1. Introduction
Cannabidiol is an antiseizure medication (ASM) used as an add-on drug for some types of pediatric epilepsies, including Dravet syndrome [
1,
2], Lennox-Gastaut syndrome [
3,
4], and tuberous sclerosis complex [
5]. Although considered effective and safe, cannabidiol is currently not recommended for other types of epilepsy in humans. However, cannabidiol is also under investigation in the field of veterinary medicine, and one recent trial suggested that this ASM is effective for dogs with idiopathic epilepsy. In these animals, cannabidiol was used as an add-on drug with positive findings, but with significant adverse events, mainly consisting of reduced food intake and vomiting [
6]. In children, cannabidiol was also associated with adverse events, including diarrhea, increased seizures, or sedation as the most frequent. Reduced appetite and vomiting were also noted in the same cohort [
7].
Clinical investigations on the effects of cannabidiol in patients with temporal lobe epilepsy (TLE) are missing, despite the fact that this type of epilepsy is the most prevalent and also poorly responsive to ASMs in adults with epileptic disorders [
8]. It has to be mentioned that cannabidiol has been investigated in various animal models of TLE, including amygdala [
9] and hippocampal kindling [
10], or pilocarpine [
11] and kainic acid [
12] administration to induce a
status epilepticus. These various models and our recent experiment [
13] documented the antiseizure properties of cannabidiol in the animals reproducing the human TLE.
The possible interest in the clinical use of cannabidiol in TLE is related to the problem of drug refractoriness, which is frequently found in this type of epilepsy [
8]. First-line drugs such as levetiracetam were shown to be poorly effective in controlling seizures in as much as 50% of patients with epilepsy [
14]. Consistent with these clinical data, levetiracetam was only partially effective in epileptic rats that were previously treated with kainic acid [
15]. In this experiment, levetiracetam produced significant effects mainly on seizure duration and not on seizure frequency, at least in non-responsive rats. Instead, in the same model, cannabidiol reduced not only the seizure duration but also the overall seizure occurrence, when used at high doses (120 mg/kg
bis in die,
b.i.d.) [
13]. Because of these partially positive findings obtained with levetiracetam, we hypothesized that there could be a potentiation of levetiracetam when combined with cannabidiol. Moreover, we also decided to test a lower cannabidiol dose by performing just one instead of two daily injections of this ASM (at 120 mg/kg/die), so as to evaluate a condition in which the adverse effects of cannabidiol could be less probable. Here we illustrate an interesting interaction between the two investigated ASMs, at a cannabidiol dose that resulted in a subthreshold.
2. Results
Twenty-two rats injected with kainic acid (15 mg/kg, intraperitoneally, i.p.) were available (four additional rats were non-responders; all of them survived to the kainic acid administration). After having induced the
status epilepticus, epileptic activity was continuously recorded using video electrocorticography (vECoG) until the animals were implanted with osmotic minipumps releasing either saline or levetiracetam [
13]. This recording continued for one week following the treatment period. Subgroups of these rats were also treated with a single daily injection of cannabidiol (120 mg/kg, subcutaneously, s.c.) for 7 days, matching the full-time infusion interval by the minipumps. This protocol resulted in four sets of animals (saline released by minipumps, n=6; levetiracetam (by minipumps), n=7; saline (by minipumps)+cannabidiol (s.c.), n=4; cannabidiol (s.c.)+levetiracetam (by minipumps), n=5). Seizure occurrence and duration were analyzed starting one week before and continuing during and after treatment administration.
2.1. Characterization of the Overall Number of Spontaneous Recurrent Seizures (SRSs)
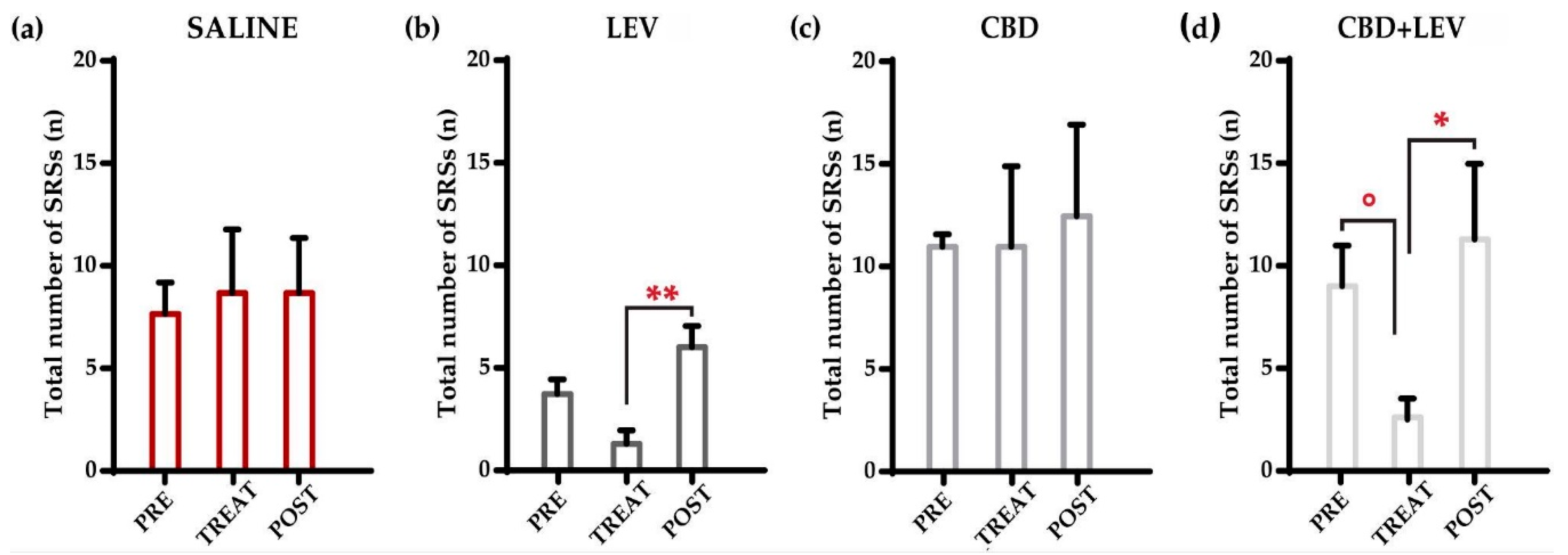
All rats were nonresponsive to ASMs administered in this study, meaning that SRSs did not disappear during treatment in nobody of the animals. Following the Shapiro-Wilk test, a two-way (treatment x interval time) repeated measures analysis of variance (ANOVA) was performed. As shown in
Figure 1a-d, we initially evaluated all the seizures that occurred during the different treatment conditions (i.e., preceding the minipump implantation, during saline or drug delivery for one week (implanted minipumps), and after the removal of minipumps (treatment withdrawal). Total SRSs decreased significantly (p<0.01) within treatment groups. Notably, SRSs showed a significant reduction (-75%) in comparison to the pretreatment period only when levetiracetam was combined with cannabidiol (°p<0.05 according to Duncan’s new multiple range test, MRT). No significant changes were observed in the other treatment groups by comparing pretreatment and treatment values. However, the comparison of posttreatment and treatment values revealed significant differences in both the levetiracetam (**p<0.01) and cannabidiol+levetiracetam (*p<0.05) groups, thus suggesting an effect due to drug withdrawal in both groups receiving the ASMs.
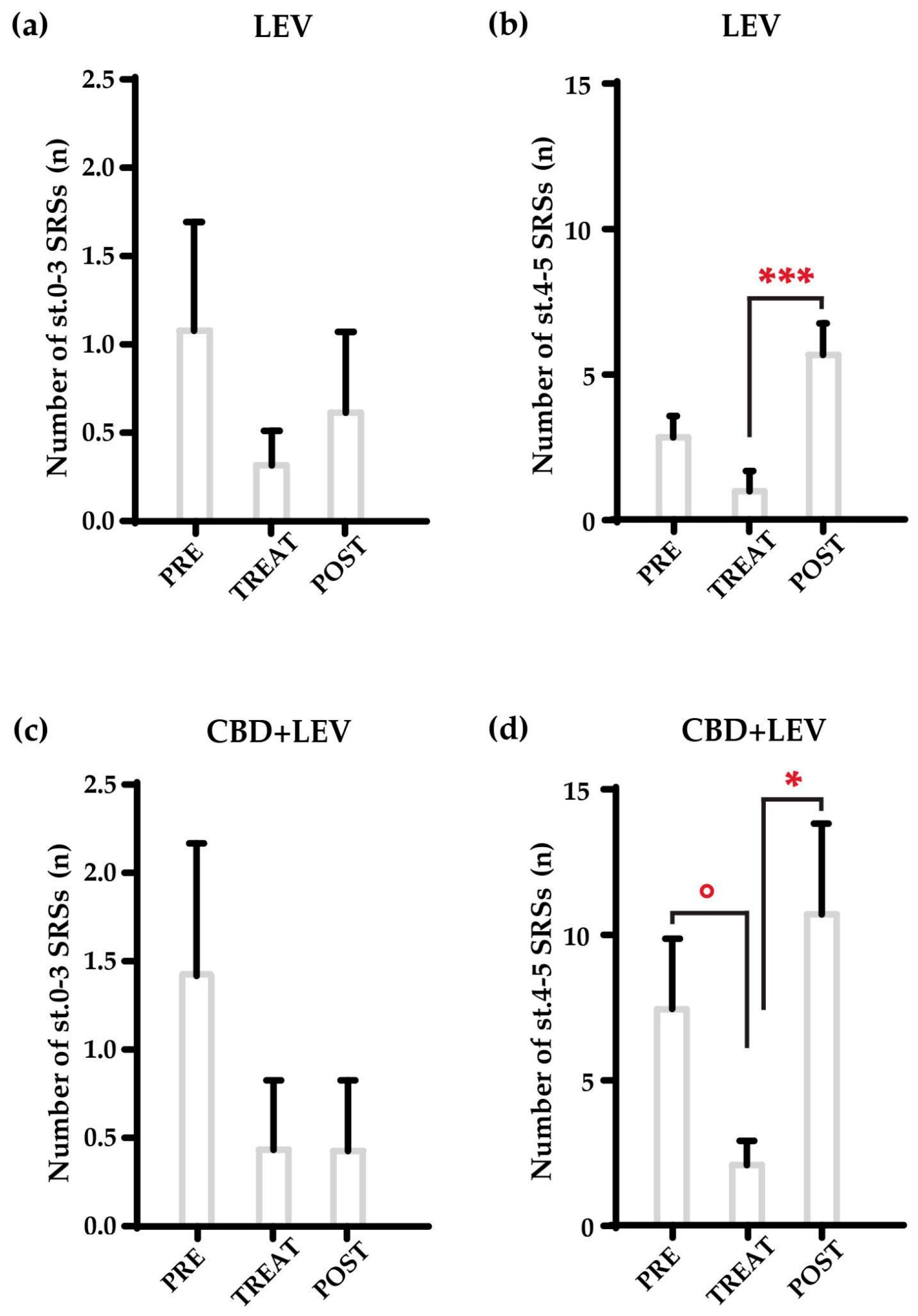
We further analyzed changes in the overall seizure occurrence found in the treatment groups that received levetiracetam and its combination with cannabidiol, by separately analyzing nonconvulsive (stages, st. 0-3 in
Figure 2) and convulsive (tonic-clonic, st. 4-5) seizures (
Figure 2a-d). The combination treatment primarily affected the number of convulsive seizures (
Figure 2d), as the MRT only revealed a significant reduction in the number of tonic-clonic SRSs during the treatment (°p<0.05 vs. pretreatment values in the same group). Additionally, we observed a significant increase in the occurrence of convulsive SRSs after the removal of minipumps loaded with levetiracetam (***p<0.001, MRT), with no changes for non-convulsive SRSs (
Figure 2 a,b). No changes at all were found for cannabidiol or saline treatments for both nonconvulsive and convulsive seizures (not shown).
2.2. Characterization of the Total Duration of SRSs
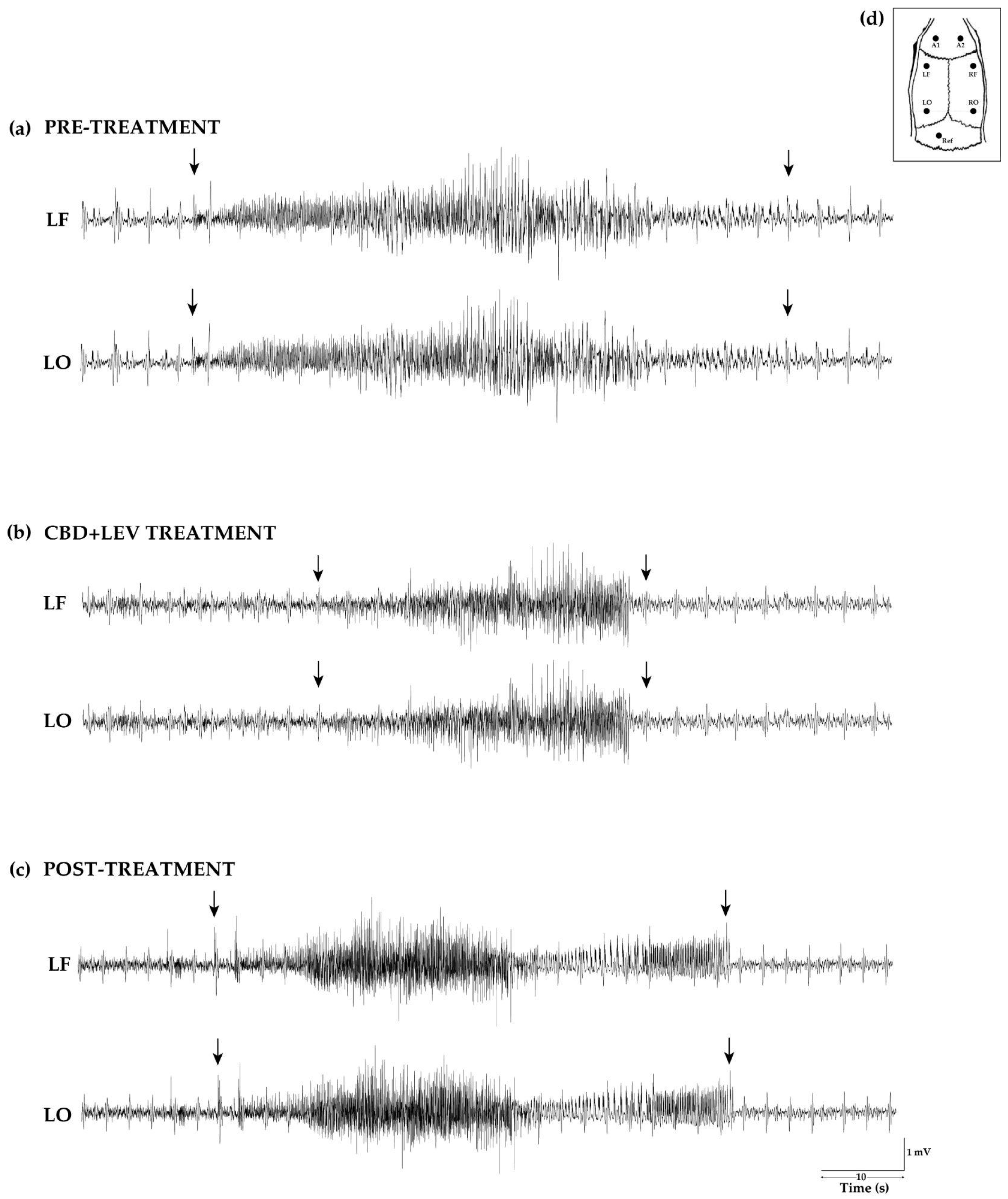
Visual inspection revealed that the duration of SRSs observed in the week before minipump implantation, and the week following the treatments, differed from that characterized during the treatment period (
Figure 3).
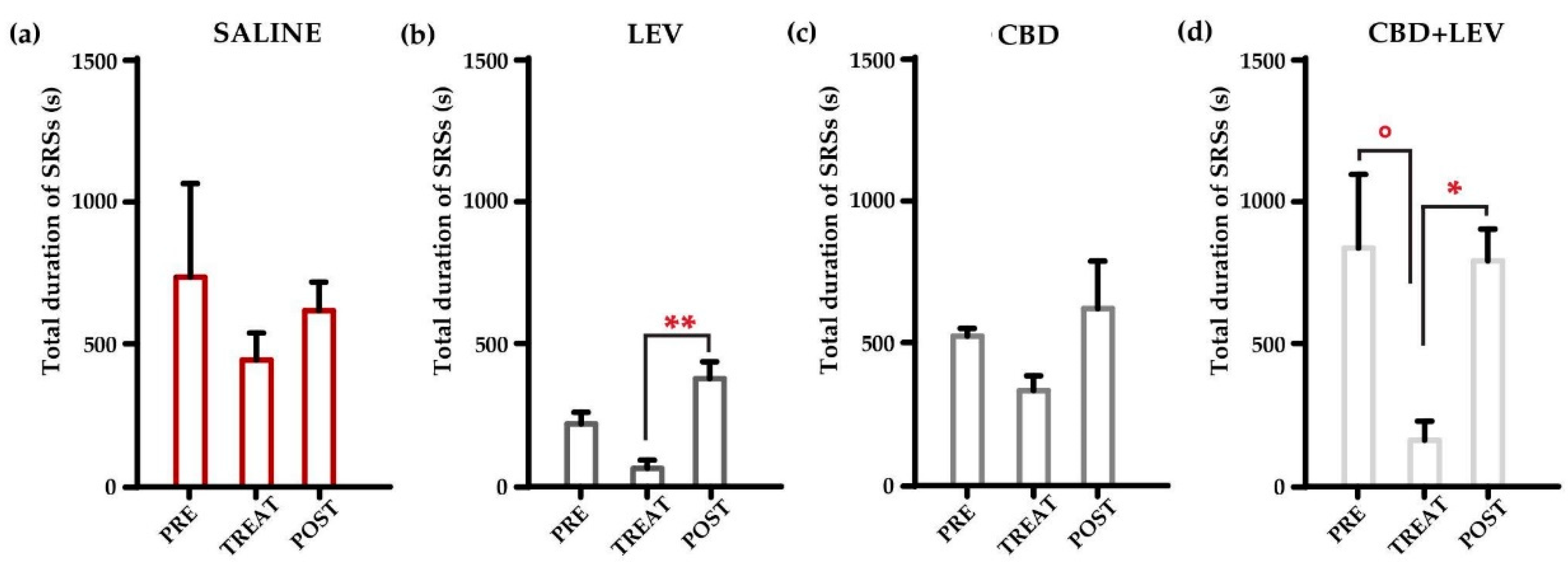
Changes under different treatment conditions were evaluated through a two-way (treatment x interval time) repeated measures ANOVA. This analysis revealed significant variations (p<0.001) in the total duration of SRSs (
Figure 4a-d). According to MRT, the SRSs’ overall duration considerably decreased (-82%) during the combined treatment with cannabidiol and levetiracetam (°p<0.05), compared to the pretreatment period (
Figure 4d). Then, the prompt cessation of combined treatment led to a significant increase (+520%, *p<0.05) in the overall duration of the SRS (
Figure 4d). Additionally, the rapid discontinuation of levetiracetam administration induced an abrupt increase in the overall duration of SRSs, up to 600% of values measured during the treatment (**p<0.01,
Figure 4b). In contrast, cannabidiol administration or its withdrawal did not significantly modify the overall seizure duration (
Figure 4c). No differences were found in the saline group at all the considered time intervals (
Figure 4a).
3. Discussion
Our study resulted in two major findings: i) cannabidiol is ineffective when administered as a single, although high dose in rats developing epilepsy after a
status epilepticus induced by i.p. kainic acid. This finding contrasts sharply with our previous study, which showed a beneficial effect of cannabidiol administered
b.i.d. at 120 mg/kg [
13]; ii) although at a subthreshold dose, cannabidiol potentiated the effects of levetiracetam, which we previously found to be incompletely effective in epileptic nonresponsive rats [
15]. This finding is of interest because it shows that an ASM could be therapeutically useful even at a subthreshold dose when combined with a first-line drug toward which is present refractoriness. This allows the use of a dose regimen that could prevent the most serious adverse effects of an additional ASM.
This observation raises the question of the possible basis for the potentiation of levetiracetam by cannabidiol. While we do not have an answer, a suggestive hypothesis could be based on our previous results [
15]. We previously noticed that levetiracetam significantly increases the tissue levels of allopregnanolone in the cerebral neocortex of epileptic rats. Allopregnanolone is the major representative of a class of molecules, known as neurosteroids, that act as modulators of neuronal excitability [
17]. Interestingly, allopregnanolone has been recognized as an anticonvulsant in TLE [
18], and it was exploited to develop an ASM, ganaxolone, actually in use for a particular type of pediatric epilepsy [
19], specifically for seizures associated with the cyclin-dependent kinase-like 5 deficiency disorder [
20,
21].
Since cannabidiol can interact with a variety of proteins in the brain [
22], there could be multiple mechanisms for the hypothesized interaction of cannabidiol and allopregnanolone. Recently, cannabidiol was found to modulate the γ-aminobutyric type A (GABA
A) receptor-mediated currents [
23] generated in
Xenopus laevis oocytes transplanted with GABA
A receptors, obtained from the neocortex of patients with TLE. GABA
A receptors are well-known targets for allopregnanolone, which provokes a positive allosteric modulation of these channel-linked receptors [
24], thus potentiating inhibition in the brain. Interestingly, cannabidiol mainly modifies currents produced by α1-6βγ2 receptors, in the low micromolar range. The α2-containing GABA
A receptors appeared to be those more sensitive to cannabidiol, leading to a 4-fold increase of the current. A β-subunit selectivity was also reported by the same investigators, with a prevalence of β2/β3 over β1 subunits [
22]. Several binding sites have been identified in the GABA
A receptor for allopregnanolone, both in α and β subunits [
25], suggesting the possibility that the modulation of these subunits could underpin the reported synergism between cannabidiol and the allopregnanolone analog ganaxolone [
26].
4. Materials and Methods
4.1. Animals
The study protocol was authorized by the Italian Ministry of Health (729/2021-PR), after approval by the university Animal Welfare Body. All experiments were performed by the European Directive 2010/63/EU and the consequent Italian act (DM 26/2014). Twenty-six adult Sprague-Dawley male rats (Charles River, Calco, Italy) were housed in a pathogen-free facility with a controlled environment and unlimited access to food and water. A total of 22 rats, with an initial weight of 175-200 g, were used in this study. Every effort was made to refine procedures, improve animal welfare, and minimize the number of animals utilized in experiments.
4.2. Experimental Design
Status epilepticus was induced by an i.p. injection of kainic acid (15 mg/kg, in saline; Cayman Chemical, Ann Arbor, MI, US) one week after electrode implantation. To minimize discomfort caused by the
status epilepticus, a s.c. injection of Ringer’s lactate solution (3-5 mL) along with softened rat chow was administered. Six weeks after kainic acid administration, rats were randomly divided into four groups: i) saline (n=6), ii) levetiracetam (Cayman Chemical) (n=7), iii) cannabidiol (Farmabios, Gropello Cairoli, Italy) (n=4), and iv) cannabidiol+levetiracetam (n=5). All rats were anesthetized and implanted subcutaneously with a minipump delivering continuous dosing over one week (2ML1 ALZET, flow rate: 10 µL/h, DURECT Corporation, Cupertino, CA, US) of saline (i, iii) and levetiracetam at 300 mg/kg/day [
15] (ii, iv). Since this study aimed to evaluate the combination of levetiracetam with a subthreshold dose of cannabidiol, group (iv) was treated at the same time with cannabidiol (120 mg/kg, s.c.) dissolved in MCT oil (USP pharmaceutical grade MCT Lean; provided by MCT Foods, Glencoe, IL, USA). The last group (iii) consisted of epileptic rats (n=4) treated with cannabidiol dissolved in MCT oil (120 mg/kg, s.c) once a day for 7 days, and implanted with a minipump loaded with saline. In all animals, SRSs were continuously video-ECoG monitored.
4.3. Electrode Implantation and Video-Electrocorticography Analysis
As previously described [
13,
15], rats were implanted with epidural electrodes in the frontal (bregma 0 mm, 3.5 mm lateral from midline) and occipital cortices (bregma −6.5 mm, 3.5 mm lateral from midline). One electrode was implanted below the lambda in the midline and used as a reference. The ECoG data were recorded via a cable connection between the headset and preamplifiers. Electrical activity was digitally filtered (0.3 Hz high-pass, 500 Hz low-pass) and acquired a 1 kHz per channel. All data was stored on a personal computer after the mathematical subtraction of traces of recording electrodes from the trace of reference electrodes by using a PowerLab8/30 amplifier connected to 4 BioAmp preamplifiers (ADInstruments; Dunedin, Otago, New Zealand). Videos were digitally recorded through a camera connected to the dedicated computer and synchronized to the ECoG traces through the LabChart 8 PRO internal trigger.
Using LabChart 8 PRO (ADInstruments), offline ECoG signals were digitally filtered (band-pass: high, 50 Hz; low, 1 Hz) and manually examined. The ECoG traces were used to identify seizures, and the synchronized video recordings were used to evaluate the animal behaviors. As regards seizures, they were scored as follows: stage 0 if a clear epileptiform ECoG signal was observed without behavioral changes in the video; stage 1–2 in the presence of absence-like immobility, “wet-dog shakes,” facial automatisms, and head nodding; stage 3 when presenting with forelimb clonus and lordosis; stage 4 corresponding to generalized seizures and rearing; and stage 5 when seizures consisted of rearing with the loss of posture and/or wild running, followed by generalized convulsions [
16].
4.4. Statistical Analysis
We compared the data for all groups using repeated-measures two-way ANOVA, followed by MRT. Specifically, after having tested the normality distribution (Shapiro-Wilk), data on the total number and the duration of SRSs were analyzed by considering treatments and time intervals as the main factors. To evaluate the differences in the type of seizure (nonconvulsive or convulsive), a three-way analysis of variance considered as factors treatments, time intervals, and the seizure type, followed by the MRT. All statistical analyses were carried out using SigmaPlot 11 (Systat Software, San Jose, CA, U.S.A.). Data are presented as mean ± standard error of the mean (SEM) and were regarded as significantly different at p<0.05.
Author Contributions
Conceptualization, G.B. and G.C.; methodology, C.L., A.M.C.; software, C.L, A.K.A.M.S.; validation, G.B. and C.L.; formal analysis, C.L, A.K.A.M.S., and M.M.; investigation, C.L, A.K.A.M.S., A.M.C., and M.M.; resources, G.C., and G.B.; data curation, C.L.; writing—original draft preparation, C.L., and G.B.; writing—review and editing, C.L, G.B., G.C., and A.M.C.; visualization, C.L, A.K.A.M.S., A.M.C., G.B., G.C., and M.M.; supervision, C.L., and G.B.; project administration, G.B., and G.C.; funding acquisition, G.B., and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by BPER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We thank Farmabios S.p.A. (Gropello Cairoli , Italy) for providing the synthetic cannabidiol.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in Patients with Seizures Associated with Lennox-Gastaut Syndrome (GWPCARE4): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. The Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285. [Google Scholar] [CrossRef]
- Rozental, A.J.; Weisbeck, B.G.; Corsato Alvarenga, I.; Gustafson, D.L.; Kusick, B.R.; Rao, S.; Bartner, L.R.; McGrath, S. The Efficacy and Safety of Cannabidiol as Adjunct Treatment for Drug-resistant Idiopathic Epilepsy in 51 Dogs: A Double-blinded Crossover Study. J. Vet. Intern. Med. 2023, 37, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Devinsky, O.; Lopez, M.; Park, Y.D.; Zentil, P.P.; Patel, A.D.; Thiele, E.A.; Wechsler, R.T.; Checketts, D.; Sahebkar, F. Long-term Efficacy and Safety of Cannabidiol in Patients with Treatment-resistant Epilepsies: Four-year Results from the Expanded Access Program. Epilepsia 2023, 64, 619–629. [Google Scholar] [CrossRef]
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Res. Treat. 2012, 2012, 630853. [Google Scholar] [CrossRef]
- Fallah, M.S.; Dlugosz, L.; Scott, B.W.; Thompson, M.D.; Burnham, W.M. Antiseizure Effects of the Cannabinoids in the Amygdala-kindling Model. Epilepsia 2021, 62, 2274–2282. [Google Scholar] [CrossRef]
- Reddy, D.S. Therapeutic and Clinical Foundations of Cannabidiol Therapy for Difficult-to-Treat Seizures in Children and Adults with Refractory Epilepsies. Exp. Neurol. 2023, 359, 114237. [Google Scholar] [CrossRef]
- Patra, P.H.; Barker-Haliski, M.; White, H.S.; Whalley, B.J.; Glyn, S.; Sandhu, H.; Jones, N.; Bazelot, M.; Williams, C.M.; McNeish, A.J. Cannabidiol Reduces Seizures and Associated Behavioral Comorbidities in a Range of Animal Seizure and Epilepsy Models. Epilepsia 2019, 60, 303–314. [Google Scholar] [CrossRef]
- Thomson, K.E.; Metcalf, C.S.; Newell, T.G.; Huff, J.; Edwards, S.F.; West, P.J.; Wilcox, K.S. Evaluation of Subchronic Administration of Antiseizure Drugs in Spontaneously Seizing Rats. Epilepsia 2020, 61, 1301–1311. [Google Scholar] [CrossRef]
- Costa, A.-M.; Russo, F.; Senn, L.; Ibatici, D.; Cannazza, G.; Biagini, G. Antiseizure Effects of Cannabidiol Leading to Increased Peroxisome Proliferator-Activated Receptor Gamma Levels in the Hippocampal CA3 Subfield of Epileptic Rats. Pharmaceuticals 2022, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Tate, S.K.; Kinirons, P.; Weale, M.E.; Cavalleri, G.L.; Depondt, C.; Murphy, K.; O’Rourke, D.; Doherty, C.P.; Shianna, K.V.; et al. No Major Role of Common SV2A Variation for Predisposition or Levetiracetam Response in Epilepsy. Epilepsy Res. 2009, 83, 44–51. [Google Scholar] [CrossRef]
- Costa, A.-M.; Lucchi, C.; Malkoç, A.; Rustichelli, C.; Biagini, G. Relationship between Delta Rhythm, Seizure Occurrence and Allopregnanolone Hippocampal Levels in Epileptic Rats Exposed to the Rebound Effect. Pharmaceuticals 2021, 14, 127. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of Seizure Activity by Electrical Stimulation: II. Motor Seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Biagini, G.; Panuccio, G.; Avoli, M. Neurosteroids and Epilepsy. Curr. Opin. Neurol. 2010, 23, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Biagini, G.; Avoli, M. Neurosteroids and Focal Epileptic Disorders. Int. J. Mol. Sci. 2020, 21, 9391. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids as Novel Anticonvulsants for Refractory Status Epilepticus and Medical Countermeasures for Nerve Agents: A 15-Year Journey to Bring Ganaxolone from Bench to Clinic. J. Pharmacol. Exp. Ther. 2024, 388, 273–300. [Google Scholar] [CrossRef]
- Knight, E.M.P.; Amin, S.; Bahi-Buisson, N.; Benke, T.A.; Cross, J.H.; Demarest, S.T.; Olson, H.E.; Specchio, N.; Fleming, T.R.; Aimetti, A.A.; et al. Safety and Efficacy of Ganaxolone in Patients with CDKL5 Deficiency Disorder: Results from the Double-Blind Phase of a Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Neurol. 2022, 21, 417–427. [Google Scholar] [CrossRef]
- Olson, H.E.; Amin, S.; Bahi-Buisson, N.; Devinsky, O.; Marsh, E.D.; Pestana-Knight, E.; Rajaraman, R.R.; Aimetti, A.A.; Rybak, E.; Kong, F.; et al. Long-term Treatment with Ganaxolone for Seizures Associated with Cyclin-dependent Kinase-like 5 Deficiency Disorder: Two-year Open-label Extension Follow-up. Epilepsia 2024, 65, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; Van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABA A Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Gaeta, A.; Cannata, B.; Pinzaglia, C.; Aronica, E.; Morano, A.; Cifelli, P.; Palma, E. GABAergic Neurotransmission in Human Tissues Is Modulated by Cannabidiol. Life 2022, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.G.; Cunningham, L.; Mitchell, S.G.; Swinny, J.D.; Lambert, J.J.; Belelli, D. GABAA Receptor-Acting Neurosteroids: A Role in the Development and Regulation of the Stress Response. Front. Neuroendocrinol. 2015, 36, 28–48. [Google Scholar] [CrossRef]
- Chintala, S.M.; Tateiwa, H.; Qian, M.; Xu, Y.; Amtashar, F.; Chen, Z.; Kirkpatrick, C.C.; Bracamontes, J.; Germann, A.L.; Akk, G.; et al. Direct Measurements of Neurosteroid Binding to Specific Sites on GABA A Receptors. Br. J. Pharmacol. [CrossRef]
- Golub, V.; Ramakrishnan, S.; Reddy, D.S. Isobolographic Analysis of Adjunct Antiseizure Activity of the FDA-Approved Cannabidiol with Neurosteroids and Benzodiazepines in Adult Refractory Focal Onset Epilepsy. Exp. Neurol. 2023, 360, 114294. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).