Submitted:
08 August 2024
Posted:
08 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
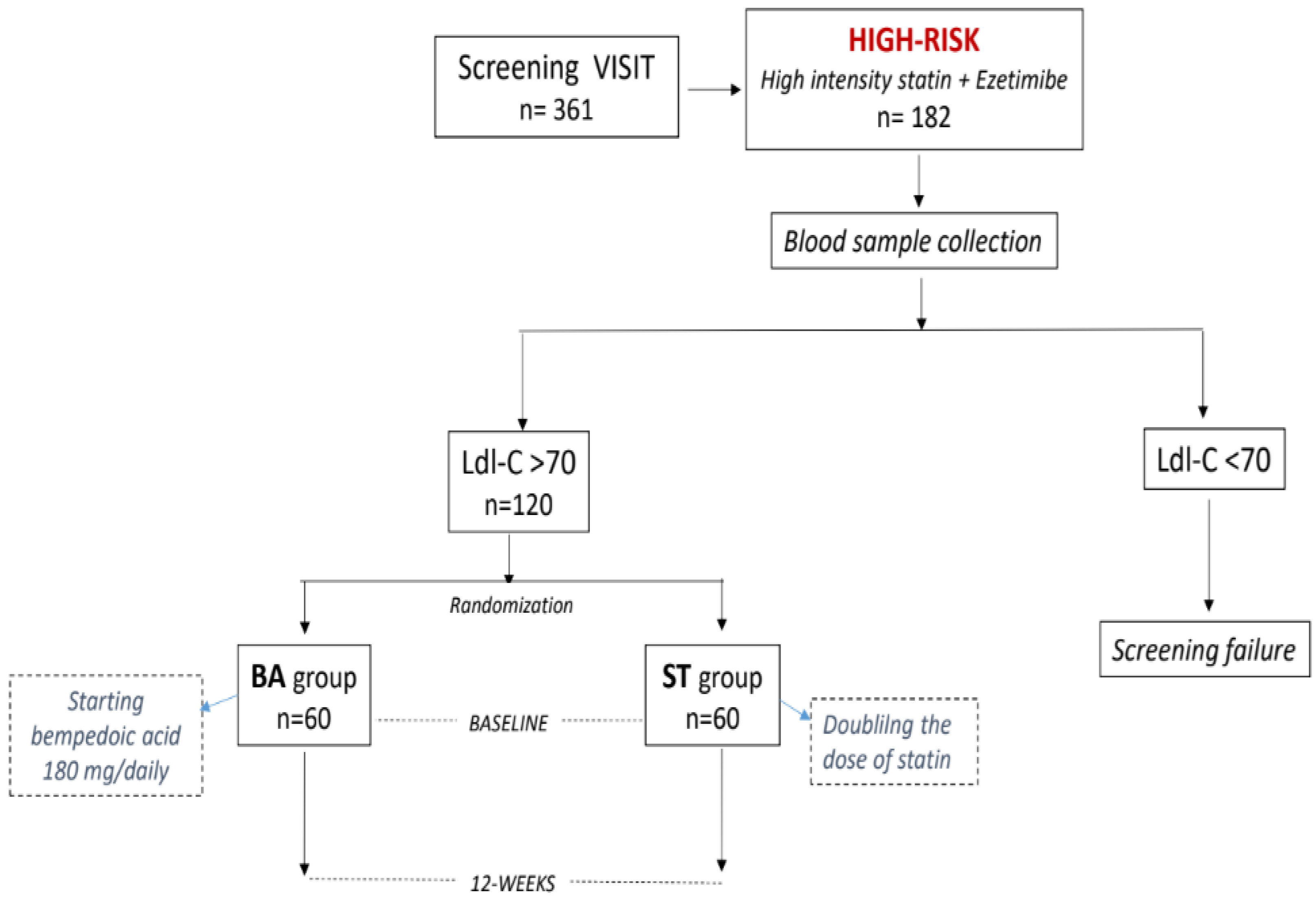
2.1. Population
2.2. Study Design
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016 Feb 19;118(4):535-46. [CrossRef]
- Parini P., Frikke-Schmidt R., Tselepis A.D., Moulin P., von Eckardstein A., Binder C.J., Catapano A.L., Ray K.K., Tokgözoğlu L. Taking action: European Atherosclerosis Society targets the United Nations Sustainable Development Goals 2030 agenda to fight atherosclerotic cardiovascular disease in Europe. Atherosclerosis. 2021;322:77–81. [CrossRef]
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep 7;42(34):3227-3337. [CrossRef]
- Baigent C. Cholesterol treatment trialists’ (CTT) collaborators: efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366: 1267–1278. [CrossRef]
- LaRosa, JC, Grundy, SM, Waters, DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-1435. [CrossRef]
- Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005 Nov 16;294(19):2437-45. [CrossRef]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008 Nov 20;359(21):2195-207. [CrossRef]
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Wong JB. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2022 Aug 23;328(8):746-753. [CrossRef]
- Cannon C.P., Blazing M.A., Giugliano R.P., McCagg A., White J.A., Théroux P., Darius H., Lewis B.S., Ophuis T.O., Jukema J.W., et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015;372:2387–2397. [CrossRef]
- Ezhov MV, Sergienko IV, Kryzhanovskiy SM, Manko KS, Timoshina EV. Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting. Diseases. 2023 Nov 13;11(4):168. [CrossRef]
- Lee J, Lee SH, Kim H, Lee SH, Cho JH, Lee H, Yim HW, Yoon KH, Kim HS, Kim JH. Low-density lipoprotein cholesterol reduction and target achievement after switching from statin monotherapy to statin/ezetimibe combination therapy: Real-world evidence. J Clin Pharm Ther. 2021 Feb;46(1):134-142. [CrossRef]
- Daskalopoulou SS, Mikhailidis DP. Reaching goal in hypercholesterolaemia: dual inhibition of cholesterol synthesis and absorption with simvastatin plus ezetimibe. Curr Med Res Opin. 2006 Mar;22(3):511-28. [CrossRef]
- Biolo G, Vinci P, Mangogna A, Landolfo M, Schincariol P, Fiotti N, Mearelli F, Di Girolamo FG. Mechanism of action and therapeutic use of bempedoic acid in atherosclerosis and metabolic syndrome. Front Cardiovasc Med. 2022 Oct 28;9:1028355. [CrossRef]
- Thompson PD, Rubino J, Janik MJ, et al. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J ClinLipidol 2015; 9:295–304.
- Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis. (2021) 320:122–8. 10.1016/j.atherosclerosis.2020.12.023.
- Lalwani ND, Hanselman JC, MacDougall DE, Sterling LR, Cramer CT. Complementary low-density lipoprotein-cholesterol lowering and pharmacokinetics of adding bempedoic acid (ETC-1002) to high-dose atorvastatin background therapy in hypercholesterolemic patients: a randomized placebo-controlled trial. J ClinLipidol. (2019) 13:568–79. 10.1016/j.jacl.2019.05.003.
- Nissen SE, Menon V, Nicholls SJ, Brennan D, Laffin L, Ridker P, Ray KK, Mason D, Kastelein JJP, Cho L, Libby P, Li N, Foody J, Louie MJ, Lincoff AM. Bempedoic Acid for Primary Prevention of Cardiovascular Events in Statin-Intolerant Patients. JAMA. 2023 Jul 11;330(2):131-140. [CrossRef]
- SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. EurHeart J. 2021 Jul 1;42(25):2439-2454. [CrossRef]
- Tremblay AJ, Morrissette H, Gagné J-M, Bergeron J, Gagné C, Couture P. Validation of the Friedewald formula for the determination of low-density lipoprotein cholesterol compared with β-quantification in a large population. Clinical Biochemistry. 2004;37(9):785–790.
- Mhaimeed O, Burney ZA, Schott SL, Kohli P, Marvel FA, Martin SS. The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: Lower for longer is better. Am J Prev Cardiol. 2024 Mar 18;18:100649. [CrossRef]
- Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LR, Kelly S, Stroes ESG. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019 Apr 2;8(7):e011662. [CrossRef]
- Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, Leiter LA. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: A randomized, placebo-controlled study. Atherosclerosis. 2018 Oct;277:195-203. [CrossRef]
- Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL, Ballantyne CM; CLEAR Harmony Trial. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N Engl J Med. 2019 Mar 14;380(11):1022-1032. [CrossRef]
- Goldberg AC, Leiter LA, Stroes ESG, Baum SJ, Hanselman JC, Bloedon LT, Lalwani ND, Patel PM, Zhao X, Duell PB. Effect of Bempedoic Acid vs Placebo Added to Maximally Tolerated Statins on Low-Density Lipoprotein Cholesterol in Patients at High Risk for Cardiovascular Disease: The CLEAR Wisdom Randomized Clinical Trial. JAMA. 2019 Nov 12;322(18):1780-1788. [CrossRef]
- Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW; STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003 Jul 15;92(2):152-60. [CrossRef]
- McQueen RB, Baum SJ, Louie MJ, Sasiela WJ, Bilitou A, Shah H, Nash B, Gillard KK, Ray KK. Potential Cardiovascular Events Avoided with Bempedoic Acid Plus Ezetimibe Fixed-Dose Combination Compared with Ezetimibe Alone in Patients with Atherosclerotic Cardiovascular Disease Taking Maximally Tolerated Statins. Am J Cardiovasc Drugs. 2023 Jan;23(1):67-76. [CrossRef]
- Ballantyne CM, Laufs U, Ray KK, Leiter LA, Bays HE, Goldberg AC, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J PrevCardiol. 2020;27:593–603. [CrossRef]
- Thompson PD, MacDougall DE, Newton RS, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J ClinLipidol 2016; 10:556–567.
- Bays HE, Banach M, Catapano AL, Duell PB, Gotto AM Jr, Laufs U, Leiter LA, Mancini GBJ, Ray KK, Bloedon LT, Sasiela WJ, Ye Z, Ballantyne CM. Bempedoic acid safety analysis: Pooled data from four phase 3 clinical trials. J ClinLipidol. 2020 Sep-Oct;14(5):649-659.e6. [CrossRef]
| BA group (n=60) | ST group (n=60) | |
| Clinical profile | ||
| Age, y | 61.7.4±11.2 | 61.9±7.4 |
| BMI, kg/m2 | 27.7±6.4 | 28.0±7.2 |
| Males, n (%) | 32 (53) | 34 (56) |
| Hypertension, n (%) | 39 (65) | 41 (68) |
| Diabetes, n (%) | 33 (55) | 32 (53) |
| Active smokers, n(%) | 18 (30) | 17 (28) |
| Laboratory | ||
| eGFR, ml/min per 1.73 m2 | 89.3±11.6 | 79.8±14.1 |
| ALT, U/l | 30.7±5.3 | 31.0±7.9 |
| AST, U/l | 30.2±8.2 | 29.8±8.6 |
| Creatinine, mg/dl | 0.91±0.7 | 0.87±0.3 |
| Uric acid, mg/dl | 6.2±1.5 | 5.8±2.1 |
| CK, U/l | 68.3±13.4 | 75.2±18.1 |
| Glucose, mg/dl | 101.2±26.7 | 97.8±21.84 |
| Treatments | ||
| Atorvastatin ,n (%) | 33 (55) | 31 (54) |
| Betablockers, n (%) | 14 (93.3) | 14 (93.3) |
| ACE-Is /ARBs, n (%) | 23 (38) | 21 (35) |
| CCAs, n (%) | 16 (27) | 19 (32) |
| Acetylsalicylic acid, n (%) | 7 (12) | 9 (15) |
| Clopidogrel, n (%) | 3 (5) | 4 (6) |
| Metformin, n (%) | 22 (37) | 23 (38) |
| SGLT2-Is, n (%) | 18 (30) | 17 (28) |
| Sitagliptin, n (%) | 6 (10) | 8 (13) |
| BA group (n=60) | Δ | ST group (n=60) | Δ | |||
| Baseline | 12 weeks | Baseline | 12 weeks | |||
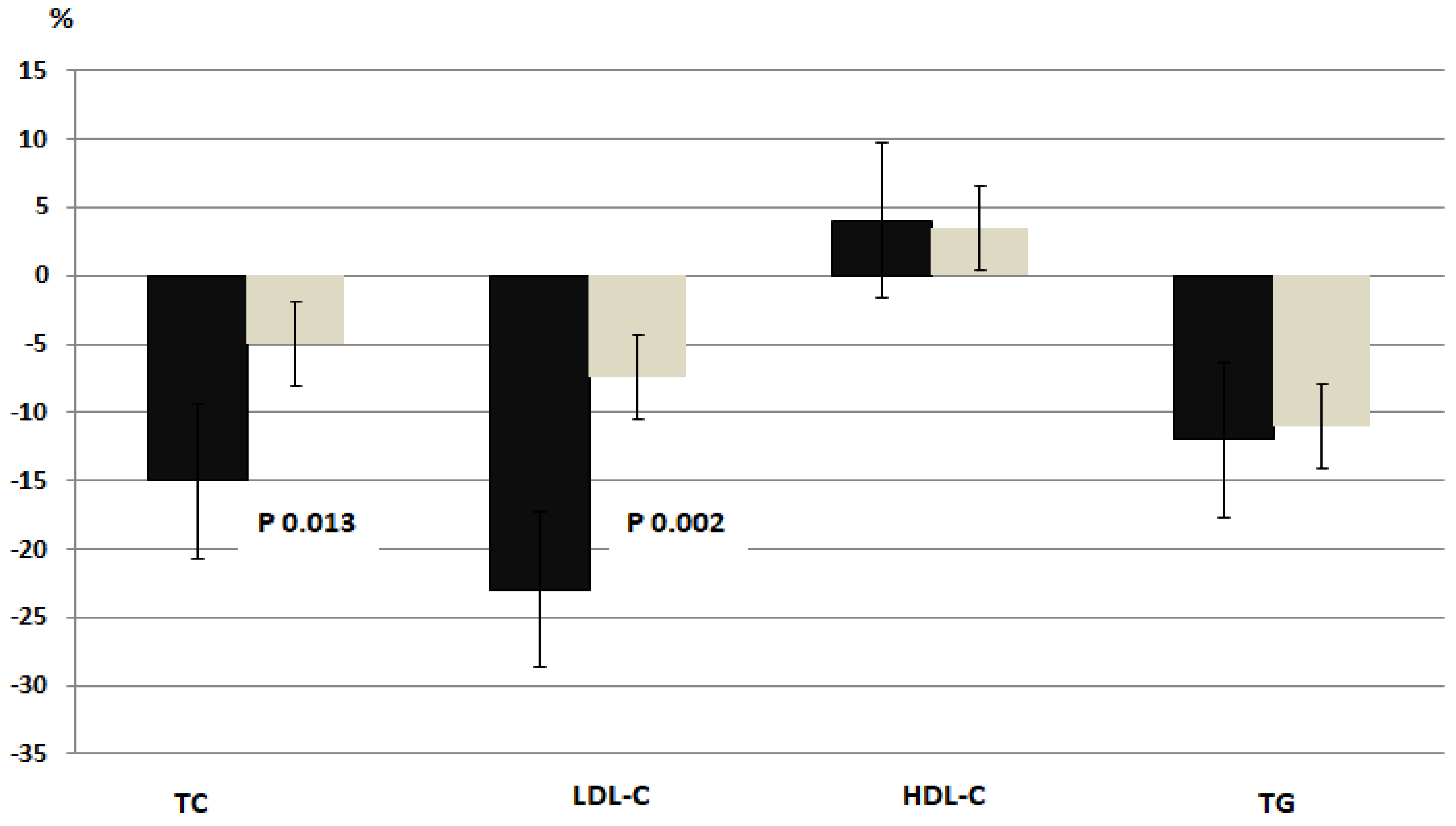
| Total-Chol, mg/dl | 148.8±44.5 | 126.9±52.1 | -21.9±6.2* | 146.0±48.6 | 139.5±55.2 | -6.5±2.4 |
| LDL-Chol, mg/dl | 89.9±7.9 | 69.4±6.5* | - 20.5±7.3* | 87.5±8.8 | 80.8±8.5 | -6.7±2.5 |
| HDL-Chol, mg/dl | 37.2±4.6 | 39.0±3.8 | 1.8±0.6 | 36.9±4.2 | 38.3±5.7 | 1.4±0.7 |
| TG, mg/dl | 108.4±32.4 | 95.9±39.1 | -12.5±3.7 | 109.2±33.7 | 99.1±26.4 | -10.2±3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





