1. Introduction
Cestodes, or tapeworms, are an ample and diversified group of obligate endoparasites able to infect a multitude of animal species worldwide. Several members of the class Cestoda are of medical importance since they use humans and other animals as definitive and/or intermediate hosts, causing serious diseases. Among them,
Echinococcus granulosus sensu lato and
Echinococcus multilocularis are the etiological agents of cystic and alveolar echinococcosis, respectively; and
Taenia solium causes cysticercosis. These diseases are among the 20 neglected tropical diseases prioritized by the World Health Organization, that mainly affect people living in poverty principally in tropical and subtropical regions [
1]. Currently, the chemotherapeutic options to treat these parasitosis are very limited, relying mainly in the benzimidazoles albendazole and mebendazole, and praziquantel. Benzimidazoles are heterocyclic aromatic compounds formed by the fusion of benzene and imidazole rings. The main mode of action is the selective binding to helminth β-tubulin thereby inhibiting its polymerization and the formation of microtubules, hampering glucose uptake and other cellular functions [
2]. Praziquantel is a derivative of pyrazinoquinoline. The mechanism of action of praziquantel is not completely defined. It is known that praziquantel induces high rates of calcium ion influx which results in uncontrolled muscle contraction and paralysis of the worms. The postulated drug targets that trigger this calcium ingress are voltage-gated calcium channels [
3], or more recently a transient receptor potential melastatin ion channel (TRPM) [
4]. These drugs present some disadvantages in terms of safety, tolerance, efficacy, length of treatment and access for the affected population. For example, 20%-40% inefficacy was reported for benzimidazoles in the treatment of human cystic echinococcosis [
5,
6], and approximately 60% for alveolar echinococcosis [
7]. Albendazole is only parasitostatic and not parasiticide for
E. multilocularis, implying that a long-term treatment, often life-long, with concomitant adverse effects, is needed to treat alveolar echinococcosis. Currently, the availability of these drugs is low and the cost is high in many endemic countries [
8]. This scenario highlights the need for new, effective, safe and affordable drugs to control these cestodiases.
One of the main limitations of research on cestodes is the low availability of parasitic material. Some zoonotic cestodes, such as
E. granulosus s. l., cannot be reproduced continuously in experimental models being obtained only from natural infections, which makes the implementation of systematic drug evaluation studies difficult.
Mesocestoides vogae is a cestode whose tetrathyridium (TTy) larval stage can multiply continuously in laboratory animals, providing a continuous and reproducible source of material for biological assays. Furthermore, it is easily cultivated in vitro and is considered non-zoonotic so it can be handled safely [
9,
10]. This parasite has been validated as a laboratory model [
11], being used for development studies [
12,
13,
14], and for the identification of new cestocidal compounds in pharmacological studies [
15,
16].
Natural products have made a significant contribution to the development of medicines against a variety of pathologies. It is estimated that more than 50% of the drugs currently on the market are derived from natural sources or were inspired by nature [
17]. In this sense, natural products represent an extensive source of molecules. It is estimated that out of the total of 300,000 plant species inhabiting the Earth, only 6% have been investigated for their pharmacological properties. Furthermore, the molecular complexity and diversity of these compounds, inherent to the biosynthetic machinery of a living organism, are incomparable to the structural possibilities of synthetic molecules. Molecular complexity is often accompanied by highly selective and specific biological activities, as seen in cases such as taxol and artemisinin [
18]. These properties make natural products highly attractive for the discovery of new lead molecules in drug design for the development of new medicinal treatments.
The
Stevia genus (Asteraceae) consists of more than 230 plant species which are mainly distributed from the Southern United States to the South American Andean region.
Stevia rebaudiana Bertoni is the most popular member of this genus since it is known to produce the diterpene glycoside sweetener, stevioside. Beyond
S. rebaudiana, numerous other species belonging to the
Stevia genus are recognized for their medicinal properties [
19]. Ethnobotanical records of
Stevia are described in the book “Natural History of Plants of the New Spain, written between 1570 and 1576 by Francisco Hernandez [
20].
Stevia species have been popularly employed for the treatment of various ailments since the 18th century [
19]. Popularly drinks such as decoctions and infusions have been used as febrifuges, to treat inflammation, to cure wounds and for intestinal upsets due to parasites, among other uses [
20].
Stevia genus is characterized by the presence of sesquiterpene lactones, diterpenes, longipinanes, and flavonoids as main phytochemical constituents. Extracts and compounds isolated from species of this genus have demonstrated diverse pharmacological activities, being antioxidant, antiviral, anti-inflammatory, and antiproliferative, the most frequently reported [
21]. Within the genus, several species have shown antiparasitic activity, including phytochemicals isolated from
S. satureiifolia var. satureiifolia,
S. alpina and
S. maimarensis that have demonstrated trypanocidal properties [
22,
23,
24,
25] and the extracts of
S. satureiifolia var. satureiifolia,
S. entreriensis,
S. multiaristata and
S. aristata which have exerted trypanocidal, leishmanicidal, and/or anti-
Echinococcus granulosus activities [
25,
26,
27].
Considering the potential of natural products in the drug discovery process and the antiparasitic activity demonstrated by some species of Stevia genus, the aim of this work was to analyze the effect of Stevia extracts and isolated compounds on M. vogae in the search for selective and effective drugs to treat neglected tropical diseases caused by cestodes.
2. Results
2.1. Stevia Extracts
Before the evaluation of the anti-DENV-2 activity, the pure natural compounds were
The dichloromethane extracts of
S. alpina,
S. multiaristata,
S. maimarensis and
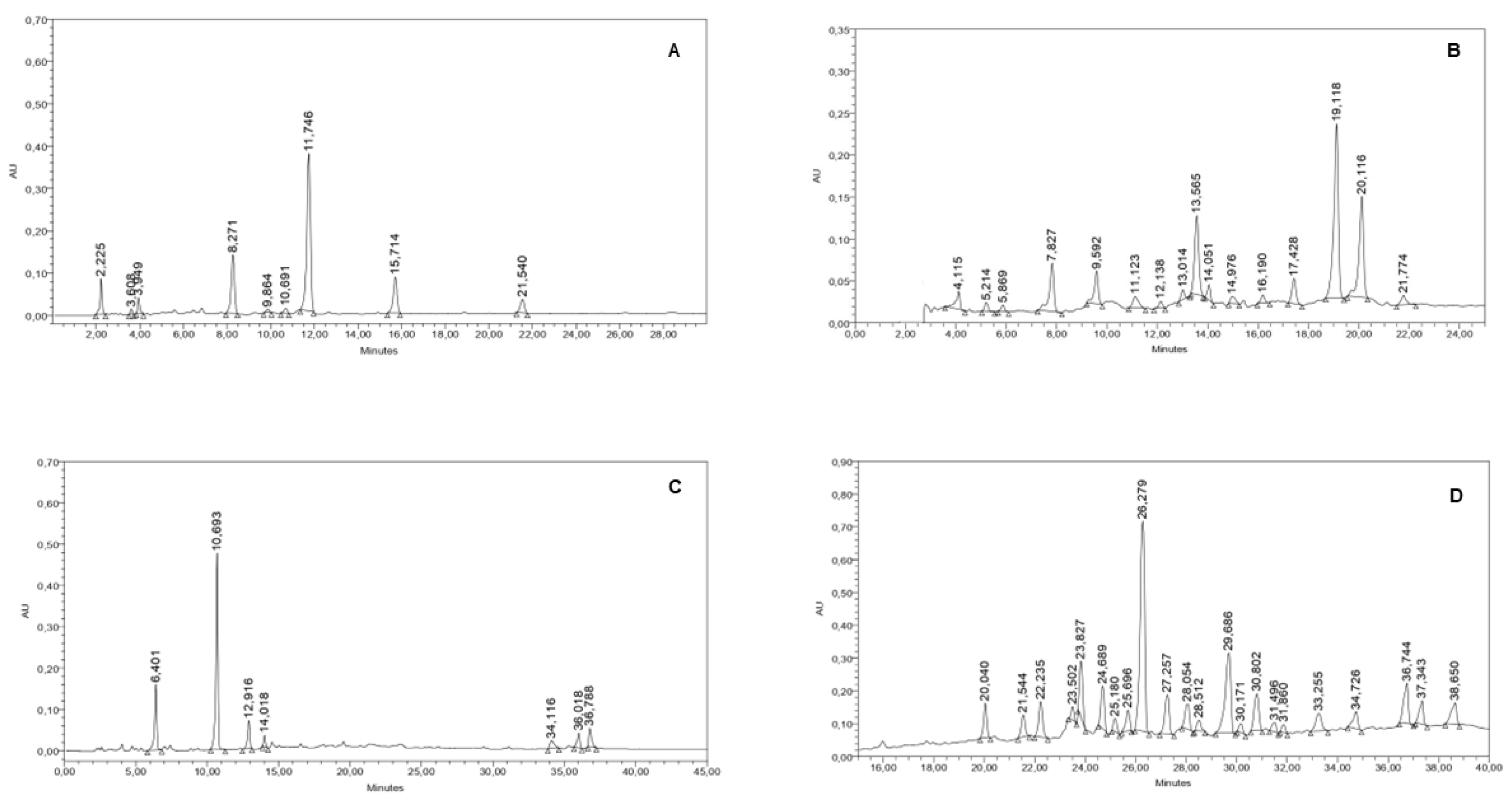
S. aristata showed yields from the dried plant material of 5.0, 16.6, 5.3 and 2.5%, respectively. The analysis of the chemical composition of the extracts was performed by HPLC coupled with a UV-diode-array detector (DAD). The chromatograms obtained can be seen in
Figure 1.
The dichloromethane crude extract of
S. alpina showed nine peaks. A major peak of 52.43% of the total peaks area was observed at a Rt (Retention time) of 11.746 min (
Figure 1A). This compound showed an UV spectrum with a maximum absorption (λmax) at 210 nm. After processing the extract as described in subsection 2.4, the major component of
S. alpina was purified and identified as compound 1. The chromatogram of
S. multiaristata organic crude extract showed sixteen peaks. Three major peaks at Rts of 19.119, 20.116 and 13.565 min, representing 32.39, 18.76 and 13.56 % of the total peaks area, were detected (
Figure 1B). The dichloromethane crude extract of
S. maimarensis showed seven peaks with one major peak that represented 53.35% of the total peaks area at a Rt of 10.693 min (
Figure 1C). The UV spectrum of this peak did not show a specific pattern with a λmax at 212 nm. This phytochemical was later purified as explained in subsection 4.4 and identified as compound 2. The
S. aristata organic crude extract presented 22 peaks. A major peak which represented 28.25 % of the total peak area was observed at a Rt of 26.279 min. This compound showed an UV spectrum with maximum absorptions at 276 and 340 nm (
Figure 1D).
2.2. Pure Phytochemicals Isolated from Stevia Alpina and S. maimarensis
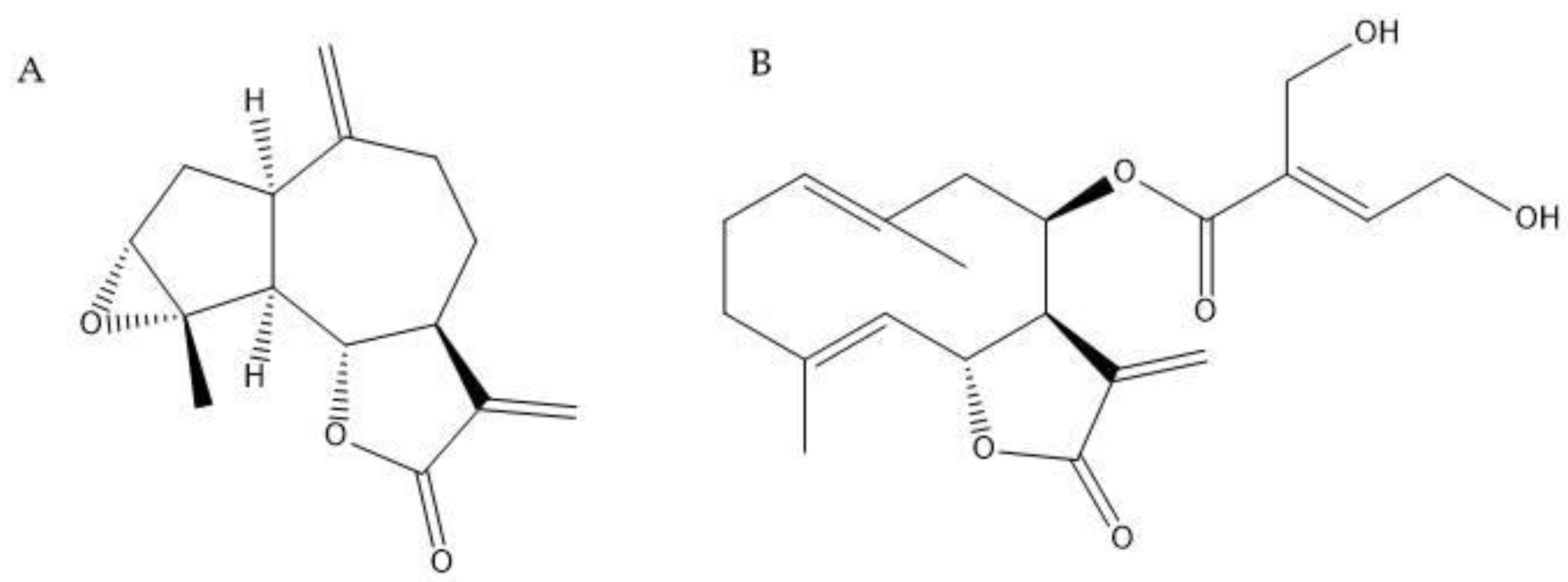
The sesquiterpene lactones estafietin (compound 1) and eupatoriopicrin (compound 2) were isolated from the aerial parts of
S. alpina and
S. maimarensis, respectively. These compounds were identified by spectroscopic methods comparing the experimental spectra with those found in the literature [
28,
29]. The chemical structures of 1 and 2 can be seen in
Figure 2. The purity levels of these phytochemicals were 93.3 and 94.6 % for compounds 1 and 2, respectively.
2.3. Effect of Stevia Extracts and Compounds on Cestode Viability
As detailed in subsection 4.9, the cestocidal activity of
Stevia species extracts and compounds was evaluated with a worm tracker device that quantifies
M. vogae TTy motility. Additionally, the alterations on TTy structures were recorded daily with a camera attached to an inverted optical microscope. The morphology of
M. vogae TTy larvae, when obtained from the mouse peritoneal cavity, consists of an anterior scolex with four suckers as attachment organs; an internal region with a compact, acoelomate and non-segmented parenchyma; and a well-defined external syncytial (multinucleate) tegument that covers the body [
30].
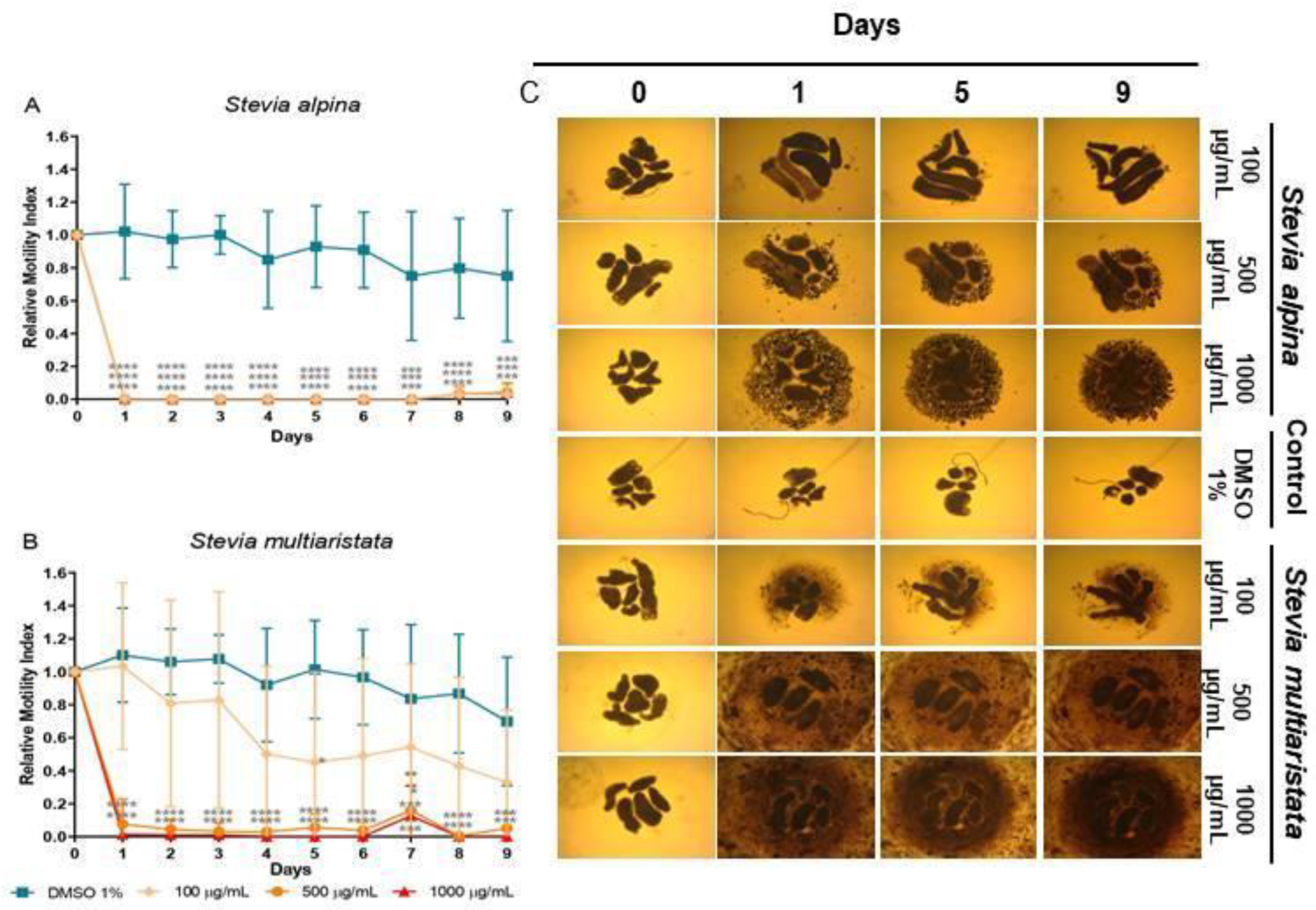
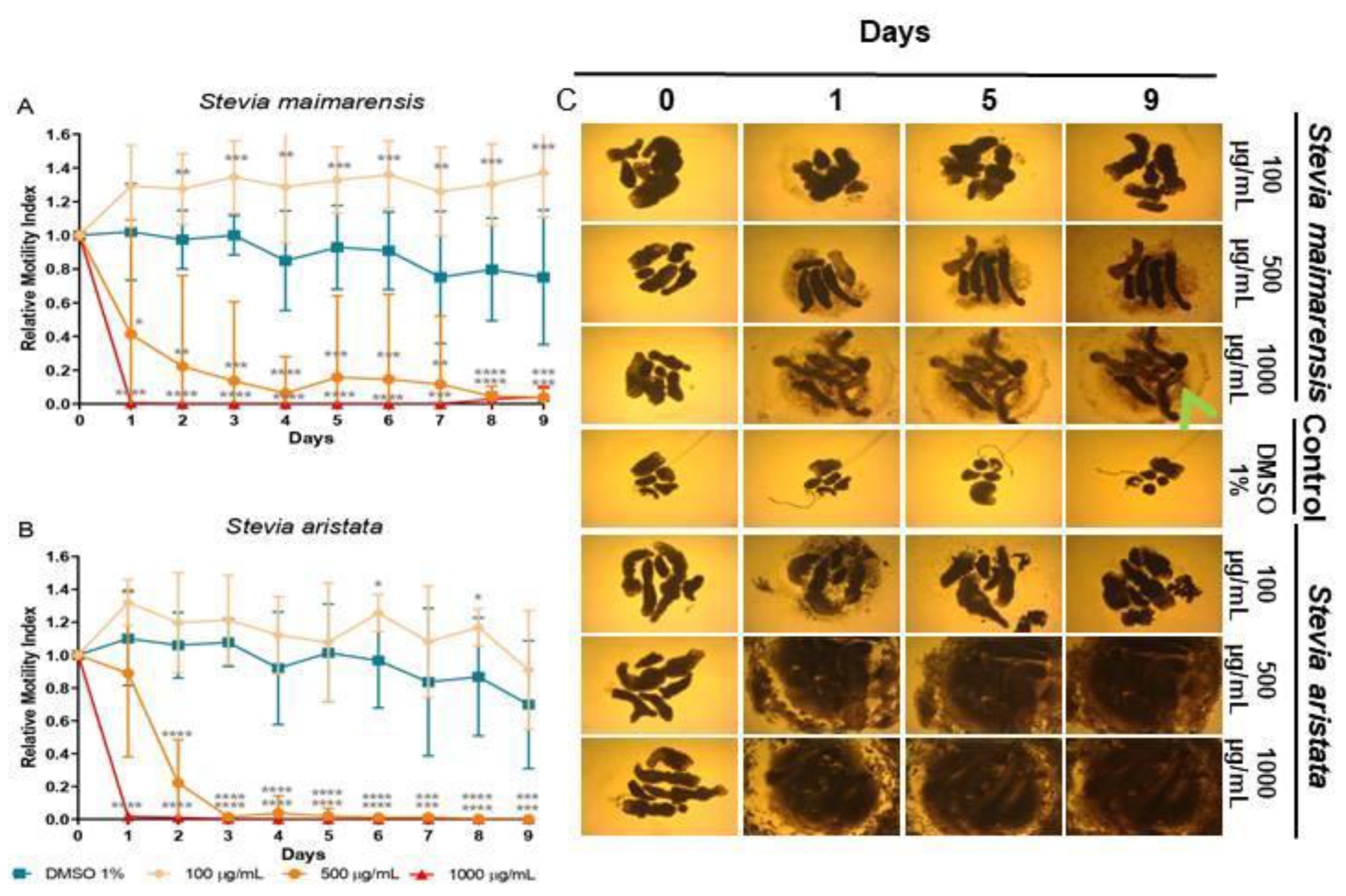
Stevia alpina extract showed the highest cestocidal activity of the four species analyzed here. This extract showed almost complete reduction of viability from day 1 at all concentrations tested (96-100%, p<0.0001 days 1-9 for all concentrations) (
Figure 3A). The other three extracts showed a dose-dependent response. At high concentration (1000 μg/mL) they killed TTy from day 1 (96-100%, p<0.001-P<0.0001, days 1-9) (
Figures 3B, 4A and 4B).
S. multiaristata showed a high cestocidal activity also at 500 μg/mL (84-97%, p<0.001-p<0.0001, days 1-9) (
Figure 3B).
S. maimarensis and
S. aristata, showed a similar cestocidal activity. At 500 μg/mL it was observed an initial reduction of viability (
S. maimarensis: 59%, p<0.05, day 1; 78%, p<0.01, day 2;
S. aristata: 78%, p<0.0001, day 2) and a delayed high cestocidal action (days 3-9:
S. maimarensis: 84-96%, p<0.01-p<0.001;
S. aristata: 96-100%, p<0.001-p<0.0001). An unexpected stimulatory effect was displayed at 100 μg/mL (
S. maimarensis: 26-37% of increase, p<0.01-p<0.001, days 2-9) (
Figure 4A) (S. aristata: 8% of increase on days 6 and 8, p<0.05) (
Figure 4B).
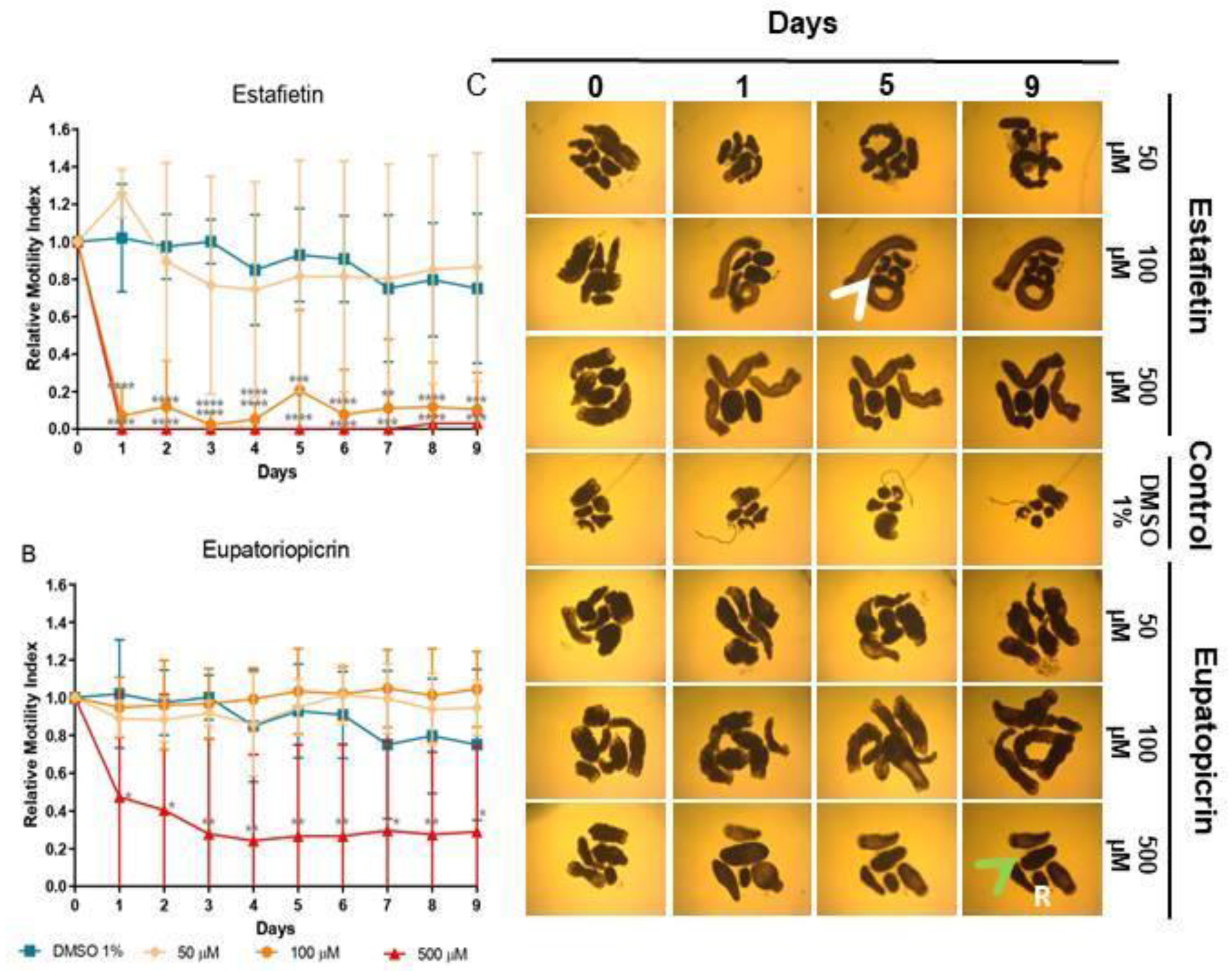
Estafietin, the main compound of
S. alpina, induced a strong dose-dependent reduction of viability at 500 (97-100%, p<0.001-p<0.0001) and 100 μM (79-97%, p<0.001-p<0.0001), with no effect at 50 μM (
Figure 5A). Eupatoriopicrin was less effective than estafietin. This compound, obtained from
S. maimarensis, showed at 500 μM a delayed reduction of viability (53%, day 1 60%, day 2; 71-76%, days 3-9; p<0.05-p<0.01). Unlike the parent extract, that produced an 8% increase of viability, TTy treated with lower concentrations of this terpene displayed similar values as negative control parasites (
Figure 5B).
The results of microscopical observations confirmed those obtained with the WMicrotracker. At concentrations of extracts or compounds that reduced viability, we observed a lack of motility with an evident alteration of general morphology, compared with untreated parasites (Figures 3C, 4C and 5C). The main change observed in S. alpina, S. maimarensis and S. aristata was an elongation of the body, in addition to a loss of tegumental definition. Also, cellular debris was observed in the culture medium. TTys treated with S. multiaristata looked more compact (tegumental debris couldn’t be observed because of the extract precipitation), and some alterations of morphology and extensive damage in the tegument that lines the worms were observed.
3. Discussion
The scarcity of safe, effective and affordable drugs to treat diseases caused by cestodes such as echinococcosis and cysticercosis, which mainly affect vulnerable populations, highlights the importance of finding new treatment alternatives. In previous works, we assessed the effect of extract of plants from the Asteraceae family on
E. granulosus sensu stricto (s.s.) protoscoleces, and cysts obtained from the murine experimental model and observed that the extracts from
S. aristata [
26] and
S. multiaristata [
27] showed
in vitro protoscolicidal potential. Both
Stevia extracts also caused damage to the germinal layer of murine cysts and produced a significant reduction in the parasitic mass obtained from mice infected with
E. granulosus s.s. protoscoleces. Based on these findings, we hypothesized that major compounds of
Stevia extracts could have cestocidal potential. To test this hypothesis, we evaluated the potential of the mentioned
Stevia extracts and two additional ones from
S. alpina and
S. maimarensis, to reduce the viability of
M. vogae TTy. Then, we isolated the major compounds of two of these extracts and evaluated their cestocidal potential on these parasites. The cestocidal effect was measured by the quantification of worm motility using the worm microtracker device [
31] adapted to cestodes [
32,
33,
34,
35]. This method allows the simultaneous evaluation of a high number of compounds, objectively and quantitatively. It also enables continuous, real-time, and non-invasive measurements, facilitating the assessment of parasitic viability on the same plate throughout the entire testing period. For the assay, we used
M. vogae TTy, since this is a cestode laboratory model that allows the implementation of systematic drug evaluation studies. The results obtained showed that all the extracts tested produced a high and significant reduction of
M. vogae TTy viability at the highest concentration tested (1000 µg/mL). At lower concentrations
S. alpina was the most potent, reducing parasite viability by more than 95%, from the first day of incubation.
Estafietin, the major compound present in
S. alpina extract, showed a high cestocidal potential being able to kill 100% of parasites at the highest concentration tested -500 µM- and more than 76% of parasites at 100 µM, even from the first day of incubation. On Vero cells, this compound presented a 50% cytotoxicity concentration (CC
50) value of 800.8 µM [
36], thus indicating it does not show overt toxicity against this human cell line. The early effect of this compound suggests that it could help shorten cestocidal treatments. This is of major importance taking into consideration the need for prolonged treatment in echinococcosis treatments with the currently used drug albendazole. Using the same parasite model and motility measure method as in this work, we have previously shown a delayed effect for albendazole at 20 µM which induced 50% viability reduction only from day 4 of incubation [
28]. Eupatoriopicrin, isolated from
S. maimarensis extract, showed cestocidal capacity although it was less potent and selective than estafietin (CC
50=257.7 µM) [
36]. This compound reduced parasite motility or did not change it, according to the concentration used. It did not increase parasite motility, suggesting that the low increase in motility observed with
S. maimarensis could be due to other/s compounds present in the extract. Both estafietin and eupatoriopicrin are sesquiterpene lactones (STLs), which are a class of terpenoid compounds, mainly found in species of the Asteraceae family. This group of phytochemicals presents a fifteen-carbon backbone (C15) with a ɣ-lactone ring closed toward C6 or C8 and a methylene group conjugated to the carbonyl group, forming an ɑ,β-unsaturated ɣ-lactone moiety [
37]. According to their skeletal arrangement estafietin and eupatoriopicrin are classified as guaianolide and germacranolide type, respectively. STLs have been thoroughly studied due to their wide range of biological activities. These activities include antimicrobial, antitumor, anti-inflammatory, molluscicidal, antihelminthic, antiprotozoal, among others [
29]. Moreover, STLs play a crucial role in plant-insect interactions, serving as attractants, deterrents, and antifeedants. Initially considered highly cytotoxic, chemical modifications have enhanced their biological activities while reducing their cytotoxicity, renewing interest in them as lead compounds in the drug discovery process.
Several studies investigated the anthelmintic properties of plant extracts or essential oils [
38]. However, only a few of them have focused on the effect of isolated compounds from plants. Concerning STLs, some studies of their effect on
E. multilocularis were reported. Since dihydroartemisinin and artesunate were effective against
E. multilocularis metacestodes
in vitro but not
in vivo (mouse model), the
in vitro effects of synthetic ozonides (1,2,4-trioxolanes) were investigated. These compounds were shown to induce structural alterations in the parasites [
39]. As far as we know there are no further reports of the effects of STLs on cestodes.
Herein, we showed that estafietin, as well as its parent extract of S. alpina, produced an early and potent in vitro cestocidal effect. Experiments to assess their in vivo effect in animal models will be conducted in the future. The results of this work suggest that sesquiterpene lactones, terpenoid compounds present in Asteraceae, such as Stevia species, alone or in combination with currently used drugs, could be considered potential candidates for the development of new medicines to treat neglected diseases caused by cestode parasites.
4. Materials and Methods
4.1. Plant Materials
The aerial parts of the
Stevia species used in this work were collected from different locations within Argentine territory. In order to preserve the genetic resource and the natural ecosystem, these plant materials were harvested conservatively, cutting 10-15% of the aerial parts with scissors [
24].
Stevia maimarensis (Hieron.) Cabrera was collected in Jujuy Province, Tilcara Department: Perchel, in March 2017.
Stevia alpina Griseb. was collected in Catamarca Province, Provincial Route 307, Km 49, in April 2015.
Stevia aristata D. Don ex Hook. & Arn. was collected in Entre Ríos Province, La Paz Department, Provincial Route 1, Esquivel stream, in December 2012.
Stevia multiaristata Spreng. was collected in Entre Ríos Province, Paraná Department, Pueblo Brugo, Paraná River in December 2012. The identification of these wild species was conducted by the renowned taxonomists Dr. Gustavo Giberti and Hernan Bach. Voucher specimens are available at the Museo de Farmacobotánica “Juan A. Domínguez”, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Argentina, under the identification numbers BAF12264, BAF12266, BAF797 and BAF798, respectively.
The plant materials collected from each species were dried at room temperature protected from sunlight and humidity and afterwards manually grounded to be stored until used.
4.2. Extract Preparation
Once dried and grounded, the plant materials were extracted to obtain the crude organic extract from each species. Consequently, the material was extracted twice by maceration with dichloromethane (CH2Cl2) (10% w/v) for 5 minutes. The resulting extracts were filtered (Schleicher and Schuell-Whatman, grade 0859, medium, smooth, 90 mm) and taken to dryness at 40°C under vacuum in a rotary evaporator (Buchi R-134). The yield of the extraction process was calculated as: yield (%) = (weight of the obtained extract (g) × 100)/weight of initial plant material (g).
4.3. Chromatographic Analysis of the Stevia Crude Extracts
The chromatographic analysis of the crude extracts of Stevia species was performed by high-performance liquid chromatography (HPLC) with a Waters equipment coupled to a photodiode array detector (Waters 2996), a Rheodyne injection valve (20 μL), pump (Waters Delta 600), Waters 600 controller, and in-line degasser. A reversed-phase column (Agilent Eclipse Plus C-18, 4.6 × 250 mm, 5 μm particle size) was used, and the photodiode array detector was set at 210 nm. The extracts were dissolved in water: acetonitrile (1:1) at 10 mg/mL concentration. The solutions were filtered with a nylon filter (0.45 µM, Agilent) and eluted with a gradient of water (A) and acetonitrile (B) in 40 min. The specific gradients used were 0-98% B for the extract S. aristata, 35-95% B for S. multiaristata, S. maimarensis and S. alpina. The flow rate was 1.0 mL/min, and the elution was performed at room temperature. Chromatograms were recorded and processed using the Empower Pro 3 software. The water employed to prepare the mobile phase was of ultrapure quality (Milliq). Acetonitrile (HPLC) J. T. Baker and methanol (HPLC) J. T. Baker were used.
4.4. Isolation and Purification of Phytochemicals
The crude extract of S. alpina (1.5 g) was fractionated by Silicagel 60 column chromatography (40 × 3 cm, 80 g, 230–400 mesh) eluted with CH2Cl2: ethyl acetate (EtOAc) (9.5:0.5). Fractions of 75 mL each were collected and tested by TLC (SP: Silicagel F254; MP: CH2Cl2:EtOAc (9.5:0.5); SR: sulfuric anisaldehyde). From fractions 3-6 a major compound precipitated in the form of white sharp needles. This precipitate was dissolved in the minimum volume of a heated mixture of heptane: EtOAc (2:1) to be cooled to 4°C for 24 h in order to facilitate the crystallization process. Afterwards, the crystals were separated from the solution and taken to dryness under vacuum to afford compound 1.
The crude extract of Stevia maimarensis was submitted to a dewaxing process to eliminate sterols and lipids. The extract obtained as described in subsection 2.2 was suspended in 200 mL of ethanol: water (70:30) to be partitioned three times with 60 mL of hexane (Hx). The remaining hydroalcoholic suspension was extracted thrice with 60 mL of CH2Cl2. The resulting dichloromethane sub-extracts were gathered, dried with anhydrous sodium sulfate (Biopack), filtered, and taken to dryness under reduced pressure. The dewaxed extract of S. maimarensis (10 g) was fractionated by column chromatography (50 × 5 cm) using Silicagel 60 (180 g, 230–400 mesh) as stationary phase (SP) and CH2Cl2:EtOAc (1:2) as mobile phase (MP). 40 fractions of 50 mL each were collected. The fractions were tested by Thin Layer Chromatography (TLC) [SP: Silicagel F254; MP: Hx:EtOAc (5:5); SR (spraying reagent): sulfuric anisaldehyde]. Between fractions 17 and 29 one pure compound was detected. These fractions were gathered and taken to dryness under reduced pressure to afford yellow crystals corresponding to compound 2.
4.5. Purity Assessment and Identification of the Isolated Compounds
The purity analysis of compounds 1 and 2 was carried out using HPLC. A Waters chromatograph equipped with a UV-visible diode array detector (Waters 2996) and a pump (Waters Delta 600) was utilized. An Agilent Eclipse Plus C-18 analytical column of 4.6 × 250 mm and 5 μm particle size was employed. Gradients of water:acetonitrile (A:B) were used as MP (45-95% B and 35-95% B, for compounds 1 and 2, respectively.) The compounds were dissolved in a mixture of A:B (1:1) using an ultrasonic bath. Subsequently, the solutions were filtered using 0.45 μm nylon filters. In all cases, a 20 μL loop and a flow rate of 1 mL/min were used. The runtime was set to 30 min. A run for the solvent was performed by injecting the solvent mixture used to dissolve the compounds. The purity was calculated as: purity (%) = (area of the major peak × 100) / ∑ area of every peak.
The identities of the isolated compounds were determined by spectroscopic methods: proton nuclear magnetic resonance (
1H-NMR) and carbon nuclear magnetic resonance (
13C-NMR), heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), correlated spectroscopy (COSY) (Bruker Avance 600) (600 MHz in CDCl3), electron impact mass spectrometry (EI-MS), and spectrophotometry (UV), comparing experimental spectra with literature data [
11,
12].
4.6. Drugs Preparation for Biological Assays
Stock solutions of the isolated Stevia compounds and the crude extracts were prepared using dimethyl sulfoxide as the vehicle (DMSO) at concentrations of 30 mg/mL and 100 mg/mL, respectively. The solutions were afterward fractionated and stored at -18ºC and thawed in an ultrasonic bath at 40ºC to later be diluted as necessary for each biological assay.
4.7. Ethics Statement Assays
Experiments involving the use of experimental animals were conducted strictly in accordance with the protocols approved by the Comité Institucional para el Cuidado y Uso de Animales de Laboratorio (CICUAL), Facultad de Medicina, Universidad de Buenos Aires (UBA), Argentina (protocols: ‘‘In vivo passages of cestode parasites from Mesocestoides vogae” CD Nº 1127/2015 and ‘‘Histone modifying enzymes in flatworms: study of their potential as new drug targets in diseases of importance in veterinary medicine and human health” CD Nº 187/2020).
4.8. Parasite Material
The
M. vogae tetrathyridia (TTy) used in this work were maintained in the laboratory by alternate intraperitoneal infection in Wistar rats and BALB/c mice, as described previously [
40]. The experimental animals were bred and housed in a temperature-controlled light cycle room with food and water
ad libitum at the animal facilities of Instituto de Investigaciones en Microbiología y Parasitología Médica (IMPaM), Universidad de Buenos Aires (UBA) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires, Argentina. After 3 months of infection, mice were euthanized by CO
2 inhalation. TTy were collected from the peritoneal cavity, using standard aseptic techniques, and washed three times with sterile PBS solution, pH 7.2. Finally, before being employed in experiments, TTy were size-selected using monofilament polyester meshes to a final size of 150 – 250 µm and incubated for 24 h in 5 mL of MvRPMI medium –a modified RPMI 1640 medium without phenol red (Sigma-Aldrich, USA) complemented with 10% v/v inactivated fetal bovine serum (INTERNEGOCIOS SA, Argentina), 2.4 g/L of HEPES Free acid (JT Baker, USA), 2.5 g/L of glucose (4.5 g/L final concentration, Britania, Argentina), 2 g/L of Sodium Bicarbonate (Anedra, Argentina) and 1% v/v Pen/Strep (Penicillin-Streptomycin 10,000 U/mL, Gibco, USA) at 37ºC under 5% CO
2 atmosphere.
4.9. In Vitro Anthelmintic Assays
The
in vitro effect of each extract and compound on parasite viability was studied as described before by our group [
32,
33,
34,
35] with minimum modifications. Viability was evaluated with a motility assay employing a worm tracker device (WMicrotracker MINI, Designplus SRL, Argentina) [
31], that was previously adapted to measure the movement of
M. vogae TTy [
33]. Briefly, the 24 h incubated TTy, as described in 4.8, were distributed in U-shaped 96-well microplates (Greiner Bio-One, Germany) (five TTy per well) with 150 µL of MvRPMI medium at 37ºC under 5% CO
2 atmosphere and incubated an additional 24h. Incubations with extracts or compounds were performed the following day. Extracts were tested at concentrations of 100, 500 and 1000 µg/mL and pure compounds at concentrations of 50, 100 and 500 µM. As positive controls, pre-treated parasites with ethanol 70% for 30 min and the antiparasitic drug praziquantel at 20 µM were used. All motility assays were performed using an equal amount of the drug vehicle (1% DMSO final concentration) and the corresponding negative control (1% DMSO). To determine the effects of the treatments, TTy were incubated for nine days without changing the medium in the same conditions as described above. Measurements of motility with the WMicrotracker and microscopical observations were performed before adding the compounds (day 0) and daily afterward. The data was collected from three independent biological replicates, each corresponding to TTy obtained from a different mouse, in quadruplicate for each tested condition. Relative motility indices (RMI) of each well respective to its motility before adding the compounds were calculated as described previously [
32,
33,
34,
35]. Statistical analyses were carried out using GraphPad Prism 8.0.2. Repeated two-way ANOVA tests were used to analyze the effects of the compounds on TTy motility. Significant differences (P<0.05) were determined by Dunnett’s comparisons post-tests, comparing each treatment concentration with the negative control group (each run on each day). The percentages of reduction (or increase) of viability, as described in the Results section, were taken from the relative motility index by using the formula % reduction = (1-RMI)*100 (for example, an RMI of 0.2 corresponds to 80% of viability reduction. Microscopical observation of motility and morphological changes of the TTy were also employed in all assays. The observation was performed with an inverted microscope (Primo Vert, Carl Zeiss, Germany) and images were taken using a digital video camera (AxioCam ERc5c, Carl Zeiss, Germany).
5. Conclusions
This study provides laboratory evidence of the in vitro effect of the STL estafietin, as well as its parent extract of S. alpina on cestodes. The early and potent cestocidal effects observed suggest that Stevia extracts and compounds can be explored as new therapeutic alternatives, to be applied alone or in combination with classical medicines such as albendazole to treat tropical neglected diseases caused by these types of parasites.
Author Contributions
Conceptualization, A.M.C., M.C.R., V.P.S.; Methodology, M.P.C.C., J.B., A.M.C., O.A., H.B., C.A.N.C. ,A.E.B., H.R.V., M.C.R.,V.P.S., validation, M.P.C.V., J.B., A.M.C., formal analysis, A.M.C., M.P.C.C., J.B.; investigation,. M.P.C.C., J.B., A.M.C, M.C.R.,V.P.S; resources, M.P.C.C., J.B., C.A.N.C, M.C.R.,V.P.S.; data curation, A.M.C., M.P.C.C., J.B.; writing—original draft preparation, M.P.C.C., J.B., M.C.R.; writing—review and editing, M.P.C.C., J.B., A.M.C., H.R.V., A.EB, V.P.S..; visualization, M.P.C.C., A.M.C., M.C.R.; supervision, V.P.S.. M.C.R.; project administration, V.P.S., M.C.R.; funding acquisition, V.P.S., M.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the University of Buenos Aires (Grant UBACYT 20020220300118BA), The National Agency for Science and Technology Promotion (PICT 2020 SERIEA-03061 and PICT 2019 Nº3367) and CONICET. This investigation is part of the activities carried out within the “Research Network Natural Products against Neglected Diseases” (ResNet NPND):
http://www. resnetnpnd.org/.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Eugenia Santana, Marianela Lewicki and Eduardo Giménez from the Instituto de Investigaciones en Microbiología y Parasitología Médica (IMPaM), Facultad de Medicina, Universidad de Buenos Aires (UBA, Argentina), for their excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030 (2020). https://www.who.int/publications/i/item/9789240010352. [accessed 2024-05-30].
- Lacey E. Mode of action of benzimidazoles. Par Today 1990; 6(4): 112-115. [CrossRef]
- Jeziorski MC, Greenberg RM. Voltage-gated calcium channel subunits from platyhelminths: Potential role in praziquantel action. Int J Parasitol 2006; 36: 625-632. [CrossRef]
- Park SK, Friedrich L, Yahya NA, Rohr CM, Chulkov EG, Maillard D, Rippmann F, Spangenberg T, Marchant JS. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci Transl Med 2021; 13(625): eabj5832. [CrossRef]
- Eckert J, Thompson RC. Historical aspects of echinococcosis. Adv Parasitol 2017; 95: 1–64. [CrossRef]
- Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, Nicolaidou P, Cobanoglu N, Junghanss T. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis 2009; 3(9): e524. [CrossRef]
- Ammann RW, Stumpe KD, Grimm F, Deplazes P, Huber S, Bertogg K, Fischer DR, Müllhaupt B. Outcome after discontinuing long-term benzimidazole treatment in 11 patients with non-resectable alveolar echinococcosis with negative FDG-PET/CT and anti-EmII/3-10 serology. PLoS Negl Trop Dis 2015; e0003964. [CrossRef]
- Lightowlers MW, Gasser RB, Hemphill A, Romig T, Tamarozzi F, Deplazes P, Torgerson PR, Garcia HH, Kern P. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int. J Parasitol 2021; 51(13-14): 1167-1192. [CrossRef]
- Thompson R, Jue Sue LP, Buckley SJ. In vitro development of the strobilar stage of Mesocestoides corti. Int J Parasitol 1982; 12: 303–314. [CrossRef]
- Hrčková G, Velebný S, Corba J. Effects of free and liposomized praziquantel on the surface morphology and motility of Mesocestoides vogae tetrathyridia (syn. M. corti ; Cestoda: Cyclophyllidea) in vitro. Parasitol Res 1998; 84: 230–238. [CrossRef]
- Hemphill A. Development and applications of cestode and trematode laboratory models. Parasitology 2010; 137(3): 329-333. [CrossRef]
- Saldaña J, Marín M, Fernández C, Domínguez L. In vitro taurocholate-induced segmentation and clustering of Mesocestoides vogae (syn. corti) tetrathyridia (Cestoda)–inhibition by cestocidal drugs. Parasitol Res 2001; 87(4): 281-286. [CrossRef]
- Koziol U, Domínguez M F, Marín M, Kun A, Castillo E. Stem cell proliferation during in vitro development of the model cestode Mesocestoides corti from larva to adult worm. Front Zool 2010; 7: 1-12. [CrossRef]
- Grecco A, Macchiaroli N, Pérez MG, Casulli A, Cucher MA, Rosenzvit MC. microRNA silencing in a whole worm cestode model provides insight into miR-71 function. Int. J Parasitol 2023; 53(13): 699-710. [CrossRef]
- Maggiore M, Elissondo MC. In vitro cestocidal activity of thymol on Mesocestoides corti tetrathyridia and adult worms. Interdiscip Perspect Infect Dis 2014; ID 268135:1–8. [CrossRef]
- De Lange A, Mahanty S, Raimondo J V. Model systems for investigating disease processes in neurocysticercosis. Parasitology 2019; 146(5): 553–562. [CrossRef]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 2020; 83: 770–803. [CrossRef]
- Cragg G, Newman D. Natural products and drug discovery and development: A history of success and continuing promise for the future. Planta Med 2014; 80: IL1. [CrossRef]
- Kinghorn AD. Overview. In: Kinghorn AD, editor. Stevia: The genus Stevia, London, UK: Taylor and Francis; 2002, Chapter 1: p. 1-17.
- Soejarto DD. Ethnobotany of Stevia and Stevia rebaudiana. In: Kinghorn AD, editor. Stevia: The genus Stevia, London, UK: Taylor and Francis; 2002, Chapter 3: p. 40-67.
- Borgo J, Laurella LC, Martini F, Catalán CAN, Sülsen VP. Stevia genus: Phytochemistry and biological activities update. Molecules 2021; 26: 2733. [CrossRef]
- Elso OG, Puente V, Barrera P, Sosa-Escudero MA, Sülsen VP, Lombardo ME. Mode of action of the sesquiterpene lactones eupatoriopicrin and estafietin on Trypanosoma cruzi. Phytomedicine 2022; 96: 153900. [CrossRef]
- Sülsen V, Lizarraga E, Elso O, Cerny N, Sanchez Alberti A, Bivona A, Malchiodi E, Cazorla S, Catalán C. Activity of estafietin and analogues on Trypanosoma cruzi and Leishmania braziliensis. Molecules 2019; 24: 1209. [CrossRef]
- Borgo J, Elso OG, Gomez J, Coll M, Catalán CAN, Mucci J, Alvarez G, Randall LM, Barrera P, Malchiodi EL.; et al. Anti-Trypanosoma cruzi properties of sesquiterpene lactones isolated from Stevia spp.: In vitro and in silico studies. Pharmaceutics 2023; 15: 647. [CrossRef]
- Beer MF, Frank FM, Elso OG, Bivona A, Cerny N, Giberti G, Malchiodi EL, Martino SV, Alonso MR, Sülsen VP, et al. Trypanocidal and leishmanicidal activities of flavonoids isolated from Stevia satureiifolia var. satureiifolia. Pharm Biol 2016; 54: 2188–2195. [CrossRef]
- Albani CM, Borgo J, Fabbri J, Pensel P, Paladini A, Beer MF, Laurella L, Elso O, Farias N, Elissondo N, Gambino G, Sülsen V, Elissondo MC. Antiparasitic effects of Asteraceae species extracts on Echinococcus granulosus s.s. Evid Based Complement Alternat Med 2022; 2022: e6371849. [CrossRef]
- Albani CM, Borgo J, Fabbri J, Pensel P, Fasciani L, Elso O, Papademetrio D, Grasso D, Paladini A, Beer MF, Farias NE, Elissondo N, Gambino G, Zoppi Sülsen V, Elissondo MC. Anthelmintic activity of Stevia multiaristata extract against Echinococcus granulosus sensu stricto. Parasitology 2022; 149(4): 519–528. [CrossRef]
- de Heluani CS, de Lampasona MP, Catalán CAN, Goedken VL, Gutiérrez AB, Herz W. Guaianolides, heliangolides and other constituents from Stevia alpina. Phytochemistry 1989; 28: 1931–1935. [CrossRef]
- Borgo J, Wagner MS, Laurella LC, Elso OG, Selener MG, Clavin M, Bach H, Catalán CAN, Bivona AE, Sepúlveda CS, et al. Plant extracts and phytochemicals from the Asteraceae family with antiviral properties. Molecules 2024; 29: 814. [CrossRef]
- Cabrera G, Espinoza I, Kemmerling U, Galanti N. Mesocestoides corti: Morphological features and glycogen mobilization during in vitro differentiation from larva to adult worm. Parasitology 2010; 137: 373-384. [CrossRef]
- Simonetta SH, Golombek DA. An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. J Neurosci Methods 2007; 161: 273–80. [CrossRef]
- Camicia F, Celentano AM, Johns ME, Chan JD, Maldonado L, Vaca H, Di Siervi N, Kamentezky L, Gamo, AM, Ortega-Gutierrez S, Martin-Fontecha M, Davio C, Marchant JS, Rosenzvit MC. Unique pharmacological properties of serotoninergic G-protein coupled receptors from cestodes. PLoS Negl Trop Dis 2018; 12: e0006267 . [CrossRef]
- Vaca HR, Celentano AM, Macchiaroli N, Kamenetzky L, Camicia F, Rosenzvit MC. Histone deacetylase enzymes as potential drug targets of Neglected Tropical Diseases caused by cestodes. IJP: Drugs and Drug Resistance 2019; 9: 120-132. [CrossRef]
- Vaca HR, Celentano AM, Toscanini MA, Heimburg T, Ghazy E, Zeyen P, Thomas Hauser A, Oliveira G, Elissondo MC, Jung M, Sippl W, Camicia F, Rosenzvit MC. The potential of histone deacetylase (HDAC) inhibitors as cestocidal drugs. PLoS Negl Trop Dis 2021; 15: e0009226. [CrossRef]
- Vaca HR, Celentano AM, Toscanini MA, Thomas Hauser A, Macchiaroli N, Cuestas ML, Nusblat AD, Sippl W, Elissondo MC, Jung M, Camicia F, Rosenzvit MC.Identification and characterization of sirtuin enzymes in cestodes and evaluation of sirtuin inhibitors as new cestocidal molecules. Int J Parasitol 2022; 52: 317-329. [CrossRef]
- Elso OG, Bivona AE, Sanchez Alberti A, Cerny N, Fabian L, Morales C, Catalán CAN, Malchiodi EL, Cazorla SI, Sülsen VP. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020; 25: 2014. doi: 10.3390/molecules25092014.
- Sulsen V, Martino V. Overview. In: Sulsen V, Martino V, editors. Sesquiterpene lactones. Advances in their chemistry and biological aspects, Cham, Switzerland: Springer; 2018, p. 3–17.
- Alvi MA, Khan S, Ali RMA, Qamar W, Saqib M, Faridi NY, Li L, Fu BQ, Yan HB, Jia WZ. Herbal medicines against hydatid disease: A systematic review (2000-2021). Life (Basel) 2022; 12(5): 676. [CrossRef]
- Küster T, Kriegel N, Stadelmann B, Wang X, Dong Y, Vennerstrom JL, Keiser J, Hemphill A. Amino ozonides exhibit in vitro activity against Echinococcus multilocularis metacestodes. Int J Antimicrob Agents. 2014; 43(1): 40-6. [CrossRef]
- Markoski MM, Bizarro CV, Farias S, Espinoza I, Galanti N, Zaha A, Ferreira HB. In vitro segmentation induction of Mesocestoides corti (Cestoda) tetrathyridia. J Parasitol 2003; 89: 27–34. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).