1. Introduction
Frequent daily urinary catheterization (self or staff-assisted) is a commonly employed procedure used by individuals with impaired bladder emptying, particularly those with central nervous system impairments such as multiple sclerosis, spina bifida, or spinal and brain injuries. Spinal cord injury (SCI) is one of the most common causes of neurogenic lower urinary tract dysfunction, which affects more than 291,000 individuals in the United States and an annual incidence rate of 17,730 cases. It is estimated that 70%-84% of SCI patients have neurogenic lower urinary tract dysfunction. Each year, thousands of new cases of neurogenic lower urinary tract dysfunction (NLUTD) are reported worldwide [1a–d]. Patients with NLUTD universally have bacteria present in their urine due to asymptomatic colonization. Bacteriuria, is a non-specific term that refers to the presence of bacteria in the urine, is common in people with neurogenic bladders and their urine is mostly devoid of Lactobacilli [2]. These changes and imbalance in the urinary microbiome often contribute to the urinary symptoms experienced by these patients, making the diagnosis of urinary tract infections (UTIs) a challenge [3].
Catheter lubricants are commonly used during the insertion of urinary catheters to facilitate the process. Therefore, incorporating beneficial Lactobacillus microflora into the catheter lubricant may prove more user-friendly, as patients are already accustomed to using lubricants. Direct bladder instillations, on the other hand, are a burdensome and demanding procedure, requiring elevated levels of commitment and compliance from participants.
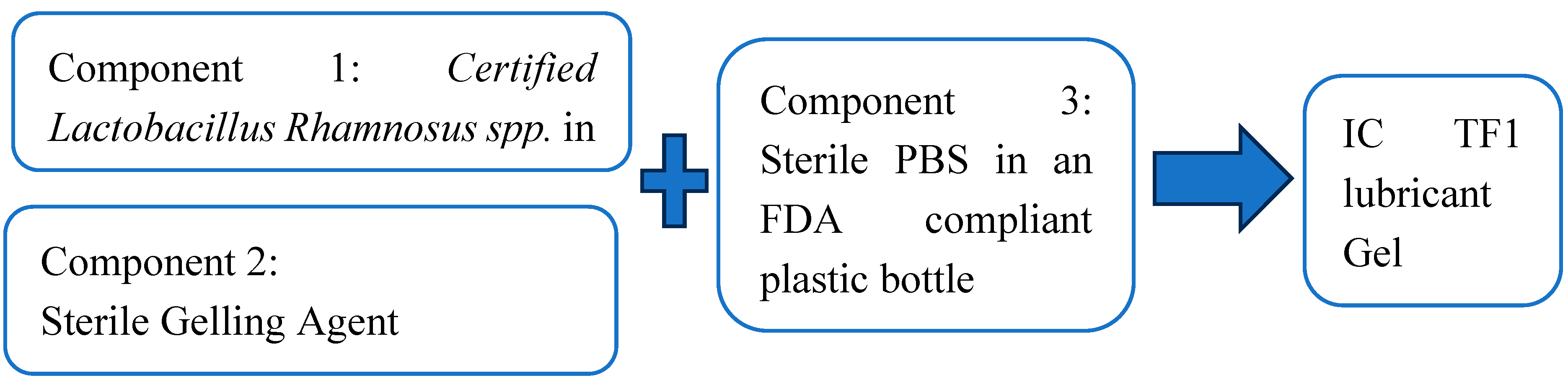
Recent on-going clinical studies are exploring the use of self-administered direct bladder instillation of Lactobacillus spp. (specifically Lactobacillus Rhamnosus GG® (LGG) in patients with neurogenic bladder [4]. Initial studies have demonstrated the safety and tolerance of this approach in both children and adults, with the potential to alleviate urinary symptoms in adult patients [5,6,7,8,9,10]. This research study introduces a novel instant gel catheter lubricant kit comprising three components: a dry packaged formulation containing Lactobacillus spp. as a Generally Recognized as Safe (GRAS) ingredient, a pre-filled sterile gelling agent utilizing a pharmaceutical-grade, water-soluble polymer (also GRAS), and premeasured sterile saline.
Rationale for Single-Use Catheter Lubrication Units
The instant gel formation method overcomes Lactobacillus viability issues (by using a dry premix in a package containing the potency certified probiotics with the pre-measured sterile buffer provided in a disposable plastic bottle) until the consumer chooses to make the gel.2.Materials and Methods
2.1. Materials
A USP grade, pharmaceutically approved, gelling polymer was purchased from Spectrum Chemical (New Brunswick, NJ). Proprietary capsules containing a Lactobacillus Rhamnosus spp. composition were obtained from a reputable commercial supplier. MRS (De Man Rogosa Sharpe) agar was obtained from Merck (Rahway, NJ). The viscosity standard, fluid 30,000 was obtained from Brookfield Ametek (Middleborough, MA). HR Lubricating Jelly was purchased from HR Pharmaceuticals (York, PA). EZ Lubricating Jelly was purchased from Medline Industries.
Simulated urine (SU) medium was adapted from Brooks, et al. [11]. Human urine (HU) was a fresh clean human urine sample collected from an individual, it was sterile filtered (Thermo Scientific Nalgene Rapid-Flow Filter, 0.45 µm a PES membrane) prior to use.
2.2. Culture Preparation
Four clinical bacterial strains and one fungal strain E. coli 700928, E. faecium 5129, K. pneumoniae 700603, P. mirabilis 2924 and C. albicans 90028(ATCC, American Type Culture Collection) were cultured on tryptic soy agar (TSB) and potato dextrose agar (PDB) respectively. The cultures were incubated at 37˚C (for bacteria) or 30˚C (for fungi) in shaking incubator at 200 rpm. The optical density was measured at 600 nm to determine CFU/ml. The bacterial cultures were diluted with PBS to make serial dilutions.
2.3. Porcine Tissue Procurement and Preparation
Normal porcine vaginal mucosa and bladder tissue were transported to Extherid Bioscience (formerly, now Perfectus Biomed LLC laboratories) on wet ice within 4 hours after slaughter. Uniform-sized tissue explants were obtained using a 5 mm biopsy punch and trimmed connective tissue with a scalpel. The tissue explants were sterilized in RPMI 1640 without FCS (Caisson Labs) + 2% penicillin/streptomycin (ABX, Antibiotics; Sigma) for 20±5 min. To remove antibiotics, the explants were washed three times in RPMI (no ABX, no FCS), incubated in fresh RPMI (no ABX, no FCS) at 37±2°C for 30±5 min, and washed again in three RPMI changes (no ABX, no FCS). The cells were conditioned in a 1:1 RPMI / Surine TM Negative Urine Control (Sigma) mixture for 10±5 min to simulate bladder conditions in vivo and transferred in triplicate onto a PET track-etched 0.4 μm cell culture insert in 6-well plates with 2.0±0.1 mL of RPMI (no ABX, no FCS) per well. Ex vivo tests were conducted by Perfectus Biomed LLC.
2.4. Methods Rationale
The IC TF1 gel is to be prepared at the time of use. The instant gel formation method involves mixing three
individually packed, components as illustrated in
Figure 1:
1. a dry premix of probiotics Lactobacillus Rhamnosus spp. with certified potency with
2. pre-measured PBS buffer provided in the container and
3. a gelling agent in a needleless syringe. Prior to the mixing of these three components before use, the components are contained in their own packaging.
The gel formation rate was optimized to ensure the timing for reaching the maximum viscosity of the gel at room temperature. (18-25⁰C). The syringe fill volume of the gelling agent was optimized to obtain the desired viscosity needed to cover the catheter.
2.3. Method Description
2.3.1. Gelling Agent Preparation
A pharmaceutical grade water-soluble cellulose type polymer was used to create a homogenous material that was filled in disposable sterile syringes to disperse a predetermined precise volume such that the desired viscosity is obtained upon mixing with the phosphate buffered solution.
2.3.2. IC TF1 Container Preparation
Disposable LDPE containers were filled with precise volumes of phosphate buffered saline (PBS).
2.3.3. Sterilization, Packaging and Manufacturability
The prefilled syringes containing the gelling agent and the prefilled IC TF1 containers were packaged into individual medical grade pouches prior to sterilization. The prefilled syringes containing the gelling agent were steam sterilized (121⁰C, 30 min). The IC TF1 containers prefilled with PBS were sterilized by Gamma at a predetermined dose. (Steris corporation).
The feasibility of scale up was demonstrated by producing batches of 400 units of IC TF1 containers and prefilled syringes followed by sterilization validation and packaging using medical contractual services per the FDA standards. (US FDA IND pending)
2.3.4. IC TF1 Catheter Lubricant Gel Preparation
The IC TF1 Gel was prepared mixing the three components, i.e., by emptying the capsule contents into the sterile PBS in the container, followed by the addition of the gelling agent from the syringe and shaking well to form the instant catheter lubricant gel containing Lactobacillus with precise viability that sustains for 24 hours at room temperature (18-25⁰C).
2.3.5. hysical Properties of the IC TF1 Gel
Visual Appearance
The IC TF1 gel yielded a uniformly smooth-textured, lubricious opaque gel, achieving consistent viscosities after 15-30 minutes at room temperature. The gel was visually and microscopically examined for color, uniformity, and homogeneity.
pH
The pH of the gels was measured using Hydrion® pH strips.
Viscosity
The viscosity of IC TF1 Gel immediately after mixing per a simple protocol, was monitored for gel formation rate at room temperature until no change was noted. The viscosity typically stabilized in 20-30 minutes after preparation. All viscosity measurements were obtained using the Brookfield Synchro-Lectric Viscometer, Model RVF-100, Spindle 6, and Speed 10. The viscometer was calibrated using the viscosity standard, fluid 30,000 and comparison to HR Lubricating JellyTM and EZ Lubricating JellyTM.
Osmolality
The method of freezing point depression was used to measure this colligative property. (Advanced Instruments, AI, Norwood, MA) and their validated standards and reference solutions were used. Advanced Instruments completed internal linearity testing and made measurements on triplicate IC TF1 samples at room temperature and 37⁰C, at two time points.
Lactobacillus assay in IC TF1 gel for Viability
IC TF1 Gel was assayed 30 minutes after preparation. As a control for comparison, the assays of the Lactobacillus capsules were conducted for verifying manufacturer’s specifications. The assay method involved triplicate aliquots of IC TF1 gel samples using a standardized dilution scheme and plating. Lactobacillus viability was recorded after incubation in MRS agar plates for 48 hours at 37⁰C.
Lactobacillus assay in IC TF1 gel for Viability at three temperature conditions
The Effect of Temperature on IC TF1 Gel (“neat” as prepared)
The reproducibility of Lactobacilli viability was assayed 30 minutes after preparation. Additional sets of IC TF1 Gel were exposed to ambient room temperature, 37⁰C and refrigerator temperatures (1-10⁰C) for 24 hours. Aliquots from these respective sets were assayed at 24 hours using a standardized dilution scheme. The viable colonies were enumerated after growth on MRS agar plates.
2.3.6. Antimicrobial Activity
Agar Spot on Lawn (ASL) Method
100 μl of ICET formulations were spotted on MRS agar plates. The plates were incubated overnight at room temperature to let formulation dry. A soft agar (0.7%) was prepared with Tryptic Soy Agar) TSB for bacteria and PDA (Potato dextrose Agar) for C. albicans. The bacterial or fungal culture dilution were prepared to make ~1x 106 CFU/ml and 7.7 ml of the bacterial or fungal culture dilutions (~1×106 CFU/ml) were added. The mixture was overlaid on MRS agar plate containing the test samples and incubated at 37ᵒC for 24 hours.
2.3.7. Biocompatibility with Mouse Embryonic and Human Urethra Epithelial Cells
2.3.8. Biocompatibility with Ex Vivo Porcine Bladder Tissue
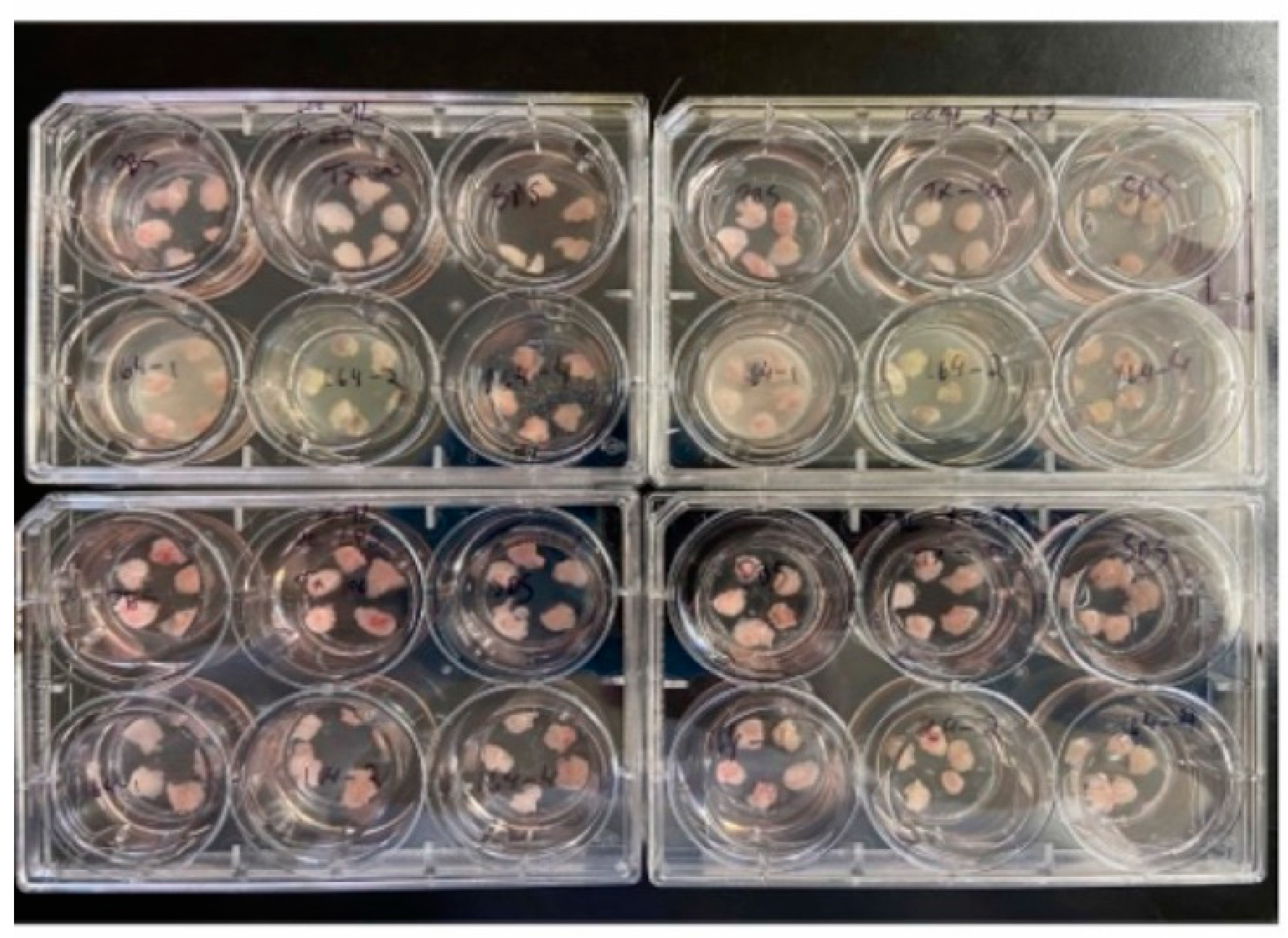
This test uses a well-established ex vivo porcine bladder and vaginal and mucosal model [12,13,14] due to its histological similarity to human tissue, weekly availability, reproducibility, predictive value, and robustness compared to cell culture models [15]. In addition, to address intraday variability, all cytotoxicity testing and biomarker studies are conducted with the same donor tissue and replicas of 6 explants per tissue. Normal porcine vaginal mucosa and bladder tissue were transported to laboratories on wet ice within 4 hours of slaughter. The tissue was obtained within 4 hours of slaughter. The mucosal surfaces are directly exposed to the formulations without any dilution, so direct toxicities are obtained.
The cells were conditioned in a 1:1 RPMI/Surine™ Negative Urine Control (Sigma) mixture for 10±5 min to simulate bladder conditions in vivo and transferred in triplicate onto a PET track-etched 0.4 μm cell culture insert in 6-well plates with 2.0±0.1 mL of RPMI (no ABX, no FCS) per well. 10 µl of formulation (52 μL/cm
2) was applied per explant (24-hour contact time). After 24 h incubation, explants are washed with PBS in three sequential wells of PBS in the 96-well plate (3 times), refer to
Figure 2. The washed explants were used to perform the MTT assay. The MTT assay is a measure of cellular metabolic activity as an indicator of cell viability, proliferation, and cytotoxicity. This colorimetric assay is based on the reduction of a yellow tetrazolium salt (3- (4-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide or MTT) to purple formazan crystals by metabolically active cells. Results are expressed as percentage of viability against a validated control, PBS. Any color changes of the methyl red indicator in the tissue-well were noted to assess acidity produced by the growth of
Lactobacilli on the ex vivo tissue.
3. Data Analysis
All studies were carried out in triplicate (n=3 explants) per formulation on multiple dates to assess reproducibility. Ex vivo studies were analyzed utilizing Graph Pad Prism. Statistical analysis was conducted with a one-way ANOVA followed by Dunnett’s multiple comparison test.
4. Storage and Sterility of Packaging
Triplicate sets of IC TF1 lots prepared from stored IC TF1 kit components were analyzed for physical properties using the previously described test methods. The individually packed Lactobacillus blister capsules were stored at room temperature. The capsule has a shelf life of one year per manufacturer. The individually packaged and sterilized syringes containing the gelling agent and the PBS filled container were stored at room temperature for up to 12 months. Standard sterility and dye penetration tests for packaging seal integrity were conducted per standard protocols (Ethide Labs, RI, USA) to ensure sterility of the gelling agent and the PBS filled bottles. (Bacteriostasis/Fungistasis method Validation Testing—one media-SCD [Soy-bean Casein Digest] AAMI, three organisms, sterility by Direct Immersion, and sterility barrier testing using ASTM Standard Method Designation: F 1929-15)
Comparisons were made between non-aged IC TF1 components and aged components in terms of their physical and chemical properties including visual appearance, pH, viscosity, and viability and compared to fresh data.
5. Results
5.1. Physical Properties of the IC TF1 Gel
The IC TF1 catheter lubricant gel (also known as the IC TF1 Gel) was prepared. A summary of the physical properties is found in
Table 1.
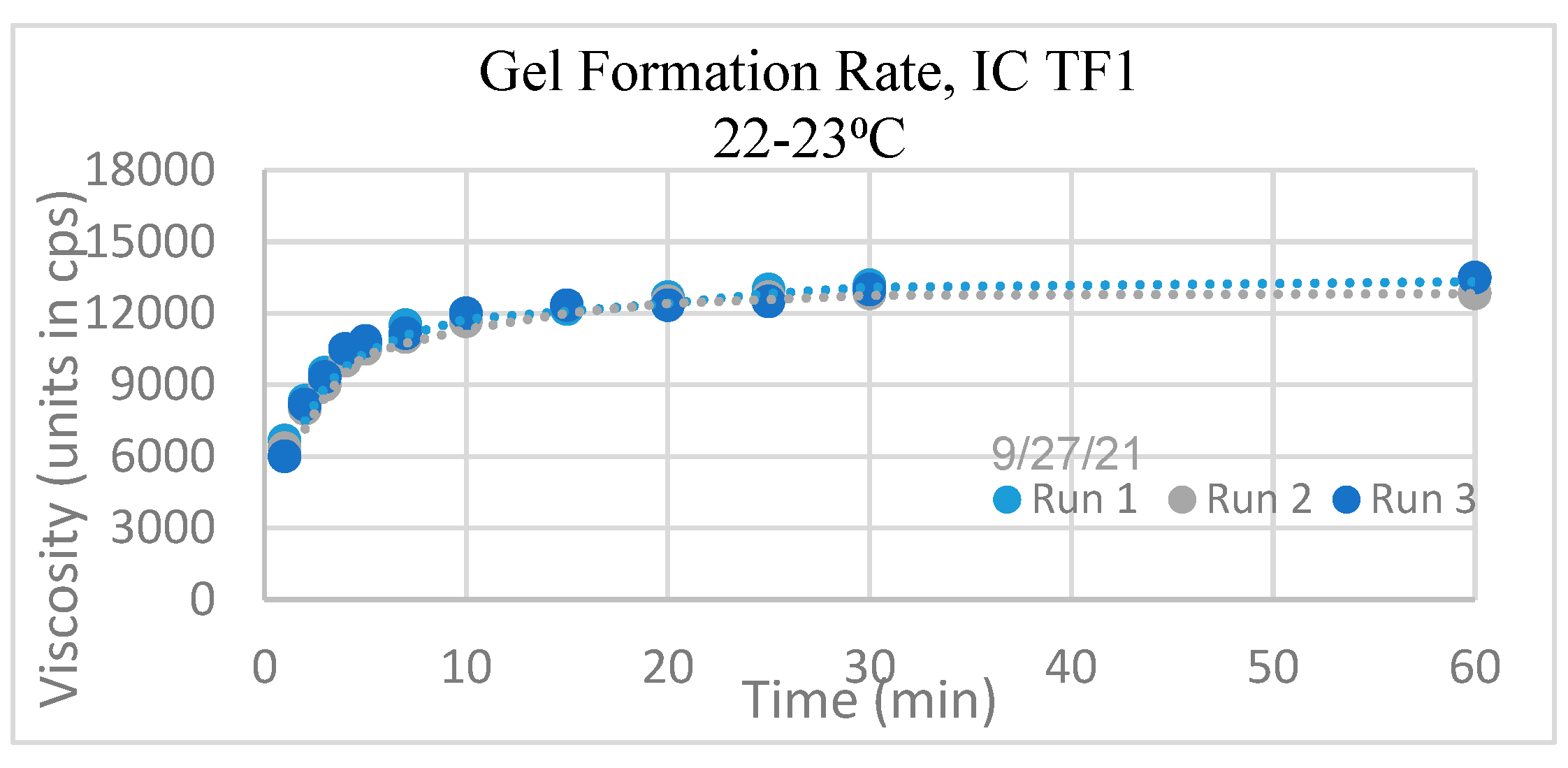
Gel Formation Rate: The viscosity of the IC TF1 Gel quickly increased and plateaued by 30 minutes in each of the three runs, as seen in
Figure 3. No notable change was observed after 30 minutes; therefore, all initial tests of the IC TF1 gel were performed 30 minutes after preparation.
pH
The pH of the IC TF1 Gel was consistently in the range of 6-7. No aberrations in gel pH were observed between lots, leading to the conclusion of consistent and stable gel pH from various preparations.
Viscosity
The viscosity of the IC TF1 gel was consistent at room temperature. Viscosity, in the context of lubrication, is an important physical property to ensure that the catheter is evenly coated, and the lubricating gel does not drip. The viscosity values of IC TF1 Gel were highly reproducible and consistent across various lots. The texture of IC TF1 Gel allows it to adhere to an intermittent catheter without dripping.
Osmolality
The average osmolality as summarized for IC TF1 Gel in
Table 2 did not change over 24 hours at room temperature or 37⁰C. The average osmolality of the IC TF1 gel was <1100 mOsm/kg. The commercial gels tested showed higher osmolality compared to our gel.
Distribution of Viable Lactobacilli Numbers at the Time of Preparation
The Lactobacillus viability observed in the IC TF1 Gel demonstrate reproducibility of the CFU concentration. This supports the uniform blending and distribution of Lactobacillus in the IC TF1 gel. Uniform mixing and distribution ensured that a consistent dose will be administered.
The Effect of Temperature on Viable Lactobacilli in the IC TF1 Gel
It is important to determine the effect of storage of the IC TF1 Gel at various temperatures on the viability of Lactobacillus. The Lactobacilli determined to remain stable and viable for 24 hours under refrigeration or at room temperature not exceeding 25°C.
5.2. Antimicrobial activity
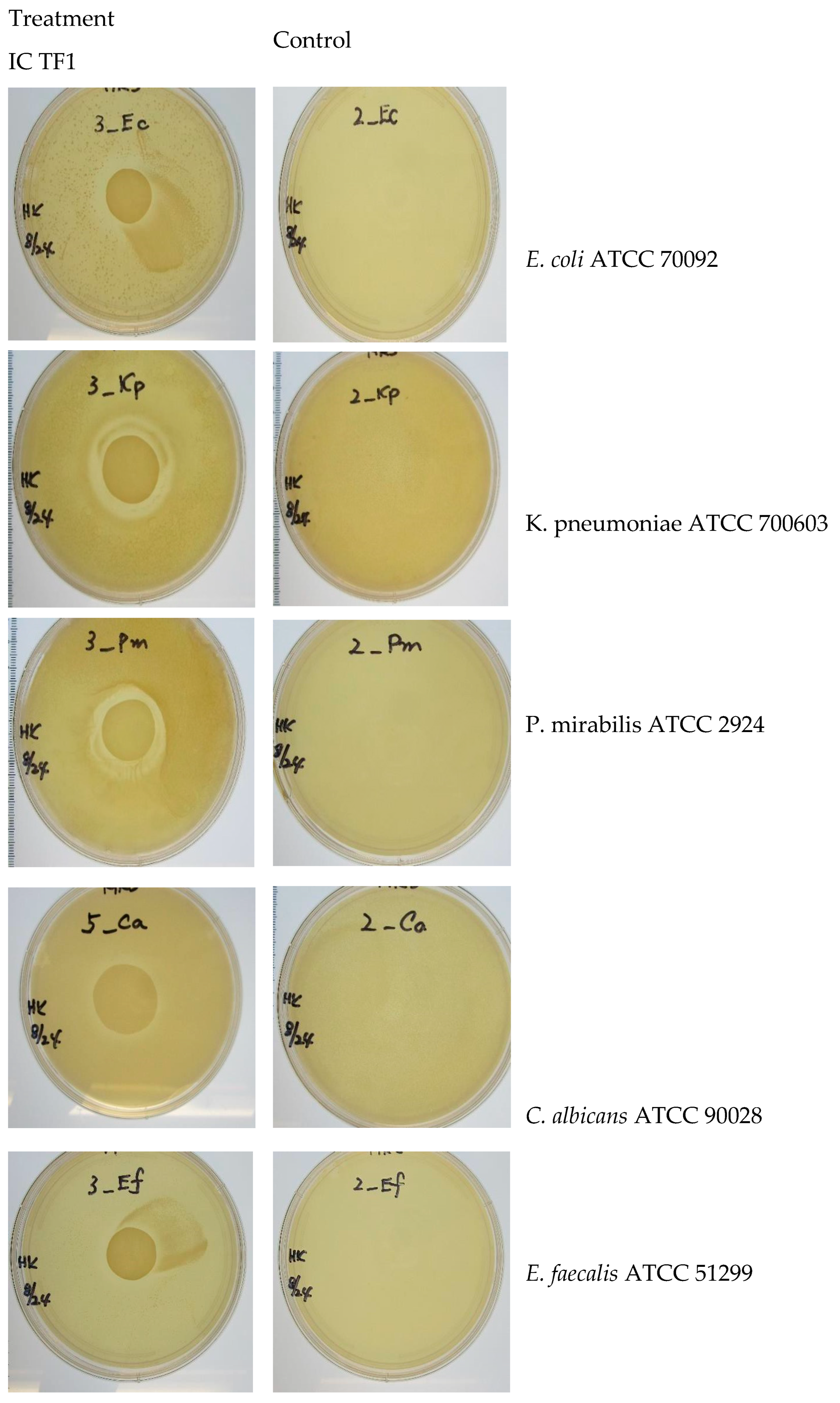
Zone of inhibition results in the presence and absence of IC TF1 against five uropathogens can be found in
Figure 4 and
Table 3 (ZOI measurements).
IC TF1 gel shows antimicrobial activity against the selected uropathogens under the zone of inhibition test conditions.
5.3. Biocompatibility
In vitro direct contact cytotoxicity testing Three direct contact in vitro cytotoxicity tests were performed to ensure suitability for human use.
Table 4 illustrates the results of all three tests, showing cytotoxicity ranges of 0-2 for the control gel (without
Lactobacillus) and the IC TF1 gel. The IC TF1 gel is not cytotoxic and was rated in a similar cytotoxicity level to two commercial FDA-cleared catheter lubricant gels.
-
Test 1: Direct Contact Cytotoxicity (Toxikon, MA)
- ○
Cytotoxicity- L929 direct contact test—ISO 10993-5
- ○
Test Article: Control gel (No Lactobacillus), HR Lubricating Jelly (commercial)
-
Test 2: Direct contact cytotoxicity testing (ISO 10993-5, modified without antibiotics) Integrated Pharma Services, MD
- ○
Test Article: ICET, Inc. Gel Formulations, Commercial lubricants.
-
Test 3: Human urethral cell line direct cytotoxicity (ISO 10993-5, modified without antibiotics) Integrated Pharma Services,
- ○
Test Article: ICET Gel Formulation, Commercial lubricants.
Direct-contact biocompatibility with ex vivo porcine bladder tissue. The results are summarized in
Table 5.
The control Gel is without Lactobacillus.
5.4. The influence of storage of IC TF1 kit.
The shelf life of the Lactobacillus capsule by itself is one year which is a separate component. The PBS buffer and the gelling agent were stored for one year. Fresh gels made from these individual components were tested for reproducible properties and compared to non-aged lots. Triplicate lot testing of IC TF1 Gel prepared from the one-year stored kits were found to show consistent sterility, with the package maintaining sterility barrier, viscosity, pH, and viability comparing to freshly tested IC TF1 Gel samples. This demonstrates storage of at least one year for the IC TF1 gel components, though higher shelf life is projected for the PBS container and the gelling agent. The viable Lactobacilli dose in the gel were found to match the supplier’s specifications.
6. Discussion
Neurogenic bladder is a chronic condition that has significant medical and quality of life implications for patients [16]. Individuals with spinal cord injuries are particularly vulnerable to urinary tract infections due to urethral colonization. Several factors contribute to the risk of UTIs in this population, including catheterization technique, fluid intake, frequency of catheterization, and bowel management. Additionally, individuals with limited mobility or restricted access to clean restroom facilities face an even higher risk of developing UTIs. Unfortunately, commonly used non-antibiotic options for UTI prevention, such as methenamine-hippurate and cranberry tablets, are ineffective in this population [17]. The treatment of UTIs among neurogenic bladder patients with simple oral antibiotics is often ineffective, leading to an increase in multidrug resistant organisms because of regular antimicrobial prophylaxis [18]. Oral probiotics, such as RC14-GR1 or LGG-BB12, have not been proven to be an effective UTI prevention strategy [20].
Recent research has explored the potential of Lactobacilli-based vaginal gels as a treatment for vulvovaginal candidiasis [21]. Human trials investigating the use of LACTIN-V, a gel formulation containing Lactobacillus Crispatus, have shown promising outcomes in the management of vaginal infections [22]. Additionally, studies have demonstrated the antimicrobial activity of intra-urethrally administered probiotic Lactobacillus casei strain against Escherichia coli in a murine urinary tract infection model, resulting in a reduction in UTI organisms and inflammation [23].
A staunch support and inspiration for our local delivery approach comes from the recent self- administered direct Bladder instillation studies of Lactobacillus Rhamnosus GG, referenced earlier, which was found to reduce urinary symptoms without causing bacteremia or sepsis. Adverse events were not found to be related to the frequency or quantity of Lactobacillus instilled [6,7,8,9,10].
A recent paper reported that among people with spinal cord injury/disease (SCI/D) who manage their bladders with intermittent catheterization (IC), intravesical Lactobacillus alters the bacterial composition and diversity of the urine ecosystem, potentially disrupting the uropathogenic urobiome. A decline in Escherichia/Shigella predominance (p < .001) and altered bacterial diversity (p < .05) resulted in a pilot clinical trial [24]. Several Phase 2 and 3 trials have been initiated (NCT04373512, NCT04323735, and NCT05230511).
When it comes to intermittent catheterization, urine drainage is typically done 3-7 times a day depending on individual needs [25]. A lubricant is applied to the catheter before insertion into the urethra. The amount of gel introduced or retained in the urethra or perineal area is not measurable, as it depends on individual technique and preference. The IC TF1 gel has a smooth texture like commercial lubricants and adheres to urinary catheter materials without dripping, providing lubrication and a steady concentration of Lactobacillus Rhamnosus spp.
The IC TF1 Gel has demonstrated stable viability of Lactobacillus up to 24 hours when stored at room temperature (25°C). Importantly, the gel has shown consistent viability even when incubated at 37°C with synthetic or human urine (in high and low gel/urine ratio), indicating its robust survival in urine environments.
Osmolality is an important property with a value below 1200 preferred as hyperosmolar gel materials may cause damage to the epithelium [26]. The average osmolality values of the IC TF1 Gel remained consistent at around 1080 mOsm/kg over a 24-hour period, a level preferred for maintaining homeostasis in the urinary tract. The pH of the gel has consistently been within the range of 6-7, without any observed changes during ex vivo studies. A decrease in local pH would have produced a cytotoxic tissue response.
Biocompatibility studies have been conducted using mouse and human cell lines, as well as on porcine ex vivo bladder tissues to assess the cell and tissue toxicity of the IC TF1 Gel. These studies compared the gel to commercially FDA-approved lubricants and demonstrated promising results, indicating its potential for clinical application.
Furthermore, in vitro antimicrobial activity of the gel has shown promise, including activity against Candida. However, in vivo, the beneficial Lactobacilli must compete with pathogenic organisms for nutrients and overcome their fast growth rates to establish and maintain viability [27] whether instilled or introduced via the lubricant. Lactobacilli have been extensively studied for their modes of action, which include adhering to mucosal surface cells, reducing pathogenic adherence, persisting in the environment, and producing substances that inhibit pathogen growth. These properties have been widely studied in the context of probiotic oral supplements that come into direct contact with the gastrointestinal mucosa. Historically, probiotic supplements need to be replenished as its in vivo persistence is low [28]. For example, Lactobacilli does not permanently establish itself on the gastrointestinal mucosa and does not become a fortified species and requires constant replenishment and is sold as a daily supplement. In chronic catheter populations who are asymptotically colonized, the microbial environment in the urinary tract has less complexity of microbial diversity compared to the gut [29].
Mechanisms, such as the production of bacteriocins and modulation of the immune response, may not necessarily depend on colonization. Instead, transient Lactobacilli could potentially actively combat pathogens by preventing their adherence to urinary tract surfaces and even directly inhibiting their growth. The presence of Lactobacilli in the urinary tract, even for a brief period, could confer significant therapeutic benefits, if replenished periodically
While the effectiveness of transient Lactobacilli in combating urinary tract infections requires further investigation, their potential therapeutic benefits should not be dismissed. Different strains may possess varying abilities to produce antimicrobial substances, compete with pathogens, or modulate the immune response.
Clinical Implications and Future Directions
The acceptance of the product involving a mixing step to create an instant gel was not perceived as a significant challenge based on the feedback from the small number of patients we interviewed. They acknowledged the potential benefits of the IC TF1 gel balancing for the extra effort, although they expressed a preference for a premade gel due to its ease of use. The lubricant method, however, was considered undeniably more convenient compared to self-administered bladder instillation.
The IC TF1 Gel is proposed for use during the last catheterization prior to sleep and the initial catheterization upon awakening in the morning. To identify the most effective frequency of application, assessments will be conducted through rigorous evaluation during human trials.
Efforts are also currently underway to explore stabilized forms of Lactobacillus Rhamnosus spp in non-aqueous media. However, the regulatory approval process presents its own set of challenges that need to be addressed.
7. Conclusions
The study presented in this report focuses on the design and characteristics of a kit called IC TF1, which allows for the instant preparation of a disposable, a 24 hr use, urinary catheter lubricant containing live Lactobacilli. The physical properties and biocompatibility of the lubricant with ex vivo porcine tissues have shown promising results, justifying further testing in pre-clinical models and human trials.
Long-term studies are warranted to assess the impact of regular probiotic supplementation through this method on urological conditions, specifically urinary tract infections and urethral strictures. Additionally, the development of tailored probiotic formulations and standardized protocols for catheter lubrication intervals would further advance the clinical application of this approach. By optimizing the formulation and providing clear usage guidelines, healthcare practitioners would be able to deliver more effective and individualized patient care.Human trials will help determine the safety and efficacy of the product and its potential for addressing urinary tract infections (UTIs) and perineal hygiene in a wider range of populations non-catheter populations as well. Moreover, the product may also complement other treatment modalities for individuals with neurogenic bladder and those who require chronic catheter use. With continued research and refinement, it has the potential to significantly impact the lives of individuals with various urinary conditions.
Funding
This research was supported by a Small Business Innovation Research Grant (SBIR) 90BISB0017 from the National Institute of Disability, Independent Living, and Rehabilitation Research (NIDILRR).
Data Availability Statement
Upon reasonable request.
Conflicts of Interest
None.
References
- Manack A, Motsko SP, Haag-Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn. 2011; 30:395–401. [CrossRef]
- Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, et al. Traumatic spinal cord injury in the United States, 1993-2012. JAMA 2015; 313:2236-43. [CrossRef]
- Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014; 52:110–6.
- Stoffel, John T. (2016). “Detrusor sphincter dyssynergia: a review of physiology, diagnosis, and treatment strategies”. Translational Andrology and Urology. 5 (1): 127–135.
- Groah S, Perez-Losada M, Caldovic L, et al. MP20–08 pyuria and asymptomatic bacteriuria is associated with novel and specific urine microbiomes. J Urol 2015; 193: e226. [CrossRef]
- Groah SL. Neurogenic bladder and the urine microbiome: the good, the bad and the ugly. Presented at the American Spinal Injury Association Annual Meeting, April 2017, Albuquerque, NM.
- https://clinicaltrials.gov/study/NCT04373512?term=Suzanne%20Groah&rank=2.
- Groah SL, Pérez-Losada M, Caldovic L, et al. Redefining Healthy Urine: A Cross-Sectional Exploratory Metagenomic Study of People with and Without Bladder Dysfunction. J Urol. 2016 Aug;196(2):579-587. [CrossRef]
- Tractenberg, R.E., Groah, S.L., Frost, J.K., Rounds, A.K., Davis, E., Ljungberg, I.H. and Schladen, M.M. (2021), Effects of Intravesical Lactobacillus spp. on Urinary Symptom Burden in People with Neurogenic Lower Urinary Tract Dysfunction. PM&R, 13: 695-706. [CrossRef]
- Groah S, Ljungberg I, Tractenberg R, et al. Self-Management of Urinary Symptoms Using a Probiotic in People with Spinal Cord Injuries, Spina Bifida, and Multiple Sclerosis [Internet]. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Dec.
- Tractenberg, R.E., Frost, J.K., Yumoto, F. et al. Reliability of the Urinary Symptom Questionnaires for people with neurogenic bladder (USQNB) who void or use indwelling catheters. Spinal Cord 59, 939–947 (2021). [CrossRef]
- Tractenberg, R.E., Groah, S.L., Rounds, A.K., Davis, E.F., Ljungberg, I.H., Frost, J.K. and Schladen, M.M. (2021), Clinical Profiles and Symptom Burden Estimates to Support Decision-Making Using the Urinary Symptom Questionnaire for People with Neurogenic Bladder (USQNB) using Intermittent Catheters. PM&R: The Journal of Injury, Function and Rehabilitation, 13: 229-240. [CrossRef]
- Forster CS, Hsieh MH, Pérez-Losada M, Caldovic L, Pohl H, Ljungberg I, Sprague B, Stroud C, Groah S. A single intravesical instillation of Lactobacillus rhamnosus GG is safe in children and adults with neuropathic bladder: A phase Ia clinical trial. J Spinal Cord Med. 2021 Jan;44(1):62-69. [CrossRef]
- Brooks, T and Keevil, CW. A simple artificial urine for the growth of urinary pathogens. Letters in Applied Microbiology 1997, 24, 203–206. [CrossRef]
- Anderson, M.J., Parks, P.J., and M. L. Peterson. A mucosal model to study microbial biofilm development and antibiofilm therapeutics. J. Micro. Methods 2013 Feb 15;92(2):201-8. [CrossRef]
- Anderson, M.J., Scholz, M.T., Parks, P.J., and M. L. Peterson Ex vivo porcine vaginal mucosal model of infection for determining effectiveness and toxicity of antiseptics. J. Applied Microbiol. 2013 Sep;115(3):679-88.
- Anderson, M.J., David, M.L., Scholz, M., Bull, S.J., Morse, D., Hulse-Stevens, M., and M. L. Peterson. Efficacy of Skin and Nasal Povidone-Iodine Preparation against mupirocin resistant MRSA and Staphylococcus aureus within the anterior nares. Antimicrob. Agents Chemother. 2015 May; 59(5):2765-73.
- Parsons BA, Drake MJ, Gammie A, Fry CH, Vahabi B. The validation of a functional, isolated pig bladder model for physiological experimentation. Front Pharmacol. 2012 Mar 30;3:52. [CrossRef]
- Lucas E. Medical Management of Neurogenic Bladder for Children and Adults: A Review. Top Spinal Cord Inj Rehabil. 2019 Summer; 25(3):195-204. [CrossRef]
- Lee B, Haran M, Hunt L, Simpson J, Marial O, Rutkowski S, et al. Spinal-injured neuropathic bladder antisepsis (SINBA) trial. Spinal Cord. 2007; 45:542–50. [CrossRef]
- Morton SC, Shekelle PG, Adams JL, Bennett C, Dobkin BH, Montgomerie J, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002; 83:129–38. [CrossRef]
- Toh SL, Boswell-Ruys CL, Lee BSB, Simpson JM, Clezy KR. Probiotics for preventing urinary tract infection in people with neuropathic bladder. Cochrane Database Syst Rev. 2017;9 CD010723.
- Falagas ME, Betsi GI, Tokas T, Athanasiou S. Probiotics for prevention of recurrent urinary tract infections in women. Drugs. 2006; 66:1253–61. [CrossRef]
- Oerlemans, E.F.M., Bellen, G., Claes, I. et al. Impact of a Lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci Rep 10, 7976 (2020). [CrossRef]
- Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, Green L, Miller S, Powell J, Parks T, Hemmerling A. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N Engl J Med. 2020 May 14;382(20):1906-1915.
- Asahara T, Nomoto K, Watanuki M, Yokokura T. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother. 2001 Jun;45(6):1751-1760.
- Groah SL, Rounds AK, Pérez-Losada M. Intravesical Lactobacillus rhamnosus GG Alters Urobiome Composition and Diversity Among People with Neurogenic Lower Urinary Tract Dysfunction. Top Spinal Cord Inj Rehabil. 2023 Summer;29(3):44-57. [CrossRef]
- Dauw CA, Wolf JS. Fundamentals of urinary tract drainage. In: Partin AW, Dmochowski RR Kavoussi LR, Peters CA, eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021: chap 12.
- World Health Organization. (2012). Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360: advisory note. World Health Organization.
- Caballero-Flores, G., Pickard, J.M. & Núñez, G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol 21, 347–360 (2023). [CrossRef]
- Lebeer, S., Verhoeven, T., Claes, I., De Hertogh, G., Vermeire, S., Buyse, J., Van Immerseel, F., Vanderleyden, J. and De Keersmaecker, S. (2011), FISH analysis of Lactobacillus biofilms in the gastrointestinal tract of different hosts. Letters in Applied Microbiology, 52: 220-226.
- Fouts, D.E., Pieper, R., Szpakowski, S., Pohl, H., Knoblach, S., Suh, M., Huang, S., Ljungberg, I., Sprague, B.M., Lucas, S.K., Torralba, M., Nelson, K.E., Groah, S.L. (2012). Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. Journal of Translational Medicine, 10:174. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).