1. Introduction
Infection by the SARS-CoV-2 virus is a major cause of morbidity and mortality and resulted in a severe pandemic [
1,
2,
3]. The virus attaches to the angiotensin converting enzyme (ACE)-2, which is found as an ectoenzyme on arterial, venous, and capillary endothelial cells. In addition to injuring the endothelium, leading to many types of vascular complications, the subsequent corporeal inflammatory reaction magnifies the injury and the severity of the systemic illness [
4]. The lung is particularly affected, possibly because the major method of transmission of the virus is via inhalation, but the lung circulation is also particularly rich in ACE-2. Affected patients may have a mild respiratory illness, or they may progress to severe lung injury and acute respiratory distress syndrome (ARDS), which carries a high mortality [
2]. Several studies have shown that the pathophysiology of COVID-19-induced ARDS is more complex and diverse compared to typical ARDS that is characterized by “reduced lung volume and decreased compliance” [
5,
6,
7].
Endothelial dysfunction often results in decreased nitric oxide (NO) synthesis and increased release of vasoconstrictors, including endothelin-1 (ET-1) and angiotensin II [
8,
9]. Endothelin-1 is a potent vasoconstrictor produced by endothelial cells throughout the body. Circulating plasma ET-1 levels are generally low, but they rise in disease states, including cardiovascular diseases, cancer, chronic pain, and asthma [
10]. The major site of circulating endothelin clearance is in the lung circulation, via endothelial endothelin type B (ETB) receptors [
11,
12]. Normally, the plasma ET-1 ratio for blood entering the lung compared to that leaving the lung appears to be equal to unity [
12]. This occurs since approximately 50% of circulating ET-1 is removed by the human lung, however, a comparable amount is released back into circulation by the lung, explaining the absence of an arteriovenous ET-1 gradient across the pulmonary circulation [
13]. In healthy control subjects, plasma ET-1 ratio has been found to be 0.59-0.7 [
14,
15]. Decreased clearance of ET-1 has been described in pulmonary hypertension, pulmonary fibrosis, coronary disease, asthma, ARDS, septic shock, and collagen disorders among others [
14,
15,
16].
In addition to its vasoconstrictive action, ET-1 is a potent mitogen for smooth muscle cells and fibroblasts, and ET-1-induced cytokine release causes activation of the inflammatory cascade, leading to increased vascular permeability, and eventually multi-organ failure [
17].
In view of the above, we hypothesized that with the pulmonary endothelial dysfunction seen in COVID-19-induced ARDS patients, there would be imbalance between the pulmonary synthesis and clearance of ET-1. We therefore measured ET-1 levels in the central venous and systemic arterial circulation of mechanically ventilated COVID-19-induced ARDS patients. Based on previous studies that showed high circulating ET-1 levels in COVID-19, we explored whether they were related to abnormal ET-1 clearance, or net synthesis, and whether any abnormal pulmonary ET-1 handling would reverse in patients who show clinical improvement.
2. Results
During the screening period, no patients were excluded based on the study’s criteria. Eventually, 18 COVID-19-induced ARDS Caucasian patients were enrolled with a median age of 73 (66-78) years and 72% were male. On ICU admission, the APACHE II score was 16 (10-21) and the SOFA score 8 (6-9). Two mechanically-ventilated critically ill control groups were also included; non-COVID-19 ARDS patients (N= 14) and non-COVID-19/non-ARDS patients (N= 20).
Table 1 shows the demographics, clinical characteristics, biochemical and laboratory data of the 3 patient groups on ICU admission.
The patient groups were matched for age, sex, and critical illness severity scores. The non-COVID-19 ARDS patients had a respiratory infection (mostly caused by Gram-negative bacteria), whereas the non-COVID-19 non-ARDS patients were mainly surgical/trauma patients. As expected, the two ARDS groups had a lower PaO2/FiO2 ratio, a higher respiratory rate, and a higher positive end-expiratory pressure (PEEP) compared to the non-ARDS patients. With regard to surrogate endothelial markers, plasma soluble intercellular adhesion molecule 1 (sICAM-1) levels were higher in the two groups of ARDS patients, while sE-selectin levels were higher in the non-COVID-19 ARDS patients.
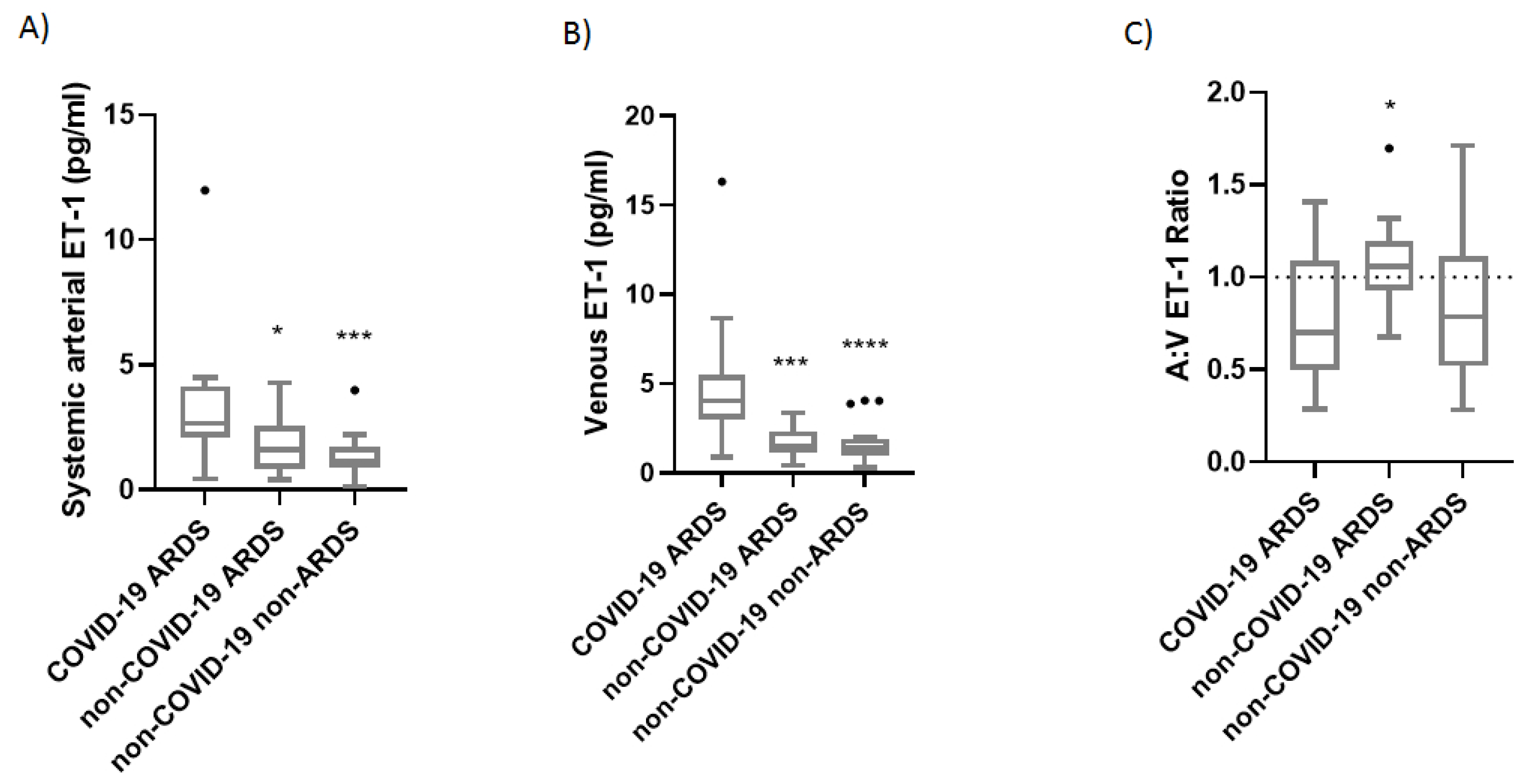
We then proceeded to concurrently measure the venous and arterial ET-1 levels in 18 COVID-19-induced ARDS patients, 14 non-COVID-19 ARDS patients, and 20 critically ill patients, on Days 1 and 3 from ICU admission. COVID-19-induced ARDS patients had higher systemic arterial and venous ET-1 levels on ICU admission compared to the non-COVID-19 ARDS and the non-COVID-19 critically ill patients [2.66 (2.11-4.14) pg/mL vs. 1.59 (0.85-2.54) pg/mL and 1.12 (0.89-1.72) pg/mL, respectively] (
Figure 1A; p< 0.05 and p< 0.001; respectively]. Levels of venous ET-1 were 4.04 (2.99-5.48) pg/mL vs. 1.52 (1.12-2.33) pg/mL and 1.40 (0.95-1.93) pg/mL, respectively (
Figure 1B; p< 0.001 and p< 0.0001, respectively).
The arterial:venous (A:V) ratio was 0.63 (0.49-1.02) in our COVID-19-ARDS cohort, 1.06 (0.93-1.20) in the non-COVID-19 ARDS patients, and 0.79 (0.52-1.11) in the non-COVID-19 critically ill patients on ICU admission (
Figure 1C; p< 0.05).
On Day 3 in the COVID-19 patients (N= 10), systemic arterial ET-1 levels remained unchanged as compared to admission [2.31 (1.78-4.60) pg/mL], whereas venous ET-1 levels decreased [2.62 (1.43-5.67) pg/mL], albeit not statistically significantly (p> 0.05). This resulted in a small rise in the A:V ratio [0.98 (0.60-1.17)]. In the two control groups, the A:V ET-1 ratios were 1.00 (0.69-1.16) in the non-COVID-19 ARDS patients (N= 13) and 0.89 (0.76-1.24) in the non-COVID-19 critically ill patients (N= 17) (all p> 0.05).
Figure 2 shows the time progression of the A:V ET-1 ratio in the three groups.
The COVID-19-induced ARDS patient group was then subdivided into survivors (N= 12) and non-survivors (N= 5), according to 28-day ICU mortality. For one patient we did not have the outcome since the patient was transferred to another facility on Day 20 from ICU admission.
Table 2 lists the demographics, clinical characteristics, and laboratory data of survivors and non-survivors on ICU admission. The two patient subgroups differed only in the respiratory rate. The critical illness severity scores, APACHE II and SOFA scores, and PaO
2/FiO
2 did not differ on ICU admission between survivors and non-survivors.
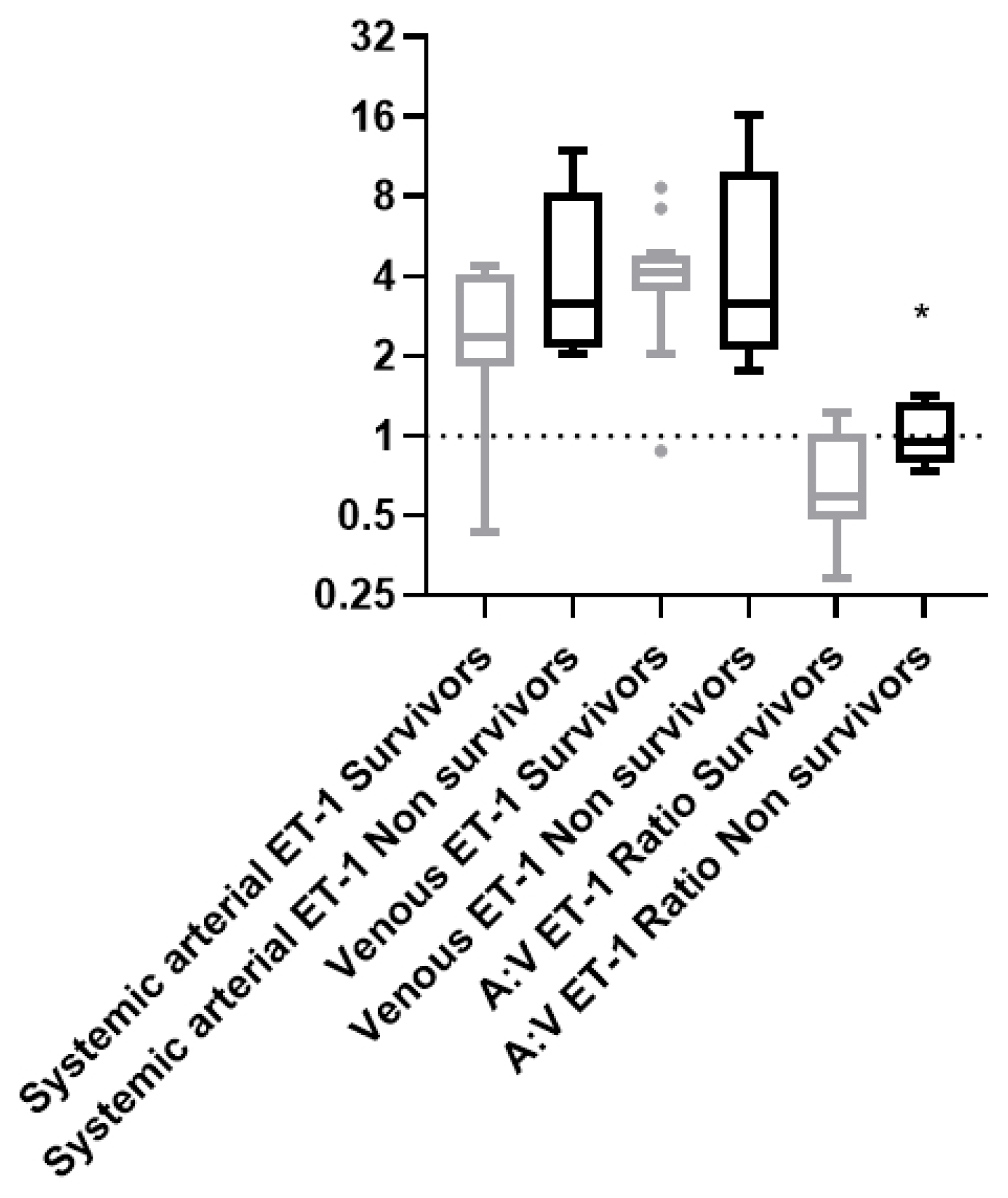
The ET-1 arterial and venous levels by themselves did not differ between survivors and non-survivors [A: 2.37 (1.82-4.09) pg/mL and V: 4.10 (3.54-4.82) pg/mL vs. 3.18 (2.14-8.22) pg/mL and 3.17 (2.10-9.84) pg/mL, respectively, p> 0.05,
Figure 3). However, the A:V ET-1 ratio was higher upon ICU admission in the non-survivors [0.95 (0.78-1.34)] vs 0.57 (0.48-0.92), p= 0.027,
Figure 3].
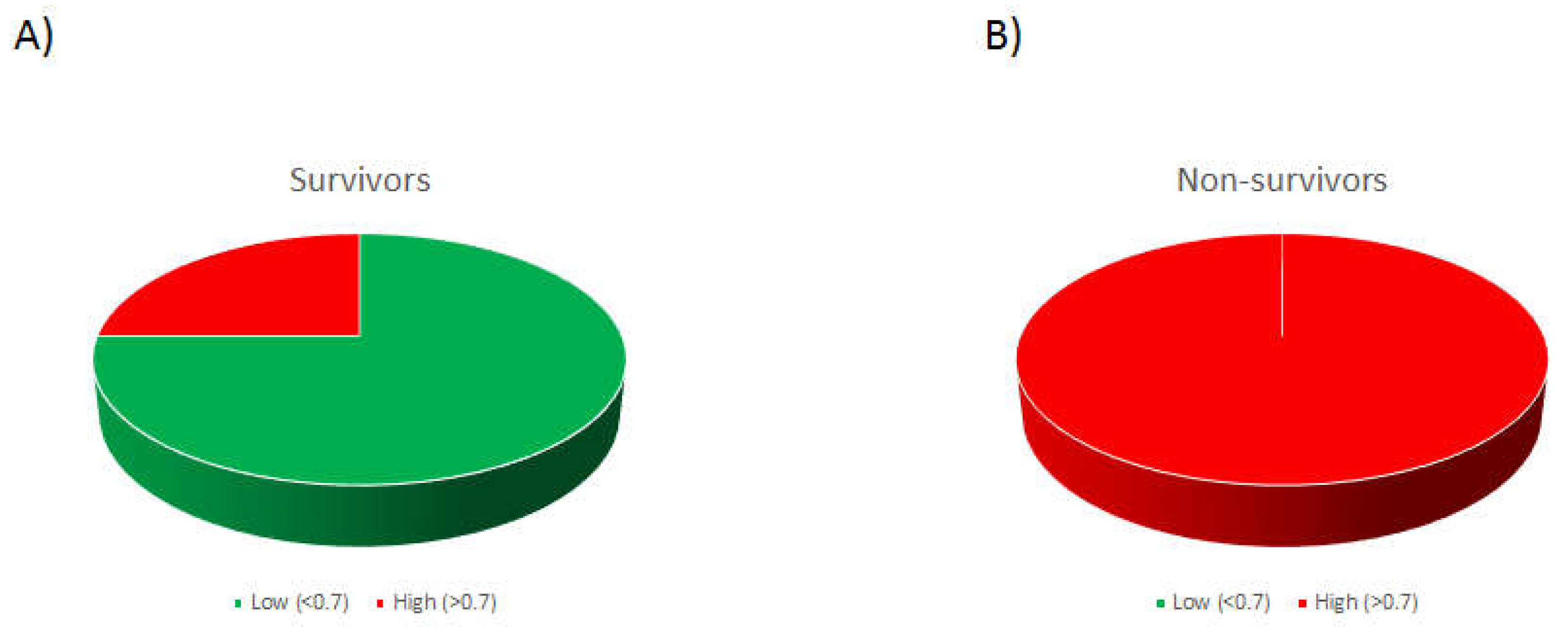
A ROC curve was subsequently generated to test the prognostic value of the A:V ET-1 ratio in 28-day ICU mortality. A cut-off of 0.701 showed a sensitivity of 100% and a specificity of 75%, generating an area under the curve (AUC) of 0.85 (0.66-1.00), p= 0.027. If the patients were assigned to high (above the cut-off) and low (below the cut-off) groups, 75% of the patients who eventually survived were in the low group, whereas 100% of the patients who died were in the high group (chi-square, p= 0.009;
Figure 4).
3. Discussion
There has been extensive previous research on COVID-19 and lung injury, but few reports on ET-1 levels in COVID-19 infection [
18,
19,
20,
21,
22]. However, to our knowledge, the effect of COVID-19 on transpulmonary ET-1 handling, and how abnormalities might relate to prognosis, has not been explored.
Increased plasma ET-1 levels were found in patients hospitalized with COVID-19 [
19], while autoantibodies against the ETA receptor were found in COVID-19 patients with an unfavorable disease course [
18]. Plasma ET-1 levels were significantly higher in acute COVID-19 compared to control subjects and were further elevated 3 months post-COVID-19 [
20]. Blood plasma ET-1 levels were associated with an increased mortality risk during the acute phase of COVID-19, while the association of increased plasma ET-1 levels with COVID-19 mortality risk did not persist after 12 months [
21]. In a randomized, double blind, placebo-controlled trial it was shown that early administration of bosentan, a dual endothelin receptor antagonist (ERA), prevented disease progression and thromboembolic events in high-risk COVID-19 outpatients [
22].
In the present study we found that in COVID-19-induced ARDS, both systemic arterial and venous ET-1 levels were elevated compared to the two control groups, however the COVID-19-induced ARDS group’s A:V ratio was less than 1. In contrast to the study by Turgunova et al. [
21], we were not able to find elevated venous ET-1 levels in the non-survivors, possibly due to our small sample size. However, we were able to differentiate survivors and non-survivors among COVID-19-induced ARDS patients based on their A:V ET-1 ratio on ICU admission. We showed that the ratio was higher on ICU admission in patients who will eventually die. Based on the ROC curve generated for 28-day mortality, the cut-off value for the A:V ET-1 ratio was 0.7. All patients who died had an A:V ratio > 0.7.
Three endothelin subtypes have been recognized, endothelin-1, 2 & 3, each with varying functions that depend on the receptor they bind [
4]. Endothelin-1, a potent vasoconstrictor peptide produced by endothelial cells and degraded predominantly in the pulmonary vasculature, is considered a marker of lung injury. It functions via its binding to the endothelin type A and B receptors (ETA, ETB) of the smooth muscle cells. In general, the largest part of its contractile function acts through the ETA receptors, while in normal conditions, ET-1 is removed from the pulmonary circulation via the ETB receptors found on endothelial cells.
Endothelin-1 has been mostly studied in pulmonary arterial hypertension (PAH), a disease characterized mainly by tissue remodeling of the precapillary pulmonary vasculature, with subsequent right heart failure. In that disorder, ET-1 levels are high, mainly due to excess synthesis rather than reduced clearance [
14,
16,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36].
Increased plasma ET-1 levels were found in ARDS patients compared to healthy control subjects [
15,
37], as a result of either increased synthesis or decreased clearance. These abnormalities reversed in the patients who recovered [
15]. In critically ill patients with sepsis, including ARDS subjects, increased endothelin production contributed to local increases in vascular resistance, hypoperfusion, and the development of organ failure [
38]. It has also been suggested that high circulating ET-1 levels may partly contribute to the development of pulmonary vasoconstriction and bronchoconstriction associated with acute respiratory failure [
39]. Increased ET-1 immunohistochemical staining was seen in the lungs of subjects who died with ARDS [
40], and it was demonstrated that in patients with ARDS, ET-1 is produced mainly in the lung and is associated not only with pulmonary vasoconstriction but also with the development of permeability edema, leading to the impairment of oxygenation [
41]. Studies in animal models have also provided evidence for the involvement of ET-1 in lung injury and the use of endothelin receptor antagonists as potential treatments for human inflammatory lung diseases [
42,
43,
44,
45].
ET-1 levels may fluctuate in chronic pulmonary hypertension. In the setting of ARDS, the patients frequently present with acute pulmonary hypertension and hypoxemia. Studies indicate that ET-1 may contribute to hypoxia-induced pulmonary vasoconstriction [
46,
47]. Positive end-expiratory pressure and positive pressure ventilation may affect ET-1 levels. The two ARDS groups used had similar ventilator settings, suggesting that the differences observed in ET-1 levels may be due to the sequelae of the viral infection itself. Indeed, several studies have shown that the pathophysiology of ARDS caused by COVID-19 is more complex and diverse compared to typical ARDS of “reduced lung volume and decreased compliance” [
5]. Moreover, data presented indicate that the inflammatory profile in COVID-19 patients is different than that observed in patients with either ARDS or sepsis, with concentrations of inflammatory cytokines in both severely and critically ill COVID-19 patients being significantly lower than those in even patients meeting criteria for "hypo-inflammatory ARDS" [
48,
49,
50,
51,
52,
53,
54].
As a surrogate of endothelial dysfunction, we also measured various soluble endothelial activation/damage indices. Our results showed an increase in sICAM-1 in COVID-19 and non-COVID-19 ARDS, however we were not able to establish a correlation between endothelial biomarker levels and ET-1 levels. We believe that this may be since ET-1 levels reflect the synthesis/clearance balance that is mainly pulmonary, while other biomarker levels, measured in peripheral venous blood, might be affected by systemic vascular injury and inflammation.
While ET-1 is taken up and released by the pulmonary circulation, it is also metabolized by hepatocytes. However, this represents a minor component of ET-1 clearance, the great bulk of which is pulmonary. Since most of the central lines were placed in the superior vena cava, it is possible that the blood sampled will not adequately reflect venous return from the lower extremities or the portal circulation, and therefore, any hepatic effects might be missed. None of our patients had overt liver disease, and we hypothesize that its clearance is mainly by the pulmonary circulation.
The limitations of our study must be mentioned. The main limitation was the small sample size but, despite that, we found significant differences amongst the clinical groups. To further assist in understanding our findings, we used two different critically ill cohorts, matched for age, sex, and critical illness severity, to validate our analysis of differences between the groups. Recruitment was hampered by the initial overwhelming patient load and resultant diversion of resources away from research, then by the reduced incidence of lung injury with the arrival of COVID-19 vaccines. Another limitation is that we did not collect swab samples from our patients to isolate and identify the particular variant. Finally, blood sampling in the COVID-19 patients occurred following dexamethasone administration, hence we could not measure cytokines to compare the inflammatory status of the patient groups. Even though COVID-19 has subsided, ICU admissions and mortality due to COVID-19 still exist, and our findings could be applicable to any future strain that becomes more virulent and causes fulminant lung injury as was seen in the pre-vaccine era.
4. Materials and Methods
This observational, case-control, single-center study included consecutive adult, mechanically ventilated patients hospitalized in our intensive care unit (ICU, N= 18) with COVID-19-induced ARDS. Two groups matched for age, sex, and critical illness severity, as assessed by the acute physiology and chronic health evaluation (APACHE) II and sequential organ failure (SOFA) scores, were also included as control groups; a group of SARS-CoV2-negative mechanically ventilated non-ARDS patients (N= 20), mostly with central nervous system (CNS)-related injuries, and a group of SARS-CoV2-negative mechanically ventilated ARDS patients (N= 14). SARS-CoV-2 infection was diagnosed by real-time reverse transcription PCR (RT-PCR) in nasopharyngeal swabs. The only exclusion criterion was the presence of acquired immunodeficiency syndrome. Based on a previous study on ET-1 clearance in ARDS 15, to detect a 40% increase in the systemic arterial: venous ET-1 ratio with a Cohen-d effect size of 0.9, an α-error probability <0.05, and a power >80%, it was estimated that a cohort of 16 critically ill patients and a group of 16 matched control subjects would suffice.
The enrolment took place between September 2021 to September 2023. The study was approved by the Hospital’s Research Ethics Committee (418/9-9-2021) and all procedures carried out on patients were in compliance with the Helsinki Declaration. Informed written consent was obtained from the patients’ next-of-kin.
Criteria for ARDS (Berlin definition) included PaO
2/FiO
2<300 mmHg and bilateral infiltrates in the chest X-ray [
55]. Since ET-1 levels are known to fluctuate in patients with pulmonary hypertension [
14], pre-existing pulmonary hypertension was recorded from the patient’s medical history. Patients with human immunodeficiency virus infection were excluded from the study. Demographics, vital signs, mechanical ventilation settings, arterial blood gases, acute physiology and chronic health evaluation (APACHE II) and sequential organ failure (SOFA) score, comorbidities, pharmaceutical treatment, and routine laboratory data were recorded for all patients enrolled in the study.
A simultaneous blood draw was performed in each patient from a central venous line (jugular or subclavicular vein) and from a peripheral arterial line catheter (radial artery) with a slow 3-minute draw to avoid sample hemolysis, on ICU admission (within 24-hours) and on Day 3 from admission, as has been described [
14,
15]. A second blood draw was not obtained if the patient was discharged earlier, was extubated, or if death occurred. The blood samples were collected in pre-chilled BD Vacutainer® EDTA Tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), were centrifuged, and the resulting plasma was aliquoted and stored at -70οC until use.
Endothelin-1 plasma levels were measured in the venous and systemic arterial blood samples obtained on ICU admission (within 24-hours) and on Day 3 after admission, by enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Minneapolis, MN, USA). The intra-assay coefficient of variability (CV) of the assay was 2.7%, the inter-assay CV 6.3%, with a detection limit of 0.207 pg/mL. The researcher who performed the measurements was blinded to the samples measured.
Soluble (s)E-selectin (intra-assay coefficient of variability (CV) 5.8%, detection limit 0.027 ng/mL), soluble intercellular adhesion molecule 1 (sICAM-1) (CV 4.6%, detection limit 0.254 ng/mL), soluble vascular cell adhesion molecule-1 (sVCAM-1) (CV 3.1%, detection limit 1.26 ng/mL) (R&D Systems Inc., Minneapolis, MN, USA) and von Willebrand factor (vWf) (CV 5.2%, detection limit <50 pg/mL, OriGene Technologies, Inc., Rockville, MD, USA) were measured in plasma samples by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers’ instructions. The assays use two different polyclonal antibodies against the molecules as catching and tagging antibody. The researcher who performed the measurements was blinded to the samples measured.
Data are presented as individual values with percentages (N, %), mean ± standard deviation (SD) for normally distributed variables, and median with interquartile range (IQR) for variables with skewed distribution. Between group comparisons were performed by the t-test or the non-parametric Mann-Whitney test, as appropriate. One-way ANOVA followed by Kruskal-Wallis was performed for more than two group comparison. Associations between qualitative variables were examined by the chi-square test. A receiver operating characteristic (ROC) curve was plotted using 28-day ICU mortality as the classification variable and A:V ET-1 ratio on ICU admission as the prognostic variable. The optimal cut-off value for predicting 28-day mortality was calculated as the point with the greatest combined sensitivity and specificity. All p-values are two-sided; p< 0.05 was considered significant.
5. Conclusions
In our COVID-19-induced ARDS cohort we found high circulating plasma levels of ET-1, but a normal A:V ratio, suggesting that pulmonary ET-1 clearance was intact. However, on ICU admission, the A:V ET-1 ratio was higher in non-survivors compared to survivors, indicating net pulmonary ET-1 release. Our findings suggest that early measurement of pulmonary ET-1 handling may be useful in stratifying outcome in critically ill patients with COVID-19.
Author Contributions
Conceptualization, A.G.V., D.L., and S.E.O..; methodology, A.R., C.K., N.A., S.T., E.J., C.S.V., V.G., A.H., and G.F.; validation, A.G.V., A.R., I.D., A.K., D.L. and S.E.O.; formal analysis, A.G.V., A.R., and C.K.; investigation, A.G.V., A.R., and S.E.O.; resources, I.D., A.K., and S.E.O.; data curation, A.G.V., A.R., C.K., N.A., S.T., E.J., C.S.V., V.G., A.H., and G.F.; writing—original draft preparation, A.R., C.K., N.A., S.T., E.J., C.S.V., V.G., A.H., and G.F.; writing—review and editing, A.G.V., I.D., A.K., D.L. and S.E.O.; visualization, A.G.V., D.L., and S.E.O..; supervision, A.G.V., I.D., A.K. and S.E.O.; project administration, A.G.V., I.D., A.K. and S.E.O.; funding acquisition, A.K. and S.E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of EVANGELISMOS HOSPITAL (protocol code 418 and date of approval 9/9/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank the Nursing Staff of the 1st Department of Intensive Care Medicine for their help and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Jama 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, D.K.; Titus, A.; Caulfield, T.R.; David Freeman, W. Endotheliitis, endothelin, and endothelin receptor blockers in COVID-19. Medical hypotheses 2021, 150, 110564. [Google Scholar] [CrossRef]
- Lu, S.; Huang, X.; Liu, R.; Lan, Y.; Lei, Y.; Zeng, F.; Tang, X.; He, H. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: Similarities and Differences. Front Med (Lausanne) 2022, 9, 829771. [Google Scholar] [CrossRef] [PubMed]
- Beloncle, F.M. Is COVID-19 different from other causes of acute respiratory distress syndrome? J Intensive Med 2023, 3, 212–219. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997, 100, 2153–2157. [Google Scholar] [CrossRef]
- Mather, K.J.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes 2004, 53, 2060–2066. [Google Scholar] [CrossRef]
- Banecki, K.; Dora, K.A. Endothelin-1 in Health and Disease. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- D'Orléans-Juste, P.; Labonté, J.; Bkaily, G.; Choufani, S.; Plante, M.; Honoré, J.C. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther 2002, 95, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Stewart, D.J.; Cernacek, P.; Gosselin, G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation 1996, 94, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Cernacek, P.; Tardif, J.C.; Stewart, D.J.; Gosselin, G.; Dyrda, I.; Bonan, R.; Crépeau, J. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am Heart J 1998, 135, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Levy, R.D.; Cernacek, P.; Langleben, D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 1991, 114, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D.; DeMarchie, M.; Laporta, D.; Spanier, A.H.; Schlesinger, R.D.; Stewart, D.J. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis 1993, 148, 1646–1650. [Google Scholar] [CrossRef]
- Langleben, D.; Dupuis, J.; Hirsch, A.; Giovinazzo, M.; Langleben, I.; Khoury, J.; Ruel, N.; Caron, A. Pulmonary endothelin-1 clearance in human pulmonary arterial hypertension. Chest 2005, 128, 622s. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin 2023, 44, 695–709. [Google Scholar]
- Miedema, J.; Schreurs, M.; van der Sar-van der Brugge, S.; Paats, M.; Baart, S.; Bakker, M.; Hoek, R.; Dik, W.A.; Endeman, H.; Van Der Velden, V.; et al. Antibodies Against Angiotensin II Receptor Type 1 and Endothelin A Receptor Are Associated With an Unfavorable COVID19 Disease Course. Frontiers in immunology 2021, 12, 684142. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.R.; Kuc, R.E.; Althage, M.; Greasley, P.J.; Ambery, P.; Maguire, J.J.; Wilkinson, I.B.; Hoole, S.P.; Cheriyan, J.; Davenport, A.P. Endothelin-1 is increased in the plasma of patients hospitalised with Covid-19. Journal of molecular and cellular cardiology 2022, 167, 92–96. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.H.; Groh, L.A.; Leentjens, J.; Janssen, N.A.F.; Netea, M.G.; Thijssen, D.H.J.; Hannink, G.; et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res 2022, 209, 106–114. [Google Scholar] [CrossRef]
- Turgunova, L.; Mekhantseva, I.; Laryushina, Y.; Alina, A.; Bacheva, I.; Zhumadilova, Z.; Turmukhambetova, A. The Association of Endothelin-1 with Early and Long-Term Mortality in COVID-19. J Pers Med 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Vahdat Shariatpanahi, Z.; Shahbazi, E. Bosentan for high-risk outpatients with COVID-19 infection: a randomized, double blind, placebo-controlled trial. EClinicalMedicine 2023, 62, 102117. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993, 328, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Langleben, D.; Barst, R.J.; Badesch, D.; Groves, B.M.; Tapson, V.F.; Murali, S.; Bourge, R.C.; Ettinger, N.; Shalit, E.; Clayton, L.M.; et al. Continuous infusion of epoprostenol improves the net balance between pulmonary endothelin-1 clearance and release in primary pulmonary hypertension. Circulation 1999, 99, 3266–3271. [Google Scholar] [CrossRef]
- Michel, R.P.; Langleben, D.; Dupuis, J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol 2003, 81, 542–554. [Google Scholar] [CrossRef]
- Langleben, D.; Dupuis, J.; Langleben, I.; Hirsch, A.M.; Baron, M.; Senécal, J.L.; Giovinazzo, M. Etiology-specific endothelin-1 clearance in human precapillary pulmonary hypertension. Chest 2006, 129, 689–695. [Google Scholar] [CrossRef]
- Langleben, D. Endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Clin Chest Med 2007, 28, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Amoura, Z.; Langleben, D. [Treatment of pulmonary arterial hypertension by endothelin receptor antagonists in 2008]. Rev Med Interne 2008, 29, 283–289. [Google Scholar] [CrossRef]
- Star, G.P.; Giovinazzo, M.; Langleben, D. Bone morphogenic protein-9 stimulates endothelin-1 release from human pulmonary microvascular endothelial cells: a potential mechanism for elevated ET-1 levels in pulmonary arterial hypertension. Microvasc Res 2010, 80, 349–354. [Google Scholar] [CrossRef]
- Horn, E.M.; Chakinala, M.; Oudiz, R.; Joseloff, E.; Rosenzweig, E.B. Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm Circ 2020, 10, 2045894020922799. [Google Scholar] [CrossRef]
- Badagliacca, R.; Sciomer, S.; Petrosillo, N. Endothelin receptor antagonists for pulmonary arterial hypertension and COVID-19: Friend or foe? The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2020, 39, 729–730. [Google Scholar] [CrossRef]
- Nabeh, O.A.; Matter, L.M.; Khattab, M.A.; Esraa, M. "The possible implication of endothelin in the pathology of COVID-19-induced pulmonary hypertension". Pulmonary pharmacology & therapeutics 2021, 71, 102082. [Google Scholar] [CrossRef]
- Shilin, D.S.; Shapovalov, K.G. Changes in Some Vascular Biomarkers in Patients with Severe COVID-19 with Various Degrees of Pulmonary Hypertension. Bull Exp Biol Med 2022, 173, 433–436. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, X.; Xu, S. Aprocitentan, a dual endothelin-1 (ET-1) antagonist for treating resistant hypertension: Mechanism of action and therapeutic potential. Drug Discov Today 2023, 103788. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yuan, T.; Wang, R.; Gong, D.; Wang, S.; Du, G.; Fang, L. Insights into Endothelin Receptors in Pulmonary Hypertension. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Kanda, S.; Chatha, U.; Odoma, V.A.; Pitliya, A.; AlEdani, E.M.; Bhangu, J.K.; Javed, K.; Manshahia, P.K.; Yu, A.K. Current Status of Endothelin Receptor Antagonists in Pulmonary Arterial Hypertension: A Combined Study Results and Pharmacology-Based Review. Cureus 2023, 15, e42748. [Google Scholar] [CrossRef] [PubMed]
- Druml, W.; Steltzer, H.; Waldhäusl, W.; Lenz, K.; Hammerle, A.; Vierhapper, H.; Gasic, S.; Wagner, O.F. Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis 1993, 148, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Sanai, L.; Haynes, W.G.; MacKenzie, A.; Grant, I.S.; Webb, D.J. Endothelin production in sepsis and the adult respiratory distress syndrome. Intensive Care Med 1996, 22, 52–56. [Google Scholar] [CrossRef]
- Mitaka, C.; Hirata, Y.; Nagura, T.; Tsunoda, Y.; Amaha, K. Circulating endothelin-1 concentrations in acute respiratory failure. Chest 1993, 104, 476–480. [Google Scholar] [CrossRef]
- Albertine, K.H.; Wang, Z.M.; Michael, J.R. Expression of endothelial nitric oxide synthase, inducible nitric oxide synthase, and endothelin-1 in lungs of subjects who died with ARDS. Chest 1999, 116, 101S–102S. [Google Scholar] [CrossRef]
- Nakano, Y.; Tasaka, S.; Saito, F.; Yamada, W.; Shiraishi, Y.; Ogawa, Y.; Koh, H.; Hasegawa, N.; Fujishima, S.; Hashimoto, S.; et al. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology 2007, 12, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, T.M.; Cerreta, J.M.; Liu, M.; Reznik, S.E.; Cantor, J.O. Phosphoramidon, an endothelin-converting enzyme inhibitor, attenuates lipopolysaccharide-induced acute lung injury. Exp Lung Res 2008, 34, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Magder, S.; Cernacek, P.; Goldberg, P.; Guo, Y.; Hussain, S.N. Endothelin receptor blockade attenuates lipopolysaccharide-induced pulmonary nitric oxide production. Am J Respir Crit Care Med 2000, 161, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, V.; Kirov, M.; Sovershaev, M.; Andreasen, T.; Ingebretsen, O.C.; Ytrehus, K.; Bjertnaes, L. Tezosentan-induced attenuation of lung injury in endotoxemic sheep is associated with reduced activation of protein kinase C. Crit Care 2005, 9, R211–217. [Google Scholar] [CrossRef]
- Manitsopoulos, N.; Nikitopoulou, I.; Maniatis, N.A.; Magkou, C.; Kotanidou, A.; Orfanos, S.E. Highly Selective Endothelin-1 Receptor A Inhibition Prevents Bleomycin-Induced Pulmonary Inflammation and Fibrosis in Mice. Respiration 2018, 95, 122–136. [Google Scholar] [CrossRef]
- Dumas, J.P.; Bardou, M.; Goirand, F.; Dumas, M. Hypoxic pulmonary vasoconstriction. Gen Pharmacol 1999, 33, 289–297. [Google Scholar] [CrossRef]
- Sato, K.; Morio, Y.; Morris, K.G.; Rodman, D.M.; McMurtry, I.F. Mechanism of hypoxic pulmonary vasoconstriction involves ET(A) receptor-mediated inhibition of K(ATP) channel. Am J Physiol Lung Cell Mol Physiol 2000, 278, L434–442. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a "Cytokine Storm" Relevant to COVID-19? JAMA Intern Med 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y.; et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. Jama 2020, 324, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, S.D.; Watchorn, J.; Trovato, F.; Napoli, S.; Mujib, S.F.; Hopkins, P.; McPhail, M. Microcirculatory, Endothelial, and Inflammatory Responses in Critically Ill Patients With COVID-19 Are Distinct From Those Seen in Septic Shock: A Case Control Study. Shock 2021, 55, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Dimopoulou, I.; Jahaj, E.; Keskinidou, C.; Mastora, Z.; Orfanos, S.E.; Kotanidou, A. Selection of the Appropriate Control Group Is Essential in Evaluating the Cytokine Storm in COVID-19. In Vivo 2021, 35, 1295–1298. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O'Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018, 6, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: the Berlin Definition. Jama 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
ICU admission ET-1 levels in the three critically ill groups. Systemic arterial (A) and venous (B) ET-1 was measured in the COVID-19 ARDS (N= 18), non-COVID-19 ARDS (N= 14), and non-COVID-19 non-ARDS (N= 20) patients by ELISA. The arterial:venous (A:V) ET-1 ratio was calculated thereafter (C). The horizontal line represents an A:V ET-1 ratio of 1. Data are presented as box plots. Line in the middle of the box, median value; box edges, 25th to 75th centiles; whiskers, range of values; bullets, outliers. One-way ANOVA followed by Kruskal-Wallis was performed to test for differences between the groups. *p< 0.05, *** p< 0.01, **** p< 0.0001.
Figure 1.
ICU admission ET-1 levels in the three critically ill groups. Systemic arterial (A) and venous (B) ET-1 was measured in the COVID-19 ARDS (N= 18), non-COVID-19 ARDS (N= 14), and non-COVID-19 non-ARDS (N= 20) patients by ELISA. The arterial:venous (A:V) ET-1 ratio was calculated thereafter (C). The horizontal line represents an A:V ET-1 ratio of 1. Data are presented as box plots. Line in the middle of the box, median value; box edges, 25th to 75th centiles; whiskers, range of values; bullets, outliers. One-way ANOVA followed by Kruskal-Wallis was performed to test for differences between the groups. *p< 0.05, *** p< 0.01, **** p< 0.0001.
Figure 2.
Arterial: venous (A:V) ET-1 ratio in the three critically ill groups at two time-points in the ICU. The arterial:venous (A:V) ET-1 ratio is shown on ICU admission (Time-point 1) in the COVID-19 ARDS (N= 18), non-COVID-19 ARDS (N= 14), and non-COVID-19 non-ARDS (N= 20), and Day 3 after admission (Time-point 2) in the COVID-19 ARDS patients (N= 10), the non-COVID-19 ARDS patients (N= 13), and the non-COVID-19 critically ill patients (N= 17). The vertical line represents an A:V ET-1 ratio of 1.
Figure 2.
Arterial: venous (A:V) ET-1 ratio in the three critically ill groups at two time-points in the ICU. The arterial:venous (A:V) ET-1 ratio is shown on ICU admission (Time-point 1) in the COVID-19 ARDS (N= 18), non-COVID-19 ARDS (N= 14), and non-COVID-19 non-ARDS (N= 20), and Day 3 after admission (Time-point 2) in the COVID-19 ARDS patients (N= 10), the non-COVID-19 ARDS patients (N= 13), and the non-COVID-19 critically ill patients (N= 17). The vertical line represents an A:V ET-1 ratio of 1.
Figure 3.
ICU admission ET-1 levels and ratio and 28-day ICU mortality. Systemic arterial, venous, and A:V ET-1 ratio in the 28-day ICU survivors and non survivors of the COVID-19 ARDS patients. Systemic arterial and venous ET-1 was measured in the COVID-19 ARDS survivors (N= 12) and non-survivors (N= 5) by ELISA. The arterial:venous (A:V) ET-1 ratio was calculated thereafter. The horizontal line represents an A:V ET-1 ratio of 1. Data are presented as box plots. Line in the middle of the box, median value; box edges, 25th to 75th centiles; whiskers, range of values; bullets, outliers. * p< 0.05 by Mann-Whitney.
Figure 3.
ICU admission ET-1 levels and ratio and 28-day ICU mortality. Systemic arterial, venous, and A:V ET-1 ratio in the 28-day ICU survivors and non survivors of the COVID-19 ARDS patients. Systemic arterial and venous ET-1 was measured in the COVID-19 ARDS survivors (N= 12) and non-survivors (N= 5) by ELISA. The arterial:venous (A:V) ET-1 ratio was calculated thereafter. The horizontal line represents an A:V ET-1 ratio of 1. Data are presented as box plots. Line in the middle of the box, median value; box edges, 25th to 75th centiles; whiskers, range of values; bullets, outliers. * p< 0.05 by Mann-Whitney.
Figure 4.
Distribution of ICU admission A:V ET-1 ratio in COVID-19 ARDS survivors and non-survivors. Distribution of A:V ET-1 ratio in COVID-19 ARDS survivors (A) and non-survivors (B). COVID-19 ARDS patients were assigned in two groups, low (green colour) and high (red colour), based on the cut-off value generated by the ROC curve (0.701). Distribution is shown as a 3D pie chart. p= 0.009 by chi square test.
Figure 4.
Distribution of ICU admission A:V ET-1 ratio in COVID-19 ARDS survivors and non-survivors. Distribution of A:V ET-1 ratio in COVID-19 ARDS survivors (A) and non-survivors (B). COVID-19 ARDS patients were assigned in two groups, low (green colour) and high (red colour), based on the cut-off value generated by the ROC curve (0.701). Distribution is shown as a 3D pie chart. p= 0.009 by chi square test.
Table 1.
Demographics, clinical characteristics, biochemical and laboratory data of the 3 patient groups on ICU admission.
Table 1.
Demographics, clinical characteristics, biochemical and laboratory data of the 3 patient groups on ICU admission.
| Characteristics |
COVID-19 ARDS |
Non-COVID-19 ARDS |
Non-COVID-19 non-ARDS |
| Number of patients, N |
18 |
14 |
20 |
| Age (years) |
73 (66-78) |
64 (48-76) |
62 (45-73) |
| Sex, N (%) |
|
|
|
| Male |
13 (72.2) |
7 (50.0) |
11 (55.0) |
| Female |
5 (27.8) |
7 (50.0) |
9 (45.0) |
| Comorbidities, N (%) |
|
|
|
| Hypertension |
11 (61.1) |
7 (50.0) |
6 (30.0) |
| Dyslipidemia |
4 (22.2) |
4 (28.6) |
5 (25.0) |
| Diabetes |
4 (22.2) |
6 (42.9) |
3 (15.0) |
| Coronary disease |
4 (22.2) |
2 (14.3) |
2 (10.0) |
| Cancer |
3 (16.7) |
1 (7.1) |
4 (20.0) |
| COPD |
2 (11.1) |
0 (0.0) |
1 (5.0) |
| Chronic renal failure |
0 (0.0) |
2 (14.3) |
0 (0.0) |
| Liver failure |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| APACHE II |
16 (10-21) |
16 (13-23) |
15 (11-20) |
| SOFA |
8 (6-9) |
10 (9-10) |
7 (6-10) |
Admission diagnosis
Medical
Surgical
Trauma |
18 (100.0)
0 (0.0)
0 (0.0) |
3 (21.4)
11 (78.6)
0 (0.0) |
7 (35.0)
6 (30.0)
7 (35.0) |
| Mean arterial pressure (mmHg) |
92 (85-102) |
80 (76-88) ##
|
85 (79-90) #
|
| White blood cell count (per μL) |
9930 (8165-13175) |
12545 (7960-16590) |
14680 (8650-18450) |
| Neutrophlils (%) |
88.2 (81.2-92.3) |
86.3 (76.3-90.9) |
89.6 (76.0-94.5) |
| Platelets (per μL) |
265500 (118000-371250) |
220000 (158500-321500) |
232500 (169250-281500) |
| PO2 (mmHg) |
98.3 (76.6-117.8) |
108.6 (85.6-145) |
124.5 (92.2-167.0) |
| HCO3 (mEq/L) |
22.9 (20.0-25.8) |
22.1 (19.7-24.6) |
21.4 (19.4-23.2) |
| PaO2/FiO2 (mmHg) |
131 (95-177) |
175 (100-209) |
310 (202-381) #
|
| PCO2 (mmHg) |
44.3 (40.8-50.8) |
51.6 (38.7-56.0) |
37.3 (34.5-42.3) # §
|
| Urea (mg/dL) |
55 (47-67) |
56 (33-87) |
32 (26-43) # §
|
| Creatinine (mg/dL) |
0.95 (0.68-1.33) |
1.10 (0.68-2.88) |
0.80 (0.60-0.98) |
| Total bilirubin (mg/dL) |
0.69 (0.39-1.37) |
0.55 (0.30-1.10) |
0.63 (0.52-0.80) |
| LDH (U/L) |
431 (449-615) |
228 (215-489) ##
|
328 (255-425) #
|
| CRP (mg/dL) |
9.8 (6.2-13.4) |
17.3 (6.3-28.0) |
6.5 (2.0-13.8) §
|
| Fibrinogen (mg/dL) |
623 (527-718) |
575 (424-763) |
418 (255-535) ### §
|
| pH |
7.33 (7.29-7.36) |
7.29 (7.24-7.38) |
7.37 (7.31-7.42) |
| Inotrope dose (mL/h) |
5 (4-7) |
12 (4-25) #
|
12 (4-20) |
| Tidal volume (VT) (mL) |
420 (408-463) |
450 (380-500) |
440 (405-480) |
Respiratory rate (RR)
(mechanical breaths/min) |
27 (22-29) |
24 (20-30) |
22 (18-26) #
|
| Positive end-expiratory pressure (PEEP) (cmH2O) |
12 (10-13) |
11 (9-15) |
8 (6-10) #### §
|
| VC, N (%) |
18 (100.0) |
14 (100.0) |
19 (95.0) |
| sICAM-1 (ng/mL) |
630 (482-1014) |
595 (490-1464) |
447 (293-650) # §
|
| sVCAM-1 (ng/mL) |
1488 (797-2166) |
1395 (933-2414) |
1147 (484-1703) |
| sE-selectin (ng/mL) |
78.9 (60.4-108.8) |
142.9 (107.3-226.5) ##
|
67.6 (57.1-117.9) |
| vWf (pg/mL) |
12313 (4581-15254) |
7558 (4637-18020) |
10632 (3723-17280) |
| ICU Outcomes |
|
|
|
| Length of ICU stay |
28 (6-40) |
25 (8-51) |
14 (9-25) |
| Mortality, N (%) |
5 (27.8) |
4 (28.6) |
2 (10.0) |
Table 2.
Demographics, clinical characteristics, and laboratory data of the COVID-19 ARDS survivors and non survivors on ICU admission.
Table 2.
Demographics, clinical characteristics, and laboratory data of the COVID-19 ARDS survivors and non survivors on ICU admission.
| Characteristics |
Survivors |
Non-survivors |
p-value |
| Number of patients, N |
12 |
5 |
|
| Age (years) |
73 (63-79) |
74 (65-78) |
>0.99 |
| Sex, N (%) |
|
|
>0.99 |
| Male |
8 (66.7) |
4 (80.0) |
|
| Female |
4 (33.3) |
1 (20.0) |
|
| Comorbidities, N (%) |
|
|
>0.99 |
| Hypertension |
8 (66.7) |
3 (60.0) |
|
| Dyslipidemia |
3 (25.0) |
1 (20.0) |
|
| Diabetes |
3 (25.0) |
0 (0.0) |
|
| Coronary disease |
3 (25.0) |
1 (20.0) |
|
| Cancer |
2 (16.7) |
1 (20.0) |
|
| COPD |
1 (8.3) |
1 (20.0) |
|
| APACHE II |
15 (10-19) |
20 (9-23) |
0.51 |
| SOFA |
7 (4-9) |
7 (6-12) |
0.44 |
| Mean arterial pressure (mmHg) |
92 (88-101) |
100 (81-118) |
0.65 |
| White blood cell count (per μL) |
9930 (9025-12498) |
12870 (4920-24895) |
0.72 |
| Neutrophlils (%) |
87.2 (81.5-91.3) |
92.9 (73.9-93.5) |
0.16 |
| Platelets (per μL) |
265500 (135500-385750) |
317000 (54000-604500) |
0.89 |
| PCO2 (mmHg) |
44.3 (41.4-48.3) |
41.0 (36.3-65.0) |
0.65 |
| HCO3 (mEq/L) |
21.9 (19.7-26.6) |
25.2 (21.0-25.9) |
0.57 |
| PaO2/FiO2 (mmHg) |
125 (83-193) |
132 (107-195) |
0.88 |
| Glucose (mg/dL) |
179 (139-220) |
137 (120-188) |
0.16 |
| Urea (mg/dL) |
52 (40-57) |
65 (57-80) |
0.06 |
| Creatinine (mg/dL) |
0.8 (0.6-1.3) |
1.2 (1.0-1.3) |
0.13 |
| Total bilirubin (mg/dL) |
0.7 (0.4-0.9) |
1.5 (0.4-1.9) |
0.51 |
| LDH (U/L) |
431 (344-589) |
566 (372-4170) |
0.51 |
| CRP (mg/dL) |
10.9 (6.5-14.1) |
8.2 (5.4-24.8) |
0.57 |
| pH |
7.32 (7.29-7.36) |
7.35 (7.24-7.40) |
0.51 |
| Inotrope dose (mL/h) |
5 (3-5) |
4 (3-31) |
0.79 |
| Tidal volume (VT) (mL) |
430 (420-465) |
410 (375-465) |
0.51 |
| Respiratory rate (RR) (mechanical breaths/min) |
24 (21-28) |
28 (28-30) |
0.04* |
| Positive end-expiratory pressure (PEEP) (cmH2O) |
12.0 (10.3-14.8) |
10.0 (8.0-12.5) |
0.23 |
| Dexamethasone administration, N (%) |
10 (83.3) |
4 (80.0) |
>0.99 |
| Day of death |
N/A |
6 (4-23) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).