1. Introduction
Generalised Modules for Membrane Antigens (GMMA) are outer membrane vesicles (OMV) of Gram-negative bacteria, spontaneously released while growing(Piccioli, Bartolini et al. 2022). As such, GMMA represent bacterial subunits containing surface exposed antigens in the native environment and can be purified by the bacterial culture supernatant with relatively straightforward and inexpensive methods of purification(Piccioli, Bartolini et al. 2022). For these reasons, GMMA have been exploited to design affordable vaccines, which is a relevant aspect for immunization campaigns in low and middle-income countries(Piccioli, Bartolini et al. 2022). The bacterial species used as source of GMMA can be genetically manipulated to enhance the vesicle blebbing, increasing the yield of GMMA purification and facilitating the manufacturing of GMMA-based vaccines(Piccioli, Bartolini et al. 2022). This is not the only option of genetic manipulation which can be introduced(Piccioli, Bartolini et al. 2022). Indeed, the bacteria can be modified, either to overexpress specific antigens of interest or to eliminate/modify antigens that can be detrimental for vaccine design(Piccioli, Bartolini et al. 2022). One key example of the latter, is the genetic modification which leads to penta-acylated Lipid A of the lipopolysaccharide (LPS), resulting in a lower engagement of the Toll-like Receptor 4 (TLR4) and a reduced reactogenic profile of GMMA-based vaccines upon injection (called also GMMA detoxification)(Park, Song et al. 2009, Rossi, Pesce et al. 2014, Rossi, Caboni et al. 2016, Mancini, Rossi et al. 2020). GMMA are highly immunogenic and have been described to be able to promote a potent and effective humoral immune response against protein or saccharidic antigens localized on their surface(Micoli, Alfini et al. 2020, Piccioli, Bartolini et al. 2022). Indeed, GMMA can also be exploited as carrier for heterologous antigens, by making possible the design of combination vaccines(Micoli, Alfini et al. 2020, Piccioli, Bartolini et al. 2022).
We recently found that the potent GMMA immunogenicity is associated to an improved quality of the antigen-specific antibody response(Piccioli, Buricchi et al. 2023). Indeed, when an antigen is displayed on the GMMA surface, both affinity maturation and isotype switching of antigen-specific IgG are stimulated(Piccioli, Buricchi et al. 2023). We also previously discovered that the optimal immunogenic potential of GMMA is associated to antigen presentation by Follicular Dendritic Cells (FDC) to cognate B cells(Piccioli, Alfini et al. 2022). In addition, we showed that, as expected, the engagement of TLR4 plays an important role for GMMA immunogenicity, although less critical compared to the antigen presentation by FDC, whereas TLR2 has surprisingly no role(Piccioli, Alfini et al. 2022). This latter is an intriguing finding as both TLR4 and TLR2 possess the capacity to stimulate innate immune responses that can drive the adaptive immunity(Akira, Takeda et al. 2001, Iwasaki and Medzhitov 2010, Kawai and Akira 2010). However, the proinflammatory properties of TLR4 and TLR2 stimulation are obviously not only linked to immunogenicity, but also to reactogenicity, particularly for TLR4(Schromm, Brandenburg et al. 2000). In fact, the GMMA detoxification is essentially aimed at reducing the engagement of TLR4 and not of TLR2, despite also this TLR possesses immunostimulatory capacity(Kawai and Akira 2010, Rossi, Pesce et al. 2014, Rossi, Caboni et al. 2016, Piccioli, Bartolini et al. 2022). In any case, beyond the immunogenicity, GMMA can promote inflammation and consequently may provoke reactogenicity, either at local or systemic level(Mancini, Rossi et al. 2020, Piccioli, Bartolini et al. 2022).
The GMMA-based vaccines are usually formulated with the adjuvant aluminum hydroxide (Alum) by adsorbing the GMMA particles on Alum to limit the systemic exposure of the vesicles, improving in this way the reactogenic profile of the GMMA-based vaccines(Brito and O'Hagan 2014, Oleszycka and Lavelle 2014, Wu, Singh et al. 2014, Cortez, Li et al. 2016, Piccioli, Bartolini et al. 2022). Thus, in the case of GMMA, the Alum is not used as adjuvant to enhance the immune response of the vaccines that are already highly immunogenic, but rather as adsorbant to potentially further improve the safety profile of the GMMA-based vaccines(Piccioli, Bartolini et al. 2022). Indeed, the presence of TLR2 and TLR4 agonists on GMMA, such as lipoproteins for TLR2 or LPS for TLR4, are believed to be able to induce systemic reactogenicity if GMMA are exposed in the circulation because of their proinflammatory ability(Rosenqvist, Hoiby et al. 1998, Kawai and Akira 2010, Rossi, Pesce et al. 2014, Rossi, Caboni et al. 2016, Mancini, Rossi et al. 2020, Piccioli, Bartolini et al. 2022). However, this hypothesis has never been proven and, along this line, the reactogenic capacity of the detergent extracted OMV of Meningococcus B, not adsorbed on Alum, is not fully clear(Rosenqvist, Hoiby et al. 1998).
Interestingly, preclinical studies revealed that the added value of Alum as adjuvant for the already highly immunogenic GMMA was controversial and not fully clear(Micoli, Alfini et al. 2020, Micoli, Alfini et al. 2021, Piccioli, Alfini et al. 2022, Mancini, Alfini et al. 2023, Piccioli, Buricchi et al. 2023). Recently, the hypothesis that Alum-based adjuvants do not provide a real added value for immunogenicity of GMMA has been consolidated in both mouse and rabbit models by using Shigella sonnei GMMA(Di Benedetto, Mancini et al. 2023, Mancini, Caradonna et al. 2024).
In addition, in this recent work, on the contrary of what observed for detergent extracted OMV of Neisseria meningitidis, it has been shown that Shigella sonnei detoxified GMMA not adsorbed on Alum, did not lead to weight decrease and a substantial temperature rise after injection in the rabbit model(Mancini, Caradonna et al. 2024). Indeed, only a mild and transient increase of temperature was observed(Mancini, Caradonna et al. 2024). Thus, in the appropriate animal model, although not via a proper toxicology study, GMMA injected in absence of Alum formulation did not show signs of concerning systemic reactogenicity and an unacceptable safety profile(Mancini, Caradonna et al. 2024).
We believe that understanding whether Alum is necessary or not in the formulation of GMMA-based vaccines for their safety and/or immunogenic profile is a critical information for the design of most effective and affordable vaccines made with GMMA and that clinical investigation is needed to arrive to a definitive answer.
Here, we used GMMA formulations similar to those already utilized in previous immunogenicity studies, in order to assess the reactogenicity of these formulations at the injection site, in the mouse muscle. We found that the Alum is the major driver of inflammation within the muscle, whereas highly immunogenic GMMA showed only a mild reactogenic profile.
2. Materials and Methods
Immunogens and formulation. Neisseria meningitidis GMMA, and Factor H Binding Protein variant 3 (hereinafter referred as fHbp or fHbp-v3) were produced and analyzed as previously described(Piccioli, Buricchi et al. 2023). Synthesis and characterization of the fHbp-GMMA conjugates and formulations were performed as previously described(Piccioli, Buricchi et al. 2023).
Animals and injections. Animal studies were carried out at the GSK Animal Facility in Siena, Italy, in compliance with the Italian D. Lgs. n. 26/14, the European Directive 2010/63/UE and the GSK Policy and Standards on the Care, Welfare and Treatment of Animals. The animal protocols used for these studies were ethically reviewed by the Animal Welfare Body of GSK Vaccines, Siena, Italy and approved by the Italian Ministry of Health. C57BL/6 female mice, 6/8 weeks old at day 0 of the study, were injected in the calf muscle of one leg with 25 l of inoculum volume. The animals were treated with 0.1 g of fHbp physically mixed with 1.9 g of GMMA or with 2 g of GMMA-fHbp conjugates containing 0.1 g of fHbp chemically conjugated to GMMA, either adsorbed or not to Alum (at concentration of 3 mg/ml of aluminum hydroxide). As control, the animals were treated with 0.1 g of fHbp adsorbed or not to Alum (at concentration of 3 mg/ml of aluminum hydroxide) or with the formulation buffer alone, in absence of Alum. Groups of 20 mice per treatment were used. Mice were immunized one time at day 0 and both the injected muscle and the draining lymph nodes of 5 animals per each group of treatment were collected 3 hours, 24 hours, 3 days and 7 days after the immunization. Tissue samples were treated for histopathological assessment and then analyzed in blind to assign the inflammation score.
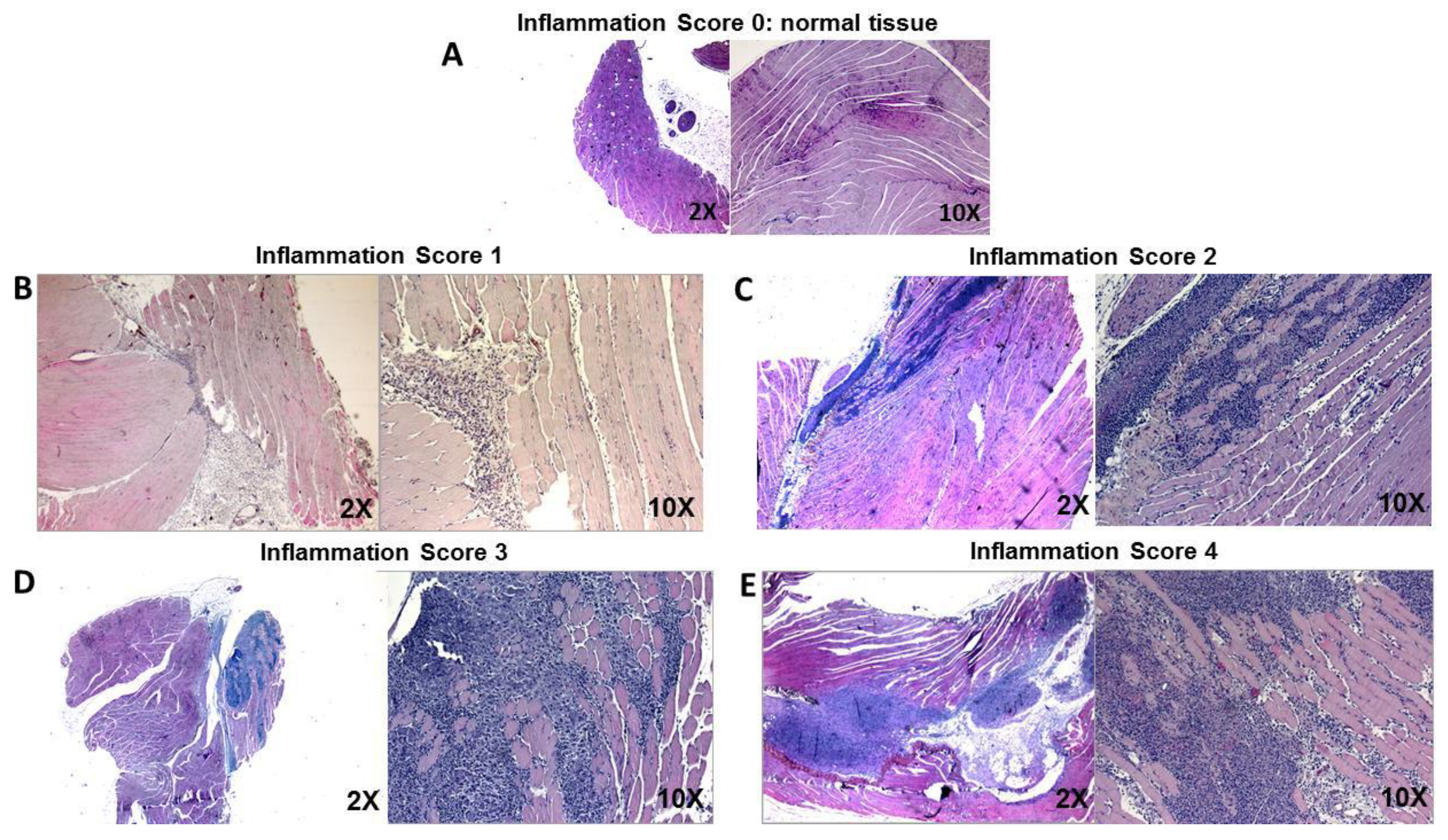
Histopathology. Muscles and draining lymph nodes from each mouse (2-4 sections/animal) were fixed for 18–24 h in 4% buffered formaldehyde (Carlo Erba). The organs were washed and embedded in paraffin (formaldehyde-fixed and paraffin embedded, FFPE) and then, 5 µm-thick sections were cut and stained with hematoxylin and eosin (Leica Kit Infinity stained in Leica ST501 slides autostainer) following standard procedures. Tissue slides were examined blindly by a histopathologist. In the muscles, pathological changes were assessed according to a semi-quantitative scoring system, by analysing the whole of the tissue present in the section at inoculation site level. Tissue pathology was scored assessing cellular infiltrate (type of inflammatory cells and degree of infiltration), and muscle fibers pathology (degeneration, necrosis, re-generation). Muscle sections were scored as follows: score 0: normal muscle tissues; score 1: normal muscle tissue with light polymorphonucleated cellular infiltrate; score 2: mild muscle degeneration with light to moderate mixed (polymorphonucleated and lymphomonocytic) cell infiltration; score 3: moderate muscle degeneration, with scattered small areas of necrosis and mild loss of architecture accompanied by a moderate to locally severe cellular infiltrate formed by mixed (polymorphonucleated and lymphomonocytic) cells; score 4: severe mixed cellular infiltrate with extensive loss of tissue architecture, tissue degeneration, necrotic muscle fibers with scattered fiber regeneration. For sections of lymph nodes, the following were evaluated: general architecture, follicle numbers and structure (with or without germinal centre and mantle), cortex and medulla appearance and cellular composition. Scores were assigned as follows: score 0: normal tissues; score 1: normal node architecture with mildly enlarged follicles, with or without mantle; normal to minimally enlarged cortex and medulla; score 2: several enlarged follicles, with prominent mantles, enlarged and active cortex and / or medulla; node architecture maintained or slightly disrupted; score 3: large blending follicles/enlarged cortex and/or medulla, with obliteration of normal architecture in most of the tissue.
Statistical analysis. The animal study was exploratory, and no statistical success criteria were pre-defined. The sample size was not computed to ensure a target power, but to manage the experiments by obtaining indicative results at the same time, basing on past experience.
3. Results and Discussion
To evaluate the local reactogenic profile of GMMA, we performed histopathological analysis of the injection site at different time points following immunization, using formulations similar to those already tested in previous in vivo studies and known as highly immunogenic(Piccioli, Buricchi et al. 2023). In this study we showed that, at the utilized antigen dosage, the antigen alone or administered mixed with GMMA, formulated or not with Alum, did not induce a potent antibody response after three administrations, whereas, when the antigen was localized in the surface of GMMA, the antigen-specific humoral immunogenicity was substantially increased.
In the study presented here, following a study design similar to those utilized to evaluate the immunogenicity, we wanted to investigate the local reactogenicity of the same formulations. Mice were immunized intramuscularly via only one injection of GMMA chemically conjugated or physically mixed to fHbp, either adsorbed or not to Alum. As controls, mice were treated with fHbp alone, adsorbed or not to Alum, or simply with the buffer.
Mice were sacrificed 3 hours, 24 hours, 3 days and 7 days after the injection and both the muscle tissues and the draining lymph nodes were collected to investigate the histological presentation.
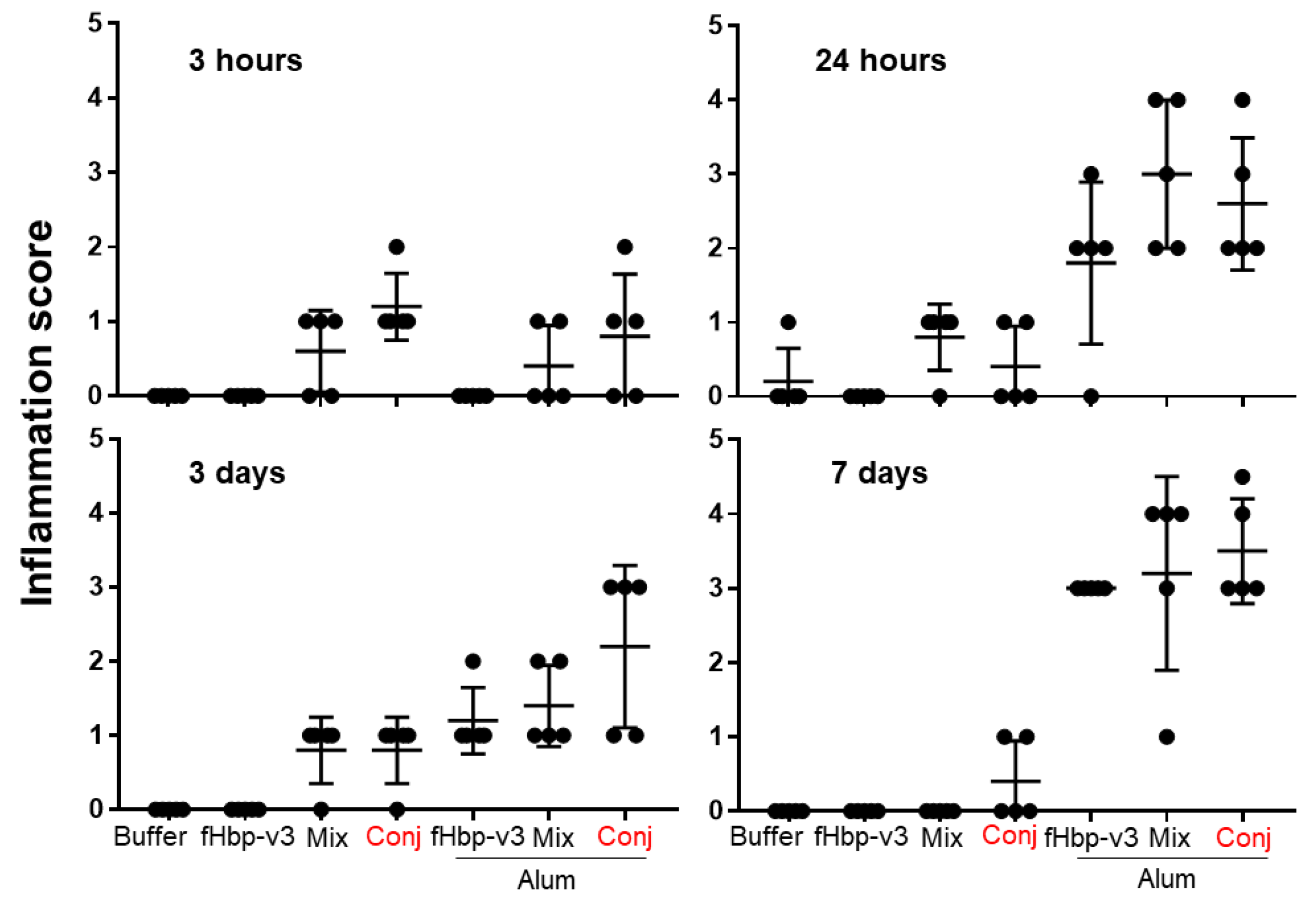
Within the muscle, injection of GMMA containing formulations in absence of Alum never lead to an inflammation score superior to 1 over the course of the 7 days, with the exception of one animal in which an inflammation score equal to 2 has been detected (
Figure 1). Score 1 represents mild inflammation, associated to a diffuse or locally scattered cellular infiltrate which is prevalently constituted by polymorphonuclear cells at early time points after the treatment, whereas lymphomonocytic cells can be found at later time points (
Figure 2). Score 2 reflects an overall inflammation, associated to a moderate to severe cell infiltration usually formed by moderate to large areas of a mix of polymorphonuclear and lymphomonocytic inflammatory cells with the detection of limited muscle fiber degeneration, in particular in areas adjacent to the inflammatory infiltration (
Figure 2).
Instead, the presence of Alum, regardless formulation with GMMA or fHbp alone, was able to promote severe inflammation during time, with the inflammation score reaching values ranging from 3 to 4 or higher (
Figure 1). Score 3 is associated to large cellular infiltrates formed by mixed polymorphonucleated and lymphomonocytic cells with loss of tissue architecture and marked muscular tissue degeneration (
Figure 2). Score 4 is determined by a very large mixed cellular infiltrate with extensive loss of tissue architecture, muscle tissue degeneration, areas of necrotic muscle fibers, macrophages and scattered muscle fiber regeneration (
Figure 2).
In all cases, lymph nodes were not affected by changes (all samples scored 0).
Very early after immunization (3 hours) no differences were observed when immunizing in absence or presence of Alum (
Figure 1). However, from 24 hours along the 7 days, Alum was demonstrated the major driver of inflammation at injection site, consistently to what has been previously shown(Kashiwagi, Maeda et al. 2014) (
Figure 1). The observed experimental variability of inflammation among different animals, which is expected, can be the reason why the overall inflammation at 3 days appeared less severe than at 24 hours, considering also that different animals are sacrificed at the indicated time points (
Figure 1).
The overall inflammation score associated to Alum injection was more severe in presence of GMMA compared to fHbp alone (
Figure 1). This observation is consistent with the fact that GMMA preparations are reactogenic per se, even though at low level, whereas fHbp is not.
In conclusion, GMMA by themselves displayed mild reactogenic potential locally, when injected intramuscularly. Noteworthy, the formulation of GMMA with Alum appeared to lead to an increased local reactogenicity compared to Alum in absence of GMMA.
Signs of solicited reactogenicty due to the generation of the humoral immune response, such as enlarged follicles with detection of germinal centers, were not expected to be observed in the lymph nodes, as the histolological assessment has been done after only one immunization. Thus, this experimental setting is particularly suitable to observe signals of the reactogenic response due to the inflammatory activity of GMMA. Interestingly, no signs of inflammation were detected within the draining lymph nodes for any type of treatment, in any experimental time points, strongly suggesting that GMMA does not promote systemic reactogenicity, even when not adsorbed on Alum and expected to move more quickly into lymphatic circulation toward lymph nodes, instead of remaining entrapped at injection site (data not shown).
In presence of mild inflammation, a cellular infiltrate prevalently constituted of polymorphonuclear cells within the injection was expected at early time points, as neutrophils are usually the first cell types that extravasate to reach inflamed tissues(Goldsby, Kindt et al. 2002, Kolaczkowska and Kubes 2013). Similarly, a more mixed cellular infiltrate with lymphomonocytic cells, observed at later time points, was expected too, because lymphocytes, monocytes, and dendritic cells recruitment within the muscle is delayed compared to neutrophils(Goldsby, Kindt et al. 2002, Calabro, Tortoli et al. 2011). Also, in the presence of moderate to severe inflammation, the mixed cellular infiltrate was expected, as the presence of pro-inflammatory signals, including chemokines, is much higher in this case and the infiltration of muscle with lymphocytes, monocytes and dendritic cells can be accelerated(Goldsby, Kindt et al. 2002, Calabro, Tortoli et al. 2011).
The histopathology results presented here are in line to the proposed mode of action of the Alum adjuvant, whose fibers should remain localized at the injection site by promoting local inflammation over time by inducing recruitment of immune cells, especially of the innate immune system and the release of pro-inflammatory mediators(Brito and O'Hagan 2014, Kashiwagi, Maeda et al. 2014, Oleszycka and Lavelle 2014). Indeed, the inflammation induced by Alum after the acute phase can last for months after the injection within the muscle in the form of inflammatory nodules(Kashiwagi, Maeda et al. 2014).
Instead, GMMA appeared to promote mild inflammation, which last few days and then remitted. This mild inflammation induced by GMMA alone is not only consistent with a low reactogenic profile of these vesicles, but intriguingly also with the adsorbant mechanism of Alum. In fact, GMMA not adsorbed on Alum are predicted to quickly move toward draining lymph nodes, not remaining entrapped at the site of injection and therefore not stimulating strong local inflammatory responses. In this context, as mentioned above, the absence of inflammation within the draining lymph nodes confirmed the low reactogenic potential of GMMA themselves.
In conclusion, our study, conducted in the mouse model, confirmed that Alum is a major driver of local inflammation and reactogenicity in GMMA-based vaccines, whereas GMMA appears able to promote only mild inflammation at injection site and thus to possess low local reactogenic potential.
This finding is particularly interesting because the Alum has not been proposed as adjuvant to improve immunogenicity for GMMA, but mainly as adsorbant to avoid any potential systemic exposure of the bacterial vesicles(Piccioli, Bartolini et al. 2022). However, preliminary data demonstrated that GMMA injected without Alum do not promote concerning systemic reactogenicity in the rabbit model(Mancini, Caradonna et al. 2024) and now our work demonstrated that GMMA injected without Alum induced only mild inflammation at injection site and no signs of inflammation within the draining lymph nodes, in the mouse model. Thus, data obtained with relevant animal models, despite the absence of a proper toxicology study so far, show that GMMA-based vaccines might be formulated in absence of Alum without affecting both immunogenicity and safety and support the rationale to confirm this observation in clinical development. As the absence of Alum in GMMA formulation further reduces the complexity of the GMMA-based vaccines, we believe that a phase 1 first-in-human clinical trial evaluating the safety and immunogenicity of a GMMA-based vaccine comparing formulations with and without Alum is a priority, as already proposed(Mancini, Caradonna et al. 2024). Results from such a clinical trial might bring to a substantial advancement for the design of GMMA-based vaccines. In fact, if Alum would result dispensable for the safety of GMMA-based vaccines, this would lead to a simplified vaccine formulation, also facilitating the design of more complex combination vaccines, for which GMMA appear particularly suitable(Piccioli, Bartolini et al. 2022). In turn, the easier manufacturing of GMMA-based vaccines not containing Alum in the formulation, would have a positive impact on the supply and ultimately on the access of vaccination for low and middle-income countries.
Author Contributions
DP: Conceptualization, Formal Analysis, Writing – Original Draft Preparation, Writing – Review & Editing, Visualization, Supervision. RC: Formal Analysis, Methodology, Validation, Investigation, Data Curation, Writing – Review & Editing. SM, RA, RDB, SG, EC, SM, GR: Methodology, Validation, Investigation, Data Curation, Writing – Review & Editing. EB: Writing – Review & Editing. FM: Writing – Review & Editing, Supervision, Project Administration.
Funding
This research received no external funding. This work was sponsored by GlaxoSmithKline Biologicals SA which was involved in all stages of the study conduct and analysis.
Institutional Review Board Statement
Animal studies were carried out at the GSK Animal Facility in Siena, Italy, in compliance with the Italian D. Lgs. n. 26/14, the European Directive 2010/63/UE and the GSK Policy and Standards on the Care, Welfare and Treatment of Animals. The facility is AAALAC-accredited. The animal protocol used for these studies, 804/2015-PR, was ethically reviewed by the Animal Welfare Body of GSK Vaccines, Siena, Italy and approved by the Italian Ministry of Health.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to GSK policy for protection of intellectual property.
Conflicts of Interest
Diego Piccioli, Raffaella Cecchi, Silvia Maccari, Renzo Alfini, Roberta Di Benedetto, Simona Gallorini, Elena Cartocci, Sara Marchi, Giacomo Romagnoli, Erika Bartolini and Francesca Micoli are employees of the GSK group of companies. Diego Piccioli, Raffaella Cecchi, Roberta Di Benedetto, Elena Cartocci, Erika Bartolini and Francesca Micoli own GSK shares.
References
- Akira, S., et al. (2001). "Toll-like receptors: critical proteins linking innate and acquired immunity." Nat Immunol 2(8): 675-680. [CrossRef]
- Brito, L. A. and D. T. O'Hagan (2014). "Designing and building the next generation of improved vaccine adjuvants." J Control Release 190: 563-579. [CrossRef]
- Calabro, S., et al. (2011). "Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes." Vaccine 29(9): 1812-1823. [CrossRef]
- Cortez, A., et al. (2016). "Incorporation of Phosphonate into Benzonaphthyridine Toll-like Receptor 7 Agonists for Adsorption to Aluminum Hydroxide." J Med Chem 59(12): 5868-5878. [CrossRef]
- Di Benedetto, R., et al. (2023). "Comparison of Shigella GMMA and glycoconjugate four-component formulations in animals." Front Mol Biosci 10: 1284515.
- Goldsby, R. A., et al. (2002). Kuby immunology. New York, W.H. Freeman.
- Iwasaki, A. and R. Medzhitov (2010). "Regulation of adaptive immunity by the innate immune system." Science 327(5963): 291-295. [CrossRef]
- Kashiwagi, Y., et al. (2014). "Inflammatory responses following intramuscular and subcutaneous immunization with aluminum-adjuvanted or non-adjuvanted vaccines." Vaccine 32(27): 3393-3401. [CrossRef]
- Kawai, T. and S. Akira (2010). "The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors." Nat Immunol 11(5): 373-384.
- Kolaczkowska, E. and P. Kubes (2013). "Neutrophil recruitment and function in health and inflammation." Nat Rev Immunol 13(3): 159-175. [CrossRef]
- Mancini, F., et al. (2023). "Exploring the Role of GMMA Components in the Immunogenicity of a 4-Valent Vaccine against Shigella." Int J Mol Sci 24(3). [CrossRef]
- Mancini, F., et al. (2024). "Testing S. sonnei GMMA with and without Aluminium Salt-Based Adjuvants in Animal Models." Pharmaceutics 16(4).
- Mancini, F., et al. (2020). "OMV Vaccines and the Role of TLR Agonists in Immune Response." Int J Mol Sci 21(12). [CrossRef]
- Micoli, F., et al. (2021). "Generalized Modules for Membrane Antigens as Carrier for Polysaccharides: Impact of Sugar Length, Density, and Attachment Site on the Immune Response Elicited in Animal Models." Front Immunol 12: 719315.
- Micoli, F., et al. (2020). "GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines." Vaccines (Basel) 8(3). [CrossRef]
- Oleszycka, E. and E. C. Lavelle (2014). "Immunomodulatory properties of the vaccine adjuvant alum." Curr Opin Immunol 28: 1-5. [CrossRef]
- Park, B. S., et al. (2009). "The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex." Nature 458(7242): 1191-1195. [CrossRef]
- Piccioli, D., et al. (2022). "Antigen presentation by Follicular Dendritic cells to cognate B cells is pivotal for Generalised Modules for Membrane Antigens (GMMA) immunogenicity." Vaccine 40(44): 6305-6314. [CrossRef]
- Piccioli, D., et al. (2022). "GMMA as a 'plug and play' technology to tackle infectious disease to improve global health: context and perspectives for the future." Expert Rev Vaccines 21(2): 163-172. [CrossRef]
- Piccioli, D., et al. (2023). "Enhanced Systemic Humoral Immune Response Induced in Mice by Generalized Modules for Membrane Antigens (GMMA) Is Associated with Affinity Maturation and Isotype Switching." Vaccines (Basel) 11(7). [CrossRef]
- Rosenqvist, E., et al. (1998). "Effect of aluminium hydroxide and meningococcal serogroup C capsular polysaccharide on the immunogenicity and reactogenicity of a group B Neisseria meningitidis outer membrane vesicle vaccine." Dev Biol Stand 92: 323-333.
- Rossi, O., et al. (2016). "Toll-Like Receptor Activation by Generalized Modules for Membrane Antigens from Lipid A Mutants of Salmonella enterica Serovars Typhimurium and Enteritidis." Clin Vaccine Immunol 23(4): 304-314. [CrossRef]
- Rossi, O., et al. (2014). "Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid A modifications: relative activation of TLR4 and TLR2 pathways in different mutants." J Biol Chem 289(36): 24922-24935.
- Schromm, A. B., et al. (2000). "Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion." Eur J Biochem 267(7): 2008-2013. [CrossRef]
- Wu, T. Y., et al. (2014). "Rational design of small molecules as vaccine adjuvants." Sci Transl Med 6(263): 263ra160. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).