2.1. Transition Metal Nitrides as Catalysts for Ammonia Synthesis
Transition metal nitrides have gained considerable attention in ammonia synthesis research due to their unique catalytic properties and the fact that they can utilise nitrogen vacancies on their surface as catalytic centers for activating dinitrogen [
9]. These materials can facilitate nitrogen reduction, which is a crucial step in ammonia synthesis. The following sections provide an in-depth look at specific transition metal nitrides, their catalytic mechanisms, and their performance in ammonia synthesis.
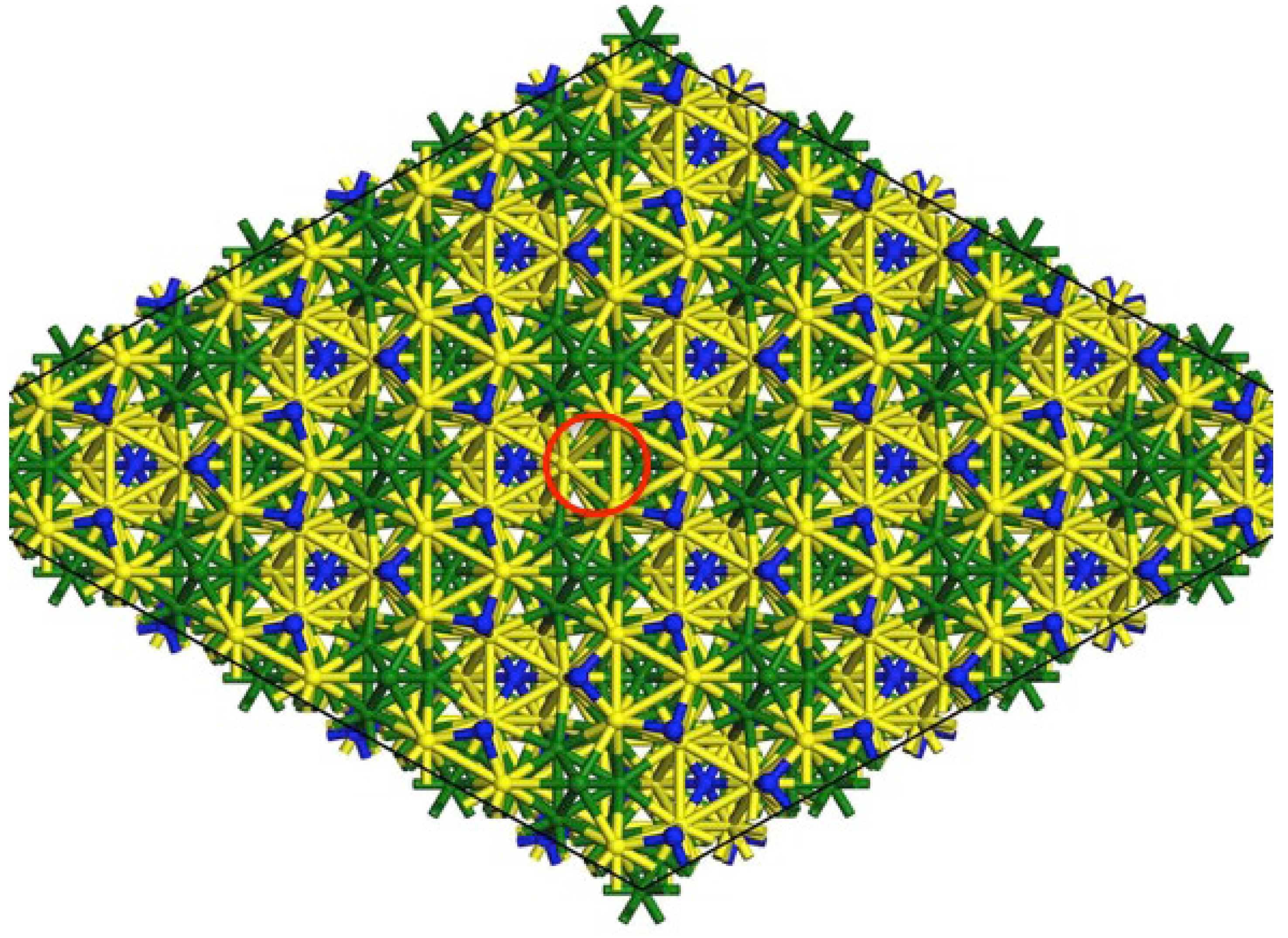
Cobalt molybdenum nitride (Co
3Mo
3N), whose structure is shown in
Figure 1, along with similar tertiary nitrides (M
xM’
yN, where M is a group VIB metal and M’ is a group VIII metal) synthesized according to Topsøe’s patented method, are known for their activity in ammonia synthesis at 400°C and high pressures using a 3:1 hydrogen to nitrogen mixture [
10]. The structure of cobalt molybdenum nitride is like a hexagonal array of Co
8 clusters embedded into a molybdenum nitride framework. This makes the catalyst bifunctional where both metal and metal support interactions are present. This type of catalyst is very versatile due to the unusual structure these metal nitrides have. Co₃Mo₃N has been identified as a highly active catalyst for ammonia synthesis by various experimental studies [
11,
12].
The study by Zeinalipour-Yazdi et al. explores the mechanisms of ammonia synthesis on Co₃Mo₃N surfaces, emphasizing the role of surface defects such as nitrogen vacancies and intrinsic surface cavities. Using dispersion-corrected DFT calculations, the research compares the Langmuir–Hinshelwood (dissociative) and Eley–Rideal/Mars–van Krevelen (associative) mechanisms. The findings highlight that, apart from the conventional dissociative mechanism, an associative mechanism involving hydrazine and diazane intermediates also exists, where hydrogen reacts directly with surface-activated nitrogen to form ammonia under milder conditions. This associative mechanism exhibits lower activation barriers for the hydrogenation steps compared to the dissociative mechanism, making it kinetically favorable. The study underscores that through surface defects, ammonia synthesis activity on Co₃Mo₃N can be significantly enhanced at lower temperatures, providing insights into optimizing this catalyst for more efficient ammonia production [
13].
Further, the study by Zeinalipour-Yazdi and Catlow employs DFT to investigate the associative mechanisms for ammonia and hydrazine synthesis on Co₃Mo₃N surfaces. The research identifies that the nitrogen intermediate, NNH
2, can form readily on Co₃Mo₃N surfaces through Eley–Rideal chemisorption of H
2 on pre-adsorbed N
2 at nitrogen vacancies. This mechanism is energetically favorable, with the highest relative barrier being 213 kJ/mol, indicating a low-energy process for hydrazine synthesis via heterogeneous catalysis. The study presents a potential energy diagram showing that the associative mechanism involves two high-barrier hydrogenation steps in the gas phase, contrasting with a significantly lower barrier for the formation of hydrazine on Co₃Mo₃N. The research emphasizes the efficient activation of N
2 at surface nitrogen vacancies, making Co₃Mo₃N a promising catalyst for synthesizing hydrazine under mild conditions. The findings highlight that the Eley–Rideal mechanism could facilitate the production of hydrazine and ammonia, enhancing catalytic activity through surface defects [
14].
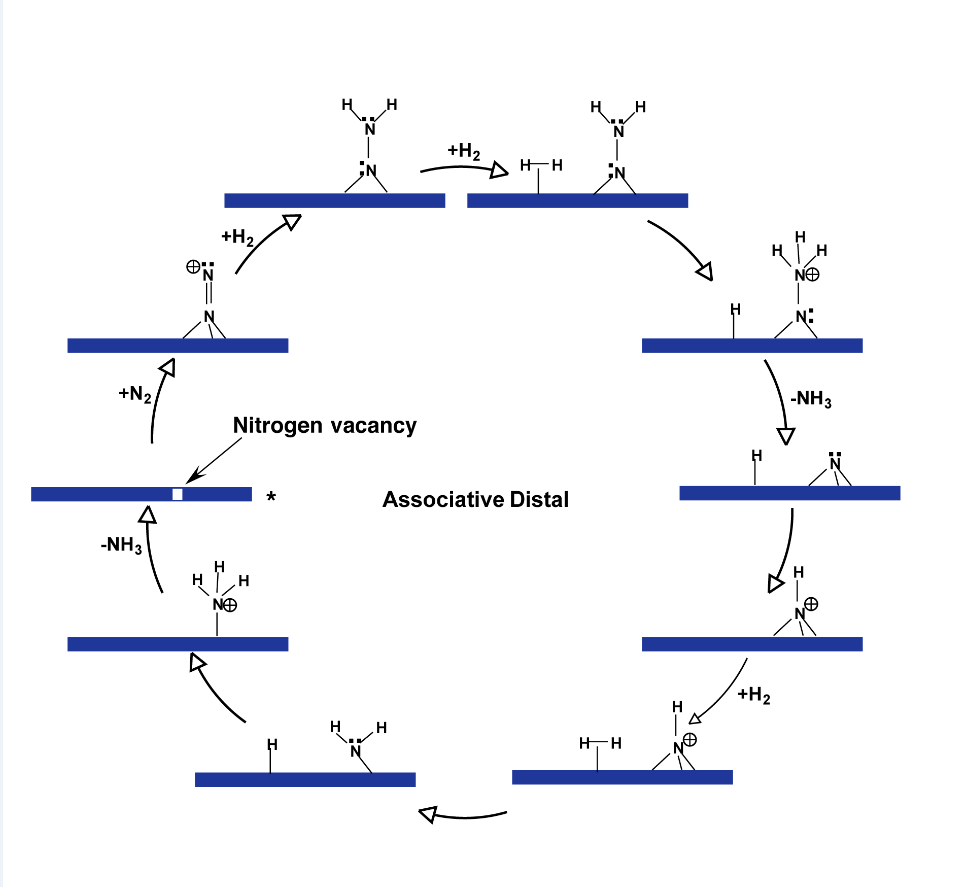
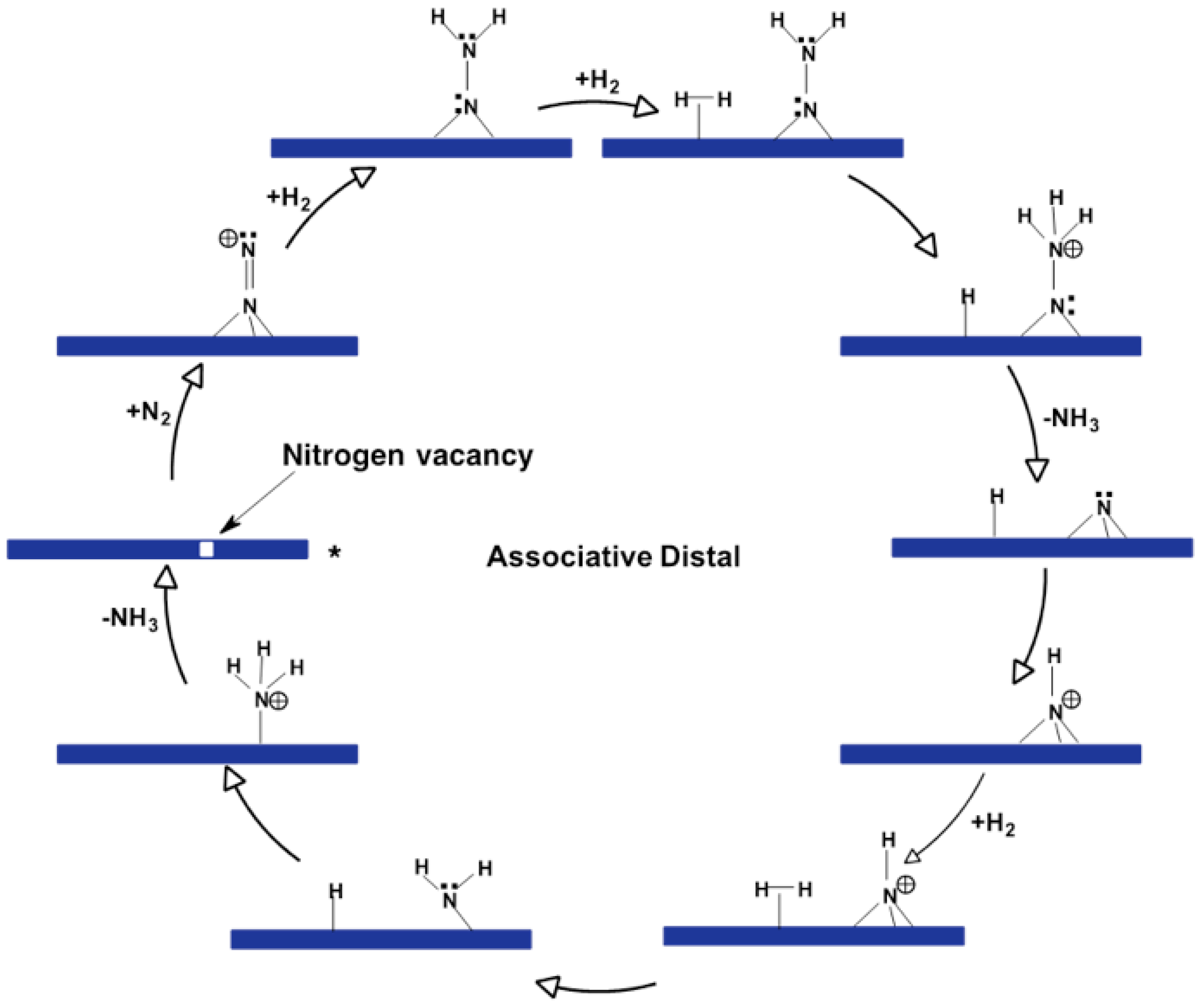
This catalyst can operate via two primary mechanisms: the Eley–Rideal/Mars–van Krevelen mechanism and the Langmuir–Hinshelwood mechanism, which have been described by DFT in VASP. In this mechanism, which is shown in
Figure 2, the active site is a nitrogen vacancy on the molybdenum nitride framework. Molecular nitrogen chemisorbs at the N-vacancies in an end-on configuration, followed by the dissociative chemisorption of molecular hydrogen. This leads to the formation of diazene and hydrazine intermediates, eventually producing ammonia. This hydrazine intermediate has a low barrier for decomposition into ammonia which is the first stoichiometric ammonia molecule produced through this mechanism. The second stoichiometric ammonia molecule is produced in higher activated steps as it involves the hydrogenation of surface nitrogen that is now strongly absorbed to the surface of the metal nitride. So one disadvantage of this catalyst could be that that the ammonia is produced on its surface by it is strongly bound to the surface. The energy profile for this mechanism indicates that the hydrogenation steps have lower barriers compared to the Langmuir–Hinshelwood mechanism, making it kinetically faster for ammonia synthesis on Co₃Mo₃N [
15].
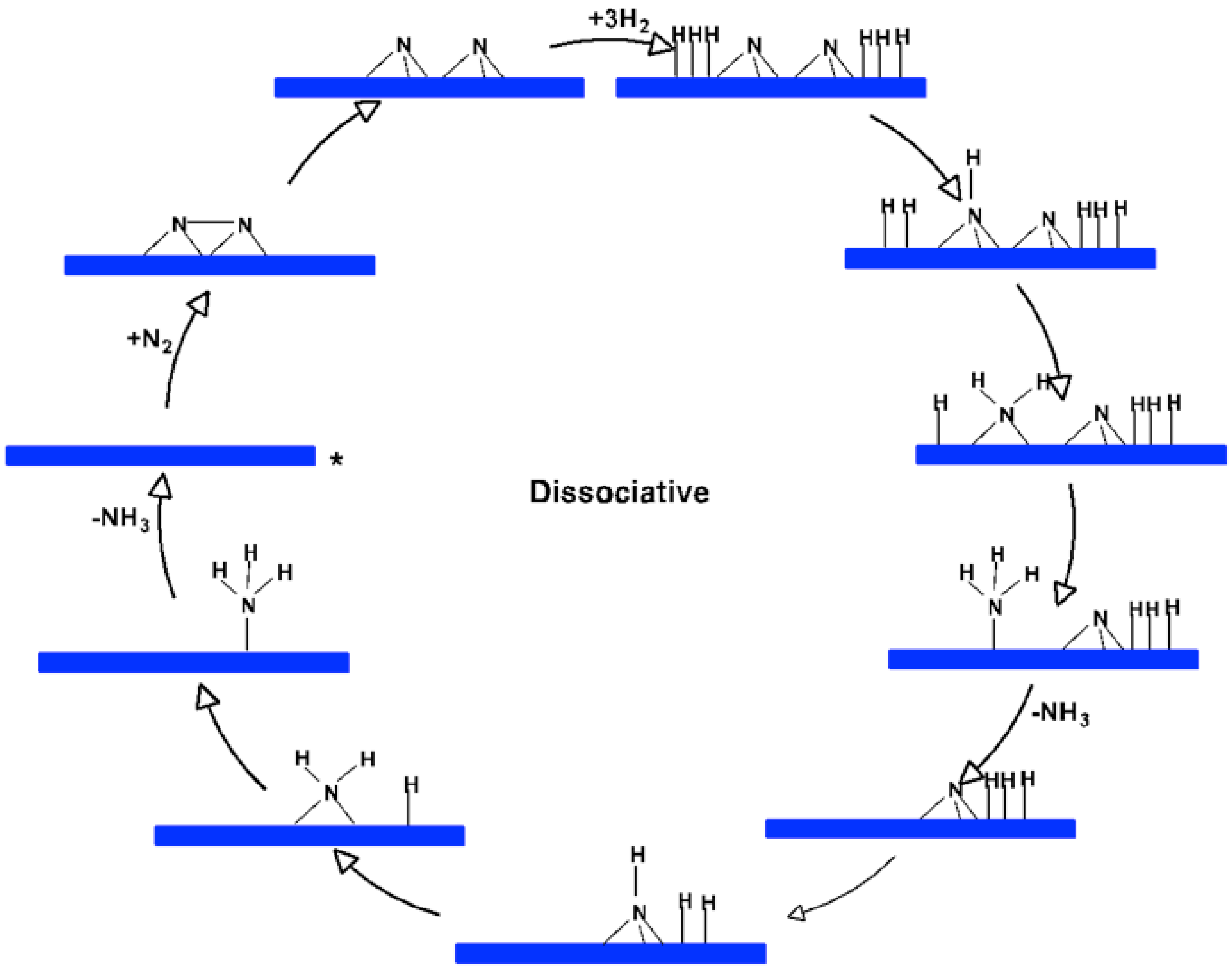
In
Figure 3, we illustrate a simplified schematic of the dissociative Langmuir-Hinshelwood mechanism of ammonia synthesis on a catalytic surface. The process begins with nitrogen molecules (N₂) adsorbing onto the catalyst surface.
Subsequently, hydrogen molecules (H₂) also adsorb onto the surface, dissociating into individual hydrogen atoms. The nitrogen atoms then react with the hydrogen atoms in a stepwise manner, forming intermediates such as NH, NH₂, and eventually NH₃ (ammonia), which desorbs from the surface. The cycle repeats as new nitrogen and hydrogen molecules continue to adsorb and react on the catalyst surface. The nitrogen and hydrogen atoms are shown interacting through a series of intermediate steps leading to the formation of ammonia.
The Langmuir–Hinshelwood mechanism for ammonia synthesis involves a series of well-defined steps starting with the adsorption of molecular nitrogen (N₂) onto the surface of the catalyst. In this mechanism, nitrogen molecules are adsorbed in a side-on configuration at the active sites of the catalyst. This adsorption mode allows the nitrogen molecule to interact simultaneously with multiple active sites, leading to a significant activation of the N≡N triple bond.
Once adsorbed, the nitrogen molecule undergoes dissociation into two nitrogen atoms, each of which is further adsorbed onto the catalyst surface in a bridged configuration. These bridged nitrogen species are highly reactive intermediates that play a crucial role in the subsequent steps of the reaction. The dissociation of the nitrogen molecule is facilitated by the strong interaction with the catalyst surface, which lowers the activation energy required to break the N≡N bond.
Following the dissociation, hydrogen molecules (H₂) adsorb onto the catalyst surface and dissociate into individual hydrogen atoms. These hydrogen atoms then migrate across the surface and react with the bridged nitrogen species in a stepwise manner. This stepwise hydrogenation leads to the formation of various nitrogen-hydrogen intermediates such as NH, NH₂, and ultimately NH₃ (ammonia).
The final product, ammonia, desorbs from the catalyst surface, freeing up active sites for new nitrogen and hydrogen molecules to be adsorbed, thereby continuing the catalytic cycle. The efficiency of this mechanism depends on the ability of the catalyst to facilitate the adsorption, activation, and dissociation of nitrogen and hydrogen molecules, as well as the subsequent hydrogenation steps.
These species then undergo hydrogenation steps to form ammonia. The relative energy diagram shows that the hydrogenation steps in this mechanism have higher barriers compared to the associative distal Eley–Rideal/Mars–van Krevelen mechanism, making it less favorable kinetically [
15].
Building on the understanding of nitrogen activation mechanisms, the study by Zeinalipour-Yazdi et al. presents a dispersion-corrected density functional theory (DFT-D3) investigation into the adsorption and activation of molecular nitrogen (N₂) and hydrogen (H₂) on Co₃Mo₃N surfaces. The research identifies two primary activation sites for N₂, a Mo₃ triangular cluster at 3f nitrogen vacancies and a surface cavity where N₂ is activated by Co₈ clusters. The study reveals that H₂ can adsorb both molecularly on the Mo₃N framework and dissociatively on Co₈ or Mo₃ clusters exposed due to N-vacancies. N₂ adsorption occurs in three configurations: side-on, end-on, and an unusual tilt end-on (155°), with the latter being particularly efficient for activation. The findings suggest that the Co₃Mo₃N surface with high nitrogen vacancy density (∼10¹³ cm⁻²) is highly effective in activating N₂ by weakening the triple bond, facilitating the Mars–van Krevelen mechanism for ammonia synthesis. This study highlights the potential of Co₃Mo₃N as a highly active catalyst for ammonia synthesis, with the unique tilt end-on configuration playing a crucial role in the activation process [
16]
Following the previous findings regarding the role of crystallographic orientation in catalytic activity, the study by Gudmundsson et al. investigated the nitrogen reduction reaction (NRR) on the (110) facets of transition metal nitrides (TMNs) using DFT. The study compared the (110) facets with the previously examined (100) facets and finds that the (110) facets generally exhibit higher overpotentials (OPs) and lower catalytic activity. Specifically, vanadium nitride (VN) shows the most promise among the tested TMNs, with a relatively low OP of -0.67 V and a favorable single vacancy mechanism for ammonia formation. The research highlights that while the (110) facets of VN demonstrate some catalytic efficiency, they are still less active and selective compared to the (100) facets. Additionally, the study emphasizes the importance of careful surface engineering to ensure the presence of optimal surface orientations, as polycrystalline samples with mixed facets could negatively impact overall catalytic performance. This investigation underscores the critical need for precise control over the crystallographic orientation in developing effective TMN catalysts for ammonia synthesis [
17].
A study by Al Sobhi et al. investigates the impact of substituting molybdenum (Mo) with tungsten (W) in Co₃Mo₃N and Ni₂Mo₃N on ammonia synthesis activity and lattice nitrogen reactivity. The research found that tungsten substitution decreases the catalytic performance of both Co₃Mo₃N and Ni₂Mo₃N, with Co₃Mo₃N exhibiting a transformation to Co₆Mo₆N, indicating significant lattice nitrogen loss, while Ni₂Mo₃N retained its lattice nitrogen, showing limited reactivity. Computational calculations revealed that tungsten has minimal impact on the formation energy of surface nitrogen vacancies and the adsorption and activation of nitrogen molecules. Despite lower ammonia synthesis activity in tungsten-doped variants, both materials maintained stable performance over extended periods. The findings suggest that while Co₃Mo₃N is a more promising candidate due to its higher lattice nitrogen reactivity, further modifications are needed to enhance its catalytic efficiency for industrial applications [
18].
Expanding on the examination of lattice nitrogen reactivity in transition metal nitrides, the study by Daisley et al. focuses on the ternary molybdenum nitrides Co₂Mo₃N and Fe₃Mo₃N, comparing them with Co₃Mo₃N. The research investigates these materials under a 3:1 H₂/Ar mixture at temperatures up to 900°C. The η-carbide structured Co₃Mo₃N demonstrated high reactivity, with significant nitrogen loss and transformation into Co₆Mo₆N, consistent with the Mars-van Krevelen mechanism. In contrast, the filled β-Mn structured Co₂Mo₃N showed stability up to 800°C, decomposing only at 900°C, suggesting a lower reactivity of its lattice nitrogen. Fe₃Mo₃N exhibited minimal nitrogen loss up to 800°C but decomposed at 900°C, forming iron nitride and molybdenum metal. The study highlights that both metal composition and phase significantly influence the bulk lattice nitrogen reactivity in these ternary nitrides, with Co₃Mo₃N showing the highest reactivity due to its optimal nitrogen binding energy and favorable structural properties for nitrogen activation [
19].
The study by Higham
et al. investigates the catalytic ammonia synthesis mechanisms in Fe₃Mo₃N, comparing it with the previously studied Co₃Mo₃N. Using plane-wave DFT, the research examines surface nitrogen vacancy formation and two distinct ammonia synthesis mechanisms: the associative Mars–van Krevelen (ER–MvK) and the dissociative Langmuir–Hinshelwood (LH) mechanisms. The findings reveal that nitrogen vacancy formation on Fe₃Mo₃N is thermodynamically more demanding than on Co₃Mo₃N, yet still feasible, suggesting that surface lattice nitrogen vacancies in Fe₃Mo₃N can facilitate ammonia synthesis. The calculations show that nitrogen activation is enhanced on Fe₃Mo₃N compared to Co₃Mo₃N, with adsorption at and adjacent to the vacancy being more favorable. The associative ER–MvK mechanism provides a less energy-demanding pathway for ammonia synthesis, particularly for the initial hydrogenation processes. However, the LH mechanism shows high activation barriers for subsequent hydrogenation steps, indicating that while both mechanisms are viable, the ER–MvK pathway is more kinetically accessible. Additionally, Fe₃Mo₃N shares the same associative distal mechanism for ammonia synthesis as the Co₃Mo₃N catalyst but is somewhat less active overall. These insights highlight the potential of Fe₃Mo₃N as a catalyst for ammonia synthesis, particularly in the context of optimizing the Co₃−ₓFeₓMo₃N system to enhance catalytic activity under milder conditions [
20].
Extending the exploration of anti-perovskite nitrides for ammonia synthesis, a study by Daisley et al. investigated the catalytic activities of Co₃CuN, Ni₃CuN, and Co₃MoN. Experimental and theoretical analyses reveal that Co₃CuN demonstrates a higher conversion of lattice nitrogen to ammonia compared to Ni₃CuN, exhibiting activity at lower temperatures. The loss of lattice nitrogen in Co₃CuN was topotactic, resulting in the formation of Co₃Cu, whereas Ni₃CuN showed minimal activity at lower temperatures and required higher temperatures for significant nitrogen loss. The study suggests that the metal composition influences the stability and activity of these nitrides, with computational modeling showing differences in nitrogen vacancy formation energies and the density of states at the Fermi level. Co₃MoN, unlike Co₃CuN and Ni₃CuN, maintained steady-state catalytic activity at 400°C with a rate of 92 ± 15 mmol h⁻¹ g⁻¹, highlighting the impact of metal composition on catalytic performance. These findings underscore the potential of anti-perovskite nitrides in ammonia synthesis through chemical looping, though regeneration using N₂ remains challenging, indicating a need for further optimization [
21].
Ammonia synthesis on manganese nitride (Mn
6N
5) begins with nitrogen vacancies on the catalyst's surface, serving as active sites for the reaction. Molecular nitrogen (N
2) adsorbs at these vacancies in an end-on configuration, allowing one nitrogen atom to interact directly with the nitrogen vacancies [
22]. This interaction significantly weakens the N≡N triple bond. Concurrently, molecular hydrogen (H
2) adsorbs directly from the gas phase onto the end-on adsorbed nitrogens forming >NNH
2 species. Two such >NNH
2 species react with a hydrogen coming from the gas phase forming two >NNH
3 species. These >NNH
3 species dissociate forming ammonia and leaving behind surface nitrogen, which is adsorbed at the nitrogen vacancy. These nitrogen species become hydrogenated forming NH
2 and then NH
3 that desorbs from the surface. The NH
3 desorbs from the surface, freeing up active sites for new nitrogen molecules and perpetuating the catalytic cycle. This mechanism underscores the essential role of nitrogen vacancies and the stepwise hydrogenation process on Mn
6N
5, highlighting the importance of tailoring the surface properties and active sites in order to achieve efficient ammonia synthesis [
23].
Building on previous research, the study by Zeinalipour-Yazdi
et al. employs dispersion-corrected density functional theory (DFT-D3) to investigate the chemisorption of H₂ and N₂ on cobalt-promoted Ta₃N₅ surfaces, focusing on the (100), (010), and (001) facets. The study reveals that nitrogen adsorbs mostly molecularly in side-on, end-on, and tilt configurations, with the formation of azide functional groups at bridging nitrogen sites, exhibiting a formation energy of 205 kJ/mol. Hydrogen was found to chemisorb molecularly with adsorption energies ranging from -81 to -91 kJ/mol, but at bridging nitrogen sites, it dissociates to form 4NH groups with an exothermic formation energy of -175 kJ/mol per H₂ molecule. The presence of cobalt promoters significantly enhances the dissociation of molecular hydrogen on the Ta₃N₅ surface, primarily at nitrogen-rich sites, with adsorption energies between -200 and -400 kJ/mol. This enhancement in dissociation suggests that cobalt promoters play a critical role in facilitating the hydrogenation steps of ammonia synthesis on Ta₃N₅ by lowering the formation energy of nitrogen vacancies, thus increasing the catalyst's overall reactivity and efficiency [
24].
Iron molybdenum nitride (Fe₃Mo₃N) follows a similar mechanism to Co₃Mo₃N for ammonia synthesis but exhibits lower reactivity. The Eley–Rideal/Mars–van Krevelen mechanism on Fe₃Mo₃N involves nitrogen vacancies and the subsequent hydrogenation of nitrogen species. Despite following a similar pathway as Co₃Mo₃N, Fe₃Mo₃N has higher activation barriers for the hydrogenation steps. The desorption of ammonia from the catalyst surface requires less energy compared to the hydrogenation steps, but overall, Fe₃Mo₃N is less reactive than Co₃Mo₃N and Mn₆N₅ [
15].
Recent studies have emphasized the significance of nitrogen vacancies in the catalytic activity of metal nitrides. Nitrogen vacancies act as active sites for the adsorption and activation of N₂ molecules. For example, in Co₃Mo₃N, the presence of nitrogen vacancies facilitates the associative Eley–Rideal mechanism, which has been shown to have lower activation barriers compared to the Langmuir–Hinshelwood mechanism [
25]. This suggests that designing catalysts with a high concentration of nitrogen vacancies could enhance ammonia synthesis rates. In the Eley–Rideal/Mars–van Krevelen Mechanism nitrogen vacancies on the Co₃Mo₃N surface adsorb N₂ molecules end-on, which then react with dissociated hydrogen to form ammonia. This mechanism is favored due to its lower activation barriers
We observe that for the mechanism suggested for the iron and ruthenium catalyst, which is the Langmuir–Hinshelwood mechanism. That this involves the side-on adsorption of N₂ and its subsequent dissociation and hydrogenation on the catalyst surface. This mechanism has barriers that are higher for the hydrogenation steps than the associative distal Eley-Rideal/ Mars-van Krevelen mechanism. Clearly suggesting that the kinetically faster mechanism on Co3Mo3N and Fe3Mo3N is a different mechanism that what has been suggested for iron and ruthenium.
The manuscript by Hargreaves and Daisley provides an insightful overview of recent advancements in the development of nitrides, hydrides, and carbides as alternative heterogeneous catalysts for ammonia synthesis [
26]. The paper emphasizes the potential of these materials to facilitate sustainable ammonia production through mechanisms that are more akin to enzymatic nitrogen activation, thus potentially allowing for milder reaction conditions compared to the traditional Haber-Bosch process. The authors highlight that metal nitrides, such as Co₃Mo₃N, can operate via a Mars-van Krevelen mechanism, involving the direct hydrogenation of lattice nitrogen, which then leads to nitrogen vacancies that can be replenished during the reaction cycle. This process can be enhanced by incorporating elements like cobalt, which helps balance nitrogen adsorption strengths, thereby improving catalytic efficiency. The study also explores the synergistic effects of combining nitrides with other materials, such as hydrides, to overcome scaling limitations through dual-site mechanisms. For instance, nickel/lanthanum nitride catalysts exhibit high activity and stability by leveraging nitrogen vacancies for nitrogen activation and using nickel for hydrogen dissociation. The authors note that the integration of alkali metal doping and hydrogen activation remains a relatively underexplored yet promising area for catalyst development. Overall, this work underscores the exciting potential and ongoing challenges in designing novel catalysts for localized, sustainable ammonia synthesis, capable of operating under lower temperatures and pressures while maintaining high efficiency and durability [
26].
Integration of experimental and computational approaches has been crucial in understanding the role of nitrogen vacancies and optimizing catalytic performance. Studies involving isotopic nitrogen exchange and computational modeling have shown that nitrogen vacancies are effective sites for N₂ activation and that the presence of surface defects can significantly influence catalytic activity [
25]. Isotopic nitrogen exchange have been recently performed to better understand the role on nitrogen adsorbed to the surface of the catalyst on the kinetics of the reaction. Experiments have demonstrated the participation of lattice nitrogen in the ammonia synthesis reaction, indicating that nitrogen vacancies are replenished during the reaction cycle.
Computational Modelling DFT calculations have supported experimental observations, showing that nitrogen vacancies have lower formation energies and are effective for N₂ activation at synthesis temperatures but the exact role of the removal of lattice nitrogen is not clearly understood. Further computational studies are necessary that can identify the exact role of the surface versus lattice nitrogen removal and its participation in the mechanism of ammonia synthesis [
25].
The addition of promoters to metal nitrides can further enhance their catalytic activity. For instance, doping Ta₃N₅ with cobalt has been shown to increase ammonia synthesis rates by facilitating hydrogen dissociation and nitrogen activation [
24]. Similarly, lithium-doped Mn₆N₅ has exhibited improved activity and stability, highlighting the potential of dopants to modify surface chemistry and catalytic performance [
27]. However the role of dopants in the mechanism for ammonia synthesis on metal nitrides is currently not well understood on an atomistic basis and further computational studies in this direction are necessary to find the exact formulation of metal nitride/promoter system that will have greater stability and activity for ammonia synthesis.
Chemical looping is an emerging approach in ammonia synthesis that leverages the Mars-van Krevelen mechanism. In this process, the lattice nitrogen in metal nitrides acts as an active species, which can be replenished in a separate regeneration step. Recent studies have explored various metal nitrides, such as manganese nitride and cerium nitride, for their potential in chemical looping systems [
28].
Manganese nitride has been identified as a promising candidate for chemical looping due to its ability to form and utilize nitrogen vacancies. Doping with lithium has been shown to enhance the reactivity of lattice nitrogen, thereby improving ammonia formation rates [
28].
Cerium nitride (CeN), especially when supported on nickel (Ni/CeN), exhibits favorable formation of nitrogen vacancies, which play a crucial role in enhancing its catalytic activity for ammonia synthesis. The ammonia synthesis process on CeN involves both dissociative and associative pathways. In the dissociative pathway, nitrogen molecules adsorb at the nitrogen vacancies and dissociate into atomic nitrogen, which subsequently reacts with hydrogen to form ammonia. In the associative pathway, nitrogen molecules are hydrogenated stepwise without prior dissociation, forming intermediates such as NH and NH₂ before finally producing NH₃ [
29] This dual-pathway mechanism enhances the overall performance of the catalyst by allowing it to utilize multiple reaction routes.
The chemical looping process further contributes to the high activity of CeN in ammonia synthesis. This process involves the continuous cycling of nitrogen between the catalyst and the reaction environment, facilitated by the Mars-van Krevelen mechanism. In this mechanism, lattice nitrogen in CeN is involved in the reaction cycle, participating in the formation of ammonia and subsequently being replenished by nitrogen from the gas phase. This ensures a steady supply of active sites and maintains high catalytic efficiency over extended periods.
Chorkendorff and co-workers have investigated the spin-mediated promotion of a cobalt catalyst for ammonia synthesis [
30]. They find that suppressing the magnetism in the catayst may be the key for lower temeprature operation of the catalyst. They show that one can use lanthanum to quench the magnetic moment of cobalt atoms on the surface of the catalyst. This results in a lower activation barrier for nitrogen cleavage compared to the barrier found on the iron catalyst.
Recent research has further expanded the understanding of molybdenum-based metal nitrides, specifically focusing on the lattice nitrogen-mediated ammonia production. This research employs a multistage design strategy to correlate the intrinsic activity of these catalysts with their electronic structures, utilizing DFT calculations as a critical tool. The study conducted by Qian
et al. uses DFT to investigate the electronic properties and catalytic behavior of molybdenum-based nitrides, such as Mo
2N and MoN. These nitrides are analyzed to understand their ability to activate nitrogen molecules and facilitate the ammonia synthesis process. The findings reveal that the lattice nitrogen in these materials plays a crucial role in the reaction mechanism, participating directly in the formation of ammonia through both associative and dissociative pathways. The associative mechanism involves the stepwise hydrogenation of adsorbed nitrogen species, forming intermediates like NH and NH
2, while the dissociative mechanism involves the breaking of the N≡N bond to form atomic nitrogen, which then reacts with hydrogen to produce ammonia. The study's computational approach provides detailed insights into the energy profiles and transition states of these reactions, highlighting the importance of electronic structure in determining the catalytic efficiency of molybdenum-based nitrides. The research underscores the potential of these materials in sustainable ammonia production, offering a pathway to optimize catalysts for industrial applications [
31].
Molybdenum-based nitrides, including Co₃Mo₃N, Fe₃Mo₃N, Ni₃Mo₃N, and Mo₂N, were employed as model catalysts. These materials were chosen to decouple the effects of electronic properties and geometrical features on catalytic activity.
It is obvious that still alot can be discovered in the thermal ammonia synthesis that utilises nitrogen vacancies. In the following sections we shall explore further the electrochemical synthesis of ammonia and the photocatalytic synthesis.
The study by Hanifpour
et al. investigates the electrochemical nitrogen reduction reaction (NRR) on transition metal nitride (TMN) thin films, specifically VN, CrN, NbN, and ZrN, using a micro-reactor flow-cell setup under ambient conditions. Employing chronoamperometry for ammonia production analysis, the research reveals that ZrN shows promising catalytic behavior, producing ammonia with higher reaction rates and current efficiencies in the presence of N₂ compared to Ar. Conversely, VN and NbN initially produce ammonia through the reaction of surface nitrides but become inactive afterward, and CrN does not produce any detectable ammonia. The study utilizes electrochemical impedance spectroscopy (EIS), X-ray reflectivity (XRR), and X-ray photoelectron spectroscopy (XPS) to provide detailed insights into the stability and surface characteristics of the TMNs, emphasizing the critical role of nitrogen vacancies and surface dynamics in catalytic performance. These findings highlight the importance of precise control and characterization in developing efficient electrochemical catalysts for ammonia synthesis [
32].
The study by Abghoui
et al. employs DFT to investigate the electrochemical reduction of nitrogen to ammonia at ambient conditions on transition metal nitride surfaces, specifically VN, ZrN, NbN, and CrN. The study identifies these nitrides as promising catalysts for ammonia synthesis due to their higher activity towards nitrogen reduction compared to the competing hydrogen evolution reaction (HER). The research highlights that VN, with its rocksalt (100) facet, can effectively reduce nitrogen to ammonia via a Mars−van Krevelen mechanism with only a -0.5 V overpotential, avoiding catalyst decomposition. Additionally, the study underscores the stability of these nitrides against poisoning and their potential to achieve sustainable and energy-efficient ammonia production at low temperatures and ambient pressures, offering a viable alternative to the traditional Haber-Bosch process. The comprehensive DFT analysis provides insights into the thermodynamics and kinetics of the nitrogen reduction reaction on these surfaces, guiding the development of efficient electrochemical ammonia synthesis catalysts [
33].
The study by Lu
et al. focuses on the development of chemically durable nickel and cobalt lanthanum-nitride-based catalysts for ammonia synthesis. The authors address the challenge of high sensitivity to air and moisture in metal nitride complexes by introducing aluminum into the LaN lattice, forming La₃AlN. This modification creates La-Al metallic bonds that enhance chemical stability while retaining catalytic functionality. The resulting Ni/La₃AlN and Co/La₃AlN catalysts demonstrated significant catalytic activity without degradation after exposure to air and moisture, achieving reaction rates of 2410 μmolg⁻¹h⁻¹ for Ni/La₃AlN and 2735 μmolg⁻¹h⁻¹ for Co/La₃AlN at 400°C and 0.1 MPa. The study highlights the dual active site mechanism, where La₃AlN facilitates N₂ absorption and activation, offering a promising approach to developing stable and efficient catalysts for ammonia synthesis [
34].
The study by Bin Liu
et al. investigates the manipulation of geometric and electronic structures of manganese nitrides (Mn₄N and Mn₂N) for improved ammonia synthesis. This research highlights that modifying Mn nitrides with transition metal heteroatoms (Cr, Fe, Co, Ni, Mo) can significantly influence the binding energies of intermediate hydrogenation products and the overall ammonia formation process. DFT calculations reveal that while the binding of NH on Mn nitride surfaces follows a linear relationship with lattice nitrogen, the binding of NH₂ and NH₃ is more sensitive to changes in geometric and electronic structures. The study also identifies that the rate-determining step for ammonia synthesis involves the diffusion of lattice nitrogen, which can be facilitated by introducing single-atom dopants. This comprehensive approach provides insights into optimizing Mn nitrides as nitrogen carriers for efficient ammonia production, emphasizing the critical role of electronic structure modifications in enhancing catalytic performance [
35].
2.2. Electrochemical Ammonia Synthesis
Electrochemical ammonia synthesis is a promising alternative to traditional thermal methods, offering the potential for ammonia production at ambient conditions using renewable energy sources. This approach not only reduces the energy requirements but also provides a more sustainable pathway by potentially eliminating carbon emissions. The electrochemical nitrogen reduction reaction (NRR) involves the stepwise reduction of nitrogen (N₂) to ammonia (NH₃) at the cathode, with hydrogen (H⁺) ions being supplied from the electrolyte. The basic reactions can be summarized as follows:
N2 + 6H+ + 6e- → 2NH3
This process competes with the hydrogen evolution reaction (HER), which often occurs concurrently, thus affecting the selectivity and efficiency of ammonia production. Significant contributions have been made by various research groups, notably the group led by Skúlason and co-workers. They have developed detailed thermochemical data and computational models to identify potential catalysts for nitrogen reduction reactions. One notable study provided a comprehensive analysis of nitrogen-binding-energy descriptors, establishing new insights into the limiting potentials required for effective electrochemical ammonia synthesis [
36]. Mo-based catalysts have shown promise due to their moderate nitrogen binding energy, which favors the NRR over HER. Studies indicate that molybdenum can serve as a robust catalyst under appropriate conditions [
36] Iron (Fe) is commonly investigated due to its abundance and catalytic properties. However, Fe also promotes HER, making it less selective for NRR in aqueous media [
36]. Ruthenium (Ru) and Rhodium (Rh) have shown high activity for NRR but also suffer from significant HER competition, requiring further optimization for selectivity [
36,
37].
Recent studies have identified several overlooked catalysts that show potential for selective NRR. Manganese (Mn) has emerged as a highly reactive and selective catalyst for NRR, particularly at higher pH levels where HER is less favored [
36]. Some post-transition metals such as gallium (Ga) and indium (In) have shown promise due to their poor HER promotion and strong nitrogen binding, making them suitable candidates for selective NRR in both aqueous and non-aqueous media [
36] Tantalum oxide (TaO₂), rhenium oxide (ReO₂), and osmium oxide (OsO₂) have been studied in recent computational studies and it was found that these rutile oxides have a potential for electrochemical ammonia formation. These oxides were found to have significantly lower potential determining steps (PDS) than previously reported, indicating their strong candidacy for NRR. TaO₂, in particular, was identified as binding NNH stronger than H, making it a promising candidate for selective ammonia synthesis [
38]. Recent DFT calculations on niobium carbonitride (NbCN) have identified NbCN as a highly effective catalyst for electrochemical NRR. It exhibits both activity and stability, capable of self-regeneration and nitrogen-to-ammonia activation with a low potential-determining step energy of 0.58 eV. NbCN facilitates ammonia formation via a mixed associative Mars-van Krevelen (MvK) mechanism [
39]. Tungsten carbonitride (WCN) has also shown promise for efficient ammonia synthesis, but it faces challenges related to higher activation energies and potential poisoning by other species in the electrolyte [
39]. FexOy Co-deposited on amorphous MoS₂: Recent research has shown that FexOy co-deposited on amorphous MoS₂ supported on a gas diffusion layer electrode (GDL) significantly enhances NRR activity. The GDL/MoS₂-Fe-1 catalyst exhibited an NH₃ yield of 7.38 µmol h⁻¹ cm⁻² and a Faradaic efficiency of 54.9% at -0.2 V vs. RHE at 25°C. This performance is attributed to the amorphous structure of MoS₂ providing abundant active sites, and Fe
xO
y enhancing electron transfer and nitrogen adsorption [
40].
Recent DFT calculations have provided insights into the mechanistic pathways of NRR on the Ru(0001) surface. The study elucidates that the initial activation of N₂ to form NNH is the rate-limiting step, with an energy barrier of 0.8 eV at an applied potential of -0.6 V. The protonation steps involve low barriers of 0.0-0.25 eV, indicating efficient proton-electron transfer processes. The preferred pathway follows an associative distal mechanism, where the N-N bond is cleaved after the third proton-electron transfer, resulting in ammonia formation [
37]. Homogeneous catalysts, typically molecular complexes with transition metals as their central atoms, are dissolved in the electrolyte. These catalysts operate through cyclic mechanisms where the metal centers coordinate with nitrogen, store charges, and facilitate the reduction process. However, the challenge lies in their separation and reuse post-reaction, which can hinder commercial applications [
41]. The use of biological catalysts, inspired by the natural nitrogenase enzyme, is an emerging area in electrochemical NRR. These catalysts mimic the natural process of nitrogen fixation and offer a potentially sustainable approach to NH₃ synthesis. However, their practical application requires overcoming challenges related to stability and efficiency under electrochemical conditions [
41].
A recent study demonstrated the effectiveness of nitrogen and phosphorus co-doped porous carbon (NPC) as an electrocatalyst for NRR. The NPC was prepared by pyrolyzing polyaniline aerogels in the presence of phytic acid. This co-doping introduced defects and heteroatoms that enhanced the catalyst's activity by providing more active sites and improving electron transfer [
42]. The electrochemical performance of the N, P co-doped carbon mater (NPC) catalyst exhibited a high NH₃ yield of 7.38 µmol h⁻¹ cm⁻² and a Faradaic efficiency of 54.9% at -0.2 V vs. RHE in 0.1 M HCl electrolyte. The high surface area and porosity of NPC, combined with the synergistic effects of N and P co-doping, contributed to its superior performance [
42]. Atomic structure modifications, such as defect engineering, surface orientation, and amorphization, have shown significant promise in improving the efficiency of electrochemical NRR. A recent review by Chen
et al. summarizes the progress in these areas, highlighting strategies like heteroatom doping and creating atom vacancies to introduce extra active sites and enhance the intrinsic activity of catalysts. For example, heteroatom doping (e.g., metal atoms like Fe and non-metal atoms like B) can increase active sites and reduce the energy barrier for the rate-determining step of NRR by forming extraordinary coordination environments and electronic structures [
43]. Additionally, surface orientation and amorphization of catalysts can significantly influence their catalytic performance. Stepped facets and amorphous structures provide abundant active sites and unique electronic properties that enhance NRR efficiency [
43]. Traditional metal electrodes like platinum and gold are often used, but their tendency to promote HER limits their effectiveness for NRR. Emerging materials such as metal nitrides and carbides are being explored for better selectivity and efficiency.
Aqueous electrolytes commonly used due to their availability and ease of handling. However, the high proton concentration favors HER, necessitating the search for alternative electrolytes. Non-aqueous electrolytes such as ionic liquids, offer a reduced proton environment, thus favoring NRR over HER. They are gaining attention for their ability to improve the selectivity of ammonia synthesis [
36]. Optimal reaction conditions are crucial for maximizing the efficiency of electrochemical ammonia synthesis. Lower temperatures and ambient pressures are typically favorable, aligning with the principles of electrochemical processes. The applied potential must be carefully controlled to favor NRR while minimizing HER. Recent research has provided detailed guidelines on the appropriate potentials for various catalysts [
36]. The mechanistic pathways for NRR on different catalysts can follow either an associative or dissociative mechanism. Associative mechanism, involves the stepwise addition of protons and electrons to adsorbed N₂, forming NH₃ via intermediates such as N₂H and NH. Dissociative mechanism, involves the initial cleavage of the N≡N bond, followed by protonation of the resulting nitrogen atoms. This pathway is less favored at ambient conditions due to the high energy barrier for N₂ dissociation [
36]. Despite the progress, several challenges remain, one is the Faradaic efficiency, the efficiency of converting electrical energy to chemical energy in the form of NH₃ remains low due to competing HER. Long-term stability of catalysts in the electrochemical environment is a critical issue, with many catalysts degrading over time. The cost of developing and scaling up new catalyst materials and processes needs to be addressed for commercial viability.
The study by Nørskov and Chorkendorff and co-workers emphasises that special care needs to be taken in studies of electrochemical ammonia synthesis to avoid false positives [
44]. In these experiments the ammonia attributed to electrochemical nitrogen fixation was found to be due to contamination from ammonia in the air, human breath or ion conducting membranes, or generated from labile nitrogen-containing compounds (for example, nitrates, amines, nitrites and nitrogen oxides). They continue to provide a protocol that should prevent false positives in experimental studies of the reduction of nitrogen to ammonia.
A study by Iqbal
et al. explored the potential of transition metal carbonitrides (TMCNs) as electrocatalysts for the nitrogen reduction reaction (NRR) under ambient conditions. Using DFT, the research examined the (111) facets of TMCNs, including VCN, NbCN, and WCN. The findings revealed that VCN and NbCN are particularly promising for NRR via the Mars-van Krevelen mechanism, requiring low onset potentials of -0.52 V and -0.53 V vs. RHE, respectively. This low energy requirement makes them efficient catalysts for ammonia synthesis at ambient conditions. The study also highlighted that while WCN showed potential, it was more susceptible to poisoning in electrochemical environments. The research underscored the importance of selecting materials with the right balance of activity and stability to enhance ammonia production efficiency. This comprehensive investigation into TMCNs provides valuable insights for developing sustainable and efficient electrocatalysts for ammonia synthesis [
39].
A paper by Liu
et al. reviews the development of electrocatalysts for the efficient nitrogen reduction reaction (e-NRR) under ambient conditions, emphasizing the need for novel catalysts to enhance practical applications. The study highlights the mechanisms of NH3 electrochemical synthesis and outlines strategies to improve catalytic performance by increasing exposed active sites or tuning electronic structures. Various novel electrocatalysts, including noble metal-based materials, single-metal-atom catalysts, non-noble metals, and metal-free materials, are systematically summarized. The review also discusses the role of surface control, defect engineering, and hybridization in improving catalytic efficiency. These insights provide valuable guidance for designing advanced catalytic systems for sustainable and efficient ammonia production through e-NRR, aiming to achieve higher energy efficiency and support decentralized ammonia production to reduce transportation costs and environmental impact [
45].
A study by Li
et al. investigated the electrochemical promotion of ammonia formation on Fe-based electrode catalysts using proton-conducting-electrolyte-supported cells with H
2–Ar and H
2–N
2 at temperatures between 550 °C and 600 °C under ambient pressure. The research examines the ammonia formation rate using two types of cathodes: a porous pure Fe electrode with a shorter triple phase boundary (TPB) length and a cermet electrode consisting of Fe–BCY (or W–Fe–BCY) with a longer TPB length. The results demonstrate that the porous pure Fe electrode outperforms the Fe–BCY cermet electrode, indicating that ammonia formation is primarily accelerated by the electrochemical promotion of catalysis (EPOC) effect on the Fe surface rather than the charge-transfer reaction at the TPB. The mechanism is dominated by a dissociative process, where direct N2 bond dissociation is accelerated with cathodic polarization on the Fe surface, with a minor contribution from a proton-assisted associative mechanism at the TPB. Despite the relatively short TPB length, the porous pure Fe cathode achieves a high ammonia formation rate of 1.4 × 10
−8 mol cm
-2 s
-1 (450 μg h
-1 mg
-1) under optimal conditions, suggesting that the effective double layer extends widely on the Fe electrode surface. This study highlights key processes for improving ammonia formation and underscores the effectiveness of the porous pure Fe electrode for electrochemical ammonia synthesis [
46].
A study by Chen
et al. presents a novel approach for enhancing electrochemical nitrogen reduction reaction (NRR) performance using non-noble metal sulfide catalysts integrated with a conductive matrix. The research highlights the self-organized growth of flower-like SnS
2 and forest-like ZnS nanoarrays on nickel foam through solvothermal conditions. These innovative structures exhibit strong nitrogen activation abilities, further enhanced by their formation on 3D porous Ni foam, which provides a large surface area and facilitates easy electrolyte permeation. Nickel foam is chosen for its superior electrical conductivity and mechanical robustness, significantly outperforming carbon-based materials. The resulting SnS
2@Ni and ZnS@Ni foams demonstrate high ammonia yields and faradaic efficiencies, rivaling or surpassing those of noble-metal-based catalysts. This work underscores the potential of integrating metal sulfides with conductive matrices to develop advanced hybrid catalysts for efficient and scalable ammonia synthesis [
47].
A study by Cui
et al. reviewed the progress and challenges in the electrocatalytic reduction of dinitrogen (N₂) to ammonia (NH₃) under ambient conditions. The paper highlights the urgent need for sustainable and eco-friendly energy pathways, given the increasing global population and depletion of fossil fuels. The authors focus on the development of heterogeneous electrocatalysts capable of facilitating the nitrogen reduction reaction (NRR) at room temperature and atmospheric pressure. They summarize the prevailing theories and mechanisms for NRR, computational screening of promising materials, and the design of electrochemical systems to enhance activity, selectivity, efficiency, and stability. The review emphasized the importance of overcoming challenges such as high energy consumption and negative environmental impacts associated with traditional ammonia synthesis methods like the Haber-Bosch process. Promising strategies to improve NRR electrocatalysts, such as cationic/anionic regulation, heteroatom doping, and defect/strain engineering, are proposed, along with the rational design of catalyst/electrolyte and electrode/catalyst interfaces to suppress competing hydrogen evolution reactions. The study provides a comprehensive overview of recent advancements and offers insights for future research directions in the field of electrocatalytic ammonia synthesis [
48].
2.3. Additional Studies Performing Mechanistic Studies of Ammonia Synthesis
Recent research has further expanded the understanding of molybdenum-based metal nitrides, specifically focusing on lattice nitrogen-mediated ammonia production. This research employs a multistage design strategy to correlate the intrinsic activity of these catalysts with their electronic structures, utilizing DFT calculations as a critical tool. A study conducted by Qian
et al. uses DFT to investigate the electronic properties and catalytic behavior of molybdenum-based nitrides, such as Mo
2N and MoN. These nitrides are analyzed to understand their ability to activate nitrogen molecules and facilitate the ammonia synthesis process. The findings reveal that the lattice nitrogen in these materials plays a crucial role in the reaction mechanism, participating directly in the formation of ammonia through both associative and dissociative pathways. The associative mechanism involves the stepwise hydrogenation of adsorbed nitrogen species, forming intermediates like NH and NH
2, while the dissociative mechanism involves the breaking of the N≡N bond to form atomic nitrogen, which then reacts with hydrogen to produce ammonia. The study's computational approach provides detailed insights into the energy profiles and transition states of these reactions, highlighting the importance of electronic structure in determining the catalytic efficiency of molybdenum-based nitrides. The research underscores the potential of these materials in sustainable ammonia production, offering a pathway to optimize catalysts for industrial applications [
31].
The Langmuir–Hinshelwood mechanism for ammonia synthesis involves a series of well-defined steps starting with the adsorption of molecular nitrogen (N2) onto the surface of the catalyst. In this mechanism, nitrogen molecules are adsorbed in a side-on configuration at the active sites of the catalyst. This adsorption mode allows the nitrogen molecule to interact simultaneously with multiple active sites, leading to significant activation of the N≡N triple bond. Once adsorbed, the nitrogen molecule undergoes dissociation into two nitrogen atoms, each of which is further adsorbed onto the catalyst surface in a bridged configuration. These bridged nitrogen species are highly reactive intermediates that play a crucial role in the subsequent steps of the reaction. The dissociation of the nitrogen molecule is facilitated by the strong interaction with the catalyst surface, which lowers the activation energy required to break the N≡N bond. Following the dissociation, hydrogen molecules (H2) adsorb onto the catalyst surface and dissociate into individual hydrogen atoms. These hydrogen atoms then migrate across the surface and react with the bridged nitrogen species in a stepwise manner. This stepwise hydrogenation leads to the formation of various nitrogen-hydrogen intermediates such as NH, NH2, and ultimately NH3 (ammonia). The final product, ammonia, desorbs from the catalyst surface, freeing up active sites for new nitrogen and hydrogen molecules to be adsorbed, thereby continuing the catalytic cycle. The efficiency of this mechanism depends on the ability of the catalyst to facilitate the adsorption, activation, and dissociation of nitrogen and hydrogen molecules, as well as the subsequent hydrogenation steps.
Continuing the investigation into transition metal catalysts for nitrogen reduction, the study by Ellingsson et al. focuses on the activity of transition metal carbide (TMC) surfaces in the (100) facets of the rocksalt (RS) structure as potential catalysts for the nitrogen reduction reaction (NRR). Using DFT, the research models reaction pathways, estimates stability, assesses kinetic barriers, and compares adsorbate energies to determine the overall performance of various TMC surfaces. The study found that pristine TMC surfaces without defects generally did not possess both exergonic nitrogen adsorption and the capability to selectively protonate nitrogen to form ammonia in an aqueous solution. However, ZrC showed potential as a catalyst in non-aqueous electrolytes. Introducing carbon vacancies provided high coordination active sites on the surface, significantly improving nitrogen adsorption, selectivity towards ammonia, and lowering overpotential (OP) for NbC and WC. Despite this, NbC displayed an unfeasible kinetic barrier for nitrogen dissociation at ambient conditions, suggesting its suitability for high temperature/pressure ammonia synthesis. WC and VC emerged as promising materials for further experimental investigation in aqueous electrolytes due to their efficient nitrogen adsorption and favorable reaction pathways [
49].
Electronic Structure Analysis in a study by Qian
et al. reveals that electron transfer between molybdenum and magnetic metals, along with the spin polarization characteristics of magnetic metals, are key descriptors of intrinsic catalytic activity. The modulation of these electronic properties through incorporating magnetic metals significantly enhances lattice nitrogen activity. This enhancement occurs because electron transfer increases the density of states at the Fermi level, facilitating nitrogen molecule activation by weakening the N≡N bond. Additionally, spin polarization stabilizes reaction intermediates, lowering the activation energy for nitrogen dissociation and subsequent hydrogenation steps. These factors collectively improve the catalyst's ability to convert nitrogen to ammonia efficiently [
31].
Expanding on the role of nitrogen vacancies in catalytic activity, the study by Abghoui et al. investigates the influence of incorporating titanium nitride (TiN) into the structure of chromium, vanadium, niobium, and zirconium nitrides. Using DFT analyses, the study finds that combining TiN with vanadium nitride enhances the potential-determining step of the nitrogen reduction reaction (NRR) by up to 20% compared to pure vanadium nitride, while maintaining a similar number of proton-electron transfer steps for forming ammonia. For chromium nitride, TiN incorporation improves the rate-determining step related to nitrogen adsorption and catalyst regeneration by around 90%. However, the integration negatively impacts niobium and zirconium nitrides, increasing the potential-determining step for niobium nitride and shifting the reaction pathway of zirconium nitride towards hydrogen evolution rather than nitrogen reduction. These results suggest that while TiN can enhance stability and reactivity for certain nitrides, it can detrimentally affect others, highlighting the need for tailored approaches in catalyst design [
50].
A recent mini-review by Gao
et al. revisits the potential of group 4–7 transition metals for heterogeneous catalytic ammonia synthesis, highlighting their ability to activate nitrogen under milder conditions than the traditional Haber-Bosch process. This review emphasizes that, despite the tendency of these metals to form nitrides that are difficult to hydrogenate, their high affinity for N2 makes them promising candidates for sustainable ammonia production. The review covers recent advances in activating these metals through various strategies, including alloying, incorporating non-transition metal elements, and employing external fields like electricity and light. These approaches aim to enhance the catalytic activity by weakening strong metal-nitrogen bonds and facilitating nitrogen hydrogenation. This renewed focus on group 4–7 transition metals could lead to the development of more efficient materials for ammonia synthesis, aligning with the goals of decentralized and flexible production systems [
51].
The study conducted by Iqbal
et al. explores the potential of transition metal carbonitrides (TMCNs) as electrocatalysts for the nitrogen reduction reaction (NRR) to produce ammonia under ambient conditions. Using DFT calculations, the researchers examined the thermodynamic feasibility and activity of various TMCNs with rocksalt (RS) structures, focusing on their (100) facets. The study identified VCN and NbCN as the most promising candidates for ammonia production via the Mars-van Krevelen (MvK) mechanism, with low onset potentials of -0.52 V and -0.53 V versus the reversible hydrogen electrode (RHE). These carbonitrides demonstrated a preference for NRR over the hydrogen evolution reaction (HER), making them suitable for efficient ammonia synthesis. The comprehensive analysis included free energy diagrams, adsorption energies, and kinetic barriers, providing valuable insights into the electrocatalytic behavior and stability of TMCNs. The results highlight the potential of VCN and NbCN as effective catalysts for sustainable ammonia production at ambient conditions, paving the way for further experimental validation and development [
52].
The study by Abghoui
et al. investigates the catalytic potential of transition metal sulfides (TMSs) for electrochemical nitrogen reduction reaction (NRR) to ammonia under ambient conditions. Using DFT calculations, the authors evaluated the performance of 18 different TMSs, including YS, ScS, ZrS, TiS, VS, CrS, NbS, NiS, FeS, MnS2, CoS2, IrS2, CuS2, OsS2, FeS2, RuS2, RhS2, and NiS2, in various crystal structures and facets. The study focused on both associative and dissociative mechanisms, analyzing the adsorption free energies of key intermediates like NNH and H to determine their selectivity for NRR over hydrogen evolution reaction (HER). RuS2 was identified as the most active sulfide for the associative mechanism with an overpotential of around 0.3 V, while TiS, VS, NbS, and CrS showed promise for both mechanisms with overpotentials ranging from 0.7 to 1.1 V. The results highlight the importance of material selection and the potential of TMSs to provide efficient and sustainable pathways for ammonia synthesis, potentially mimicking the natural enzymatic process. The study underscores the need for further experimental validation and optimization of these materials to enhance their catalytic performance for industrial applications [
53].
In a study by Kitano
et al., the electrochemical reduction of nitrogen to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V was investigated using DFT. The study revealed that ZrN, NbN, CrN, and VN possess significant potential for catalyzing the nitrogen reduction reaction (NRR) due to their ability to stabilize intermediate species and facilitate the dissociation of nitrogen. The catalytic activities of these nitrides were evaluated based on their electronic properties, surface adsorption energies, and reaction pathways. The findings suggested that these materials could lower the energy barriers for nitrogen activation and subsequent hydrogenation steps, making them promising candidates for ammonia synthesis under mild conditions. The theoretical insights provided by this study highlight the potential of transition metal nitrides as efficient and robust catalysts for electrochemical ammonia production at ambient conditions [
54].
A study by Roy
et al. demonstrates the efficacy of using dual active sites in bimetallic catalysts for ammonia synthesis under ambient pressure. The research focused on Fe-based catalysts mixed with Ru, Co, and Ni, synthesized using a mechanical milling method. Experimental results showed that Ru/Fe and Co/Fe catalysts significantly enhance ammonia synthesis at temperatures ranging from 400°C to 550°C, with activation energies of 45.73 kJ mol
−1 and 46.38 kJ mol
−1, respectively. The kinetic studies revealed that the inclusion of Ru or Co to Fe shifts the rate-determining step to NH
x formation, facilitated by electron donation from Fe to the co-catalysts. This study highlights the potential of bimetallic catalysts to operate efficiently under mild conditions, providing a promising approach for energy-efficient ammonia production [
55].
The study by Araia
et al. investigates the effects of metal loading and support particle size on the performance of Cs-promoted Ru catalysts supported on cerium oxide (CeO₂) for microwave-assisted ammonia synthesis at ambient pressure. The research demonstrates that smaller CeO₂ support sizes (25 nm) result in higher ammonia production rates due to better dispersion and smaller Ru particle sizes, leading to more active sites. In contrast, larger support sizes (5 μm) exhibit lower activity. The study also shows that increasing Ru loading enhances ammonia synthesis up to a point, after which the activity plateaus due to particle agglomeration. The findings highlight the potential of microwave-assisted synthesis to lower reaction temperatures and pressures, providing a more energy-efficient alternative to traditional methods [
56].
The study by Goto
et al. investigates the potential of anti-perovskite nitrides (Co₃ZnN, Ni₃ZnN, Co₃InN, and Ni₃InN) as nitrogen storage materials for chemical looping ammonia production. These nitrides were synthesized via ammonolysis of their respective precursor oxides and subsequently evaluated for their ammonia production capabilities under hydrogen. The findings revealed that all the nitrides produced significant amounts of ammonia, with Ni₃ZnN demonstrating the highest production rate. The study highlighted that Ni-containing nitrides exhibited higher ammonia production rates compared to Co-containing nitrides, likely due to the formation of intermediate phases (Ni₃ZnNx and Ni₃InNy) during the reaction, which facilitated the release of lattice nitrogen. This work emphasizes the potential of anti-perovskite nitrides in developing sustainable and efficient ammonia synthesis processes through chemical looping [
57].
2.4. Photocatalytic and Plasma Assisted Methods for Ammonia Synthesis
Photocatalytic and plasma-assisted methods for ammonia synthesis have garnered significant interest due to their potential to offer more sustainable and efficient alternatives to traditional thermal processes. These methods leverage unique mechanisms and advanced materials to enhance the ammonia production process. Photocatalytic ammonia synthesis utilizes light energy to drive the reduction of nitrogen to ammonia. This method often employs semiconducting materials that can absorb photons and generate electron-hole pairs, which then participate in the reduction and oxidation reactions necessary for ammonia formation. In this mechanism, nitrogen molecules adsorb onto the photocatalyst surface, and the generated electron-hole pairs facilitate the breaking of the strong N≡N bond, allowing for subsequent hydrogenation steps that lead to ammonia production. Various photocatalytic materials, such as titanium dioxide (TiO₂) and modified forms like doped TiO₂, have been studied for their effectiveness in harnessing solar energy to drive these reactions. Recent research has focused on enhancing the performance of these materials by extending their absorption range into the visible light spectrum and improving charge separation efficiency [
58].
On the other hand, plasma-assisted methods use non-thermal plasma to activate nitrogen and hydrogen molecules, facilitating their reaction to form ammonia at lower temperatures and pressures compared to the Haber-Bosch process. Non-thermal plasma provides a high-energy environment where electrons are energized to much higher temperatures than the gas molecules, allowing activation of nitrogen and hydrogen through collisions with energetic electrons. In these methods, nitrogen and hydrogen gases are introduced into a plasma reactor where they are exposed to an electric field, creating a highly reactive environment. The high-energy electrons in the plasma dissociate nitrogen molecules into reactive nitrogen atoms, which then react with hydrogen atoms to form ammonia. Studies have demonstrated that the synergy between plasma and catalysts can significantly improve reaction rates and reduce energy consumption. For instance, microwave-assisted ammonia synthesis using a Cs-Ru/CeO2 catalyst at ambient pressure has shown enhanced activity due to the improved dispersion of Ru particles and increased oxygen vacancies on the cerium oxide support [
54,
59].
Case studies and real-world applications of these methods have demonstrated their feasibility and potential benefits. Perovskite oxynitride-hydrides like BaCeO₃₋ₓNyHz have been shown to be efficient catalysts for ammonia synthesis, operating through unique lattice N³⁻ and H⁻ ion-mediated mechanisms [
60]. Additionally, microwave-assisted ammonia synthesis over Cs-Ru/CeO2 catalysts has demonstrated significant ammonia production rates, highlighting the potential for these methods to replace traditional ammonia synthesis processes [
59]. Recent studies have also provided insights into the mechanisms and optimization strategies for these advanced catalytic systems, further underscoring their promise in achieving sustainable and efficient ammonia synthesis.
Recently there has been interest to make ammonia synthesis more sustainable with the use of solar energy as the driving force for the synthesis of ammonia [
61].
Photocatalytic and plasma-assisted methods for ammonia synthesis have garnered significant interest due to their potential to offer more sustainable and efficient alternatives to traditional thermal processes. These methods leverage unique mechanisms and advanced materials to enhance the ammonia production process.
Photocatalytic ammonia synthesis utilizes light energy to drive the reduction of nitrogen to ammonia. This method employs semiconducting materials that can absorb photons and generate electron-hole pairs, which then participate in the reduction and oxidation reactions necessary for ammonia formation. The general mechanism involves the absorption of light by the photocatalyst, leading to the excitation of electrons from the valence band to the conduction band, leaving behind holes in the valence band. The excited electrons can reduce nitrogen (N₂) to ammonia (NH₃), while the holes can oxidize water or other sacrificial agents [
62].
Key advancements in photocatalytic ammonia synthesis have focused on developing efficient photocatalysts that can effectively absorb light and generate reactive species. Titanium dioxide (TiO₂) has been extensively studied due to its strong oxidizing power, chemical stability, and low cost. However, TiO₂ requires UV light for activation, which limits its practical application under sunlight. Modifications, such as doping with other elements or coupling with other semiconductors, have been employed to enhance its activity under visible light. For instance, modifications to traditional photocatalysts like TiO₂ and g-C₃N₄ have shown improved performance by extending their absorption range and enhancing charge separation efficiency [
58,
63].
Other semiconductor materials such as metal oxynitrides and nitrides have also been explored for their potential in photocatalytic ammonia synthesis. Perovskite oxynitride-hydrides like BaCeO₃₋ₓNyHz have shown promise as efficient catalysts. Kitano et al. reported that these materials could effectively utilize light to activate nitrogen molecules, leading to efficient ammonia synthesis under mild conditions. This process involves a Mars-van Krevelen mechanism where lattice N³⁻ and H⁻ ions are activated [
54].
Photocatalytic processes involve three main steps: light absorption, charge separation, and surface reactions. The photocatalyst absorbs light, generating excited electrons and holes. Efficient separation of these charges is essential to prevent recombination. The separated charges then participate in redox reactions on the catalyst surface, leading to nitrogen reduction and ammonia formation. Photocatalytic ammonia synthesis generally follows associative or dissociative pathways. The associative pathway involves stepwise hydrogenation of nitrogen molecules to form intermediates like hydrazine, which further reduce to ammonia. The dissociative pathway involves the initial dissociation of nitrogen molecules into nitrogen atoms, which are then hydrogenated to form ammonia.
Despite the potential, the efficiencies of these photocatalysts are still low due to their low photon utilization rate and the easy combination of excited electron-hole pairs. Further advancements in material design and synthesis are essential to improve the photocatalytic efficiency for large-scale applications [
29,
33].
Plasma-assisted methods use non-thermal plasma to activate nitrogen and hydrogen molecules, facilitating their reaction to form ammonia at lower temperatures and pressures compared to the Haber-Bosch process. Non-thermal plasma provides a high-energy environment where electrons are energized to much higher temperatures than the gas molecules, allowing activation of nitrogen and hydrogen through collisions with energetic electrons [
54].
In these methods, nitrogen and hydrogen gases are introduced into a plasma reactor where they are exposed to an electric field, creating a highly reactive environment. The high-energy electrons in the plasma dissociate nitrogen molecules into reactive nitrogen atoms, which then react with hydrogen atoms to form ammonia. Studies have demonstrated that the synergy between plasma and catalysts can significantly improve reaction rates and reduce energy consumption. For instance, microwave-assisted ammonia synthesis using a Cs-Ru/CeO
2 catalyst at ambient pressure has shown enhanced activity due to the improved dispersion of Ru particles and increased oxygen vacancies on the cerium oxide support [
64].
Microwave-assisted ammonia synthesis over Cs-Ru/CeO
2 catalysts has demonstrated significant ammonia production rates, highlighting the potential for these methods to replace traditional ammonia synthesis processes [
59]. Recent studies have also provided insights into the mechanisms and optimization strategies for these advanced catalytic systems, further underscoring their promise in achieving sustainable and efficient ammonia synthesis.
2.5. Single-Atom and Cluster Catalysts for Ammonia Synthesis
Single-atom and cluster catalysts have emerged as promising candidates for ammonia synthesis due to their unique electronic structures and high surface-to-volume ratios. These catalysts offer distinct advantages, including maximized atom efficiency and tunable electronic properties, which are crucial for the activation of nitrogen molecules and the subsequent synthesis of ammonia.
Single-atom catalysts (SACs) consist of isolated metal atoms dispersed on a support material. These catalysts exhibit unique properties that are different from their bulk counterparts. The isolated metal atoms in SACs provide uniform active sites, which can enhance the selectivity and efficiency of the catalytic process. Additionally, the strong metal-support interactions in SACs can significantly modify the electronic properties of the metal atoms, thereby influencing their catalytic activity.
Recent studies have demonstrated the potential of SACs in ammonia synthesis. For example, single-atom ruthenium (Ru) catalysts supported on carbon or metal oxides have shown remarkable activity for ammonia synthesis at relatively low temperatures and pressures. The isolated Ru atoms provide highly active sites for nitrogen activation, while the support material helps in stabilizing the active sites and facilitating electron transfer processes. This combination of factors results in improved catalytic performance compared to conventional catalysts [
54,
58].
Cluster catalysts, which consist of small aggregates of metal atoms, also show significant promise for ammonia synthesis. These clusters possess unique geometric and electronic structures that can enhance their catalytic activity. The small size of the clusters provides a high density of active sites, while the electronic interactions between the metal atoms in the cluster can facilitate the activation of nitrogen molecules.
One notable example is the use of iron (Fe) and cobalt (Co) clusters for ammonia synthesis. These metal clusters have been shown to effectively activate nitrogen and hydrogen molecules, leading to the formation of ammonia. The catalytic activity of these clusters is influenced by their size, composition, and support material. Studies have shown that optimizing these parameters can lead to significant improvements in catalytic performance [
29,
64].
The mechanisms underlying the catalytic activity of single-atom and cluster catalysts for ammonia synthesis involve several key steps. The first step is the adsorption of nitrogen molecules onto the active sites of the catalyst. This is followed by the activation and dissociation of the nitrogen molecules into reactive nitrogen atoms. The dissociated nitrogen atoms then react with hydrogen atoms to form ammonia. The efficiency of these steps is highly dependent on the electronic structure of the catalyst, which can be tuned by adjusting the size, composition, and support material of the catalyst [
65].
For single-atom catalysts, the strong metal-support interactions play a crucial role in modulating the electronic properties of the isolated metal atoms. These interactions can facilitate the transfer of electrons to the nitrogen molecules, thereby enhancing their activation. For cluster catalysts, the electronic interactions between the metal atoms in the cluster can create a highly reactive environment that promotes the dissociation of nitrogen molecules and the subsequent formation of ammonia [
29,
54].
Despite the promising results, several challenges need to be addressed to fully realize the potential of single-atom and cluster catalysts for ammonia synthesis. One major challenge is the stability of these catalysts under reaction conditions. The isolated metal atoms in SACs and the small metal clusters can be prone to agglomeration, which can lead to a loss of catalytic activity. Developing strategies to stabilize these catalysts, such as using strong metal-support interactions or encapsulating the metal atoms or clusters in a protective matrix, is crucial for enhancing their stability.
Another challenge is the scalability of these catalysts for industrial applications. While single-atom and cluster catalysts have shown excellent performance in laboratory-scale studies, scaling up these catalysts for large-scale ammonia production requires careful consideration of factors such as catalyst synthesis, reactor design, and process optimization. Future research should focus on developing new materials and strategies to address these challenges. This includes designing support materials that can provide strong stabilization for the isolated metal atoms or clusters, optimizing the size and composition of the clusters to maximize their catalytic activity, and exploring new synthesis methods to produce these catalysts on a large scale. Additionally, gaining a deeper understanding of the mechanisms underlying the catalytic activity of these catalysts through advanced characterization techniques and theoretical studies will be crucial for guiding the design of more efficient catalysts.
Single-atom and cluster catalysts offer a promising approach for ammonia synthesis, with the potential to achieve high catalytic efficiency and selectivity. By addressing the challenges associated with stability and scalability, and by continuing to advance our understanding of their catalytic mechanisms, these catalysts could play a significant role in the development of sustainable and efficient ammonia synthesis technologies [
59].
A study by Liu
et al. explores a novel Fe
3 single-cluster catalyst anchored on the θ-Al
2O
3(010) surface for ammonia synthesis. Unlike the traditional Haber-Bosch process that relies on the dissociative mechanism, this catalyst employs an associative mechanism where adsorbed N
2 is first hydrogenated to form NNH before dissociating. This process significantly reduces the N-N bond dissociation barrier, enhancing the turnover frequency (TOF) for ammonia production. The large spin polarization and low oxidation state of the iron cluster play crucial roles in N
2 activation. Microkinetic simulations indicate that the calculated TOF for the Fe
3/θ-Al
2O
3(010) catalyst is comparable to that of the Ru B5 site, known for its high catalytic activity. This innovative approach demonstrates the potential for ammonia synthesis under milder conditions, bypassing the limitations of the Brønsted–Evans–Polanyi (BEP) relation, and bridging the gap between heterogeneous and homogeneous catalysis [
66].
Work by Gutsev et al. explores the catalytic formation of ammonia on a 16-atom iron nanocluster (Fe16) using DFT with a generalized gradient approximation and an all-electron basis set. The research delves into both associative and dissociative pathways for N₂ attachment to the Fe16 cluster and examines the subsequent steps leading to ammonia formation. The findings indicate that the reaction
Fe16 + N₂ + 2H₂ → Fe16NH + NH₃
is exothermic by 1.02 eV. The study also identifies two mechanisms for NH₃ detachment, noting that spin fluctuations play a significant role in the bond formation and rupture processes on the catalyst surface. The detailed computational analysis provides valuable insights into the fundamental processes of nitrogen reduction on iron nanoclusters, which could contribute to the development of more efficient catalysts for ammonia synthesis [
67].
In their study, Fuller
et al. explore the potential of discovering significantly improved ammonia synthesis catalysts through a hierarchical high-throughput approach. This method involves a combination of machine learning, high-throughput experimentation, and catalyst optimization techniques. By systematically screening a vast array of catalyst compositions and process conditions, the researchers aim to identify promising candidates that outperform conventional catalysts. Their approach led to the discovery of catalysts that exhibited higher activity and stability under milder conditions, highlighting the importance of integrating advanced computational and experimental methods in catalyst development [
68].
The study conducted by Kamiguchi
et al. presents a novel approach for efficient ammonia synthesis using Angstrom-sized molybdenum (Mo) clusters supported on HY-zeolite. These clusters, prepared by the impregnation of a molybdenum halide cluster complex with an octahedral Mo
6 metal core, retain their small size (approximately 7 Å) after the removal of halide ligands through hydrogen activation. This innovative method allows for effective N₂ activation and continuous ammonia synthesis at relatively mild conditions of 200 °C and 5.0 MPa. The catalytic activity of these clusters is attributed to the cooperation of multiple Mo sites, which promotes N≡N bond cleavage and efficient ammonia synthesis. The study highlights the stability of these clusters during the synthesis process and the significant reduction in energy barriers for N-H bond formation compared to conventional catalysts. This research demonstrates the potential of ultra-small Mo clusters in achieving efficient and sustainable ammonia production [
69].
The study by Cao
et al. explores ammonia synthesis via an associative mechanism on the alkaline earth metal sites of Ca₃CrN₃H. The authors demonstrated that the nitride hydride compound Ca₃CrN₃H exhibits robust ammonia synthesis activity. This compound activates dinitrogen through active sites where calcium provides the primary coordination environment. DFT calculations revealed that an associative mechanism is favorable for this catalyst, distinct from the dissociative mechanism typically found in traditional Ru or Fe catalysts. The study highlights the potential of alkaline earth metal hydride catalysts and related 1D hydride/electrides for ammonia synthesis, emphasizing the significant role of calcium in the catalytic process. This research emphasizes the importance of considering alternative active sites, such as alkaline earth metals, for developing efficient catalysts for ammonia synthesis, potentially paving the way for more sustainable and effective catalytic processes [
70].
The study by Zhou
et al. reports the development of subnanometer Ru clusters (0.8 nm) anchored on hollow N-doped carbon spheres (Ru-SNCs) as a highly efficient catalyst for ammonia synthesis under mild conditions via an associative route. The Ru-SNCs catalyst exhibited a superior ammonia synthesis rate of 11.7 mmol NH
3·g cat⁻¹·h⁻¹ at 400°C and 3 MPa, outperforming traditional Ru nanoparticle catalysts. Characterization techniques, including UV-vis absorption spectroscopy and in situ DRIFTS, identified N
2H
4 species as the main intermediates, confirming the associative mechanism, which bypasses the direct dissociation of N
2. This work demonstrates the potential of Ru-SNCs catalysts in achieving efficient ammonia synthesis at reduced energy consumption and lower CO
2 emissions, aligning with the goals of sustainable and carbon-neutral chemical processes [
71].
In their study, Lu
et al. present a significant advancement in the development of lanthanum-nitride-based catalysts for ammonia synthesis. The research emphasizes the creation of chemically durable catalysts using nickel and cobalt incorporated into a lanthanum nitride (LaN) matrix. These catalysts demonstrate remarkable activity and stability under harsh reaction conditions, which are essential for efficient ammonia production. The study shows that the LaN-supported nickel and cobalt catalysts exhibit enhanced catalytic performance due to the strong interaction between the metal atoms and the lanthanum nitride support. This interaction facilitates the activation and cleavage of nitrogen molecules, which is a critical step in the ammonia synthesis process. The durability of these catalysts under reaction conditions suggests their potential for long-term industrial applications, reducing the frequency of catalyst replacement and thereby improving the overall efficiency and cost-effectiveness of the ammonia synthesis process [
72].
The study by Fang
et al. presents theoretical insights into the thermal reduction of N
2 to NH
3 over single metal atoms incorporated into nitrogen-doped graphene. Using DFT calculations, the study investigates the reaction mechanisms and catalytic activities of different metal atoms in N-doped graphene. The findings reveal that the catalytic activity is significantly influenced by the type of metal atom and its coordination environment. Specifically, CoN
4- and FeN
4-embedded graphene sheets, where the metal atom is fourfold coordinated, are inactive for N
2TR due to the poor stability of adsorbed H
2 and N
2 molecules. In contrast, CoN
3/G and FeN3/G, where the metal atom is threefold coordinated, show catalytic activity for N2TR, facilitating ammonia synthesis through an associative mechanism. The study identifies the formation of an NHNH* intermediate as a crucial step in the reaction pathway. Further analysis shows that FeN
3/G exhibits superior catalytic activity compared to CoN
3/G, attributed to its preference for a high spin-polarized state during the N
2TR process. This research provides valuable theoretical insights into the design and development of graphene-based single atom catalysts with high activity for ammonia synthesis through N
2 thermal reduction [
73].
2.7. Improving Process Conditions of Ammonia Synthesis
Ammonia production is one of the most energy-intensive chemical processes, consuming approximately 1-2% of the world’s energy production. The main operating cost influencing the price of ammonia is the cost of hydrogen, which constitutes about 70% of the total cost according to the U.S. Department of Energy. Moreover, 3% of global anthropogenic CO₂ emissions are attributed to steam methane reforming, a major route for hydrogen production in ammonia synthesis [
76].
Several strategies have been proposed to improve ammonia synthesis rates, including operating reactors under unsteady-state conditions and decreasing the hydrogen partial pressure in the feed. Studies have shown that higher ammonia synthesis rates can be achieved with lower H₂:N₂ ratios than the stoichiometric 3:1 ratio. For instance, unsteady-state reactor operation has been suggested for various types of ammonia synthesis catalysts, with rate improvements observed under cyclic operation conditions depending on the catalyst type. Additionally, the absorption of synthesized ammonia on supported absorbents, such as MgCl₂ on zeolite-Y, has been studied to enhance ammonia separation, reducing the need for high-pressure condensers [
76].
Co₃Mo₃N catalysts have shown potential as nitrogen transfer reagents, with significant ammonia synthesis rates observed at 300-400°C and atmospheric pressure. Kojima and Aika reported an ammonia synthesis rate expression for Co₃Mo₃N at 400°C and 31 bar pressure, based on power law kinetics. This expression reflects the positive dependency of the reaction rate on the reactants and the poisoning effect of the product [
76].
Simulations have been performed to evaluate the effect of lower H₂:N₂ ratios on both investment and operating costs of the ammonia synthesis process. An ammonia synthesis loop consisting of a packed bed reactor, a flash column, a compressor, and auxiliary units was devised and operated isothermally at 673 K and 100 bar pressure with different H₂:N2 ratios (3:1 and 1:1). The simulations indicated that operating with a lower H₂ ratio could reduce the reactor volume, compressor duties, and capacities while maintaining constant ammonia production [
76].
Improving the process conditions for ammonia synthesis is critical to enhancing the efficiency and sustainability of this essential industrial process. The study by Daisley et al. presents an empirical screening of various supported catalysts for ammonia synthesis, highlighting the impact of different process conditions on catalytic performance. The research explores the performance of catalysts such as Ru/Al₂O₃, Os₃(CO)₁₂/SiO₂, CoRe/MgO, and Ni₂Mo₃N/SiO₂ under varying temperatures and pressures. Among these, Ru/Al₂O₃ exhibited the highest activity, particularly when promoted with potassium (K⁺), which enhances electron density on the Ru surface, thereby improving catalytic activity. The study emphasizes the role of alkali metal promoters in enhancing the performance of Ru-based catalysts.
Operating conditions such as temperature and pressure significantly influence the activity of ammonia synthesis catalysts. The study found that increasing the reaction temperature from 400°C to 500°C generally increased the activity of most catalysts, although the thermodynamic favorability of ammonia synthesis decreases with rising temperature. For example, the ammonia synthesis activity of supported Os-based materials was prominent at 500°C, with the supported Os₃(CO)₁₂ catalysts demonstrating significant activity, comparable to Ru-based systems.
The use of different supports also plays a crucial role in catalyst performance. For instance, CoRe supported on MgO showed enhanced activity due to the basic nature of the support, which helps mitigate hydrogen inhibition and stabilize active phases. This highlights the importance of support selection in optimizing catalyst performance for ammonia synthesis.
Additionally, the study discusses the potential of ternary nitrides such as Ni₂Mo₃N/SiO₂. These materials offer high catalytic activity due to their unique structural properties and the presence of multiple active sites, which facilitate nitrogen adsorption and activation. The empirical screening conducted by Daisley et al. provides valuable insights into the structure-activity relationships of various catalysts, guiding further development and optimization of ammonia synthesis processes.
In conclusion, the improvement of process conditions for ammonia synthesis involves optimizing catalyst composition, operating temperature, pressure, and the use of promoters and supports. These strategies collectively enhance the efficiency and sustainability of ammonia production, making it more viable for industrial applications [
76,
77].
Recent research highlights the potential of manganese nitride-based materials as nitrogen transfer reagents for nitrogen chemical looping in ammonia production. The study by Laassiri et al. systematically investigates the reactivity of lattice nitrogen in various manganese nitride systems, such as Fe-Mn-N, Co-Mn-N, K-Mn-N, and Li-Mn-N, for ammonia synthesis. Among these, Li-Mn-N exhibited the highest reactivity, converting up to 15.8% of the total available lattice nitrogen into ammonia at 400°C. The enhanced reactivity is attributed to the role of lithium in facilitating nitrogen mobility and reactivity at lower temperatures. The research also indicates that while Fe, Co, and K dopants did not significantly improve nitrogen reactivity, the incorporation of lithium drastically improved performance, making Li-Mn-N a promising candidate for ammonia synthesis under mild conditions. Additionally, the study underscores the importance of lattice nitrogen mobility and the potential benefits of using manganese nitride materials in developing more efficient and sustainable ammonia synthesis processes [
78].
In their study,
Laassiri et al. investigated the nitrogen transfer properties of tantalum nitride-based materials, specifically focusing on the role of doping with transition metals such as Re, Fe, and Co. The researchers prepared various Ta3-xMxNy (M = Re, Fe, Co; x = 0, 0.25, 0.5, 1) materials with different microstructural features through high-temperature ammonolysis and soft chemistry synthesis techniques. They found that the nitrogen reactivity of tantalum nitride is predominantly influenced by the chemical composition and the nature of the transition metal dopant rather than the microstructural properties. Doping with low levels of Co significantly enhanced the reactivity at lower temperatures, making it the most effective dopant among those tested. The improved reactivity is attributed to the increased nitrogen mobility within the tantalum nitride lattice, which operates through a Mars-van Krevelen mechanism. This study highlights the potential of doping tantalum nitride with transition metals to develop highly active nitrogen transfer materials for ammonia synthesis under milder conditions [
79].
Work by Qian
et al. investigates the dynamic evolution of the Co3Mo3N bimetallic nitride surface during ammonia synthesis. Using a combination of ab initio thermodynamics and temperature-programmed experiments, the authors demonstrate that the Co3Mo3N catalyst adjusts its surface states in response to various reaction conditions. The study reveals that hydrogenated surfaces and subsurface defects are more thermodynamically stable than clean surfaces under typical ammonia synthesis conditions. This surface evolution significantly impacts the electronic structure and reaction performance of the catalyst. The presence of surface hydrogen inhibits nitrogen dissociation but does not affect lattice nitrogen hydrogenation, while subsurface nitrogen defects modulate the charge of molybdenum species, influencing the dissociation process. These insights into the realistic surface states are crucial for understanding reaction mechanisms and designing high-performance nitride catalysts [
80].
The study by Brown
et al. presents the first evidence of ammonia synthesis via bulk diffusion in cobalt molybdenum (CoMo) particles during a chemical looping ammonia synthesis (CLAS) process. The research demonstrated that CoMo bimetallics, when subjected to nitridation under pure N₂ gas, could produce ammonia without the presence of gaseous H₂. This unexpected phenomenon suggests a dynamic surface interaction involving a shrinking core and counter-diffusion mechanism, where nitrogen diffuses inward, and oxygen diffuses outward, stabilizing the core and forming H
xMoO₃. The study utilized various characterization techniques, including X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and time on stream (TOS) experiments, to investigate the surface and bulk chemistry of CoMoO₄ particles. The findings indicate that ammonia synthesis in this system follows the Mars-van Krevelen mechanism, involving both surface and bulk nitrogen interactions, potentially providing new insights into ammonia synthesis and catalyst design [
81].
In the study by Wang
et al. the effects of support and promoter on ruthenium (Ru) catalyst activity for microwave-assisted ammonia synthesis were investigated. The research highlighted that cesium (Cs)-promoted Ru/CeO₂ catalysts exhibit considerable activity at 533 K and ambient pressure. The combined theoretical and experimental approaches demonstrated that the strong interaction between Ru and CeO₂ leads to the formation of highly dispersed Ru particles, which favor ammonia synthesis. The higher electron-donating ability of CeO₂ and the lower electronegativity of the Cs promoter result in a higher electron density on Ru, reducing the N≡N dissociation barrier. This study underscores the potential of microwave-assisted catalytic processes in activating stable molecules for ammonia synthesis under mild conditions, providing a promising alternative to the energy-intensive Haber-Bosch process [
82].
In the study by Wang
et al. the ternary ruthenium complex hydrides Li₄RuH₆ and Ba₂RuH₆ are presented as efficient catalysts for mild-condition ammonia synthesis from N₂ and H₂. These catalysts differ significantly from traditional iron or ruthenium-based catalysts in terms of electronic, compositional, and structural properties, exhibiting remarkable catalytic performance under mild conditions. The study demonstrates that the dynamic and synergistic involvement of the electron-rich [RuH₆]⁴⁻ anionic centers, along with Li/Ba cations and hydridic hydrogen, facilitates an associative reaction mechanism with a narrow energy span. This results in superior ammonia production rates at temperatures below 373 K, outperforming existing conventional heterogeneous catalysts. The findings provide valuable insights into catalyst design for mild-condition ammonia synthesis, bridging the gap between heterogeneous and homogeneous nitrogen fixation at the molecular level [
83].
Investigations by Fuller
et al. explore the impact of alkali promoters on the activity and stability of ammonia synthesis catalysts. Alkali metals, such as potassium, cesium, and rubidium, were found to significantly enhance the catalytic performance by increasing the electron density on the active sites, thereby facilitating the nitrogen activation process. The presence of these promoters alters the electronic environment of the catalysts, reducing the energy barriers for nitrogen dissociation and subsequent hydrogenation steps. This modification leads to higher turnover frequencies (TOF) and improved overall catalytic efficiency. The research highlights that the optimal choice and concentration of alkali promoters are crucial for maximizing the performance of ammonia synthesis catalysts, providing valuable insights into the design of more effective catalytic systems for industrial applications [
84].
The study by Wang
et al. investigates the use of a cobalt-based catalyst for ammonia synthesis under mild conditions. The research focuses on a nitrogen-anchored cobalt single-atom catalyst (Co-N-C) that demonstrates both dynamic and steady-state active sites. The Co-N-C catalyst exhibits a high ammonia synthesis rate of 116.35 mmolNH3 gCo
−1 h
−1, facilitated by atomically dispersed cobalt coordinated with pyridine nitrogen, which reacts with surface hydrogen to produce ammonia via a chemical looping pathway. Pyrrolic nitrogen anchors the single cobalt atoms, forming Co1-N3.5, which aids in nitrogen adsorption and step-by-step hydrogenation of nitrogen to ammonia. The study emphasizes that the dual active sites in Co-N-C allow for efficient NH
3 production by bypassing the N
2 dissociation bottleneck, making it feasible under milder conditions compared to traditional catalysts [
85].
Work by Uner and co-workers examines the process economics of ammonia synthesis over Co₃Mo₃N by optimizing the hydrogen-to-nitrogen (H₂) feed stoichiometry. Through ammonia synthesis rate measurements at atmospheric pressure and 400°C, it was determined that the synthesis rate remains independent of the H₂ ratio for stoichiometries above 0.5:1. However, for ratios below 0.5:1, there is a linear dependency of the synthesis rate on the H₂ stoichiometry. Static measurements of hydrogen adsorption isotherms at various temperatures indicated that the Co₃Mo₃N surface becomes saturated with strongly bound hydrogen at around 100 Torr, corresponding to a H₂ ratio of approximately 0.5:1. These findings confirm the significant role of strongly bound hydrogen in the ammonia synthesis process. Using this data, the researchers modified an existing kinetic expression to develop a conceptual design that incorporates a late mixing strategy for the hydrogen stream. The economic analysis of this design demonstrated that employing low hydrogen stoichiometries could reduce both investment and operating costs by a factor of 2, highlighting the potential for more cost-effective ammonia production [
76].
2.8. Enhancing Ammonia Synthesis Catalyst Activity
Recent advancements in the field of ammonia synthesis have focused on the catalytic potential of confined cobalt molybdenum nitride (CoMo-N) nanoparticles. A notable study by Sfeir
et al. reports on the development of highly active CoMo-N catalysts supported on SBA-15, a mesoporous silica material known for its high surface area and thermal stability. This innovative approach leverages the dispersion of CoMo-N nanoparticles within SBA-15 to enhance their structural and textural properties, which are crucial for catalytic performance. The research demonstrated that SBA-15-supported CoMo-N catalysts exhibited significantly higher catalytic activity compared to unsupported Co3Mo3N. Specifically, the CoMo-N/SBA-15 catalysts with 10, 20, and 30 wt% metal loadings achieved ammonia synthesis rates of 1714, 1429, and 810 µmol gactive phase-1 h-1, respectively, far surpassing the rate of 259 µmol gcatalyst-1 h-1 for the bulk Co
3Mo
3N catalyst. This improvement is attributed to the increased surface area and enhanced dispersion of the active phase, which promote better nitrogen activation and hydrogenation. The study underscores the importance of nanoconfinement and structural optimization in designing efficient catalysts for sustainable ammonia production [
86]. Recent studies have also demonstrated that the catalytic activity of molybdenum nitride (Mo-N) catalysts can be significantly influenced by the interaction between the molybdenum nanoparticles and their support material. Work by Sfeir
et al. explored the catalytic performance of Mo-N catalysts supported on mesoporous silica (SBA-15) and titanium dioxide (TiO2). The research highlighted that SBA-15-supported Mo-N catalysts exhibited higher catalytic activity for ammonia synthesis compared to those supported on TiO2. The superior performance of SBA-15-supported catalysts is attributed to the high dispersion of Mo nanoparticles within the SBA-15 matrix, which provides a high surface area and thermal stability. Conversely, Mo-N catalysts supported on TiO2 showed poor catalytic activity and were prone to rapid deactivation due to strong metal-support interactions (SMSIs) that led to charge transfer from the reducible support to the active phase. This charge transfer was confirmed by X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance (EPR) analyses, which revealed changes in the oxidation states and electronic properties of the Mo species. The study concluded that the nature of the support material plays a crucial role in modulating the catalytic efficiency of Mo-N catalysts, with non-reducible supports like SBA-15 being more favorable for maintaining high activity and stability in ammonia synthesis. Switching on/off molybdenum nitride catalytic activity in ammonia synthesis through modulating metal–support interaction seems as an efficient method to enhance ammonia synthesis activity [
87].
In the studies of Hosono and co-workers in Co-Mo bimetallic nanoparticles prepared via sodium naphthalenide-reduction it was found that Co-Mo bimetallic nanoparticles (NPs) exhibit activity levels similar to Ru NPs [
88]. These Co-Mo bimetallic NPs with various compositions were prepared on CeO
2 by sodium naphthalenide driven reduction, which had much higher activity than the monometallic Co/CeO
2 and Mo/CeO
2 catalysts. This suggests that the synthesis method of the catalyst can signifcantly affect the resulting activity for ammonia synthesis in the Co-Mo systems.
In another study by Abghoui
et al. explores the potential of mononitrides as catalysts for nitrogen reduction to ammonia under ambient conditions, using DFT. The research highlights that transition metal nitrides (TMNs) such as ZrN, NbN, CrN, and VN show promise due to their ability to adsorb and activate N₂ molecules on their surfaces. Among these, ZrN exhibited the most favorable properties for N₂ adsorption and activation, with a lower energy barrier for nitrogen reduction, suggesting a higher catalytic activity for ammonia synthesis. The study provides critical insights into the electronic and structural properties of these mononitrides, contributing to the understanding of how these materials can be optimized for efficient ammonia production under mild conditions [
89].
The study conducted by Araia and co-workers explores the effects of carbon nanotubes (CNT) on a Cs−Ru/CeO
2 catalyst for microwave-assisted ammonia synthesis. The research demonstrates that mechanical mixing of Cs−Ru/CeO2 with CNT significantly enhances catalytic activity compared to chemically synthesized catalysts. The study highlights that the mechanically mixed catalyst achieved an ammonia production rate of 1822 μmol NH3/gcat h at 260°C, outperforming the individual components and chemically synthesized mixtures. This improvement is attributed to the increased dielectric properties and electrical conductivity provided by the CNT, which enhance microwave heating and facilitate electron transfer. The synergistic effect between the CNT and Cs−Ru/CeO
2 is also noted to improve interfacial polarization, further boosting catalytic performance. The research suggests that the combination of Ru-based catalysts and the use of CNT as microwave susceptors can significantly optimize ammonia synthesis under milder conditions [
90].
The study by Zhou
et al. focuses on integrating dissociative and associative mechanisms for efficient ammonia synthesis using a TiCN-promoted Ru-based catalyst. The developed Ru/3TiCN/ZrH2 catalyst demonstrated a superior ammonia synthesis rate of 25.6 mmol g
cat−1 h
−1 at 400°C and 1 MPa. The researchers found that TiCN transfers electrons to the Ru centers and reduces Ru metal particle aggregation, generating more B5 sites that facilitate N
2 dissociation. Additionally, the formation of nitrogen vacancies on TiCN, assisted by Ru, promotes the associative mechanism for N
2 hydrogenation. This dual mechanism enhances the overall catalytic performance under mild conditions. The study's findings highlight the synergistic effects of Ru and TiCN in improving N
2 activation, providing valuable insights for developing advanced catalysts for ammonia synthesis [
91].
In work by Fuller
et al. a hierarchical high-throughput catalyst screening (HHTCS) was employed to enhance the efficiency of ammonia synthesis via the Haber-Bosch process using Fe-based catalysts. The study specifically focused on the Fe(211) surface, one of the most active iron catalyst facets, and investigated 49 potential metal dopants to identify those that could significantly increase the turnover frequency (TOF) for ammonia synthesis. The screening identified cobalt (Co) as the most promising dopant, leading to a decrease in the overall reaction free-energy barrier by 0.19 eV. Kinetic Monte Carlo simulations predicted that the TOF for the Co-doped surface would increase by a factor of 2.8 compared to the undoped Fe(211) surface. This enhancement could potentially lower the operating pressure of the Haber-Bosch process from 200 atm to around 40 atm at 773 K while maintaining the same TOF, thus improving the catalytic efficiency of ammonia synthesis under industrial conditions. This study underscores the potential of Co-doped Fe catalysts in achieving more sustainable and cost-effective ammonia production processes [
92].
The paper by Inoue
et al. investigates the direct activation of cobalt catalysts by the electride 12CaO·7Al₂O₃ (C12A7−) for ammonia synthesis. The research highlights that while cobalt is known for its catalytic potential in ammonia synthesis, its activity is typically low due to insufficient nitrogen adsorption energy. However, this issue is effectively addressed by utilizing C12A7−, which has a low work function of 2.4 eV, significantly enhancing the ammonia synthesis activity of cobalt. The study demonstrates that Co/C12A7− can initiate ammonia synthesis at a remarkably low temperature of 200°C, in contrast to Co/C12A7₂− which requires 400°C. The activation energy of Co/C12A7− is approximately 50 kJ/mol, comparable to advanced cobalt-based catalysts like Co₃Mo₃N and LiH-Co. This significant reduction in activation energy is attributed to the effective electron donation from C12A7− to the cobalt nanoparticles, facilitating N₂ dissociation. This study provides valuable insights into improving the efficiency of cobalt catalysts for ammonia synthesis by leveraging the electronic properties of electride supports [
93].
The study by Singh
et al. explores the significant potential of innovative materials development strategies for improving ammonia synthesis under mild conditions, moving away from the traditional high-energy Haber-Bosch process. The authors highlight various approaches, including defect engineering, structure manipulation, and the design of single-atom catalysts (SACs), which have shown promise in enhancing catalytic performance. The review emphasizes the need for catalysts that can efficiently perform at lower pressures and temperatures, aligning with the goals of sustainable and eco-friendly ammonia production. Defects and structural manipulation in catalysts, such as introducing vacancies or doping, can significantly alter electronic structures and enhance activity. Additionally, the paper outlines some strategies to produce ammonia under mild conditions, underscoring the role of computational tools like DFT in guiding the design of both electrochemical and thermochemical catalysts. This comprehensive approach aims to reduce the energy consumption and environmental impact of ammonia synthesis, making the process more economically viable and environmentally friendly [
94].
A paper by Hosono addresses the significant challenge of synthesizing ammonia efficiently using carbon-footprint-free hydrogen under mild conditions. The review outlines various methods of N
2 activation for ammonia synthesis, highlighting the chronological progress in heterogeneous catalysts from the traditional use of iron oxide in the Haber-Bosch process to modern advancements. The article emphasizes the importance of developing support materials with low work functions to reduce the activation barrier for N
2 dissociation. Electride materials, which maintain bulk-like properties on their surfaces, are identified as promising candidates for this purpose. The key requirements for effective catalysts include high efficiency at low temperatures, compositions free of ruthenium, and chemical robustness in ambient conditions. The paper provides a comprehensive overview of the current state of catalytic N
2 activation and the technical challenges that need to be addressed to achieve green ammonia synthesis [
95].