Submitted:
11 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Performance of BiFeO3 under Different Atmospheres Annealed at 440 ± 5 °C
3.1.1. Microstructure Analysis
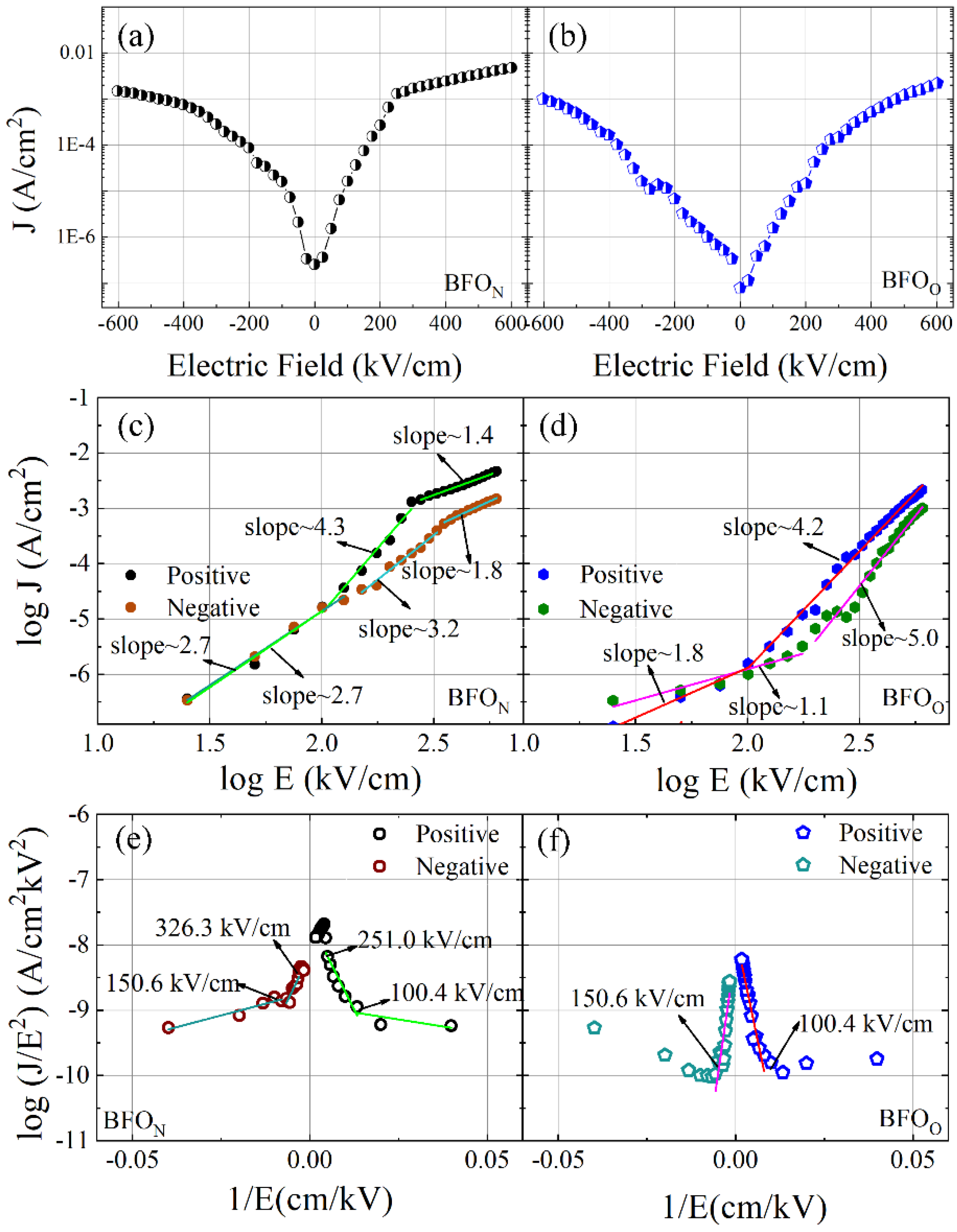
3.1.2. Room Temperature Electrical Properties
3.2. Performance of BiFeO3 Film under an N2-Rich Atmospheres Annealed at 365 ± 5 °C
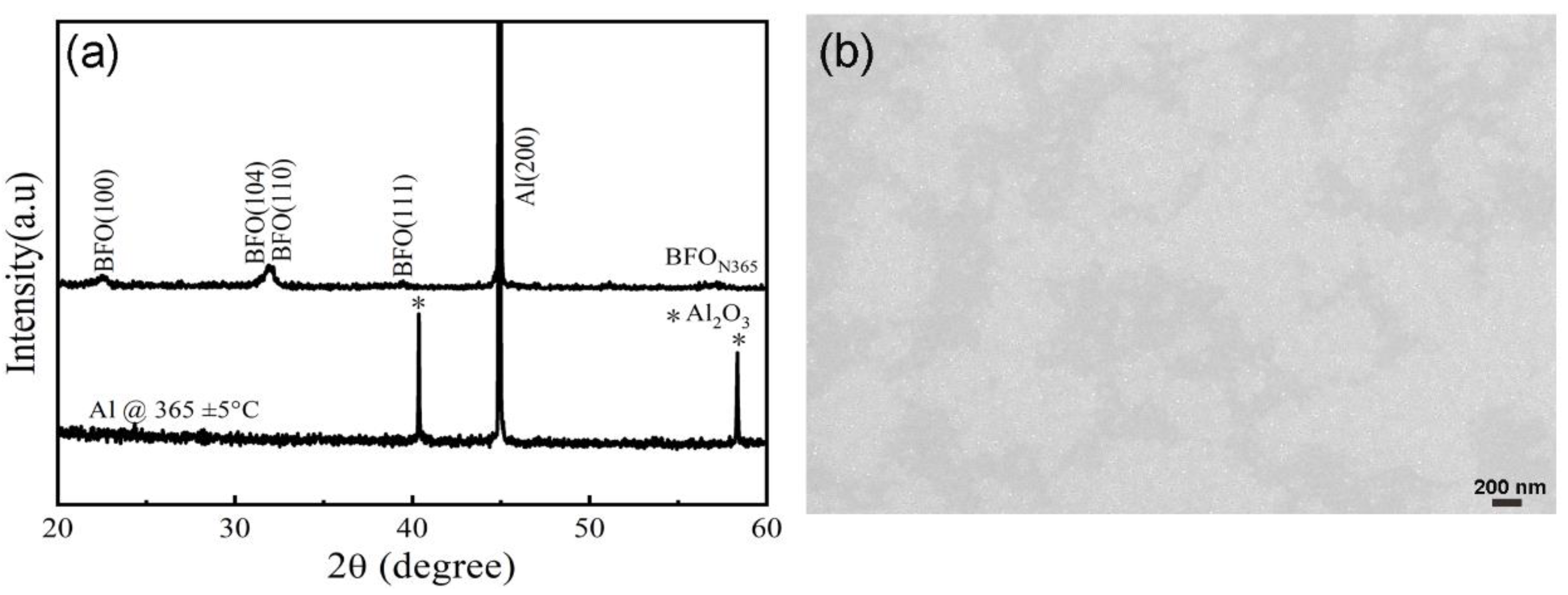
3.2.1. Microstructure Analysis
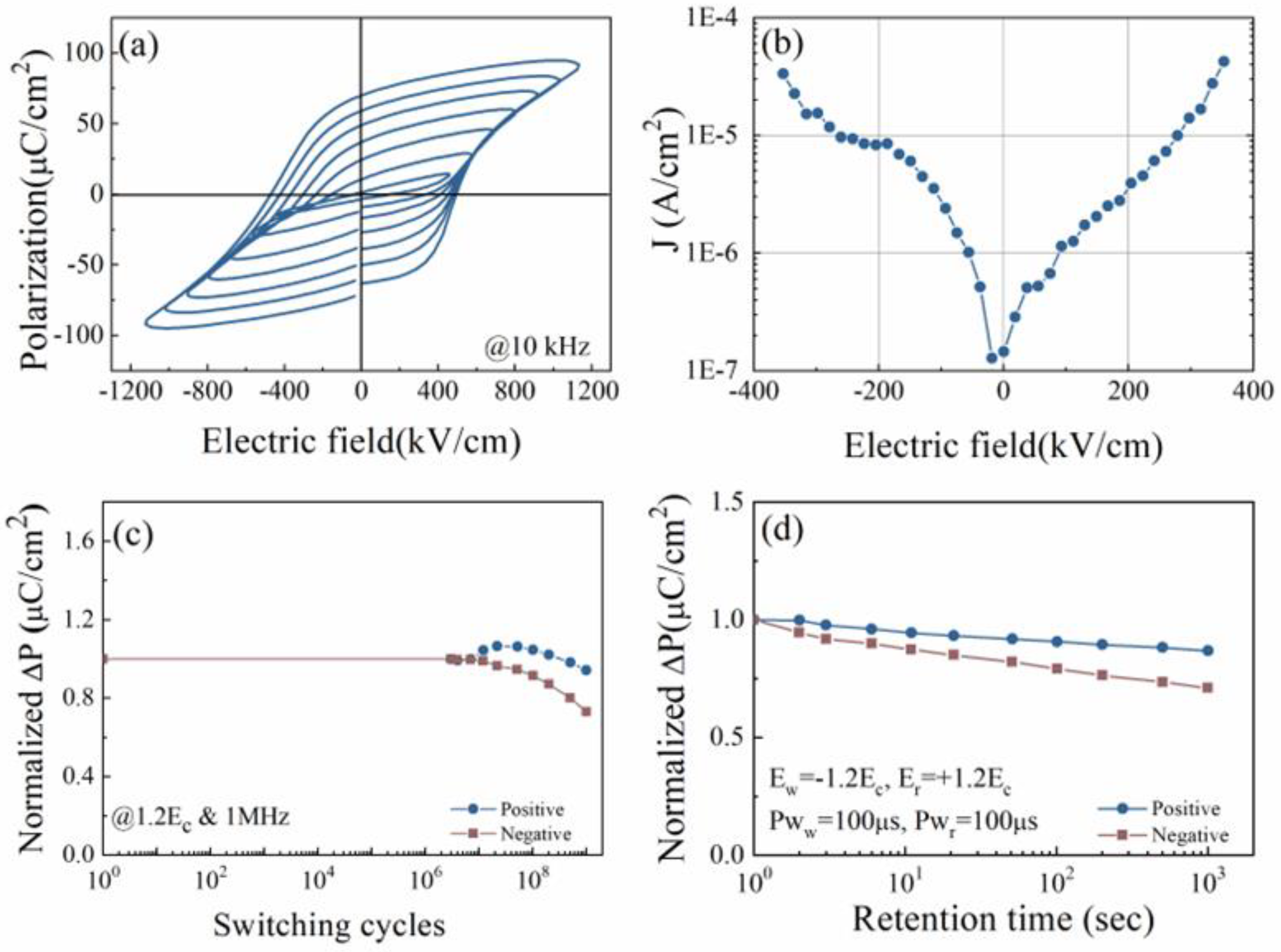
3.2.2. Room Temperature Electrical Properties
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Wang J. Orientation dependence of ferroelectric behavior of BiFeO3 thin films. J. Appl. Phys. 2009,106, 104111. [CrossRef]
- Yan J.; Ouyang J.; Cheng H.B.; Yan P. Low temperature deposition of BiFeO3 films on Ti foils for piezoelectric applications. Scripta Materialia 2021, 204, 114152. [CrossRef]
- Wang, J.; Neaton, J. B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; Spaldin, N.A.; Rabe, K.M.; Wuttig, M.; Ramesh, R. Epitaxial BiFeO3 multiferroic thin film heterostructures. Science 2003, 299, 1719–1722. [CrossRef]
- Wu, J.G.; Fan, Z.; Xiao, D.Q.; Zhu, J.G.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [CrossRef]
- Yang, B.B.; Jin, L.H.; Wei, R.H.; Tang, X.W.; Hu, L.; Tong, P.; Yang, J.; Song, W.H.; Dai, J. M.; Zhu, X.B.; Sun, Y.P.; Zhang, S.J.; Wang, X.L.; Cheng, Z.X. Chemical solution route for high-quality multiferroic BiFeO3 thin films. Small 2021, 17,1903663. [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2010, 21, 2463–2485. [CrossRef]
- Zhu, H.F.; Yang, Y.L.; Ren, W.; Niu, M.M.; Hu, W.; Ma, H.F.; Ouyang, J. Rhombohedral BiFeO3 thick films integrated on Si with a giant electric polarization and prominent piezoelectricity, Acta Materialia 2020, 200, 305–314. [CrossRef]
- 8. Tomczyk M; Bretos I.; Jiménez R.; et al. Direct fabrication of BiFeO3 thin films on polyimide substrates for flexible electronics. Journal of Materials Chemistry C, 2017, 5(47), 12529-12537. [CrossRef]
- Wen, Z.; Shen, X.; Wu, J.X; Wu, D.; Li, A.D.; Yang, B.; Wang, Z.; Chen, H.Z.; Wang, J.L. Temperature-dependent leakage current characteristics of Pr and Mn cosubstituted BiFeO3 thin films, Appl. Phys. Lett. 2010, 96,202904. [CrossRef]
- Yan, J.; Hu, G. D.; Chen, X. M.; Wu, W. B.; Yang, C. H. Ferroelectric properties, morphologies, and leakage currents of Bi0.97La0.03FeO3 thin films deposited on indium tin oxide/glass substrates, Journal of applied physics 2008, 104, 076103. [CrossRef]
- Chen, D.Y.; Chen, Z.H.; He, Q.; Clarkson, J.D.; Serrao, C. R.; Yadav, A. K.; Nowakowski, M.E.; Fan, Z.; You, L.; Gao, X.S.; Zeng, D.C.; Chen, L.; Borisevich, A.Y.; Salahuddin, S.; Liu, J.M.; Bokor, J. Interface engineering of domain structures in BiFeO3 thin films, Nano Letter 2017,17, 486−493. [CrossRef]
- Jang, H. W. ; Ortiz, D. ; Baek, S. H. ; Folkman, C. M. ; Das, R. R. ; Shafer, P. ; Chen, Y.B. ; Nelson, C. T. ; Pan, X.Q. ; Ramesh, R. ; Eom, C.B. Domain engineering for enhanced ferroelectric properties of epitaxial (001) BiFeO thin films. Advanced Materials 2009, 21, 817-823. [CrossRef]
- Yan, J.; Zhu, H. F.; Ouyang, J.; Kanno, I.; Yan, P.; Wang, Y.Y.; Onishi, K.; Nishikado, T. Highly (00l)-textured BiFeO3 thick films integrated on stainless steel foils with an optimized piezoelectric performance. Journal of the European Ceramic Society 2022, 42, 3454-3462. [CrossRef]
- Chen, X.M.; Hu, G.D.; Wang, J.C.; Cheng, L.; Yang, C.H.; Wu, W.B. Thickness effects of Bi0.89Ti0.11FeO3 thin films deposited on PbZr0.2Ti0.79Nb0.01O3 buffer layers. J. Alloy. Compd. 2011, 509, 431-434. [CrossRef]
- Li, H.; Ding, Y.W.; Ren, K. J.; Zeng, Z.X.; Chen, C.; Deng, X.L.; Gao, R.L.; Cai, W.; Wang, Z.H.; Fu, C.L.; Lei, X.; Chen, G.; Effect of rapid/slow annealing routes on the magnetic and photoelectric properties of BiFeO3/CoFe2O4 multilayer thin films. Journal of Alloys and Compounds 2024, 989, 174203. [CrossRef]
- Pan, T.M.; Chen, Z. Y.; Weng, W. C.; Her, J.L.; Electrical and structural properties of BiFeO3 thin films with four distinct buffer layers: La2O3, Pr2O3, Sm2O3, and Tm2O3. Physica B: Condensed Matter, 2024, 689, 416219. [CrossRef]
- Zhang, J.; Dai, J.Q.; Zhang, G.C.; Zhu, X. J.; Significantly improved ferroelectric properties of (Zn,Co) co-doped BiFeO3 thin films.
- 18. prepared by sol-gel method. Ceramics International 2024, 50, 28449-28457. [CrossRef]
- Wang, Y. P.; Zhou, L.; Zhang, M. F.; Chen, X. Y.; Liu, J. M.; Liu, Z. G. Room-Temperature Saturated Ferroelectric Polarization in BiFeO3 Ceramics Synthesized by Rapid Liquid Phase Sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [CrossRef]
- Palkar, V. R.; Pinto, R. BiFeO3 thin films: novel effects. Pramana J.Phys. 2002, 58, 1003–1008. [CrossRef]
- Kingon, A.I.; Srinivasan, S. Lead zirconate titanate thin films directly on copper electrodes for ferroelectric, dielectric and piezoelectric applications. Nat. Mater. 2005, 4, 233–237. [CrossRef]
- Hidnert, Peter; Krider, H. S. Thermal Expansion of Aluminum and Some Aluminum Alloys. Journal of Research of the National Bureau of Standards 1952, 48, 209-220. [CrossRef]
- Yim, W. M.; Paff, R. J. Thermal expansion of AlN, sapphire, and silicon. J. Appl. Phys. 1974, 45, 1456–1457. [CrossRef]
- Niu, M.M.; Zhu, H.F.; Wang, Y.Y.; Yan, J.; Chen, N.; Yan, P.; Ouyang, J. Integration-friendly, chemically stoichiometric BiFeO3 films with a piezoelectric performance challenging that of PZT. ACS Appl. Mater. Interfaces 2020, 12, 33899–33907. [CrossRef]
- Liu, H.Y.; Pu, Y.P.; Shi, X.; Yuan, Q.B. Dielectric and ferroelectric properties of BiFeO3 ceramics sintered in different atmospheres. Ceramics International 2013, 39, S217-S220. http://dx.doi.org/10.1016/j.ceramint.2012.10.065.
- Lee, J. S.; Joo, S. K. Analysis of grain-boundary effects on the electrical properties of Pb(Zr,Ti)O3 thin films. Applied Physics Letters 2002, 81, 2602-2604. [CrossRef]
- Yan, J.; Jiang, X. M.; Hu, G. D. Leakage mechanisms of partially self-polarized BiFeO3 film. Ceramics International 2018, 44, 13765-13772. [CrossRef]
- Sun, W.; Zhou, Z.; Luo, J.; Wang, K.; Li, J. F. Leakage current characteristics and Sm/Ti doping effect in BiFeO3 thin films on silicon wafers. J. Appl. Phys. 2017, 121, 064101-7. [CrossRef]
- Luo, J. M.; Lin, S. P.; Zheng, Y.; Wang, B. Nonpolar resistive switching in Mn-doped BiFeO3 thin films by chemical solution deposition. Appl. Phys. Lett. 2012, 101, 062902-3. [CrossRef]
- Yang, H.; Wang, Y. Q.; Wang, H.; Jia, Q. X. Oxygen concentration and its effect on the leakage current in BiFeO3 thin films. Appl. Phys. Lett. 2010, 96, 012909-3. [CrossRef]
- Hu, G.D.; Fan, S.H.; Yang, C.H.; Wu, W.B. Low leakage current and enhanced ferroelectric properties of Ti and Zn codoped BiFeO3 thin film. Appl. Phys. Lett. 2008, 92, 192905-3. [CrossRef]
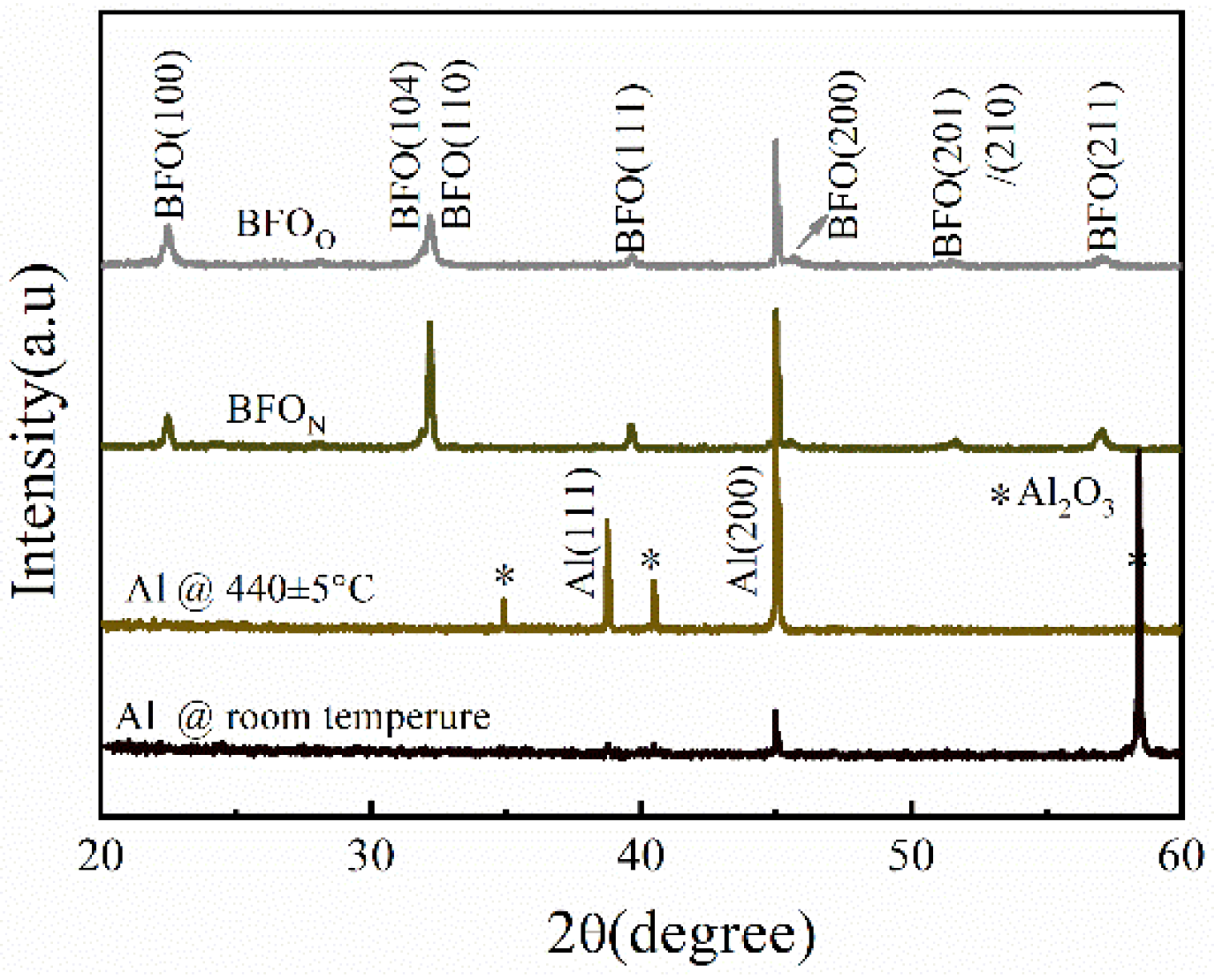
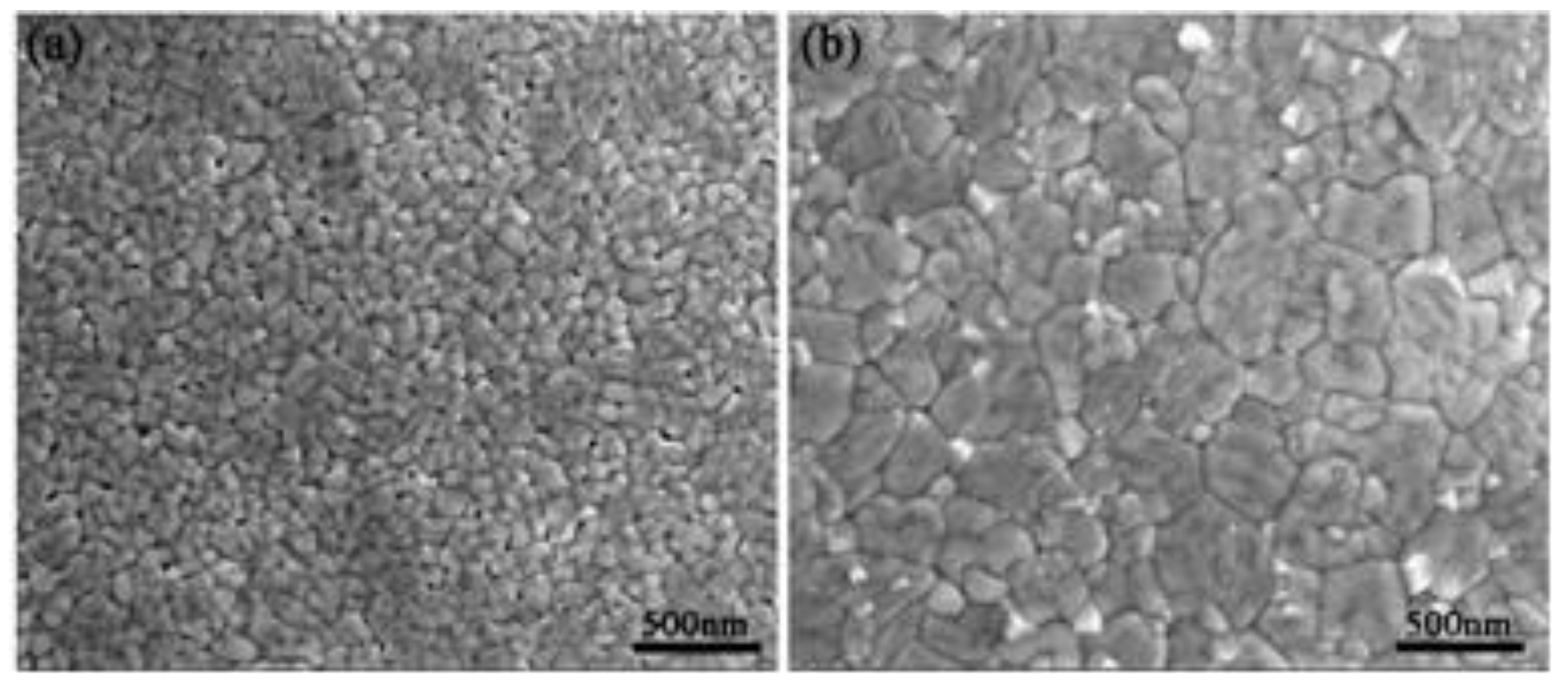
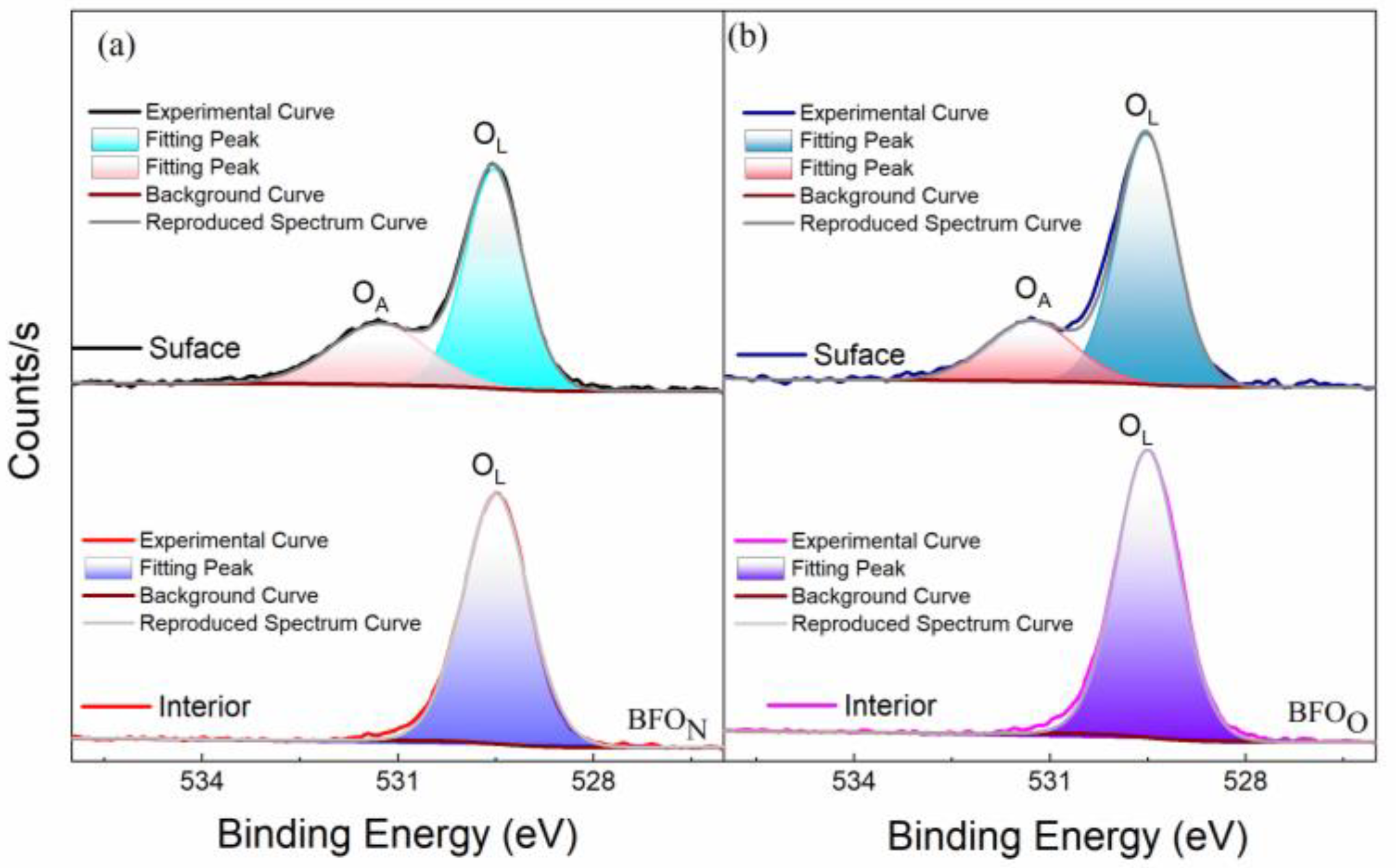
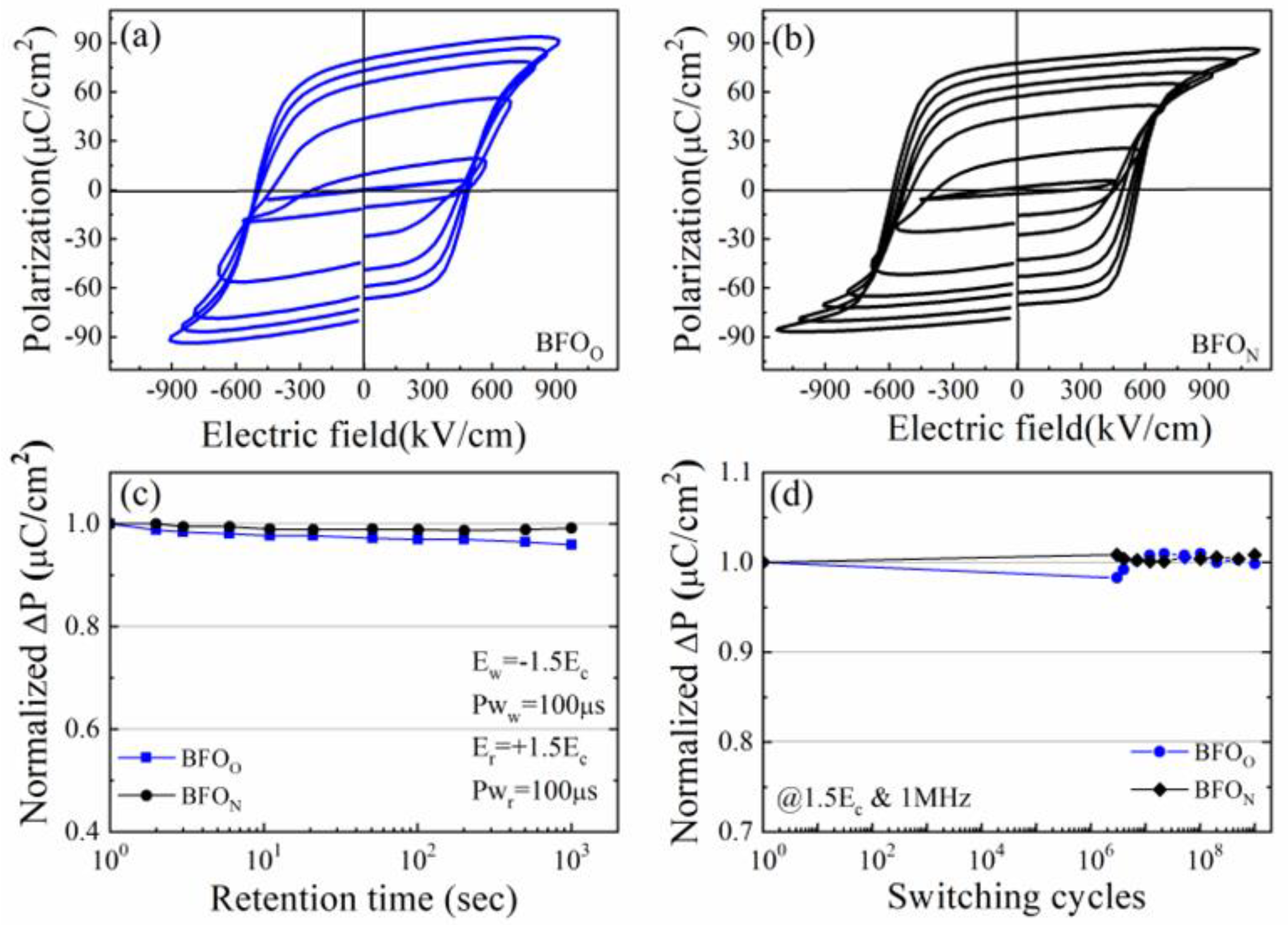
| film | BFON | BFOO | |
| the global average grain size via Scherrer formula | (100)-oriented grains | 180 nm | 120 nm |
| (111)-oriented grains | 75 nm | 50 nm | |
| (211)-oriented grains | 30 nm | 23 nm | |
| the local average grain size via SEM analysis (using 100 grains) | 105 nm | 55 nm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





