Introduction
Image Guided Superficial Radiation Therapy (IGSRT) is a novel technology which combines traditional superficial radiation therapy (SRT) with high resolution dermal ultrasound (HRDUS) to treat non-melanoma skin cancers (NMSC). Since development, IGSRT use has expanded dramatically in outpatient clinics. We review the IGSRT literature and state of the art operating principles in US dermatologic practices.
Materials and Methods
A literature search of electronic databases (Medline, Pubmed, Cochrane Library, Science Direct) combined with various authors’ published and unpublished documents, procedures and clinical experience with IGSRT were synthesized for this paper.
Results
Studies have demonstrated IGSRT consistently delivers high cure rates (99%) with low complications for early stage (stage 0, I, or II) squamous cell and basal cell carcinomas. Control rates are statistically superior to non-image guided SRT and external beam radiation (XRT) as well as Mohs Micrographic Surgery(MMS). This improvement is attributed to in-vivo dermal tumor visualization via HRDUS and using an interdisciplinary approach to deliver care. IGSRT use in the dermatologic clinic for early stage NMSCs has become common practice and continues to expand.
Conclusions
While the safety and cosmetic benefits of SRT/XRT has been long documented, IGSRT represents a significant leap forward in efficacy (statistically significant) by adding in-vivo dermal tumor imaging. Results rival and appear on one study to surpass tumor control obtained with MMS. A contributing factor to the success may be the availability and use of an interdisciplinary team approach that includes dermatologists, radiation therapists, radiation oncologists and medical physicists. The high tumor control rates, minimal side effects, favorable cosmesis and ability to treat multiple lesions per session using IGSRT is establishing this modality as a standard first line therapy for early stage NMSCs in dermatology clinics. IGSRT may represent the most effective option for the non-surgical treatment of early stage NMSC to date.
Key Summary Points:
- -
Image guided superficial radiation therapy (IGSRT) combines superficial radiation therapy (SRT) with high resolution dermal ultrasound (HRDUS).
- -
IGSRT has been used to treat non-melanoma skin cancer (NMSC) and has statistically significantly superior local control/cure rates when compared to NMSC lesions treated with SRT without image guidance or external beam radiotherapy (XRT).
- -
The improvement in local control/cure rates can be attributed to the in-vivo dermal tumor visualization before, during and after treatment and the use of an interdisciplinary team approach.
- -
IGSRT could be considered a first line non-surgical therapy option for the treatment of early stage NMSC.
Introduction
Superficial radiation therapy (SRT) has been used by dermatologists to treat non-melanoma skin cancer (NMSC) for over a century [
1]. Since 2015 there has been growing interest in a new modality called Image Guided Superficial Radiation Therapy (IGSRT), in which SRT is combined with high resolution dermal ultrasound (HRDUS). HRDUS provides crucial tumor configuration, most notably tumor thickness, that is unavailable through visual observation [
2]. IGSRT use has been used in various outpatient dermatology clinics nationwide as a non-invasive alternative to surgical removal. Due to multiple advantages including it being suitable to address a majority of NMSCs, the capability of treating multiple lesions in a single session, affordability, safety, and ease of use [
3] IGSRT has become more and more widely adopted in the dermatology clinics. We summarize the literature of published IGSRT studies as well as present the state of the art practice patterns and recommendations using IGSRT in busy dermatology clinics in the United States.
Methods
A literature search of electronic databases (Medline, Pubmed) was conducted. Studies from the electronic literature review, as well as authors’ published and unpublished documents, procedures and clinical experience with IGSRT were synthesized for this paper. All IGSRT studies reviewed in this paper utilized SRT devices embedded with HRDUS with frequencies of 20-22 megahertz (MHz). This paper reviewed studies limited geographically to the United States and therefore is based on regulations and common practices in the United States only. Although there are slightly different regulations differing amongst states, the practices are overwhelmingly consistent across the continental USA to make general observations/statements/recommendation.
A panel of experts with extensive experience and knowledge with IGSRT were invited by members of the Dermatology Association of Radiation Therapy (DART) to contribute to this paper. This included dermatologists, Mohs surgeons, radiation oncologists, physicists and radiation therapists.
Overview of Radiation Therapy (RT)
Cellular/Molecular Effects of RT
Electromagnetic energy generated from X-rays damages the DNA of rapidly dividing cancer cells, reducing the population, and activating a variety of complex signaling cascades that may result in cell cycle arrest or cellular apoptosis. The cytolytic effects of radiation therapy (RT) are countered by the activation of various DNA repair mechanisms [
4]. Clinically, patients receiving radiation experience asymptomatic erythema, scaling and mild tissue swelling or edema.
RT Dosing
RT dosing is measured in international units known as Gray (Gy) or centiGray (cGy, 1Gy = 100 cGy). The dose delivered in 1 treatment session is known as a fraction [
5]. RT dosing for NMSC ranged from 200-600 cGy or higher per fraction, with 2-5 treatments per week.
Electron-Beam Radiation
Through use of electron-beam radiation, NMSC tumors are targeted through use of negatively charged, electrons at mega-electron volt energies [
6].
Brachytherapy
Brachytherapy is a form of RT, which directly applies the radioactive source close to or into the body/organ. When treating NMSC tumors, the radioactive source or electronically generated radiation can be applied inside body tissues (interstitial), into a body cavity (intracavitary), or to a tumor-fitted surface-mold, which can be used to treat skin cancers [
7,
8]. Brachytherapy is a complex topic, and a detailed discussion is outside the scope of this paper.
Superficial Radiation Therapy (SRT)
SRT targets NMSC tumors by utilizing external radiation consisting of low energy, low penetration, photons, typically ranging from 50 to 150 kVp (peak kilovoltage). At the upper range of 200 kVp, SRT penetrates through the dermis, leaving deeper structures intact and unharmed, allowing targeting of skin tumors without disruption of deeper structures [
7].
Image Guided Superficial Radiation Therapy (IGSRT)
IGSRT utilizes an integrated high resolution dermal ultrasound (HRDUS) with SRT delivery system. The most common protocol utilized in IGSRT involves 3-4 fractions per week at a typical dose of approximately 265 to 280cGy, although doses range between 250 to 300cGy, with care to avoid doses greater than 300cGy, due to concern for excess side effects/complications. The variable energy range is suitable for treating superficial tumors (tumors <3mm in depth), including early stage (stage 0, I, and II) squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). Treatment is continued for 6-7 weeks, for a total of 20 fractions and a total dose of approximately 5,300-5,600cGy, or higher.
Description of High Resolution Dermal Ultrasound (HDRUS)—Dermal Visualization, Extent, Dermoscopy
High Resolution Dermal Ultrasound (HRDUS)
Imaging that is part of IGSRT is integral to localization, and to treatment planning. In addition to a traditional radiation dosing scheme in cGy, IGSRT uses HRDUS to visualize the exact depth and configuration of the gross NMSC tumor remaining in the dermis after the biopsy has been completed. Tumor depth is correlated with percentage depth dose (PDD) tables, which are provided and published by the IGSRT machine manufacturer in 1mm increments allowing for selection of the best energy choice (50, 70, or 100kV), both before and during treatment, whereas other publications show PDD in only 5mm increments, which is not ideal for the treatment of NMSC [
9,
10]. PDD is a concept which allows medical physicists to precisely define exactly how much deposited radiation is absorbed as it passes through each mm of dermal depth. Therefore, the precise depth measurements obtained through use of HRDUS can potentially minimize toxicity, giving dermatologists yet another advantage when treating with IGSRT.
Furthermore, HRDUS has shown us the in-vivo complexity of the interaction between radiation and NMSC tumors by revealing a series of fluctuating depth measurements throughout therapy due to edema and inflammation caused by radiation, as well as other external or internal factors such as bleeding, unintended trauma/injury, infection, excoriation, etc. This means that NMSC tumors undergoing radiation are essentially moving targets that may require adjustments in dose/fractionation energy (kV) as determined by PDD changes. These PDD measurements vary depending on depth, radiation field size, source to skin distances, and energy (50, 70, or 100 kV) used in the treatment protocol.
Due to the high definition and specific surface penetration that creates well-demarcated visuals, HRDUS is considered the first-line imaging technique for assessing and characterizing NMSC tumors, as well as monitoring treatment response and potential surgical planning [
11].
Dermal Visualization
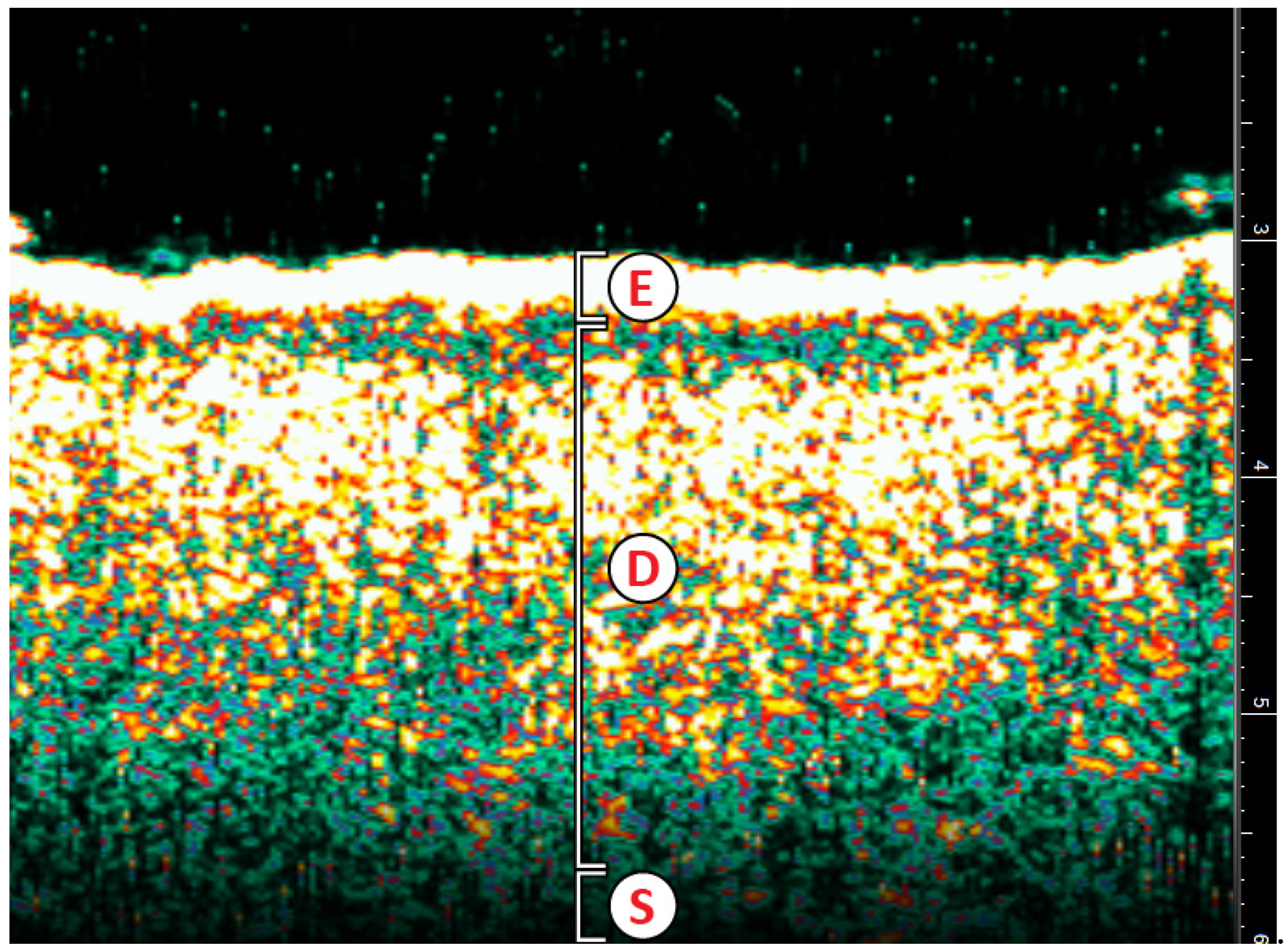
HRDUS images of normal skin anatomy are fast, accurate, and non-invasive. The epidermis, which presents as hyperechoic due to its composition of keratin, a strong reflector of ultrasound waves [
12], is visualized as a thin yellow band (
Figure 1). The papillary dermis is visualized as a thick green speckled band just below the epidermis. US probes with frequencies > 10 MHz can distinguish between the papillary and reticular dermis based on their differing compositions. The reticular dermis begins where there is a combination of green and yellow speckling in the deeper dermis. Alternatively, the hypodermis or subcutaneous layer, with the presence of fat lobules that allow passage of ultrasound waves, presents as hypoechoic [
12] and visually appears black. Of note, the doppler component of HRDUS will pick up colors where blood flow is detected. 20 MHz HRDUS penetrates a maximum depth of 7mm, which provides visualization of the epidermis and dermis (
Table 1) [
13].
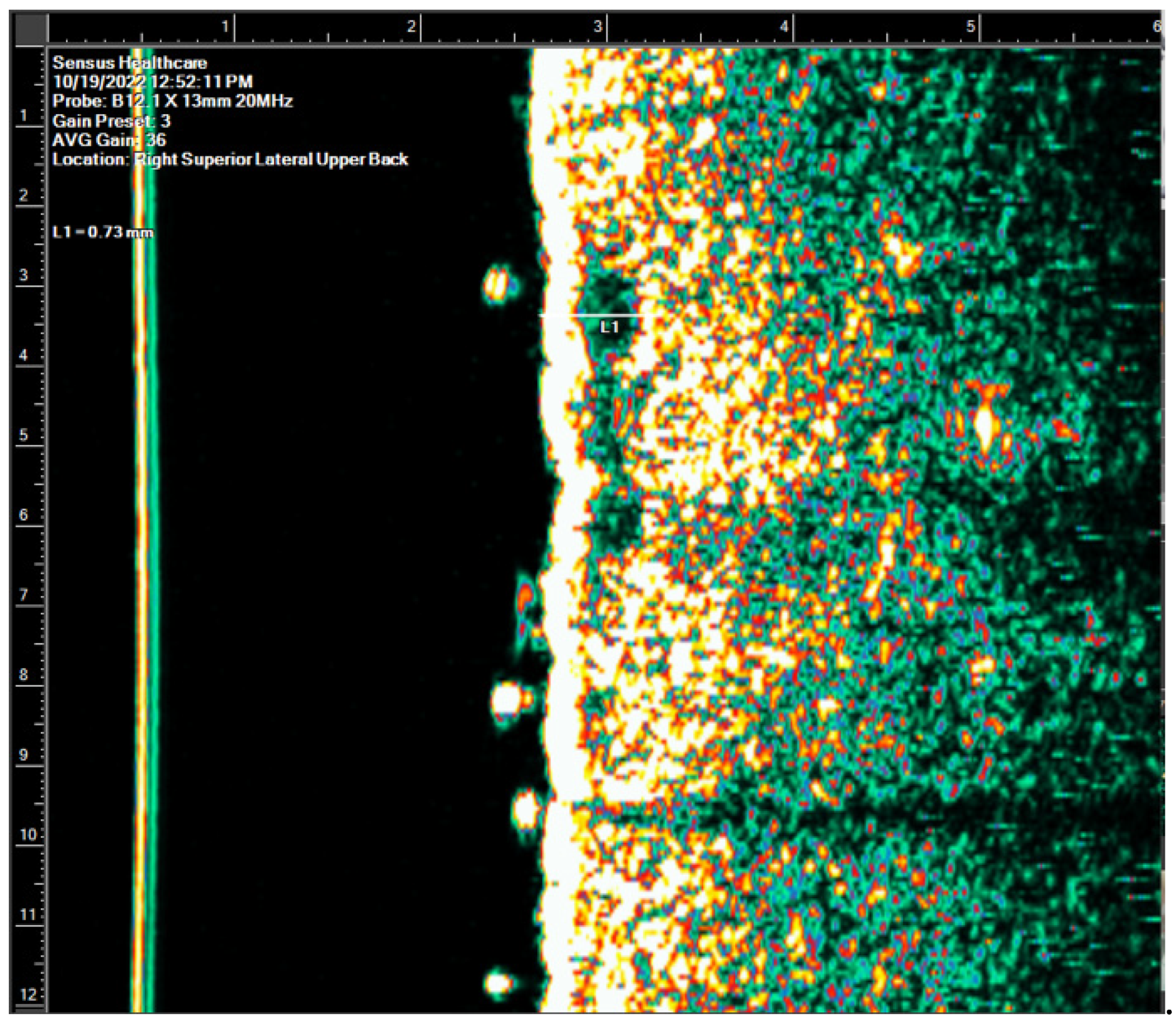
NMSC tumors appear on HRDUS images as black /hypoechoic masses hugging the underside of the epidermis (thin/yellow/hyperechoic) and expanding into the dermis (thick/green/hyperechoic). BCC presents as well-defined, irregular, or oval hypoechoic intradermal structures, which frequently contain hyperechoic spots (
Figure 2 and
Figure 3). Subtypes with high risk of recurrences (e.g micronodular, morpheaform variants) are associated with BCC tumors that contain ≳7 hyperechoic spots, which represent nests [
14,
15]. While tortuous vessels seen on HRDUS likely suggest another type of BCC which typically presents with low flow venous and arterial vessels at the bottom or within the tumor [
15]. SCC typically appears on US as irregular, heterogeneously hypoechoic without hyperechoic spots involving dermal and hypodermal structures, with deeper layers frequently involved (
Figure 4) [
14,
15]. Throughout the tumor, and specifically at the tumor margin, there is a diffuse increase in the low-flow vascular pattern [
15].
Figure 1.
Normal Cutaneous Anatomy. E=epidermis; D=dermis; S=subcutaneous layer.
Figure 1.
Normal Cutaneous Anatomy. E=epidermis; D=dermis; S=subcutaneous layer.
Figure 2.
Superficial BCC - Thin Lesion.BCC=basal cell carcinoma.
Figure 2.
Superficial BCC - Thin Lesion.BCC=basal cell carcinoma.
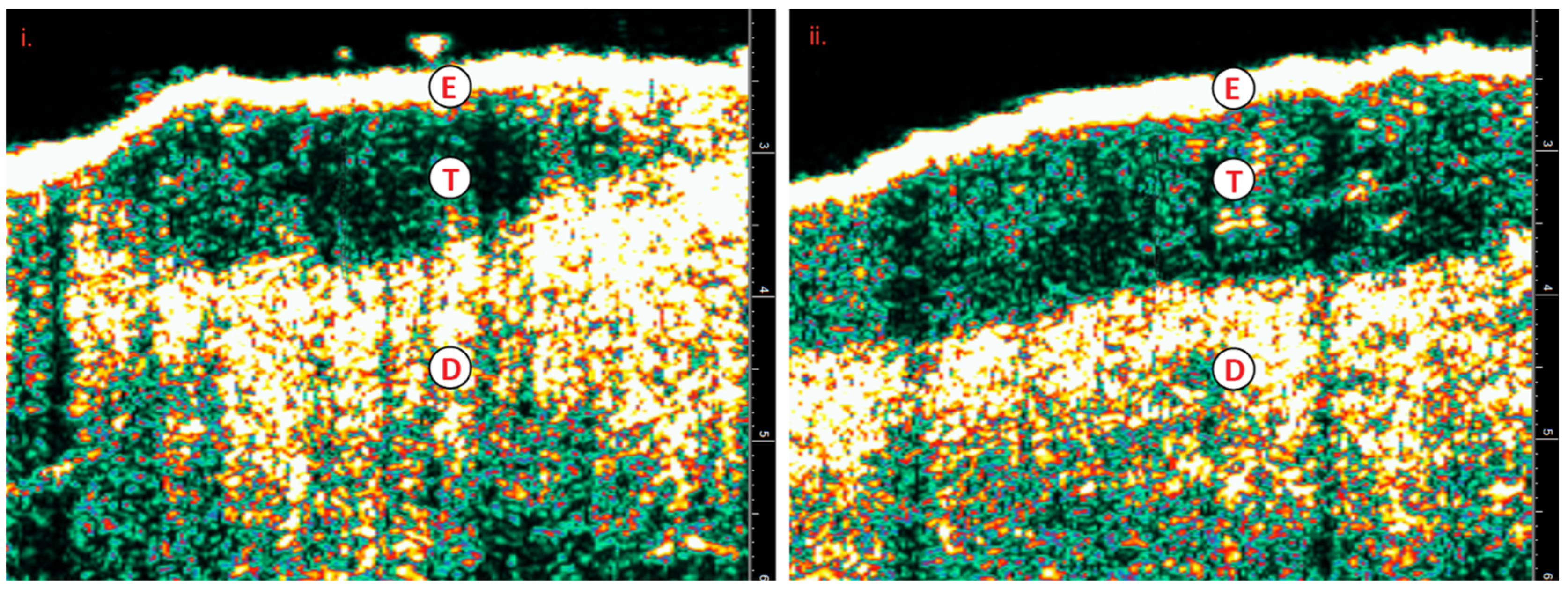
Figure 3.
i) Nodular BCC; prior to IGSRT ii) Nodular BCC with inflammatory response; mid-treatment with IGSRT.E=epidermis; T=tumor, BCC; D=dermis; BCC=basal cell carcinoma; IGSRT=image guided superficial radiation therapy.
Figure 3.
i) Nodular BCC; prior to IGSRT ii) Nodular BCC with inflammatory response; mid-treatment with IGSRT.E=epidermis; T=tumor, BCC; D=dermis; BCC=basal cell carcinoma; IGSRT=image guided superficial radiation therapy.
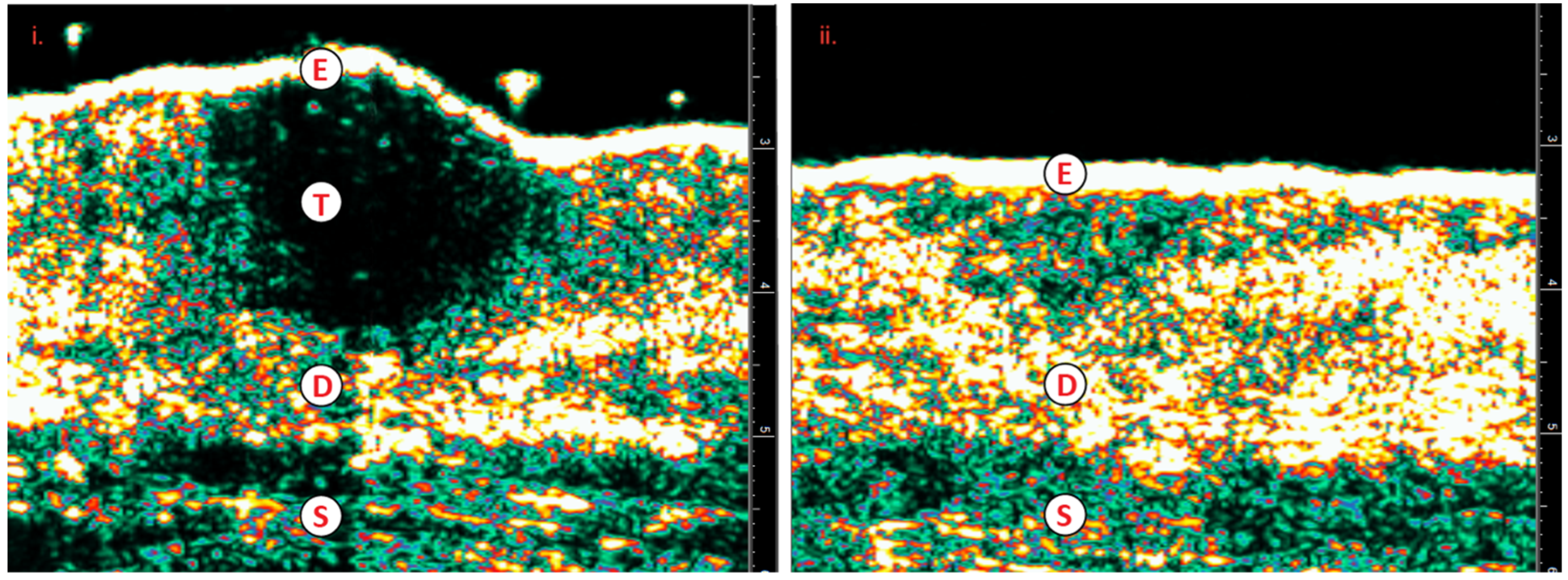
Figure 4.
i) SCC; prior to IGSRT ii) Resolving SCC, regeneration of healthy tissue; post IGSRT.E=epidermis; T=tumor, SCC; D=dermis; S=subcutaneous layer; SCC=squamous cell carcinoma; IGSRT=image guided superficial radiation therapy.
Figure 4.
i) SCC; prior to IGSRT ii) Resolving SCC, regeneration of healthy tissue; post IGSRT.E=epidermis; T=tumor, SCC; D=dermis; S=subcutaneous layer; SCC=squamous cell carcinoma; IGSRT=image guided superficial radiation therapy.
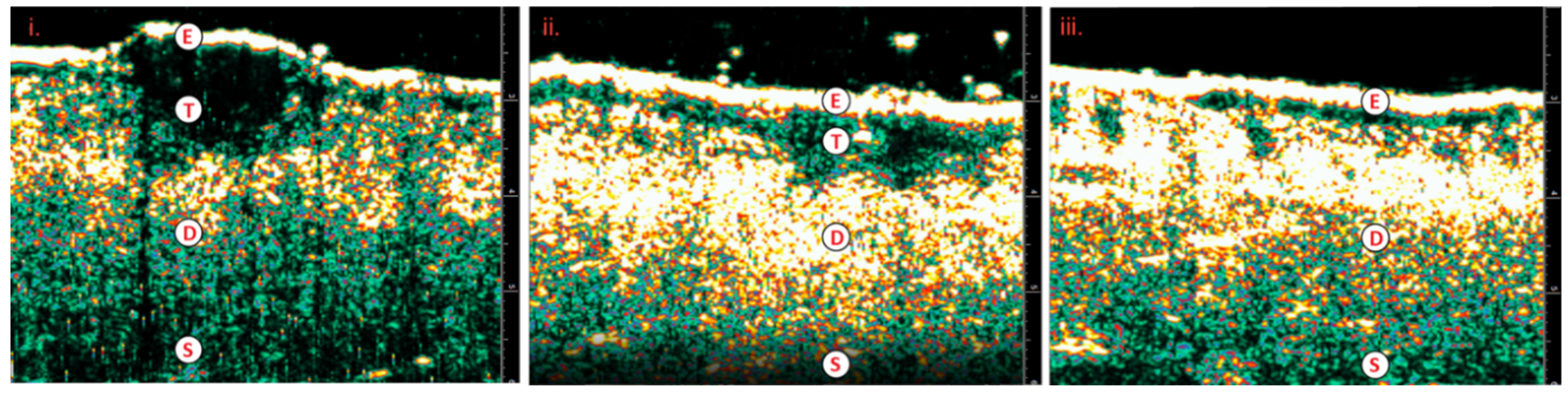
Figure 5.
i) Well-differentiated SCC; prior to IGSRT ii) Well-differentiated SCC; mid-treatment with IGSRT iii) Well-differentiated SCC; resolving 6-weeks post IGSRT.E=epidermis; T=tumor, SCC; D=dermis; S=subcutaneous layer; SCC=squamous cell carcinoma; IGSRT=image guided superficial radiation therapy.
Figure 5.
i) Well-differentiated SCC; prior to IGSRT ii) Well-differentiated SCC; mid-treatment with IGSRT iii) Well-differentiated SCC; resolving 6-weeks post IGSRT.E=epidermis; T=tumor, SCC; D=dermis; S=subcutaneous layer; SCC=squamous cell carcinoma; IGSRT=image guided superficial radiation therapy.
Tumor Configuration and Biological Dosing
HRDUS precisely measures the tumor depth and configuration from the surface of the epidermis to the deepest point of tumor involvement in the dermis and allows for gross tumor configuration identification. Based on these measurements, initial treatment planning and subsequent changes can be made in energy, dosing and time dose fractionation (TDF), and radiation field size. TDF is a numerical value, representing biological dose effect, especially useful in treating NMSC with a therapeutic range beginning at 90 and generally not exceeding 110. The TDF value can be increased for thicker tumors and decreased for thinner tumors. TDF values are a construct created to achieve a balance between the acceptable cure rate and an acceptable side effect/complication profile.
Dermoscopy
With entire books dedicated to its practice, dermoscopy, which aids in the diagnosis of BCC and SCC lesions, is a complex topic and it is beyond the scope of this paper to discuss it in detail. However, HRDUS can be augmented with dermoscopy for visualization of superficial, peripheral margins prior to IGSRT. Further, dermoscopy is may be useful in determining NMSC tumor response to treatment, in conjunction with the use of HRDUS and clinical examinations, including palpation and visual inspection.
IGSRT Literature Review
A meta-analysis by Yu et al. compared NMSC local control (LC)/cure rates from studies describing 3 different radiation modalities: external beam radiotherapy (XRT), SRT without HRDUS and IGSRT. The study found that IGSRT overall local control rates (99.3% [
9] and 99.2% [
16]) were statistically superior to those of XRT or SRT without HRDUS in all subtypes individually or collectively (LC 91-96.9% for BCC, 81.1-97% for SCC, 97.8% for SCC-IS). These results can be attributed to the use of US visualization before, during and after treatment of NMSC lesions, allowing providers to determine tumor breadth and depth to select field size, energy, and dose prior to treatment, make adjustments to therapy as needed in real-time, and confirm treatment response post-therapy. This technology provides patients a viable option for the non-surgical treatment of NMSC lesions [
17]. These findings were verified by a log regression analysis performed by Yu et al. in 2023, showed that compared to four studies utilizing non-image-guided radiotherapy (LC 90.1-96.8% for BCC, 76.7-96.9% for SCC, 97.7% for SCC-IS), LC/cure rates for all subtypes of epithelial NMSC had a statistically significant improvement through use of IGSRT (LC 98.9-99.1% for BCC, 99.2-99.3% for SCC, 99.5-99.8% for SCC-IS) [
18]. Due to the high-definition ultrasound with doppler that allows for visualization of the lesions’ overall configuration before, during, and after treatment, this statistically significant improvement was attributed to the HRDUS aspect of superficial radiotherapy. This allows the treatment provider to adjust dose and energy up to as often as daily, if needed, and confirm lesion response to treatment [
18].
Table 2 provides a summary of the following IGSRT literature review. In 2021 Yu et al. described the treatment of 1,632 patients with 2,917 NMSC tumors using IGSRT. Tumor types were as follows: Basal cell carcinoma (BCC): 48.2%, squamous cell carcinoma (SCC): 31.5% and squamous cell carcinoma in situ (SCC-IS): 20.7%. Mean follow up was 69.8 weeks or 1.34 years and the cure rate was 99.3%. Through the use of IGSRT, the depth of the tumor can be determined and correlated with the percentage depth dose (PDD) tables. This allows optimal energy selection (50, 70 or 100 kV) before and during the treatment course. As significant changes in tumor depth can be detected from one fraction to another, IGSRT allows adjustments to be made as needed. In this study, 29% of NMSC lesions were treated with multiple energies. The use of HRDUS for daily evaluation of tumor depth improves the delivery of optimal radiation energy and dose throughout the fractionated treatment scheme, as evidence by the high cure rate. To evaluate treatment related adverse events, the Radiation Therapy Oncology Group (RTOG) Acute Radiation Morbidity Scoring Criteria (
Table S1 in the electronic
Supplementary Material) was used [
19]. 0.7% and 0.2% of patients were found to have an RTOG toxicity level of grade 3 and 4, respectively [
9].
A similar study described the use of IGSRT in the treatment of 93 patients with 133 NMSC. Of the 133 NMSC lesions 67 were BCC, 17 were SCC and 49 were SCC-IS. The cure rate was 99.2% and the Kaplan Meier (KM) local control rate was 98.95% at a follow up of 2.57 years or 30.8 months. Initial energy and subsequent kV changes were selected by utilizing a combination of US imaging and clinical characteristics of the NMSC lesion. Measuring the tumor depth with US is important to treatment, as it allows the provider to select the proper penetrating energy to adequately treat the entire NMSC tumor. There were zero patients with an RTOG toxicity level of grade 3 or 4 [
20].
Tran et al. reported a study using IGSRT to treat 1,243 patients with 1,899 NMSC lesions. Tumor types were as follows: BCC (51.7%), SCC (24.6%) and SCC-IS (23.4%). The cure rate was 99.7% and the KM LC/cure rate was 99.41% at maximum follow up of 5 years or 63.6 months. HRDUS was performed prior to treatment, allowing determination of tumor breadth and depth, to select the proper width (field size), energy and dose selection. Throughout the treatment course, US was performed at each treatment to make real-time modifications if needed. Energy selection and subsequent dose modifications were made based on clinical and US imaging tumor characteristics, anatomic location, lesion depth, skin curvature, and histology. For larger, deeper, and higher risk tumors, higher doses per fraction and/or more fractions were recommended. The generally accepted premise is that most failures in NMSC lesions occur within 2-3 years, with a low likelihood of local recurrence for early stage NMSC after 2 years. This is supported by the identical 2 and 5 year absolute LC/cure rates of 99.41% which were observed in this study [
10].
Moloney et al. updated and combined the two 2021 Yu studies, leading to the largest and longest published study utilizing IGSRT in the treatment of 1,709 patients with 3,050 NMSC lesions. The overall cure rate was 99.2% at a mean follow up of 2.08 years. Cure rates by tumor type were BCC = 99.0%, SCC = 99.2% and SCC-IS = 99.85. The KM LC/cure at maximum follow up of 5.46 years or 65.56 months was 98.81%. 5 year KM LC/cure rates by tumor type were as follows: BCC = 98.17%, SCC = 99.01% and SCC-IS = 99.71% [
16].
McClure et al. performed a retrospective cohort study in 2023, which compared the 2-year recurrence probability of 5,391 early NMSC lesions treated by Mohs micrographic surgery (MMS) to 2,286 early NMSC lesions treated by IGSRT. Pooled NMSC lesions treated by IGSRT showed a statistically significant improvement in 2-year recurrence probability compared to pooled lesions treated by MMS, with p < 0.001, and to lesions treated by MMS that were separated by histologic subtype, with SCC p <0.001 and BCC p = 0.022 [
21].
A retrospective cohort study performed by McClure et al. [
22] in 2022 showed an improved overall 2-year recurrence probability of 2,880 NMSC lesions treated by IGSRT to two studies with previously existing data on NMSC lesions treated with SRT. For IGSRT, McClure et al. found an overall 0.7% 2-year recurrence rate for all NMSC lesions (1.1% BCC, 0.8% SCC, 0.0% SCCIS). In the SRT study conducted by Cognetta et al., they reported the overall 2-year recurrence rate of NMSC lesions was 1.9% (2.0% BCC, 1.8% SCC, 1.9% SCCIS) [
23]. In the other SRT study, Silverman et al. reported a 6.3% 2-year recurrence rate for BCC lesions [
24]. For IGSRT, 2-year rates of recurrence are superior to these two previous SRT studies evaluating NMSCs across all histology in the former [
23] and BCC in the latter [
24]. This difference in recurrence rates was statistically significant (P < 0.001) [
22].
Pathology Accuracy May Be Improved Using HRDUS
In 2018, Stiegel et al. recognized a discrepancy between tumor subtypes seen in initial biopsies vs. intraoperative MMS sections. They noted that tumor subtype weighs heavily into the selection of treatment modality. A retrospective chart review of cases of NMSC referred for MMS was conducted over a one-year period, recording changes between tumor subtypes observed at biopsy and those during MMS. They discovered a substantial discrepancy was observed between preoperative biopsy and intraoperative pathology, including a significant portion of tumors that became more aggressive. They reviewed 163 cases and found that 50.5% of tumor subtypes changed aggressiveness. Of these, 33% were more aggressive, whereas 17% were less aggressive. They concluded that treatment modalities without margin control may not provide adequate treatment for a considerable number of NMSC based on tumor type alone [
25].
This high rate of error of the initial biopsy to actual final aggressiveness (an extent of tumor involvement) may at least partially explain why excision without margin assessment has documented higher failure rates compared to Mohs. Similarly, it may in part account for the study findings showing traditional radiotherapy without HRDUS demonstrating statistically worse recurrence rates compared to IGSRT which uses HRDUS to assess tumor depth and extent [
17,
18]. Prior to IGSRT, dermatologists had to make SRT treatment decisions based on NMSC pathology reports that Stiegel et al. confirmed are not giving us an accurate subtype 50.5% of the time in patients referred for MMS. Shave biopsies typically include epidermis and some portion of the papillary or superficial dermis. Dermatologists often have two goals with regards to shave biopsy: diagnosis and favorable cosmesis. Given this scenario dermatopathologists can only diagnose the tissue that has been biopsied, which is limited by sampling error. Diagnostic accuracy of the tumor subtype is therefore limited by the depth of the shave biopsy technique. HRDUS benefits IGSRT because it reveals the width, depth and morphology of the tumor that remains in the patient’s dermis after the shave biopsy. For example, if a biopsy pathology report tells us that the tumor is SCC in situ and the in vivo HRDUS image reveals a residual dermal tumor that is 1mm in thickness, the in vivo HRDUS has confirmed the accuracy of that pathology report. If, on the other hand, a biopsy report tells us that a tumor is SCC in situ but in vivo HRDUS reveals a residual dermal tumor that is 3mm in thickness, we need to adjust our diagnosis to reflect at least superficial invasion. The combination of biopsy pathology and in vivo HRDUS dermal visualization of residual dermal tumor clearly gives dermatologists a much higher level of diagnostic accuracy regarding tumor subtype. This high level of diagnostic accuracy and precise HRDUS tumor depth measurement prior to each dose of radiation is the most likely reason why IGSRT’s high cure rates are statistically superior to those of SRT or XRT.
Overall, these studies (
Table S2 in the electronic supplementary material) demonstrate that IGSRT can consistently achieve ≥ 99% cure rates for NMSC in a dermatologic clinical setting, which is comparable and statistically superior on early analysis to cure rates achieved by MMS, with the educational support of an organized interdisciplinary team including dermatologists, radiation oncologists, medical physicists and certified radiation therapists. Additionally, while MMS requires surgery to determine tumor depth, HRDUS can evaluate tumor depth in a non-invasive manner, without requiring surgery. While other non-invasive therapies for NMSC such as photodynamic therapy (PDT), topical 5-FU and topical imiquimod can be useful, none of these can deliver the high cure rates of IGSRT or MMS. Further, topical applications require patient compliance for application, which could be jeopardized due to lack of clinician involvement.
Ladd-Yu NMSC IGSRT Protocol Guidelines v2
Although the Ladd-Yu NMSC IGSRT Protocol Guidelines have been modified/updated since 2019, shown below is the most recently published version of the protocol. This protocol is useful for dermatologists because it standardizes dosing schedules, provides a guideline which can be helpful for new users, and allows for better outcomes. Of note, IGSRT is not recommended for lesions that are >3-4mm deep (unless debulked), have perineural invasion, or are deeply infiltrative and should be limited to lesions that are early stage I or II NMSC or Stage 0 SCCIS with full thickness atypia. Actinic keratoses are not appropriate for treatment with IGSRT.
Ladd-Yu NMSC IGSRT Table 2019 Version 2
| |
|
NMSC IGSRT Protocol Guidelines v2 |
|
|
| Developed by Drs. Daniel Ladd and Lio Yu (2019) |
| BCC (except Nodular) and SCCIS1 Protocol for Image Guided Superficial Radiotherapy |
| Lesion Depth (mm) |
Minimum TDF (not to exceed 109) |
1st Energy (kV) (Fraction 1-10) |
2nd Energy (kV) (Fraction 11-20) |
Suggested Total Fraction # |
Suggested Fractions/week |
Suggested Daily Dose (Keep BELOW 280cGy daily) |
| 0.0 - 0.7 |
90 |
50 |
50 |
20 |
3 or 4 |
250-270 |
| 0.71 - 1.0 |
92 |
50 |
50 |
20 |
3 or 4 |
250-274 |
| 1.1 - 1.5 |
94 |
70 |
502
|
20 |
3 or 4 |
255-275 |
| 1.6 - 2.0 |
95 |
70 |
70 |
20 |
3 or 4 |
255-275 |
| 2.1 - 2.5 |
96 |
70 |
70 |
20-213
|
3 or 4 |
250-275 |
| 2.6 - 3.0 |
97 |
1004
|
702 |
20-223
|
3 or 4 |
250-275 |
| 3.1 - 3.5 |
98 |
100 |
1004
|
20-223
|
3 or 4 |
245-279 |
| 3.6 - 4.0 |
≥99 |
100 |
1004
|
20-223
|
3 or 4 |
245-279 |
| > 4.0 |
Seek consultation with SkinCure Oncology Round Table and/or Grand Rounds |
| |
|
|
|
|
|
|
| Squamous Cell & Nodular BCC Protocol for Image Guided Superficial Radiotherapy |
| Lesion Depth (mm) |
Minimum TDF (not to exceed 109) |
1st Energy (kV) Fraction 1-10) |
2nd Energy (kV) (Fraction 11-20) |
Suggested Total Fraction # |
Suggested Fractions/week |
Suggested Daily Dose (Keep BELOW 280cGy daily) |
| 0.0 - 0.5 |
94 |
70 |
70 |
20 |
3 or 4 |
255-275 |
| 0.6 - 1.0 |
94 |
70 |
70 |
20 |
3 or 4 |
255-275 |
| 1.1 - 1.5 |
95 |
70 |
70 |
20 |
3 or 4 |
255-275 |
| 1.6 - 2.0 |
96 |
70 |
70 |
20-223
|
3 or 4 |
245-279 |
| 2.1 - 2.5 |
96 |
1004
|
1004
|
20-223
|
3 or 4 |
245-279 |
| 2.6 - 3.0 |
≥97 |
1004
|
1004
|
20-223
|
3 or 4 |
245-279 |
| > 3.0 |
Seek consultation with SkinCure Oncology Round Table and/or Grand Rounds |
| |
|
|
|
|
|
|
|
Thick lesions above 3 or 4 mm thick - shave down before (or even during) IGSRT. |
|
Keratoacanthomas that are rapidly growing - consider for electrodessication and cautery right before and/or during IGSRT. |
Description of Interdisciplinary Team Members and Role
Dermatologists and Radiation Therapists
Dermatologists and radiation therapists are the primary role players in the treatment of NMSCs with IGSRT. Dermatologists are highly skilled skin experts. According to the American Academy of Dermatology, Dermatologists are experts in the diagnosis and treatment of skin cancer [
26]. Dermatologists have extensive knowledge on the multitude of subtypes of NMSCs which allow them to develop treatment plans based on the specific characteristics of each subtype. Without the expertise of a dermatologist, NMSC treatment with IGSRT poses the risk of under-treating aggressive subtypes and increases the likelihood of marginal miss if the growth pattern of the specific NMSC is not understood.
Complex dermatological diagnoses have the potential to adversely interfere with radiation therapy. Unlike other doctors, dermatologists have the expertise needed to either manage such diagnoses for the patient while they undergo radiation therapy or deem the patient as an inappropriate or non-ideal candidate for radiation therapy based on their dermatologic history. Examples of such dermatologic diagnoses may include lupus, connective tissue diseases, ataxia telangiectasia, etc. Examples where added precautions or modification to the IGSRT plan may be warranted include eruptive keratoacanthoma, pustular dermatosis of the scalp, pyoderma gangrenosum, rhinophyma, etc.
Dermatologists establish the treatment prescription for IGSRT based on medical history, histologic findings, clinical presentation, and HRDUS images acquired by the radiation therapist during simulation. Establishment of an IGSRT prescription includes the selection of margin and field size, dose, energy and fractionation to achieve a desired biologic effectiveness based on TDF. Dermatologists monitor the progress of treatment via continued review of HRDUS images that are acquired throughout the course of therapy and by periodic clinical evaluation visits with the patient. Prescription modifications can be made by the dermatologist based on their interpretation of HRDUS images and clinical findings.
As skin experts, dermatologists can offer superior management of acute skin reactions related to radiation therapy. Examples of such reactions include erythema, edema, desquamation and pruritus. Moreover, management of latent effects of skin radiation requires the expertise of the dermatologist. Latent effects of radiation include but are not limited to hyperpigmentation, hypopigmentation, atrophy, telangiectasias, ulceration, infection, and excoriation.
Radiation therapists have extensive knowledge regarding the appropriate use of radiation. This includes promotion of radiation safety procedures for patients, operators and clinical staff through in-depth knowledge for proper machine handling, utilization of appropriate shielding devices to limit exposure to surrounding critical structures and reporting of machine errors to appropriate personnel, such as state and federal regulatory authorities. Additionally, with a background in radiobiology, radiation therapists understand expected side effects for different areas of the body when radiation is delivered, leading to proper side effect management. Knowledge of dose limits and how radiation beams interact within skin and other tissue enable radiation therapists to prevent overlap of beams within the tissue, leading to significant reduction in the possibility of damage due to over-exposure.
The role of radiation therapists in IGSRT also includes operating and interpreting of HRDUS to measure lesion depth and configuration – this allows the therapist to utilize HRDUS to confirm correct field placement prior to administering radiation treatments. Radiation therapists monitor fluctuating ultrasound depths throughout the course of therapy to address concerns regarding PDD and report fluctuations in depth to the prescribing provider so that prescription adjustments can be considered according to real-time changes in lesion depth.
Additionally, radiation therapists collaborate frequently with other health care providers. This may include acquiring relevant medical records from radiation oncologists and personnel in situations that involve potential overlap with previous ionizing radiation fields. Radiation therapists acquire relevant data for review by an interdisciplinary team in clinical scenarios in which appropriateness of IGSRT is in question, or specific topographical considerations are necessary/there is adjacent critical tissue.
Furthermore, radiation therapists interact with patients frequently throughout the course of treatment, allowing them the opportunity to build trust and rapport with patients. Radiation therapists educate patients on expected side effects prior to the start of treatment and provide information for management of such side effects. Patients are continuously monitored by radiation therapists for the progression of acute side effects throughout the course of care and facilitate further care by the prescribing provider if acute side effects increase in severity. HRDUS images are shared with patients by radiation therapists to inform patients of progress that is being made in eradication of their disease. This “see and treat” approach also encourages patients to maintain compliance with their treatment schedule. Aside from side effect management and HRDUS images, radiation therapists also communicate radiation safety, efficacy, risks and benefits of radiation therapy with patients undergoing, or considering undergoing, radiation therapy.
Radiation Oncologists and Medical Physicists
While dermatologists and radiation therapists are generally the primary prescribers and treatment deliverers of IGSRT in the United States (which also is subject to individual state regulations), radiation oncologists and medical physicists should be available for additional support in specific circumstances. Patients with a history of radiation therapy require review of previous radiation therapy treatment plans to confirm the absence of overlap with planned IGSRT treatment fields. Radiation oncologists can assist with the interpretation of complex treatment plans from other radiation therapy modalities such as linear accelerator, brachytherapy and electron/photon/stereotactic/proton beam plans. Additionally, radiation oncologists are available for consultation for more advanced lesions, where the use of a multidisciplinary team is advisable. Patients with a history of cancer can present for NMSC treatment while concurrently receiving medications which are radiosensitizers or systemic/targeted agents. Radiation oncologists can offer guidance on the management of such patients. In certain instances, the patient can be considered for IGSRT while undergoing treatment with such agents, whereas, in other cases use of these agents can be altered/IGSRT modified/rule out IGSRT in favor of another modality/delay treatment, based on the judgment of the radiation oncologist.
Medical physicists should be made available for completion of initial and annual machine calibrations. While radiation therapists understand how to safely operate IGSRT machines, medical physicists ensure the safe and precise functioning of IGSRT units. Medical physicists collaborate with state officials to meet radiation safety requirements specific to the state. Dermatologists and radiation therapists can benefit from the support of medical physicists for clinically challenging scenarios, such as source to skin distance (SSD) variations due to topographically challenging anatomic areas and dose decay calculations.
Figure 4 illustrates the topographically challenging area of the nasal bridge and tip. Medical physicists collaborate with dermatologists and radiation therapists in such scenarios by determining dose variances via inverse square calculations so that a therapeutic dose is received to the entirety of the field while avoiding unnecessarily high hot spots (areas within the field receiving higher dose than prescribed) or unacceptably low cold spots. Dose decay calculations are utilized by medical physicists if a patient has an unavoidable break in therapy. These calculations serve the purpose of determining how much the prescribed biologic dose (TDF) has decayed/decreased because of the break and if it will be necessary to add a fraction(s) to bring the prescription back to a therapeutic dose range. Moreover, medical physicists complete weekly and end of treatment chart checks via a remote system to verify that prescription parameters set by the dermatologist are following suggested protocol and ensure treatments are not deviating from prescribed parameters.
Conclusions
Although the use, efficacy, safety, and cosmetic benefits of SRT for treatment of NMSC by dermatologists has been documented for over a century, the development of IGSRT represents a significant clinical advancement, largely due to the ability of HRDUS to provide in-vivo, real-time dermal tumor visualization before, during, and after therapy.
The well-demarcated, high frequency, high resolution images created by HRDUS are used after biopsy but prior to treatment to visualize the specific depth and configuration of the gross NMSC tumor, allowing the provider to correlate the measurements with PDD tables and select the best energy choice. The depth measurement can often distinguish invasive SCC as being present, despite the superficial shave biopsy reporting it as SCCis, confirming or augmenting/modifying the histologic diagnosis and staging. This can improve the treatment plan/outcome. Throughout treatment, NMSC tumors display fluctuating depth measurements due to factors such as treatment response, edema, inflammation, bleeding, infection, etc. Measurement of the changing depths throughout treatment can be performed through the use of HRDUS and is critical, as different tumor depths may require alterations in dose/fractionation and energy. Post-treatment, HRDUS can be utilized to confirm treatment response.
IGSRT proves to be an effective treatment method by consistently obtaining ≥ 99% LC/cure rates, which has been shown to be statistically superior to LC/cure rates obtained by SRT or XRT without image guidance [
17,
18]. Furthermore, LC/cure rates achieved by IGSRT are comparable to those obtained by MMS [
21], proving IGSRT as an efficacious, non-invasive, non-surgical first line treatment option for patients with early-stage NMSC. Patients with multiple previous Mohs surgeries often experience surgical fatigue. IGSRT offers a non-surgical equivalent.
To achieve optimal patient outcomes, an interdisciplinary approach to IGSRT is recommended. This begins at the training level. Increasing access, practice, and exposure to SRT and IGSRT education and hands on experience in dermatology residency programs will improve the knowledge and confidence levels of future dermatologists regarding RT for treatment of NMSC tumors. One way to increase exposure to IGSRT is through the implementation of interdisciplinary virtual grand rounds which allow medical professionals from various fields to meet and discuss complex and/or challenging IGSRT cases. At the practicing medical professional level, the insight of multiple clinicians from different fields is beneficial. This includes dermatologists, radiation oncologists, medical physicists, and radiation therapists – each of which have specialized knowledge relating to certain aspects of IGSRT therapy. The combined knowledge and insight from the interdisciplinary team can lead to decreased malpractice/legal issues, as well as improvement of treatment plan creation, patient compliance, side effect management, treatment modification, cosmesis, and overall patient outcomes.
In conclusion, due to reliable high LC/cure rates, low side effect profile, and favorable cosmesis, IGSRT should be considered a first line therapy in non-surgically treating early stage NMSC tumors, especially if utilized in the setting of an interdisciplinary team including dermatologists, radiation therapists, radiation oncologists, and medical physicists.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Peyton Harris served as chief editor, evaluating and integrating edits suggested by all co-authors. Aaron Farberg, Jeanine Hopkins, Liqiao Ma, Donna Serure, Blake Robbins, Candace Osborne, Luis Bravo, Pauline Lausser and Amanda Boatner all served as IGSRT experts and co-editors. Candace Osborne provided relevant HRDUS images.
Funding
This study did not receive any funding.
Institutional Review Board Statement
This study was based on previously performed studies and does not include any new studies with animals or human participants conducted by any of the authors.
Acknowledgments
Medical Writing, Editorial, and Other Assistance: We would like to thank Dr. Daniel Ladd and Dr. Lio Yu for providing the Ladd-Yu Protocol and IGSRT processes from their clinic.
Conflicts of Interest
Peyton Harris has no conflicts of interest to disclose. Dr. Aaron Farberg is an advisor for Castle Biosciences, Inc., Novartis, Sun Pharma, Regeneron. Dr. Janine Hopkins, Dr. Liqiao Ma, Dr. Donna Serure, Candace Osborne, Dr. Blake Robbins, Luis Bravo, Pauline Lausser and Amanda Boatner have no conflicts of interest to disclose.
Abbreviation List
IGSRT: image guided superficial radiation therapy
SRT: superficial radiation therapy
HRDUS: high resolution dermal ultrasound
NMSC: non-melanoma skin cancer
XRT: external beam radiation
MMS: Mohs Micrographic Surgery
MHz: megahertz
DART: Dermatology Association of Radiation Therapy
RT: radiation therapy
Gy: Gray
cGy: centiGray
kVp: peak kilovoltage
SCC: squamous cell carcinoma
BCC: basal cell carcinoma
PDD: percentage depth dose
kV: energy
TDF: time dose fractionation
LC: local control
SCC-IS: squamous cell carcinoma in situ
RTOG: Radiation Therapy Oncology Group
KM: Kaplan Meier
SSD: source to skin distance
Notes
| 1 |
SCCIS should have full thickness atypia. |
| 2 |
Optional if responding well. |
| 3 |
Treatment of larger areas (and in general) is best done with lower (245-265) daily doses (3 or 4 times a week) with the addition of fractions rather than larger daily fractions. |
| 4 |
May use 70kV for thin skinned area over bone (e.g., dorsum hand, forehead, pretibial, zygoma, etc.). |
References
- Cognetta AB, Jr., Wolfe CM, Goldberg DJ, Hong HG. Practice and Educational Gaps in Radiation Therapy in Dermatology. Dermatol Clin. 2016;34(3):319-33.
- Han H, Gade A, Ceci FM, Lawson A, Auerbach S, Nestor MS. Superficial radiation therapy for nonmelanoma skin cancer: A review. Dermatological Reviews. 2022;3(6):409-17.
- Wortsman, X. Common Applications of Dermatologic Sonography. Journal of Ultrasound in Medicine. 2012;31(1):97-111.
- Connell PP, Hellman S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res. 2009;69(2):383-92.
- McGregor S, Minni J, Herold D. Superficial Radiation Therapy for the Treatment of Nonmelanoma Skin Cancers. J Clin Aesthet Dermatol. 2015;8(12):12-4.
- Yu LM, Mairead; Bard, Robert. Image-Guided Superficial Radiotherapy and Other Noninvasive Modalities Used in the Treatment of Non-melanoma Skin Cancer and Keloids. In: Bard RL, editor. Image-Guided Aesthetic Treatments: Springer; 2023. p. 225-51.
- Yu LM, Mairead; Bard, Robert. Image Guided Superficial Radiotherapy and Other Non-Invasive Modalities Used in the Treatment of Non-Melanoma Skin Cancer and Keloids. 2022.
- Alam M, Nanda S, Mittal BB, Kim NA, Yoo S. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol. 2011;65(2):377-88.
- Yu L, Oh C, Shea CR. The Treatment of Non-Melanoma Skin Cancer with Image-Guided Superficial Radiation Therapy: An Analysis of 2917 Invasive and In Situ Keratinocytic Carcinoma Lesions. Oncology and Therapy. 2021;9(1):153-66.
- Tran A, Moloney M, Kaczmarski P, Zheng S, Desai A, Desai T, Yu L. Analysis of image-guided superficial radiation therapy (IGSRT) on the treatment of early-stage non-melanoma skin cancer (NMSC) in the outpatient dermatology setting. Journal of Cancer Research and Clinical Oncology. 2023;149(9):6283-91.
- Laverde-Saad A, Simard A, Nassim D, Jfri A, Alajmi A, O’Brien E, Wortsman X. Performance of Ultrasound for Identifying Morphological Characteristics and Thickness of Cutaneous Basal Cell Carcinoma: A Systematic Review. Dermatology. 2022;238(4):692-710.
- Levy J, Barrett DL, Harris N, Jeong JJ, Yang X, Chen SC. High-frequency ultrasound in clinical dermatology: a review. The Ultrasound Journal. 2021;13(1).
- Mandava A, Ravuri PR, Konathan R. High-resolution ultrasound imaging of cutaneous lesions. Indian Journal of Radiology and Imaging. 2013;23(03):269-77.
- Wortsman, X. Top applications of dermatologic ultrasonography that can modify management. Ultrasonography. 2023;42(2):183-202.
- Catalano O, Roldan FA, Varelli C, Bard R, Corvino A, Wortsman X. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound. 2019;22(4):423-31.
- Moloney M, Kaczmarksi P, Zheng S, Malik A, Ladd D, Serure D, Yu L. Updated Results of 3,050 Non-melanoma Skin Cancer (NMSC) Lesions in 1725 Patients Treated with High Resolution Dermal Ultrasound-Guided Superficial Radiotherapy, A Multi-institutional Study. Journal of Investigative Dermatology. 2022;142(8).
- Yu L, Moloney M, Tran A, Zheng S, Rogers J. Local control comparison of early-stage non-melanoma skin Cancer (NMSC) treated by superficial radiotherapy (SRT) and external beam radiotherapy (XRT) with and without dermal image guidance: a meta-analysis. Discover Oncology. 2022;13(1).
- Yu L, Moloney M, Zheng S, Rogers J. High resolution dermal ultrasound (US) combined with superficial radiation therapy (SRT) versus non-image guided SRT or external beam radiotherapy (XRT) in early-stage epithelial cancer: a comparison of studies. BMC Cancer. 2023;23(1).
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-6.
- Yu LM, Mairead; Beers, Raymond; Serure, Donna. Enhancing Cosmesis While Acheiving High Cure-Rates for Early-Stage Non-Melanoma Skin Cancer In The Outpatient Dermatology Clinic Using Novel Non-Invasive Modality. American Journal of Biomedical Science & Research. 2021;12(6):525-32.
- McClure EM, Sedor G, Jin Y, Kattan MW. Image-guided superficial radiation therapy has superior 2-year recurrence probability to Mohs micrographic surgery. Clin Transl Radiat Oncol. 2023;43:100678.
- McClure E, Sedor G, Moloney M, Jin Y, Kattan MW, Yu L. Image guidance improves freedom from recurrence in superficial radiation therapy for non-melanoma skin cancer. 2022.
- Cognetta AB, Howard BM, Heaton HP, Stoddard ER, Hong HG, Green WH. Superficial x-ray in the treatment of basal and squamous cell carcinomas: a viable option in select patients. J Am Acad Dermatol. 2012;67(6):1235-41.
- Silverman MK, Kopf AW, Grin CM, Bart RS, Levenstein MJ. Recurrence rates of treated basal cell carcinomas. Part 1: Overview. J Dermatol Surg Oncol. 1991;17(9):713-8.
- Stiegel E, Lam C, Schowalter M, Somani AK, Lucas J, Poblete-Lopez C. Correlation Between Original Biopsy Pathology and Mohs Intraoperative Pathology. Dermatol Surg. 2018;44(2):193-7.
- How can I tell if I have skin cancer? Available online: https://www.aad.org/public/diseases/skin-cancer/find/know-how#:~:text=If%20you%20find%20a%20suspicious,going%20to%2C%20Find%20a%20dermatologist.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).