Submitted:
08 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials & Method
2.2. Characterization
3. Results & Discussion
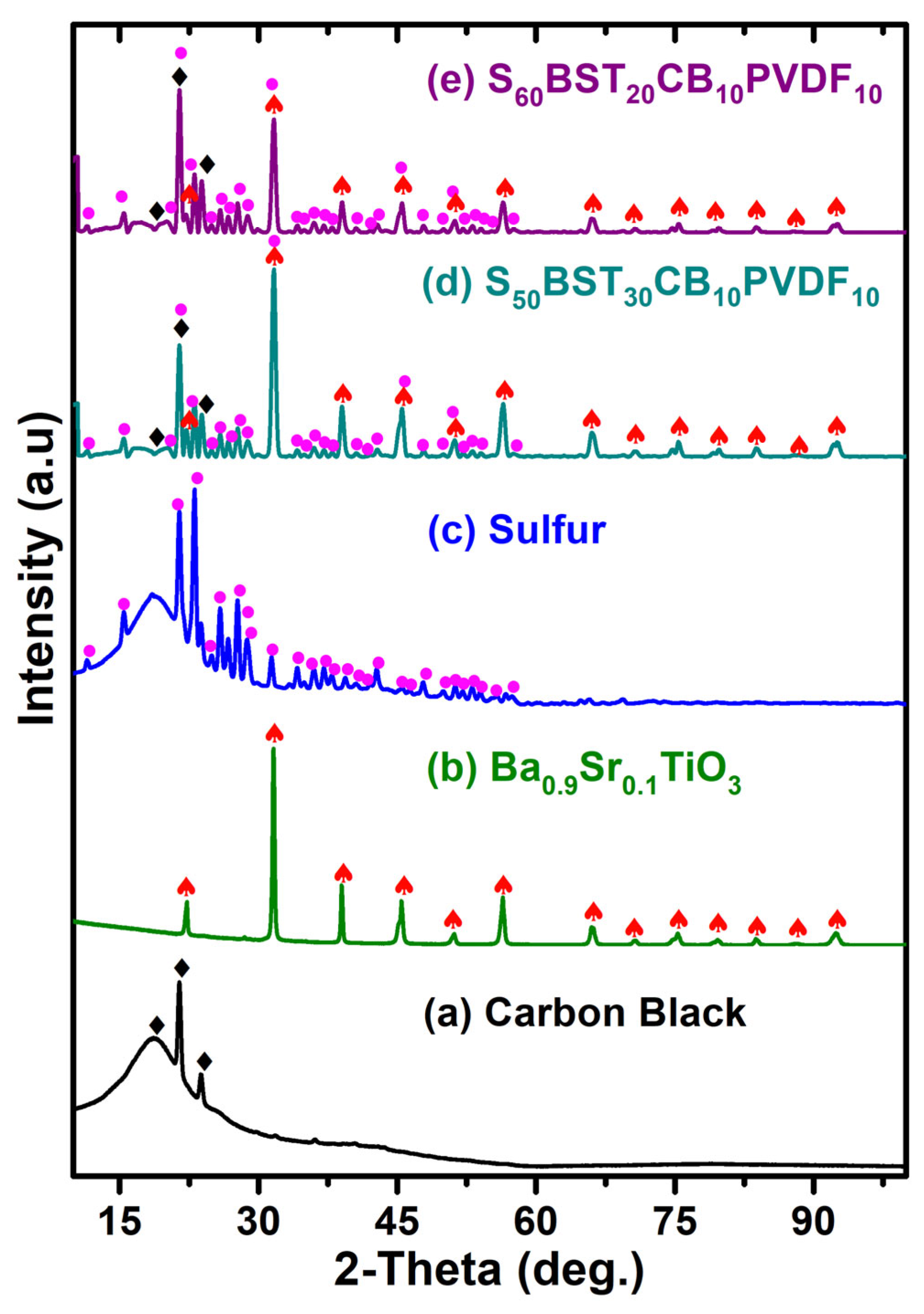
3.1. X-Ray Diffraction
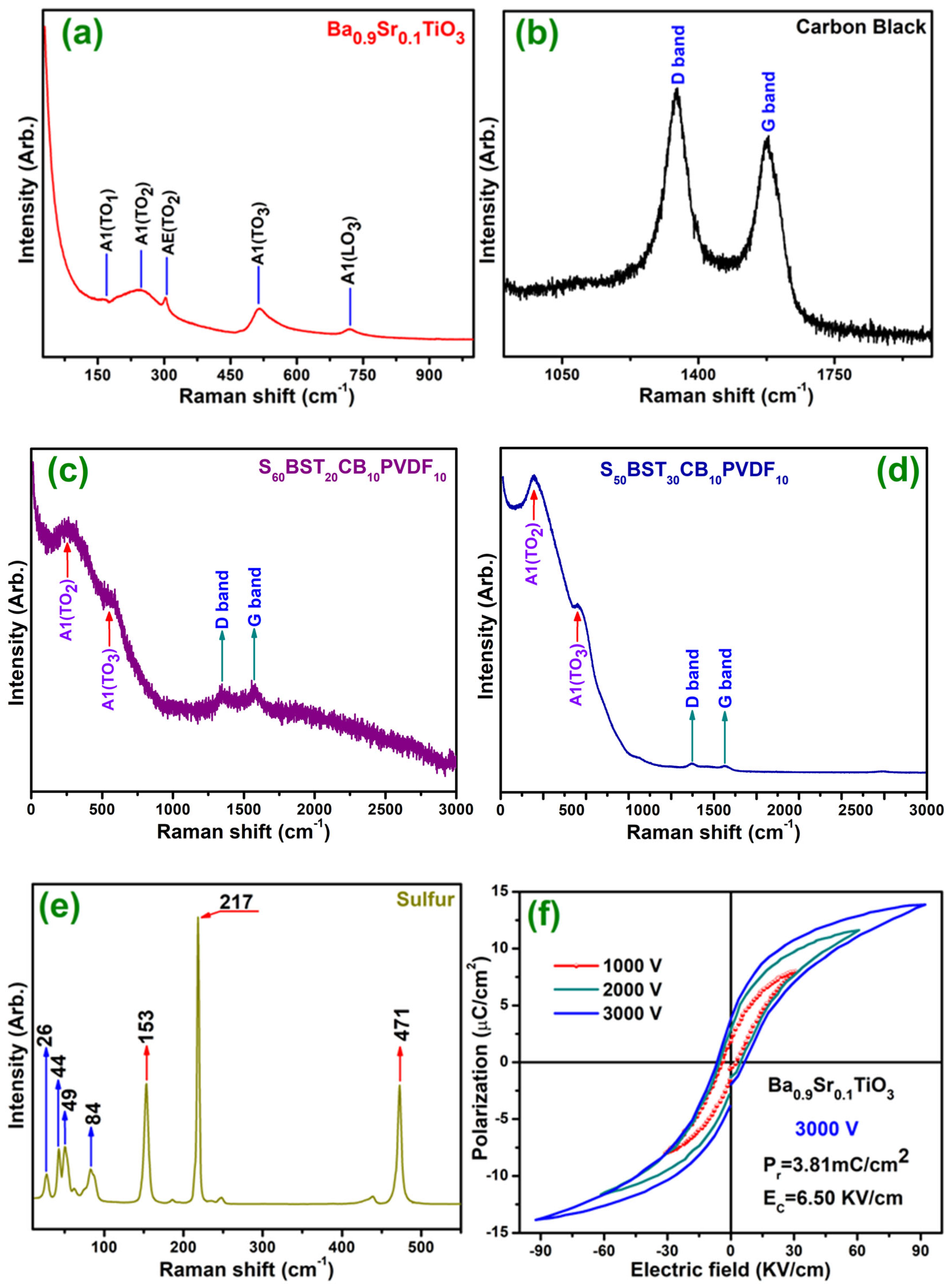
3.2. Raman Spectra
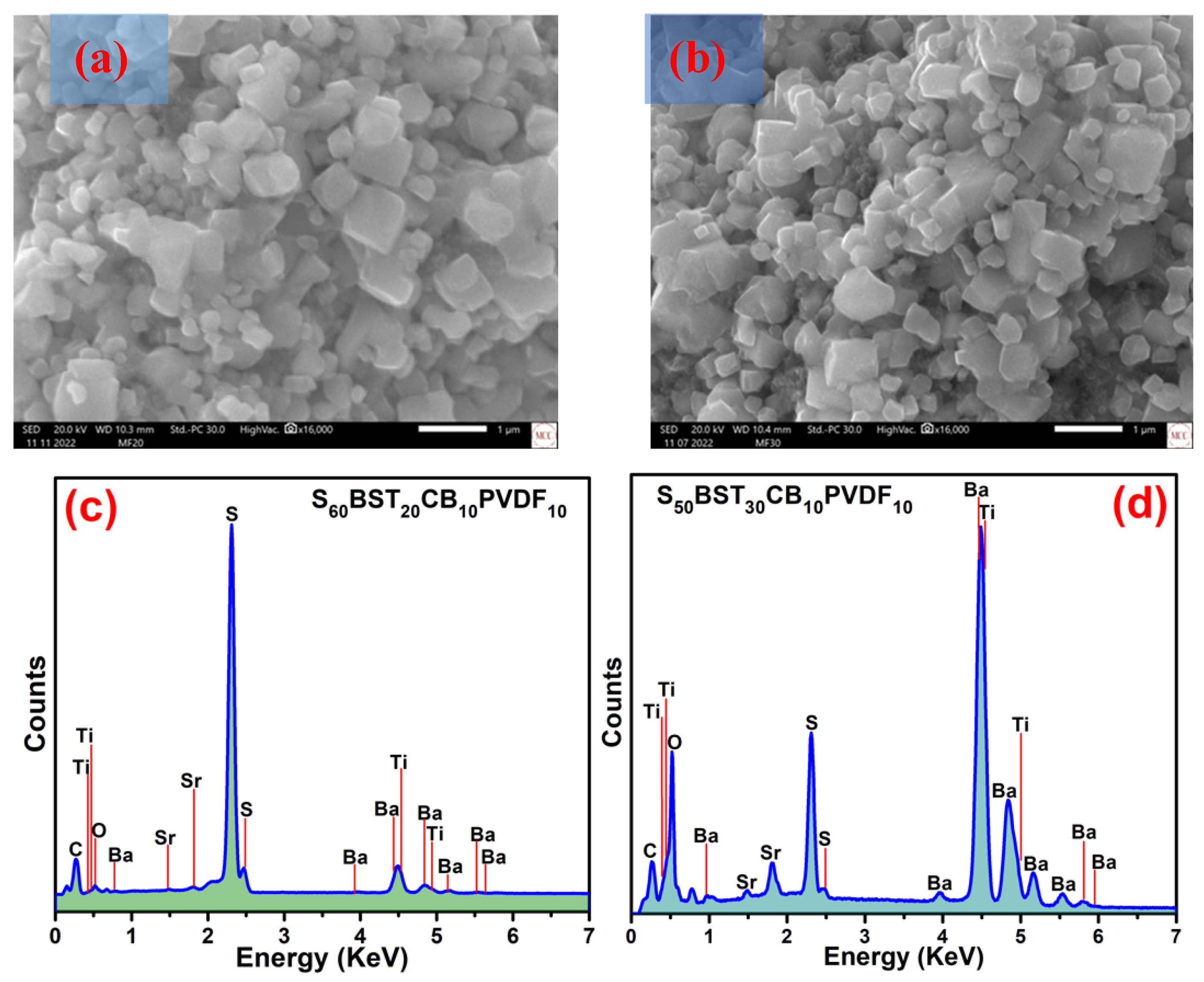
3.3. Surface Morphology
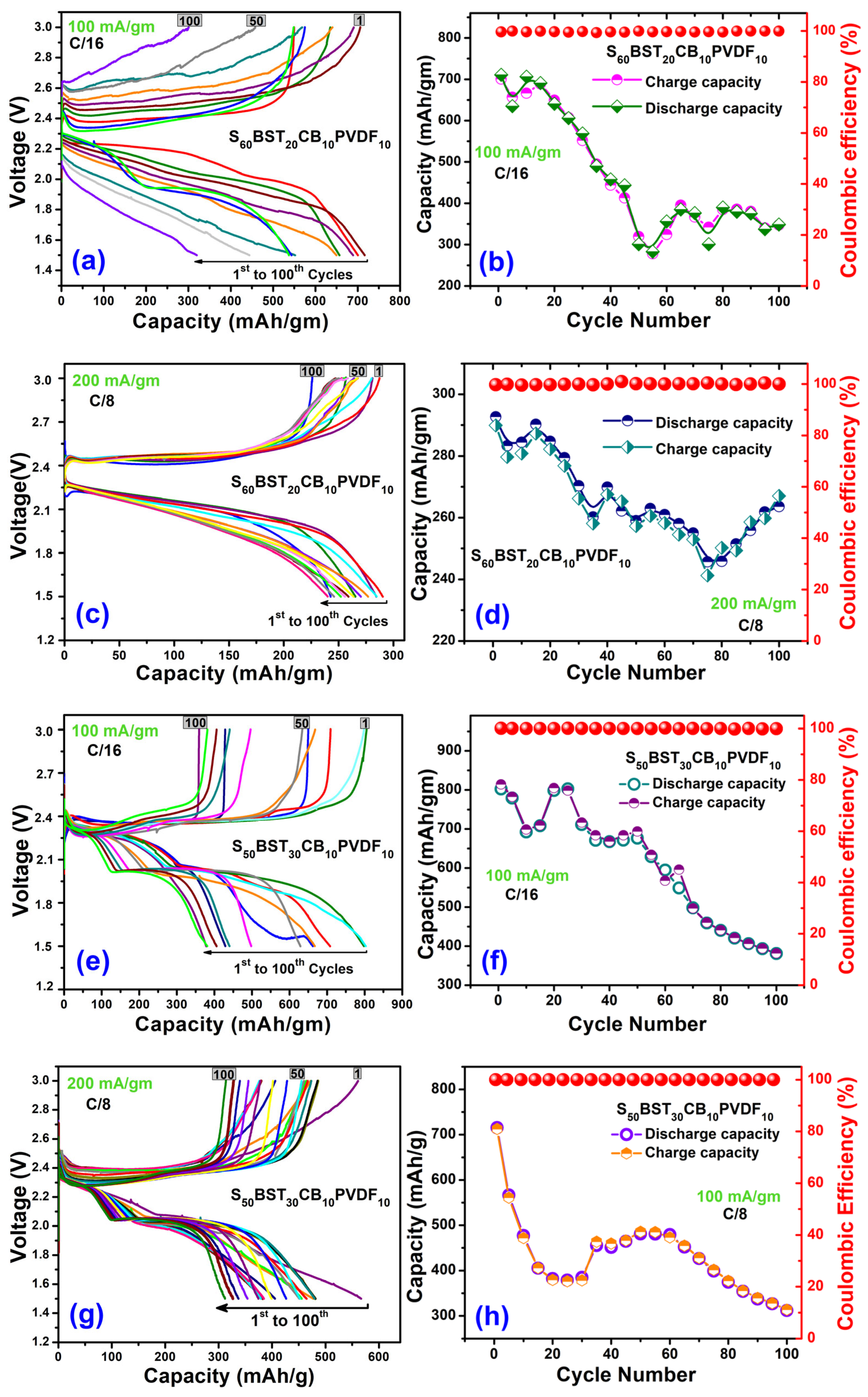
3.5.1. Charge-Discharge
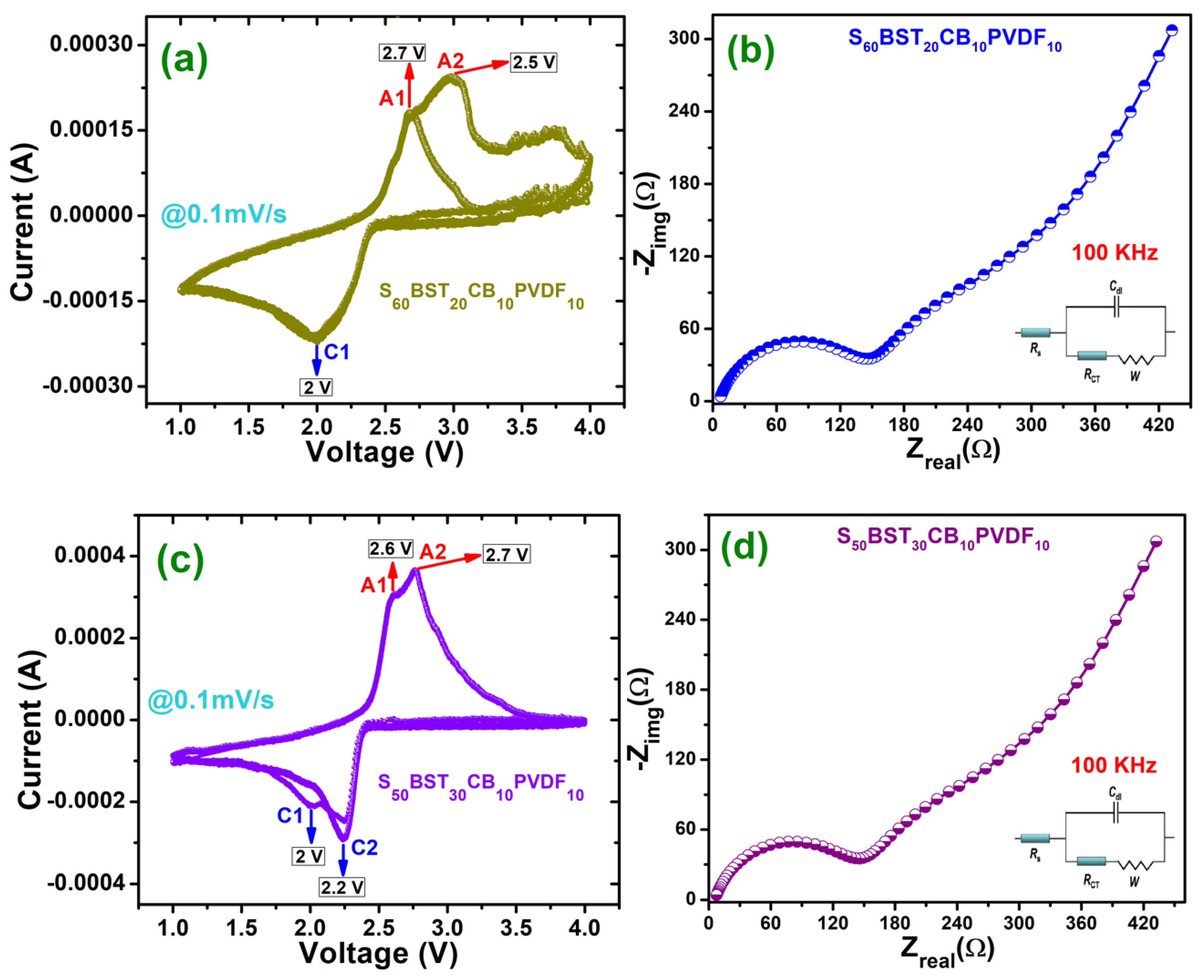
3.5.2. Cyclic voltammetry & EIS performance
4. Conclusions
Acknowledgements
References
- Li, Y. & Guo, S. Material design and structure optimization for rechargeable lithium-sulfur batteries. Matter 2021, 4, 1142–1188. [Google Scholar] [CrossRef]
- Hou, L. P. , Zhang, X. Q., Li, B. Q. & Zhang, Q. Challenges and promises of lithium metal anode by soluble polysulfides in practical lithium–sulfur batteries. Materials Today 2021, 45, 62–76. [Google Scholar]
- Yang, Y. , Zheng, G. & Cui, Y. Nanostructured sulfur cathodes. Chemical Society Reviews 2013, 42, 3018–3032. [Google Scholar] [PubMed]
- Su, Y. Challenges and Prospects of Lithium À Sulfur Batteries. 2013, 46, 1125–1134.
- Ji, X. & Nazar, L. F. Advances in Li-S batteries. Journal of Materials Chemistry 2010, 20, 9821–9826. [Google Scholar]
- Ji, X. , Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials 2009, 8, 500–506. [Google Scholar] [PubMed]
- Xin, S. , Yin, Y. X., Wan, L. J. & Guo, Y. G. Encapsulation of sulfur in a hollow porous carbon substrate for superior Li-S batteries with long lifespan. Particle and Particle Systems Characterization 2013, 30, 321–325. [Google Scholar] [CrossRef]
- Li, D.; et al. High sulfur loading cathodes fabricated using peapodlike, large pore volume mesoporous carbon for lithium-sulfur battery. ACS Applied Materials and Interfaces 2013, 5, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; et al. High efficiency immobilization of sulfur on nitrogen-enriched mesoporous carbons for LI-S batteries. ACS Applied Materials and Interfaces 2013, 5, 5630–5638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; et al. A flexible nanostructured sulphur-carbon nanotube cathode with high rate performance for Li-S batteries. Energy and Environmental Science 2012, 5, 8901–8906. [Google Scholar] [CrossRef]
- Susanne, D. W.; et al. High capacity vertical aligned carbon nanotube/sulfur composite cathodes for lithium–sulfur batteries. Chemical Communications 2012, 48, 4097–4099. [Google Scholar]
- Liu, X.; et al. Hierarchical nanostructured composite cathode with carbon nanotubes as conductive scaffold for lithium-sulfur batteries. Journal of Energy Chemistry 2013, 22, 341–346. [Google Scholar] [CrossRef]
- Hagen, M.; et al. Lithium-sulphur batteries - Binder free carbon nanotubes electrode examined with various electrolytes. Journal of Power Sources 2012, 213, 239–248. [Google Scholar] [CrossRef]
- Zhou, G.; et al. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium-sulfur batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Q.; et al. Entrapment of sulfur in hierarchical porous graphene for lithium-sulfur batteries with high rate performance from -40 to 60°C. Nano Energy 2013, 2, 314–321. [Google Scholar] [CrossRef]
- Wang, H.; et al. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Letters 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; et al. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. Journal of the American Chemical Society 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Q. , Zhang, Q., Tian, G. L., Huang, J. Q. & Wei, F. Space confinement and rotation stress induced self-organization of double-helix nanostructure: A nanotube twist with a moving catalyst head. ACS Nano 2012, 6, 4520–4529. [Google Scholar] [PubMed]
- Li, Y.; et al. One-step synthesis of nano-S/C@PANI composites for lithium-sulfur batteries with high rate and long lifespan. Journal of Materials Research and Technology 2022, 20, 3714–3722. [Google Scholar] [CrossRef]
- Yuan, H.; et al. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium–Sulfur Batteries. Advanced Energy Materials 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Xie, K.; et al. Ferroelectric-Enhanced Polysulfide Trapping for Lithium–Sulfur Battery Improvement. Advanced Materials 2017, 29, 1604724. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R. K.; et al. Role of ferroelectric nanoparticles coated separator in improvement of capacity retention at high current density on sulfur/SWCNT composite cathodes for Li-S batteries. APL Materials 2023, 11, 1–5. [Google Scholar] [CrossRef]
- Castillo, I. , Mishra, K. K. & Katiyar, R. S. Phase Transition and Energy Storage Density in Lead-Free Ferroelectric Ba1−xSrxTiO3 (x = 0.1, 0.3, and 0.7) Capacitors. Crystals 2023, 13, 630. [Google Scholar]
- Razak, K. A. , Asadov, A., Yoo, J., Haemmerle, E. & Gao, W. Structural and dielectric properties of barium strontium titanate produced by high temperature hydrothermal method. Journal of Alloys and Compounds 2008, 449, 19–23. [Google Scholar]
- Mudinepalli, V. R. , Feng, L., Lin, W. C. & Murty, B. S. Effect of grain size on dielectric and ferroelectric properties of nanostructured Ba0.8Sr0.2TiO3 ceramics. Journal of Advanced Ceramics 2015, 4, 46–53. [Google Scholar]
- Tripathi, B. , Katiyar, R. K., Morell, G., Dixit, A. & Katiyar, R. S. BiFeO3 coupled polysulfide trapping in C/S composite cathode material for Li-S batteries as large efficiency and high rate performance. Energies 2021, 14, 1–9. [Google Scholar]
- Zhang, B. , Qin, X., Li, G. R. & Gao, X. P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy and Environmental Science 2010, 3, 1531–1537. [Google Scholar]
- Singh, M. K. , Jang, H. M., Ryu, S. & Jo, M. H. Polarized Raman scattering of multiferroic BiFeO3 epitaxial films with rhombohedral R3c symmetry. Applied Physics Letters 2006, 88, 1–3. [Google Scholar]
- Xue, L.; et al. Ferroelectric polarization accelerates lithium-ion diffusion for dendrite-free and highly-practical lithium-metal batteries. Nano Energy 2021, 79, 105481. [Google Scholar] [CrossRef]
- Yim, T.; et al. Effective Polysulfide Rejection by Dipole-Aligned BaTiO3 Coated Separator in Lithium–Sulfur Batteries. Advanced Functional Materials 2016, 26, 7817–7823. [Google Scholar] [CrossRef]
- Li, G.; et al. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium-sulfur batteries. Nature Communications 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, C., Wang, Z., Wang, S. & Yu, J. Inhibition of polysulfide diffusion in lithium-sulfur batteries: Mechanism and improvement strategies. Journal of Materials Chemistry 2019, A7, 12381–12413.
- Zhao, M. , Li, B. Q., Chen, X., Xie, J., Yuan, H. & Huang, J. Q. Redox Comediation with Organopolysulfides in Working Lithium-Sulfur Batteries. Chem 2020, 6, 3297–3311. [Google Scholar] [CrossRef]
- Das, S. R. , Choudhary, R. N. P., Bhattacharya, P., Katiyar, R. S., Dutta, P., Manivannan, A. & Seehra, M. S. Structural and multiferroic properties of La-modified BiFeO3 ceramics. Journal of Applied Physics 2007, 101, 3–10. [Google Scholar]
- Ding, W. , Lu, J., Tang, X., Kou, L. & Liu, L. Ferroelectric Materials and Their Applications in Activation of Small Molecules. ACS Omega 2023, 8, 6164–6174. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




