Submitted:
07 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design, Soil Sampling, and Pretreatment
2.3. Physical and Chemical Properties of Soils
2.4. Microbial Biomass C (Cmic)
2.5. Microbial Respiration
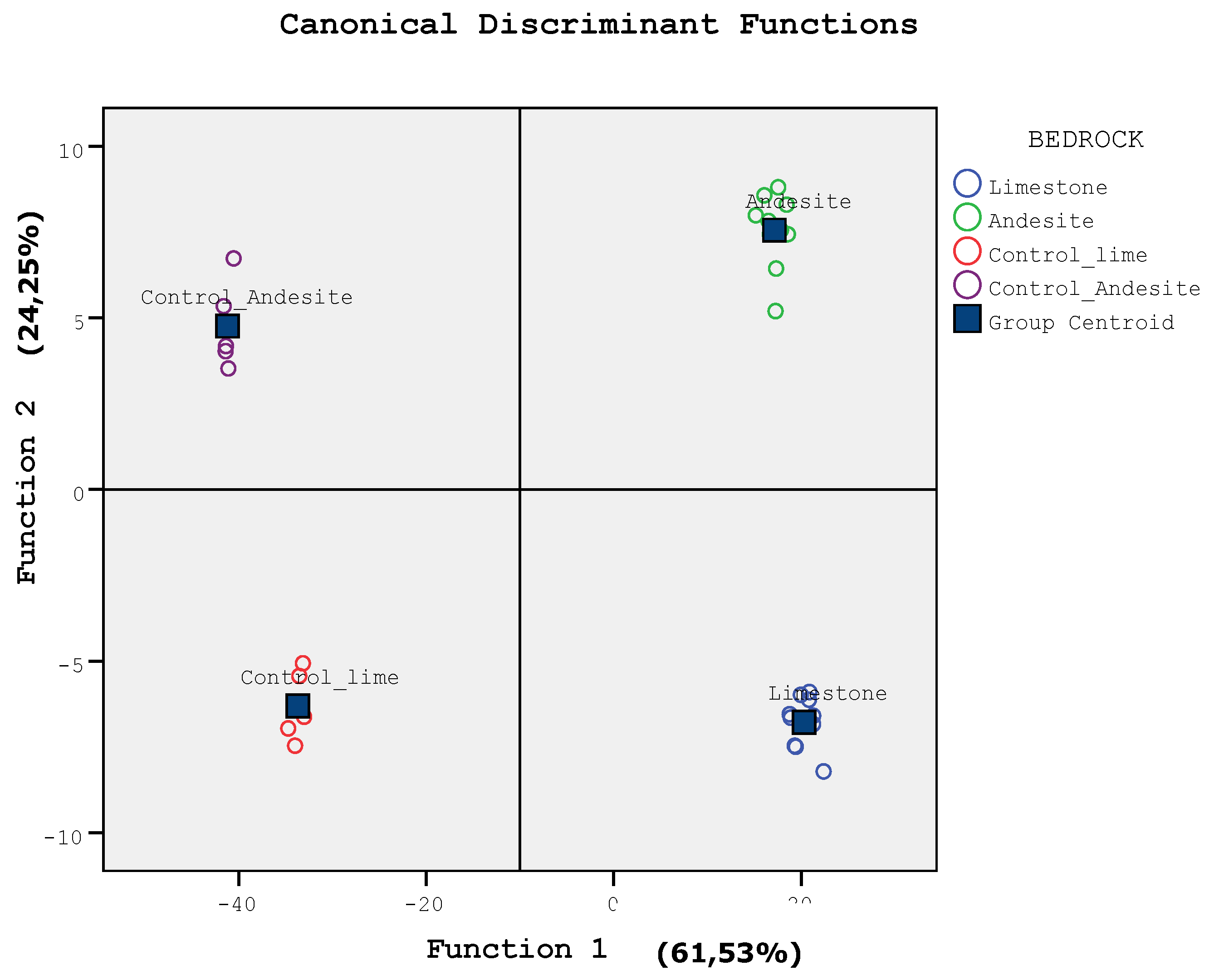
2.6. Statistical Analyses
3. Results
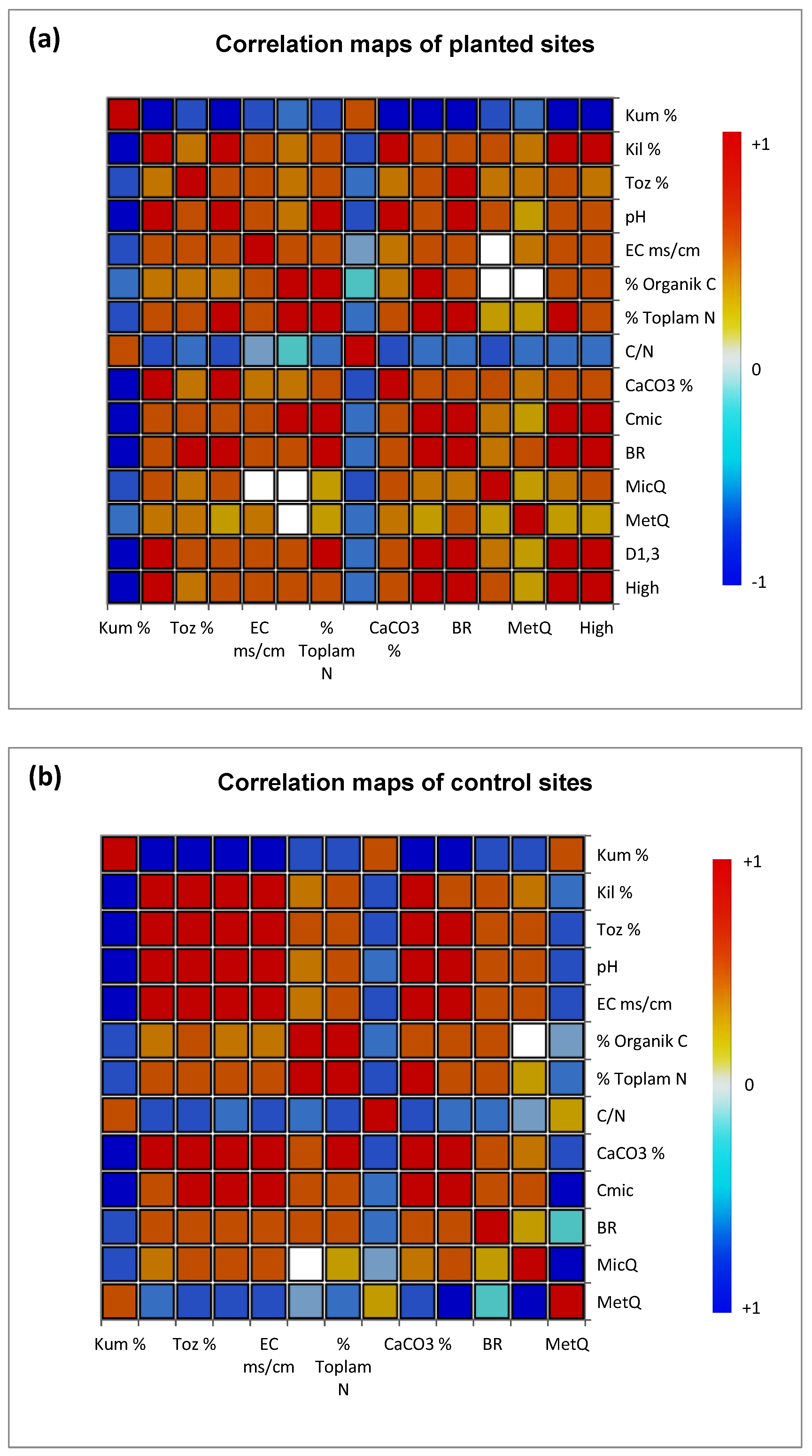
3.1. Physicochemical Properties of the Soil
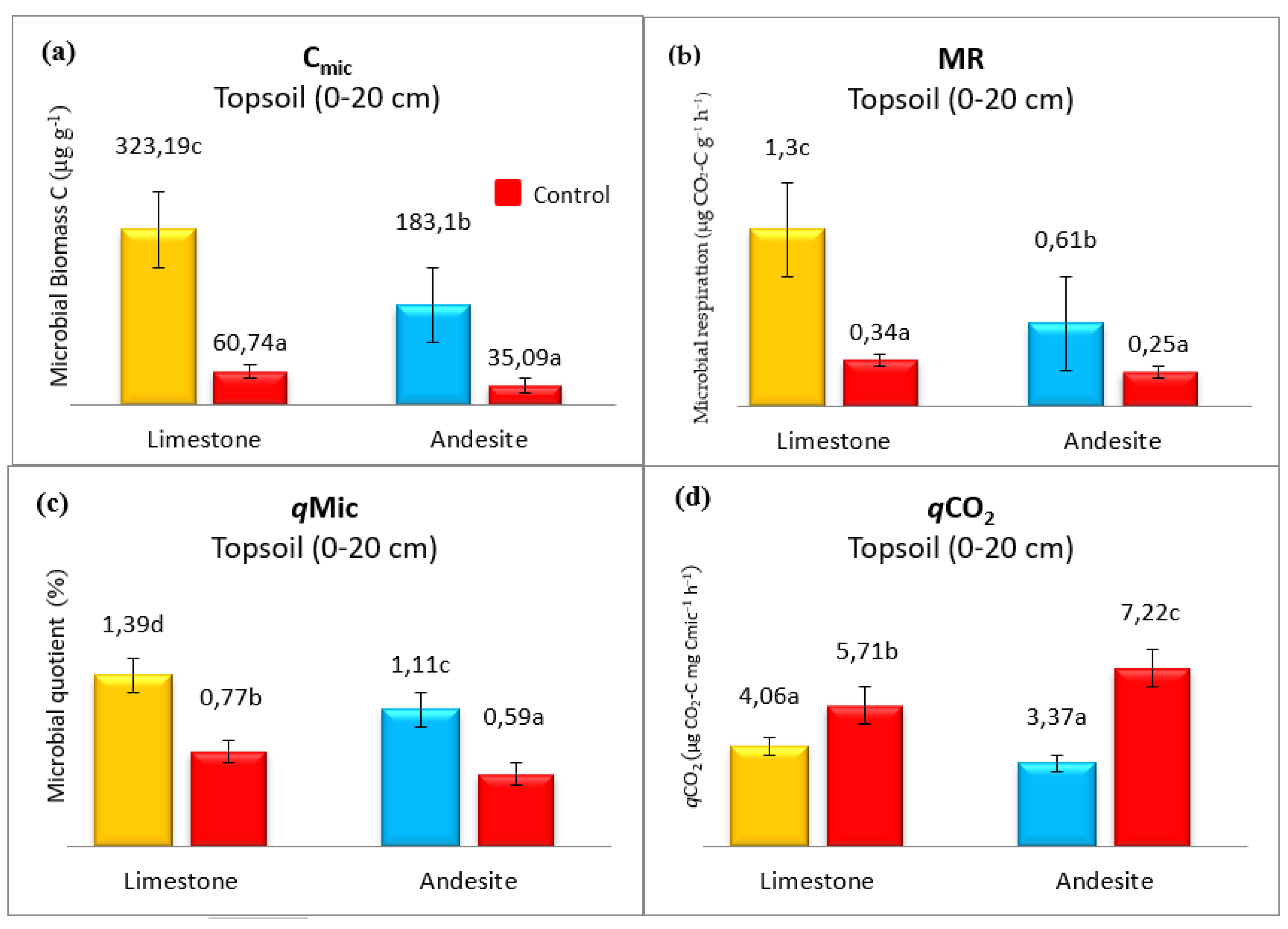
3.2. Soil Microbiological Properties
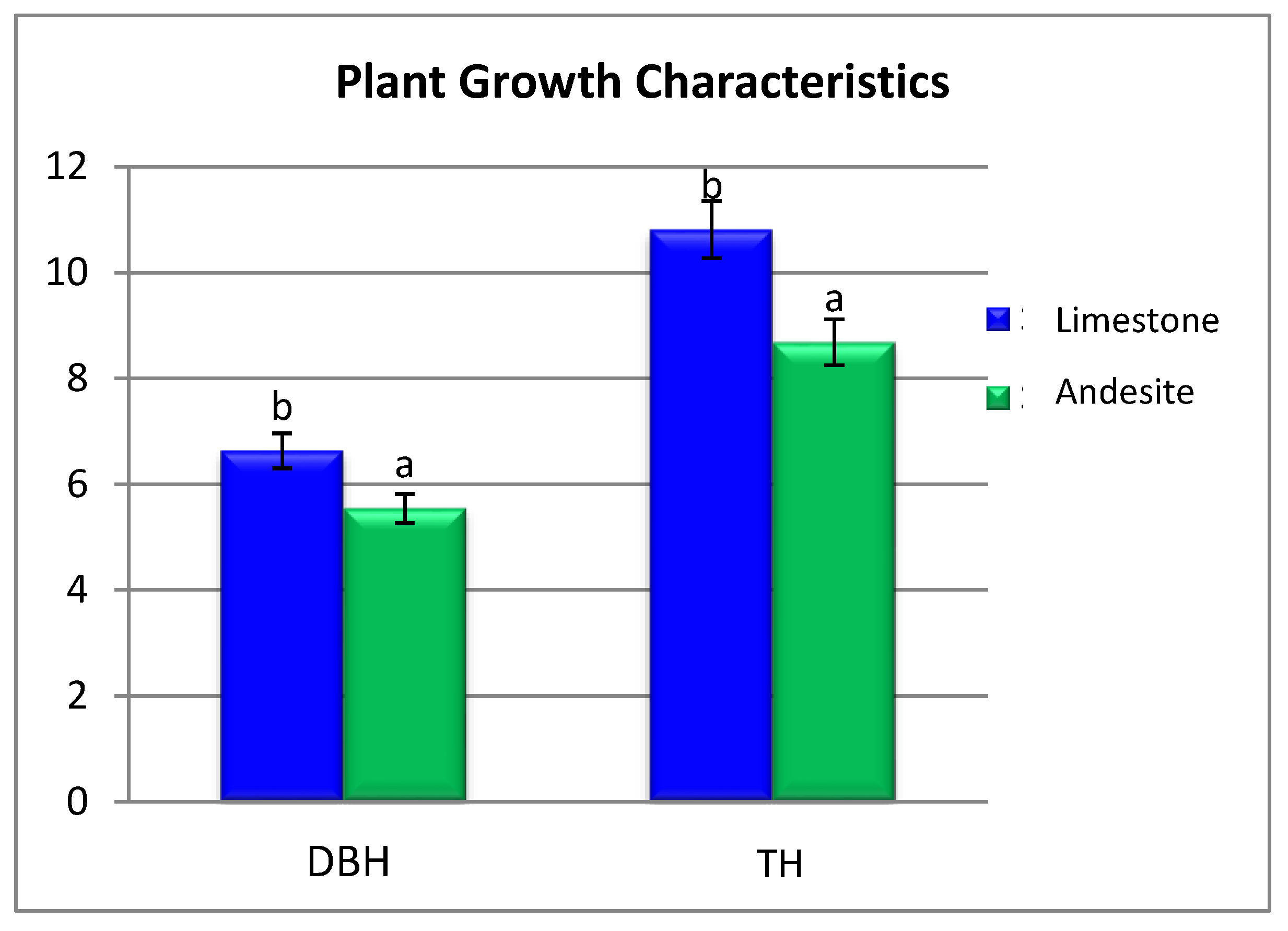
3.3. Plant Growth Characteristics
4. Discussion
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Babur, E. Effects of parent material on soil microbial biomass carbon and basal respiration within young afforested areas. Scand. J. For. Res. 2019, 34, 94–101. [Google Scholar] [CrossRef]
- Lavee, H.; Imeson, A.C.; Sarah, P. The impact of climate change on geomorphology and desertification along a Mediterranean-arid transect. Land Degrad Dev. 1998, 9, 407–424. [Google Scholar] [CrossRef]
- Kooijman, A.M.; Jongejans, J.; Sevink, J. Parent material effects on Mediterranean woodland ecosystem in NE Spain. Catena. 2005, 59, 55–68. [Google Scholar] [CrossRef]
- Gökbulak, F.; Özcan, M. Hydro-physical properties of soils developed from different parent materials. Geoderma. 2008, 145, 376–380. [Google Scholar] [CrossRef]
- Birkeland, P.W. Pedology, Weathering and Geomorphological Research; Oxford University Press: New York, 1974. [Google Scholar]
- Johnson, D.L. Darwin would be proud: Bioturbation, dynamic denudation, and the power of theory in science. Geoarchaeology, 2002, 17, 7–40. [Google Scholar] [CrossRef]
- Gabet, E.J.; Reichmann, O.J.; Seabloom, E.W. The effects of bioturbation on soil processes and sediment transport. Annu. Rev. Earth Planet. Sci. 2003, 31, 249–273. [Google Scholar] [CrossRef]
- Phillips, J.D.; Marion, D.A. Biomechanical effects, litholological variability, and pedodiversity in some forest soils of Arkansas. Geoderma 2004, 124, 73–89. [Google Scholar] [CrossRef]
- Phillips, J.D.; Marion, D.A. Biomechanical Effects of Trees on Soil and Regolith: Beyond Treethrow, Ann. Assoc. Am. Geogr., 2006, 96, 233–247. [Google Scholar] [CrossRef]
- Kara, O.; Babur, E.; Altun, L.; Seyis, M. Effects of afforestation on microbial biomass C and respiration in eroded soils of Turkey. J. Sustain. For. 2016, 35, 385–396. [Google Scholar] [CrossRef]
- Anderson, D.W. The effect of parent material and soil development on nutrient cycling in temperate ecosystems. Biogeochem. 1988, 5, 71–97. [Google Scholar] [CrossRef]
- Turkish Ministry of Agriculture and Forestry, The afforestation and erosion control national mobilization action plan (2008-2012) and combating with erosion action plan (2013- 2017) Ankara, Turkey, 2008, (accesed in 2024).
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science, 2005, 310, 1628–1632. [Google Scholar] [CrossRef]
- Silver, W.L.; Kueppers, L.M.; Lugo, A.E.; Ostertag, R.; Matzek, V. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecol Appl 2004, 14, 1115–1127. [Google Scholar] [CrossRef]
- Grayston, S.J.; Vaughan, D.; Jones, D. Rhizosphere carbon flow in trees, in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol. 1996, 5, 29–56. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroglu, T.; Danish, S.; Haggblom, M.M.; Ozlu, E.; Gozukara, G.; Uslu, O.S. Spatial responses of soil carbon stocks, total nitrogen, and microbial indices to post-wildfire in the Mediterranean red pine forest. J. Environ. Manag. 2022, 320, 115939. [Google Scholar] [CrossRef]
- Bauhus, J.D.; Pare, D.; Cote, L. Effects of tree species, stand age, and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol Biochem, 1998, 30, 1077–1089. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I. The effect of different land uses on soil microbial biomass carbon and nitrogen in Bartin Province. Turk. J. Agric. For. 2008, 32, 281–288. [Google Scholar]
- Yang, Y.; Guo, J.; Chen, G.; Yin, Y.; Gao, R.; Lin, C. Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil. 2009, 323, 153–162. [Google Scholar] [CrossRef]
- Pandey, C.B.; Singh, G.B.; Singh, S.K.; Singh, R.K. Soil nitrogen and microbial biomass carbon dynamics in native forests and derived agricultural land uses in a humid tropical climate of India. Plant Soil. 2010, 333, 453–467. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena. 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroglu, T.; Solaiman, Z.M.; Battaglia, M.L. Microbial Respiration, Microbial Biomass and Activity Are Highly Sensitive to Forest Tree Species and Seasonal Patterns in the Eastern Mediterranean Karst Ecosystems. Sci Total Environ. 2021, 775, 145868. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Y.; Wei, X.; Zhao, G.; Guo, H.; Bai, X. Effects of forest type, stand age, and altitude on soil respiration in subtropical forests of China. Scand J Forest Res. 2011, 26, 40–47. [Google Scholar] [CrossRef]
- Babur, E.; Kara, O.; Fathi, R.; Susam, Y.; Riaz, M.; Arif, M.; Akhtar, K. Wattle fencing improved soil aggregate stability, organic carbon stocks and biochemical quality by restoring highly eroded mountain region soil. J. Environ. Manag. 2021, 288, 112489. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Potts, J.M.; Millard, P. Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl Soil Ecol, 2007, 36, 147–155. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Macdonald, L.M.; Rogers, S.L.; Gregg, A.L.; Bolger, T.P.; Baldock, J.A. Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol Biochem, 2008, 40, 803–813. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Lawson, C.S.; Hedlund, K.; Edwards, A.R.; Brook, A.J.; Igual, J.M.; Van Der Putten, W.H. Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J Ecol, 2006, 94, 893–904. [Google Scholar] [CrossRef]
- Lamb, E.G.; Kennedy, N.; Siciliano, S.D. Effects of plant species richness and evenness on soil microbial community diversity and function. Plant Soil, 2011, 338, 483–495. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Rojo Serrano, L.; Jiménez, M.N.; Navarro, F.B.; Díez, M.; Martín, F.; Aguilar, J. Afforestation improves soil fertility in south-eastern Spain. Eur J For Res, 2010, 129, 707–717. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For Ecol Manag, 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena. 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroğlu, T.; Riaz, M.; Uslu, O.S. Seasonal Variations in Litter Layers’ Characteristics Control Microbial Respiration and Microbial Carbon Utilization Under Mature Pine, Cedar, and Beech Forest Stands in the Eastern Mediterranean Karstic Ecosystems. Microb. Ecol. 2022, 84, 153–167. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Tüfekçioglu, A.; Küçük, M. Comparison of Decomposition Rates of Beech (Fagus orientalis Lipsky) and Spruce (Picea orientalis (L.) Link) Litter in Pure and Mixed Stands of Both Species in Artvin, Turkey. Turk J Agric For 2005, 29, 429–438. [Google Scholar]
- Babur, E.; Dindaroğlu, T.; Roy, R.; Seleiman, M.F.; Ozlu, E.; Battaglia, M.L.; Uslu, Ö.S. Relationship between organic matter and microbial biomass in different vegetation types. In Microbial Syntrophy-Mediated Eco-Enterprising; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–245. [Google Scholar]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ectoxicol Environ Saf 2005, 62, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Akburak, S.; Son, Y.; Makineci, E.; Çakir, M. Impacts of Low-Intensity Prescribed Fire on Microbial and Chemical Soil Properties in a Quercus Frainetto Forest, J. For. Res. 2018. 29, 687–696. [CrossRef]
- Bolat, I. Microbial Biomass, Basal Respiration, and Microbial Indices of Soil in Diverse Croplands in a Region of Northwestern Turkey (Bartın), Environ. Monit. Assess. 2019. 191, 695.
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, A.I. An examination of the Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of soil analysis, Part 2 Chemical and Microbiological Properties (Ed. A.L. Page). SSSA Book series No: 9, Madison, 1982; pp. 595-622.
- Rowell, D.L. Soil science; methods and applications; Longman Publishers (Pte) Ltd.: Singapore, 1994; p. 350. [Google Scholar]
- Horwath, W.R.; Paul, E.A. Microbial biomass. In methods of soil analysis, part 2. microbiological and biochemical properties-SSSA book series, no. 5; Soil Science Society of America: Madison, WI, USA, 1994; pp. 753–773. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil, Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Dilly, O.; Munch, J.C. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol Fertil Soils. 1998, 27, 374–379. [Google Scholar] [CrossRef]
- Alef, K. Soil respiration. In Methods in applied soil microbiology and biochemistry; Alef, K., Nannipieri, P., Eds.; Academic: London, 1995; pp. 214–219. [Google Scholar]
- Mahía, J.; Pérez-Ventura, L.; Cabaneiro, A.; Díaz-Raviña, M. Soil microbial biomass under pine forests in the north-western Spain: influence of stand age, site index and parent material. Sist Rec. Forest. 2006, 15, 152–159. [Google Scholar] [CrossRef]
- Hair, J.F., Jr.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate data analysis: With readings; Macmillan: New York, NY, 1992. [Google Scholar]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. Forest Ecol Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroglu, T. Seasonal changes of soil organic carbon and microbial biomass carbon in different forest ecosystems. Environmental Factors Affecting Human Health, Ivan Uher, IntechOpen 2020.
- Rothe, A.; Cromack, K., Jr.; Resh, S.C.; Makineci, E.; Son, Y. Soil carbon and nitrogen changes under Douglas-fir with and without red alder. Soil Sci Soc Am J 2002, 66, 1988–1995. [Google Scholar] [CrossRef]
- Abdalmoula, M.M.; Makineci, E.; Özturna, A.G.; Pehlivan, S.; Şahin, A.; Tolunay, D. Soil organic carbon accumulation and several physicochemical soil properties under stone pine and maritime pine plantations in coastal dune, Durusu-Istanbul. Environ Monit Assess 2019, 191, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Oostra, S.; Majdi, H.; Olsson, M. Impact of tree species on soil carbon stocks and soil acidity in southern Sweden. Scand J Forest Res. 2006, 21, 364–371. [Google Scholar] [CrossRef]
- Yildiz, O.; Esen, D.; Karaoz, O.M.; Sarginci, M.; Toprak, B.; Soysal, Y. Effects of different site preparation methods on soil carbon and nutrient removal from Eastern beech regeneration sites in Turkey’s Black Sea region. Applied Soil Ecology 2010, 45, 49–55. [Google Scholar] [CrossRef]
- Kantarcı, D. Soil Science Lessons Book; Istanbul University, Department of Soil Science and Ecology, Publications of Istanbul University, No. 4261, Faculty of Forestry No. 462: Istanbul, 2000; p. 420. [Google Scholar]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil Microbial Biomass Carbon Stock and Its Relation with Climatic and Other Environmental Factors in Forest Ecosystems: A Review. Acta Ecologica Sinica 2023, 933–945. [Google Scholar] [CrossRef]
- Yüksek, T. The restoration effects of black locust (Robinia pseudoacacia L) plantation on surface soil properties and carbon sequestration on lower hillslopes in the semi-humid region of Coruh Drainage Basin in Turkey. Catena 2012, 90, 18–25. [Google Scholar] [CrossRef]
- Uslu, O.S.; Babur, E.; Battaglia, M.L.; et al. Effects of gyttja applications on hay yield and quality of a rangeland in the Mediterranean region. Int. J. Environ. Sci. Technol. 2023, 20, 4139–4150. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L. Soil organic matter under different forest types in Southern China. Geoderma. 2007, 142, 349–356. [Google Scholar] [CrossRef]
- Tian, J.; Fan, M.; Guo, J.; Marschner, P.; Li, X.; Kuzyakov, Y. Effects of land use intensity on dissolved organic carbon properties and microbial community structure. Euro J Soil Biol. 2012, 52, 67–72. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Marañon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial Carbon Limitation: The Need for Integrating Microorganisms into Our Understanding of Ecosystem Carbon Cycling, Glob. Chang. Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Tomar, U.; Baishya, R. Seasonality and Moisture Regime Control Soil Respiration, Enzyme Activities, and Soil Microbial Biomass Carbon in a Semi-Arid Forest of Delhi, India. Ecol Process 2020, 9, 50. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. The Role of Earthworms in Organic Matter and Nutrient Cycles. In Biology and Ecology of Earthworms; Springer US: New York, NY, 2022; pp. 233–274. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Insam, H.; Domsch, K.H. Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb Ecol. 1988, 15, 177–188. [Google Scholar] [CrossRef]
- Anderson, T.H. Microbial eco-physiological indicators to assess soil quality. Agr Ecosyst Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeoscience. 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A.; Elser, J. Carbon use efficiency of microbial communities: stoichiometry, methodology and modeling. Ecol Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef]
- Hartman, W.H.; Ye, R.; Horwath, W.R.; Tringe, S.G. A genomic perspective on stoichiometric regulation of soil carbon cycling. ISME J. 2017, 11, 2652–2665. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Xia, Y.D. Soil microbial and enzymatic activities across a chronosequence of Chinese Pine plantation development on the Loess Plateau of China. Pedosphere. 2012, 22, 1–12. [Google Scholar]
- Khalil, H.A.; Hossain, M.S.; Rosamah, E.; Azli, N.A.; Saddon, N.; Davoud Poura, Y.; Islam, M.N.; Dungani, R. The role of soil properties and it’s interaction towards quality plant fiber: a review. Renew. Sustain. Energy Rev. 2015, 43, 1006–1015. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Rossi, E.; Milbau, A.; Nijs, I. Habitat properties and plant traits interact as drivers of non-native plant species’ seed production at the local scale. Ecol. Evol. 2018. 8, 4209–4223. [CrossRef]
- Martins-Noguerol, R.; Matías, L.; Pérez-Ramos, I.M.; Moreira, X.; Francisco, M.; Pedroche, J.; DeAndrés-Gil, C.; Gutiérrez, E.; Salas, J.J.; Moreno-Pérez, A.J.; et al. Soil Physicochemical Properties Associated with the Yield and Phytochemical Composition of the Edible Halophyte Crithmum Maritimum. Sci. Total Environ. 2023, 869, 161806. [Google Scholar] [CrossRef] [PubMed]
- Yüksek, T. The restoration effects of black locust (Robinia pseudoacacia L) plantation on surface soil properties and carbon sequestration on lower hillslopes in the semi-humid region of Coruh Drainage Basin in Turkey. Catena 2012, 90, 18–25. [Google Scholar] [CrossRef]
- Sarkara, S.; Dasb, D.K.; Singhc, A.; Laikd, R.; et al. Seasonal variations in soil characteristics control microbial respiration and carbon use under tree plantations in the Middle Gangetic region. Heliyon. 2024. [Google Scholar] [CrossRef]
| Environmental Factors | Limestone | Andesite | ||
|---|---|---|---|---|
| Sample | Control | Sample | Control | |
| Longitude | 39o 33′ 19′’ | 39o 33′ 46′’ | 39o 33′ 43′’ | 39o 33′ 23′’ |
| Latitude | 40o 21′ 08′’ | 40o 21′ 23′’ | 40o 21′ 20′’ | 40o 21′ 07′’ |
| Altitude mean (m) | 1480 | 1520 | 1500 | 1450 |
| Slope degree mean (%) | 50 | 40 | 45 | 50 |
| Afforestation date | 2005 | - | 2005 | - |
| Vegetation type | Scotch Pine | Bare space | Scotch Pine | Bare space |
| Stand canopy (%) | 70-100 | - | 70-100 | - |
| Soil texture | Loamy clay | Clay loam | Sandy clay loam | Sandy loam |
| pH class | Moderately Alkaline | Slightly Alkaline | Moderately Alkaline | Slightly Alkaline |
| Sand | Silt | Clay | pH | EC | SOC | TN | C/N | CaCO3 | Cmic | MR | qMic | qCO2 | DBH | TH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent Material | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| F-values | 355.8 | 42.6 | 117,4 | 60,5 | 9.8 | 21.5 | 48.8 | 24.3 | 123.2 | 84.3 | 62.6 | 44.5 | 27.9 | 56.0 | 85.6 |
| Parent Material | |||||
|---|---|---|---|---|---|
| Limestone (n = 30) | Andesite (n = 30) | ||||
| Sample | Control | Sample | Control | ||
| SOIL PHYSICOCHEMICHAL PROPERTIES | Sand (%) | 53,50±0,94 a | 59,74±1,00 b | 72,58±,13 c | 75,28±1,55 d |
| Silt (%) | 15,40±2,89 b | 22,08±0,91 c | 10,60±1,06 a | 13,66±0,87 b | |
| Clay (%) | 31,10±2,65 c | 18,18±1,68 b | 16,82±2,15 b | 11,02±1,71 a | |
| pH (H2O) | 8,05±0,1b | 8,18±0,09 b | 7,59±0,09 a | 7,57±0,15 a | |
| EC (mS cm−1) | 0,17±0,04 b | 0,18±0,02 b | 0,12±0,02 a | 0,13±0,01 a | |
| SOC (%) | 2,40±0,8 c | 0,80±0,11 a | 1,65±0,05 b | 0,61±0,12 a | |
| TN (%) | 0,19±0,05 c | 0,04±0,00 a | 0,10±0,02 b | 0,03±0,01 a | |
| C/N | 12,61±1,88 a | 18,76±1,20 b | 16,22±2,39 b | 22,74±3,31 c | |
| CaCO3 (%) | 23,09±3,42 b | 32,90±6,31 b | 3,08±1,24 a | 4,85±2,04 a | |
| SOIL MICROBIAL PROPERTIES | Cmic | 323,19±64,96 c | 60,74±9,03 a | 183,10±9,31 b | 35,09±3,65 a |
| MR | 1,3±0,24 c | 0,34±0,04 a | 0,61±0,15 b | 0,25±0,024 a | |
| qMic | 1,39±0,20 d | 0,77±0,12 b | 1,11±0,08 c | 0,59±0,09 a | |
| qCO2 | 4,06±0,65 a | 5,71±1,23 b | 3,37±0,85 a | 7,22±0,67 c | |
| GROWTH PROPERTIES | DBH | 6,63±0,26 b | - | 5,54±0,38 a | - |
| TH | 10,81±0,70 b | - | 8,68±0,19 a | - | |
| Function | |||
| Soil variables | 1 | 2 | 3 |
| TH | 1,195 | 0,330 | 0,384 |
| DBH | 1,233 | -0,133 | -0,510 |
| Sand | -0,613 | 0,900 | -0,258 |
| Clay | -0,906 | 0,000 | -0,483 |
| pH | 0,262 | 0,103 | 0,779 |
| EC | -1,798 | -0,646 | -1,695 |
| SOC | 17,718 | 2,887 | 17,085 |
| TN | 0,936 | -0,066 | -2,746 |
| C/N | -0,512 | -0,197 | -1,344 |
| CaCO3 | -0,030 | -0,258 | 0,436 |
| Cmic | -14,303 | -2,517 | -11,114 |
| MR | -0,434 | 0,412 | 0,607 |
| qMic(Cmic/Corg) | 7,158 | 0,590 | 5,012 |
| qCO2 | 0,958 | 0,069 | 0,004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





