Introduction
Leopards (Panthera pardus) are among the most versatile and widely distributed wild cats. They can adapt to almost every habitat, from human-inhabited urban areas to dense jungles and from lush agricultural fields to deserts and mountains (Jacobson et al. 2016). However, owing to regional declines as a result of habitat fragmentation and degradation and human-wildlife conflicts (Balme et al. 2009), the leopard was recently assessed for The IUCN Red List of Threatened Species and has been listed as Vulnerable since 2022 (Stein et al. 2022). The global estimate of range reduction is 61% just in the past two decades (Stein et al. 2022). In Africa, 37% of their historical natural habitats and relatively large numbers are killed by people either by trophy hunting or retaliatory killing of actual or perceived danger to livestock (St John et al. 2011). However, the estimates of population changes in Asia are sporadic to non-existent. Most countries where the species has become extinct are from the Middle East and Southeast Asia (Shehzad et al. 2014).
The Indian leopard (P. p. fusca), prevalent beyond protected areas, is highly vulnerable to illegal wildlife trade (skins, bones, and other parts for use in traditional oriental medicine), especially young individuals (Shivakumar et al., 2023). A regional survey revealed a rate of four leopards poached per week in India for the illegal wildlife trade (Raza et al., 2012b). Furthermore, Nowell and Pervushina (2012) reported that the illegal trade of leopard parts in Asia was on par with that of tigers (P. tigris). Indeed, in India, since 2000, an average of 3.5 young leopard seizure cases per month have been reported (Stein et al., 2022).
In addition to the usual biological and ecological factors that influence their survival, Leopard cubs face unique challenges. These include predation by larger carnivores, infanticide by males who are not their sires, diseases, accidents, and starvation due to unsuccessful hunts by their mothers (Yosef et al., 2021). However, their most significant threats come from conflicts with humans, including illegal trade, poaching, and retaliatory killings. These challenges underscore the vulnerability of leopard cubs and the urgent need to identify their survival probabilities in the wild.
Little is known about the breeding cycle of the leopards in the wild (cf. Sunquist and Sunquist 2009). Captive data show that females come into estrus at any time of the year and remain in heat for up to two weeks; young are born after a gestation period of 96 days. Mating in the wild lasts a day or two, and litters average two (range one to three) cubs. Young remain in the birthing den for the first two to three months, even if the mother is absent while foraging for prey, and accompany their mother when about three months old. The young are usually independent by 12-18 months, but dispersal varies from 15-36 months (Sunquist and Sunquist 2009).
Leopard mothers play a crucial role in the survival of their cubs. They invest a significant amount of time and energy in raising their offspring, providing protection and guidance, and teaching their children essential hunting and survival skills during their formative years (Bothma and Coertze, 2004). As leopard cubs mature, they undergo a dispersal phase, leaving their mother's territory to establish their home ranges. This period is fraught with risks, as they may encounter hostile territory, competition from resident leopards, and potential conflicts with humans (Owen et al. 2010). It should also be noted that cub survival rates in leopards, like many other big cat species, can vary based on various factors such as habitat quality, availability of prey, competition, as well as human disturbances (Balme et al. 2009).
Despite some achievements in understanding the reasons for the decline in the number of this species still, the stage in the life history of a particular individual that is most crucial for their survival has yet to be determined. Therefore, conducting analyses of offspring survival from leaving the breeding ground until they become independent is one key element in determining critical periods in the lives of individuals. It is essential because even protected areas do not ensure the undisturbed functioning of the population of this species. Because the species is long-lived, it seems necessary to estimate which moment in the life of young leopards is crucial for their survival.
The aims of this study are (1) to describe the juvenile survival of the Indian Leopard during the first year of life and (2) to establish the critical period of the reproductive cycle that influences survival.
Methods
Study Area
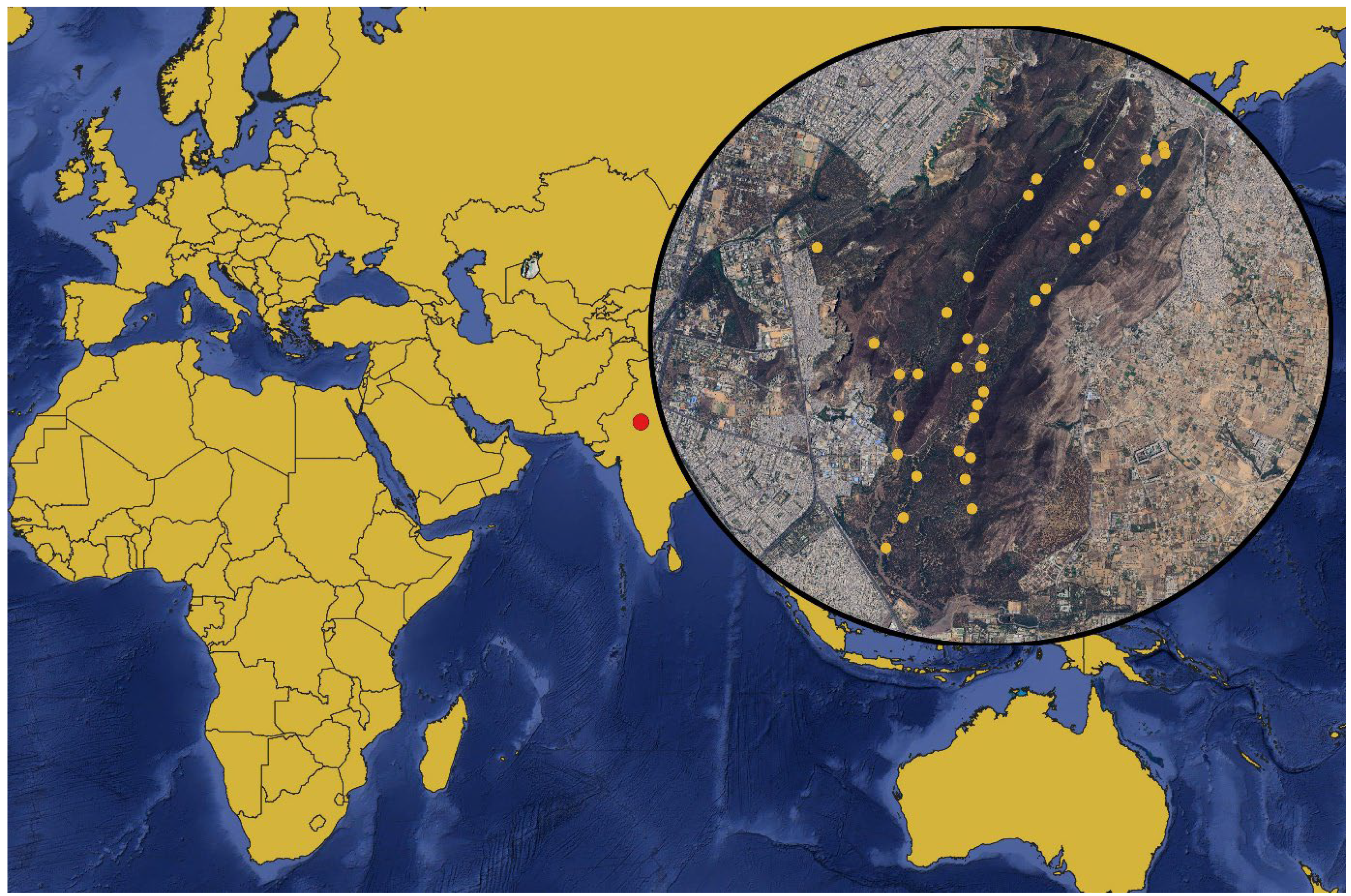
We conducted our study in the Jhalana Reserve Forest (JRF; 26º51’N, 75º49’E), located on the southeastern outskirts of Jaipur, the capital city of Rajasthan, India (Fig 1). Covering about 29 km
2, JRF sits at an altitude of 516 meters above sea level and falls under the category of Northern Tropical Dry deciduous forest. The landscape is dominated by low, flat-topped hills in the northern region, with deep erosion and dissected features (Kumbhojkar et al. 2019). Wildlife in JRF faces challenges due to the lack of natural water sources during dry months, relying on artificial waterholes created by the Rajasthan Forest Department. Despite being primarily known for its leopard population, JRF also hosts over 200 bird species. Notably, it boasts a relatively high leopard density (8.6 leopards/100 km
2; Kumbhojkar et al. 2020) compared to other regions in India (4.8/100 km
2 in Maharashtra, Athreya et al. 2013) and globally (Nepal - 1.5/100 km
2 in the Terai region, Kandell et al., 2022; 3.31 and 3.45/100 km
2 in Chitwan National Park, Lamichhane et al., 2019). Unlike many reserves, JRF lacks buffer zones and is entirely surrounded by urban and rural villages, making it a forest-island. Ecotourism is practiced in JRF, with jeep tours conducted daily, while villagers collect wood and fodder in controlled areas without noticeable impact on the reserve's predators (Kumbhojkar et al. 2019).
Figure 1.
Location of Jhalana Forest Reserve in India (red circle). The locations of the camera traps are marked on the Google maps background (yellow circles).
Figure 1.
Location of Jhalana Forest Reserve in India (red circle). The locations of the camera traps are marked on the Google maps background (yellow circles).
Camera Traps
Camera traps were strategically placed along trails and near waterholes (Fig. 2), positioned at a height of 45-50 cm above the ground. In fringe areas, the camera traps were enclosed in secure boxes attached to iron poles for extra safety measures. We deployed 18 trail cameras equipped with motion sensors (Cuddeback X-Change Color Model 1279, De Pere, WI, USA) to capture the activity of animals in JRF (Kumbhojkar et al., 2020b). No baits or lures were used during the study (du Preez et al. 2014)
Over a total of 41,312 trap hours, we collected 30,694 photos, averaging 0.74 photos per hour. Unclear pictures were excluded from the analysis (N = 123, 0.4%). Out of the 3,201 (10.4%) leopard photos captured, 1,582 (5.2%) depicted females with cubs.
Figure 2.
Sample photo from a camera trap. LK with her two cubs at a waterhole built by the locals on the roof of a local temple, Bhomiyaji Village (27o00’16’’N, 75o50’19’’E).
Figure 2.
Sample photo from a camera trap. LK with her two cubs at a waterhole built by the locals on the roof of a local temple, Bhomiyaji Village (27o00’16’’N, 75o50’19’’E).
Study Population
In this study, the leopard population is primarily influenced by Arti, her daughters, and granddaughters. The family unit includes Arti, her three daughters, two sisters (Flora, LK) born in 2013, and Sharmili born in 2015, along with their daughters, Jalebi (born to Flora in 2015) and Tim Tim (born to LK in 2016; refer to Kumbhojkar et al., 2020b for genealogy). Another family grouping comprises Nathwali and her daughter Leela, born in 2016. Additionally, independent of these two families, we also observed another female named Mrs. Khan.
Data Collection
For our analysis, we utilized data collected from 2018 to 2021, obtained through camera traps consistently positioned in the same locations throughout this period. Any breaks in monitoring were minimal, lasting only briefly when we replaced batteries. We took care not to disturb females in their dens, allowing us to capture their movements and the number of offspring they had. Therefore, in our study, the term "litters" refers to the number of offspring that accompanied the female after leaving the dens, indicating when a female was first seen with her most recent litter. It's worth noting that we may have missed cubs that were stillborn or died in the birthing dens before they emerged with their mothers and were photographed by the trail cameras. All individuals were identified by their unique facial markings and body patterning of the rosettes (see Kumbhojkar et al. 2020).
Statistical Analysis
We calculated leopard cub survival by tracking changes in the number of young individuals following their mother. If fewer cubs were observed in subsequent camera trap photos compared to the family's initial photo, we assumed that the individual had died midway between the two photographs. We established Day 1 as the start of each individual's lifespan, corresponding to the day of life on the date of the first photo, minus two months (the length of time spent in the den). The endpoint was set as day 548 (18 months old, when leopards typically separate from their mother) and extended up to day 730 (two years old) if they appeared in subsequent photos. Cub age in the first photo was determined based on field experience, assuming that females first appeared with their young at waterholes when they were two to three months old.
Survival analysis of cubs within litters was conducted using life tables (Kleinbaum 1996), tracking each cub's life history from den departure until separation from their mother, usually between 18 to 24 months old. Unlike traditional approaches that assess the proportion of surviving cubs, we treated survivability as a time-dependent function (Kleinbaum 1996; Kosicki 2012; Langowska and Zduniak 2020; Goławski et al. 2023; Sandecki and Kosicki 2024). This enabled us to identify critical time points during the breeding season when cubs depended on maternal care. Survival time for each litter was estimated using the Kaplan-Meier method (Kleinbaum 1996), while the hazard ratio, derived from life tables, helped identify critical periods indicating the likelihood of death within a specific age group (Kleinbaum 1996; Kosicki 2012; Langowska and Zduniak 2020; Goławski et al. 2023; Sandecki and Kosicki 2024). Seasonal survival comparisons were conducted using a multiple sample test, an extension of Gehan’s generalized Wilcoxon test (Gehan 1965, Peto and Peto 1972, Lee 1980). The analysis was performed using the 'survival' library (Therneau T 2024, Terry et al. 2000) in R 4.3.3 (R Development Core Team 2024).
Results
Over the course of four years (2018-2021), we monitored 16 litters born to 9 females (refer to
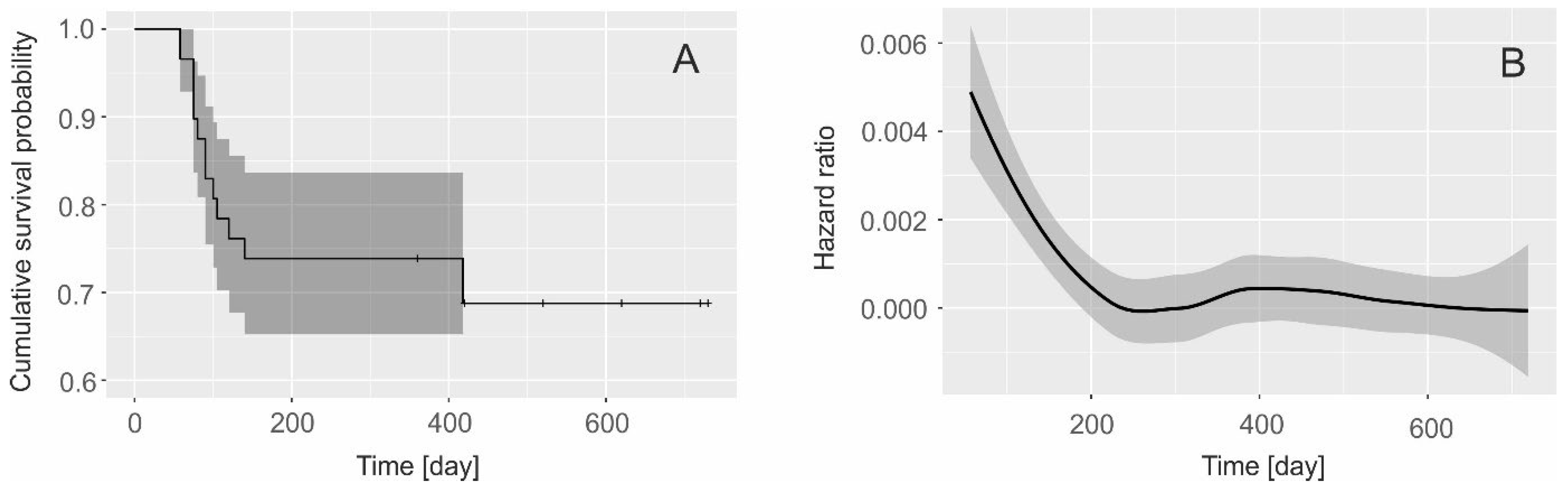
Table 1). Among these litters, a total of 33 cubs were born, but unfortunately, 13 of them died later on. Most of these fatalities (10, 30.3%) occurred within the first year of life, while only two (6, 18.1%) happened in the second year. Consequently, according to the Kaplan-Meier method, the average survival rate of cubs during their dependency on their mother (2 years) was 0.618 (95% CI: 0.526 – 0.793; see Fig. 2A). This ratio did not show any significant difference across the four-year period (chi-square = 3.8, df = 3, p = 0.3)
During the initial 12 months of a young leopard's life, the average survival rate was 0.739 (95% CI: 0.652 – 0.836), with no notable fluctuation observed across the four years (chi-square = 4.3, df = 3, p = 0.2). However, in the second year, the average survival rate rose to 0.831 (95% CI: 0.768 – 0.999). This rate was statistically higher than in the first year (Gehan–Wilcoxon test, test value = 2.39, p < 0.02), although no significant differences were observed between study years (chi-square = 4.3, df = 3, p = 0.2)
Based on the hazard ratio, the breeding success of the litter is primarily influenced during their initial year of life (Fig. 3A). The highest likelihood of death occurs shortly after birth, decreasing steadily throughout the first year and stabilizing thereafter (Fig. 3B).
Figure 3.
(A) The Kaplan-Meier survival functions for Indian Leopard (Panthera pardus fusca) cubs, (B) hazard ratio probability of death during two years of a cub’s life.
Figure 3.
(A) The Kaplan-Meier survival functions for Indian Leopard (Panthera pardus fusca) cubs, (B) hazard ratio probability of death during two years of a cub’s life.
Discussion
Our study highlights the critical period in the lives of Indian leopards, emphasizing the importance of the first 12 months. During this time frame, 30% of young individuals did not survive. Conversely, in the second year of life, the survival rate exceeded 83%, underscoring the significant impact of the female leopard's reproductive success during her cub's first year. While we did not observe infanticide by males during our study, previous reports have documented such behavior (Yosef et al. 2021).
Comparing our findings to another study, which reported combined annual survival rates for adults and subadults, revealed slight differences? In non-protected areas, the survival rate was 0.55, while in protected areas in southern Africa, it was 0.88 (Swanepoel et al., 2014). This suggests that cub survival may be an individual characteristic within a specific population or family.
A crucial aspect of our analytical approach focused on determining when leopard cubs and their siblings achieve independence from their mother. According to Owen et al. (2010), African leopard cubs typically become independent around 11.6 months old, with a range of 11 to 13 months. Our analysis aligns with this, as the majority of young leopards in our study left their mothers between 18 and 24 months of age. For instance, in April 2024, Barfi, a subadult male, separated from his mother Jalebi at 11 months old. However, we also observed that some Indian leopard cubs often stay with their mothers for up to two or three years, accompanying them within their home range (Kumbhojkar et al., 2024).
It's important to note that when cubs vanish during their first year of life (not captured by photo traps or seen again), the reasons for this mortality remain unknown, as neither we nor the rangers of the Rajasthan Forest Department found any carcasses. However, it's plausible that females conceal the carcasses of their cubs, as described by Yosef et al. (2021), who observed the mother, Flora, hiding the carcass of a deceased cub in a cluster of thor cacti (Euphorbia caducifolia) to prevent Striped Hyenas (Hyaena hyaena) or Jungle Crows (Corvus culminatus) from accessing it.
Other studies on leopards have shown that female leopards increase their hunting efficiency, especially during the first year of their cubs' lives, by targeting smaller prey more frequently (Bothma and Coertze, 2004). However, our study stands out in this aspect due to the sedentary behavior of the females in Jhalana (Kumbhojkar et al., 2020b). They primarily prey on stray dogs from the streets of neighboring Jaipur (Kumbhojkar et al., 2020a) and cattle carcasses from rural areas, ensuring a consistent food source across multiple years. This may directly contribute to the relatively high survival rate of the young and the low levels of predation observed among individual females in our study. However, the issue of disease transmission, such as rabies, warrants further analysis.
Our study has unveiled intriguing aspects of Indian leopard behavior. The relatedness of females, abundant food resources, and minimal predation have fostered the emergence of alloparenting within our leopard community (Kumbhojkar et al., 2024). This unique phenomenon suggests a distinct scenario where the leopard population has adapted by exhibiting behaviors divergent from those of other wild populations to safeguard their cubs. Such occurrences have been documented only rarely in African leopard populations (Balme et al., 2012b), adding to the fascination of our findings and warranting further exploration.
On the contrary, observations in this species have shown that females may cease parental care of current offspring to reproduce again (Balme et al., 2017). Extended parental care does not necessarily impact the subsequent breeding attempt, thus not affecting the mothers' overall fitness. Our photographs depict older cubs accompanying their mother and new litters, forming a sizable familial group, with the minimum age difference between litters being 12 months. Additionally, Owen et al. (2010) reported that females typically mate again when cubs are around ten months old, with an average interestrous period of 23 days and a gestation period of approximately 96 days, resulting in litters spaced approximately 14 months apart. Our data aligns with this to a large extent, but we observed some females having five litters in four years, suggesting that Indian leopards in JRF may have shorter interbirth intervals of 10-11 months. This is particularly noteworthy because it may be among the shortest interestrous periods observed (33 days by Bailey, 1993, and 46 days in captivity by Sadlier, 1966), potentially enabling faster recruitment of young under optimal environmental conditions in threatened populations. This phenomenon warrants further investigation in other Indian and Asiatic populations to comprehend recruitment capabilities in wild populations and assess whether the unique island ecology of JRF has influenced leopard reproductive ecology and other behaviors.
In conclusion, our study on cub survival in the Indian Leopard population within the Jhalana Reserve Forest reveals the pivotal role of the reproductive cycle in determining cub survival. The research underscores the importance of the first 12 months of a leopard cub's life, during which a significant mortality rate is observed. This understanding is essential for formulating effective conservation strategies for the vulnerable Indian Leopard population, particularly in fragmented habitats. By recognizing and addressing these dynamics, we can work towards ensuring the long-term survival and well-being of this iconic species.
Conflicts of interest
None of the authors have any conflicts of interest.
Declarations
The study was conducted under the supervision of the Deputy Conservator of Forests (Wildlife), and research permit 3(05)/2017/17 from the Chief Wildlife Warden, Rajasthan Forest Department, Jaipur.
Data availability
All data is included in
Table 1 in the manuscript. Additional data will be supplied upon reasonable request from the corresponding author.
Author Contributions
RY: SK – conceptualization, implementation; RY, SK – hypothesis; JZK, RY - Data analysis; RY, JZK - quality control; RY, JZK, SK - writing drafts and final paper.
Funding
This research received no external funding.
References
- Athreya: V., Odden, M., Linnell, J.D.C., Krishnaswamy, J. and Karanth, U. 2013. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. Plos-One 8: 1-8. [CrossRef]
- Bailey, T. N. 1993. The African leopard. Columbia University Press, New York.
- Balme, G. A., R. Slotow, L. T. B. Hunter. 2009. Impact of conservation interventions on the dynamics and persistence of a persecuted leopard (Panthera pardus) population. Biological Conservation 142:2681-2690. [CrossRef]
- Balme, G. A., A. Batchelor, N. De Woronin Britz, G. Seymour, M. Grover, L. Hes, D. W. MacDonald, L. T. B. Hunter. 2012a. Reproductive success of female leopards Panthera pardus: the importance of top-down processes. Mammal Review 43:221-237. [CrossRef]
- Balme, G., L. Hunter, N. de Woronin Britz. 2012b. A case of offspring adoption in leopards, Panthera pardus. South African Journal of Wildlife Research 42:63-66. [CrossRef]
- Balme, G. A., H. S. Robinson, R. T. Pitman, L. T. B. Hunter. 2016. Flexibility in the duration of parental care: Female leopards prioritise cub survival over reproductive output. Journal of Animal Ecology 86:1224-1234. [CrossRef]
- Bothma, J Du P., and Le Riche E. A. N. 1984.Aspects of the ecology and the behavior of the leopard Panthera pardus in the Kalahari Desert. Koede (Supplement) 1984:259-279. [CrossRef]
- Bothma, J Du P., and R. J. Coertze. 2004. Motherhood increases hunting success in southern Kalahari leopards. Journal of Mammalogy 85:756-760. [CrossRef]
- Broekhuis, F. 2018. Natural and anthropogenic drivers of cub recruitment in a large carnivore. Ecology and Evolution 8:6748-6755. [CrossRef]
- Du Preez, B. D., A. J. Loveridge, D. W. Macdonald. 2014. To bait or not to bait: A comparison of camera-trapping methods for estimating leopard Panthera pardus density. Biological Conservation 176:153-161. [CrossRef]
- Ellis, S., D. W. Franks, M. I. K. Nielsen, M. N. Weiss, D. P. Croft. 2024. The evolution of menopause in toothed whales. Nature 627:579-585. [CrossRef]
- Eltringham, S.K. 1979. The ecology and conservation of large African mammals. MacMillian, London.
- Gehan EA (1965) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52:203–223. [CrossRef]
- Golawski A, Mroz E, Golawska S, Parapura A, Zduniak P (2023) Brood survival in the Red-backed Shrike Lanius collurio in eastern Poland. Journal of Ornithology 164:921–929. [CrossRef]
- Jacobson, A. P., P. Gerngross, J. R. Lemeris, Jr., R. F. Schoonover, C. Anco, C. Breitenmoser-Wursten, S. M. Durant, M. S. Farhadinia, P. Henschel, J. F. Kamler, A. Laguardia, S. Rostro-Garcia, A. B. stein, L. Dollar. 2016. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ e1974. [CrossRef]
- Langowska A, Zduniak P (2020) No direct contact needed for drones to shorten workers lifespan in honey bee. J Apic Res 59(1):88–94. [CrossRef]
- Lee ET (1980) Statistical methods for survival data analysis. Lifetime Learning, Belmont.
- Kandel, S.R.; Lamichhane, B.R.; Subedi, N. 2020. Leopard (Panthera pardus) density and diet in a forest corridor of Terai: Implications for conservation and conflict management. Wild. Res. 47:1-8. [CrossRef]
- Kittle, A. M., A. J. Watson, T. S. P. Fernando. 2017. The ecology and behaviour of a protected area Sri Lankan leopard (Panthera pardus kotiya) population. Tropical Ecology 58:71-86.
- Kleinbaum DG (1996) Survival analysis. Springer, New York.
- Kosicki JZ (2012) Effect of weather conditions on nestling survival in the White Stork Ciconia ciconia population. Ethology Ecology & Evolution 24: 140-148. [CrossRef]
- Kumbhojkar, S., R. Yosef, B. Gujrar. 2024 Submitted. Alloparenting in leopards (Panthera pardus fusca). Acta Ethologica.
- Kumbhojkar, S., R. Yosef, Y. Benedetti and F. Morelli. 2019. Human-leopard (Panthera pardus fusca) co-existence in Jhalana Forest Reserve, India. Sustainability 11:3912. [CrossRef]
- Kumbhojkar, S., R. Yosef, J. Z. Kosicki, P. K. Kwiatkowska, P. Tryjanowski. 2020a. Dependence of the Leopard Panthera pardus fusca in Jaipur, India, on domestic animals. Oryx 55:692–698. [CrossRef]
- Kumbhojkar, S., R. Yosef, A. Mehta, and S.Rakholia. 2020b. A Camera-trap home-range analysis of the Indian Leopard (Panthera pardus fusca) in Jaipur, India. Animals 10:1600. [CrossRef]
- Lamichhane, B.R., Leirs, H., Persoon, G.A., Subedi, N., Dhakal,M., Oli, B.N., Malla, S. 2019. Factors associated with co-occurrence of large carnivores in a human-dominated landscape. Biol. Cons., 28:1473–1491. [CrossRef]
- Mills, M. G. L., and M. E. J. Mills. 2013. Cheetah cub survival revisited: a re-evaluation of the role of predation, especially by lions, and implications for conservation. Journal of Zoology 292:136-141. [CrossRef]
- Marker , L. L., and A. J. Dickman. 2005. Factors affecting leopard (Panthera pardus) spatial ecology, with particular reference to Namibian farmlands.South African Journal of Wildlife Research 35:105-115.
- Nowell, K and Pervushina, N. 2014. Review of implementation of Resolution Conf. 12.5 (Rev. CoP16) on Conservation and trade in tigers and other Appendix-I Asian big cats. IUCN and TRAFFIC report prepared for the CITES Secretariat, 65th meeting of the CITES Standing Committee, Geneva, 7-11 July. Geneva Available at: http://cites.org/sites/default/files/eng/com/sc/65/E-SC65-38-A01_0.pdf.
- Odden, M., Athreya, V., Rattan, S. and Linnell, J.D.C. 2014. Adaptable neighbors: Movement patterns of GPS-collared leopards in human dominated landscapes in India. PlosOne 9(11): 1-9. [CrossRef]
- Owen, C., S. Niemann, R. Slotow. 2010. Copulatory parameters and reproductive success of wild leopards in South Africa. Journal of Mammology 91:1178-1187. [CrossRef]
- Peto R, Peto J. (1972) Asymptotically efficient rank invariant procedures. J R Stat Soc 135:185–207. [CrossRef]
- R Core Team. (2024). R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. <https://www.R-project.org/>.
- Raza, R.H., Chauhan, D.S, Pasha, M.K.S and Sinha, S. 2012. Illuminating the blind spot: A study on illegal trade in Leopard parts in India (2001-2010). TRAFFIC India/WWF India, New Delhi, India.
- Sadlier, R. 1966. Notes on reproduction in the larger Felidae. International Zoo Yearbook 6:184–187. [CrossRef]
- Sandecki R., Kosicki J.Z. 2024. Nest survival of Crested Lark Galerida cristata in intensively used habitats in Central Poland. Journal of Ornithology (in press). [CrossRef]
- Shehzad, W., Nawaz, M.A., Pompanon, F., Coissac, E., Riaz, T., Shah, S.A. and Taberlet, P. 2014. Forest without prey: livestock sustain a leopard Panthera pardus population in Pakistan. Oryx 49(2): 248-253. [CrossRef]
- Shivakumar, S., Khettry, A., Surve, N., Rahman, H., Ghimirey, Y., Tharchen, L., Zaw, T., Waseem, M. and Jhala, Y. 2023. Panthera pardus ssp. fusca. The IUCN Red List of Threatened Species 2023(1): eT215195524A215195533.
- Stein, A.B., Athreya, V., Gerngross, P., Balme, G., Henschel, P., Karanth, U., Miquelle, D., Rostro-García, S., Kamler, J.F., Laguardia, A., Khorozyan, I. & Ghoddousi, A. 2023. Panthera pardus. The IUCN Red List of Threatened Species 2023: e.T15954A215195554. [CrossRef]
- Sunquist, M. E., & F. C. Sunquist. 2009. Family Felidae (Leopard Panthera pardus) Pp. 133-134 In Handbook of the mammals of the world (Wilson D. E., & R. A. Mittermeier, eds.). vol. 1. Carnivores. Lynx Edicions, Barcelona.
- Swanepoel, L, H., Somers, M. J., van Hoevn W, Schiess-Meier, M., Owen, C., Snyman, A., Martins, Q., Senekal, C., Camacho, G., Boshoff, W., Dalerum, F. 2014. Survival rates and causes of mortality of leopards Panthera pardus in southern Africa. Oryx 49:595-603. [CrossRef]
- Therneau T (2024). _A Package for Survival Analysis in R_. R package version 3.5-8, <https://CRAN.R-project.org/package=survival>.
- Terry M. Therneau, Patricia M. Grambsch (2000). _Modeling Survival Data: Extending the Cox Model_. Springer, New York. ISBN 0-387-98784-3.
- Yosef, R., H. Dabi & S. Kumbhojkar. 2021. Thanatological behavior of a female Leopard (Panthera pardus fusca). Acta Ethologica 24:137-140. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).