219, 1–22.
- Lim DH; Rhee JY; Park KW. Stage IV advanced diffuse large B-cell lymphoma in human immunodeficiency virus infection with achieving cure by using highly active antiretroviral therapy alone: a case report. Int. J. STD AIDS, 2017, 28, 932–936. [Google Scholar] [CrossRef]
- Atallah-Yunes SA; MurphyD; Abdelmalak R; Mantle L; Ali SS. Plasmablastic lymphoma achieving sustained remission with antiretroviral therapy alone. European journal of haematology, 2019, 103, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Monini P; Sgadari C; Toschi E; Barillari G; Ensoli B. Antitumour effects of antiretroviral therapy, Nat. Rev. Cancer 2004, 4, 861–875. [CrossRef] [PubMed]
- Maksimovic-Ivanic D; Fagone P; McCubrey J; Bendtzen K; Mijatovic S; Nicoletti F. HIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives? Int. J. Cancer. 2017, 140, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Chow WA; Jiang C; Guan M. Anti-HIV drugs for cancer therapeutics: back to the future? Lancet Oncol. 2009, 10, 61–71. [Google Scholar] [CrossRef] [PubMed]
- García-Trejo JJ; Ortega R; Zarco-Zavala M. Putative Repurposing of Lamivudine, a Nucleoside/Nucleotide Analogue and Antiretroviral to Improve the Outcome of Cancer and COVID-19 Patients. Front Oncol. 2021, 11, 66–4794. [Google Scholar]
- Akcora-Yildiz D; Gonulkirmaz N; Ozkan T; Beksac M; Sunguroglu A. HIV-1 integrase inhibitor raltegravir promotes DNA damage-induced apoptosis in multiple myeloma. Chem. Biol. Drug. Des. 2023, 102, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Şekeroğlu ZA; Şekeroğlu V; Küçük N. Effects of Reverse Transcriptase Inhibitors on Proliferation, Apoptosis, and Migration in Breast Carcinoma Cells. Int. J. Toxicol. 2021, 40, 52–61. [CrossRef] [PubMed]
- Garro HA and Pungitore, CR. Coumarins as potential inhibitors of DNA polymerases and reverse transriptases. Searching new antiretroviral and antitumoral drugs. Current Drug Discovery Technologies. 2015, 12, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Mu W; Patankar V, Kitchen S, Zhen A. Review Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses 2024, 219, 1–22.
- Rubinstein PG; Moore PC; Bimali M; Lee JY; Rudek MA; Chadburn A; Ratner L; Henry DH; Cesarman E; DeMarco CE; et al. AIDS Malignancy Consortium; Lymphoma Study Association. Brentuximab vedotin with AVD for stage II-IV HIV-related Hodgkin lymphoma (AMC 085): phase 2 results from an open-label, single arm, multicentre phase 1/2 trial. Lancet Haematol 2023, 10, e624–e632. [Google Scholar] [CrossRef] [PubMed]
- El Zarif T; Nassar AH; Adib E; Fitzgerald BG; Huang J; Mouhieddine TH; Rubinstein PG; Nonato T; McKay RR; Li M, et al. Safety and Activity of Immune Checkpoint Inhibitors in People Living With HIV and Cancer: A Real-World Report From the Cancer Therapy Using Checkpoint Inhibitors in People Living With HIV-International (CATCH-IT) Consortium. J Clin Oncol. 2023, 41, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Hattenhauer T; Mispelbaum R; Hentrich M; Boesecke C; Monin MB. Enabling CAR T-cell therapies for HIV-positive lymphoma patients – A call for action. HIV Med. 2023, 24, 957–964. [Google Scholar] [CrossRef] [PubMed]
- D'Alò F; Bellesi S; Maiolo E, Alma E, Bellisario F, Malafronte R, Viscovo M, Campana F, Hohaus S. Novel targets and advanced therapies in Diffuse Large B Cell Lymphomas. Cancers 2024, 16, 2243. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).