Submitted:
08 August 2024
Posted:
09 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Search Strategy
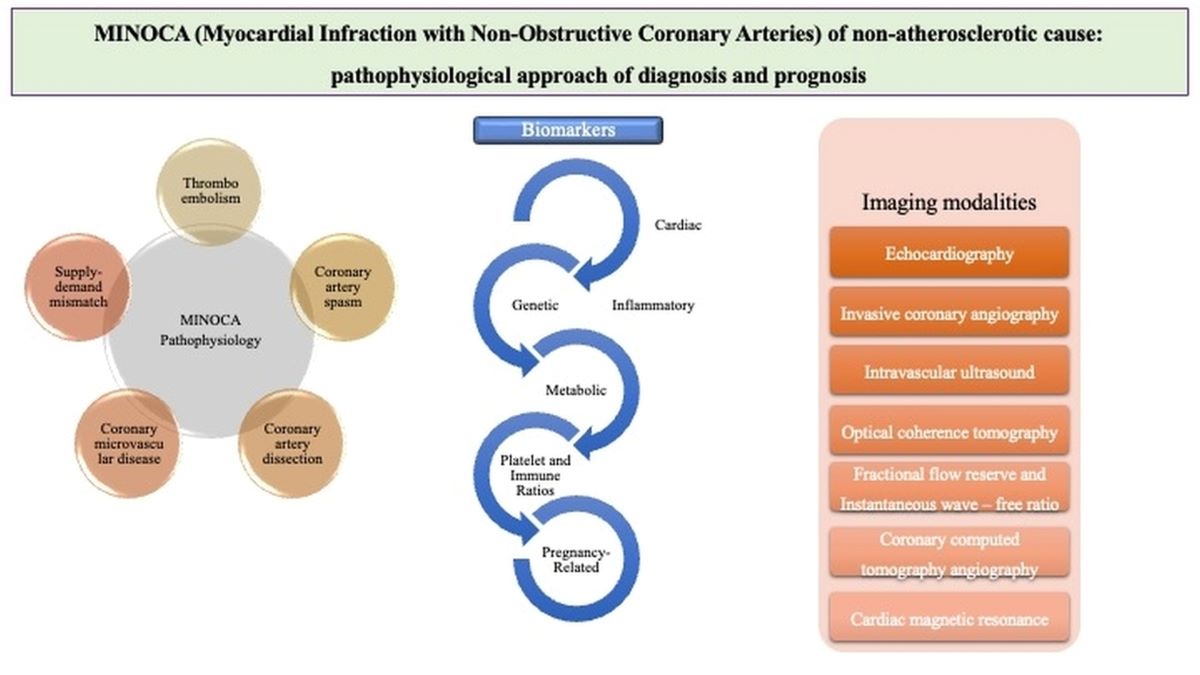
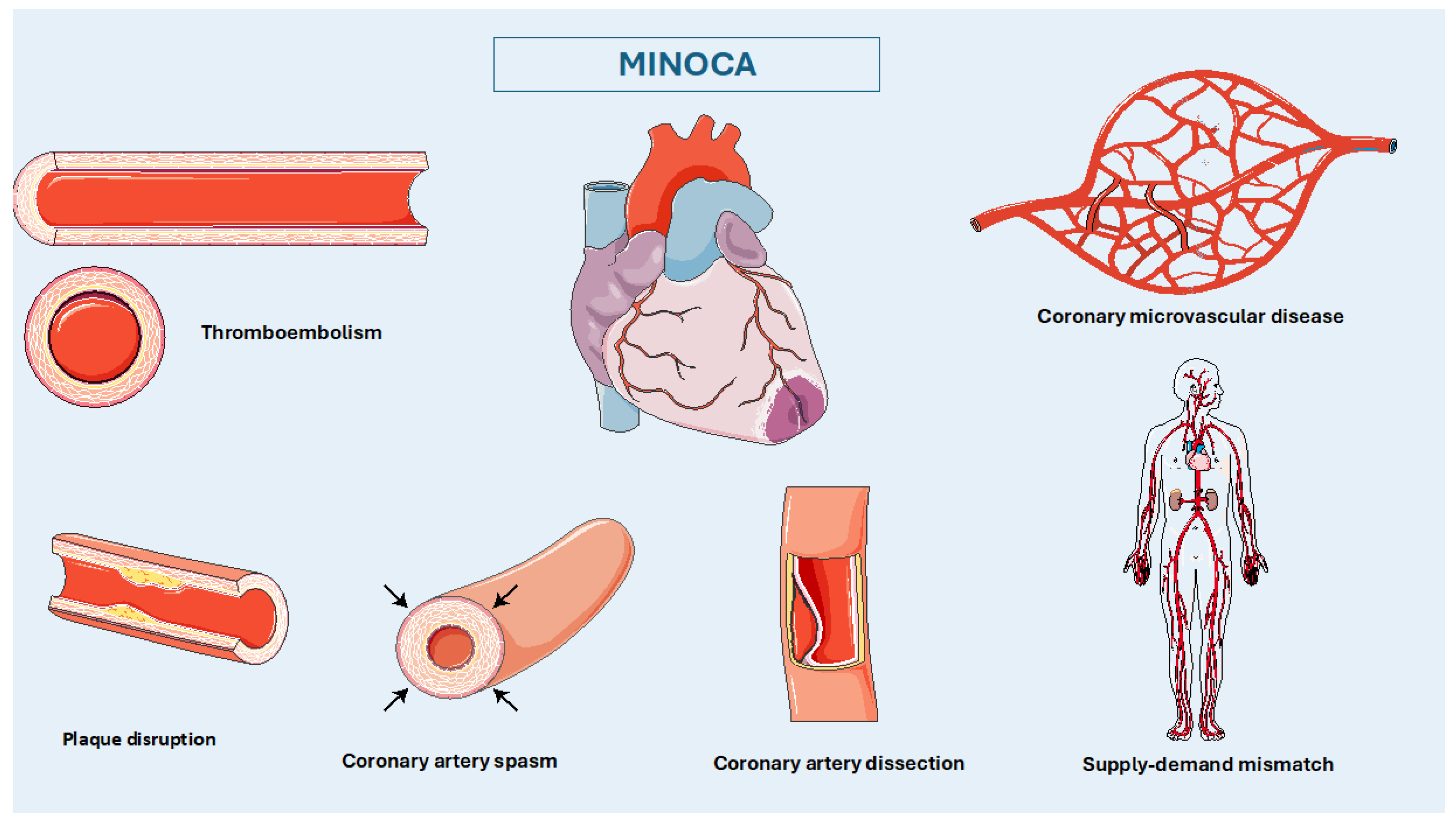
3. Pathophysiology
Thromboembolism
Coronary Artery Spasm (CAS)
Spontaneous Coronary Artery Dissection (SCAD)
Coronary Microvascular Disease (CMVD)
Supply-Demand Mismatch and Type 2 Myocardial Infarction
4. Diagnostic Approach Based on Pathophysiology
4.1. Imaging Modalities
4.1.2. Echocardiography
4.1.3. Invasive Coronary Angiography
4.1.4. Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT)
4.1.5. Fractional Flow Reserve (FFR) and Instantaneous Wave–Free Ratio (IFR)
4.1.6. Coronary Computed Tomography Angiography (CCTA)
4.1.7. Cardiac Magnetic Resonance (CMR)
4.2. Biomarkers
4.2.1. Troponin
4.2.2. Inflammatory Biomarkers
4.2.3. Natriuretic Peptides
4.2.4. Metabolic Profile
4.3. Genetic Factors/Metabolomics
5. Prognosis
5.1. Comparison with Myocardial Infarction and Obstructed CAD
5.2. Comparison with General Population
5.3. Determinants of MINOCA Prognosis
6. Current Management of MINOCA
6.1. ACEI/ARB and Statins:
6.2. Anti-Platelet Therapy:
6.3. Etiological Therapy:
7. Future Perspectives of MINOCA Personalized Therapy
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.C.S.D. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Tavella, R.; McRae, S.; Beltrame, J.F. Myocardial Infarction With Non-obstructive Coronary Arteries - Diagnosis and Management. Eur Cardiol 2015, 10, 79–82. [Google Scholar] [CrossRef]
- Boivin-Proulx, L.A.; Haddad, K.; Lombardi, M.; Chong, A.Y.; Escaned, J.; Mukherjee, S.; Forcillo, J.; Potter, B.J.; Coutinho, T.; Pacheco, C. Pathophysiology of Myocardial Infarction With Nonobstructive Coronary Artery Disease: A Contemporary Systematic Review. CJC Open 2024, 6, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.H.; Chang, K.; Lee, K.Y.; Lee, D.; Kim, J.G.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Cho, M.C.; Kim, C.J.; et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction With Non-Obstructive Coronary Arteries. J Am Heart Assoc 2019, 8, e011990. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: outcomes in a Medicare population. Eur Heart J 2020, 41, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Hjort, M.; Baron, T.; Jernberg, T.; Nordenskjold, A.M.; Tornvall, P.; Lindahl, B. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J Intern Med 2019, 285, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kaikita, K.; Sakamoto, K.; Seki, T.; Kawakami, K.; Nakai, M.; Sumita, Y.; Nishimura, K.; Miyamoto, Y.; Noguchi, T.; et al. Characteristics and in-hospital mortality of patients with myocardial infarction in the absence of obstructive coronary artery disease in super-aging society. Int J Cardiol 2020, 301, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Pasceri, V.; Niccoli, G.; Tanzilli, G.; Speciale, G.; Gaudio, C.; Crea, F.; Camici, P.G. Predictors of Mortality in Myocardial Infarction and Nonobstructed Coronary Arteries: A Systematic Review and Meta-Regression. Am J Med 2020, 133, 73–83.e74. [Google Scholar] [CrossRef] [PubMed]
- Bainey, K.R.; Welsh, R.C.; Alemayehu, W.; Westerhout, C.M.; Traboulsi, D.; Anderson, T.; Brass, N.; Armstrong, P.W.; Kaul, P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol 2018, 264, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Barr, P.R.; Harrison, W.; Smyth, D.; Flynn, C.; Lee, M.; Kerr, A.J. Myocardial Infarction Without Obstructive Coronary Artery Disease is Not a Benign Condition (ANZACS-QI 10). Heart Lung Circ 2018, 27, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Lindahl, B.; Litwin, P.; Tavella, R.; Williams, M.J.A.; Air, T.; Zeitz, C.; Smilowitz, N.R.; Reynolds, H.R.; Eggers, K.M.; et al. Survival in Patients With Suspected Myocardial Infarction With Nonobstructive Coronary Arteries: A Comprehensive Systematic Review and Meta-Analysis From the MINOCA Global Collaboration. Circ Cardiovasc Qual Outcomes 2021, 14, e007880. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Mahajan, A.M.; Roe, M.T.; Hellkamp, A.S.; Chiswell, K.; Gulati, M.; Reynolds, H.R. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017, 10, e003443. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Paolisso, P.; Gallinoro, E.; Fabbricatore, D.; Munhoz, D.; Bergamaschi, L.; Belmonte, M.; Panayotov, P.; Pizzi, C.; Barbato, E.; et al. Diagnostic and Prognostic Role of Cardiac Magnetic Resonance in MINOCA: Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging 2023, 16, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Parwani, P.; Kang, N.; Safaeipour, M.; Mamas, M.A.; Wei, J.; Gulati, M.; Naidu, S.S.; Merz, N.B. Contemporary Diagnosis and Management of Patients with MINOCA. Curr Cardiol Rep 2023, 25, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, G.; Li, Z.; Li, D.; Chen, R.; Huang, C.; Li, Y.; Li, B.; Yu, H.; Chu, X.M. MINOCA biomarkers: Non-atherosclerotic aspects. Clin Chim Acta 2023, 551, 117613. [Google Scholar] [CrossRef] [PubMed]
- Fatima, L.; Goyal, A.; Yakkali, S.; Jain, H.; Raza, F.A.; Peer, T.; Kanagala, S.G.; Sohail, A.H.; Malik, J. Precision medicine in Myocardial Infarction With Non-obstructive Coronary Disease (MINOCA): A comprehensive review. Curr Probl Cardiol 2024, 49, 102185. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021, 17, e875–e887. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Mancini, G.B.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 2011, 124, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Ren, X.; Yonetsu, T.; Kato, K.; Uemura, S.; Yu, B.; Jia, H.; Abtahian, F.; Aguirre, A.D.; Tian, J.; et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: a 3-vessel optical coherence tomography study. Am Heart J 2014, 167, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Taruya, A.; Tanaka, A.; Nishiguchi, T.; Ozaki, Y.; Kashiwagi, M.; Yamano, T.; Matsuo, Y.; Ino, Y.; Kitabata, H.; Takemoto, K.; et al. Lesion characteristics and prognosis of acute coronary syndrome without angiographically significant coronary artery stenosis. Eur Heart J Cardiovasc Imaging 2020, 21, 202–209. [Google Scholar] [CrossRef]
- Johnson, T.W.; Raber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2019, 40, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; McClure, L.A. Racial differences in venous thromboembolism. J Thromb Haemost 2011, 9, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, R.; Bellia, C.; Caruso, A.; Di Fiore, R.; Quaranta, S.; Noto, D.; Cefalu, A.B.; Di Micco, P.; Zarrilli, F.; Castaldo, G.; et al. Prothrombotic gene variants as risk factors of acute myocardial infarction in young women. J Transl Med 2012, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int J Cardiol 2019, 290, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, E.; Blet, A.; Galicier, L.; Darmon, M.; Parquet, N.; Lengline, E.; Boutboul, D.; Canet, E.; Traineau, R.; Schlemmer, B.; et al. Unresponsive thrombotic thrombocytopenic purpura in critically ill adults. Intensive Care Med 2013, 39, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M. Thrombophilia testing in women with venous thrombosis: the 4 P’s approach. Clin Chem 2014, 60, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Camma, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 2018, 39, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Sasayama, S.; Maseri, A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol 1999, 33, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Crea, F.; Meran, D.; Rodriguez, L.; Araujo, L.; Chierchia, S.; Davies, G.; Maseri, A. Local coronary supersensitivity to diverse vasoconstrictive stimuli in patients with variant angina. Circulation 1986, 74, 1255–1265. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Psaltis, P.J. The Forgotten Vascular Layer in the Forgotten Coronary Disorder. J Am Coll Cardiol 2018, 71, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Nam, P.; Choi, B.G.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; Jang, W.Y.; Kim, W.; Choi, J.Y.; Park, E.J.; et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis 2018, 270, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.H. Spontaneous Coronary-Artery Dissection. N Engl J Med 2020, 383, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.; Sandoval, J.; Lennie, V.; Dutary, J.; Medina, M.; Gonzalo, N.; Jimenez-Quevedo, P.; Escaned, J.; Banuelos, C.; Hernandez, R.; et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging 2013, 6, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv 2014, 7, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.; Rihal, C.S.; et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.H.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J Am Coll Cardiol 2020, 76, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Giacobbe, F.; Rolfo, C.; Quadri, G.; Tomassini, F.; Ferrari, F.; Mariani, F.; Anselmino, M.; Bianco, M.; Belliggiano, D.; et al. Role of Invasive and Non-invasive Imaging Tools in the Diagnosis and Optimal Treatment of Patients with Spontaneous Coronary Artery Dissection. Curr Cardiol Rep 2019, 21, 122. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Camici, P. Advances in coronary microvascular dysfunction. Heart Lung Circ 2009, 18, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arterioscler Thromb Vasc Biol 2021, 41, 1625–1637. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Limaye, S.B.; Horowitz, J.D. The coronary slow flow phenomenon--a new coronary microvascular disorder. Cardiology 2002, 97, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, R.; Srichai, M.B.; Axel, L.; Hochman, J.S.; Reynolds, H.R. Stress Cardiac MRI in Women With Myocardial Infarction and Nonobstructive Coronary Artery Disease. Clin Cardiol 2016, 39, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Ong, G.J.; Girolamo, O.C.; De Menezes Caceres, V.; Muminovic, A.; Chirkov, Y.Y.; Horowitz, J.D. Angina due to coronary artery spasm (variant angina): diagnosis and intervention strategies. Expert Rev Cardiovasc Ther 2021, 19, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Buckberg, G.D. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc 2014, 3, e000285. [Google Scholar] [CrossRef] [PubMed]
- Rafiudeen, R.; Barlis, P.; White, H.D.; van Gaal, W. Type 2 MI and Myocardial Injury in the Era of High-sensitivity Troponin. Eur Cardiol 2022, 17, e03. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial, I. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Rallidis, L.S.; Xenogiannis, I.; Brilakis, E.S.; Bhatt, D.L. Causes, Angiographic Characteristics, and Management of Premature Myocardial Infarction: JACC State-of-the-Art Review. J Am Coll Cardiol 2022, 79, 2431–2449. [Google Scholar] [CrossRef] [PubMed]
- Alves da Silva, P.; Bucciarelli-Ducci, C.; Sousa, A. Myocardial infarction with non-obstructive coronary arteries: Etiology, diagnosis, treatment and prognosis. Rev Port Cardiol 2023, 42, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Pustjens, T.F.S.; Meerman, A.; Vranken, N.P.A.; Ruiters, A.W.; Gho, B.; Stein, M.; Ilhan, M.; Veenstra, L.; Winkler, P.; Lux, A.; et al. Importance of confirming the underlying diagnosis in patients with myocardial infarction and non-obstructive coronary arteries (MINOCA): a single-centre retrospective cohort study. BMC Cardiovasc Disord 2021, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C., II; Perez-Aybar, A.E.; Roman-Ramos, J.A. MINOCA: A Working Diagnosis. Cureus 2023, 15, e49695. [Google Scholar] [CrossRef]
- Occhipinti, G.; Bucciarelli-Ducci, C.; Capodanno, D. Diagnostic pathways in myocardial infarction with non-obstructive coronary artery disease (MINOCA). Eur Heart J Acute Cardiovasc Care 2021, 10, 813–822. [Google Scholar] [CrossRef]
- Almeida, A.G. MINOCA and INOCA: Role in Heart Failure. Curr Heart Fail Rep 2023, 20, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, H.; Gibson, C.M. MINOCA: Myocardial infarction no obstructive coronary artery disease. Am Heart J Plus 2023, 33, 100312. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Smilowitz, N.R. Myocardial Infarction with Nonobstructive Coronary Arteries. Annu Rev Med 2023, 74, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Princi, G.; La Vecchia, G.; Bonanni, A.; Chiariello, G.A.; Candreva, A.; Gragnano, F.; Calabro, P.; Crea, F.; Montone, R.A. MINOCA Associated with a Myocardial Bridge: Pathogenesis, Diagnosis and Treatment. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Marrone, A.; Pavasini, R.; Scollo, E.; Gibiino, F.; Pompei, G.; Caglioni, S.; Biscaglia, S.; Campo, G.; Tebaldi, M. Acetylcholine Use in Modern Cardiac Catheterization Laboratories: A Systematic Review. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Iwanczyk, S.; Wozniak, P.; Araszkiewicz, A.; Grygier, M.; Klotzka, A.; Lesiak, M. Optical coherence tomography in the diagnosis of myocardial infarction with non-obstructive coronary arteries. Postepy Kardiol Interwencyjnej 2022, 18, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sucato, V.; Testa, G.; Puglisi, S.; Evola, S.; Galassi, A.R.; Novo, G. Myocardial infarction with non-obstructive coronary arteries (MINOCA): Intracoronary imaging-based diagnosis and management. J Cardiol 2021, 77, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Borzillo, I.; De Filippo, O.; Manai, R.; Bruno, F.; Ravetti, E.; Galanti, A.A.; Vergallo, R.; Porto, I.; De Ferrari, G.M.; D’Ascenzo, F. Role of Intracoronary Imaging in Myocardial Infarction with Non-Obstructive Coronary Disease (MINOCA): A Review. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, K.; Gasior, P.; Legutko, J.; Makowicz, D.; Kedziora, A.; Szolc, P.; Bryniarski, L.; Kleczynski, P.; Jang, I.K. OCT Findings in MINOCA. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Rinaldi, R.; D’Amario, D.; Niccoli, G. Diagnostic work-up and therapeutic implications in MINOCA: need for a personalized approach. Future Cardiol 2021, 17, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Rampidis, G.P.; Kampaktsis, P.; Kouskouras, K.; Samaras, A.; Benetos, G.; Giannopoulos, A.; Karamitsos, T.; Kallifatidis, A.; Samaras, A.; Vogiatzis, I.; et al. Role of cardiac CT in the diagnostic evaluation and risk stratification of patients with myocardial infarction and non-obstructive coronary arteries (MINOCA): rationale and design of the MINOCA-GR study. BMJ Open 2022, 12, e054698. [Google Scholar] [CrossRef] [PubMed]
- Pergola, V.; Previtero, M.; Cecere, A.; Storer, V.; Castiello, T.; Baritussio, A.; Cabrelle, G.; Mele, D.; Motta, R.; Caforio, A.P.; et al. Clinical Value and Time Course of Pericoronary Fat Inflammation in Patients with Angiographically Nonobstructive Coronaries: A Preliminary Report. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Daneshrad, J.A.; Ordovas, K.; Sierra-Galan, L.M.; Hays, A.G.; Mamas, M.A.; Bucciarelli-Ducci, C.; Parwani, P. Role of Cardiac Magnetic Resonance Imaging in the Evaluation of MINOCA. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Carisio, A.; D’Angelo, T.; Darvizeh, F.; Dell’Aversana, S.; Tore, D.; Centonze, M.; Faletti, R. Cardiovascular magnetic resonance in myocardial infarction with non-obstructive coronary arteries patients: A review. World J Cardiol 2020, 12, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Herling de Oliveira, L.L.; Correia, V.M.; Nicz, P.F.G.; Soares, P.R.; Scudeler, T.L. MINOCA: One Size Fits All? Probably Not-A Review of Etiology, Investigation, and Treatment. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Lintingre, P.F.; Nivet, H.; Clement-Guinaudeau, S.; Camaioni, C.; Sridi, S.; Corneloup, O.; Gerbaud, E.; Coste, P.; Dournes, G.; Latrabe, V.; et al. High-Resolution Late Gadolinium Enhancement Magnetic Resonance for the Diagnosis of Myocardial Infarction With Nonobstructed Coronary Arteries. JACC Cardiovasc Imaging 2020, 13, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Machanahalli Balakrishna, A.; Ismayl, M.; Thandra, A.; Walters, R.; Ganesan, V.; Anugula, D.; Shah, D.J.; Aboeata, A. Diagnostic Value of Cardiac Magnetic Resonance Imaging and Intracoronary Optical Coherence Tomography in Patients With a Working Diagnosis of Myocardial Infarction With Non-obstructive Coronary Arteries - A Systematic Review and Meta-analysis. Curr Probl Cardiol 2023, 48, 101126. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Onuma, S.; Hao, K.; Godo, S.; Shiroto, T.; Yasuda, S. Pathophysiology and diagnostic pathway of myocardial infarction with non-obstructive coronary arteries. J Cardiol 2024, 83, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Abdu, F.A.; Mohammed, A.Q.; Liu, L.; Xu, Y.; Che, W. Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): A Review of the Current Position. Cardiology 2020, 145, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; De Silva, K.; Sood, A.; Denniss, A.R.; Hsu, C.J. Predicting Patients With Troponin Positive Chest Pain and Unobstructed Coronary Arteries With Electrocardiogram, Troponin Kinetics and GRACE Score. Heart Lung Circ 2022, 31, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, A.; Kadoglou, N.P.E.; Lambadiari, V.; Alexiou, S.; Theodoropoulos, K.C.; Avraamides, P.; Kassimis, G. Prognostic role of inflammatory cytokines and novel adipokines in acute myocardial infarction: An updated and comprehensive review. Cytokine 2022, 153, 155848. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Tahmatzidis, D.K.; Giannakoulas, C.; Kapelouzou, A.; Gkontopoulos, A.; Parissis, J.; Lampropoulos, S.; Kottas, G. Serum levels of novel adipokines, omentin-1 and chemerin, in patients with acute myocardial infarction: KOZANI STUDY. J Cardiovasc Med (Hagerstown) 2015, 16, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lakic, T.G.; Lindback, J.; Budaj, A.; Cornel, J.H.; Giannitsis, E.; Katus, H.A.; Siegbahn, A.; Storey, R.F.; et al. Biomarker Concentrations and Their Temporal Changes in Patients With Myocardial Infarction and Nonobstructive Compared With Obstructive Coronary Arteries: Results From the PLATO Trial. J Am Heart Assoc 2023, 12, e027466. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lindhagen, L.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenback, C.; Frick, M.; Henareh, L.; et al. Increased Inflammatory Activity in Patients 3 Months after Myocardial Infarction with Nonobstructive Coronary Arteries. Clin Chem 2019, 65, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Ekenback, C.; Agewall, S.; Brolin, E.B.; Caidahl, K.; Cederlund, K.; Collste, O.; Eurenius, L.; Frick, M.; Younis-Hassan, S.; et al. Risk Factors and Markers for Acute Myocardial Infarction With Angiographically Normal Coronary Arteries. Am J Cardiol 2015, 116, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, V.V.; Vorobeva, D.A.; Kologrivova, I.V.; Suslova, T.E. Pro-Inflammatory Biomarkers and Progression of Atherosclerosis in Patients with Myocardial Infarction with Non-Obstructive Coronary Artery Disease: 1-Year Follow-Up. J Pers Med 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Stangret, A.; Dykacz, W.; Jablonski, K.; Wesolowska, A.; Klimczak-Tomaniak, D.; Kochman, J.; Tomaniak, M. The cytokine trio - visfatin, placental growth factor and fractalkine - and their role in myocardial infarction with non-obstructive coronary arteries (MINOCA). Cytokine Growth Factor Rev 2023, 74, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Foa, A.; Bergamaschi, L.; Donati, F.; Fabrizio, M.; Chiti, C.; Angeli, F.; Toniolo, S.; Stefanizzi, A.; Armillotta, M.; et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, H.; Ma, W.; Yuan, J.; Yu, M. Remnant Cholesterol Predicts Risk of Cardiovascular Events in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries. J Am Heart Assoc 2022, 11, e024366. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gao, S.; Huang, S.; Yuan, J.; Yu, M. Hyperuricemia as a prognostic marker for long-term outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab (Lond) 2021, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ma, W.; Huang, S.; Lin, X.; Yu, M. Effect of Lipoprotein (a) Levels on Long-term Cardiovascular Outcomes in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries. Am J Cardiol 2021, 152, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Guler, G.B.; Kizilirmak, F.; Batgerel, U.; Demir, G.G.; Gunes, H.M.; Karaca, O.; Ozcan, O.; Barutcu, I.; Turkmen, M.M.; et al. Evaluation of adiponectin and lipoprotein(a) levels in cardiac syndrome X. Herz 2015, 40 Suppl 3, 291–297. [Google Scholar] [CrossRef]
- Lin, Y.K.; Yeh, C.T.; Kuo, K.T.; Fong, I.H.; Yadav, V.K.; Kounis, N.G.; Hu, P.; Hung, M.Y. Apolipoprotein (a)/Lipoprotein(a)-Induced Oxidative-Inflammatory alpha7-nAChR/p38 MAPK/IL-6/RhoA-GTP Signaling Axis and M1 Macrophage Polarization Modulate Inflammation-Associated Development of Coronary Artery Spasm. Oxid Med Cell Longev 2022, 2022, 9964689. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Colombo, L.; Tomarelli, E.; Piccialuti, A.; Di Pietro, G.; Birtolo, L.I.; Maestrini, V.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Nour, K.R.; Zalos, G.; Quyyumi, A.A. Endogenous endothelin in human coronary vascular function: differential contribution of endothelin receptor types A and B. Hypertension 2007, 49, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Corcoran, D.; Padmanabhan, S.; Aman, A.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Maguire, J.J.; Watkins, S.; Eteiba, H.; et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J 2020, 41, 3239–3252. [Google Scholar] [CrossRef]
- Cox, I.D.; Botker, H.E.; Bagger, J.P.; Sonne, H.S.; Kristensen, B.O.; Kaski, J.C. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol 1999, 34, 455–460. [Google Scholar] [CrossRef]
- Verstraeten, A.; Perik, M.; Baranowska, A.A.; Meester, J.A.N.; Van Den Heuvel, L.; Bastianen, J.; Kempers, M.; Krapels, I.P.C.; Maas, A.; Rideout, A.; et al. Enrichment of Rare Variants in Loeys-Dietz Syndrome Genes in Spontaneous Coronary Artery Dissection but Not in Severe Fibromuscular Dysplasia. Circulation 2020, 142, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Au Yeung, K.; Sandor, G.G.; Judge, D.P.; Dietz, H.C.; van Breemen, C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res 2007, 101, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Yang, M.-L.; Trinder, M.; Tcheandjieu, C.; Xu, C.; Starovoytov, A.; Birt, I.; Mathis, M.R.; Hunker, K.L.; Schmidt, E.M.; et al. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nature Communications 2020, 11, 4432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Y.; Li, Y.; Li, Z.; Li, C.; Yu, T.; Xiao, L.; Yu, B.; Zhao, H.; Tao, M.; et al. Association of TSR1 Variants and Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2019, 74, 167–176. [Google Scholar] [CrossRef]
- Lozano-Prieto, M.; Adlam, D.; Garcia-Guimaraes, M.; Sanz-Garcia, A.; Vera-Tome, P.; Rivero, F.; Cuesta, J.; Bastante, T.; Baranowska-Clarke, A.A.; Vara, A.; et al. Differential miRNAs in acute spontaneous coronary artery dissection: Pathophysiological insights from a potential biomarker. EBioMedicine 2021, 66, 103338. [Google Scholar] [CrossRef]
- Li, L.; Liao, J.; Yuan, Q.; Hong, X.; Li, J.; Peng, Y.; He, M.; Zhu, H.; Zhu, M.; Hou, F.F.; et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci Adv 2021, 7. [Google Scholar] [CrossRef]
- Margaritis, M.; Saini, F.; Baranowska-Clarke, A.A.; Parsons, S.; Vink, A.; Budgeon, C.; Allcock, N.; Wagner, B.E.; Samani, N.J.; von der Thusen, J.; et al. Vascular histopathology and connective tissue ultrastructure in spontaneous coronary artery dissection: pathophysiological and clinical implications. Cardiovasc Res 2022, 118, 1835–1848. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10. [Google Scholar] [CrossRef]
- Franczyk, B.; Dybiec, J.; Frak, W.; Krzeminska, J.; Kucmierz, J.; Mlynarska, E.; Szlagor, M.; Wronka, M.; Rysz, J. Cellular Mechanisms of Coronary Artery Spasm. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of coronary artery spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Yasuda, S.; Aizawa, K.; Tsuburaya, R.; Ito, Y.; Takeda, M.; Nakayama, M.; Ito, K.; Takahashi, J.; Shimokawa, H. Enhanced Rho-kinase activity in circulating neutrophils of patients with vasospastic angina: a possible biomarker for diagnosis and disease activity assessment. J Am Coll Cardiol 2011, 58, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Planer, D.; Mehran, R.; Ohman, E.M.; White, H.D.; Newman, J.D.; Xu, K.; Stone, G.W. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv 2014, 7, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Jeong, M.H.; Ahn, Y.K.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; Choi, D.H.; et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol 2011, 146, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, G.; Coiro, S.; Tritto, I.; Benedetti, M.; Guerra, F.; Del Pinto, M.; Finocchiaro, G.; Cavallini, C.; Capucci, A.; Kaski, J.C.; et al. Predictors of poor clinical outcomes in patients with acute myocardial infarction and non-obstructed coronary arteries (MINOCA). Int J Cardiol 2018, 267, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Raparelli, V.; Elharram, M.; Shimony, A.; Eisenberg, M.J.; Cheema, A.N.; Pilote, L. Myocardial Infarction With No Obstructive Coronary Artery Disease: Angiographic and Clinical Insights in Patients With Premature Presentation. Can J Cardiol 2018, 34, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjold, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef]
- Andersson, H.B.; Pedersen, F.; Engstrom, T.; Helqvist, S.; Jensen, M.K.; Jorgensen, E.; Kelbaek, H.; Rader, S.; Saunamaki, K.; Bates, E.; et al. Long-term survival and causes of death in patients with ST-elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J 2018, 39, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Montenegro Sa, F.; Ruivo, C.; Santos, L.G.; Antunes, A.; Saraiva, F.; Soares, F.; Morais, J. Myocardial infarction with nonobstructive coronary arteries: a single-center retrospective study. Coron Artery Dis 2018, 29, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjold, A.M.; Baron, T.; Eggers, K.M.; Jernberg, T.; Lindahl, B. Predictors of adverse outcome in patients with myocardial infarction with non-obstructive coronary artery (MINOCA) disease. Int J Cardiol 2018, 261, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Abdu, F.A.; Liu, L.; Mohammed, A.Q.; Luo, Y.; Xu, S.; Auckle, R.; Xu, Y.; Che, W. Myocardial infarction with non-obstructive coronary arteries (MINOCA) in Chinese patients: Clinical features, treatment and 1 year follow-up. Int J Cardiol 2019, 287, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, R.; Shaw, L.J.; Chandrashekhar, Y. The Promise of Imaging in MINOCA. JACC Cardiovasc Imaging 2019, 12, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Lindahl, B.; Baron, T.; Jernberg, T.; Tornvall, P.; Eggers, K.M. Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am Heart J 2018, 200, 60–66. [Google Scholar] [CrossRef]
- Gurzau, D.; Sitar-Taut, A.; Caloian, B.; Gusetu, G.; Comsa, H.; Fringu, F.; Zdrenghea, D.; Pop, D. The Role of IL-6 and ET-1 in the Diagnosis of Coronary MicroVascular Disease in Women. J Pers Med 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Cenko, E.; Manfrini, O.; Morell, C.; Das, R.; Barth, J.H.; Hall, A.S.; Gale, C.P.; Bugiardini, R.; Methods, o.b.o.E.o.; Events, M.o.A.C. Angiotensin-converting enzyme inhibitor therapy in patients non-obstructive coronary artery disease. European Heart Journal 2013, 34. [Google Scholar] [CrossRef]
- Cespon Fernandez, M.; Abu-Assi, E.; Raposeiras Roubin, S.; Munoz Pousa, I.; Dominguez Rodriguez, L.M.; Dominguez Erquicia, P.; Cobas Paz, R.; Caneiro Queija, B.; Jamhour Chelh, K.; Perez Casares, L.E.; et al. P880 Cardiovascular mortality in patients with MINOCA and prognostic effect of statin treatment. European Heart Journal 2019, 40. [Google Scholar] [CrossRef]
- Bossard, M.; Yusuf, S.; Tanguay, J.F.; Faxon, D.P.; Boden, W.E.; Steg, P.G.; Granger, C.; Kastrati, A.; Budaj, A.; Di Pasquale, G.; et al. 2387Recurrent cardiovascular events and mortality in relation to antiplatelet therapy in patients with myocardial infarction without obstructive coronary artery disease (MINOCA). European Heart Journal 2019, 40. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Yamanaga, K.; Miyazaki, T.; Akasaka, T.; Tabata, N.; Arima, Y.; Sueta, D.; Sakamoto, K.; et al. Impact of aspirin on the prognosis in patients with coronary spasm without significant atherosclerotic stenosis. Int J Cardiol 2016, 220, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.; Gao, P.; Boden, W.; Steg, G.; Tanguay, J.F.; Joyner, C.; Granger, C.B.; Kastrati, A.; Faxon, D.; Budaj, A.; et al. Antiplatelet therapy in patients with myocardial infarction without obstructive coronary artery disease. Heart 2021, 107, 1739–1747. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Pustjens, T.F.S.; Appelman, Y.; Damman, P.; Ten Berg, J.M.; Jukema, J.W.; de Winter, R.J.; Agema, W.R.P.; van der Wielen, M.L.J.; Arslan, F.; Rasoul, S.; et al. Guidelines for the management of myocardial infarction/injury with non-obstructive coronary arteries (MINOCA): a position paper from the Dutch ACS working group. Neth Heart J 2020, 28, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Scalone, G.; Crea, F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 2015, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Ceasovschih, A.; Mantzouranis, E.; Dimitriadis, K.; Sorodoc, V.; Vlachakis, P.K.; Karanikola, A.E.; Theofilis, P.; Koutsopoulos, G.; Drogkaris, S.; Andrikou, I.; et al. Coronary artery thromboembolism as a cause of myocardial infarction with non-obstructive coronary arteries (MINOCA). Hellenic J Cardiol 2024. [Google Scholar] [CrossRef] [PubMed]
- Pirozzolo, G.; Seitz, A.; Athanasiadis, A.; Bekeredjian, R.; Sechtem, U.; Ong, P. Microvascular spasm in non-ST-segment elevation myocardial infarction without culprit lesion (MINOCA). Clin Res Cardiol 2020, 109, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Offen, S.; Yang, C.; Saw, J. Spontaneous coronary artery dissection (SCAD): A contemporary review. Clin Cardiol 2024, 47, e24236. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Humphries, K.; Aymong, E.; Sedlak, T.; Prakash, R.; Starovoytov, A.; Mancini, G.B.J. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol 2017, 70, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology 2022, 79, e21–e129. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Rasoul-Arzrumly, E.; McDaniel, M.; Mekonnen, G.; Timmins, L.H.; Lutz, J.; Guyton, R.A.; Samady, H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol 2014, 63, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Fang, C.; Jiang, S.; Wang, J.; Wang, Y.; Guo, J.; Lei, F.; Sun, S.; Pei, X.; Jia, R.; et al. Optical Coherence Tomographic Features of Pancoronary Plaques in Patients With Acute Myocardial Infarction Caused by Layered Plaque Rupture Versus Layered Plaque Erosion. Am J Cardiol 2022, 167, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pacheco Claudio, C.; Quesada, O.; Pepine, C.J.; Noel Bairey Merz, C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol 2018, 41, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Oshita, C.; Uchimura, Y.; Akazawa, R.; Orita, Y. Coronary Microvascular Vasodilatory Function: Related Clinical Features and Differences According to the Different Coronary Arteries and Types of Coronary Spasm. J Clin Med 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Handberg, E.M.; Merz, C.N.B.; Cooper-Dehoff, R.M.; Wei, J.; Conlon, M.; Lo, M.C.; Boden, W.; Frayne, S.M.; Villines, T.; Spertus, J.A.; et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J 2021, 237, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Corcoran, D.; Oldroyd, K.G.; McEntegart, M.; Rocchiccioli, P.; Watkins, S.; Brooksbank, K.; Padmanabhan, S.; Sattar, N.; Briggs, A.; et al. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Angina (CorMicA) stratified medicine clinical trial. Am Heart J 2018, 201, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Onuma, S.; Hao, K.; Godo, S.; Shiroto, T.; Yasuda, S. Pathophysiology and diagnostic pathway of myocardial infarction with non-obstructive coronary arteries. J Cardiol 2024, 83, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lakic, T.G.; Lindback, J.; Budaj, A.; Cornel, J.H.; Giannitsis, E.; Katus, H.A.; Siegbahn, A.; Storey, R.F.; et al. Biomarker Concentrations and Their Temporal Changes in Patients With Myocardial Infarction and Nonobstructive Compared With Obstructive Coronary Arteries: Results From the PLATO Trial. J Am Heart Assoc 2023, 12, e027466. [Google Scholar] [CrossRef] [PubMed]
- Hjort, M.; Eggers, K.M.; Lindhagen, L.; Agewall, S.; Brolin, E.B.; Collste, O.; Daniel, M.; Ekenback, C.; Frick, M.; Henareh, L.; et al. Increased Inflammatory Activity in Patients 3 Months after Myocardial Infarction with Nonobstructive Coronary Arteries. Clin Chem 2019, 65, 1023–1030. [Google Scholar] [CrossRef] [PubMed]

| Imaging Modality | Parameters | Diagnosis | Prognosis |
|---|---|---|---|
| Echocardiography | - Myocardial function - Wall motion abnormalities - Coronary Flow Reserve (CFR) |
- Causes of myocardial injury - Differential diagnosis of other non-cardiac diseases - CMVD |
- Initial assessment tool |
| Invasive Coronary Angiography | - Coronary artery stenosis - Coronary flow rates - Epicardial coronary vasospasm |
- Coronary arteries visualization - SCAD - Vasospasm and microvascular dysfunction -Coronary artery bridging |
- Poorer outcomes in non-obstructive coronary atherosclerotic plaques - Worse prognosis in positive provocative testing for coronary spasm |
| Intravascular Ultrasound (IVUS) | - 360-deggree imaging of coronary arteries | - Detailed characterization of coronary lesions | - Poorer prognosis in complex lesions |
| Optical Coherence Tomography (OCT) | - Visualization of luminal and superficial lesions | - High-resolution imaging for precise lesion detection (plaque disruption, SCAD, and distal embolization) | - Poorer prognosis in disrupted lesions |
| Fractional Flow Reserve (FFR) and Instantaneous Wave-Free Ratio (IFR) | - Pressure measurements | - Functional significance of epicardial stenosis - Microvascular function with CFR and IMR |
- Risk stratification in specific cases |
| Coronary Computed Tomography Angiography (CCTA) | - Plaque characteristics -Perivascular Fat Attenuation Index (pFAI) |
- Identifies extracardiac structures - Lesion changes - Differentiation coronary vs non-coronary origin of MINOCA |
- Elevated pFAI values indicate load of inflammation |
| Cardiac Magnetic Resonance (CMR) | - Myocardial perfusion - Ventricular function - T2 and LGE sequences |
- Differentiation ischemic vs. non-ischemic injuries - Identification myocardial edema, fibrosis, and microvascular obstruction |
- Prognostic value of LGE, MPI and MPRI |
| Biomarkers | Mechanism | Changes in MINOCA | Prognostic value |
|---|---|---|---|
| Troponin (cTnI and cTnT) | Myocardiocytes destroy | ↑ cTn levels > 99th percentile: myocardial injury |
|
| C-reactive protein (CRP) | Inflammation | ↑ in MINOCA | ↑ risk of all-cause mortality and MACE |
| P-selectin glycoprotein ligand-1 (PSGL-1) | Inflammation | Discriminates between MINOCA, AMI-CAD and healthy controls | poorer prognosis in MINOCA patients |
| Interleukin 6 (IL-6) | Inflammation, CMVD, CAS | ↑ in CMVD and CAS | ↑ adverse cardiovascular outcomes |
| Soluble urokinase-type plasminogen activator receptor (suPAR) | Inflammation | ↑ in MINOCA vs healthy controls | ↑ adverse cardiovascular outcomes |
| N-terminal pro-B-type natriuretic peptide (NT-proBNP) | Endo-cardiac pressure | ↑ in MINOCA and AMI-CAD vs healthy controls | Unknown |
| Lipoprotein (a) (Lp(a)) | Lipid metabolism | ↑ in MINOCA | poorer prognosis in MINOCA patients |
| Visfatin | Inflammation, endothelial function | ↑ in MINOCA and type 2 diabetes mellitus | Unknown |
| Placental growth factor (PlGF) | Endothelial function, inflammation, angiogenesis | ↑ in MINOCA | Unknown |
| Fractalkine (CX3CL-1/FKN) | Plaque formation, inflammation, endothelial dysfunction | ↑ in MINOCA | Unknown |
| White blood cell count to mean platelet-volume ratio (WMR) | Inflammation | ↑ in MINOCA | ↑ risk of MACE |
| Neutrophil-to-platelet ratio (NPR) | Inflammation | ↑ in MINOCA | ↑ risk of MACE |
| Platelet-to-lymphocyte ratio (PLR) | Inflammation | ↑ in MINOCA | ↑ risk of MACE |
| Neutrophil-to-lymphocyte ratio (NLR) | Inflammation | ↑ in MINOCA | ↑ risk of MACE |
| Uric acid | - | ↑ in MINOCA | ↑ adverse outcomes and MACE |
| HMOX1 gene | stress-induced protective enzyme against myocardial ischemia | Long promoter guanine-thymine repeats | ↑ adverse outcomes and heart dysfunction |
| ET-1 (Endothelin-1) | Regulation vascular tone and proliferation | ↑ in MINOCA | Association with CMVD |
| TGF-β (Transforming Growth Factor-β) | endothelial cell dysfunction and vascular permeability | ↑ in MINOCA | Association with SCAD in women |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





