Submitted:
08 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
Keywords:
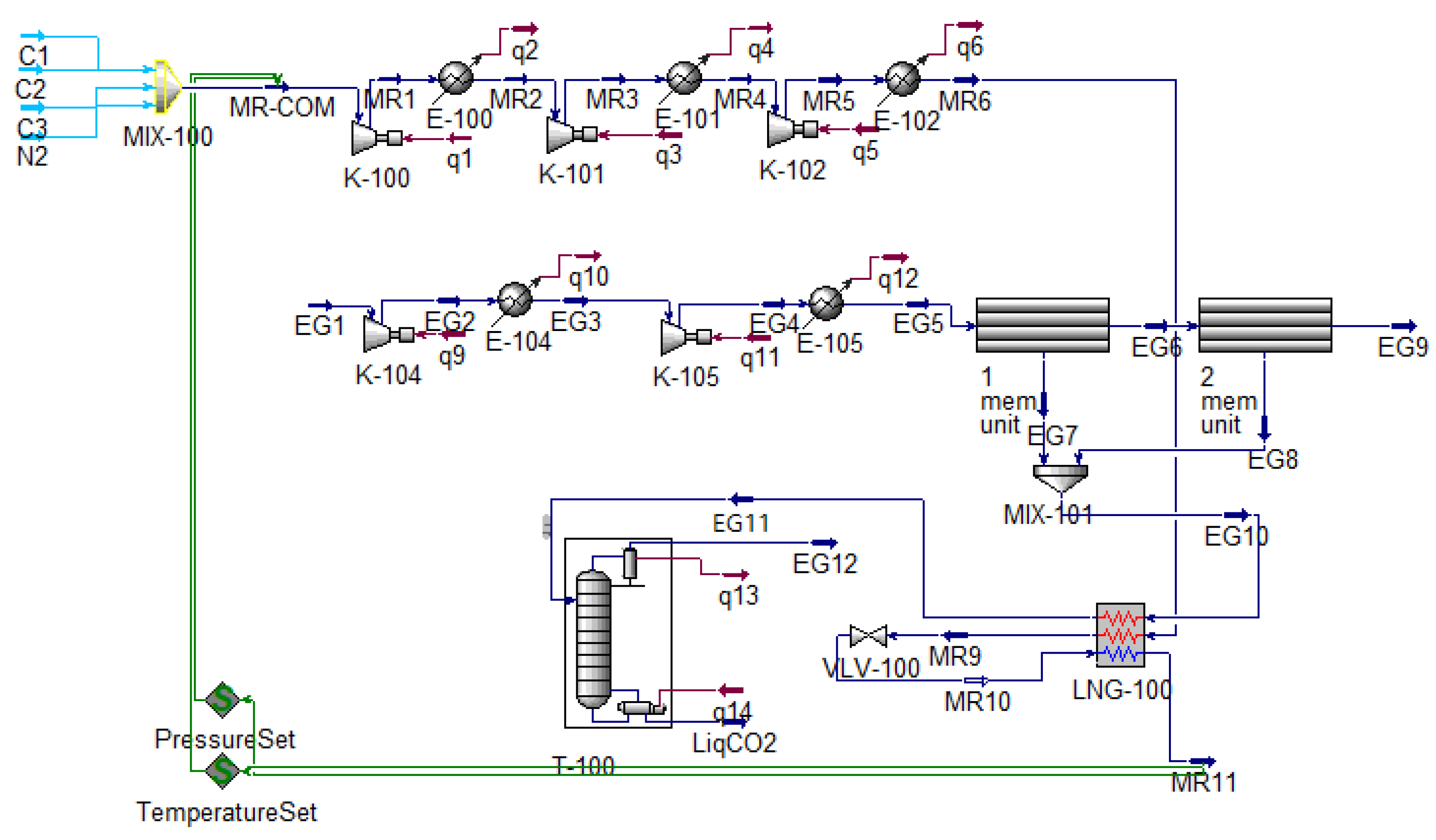
1. Design of CO2 Recovery Process of Fire-Flooding Exhaust
- (1)
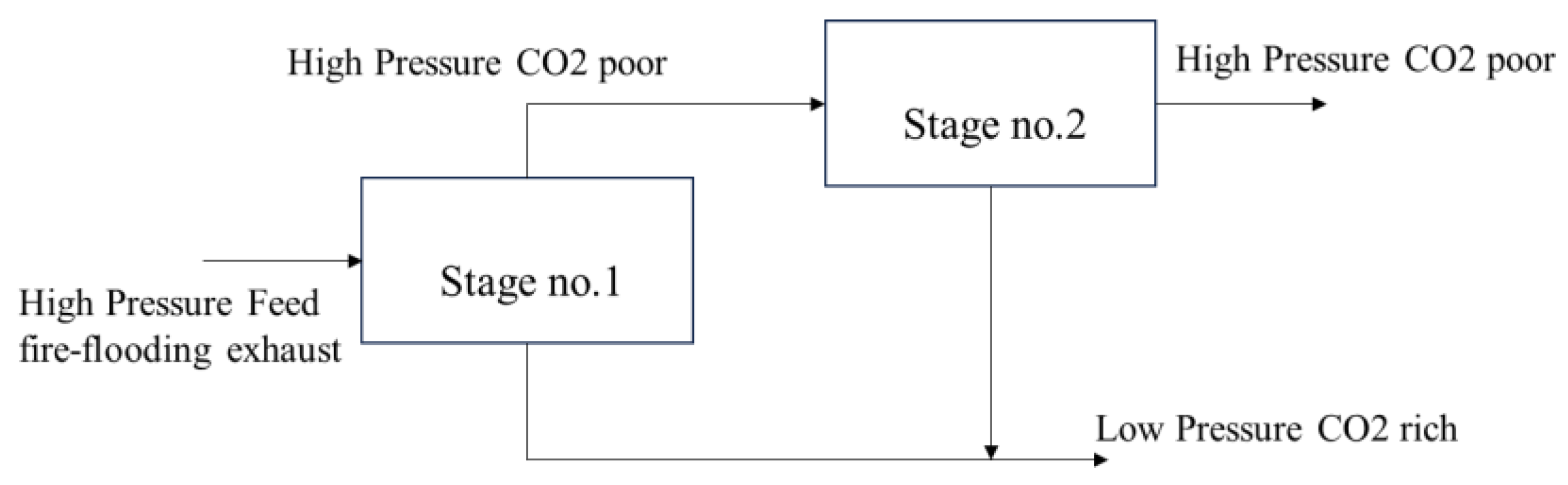
- Secondary membrane separation
- (2)
- Mixed refrigerant refrigeration cycle
- (3)
- CO2 distillation and purification
2. Analysis of Parameters of Liquefaction Process
2.1. Initial Parameter Setting and Product Requirements
2.2. Process Parameters and Performance Specifications
3. Process Optimization
3.1. Objective Functions and Constraints
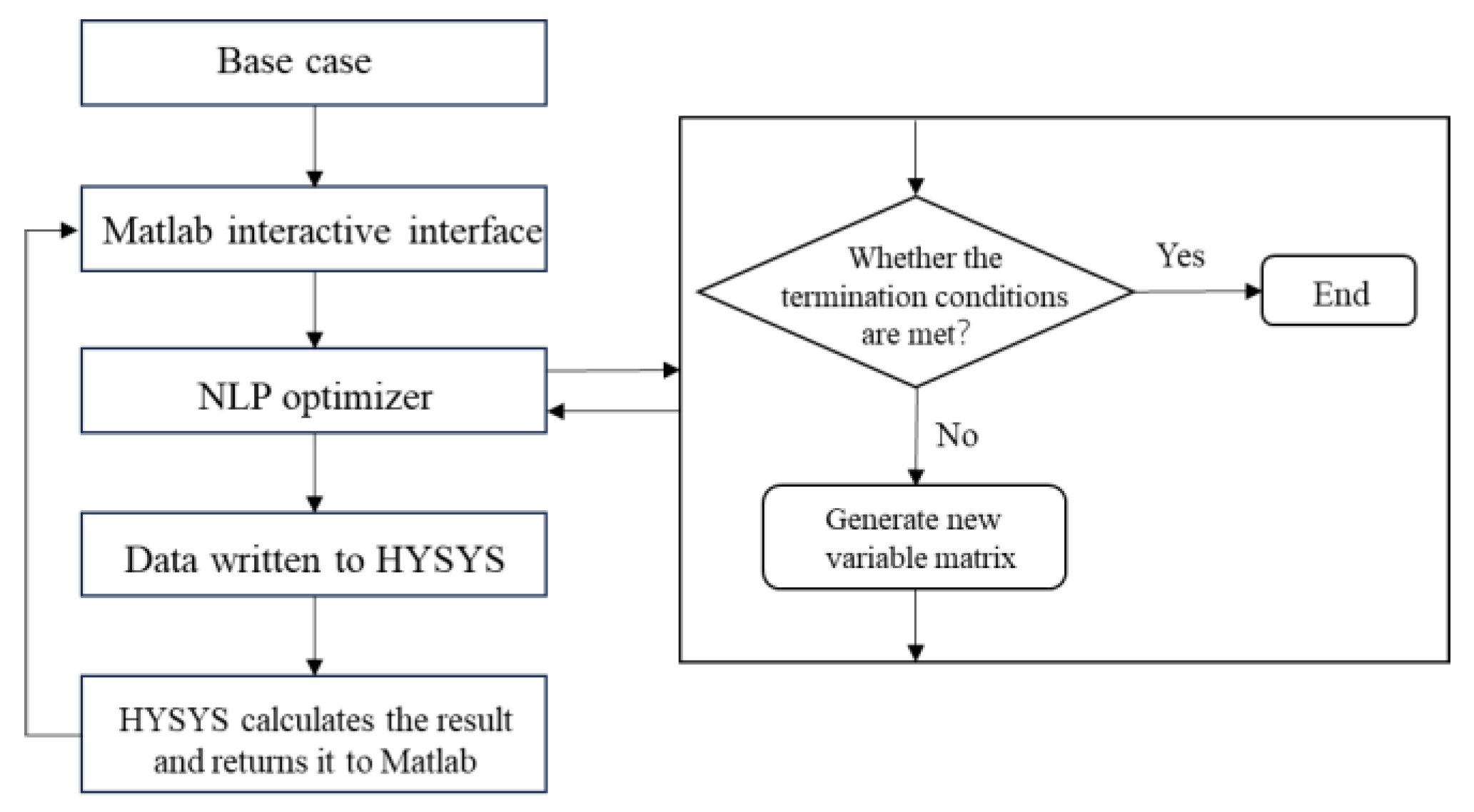
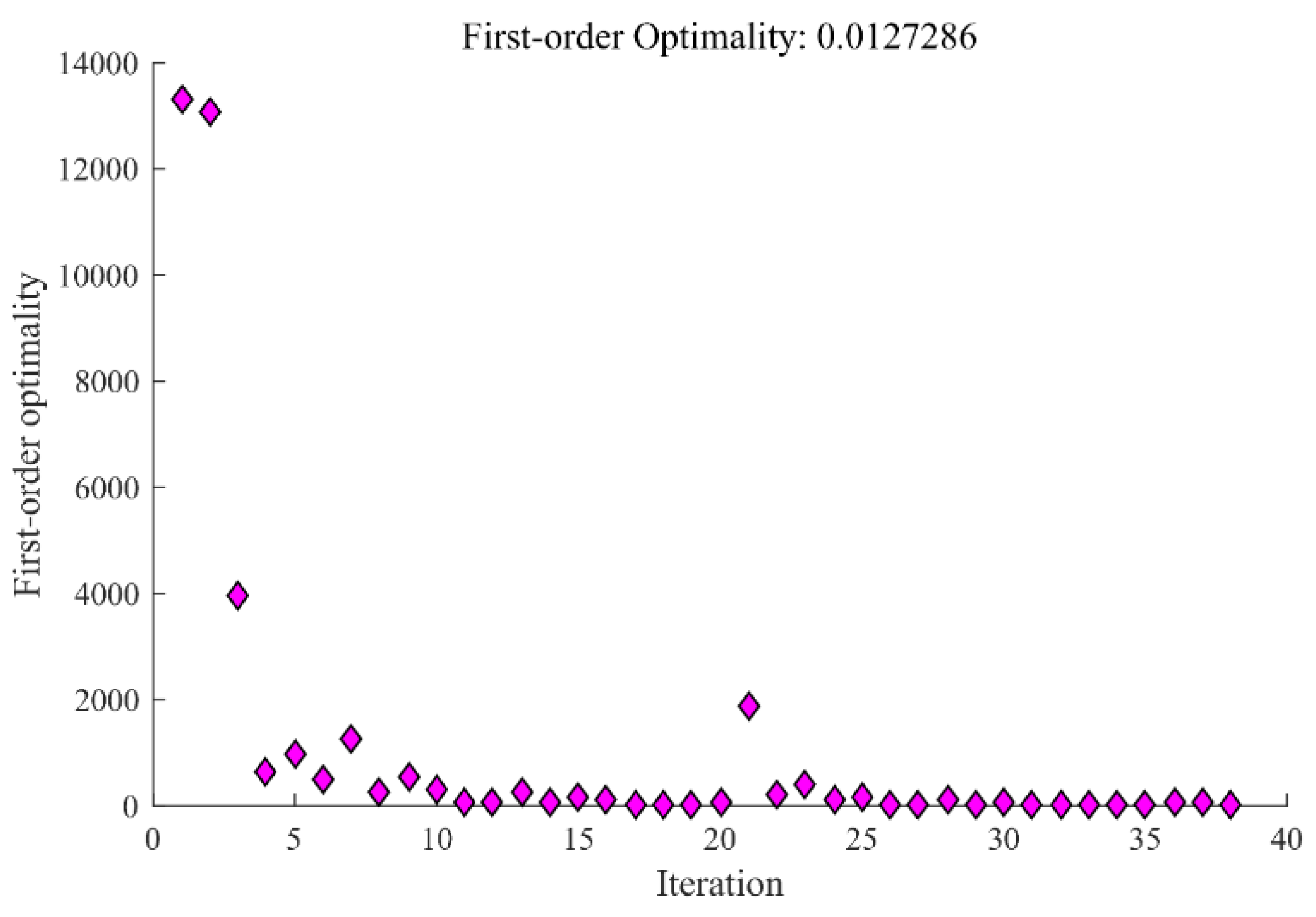
3.2. Optimization Process
3.3. Results and Analysis
4. Process Adaptability Analysis
5. Conclusions
- (1)
- The designed CO2 recovery process of fire-flooding exhaust can achieve the preparation of food-grade liquid CO2. HYSYS process simulation shows that the CO2 recovery rate is 69.02% under the basic case condition. After the optimization, the specific power consumption of the liquefaction process is reduced to 2.287 kW∙h/kg, which is 18.8% lower than before the optimization.
- (2)
- For a flow rate of 1000 kg/h, the total sales of products under optimized conditions under the components in Table 1 is S=2086.8 yuan/h. Under the conditions of N2 / CO2 components in Table 6, with the increase of CO2 content in the exhaust gas, the total sales volume of the product increases, the specific power consumption of the process decreases, and the CO2 recovery rate increases, that is, the ability of the process to recover CO2 becomes higher and higher.
- (3)
- The process adopts membrane separation and enrichment of CO2, avoids complex chemical decarbonization process, reduces the initial investment of equipment and long-term operating costs, makes full use of carbon resources in fire-flooding exhaust, and achieves the dual carbon goal of energy saving and emission reduction.
References
- Wang Y, Chang M, Chen L, et al. Evaluation of prediction models for the physical properties in fire-flooding exhaust reinjection process[J]. Energies, 2022, 15(2): 562.
- ZHANG Minglong, LI Yuying, ZHOU Xian, et al. Feasibility study of fire flooding exhaust gas reinjection EOR and storage [J]. World Petroleum Industry, 2022,29(02):61-68.
- QIN Hongyan. Research and application of fire flooding exhaust emission system[J]. Environmental protection of oil & gas fields,2018,28(02):24-26+61.
- Pan S Y , Chang E E , Chiang P C .CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications[J].Aerosol and Air Quality Research, 2016, 12(5):770-791.
- MU Zhonghua, ZHANG Ping, BAI Jianfeng, et al. Research on CO2 Capture and Liquefaction Technology in Oilfield Associated Gas [J]. Field surface engineering,2023,42(07):1-5.
- Zhao L, Riensche E, Menzer R, et al. A parametric study of CO2/N2 gas separation membrane processes for post-combustion capture[J]. Journal of membrane science, 2008, 325(1): 284-294.
- Scholes C A, Smith K H, Kentish S E, et al. CO2 capture from pre-combustion processes—Strategies for membrane gas separation[J]. International Journal of Greenhouse Gas Control, 2010, 4(5): 739-755.
- Xu J, Wang Z, Qiao Z, et al. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure[J]. Journal of membrane science, 2019, 581: 195-213.
- Zhao L, Riensche E, Blum L, et al. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses[J]. Journal of membrane science, 2010, 359(1-2): 160-172.
- Zhang X, He X, Gundersen T. Post-combustion carbon capture with a gas separation membrane: parametric study, capture cost, and exergy analysis[J]. Energy & Fuels, 2013, 27(8): 4137-4149. [CrossRef]
- Bounaceur R, Lape N, Roizard D, et al. Membrane processes for post-combustion carbon dioxide capture: a parametric study[J]. Energy, 2006, 31(14): 2556-2570.
- Hussain A, Farrukh S, Minhas F T. Two-stage membrane system for post-combustion CO2 capture application[J]. Energy & Fuels, 2015, 29(10): 6664-6669.
- Li M, Jiang X, He G. Application of membrane separation technology in postcombustion carbon dioxide capture process[J]. Frontiers of Chemical Science and Engineering, 2014, 8: 233-239.
- Ren L X, Chang F L, Kang D Y, et al. Hybrid membrane process for post-combustion CO2 capture from coal-fired power plant[J]. Journal of membrane science, 2020, 603: 118001.
- Gkotsis P, Peleka E, Zouboulis A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials[J]. Membranes, 2023, 13(12): 898.
- Lee U, Yang S, Jeong Y S, et al. Carbon dioxide liquefaction process for ship transportation[J]. Industrial & engineering chemistry research, 2012, 51(46): 15122-15131.
- Mehrpooya M, Ghorbani B. Introducing a hybrid oxy-fuel power generation and natural gas/carbon dioxide liquefaction process with thermodynamic and economic analysis[J]. Journal of cleaner production, 2018, 204: 1016-1033.
- Ghorbani B, Ebrahimi A, Ziabasharhagh M. Thermodynamic and economic evaluation of biomethane and carbon dioxide liquefaction process in a hybridized system of biogas upgrading process and mixed fluid cascade liquefaction cycle[J]. Process Safety and Environmental Protection, 2021, 151: 222-243.
- Xin Y, Zhang Y, Xue P, et al. The optimization and thermodynamic and economic estimation analysis for CO2 compression-liquefaction process of CCUS system using LNG cold energy[J]. Energy, 2021, 236: 121376.
- ZHENG Pingyang, HAO Jiahao, CHANG Hong, et al. Research progress of liquid carbon dioxide energy storage system based on different liquefaction methods [J]. Southern energy construction, 2024, 11(2): 102-111.
- Yang S, Lee U, Lim Y, et al. Process design and cost estimation of carbon dioxide compression and liquefaction for transportation[J]. Korean Chemical Engineering Research, 2012, 50(6): 988-993.
- LI Zibo, ZHANG Yong, WANG Yanjun, et al. Design and Analysis of CO2 Capture Process Based on Cryogenic Liquefaction Technology [J]. Oil and gas and new energy,2024,36(02):58-65.
- Sun H, Geng J, Na F, et al. Performance evaluation and comparison of commonly used optimization algorithms for natural gas liquefaction processes[J]. Energy Reports, 2022, 8: 4787-4800.
- SHI Huijie, XU Hong, PU Peng, et al. Pilot Scale Test on Biogas Purification Using Membrane Separation Technology [J]. China Biogas, 2015, 33(1):36-40.
- Ahsan M, Sweeney OM, Hussain A. Development of user-defined extension for the simulation of membrane process in Aspen HYSYS[J]. SIGMA Sigma Journal of Engineering and Natural Sciences Sigma Mühendislik ve Fen Bilimleri Dergisi, 2017, 35 (1), 35-45.
- HOORFAR M, ALCHEIKHHAMDON Y, CHEN B. A Novel Tool for the Modeling, Simulation and Costing of Membrane based Gas Separation Processes using Aspen HYSYS: Optimization of the CO2/CH4, Separation Process[J]. Computers & Chemical Engineering, 2018:S0098135418301509.
- AHMAD F, LAU K K, LOCK S S M, et al. Hollow fiber membrane model for gas separation: Process simulation, experimental validation and module characteristics study[J]. Journal of Industrial and Engineering Chemistry, 2015, 21:1246-1257.
- YUAN Z M, CUI M M, SONG R, et al. Evaluation of prediction models for the physical parameters in natural gas liquefaction processes[J]. Journal of Natural Gas Science and Engineering, 2015,27: 876-886.
- GONG Keqin, WANG Zhuozhi, JIA Yongying. Generalization of Thermodynamic Research of LNG and Other Mixtures Storage and Transportation Process [J]. Science Technology and Engineering, 2013(35):10549-10559.
- GAO T, LIN W S, GU A Z. Mixed refrigerant cycle liquefaction process for coalbed methane with high nitrogen content[J]. Journal of the Energy Institute, 2016, 84(4):185-191.
- SONG R, CUI M M, LIU J J. Single and multiple objective optimization of a natural gas liquefaction process[J]. Energy, 2017, 124:19-28.
- National Health and Family Planning Commission, People's Republic of China. Food safety National standard for food additives Carbon dioxide: GB 1886.228-2016[S]. Beijing: Standards Press of China, 2016.
- GEORGE G , BHORIA N , ALHALLAQ S , et al. Polymer Membranes for Acid Gas Removal from Natural Gas[J]. Separation and Purification Technology, 2016, 158:333-356.
- Khan MS, Lee M. Design optimization of single mixed refrigerant natural gas liquefaction process using the particle swarm paradigm with nonlinear constraints[J]. Energy, 2013, 49:146-155.
- Ji Zhongli, Deng Zhian, ZHAO Huijun. Pumps and Compressors [M]. Beijing: Petroleum Industry Press, 2015.
- Khan M S, Lee S, Lee M. Optimization of single mixed refrigerant natural gas liquefaction plant with nonlinear programming[J]. Asia-Pacific Journal of Chemical Engineering, 2012, 7: S62-S70.
| Components | CH4 | C2H6 | C3H8 | C4+ | N2 | CO2 | O2 | H2S |
| mol% | 1.9 | 0.14 | 0.07 | 0.08 | 78.13 | 17.59 | 2.07 | 0.02 |
| Parameters | Value | Remarks |
| Feed temperature | 30 ℃ | |
| Feed pressure | 500 kPa | |
| Feed flow | 1000 kg∙h-1 | |
| Physical properties simulation fluid package | GERG-2008 | [28,29] |
| Water cooler cooling temperature | 30 ℃ | [30,31] |
| Water cooler/heat exchanger pressure drop | 10 kPa | |
| Minimum heat transfer temperature difference | 3 ℃ | |
| Compressor adiabatic efficiency | 85% | |
| Pressure ratio | <3 | |
| refrigerant | CH4, C2H6, C3H8 and N2 | |
| Liquid CO2 purity | >99.95% | [32] |
| Acetate membrane | PCO2=2.43 Barrer, αCO2/CH4=22.1 | [33] |
| Number of units = 1, Area per unit = 10 m2 |
| Variable | MR_Com | MR5 | EG1 | EG8 | LiqCO2 |
| T / ℃ | 16.49 | 86.16 | 30 | 30 | -11.96 |
| P / MPa | 190 | 2.5 | 500 | 2500 | 2490 |
| F / kgmole∙h-1 | 193.9 | 193.9 | 32.54 | 1.659 | 3.953 |
| CH4 / mole frac | 0.2558 | 0.2558 | 0.019 | 0 | 0.0003 |
| CO2 / mole frac | 0 | 0 | 0.1758 | 0.8938 | 0.9996 |
| C2H6 / mole frac | 0.1331 | 0.1331 | 0.0007 | 0 | 0 |
| C3H8 / mole frac | 0.5467 | 0.5467 | 0.0017 | 0 | 0 |
| N2 / mole frac | 0.0645 | 0.0645 | 0.7811 | 0 | 0 |
| O2 / mole frac | 0 | 0 | 0.0207 | 0.1052 | 0 |
| H2S / mole frac | 0 | 0 | 0 | 0 | 0.0001 |
| 目标函数及变量 Objective function and variable |
优化前 Before optimization |
优化后 After optimization |
单位 Unit |
| w | 2.818 | 2.287 | kW∙h/kg |
| qC1 | 49.6 | 39.7 | kgmole/h |
| qC2 | 25.8 | 20.64 | kgmole/h |
| qC3 | 106 | 85.0 | kgmole/h |
| qN2 | 12.5 | 10.0 | kgmole/h |
| χ | 2.5 | 2.3 | MPa |
| μ | -140 | -141 | ℃ |
| Performance parameter | Optimization result |
| Overall heat transfer coefficient | 1.731e+05 kJ∙℃-1∙h-1 |
| Logarithmic mean temperature difference | 23.68 ℃ |
| Minimum heat transfer temperature difference | ℃ |
| Sample Name | MoleFrac N2/ CO2 |
| Sample 1 | 0.90 / 0.10 |
| Sample 2 | 0.80 / 0.20 |
| Sample 3 | 0.70 / 0.30 |
| Sample 4 | 0.60 / 0.40 |
| Sample 5 | 0.50 / 0.50 |
| Sample Name | S/yuan | w/ kW∙h/kg | xrec,CO2 |
| Sample 1 | 925.5 | 5.183 | 0.5189 |
| Sample 2 | 2112 | 2.27 | 0.6241 |
| Sample 3 | 3302 | 1.452 | 0.6839 |
| Sample 4 | 4504 | 1.065 | 0.7337 |
| Sample 5 | 5725 | 0.8396 | 0.7808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




