Submitted:
12 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
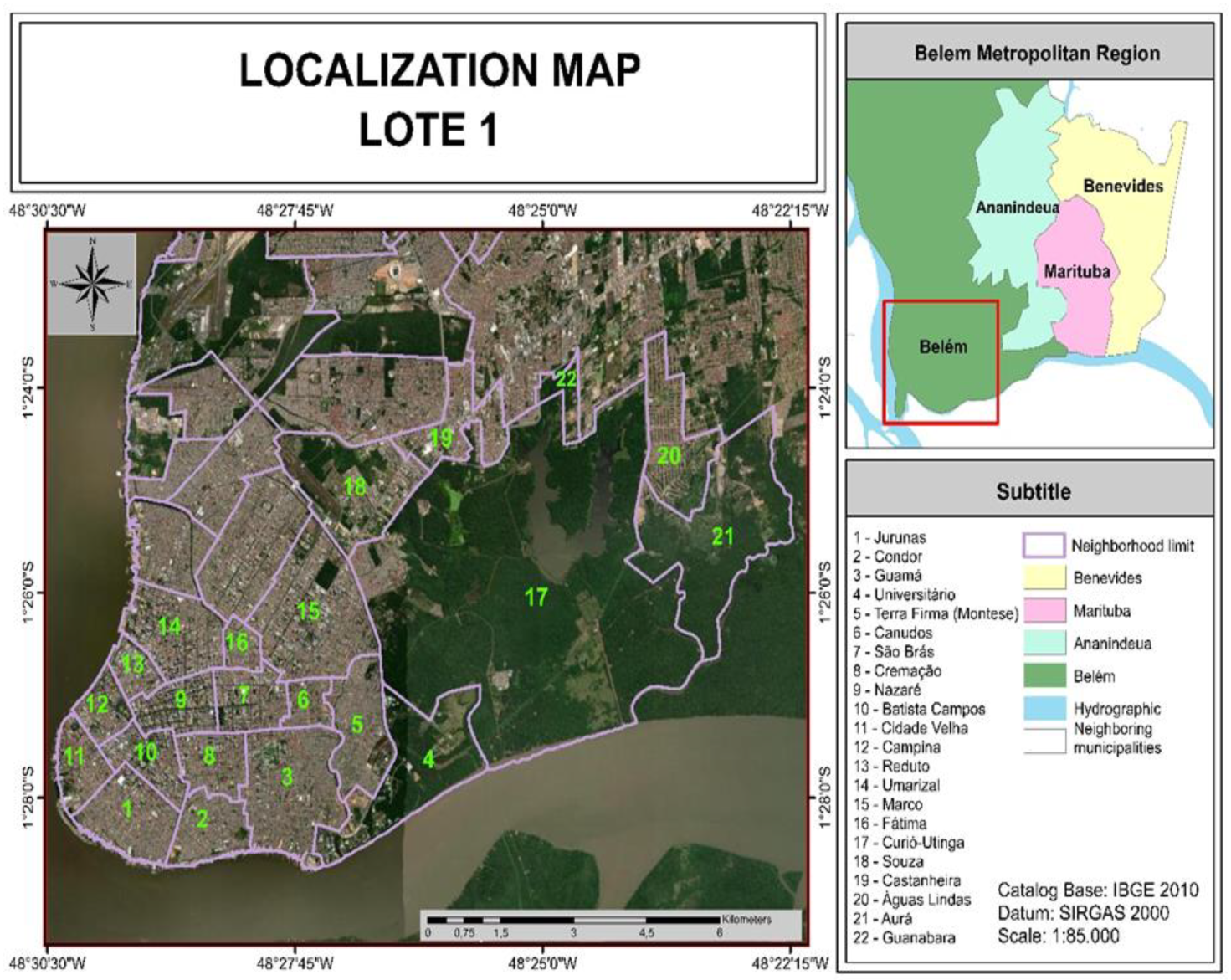
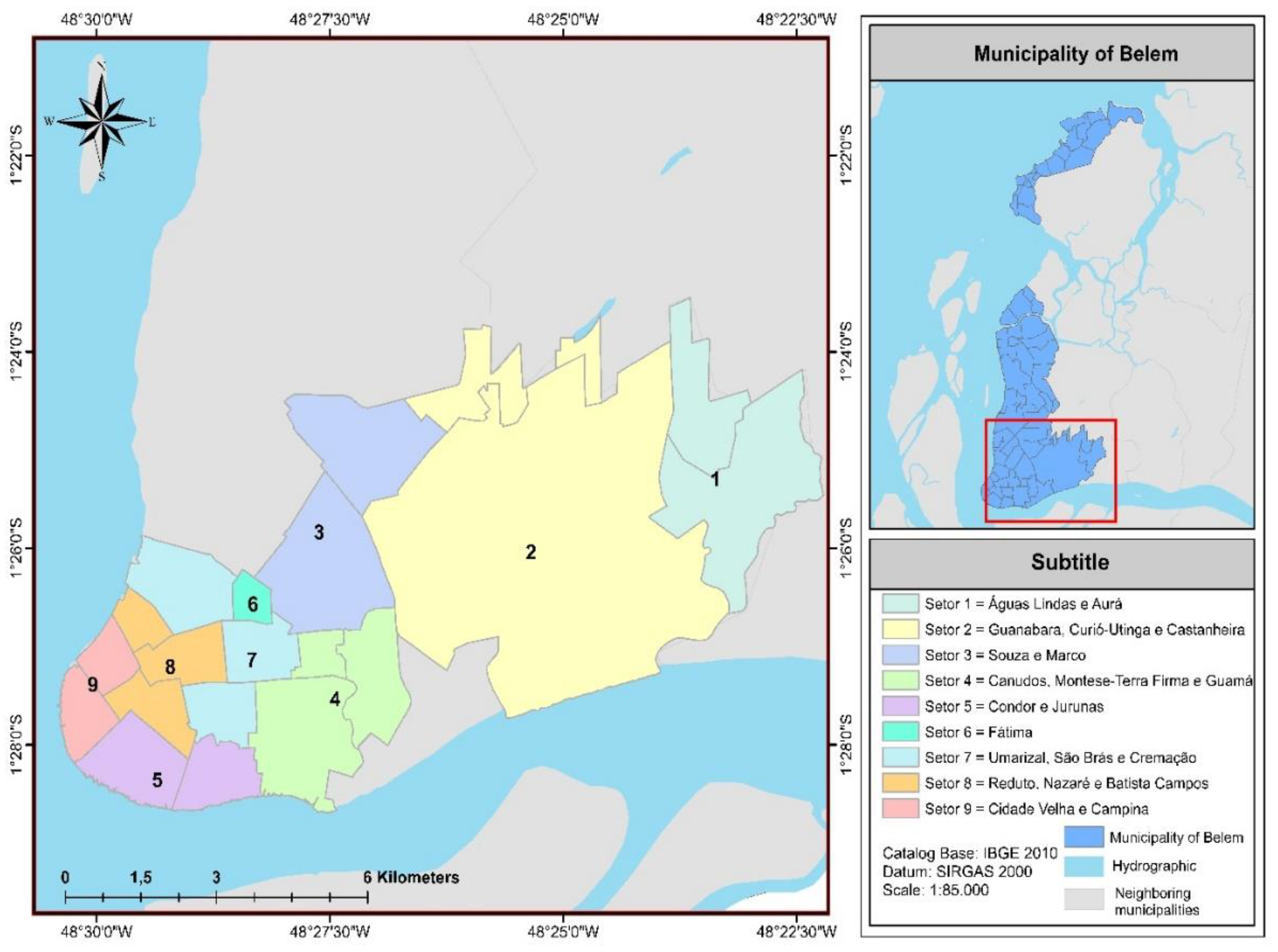
2.1. Characterization of the Study Area
| Region | Class | Sectors | Neighborhoods |
|---|---|---|---|
| 1 | E | 1, 2 e 3 | Aurá, Águas Lindas, Curió-Utinga, Guanabara, Castanheira, Souza e Marco. |
| 2 | D | 4, 5 e 6 | Canudos, Terra Firme, Guamá, Condor, Jurunas e Fátima. |
| 3 | C | 7, 8 e 9 | Umarizal, São Brás, Cremação, Batista Campos, Nazaré, Reduto, Campina e Cidade Velha. |
2.3. Calculation of the Mass Required for the Gravimetric Composition
2.4. Gravimetric Collection and Composition
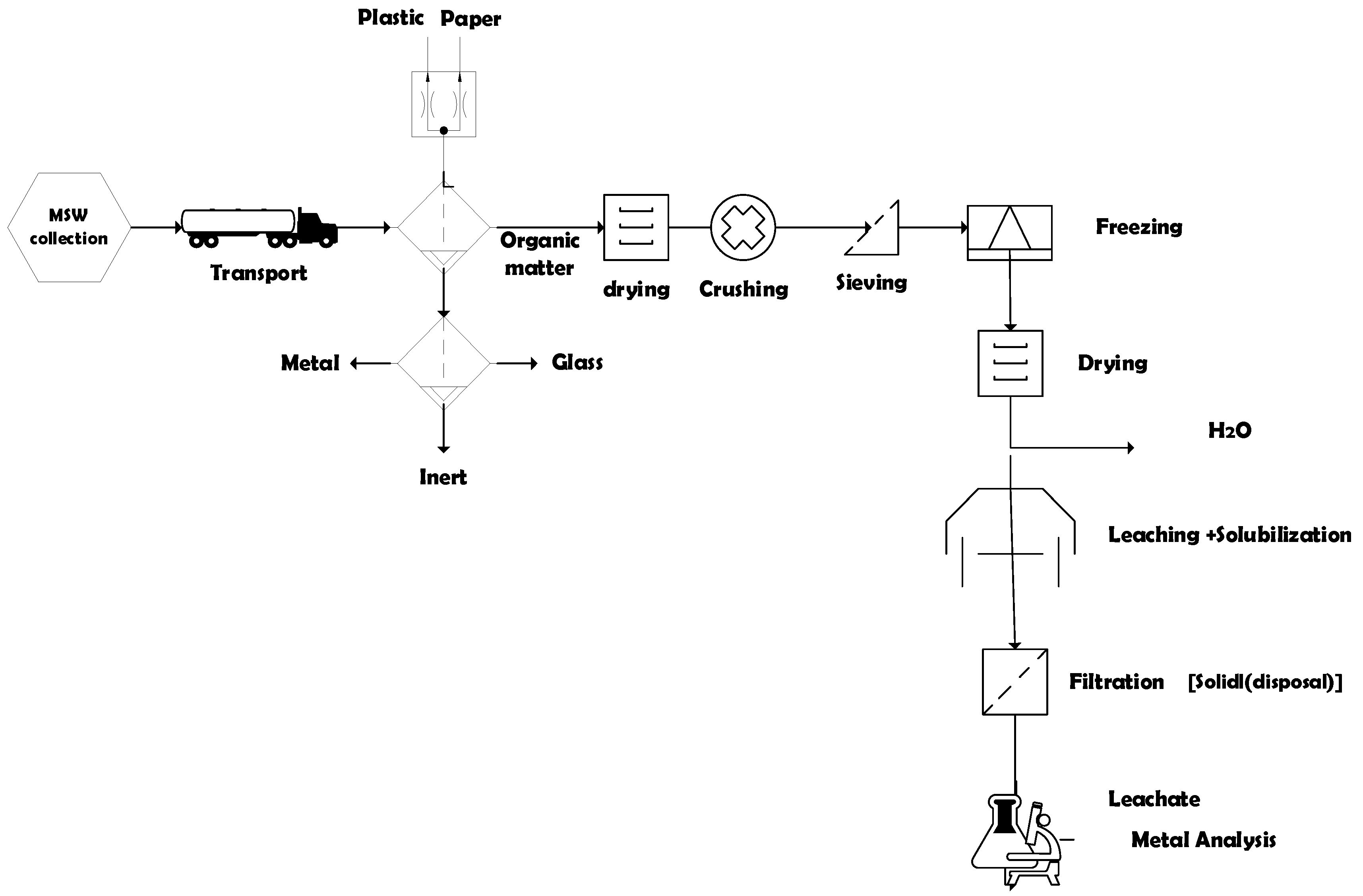
2.5. Pre-Treatment of Samples (Drying, Crushing and Sieving Content)
2.5.1. Laboratory Determinations
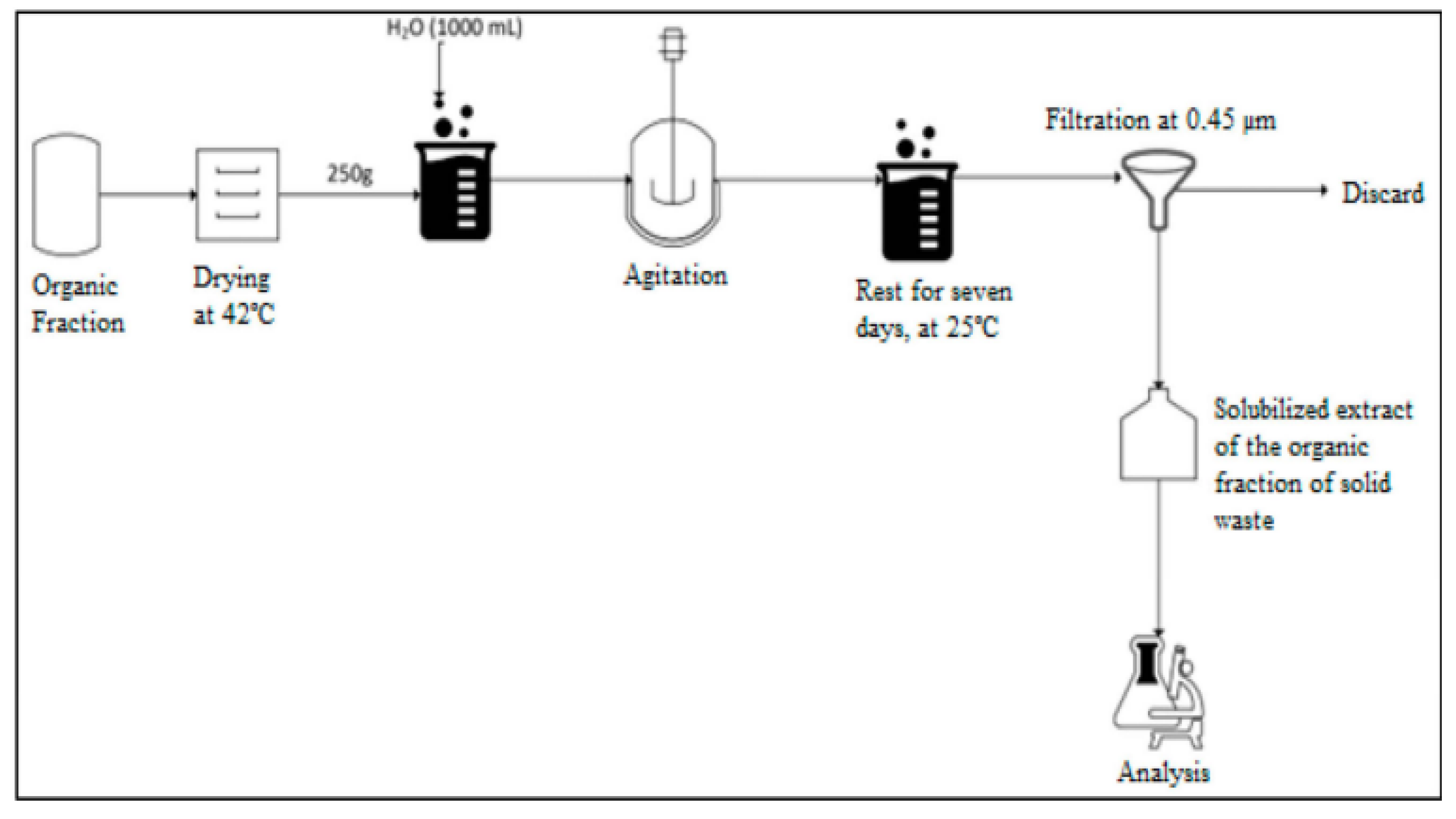
2.5.2. Method and Statistical Analysis
3. Results
3.1. Analysis of the Leachate Extract of the Organic Fraction
3.1.2. Verification of Compliance with Legal Sanitary and Environmental Standards
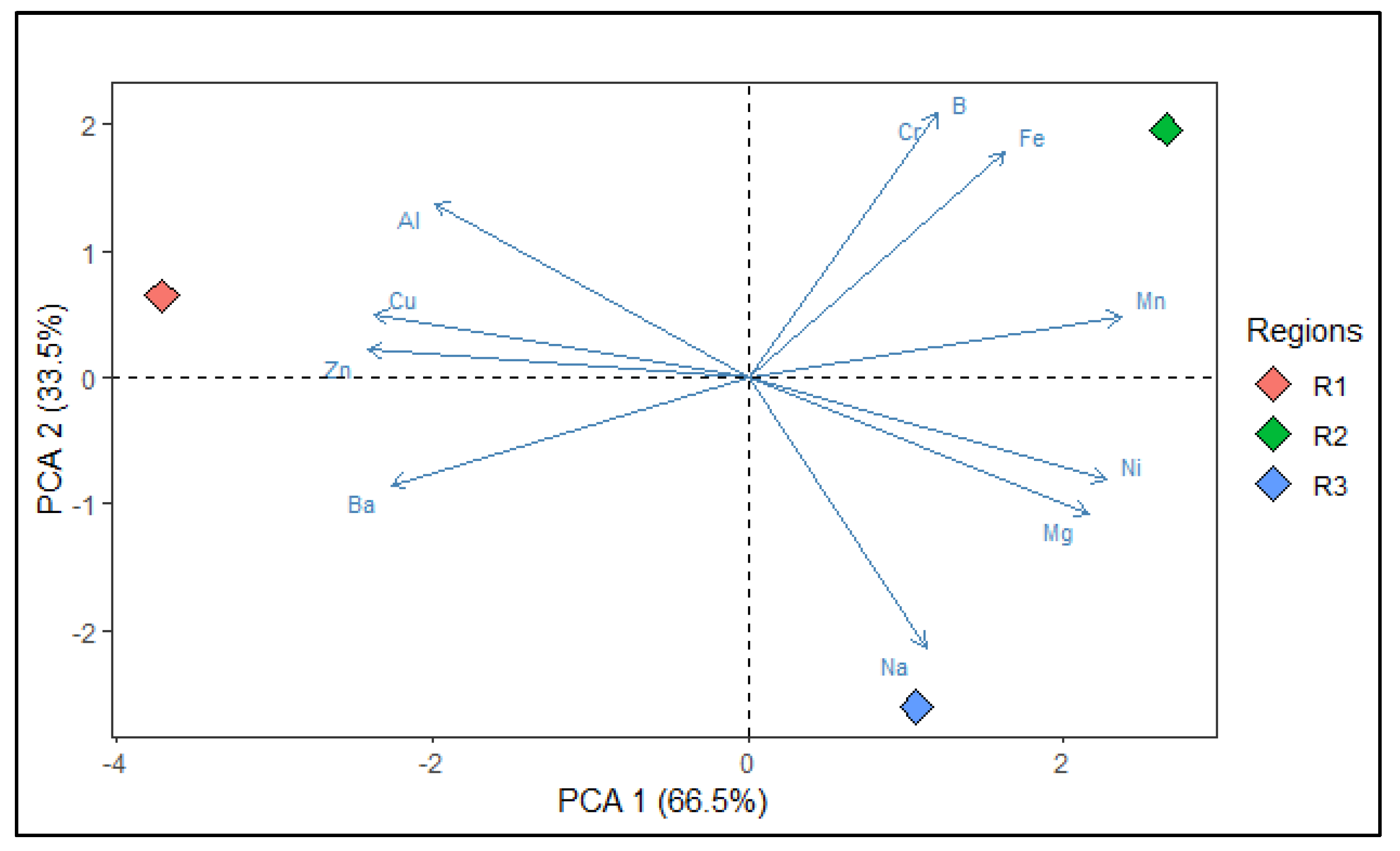
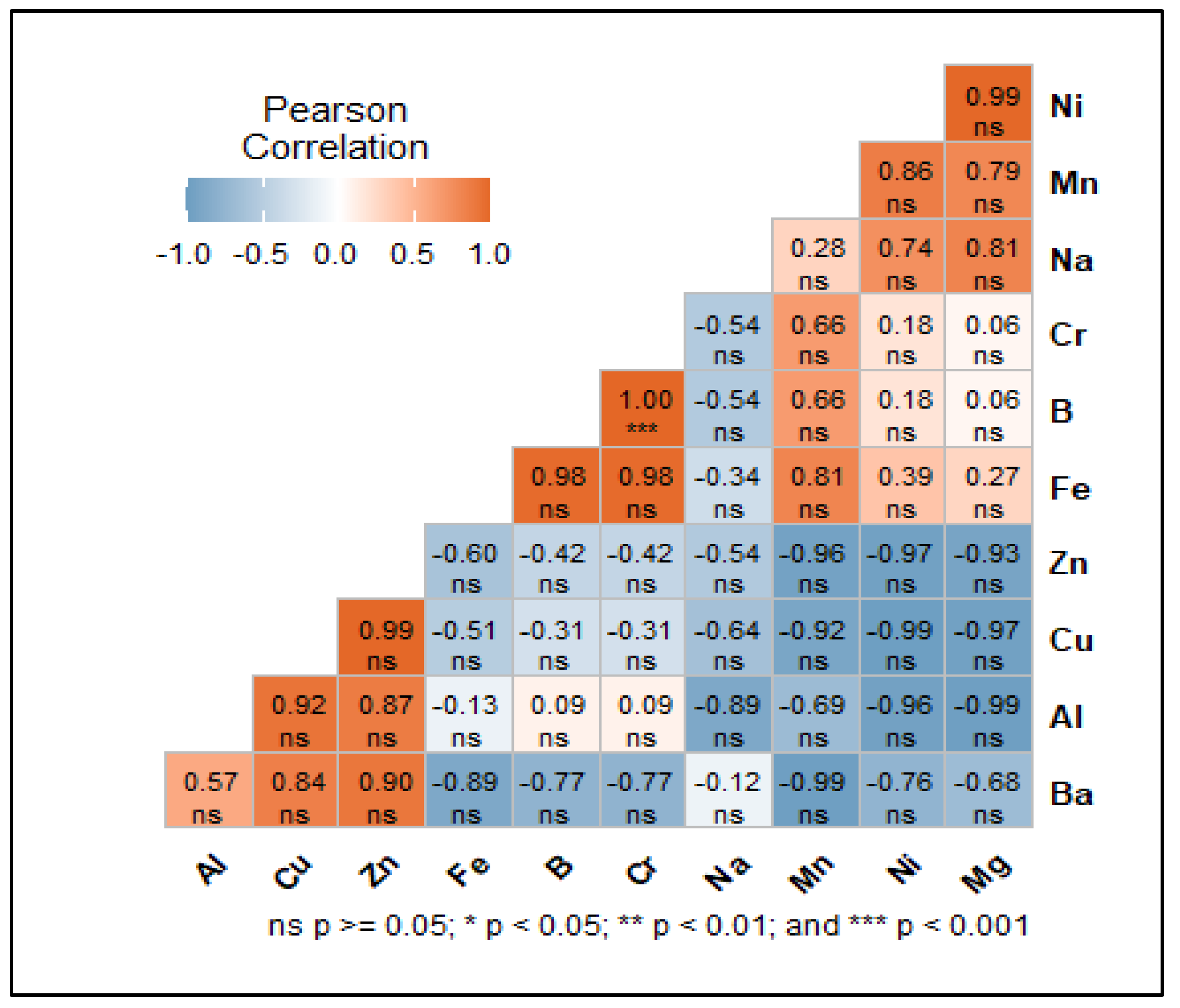
3.1.3. Pearson Correlation
| Variables | PC1 | PC2 |
|---|---|---|
| Al | -0.821 | 0.571 |
| Ba | -0.936 | -0.353 |
| B | 0.498 | 0.867 |
| Cr | 0.498 | 0.867 |
| Cu | -0.979 | 0.205 |
| Fe | 0.674 | 0.739 |
| Mn | 0.980 | 0.199 |
| Ni | 0.942 | -0.336 |
| Na | 0.466 | -0.885 |
| Zn | -0.996 | 0.090 |
| Mg | 0.895 | -0.447 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, F.M. , Bui, T.D., Tseng, M.L., Wu, K.J. (2020). A causal municipal solid waste management model for sustainable cities in Vietnam under uncertainty: a comparison. Resources, Conservation and Recycling, 154, p. 104599.
- Hoornweg, D. , & Bhada-Tata, P. (2012). What a waste: a global review of solid waste management.
- Kaza, S. , Yao, L., Bhada-Tata, P., Woerden, V.F. (2018). What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. World Bank.
- ASSOCIAÇÃO BRASILEIRA DE EMPRESAS DE LIMPEZA PÚBLICA E RESÍDUOS ESPECIAIS – ABRELPE. Panorama 2021. São Paulo: ABRELPE, 2021.
- Silva, D.R.B. da, Costa Filho, I.S., Souza, W.C.S. de, Santos, F.V. dos, Machado, P.C. de F., Brandão, I.W. de S., Pereira, F.C., Assunção, M.A. da C., Silva, R.H.Y., Russo, M.A.T., Mendonça, N.M. (2022). Aspectos Quantitativos e Qualitativos de Resíduos Sólidos Urbanos nos Municípios de Ananindeua, Belém e Marituba. In: Pereira, C., & Fricke, K. (Coords.). Cooperação Intersetorial e Inovação: ferramentas para a gestão sustentável de resíduos sólidos. Braunschweig: Technische Universität Braunschweig.
- Renou, S. , Givaudan, J.G., Poulain, S., Dirassouyan, F., Moulin, P. (2008). Tratamento de chorume de aterro: revisão e oportunidade. Journal of Hazardous Materials, 150(3), pp. 468–493.
- Bengtsson, L. , Bendz, D., Hogland, W., Rosqvist, H., Åkesson, M. (1994). Water balance for landfills of different age. Journal of Hydrology, 158(3–4), pp. 203-217.
- Mull, E.J. (2005). Approaches toward sustainable urban solid waste management: Sahakaranagar Layout. Unpublished M Sc Int Environ Sci, Lund University, Lund, Sweden, p. 37.
- Mor, S. , Ravindra, K., Dahiya, R.P., Chandra, A. (2006). Leachate characterization and assessment of groundwater pollution near municipal solid waste landfill site. Environmental Monitoring and Assessment, 118(1), pp. 435-456.
- Guerrero, L.A. , Maas, G., Hogland, W. (2013). Solid waste management challenges for cities in developing countries. Waste Management, 33(1), pp. 220-232.
- Joardar, J.C. , Halder, M. (2013). Chemical Characterization of Khulna City Waste and Its Effects on Surrounding Soils through Lateral Movement. In: Alamgir, M., Rafizul, I.M., & S.M.T. (Eds.), Proceedings of the Waste Safe 2013 – 3rd International Conference on Solid Waste Management in the Developing Countries (pp. [Page Numbers]). Khulna, Bangladesh.
- Kanmani, S. , Gandhimathi, R. (2013). Assessment of heavy metal contamination in soil due to leachate migration from an open dumping site. Applied Water Science, 3, pp. 193-205.
- Rodrigues, S.M. Henriques, B., da Silva, E.F., Pereira, M.E., Duarte, A.C., & Römkens, P.F. (2010). Evaluation of an approach for the characterization of reactive and available pools of twenty potentially toxic elements in soils: Part I-The role of key soil properties in the variation of contaminants’ reactivity. Chemosphere, 81(11), pp. 1549-1559.
- Wong, C.S. , Li, X., Thornton, I. (2006). Urban environmental geochemistry of trace metals. Environmental Pollution, 142, pp. 1-16.
- Dong, J. , Yu, M., Bian, Z., Wang, Y., & Di, C. (2011). Geostatistical analyses of heavy metal distribution in reclaimed mine land in Xuzhou, China. Environmental Earth Sciences, 62(1), pp. 127-137.
- International Agency for Research on Cancer (IARC). (2018). Chromium (IV) Compounds. IARC Monograph 100C.
- Dash, S., Borah, S.S., Kalamdhad, A. (2019). A modified indexing approach for assessment of heavy metal contamination in Deepor Beel, India. Ecological Indicators, 106, p. 105444.
- Singh, P. , Purakayastha, T.J., Mitra, S., Bhowmik, A., & Tsang, D.C. (2020). River water irrigation with heavy metal load influences soil biological activities and risk factors. Journal of Environmental Management, 110517.
- Zhang, C. , Nie, S., Liang, J., Zeng, G., Wu, H., Hua, S., Liu, J., Yuan, Y., Xiao, H., Deng, L., & Xiang, H. (2016). Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Science of the Total Environment, 557, pp. 785-790.
- Zhou, C. , Huang, H., Cao, A., & Xu, W. (2015). Modeling the carbon cycle of the municipal solid waste management system for urban metabolism. Ecological Modelling, 318, pp. 150-156.
- Stamps, B.W. , Lyles, C.N., Suflita, J.M., Masoner, J.R., Cozzarelli, I.M., Kolpin, D.W., & Stevenson, B.S. (2016). Municipal solid waste landfills harbor distinct microbiomes. Frontiers in Microbiology, 7, p. 534.
- Ma, S. , Zhou, C., Pan, J., Yang, G., Sun, C., Liu, Y., Chen, X., Zhao, Z. (2022). Leachate from municipal solid waste landfills in a global perspective: characteristics, influential factors and environmental risks. Journal of Cleaner Production, 333, Article 130234.
- Lin, Y. , Ye, Y., Hu, Y., Shi, H. (2019). The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicology and Environmental Safety, 180, pp. 557-564.
- Desai, C. , Parikh, R.Y., Vaishnav, T., Shouche, Y.S., Madamwar, D. (2009). Tracking the influence of long-term chromium pollution on soil bacterial community structures by comparative analyses of 16S rRNA gene phylotypes. Research in Microbiology, 160(1), pp. 1-9.
- Kawahigashi, F.; et al. (2014). Pós-tratamento de lixiviado de aterro sanitário com carvão ativado. Revista Brasileira de Engenharia Sanitária e Ambiental, 19(3), pp. 235-244.
- Lange et al. (2006). Tratamento de lixiviado de aterro sanitário por processo oxidativo avançado empregando reagente de Fenton. Revista Brasileira de Engenharia Sanitária e Ambiental.
- Assunção, F.P.d.C.; Pereira, D.O.; Silva, J.C.C.d.; Ferreira, J.F.H.; Bezerra, K.C.A.; Bernar, L.P.; Fer-reira, C.C.; Costa, A.F.d.F.; Pereira, L.M.; Paz, S.P.A.d.; et al. A Systematic Approach to Thermo-chemical Treatment of Municipal Household Solid Waste into Valuable Products: Analysis of Routes, Gravimetric Analysis, Pre-Treatment of Solid Mixtures, Thermochemical Processes, and Characterization of Bio-Oils and Bio-Adsorbents. Energies 2022, 15, 7971. [Google Scholar] [CrossRef]
- PEREIRA, D. O.; ASSUNÇÃO, F. P. da C.; SILVA, J. C. C. da; FERREIRA, J. F. H.; FERREIRA, R. B. P.; LOLA, Á. L.; NASCIMENTO, Í. C. P. do; CHAVES, J. P.; NASCIMENTO, M. S. C. do; SILVA GOUVÊA, T. et al. Prediction of leachate characteristics via an analysis of the solubilized extract of the organic fraction of domestic solid waste from the Municipality of Belém, PA. Sustainability, v. 15, 15456, 2023. [CrossRef]
- BELÉM. Lei Municipal nº 9.656, de 30 de dezembro de 2020. Institui a Política Municipal de Saneamento Básico do Município de Belém, o Plano Municipal de Saneamento Básico (PMSB), e o Plano de Gestão Integrada de Resíduos Sólidos (PGIRS), em atenção ao disposto no Art. 9º da Lei Federal nº 11.445/2007, com as atualizações trazidas pela Lei nº 14.026/2020, o Novo Marco do Saneamento Básico, e dá outras providências. Belém, PA, 30 dez. 2020.
- IBGE. Instituto Brasileiro de Geografia e Estatística. Censo Brasileiro de 2010; IBGE: Rio de Janeiro, Brazil, 2012. Available online: https://censo2010.ibge.gov.br/ (acesso em 1 Augusto 2022).
- FESSEHA, S. N.; BIN, F. The assessment of solid waste products management in Ethiopians municipal urban areas. International Journal of Social Sciences and Management, v. 2, p. 165-179, 2015;
- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. ANBT. NBR 10.004/2004 –Resíduos Sólidos – Classificação. Rio de Janeiro, 2004.
- ABNT. NBR 10005: Lixiviação de resíduos sólidos - Procedimento. Rio de Janeiro: ABNT, 2004b.
- ABNT. NBR 10006: Solubilização de resíduos sólidos - Procedimento. Rio de Janeiro: ABNT, 2004c.
- APHA. Standard Methods for the Examination of Water and Wastewater. 23rd ed. Washington, DC: American Public Health Association, 2017.
- Croghan, CW, Egeghy, PP (2003) Methods for Dealing with Values Below the Limit of Detection using SAS. North Carolina State University, Institute for Advanced Analytics, Raleigh, NC.
- Hewett, P and Ganser, GH (2007) A comparison of several methods for analyzing censored data. Ann. Occup. Hyg. 51(7): 611-632.
- Zhang, F. , Li, C., Shi, Y. et al. Evaluation on leachability of heavy metals from tailings: risk factor identification and cumulative influence. Environ Sci Pollut Res 30, 64565–64575 (2023). [CrossRef]
- Jiang, W. , Liu, H., Sheng, Y. et al. Distribution, Source Apportionment, and Health Risk Assessment of Heavy Metals in Groundwater in a Multi-mineral Resource Area, North China. Expo Health 14, 807–827 (2022). [CrossRef]
- Kumari, P. , Gupta, N.C., Kaur, A., Singh, K., 2019. Application of principal componentanalysis and correlation for assessing groundwater contamination in and aroundmunicipal solid waste landfill of Ghazipur, Delhi. J. Geol. Soc. India 94, 595–604. [CrossRef]
- KRUGEL, J. Estudo da concentração do percolado de aterro industrial por evaporação visando à redução da carga poluidora. 2013. Dissertação (Mestrado em Engenharia Ambiental) – Universidade Federal do Paraná, Curitiba, 2013.
- SAMADDER, S. R.; PRABHAKAR, R.; KHAN, D.; KISHAN, D.; CHAUHAN, M. S. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Science of The Total Environment, v. 580, p. 593-601, 2017. ISSN 0048-9697. [CrossRef]
- BRASIL. CONSELHO NACIONAL DO MEIO AMBIENTE. Resolução nº 396, de 03 de abril de 2008. Dispõe sobre a classificação e diretrizes ambientais para o enquadramento das águas subterrâneas e dá outras providências. Diário Oficial da União, Brasília, DF, 04 abr. 2008. Seção 1, p. 89.
- BRASIL. Conselho Nacional de Meio Ambiente. Resolução nº 430, de 13 de maio de 2011. Dispõe sobre as condições e padrões de lançamento de efluentes. CONAMA: Brasília, 2005.
- FAO. Plant nutrition for food security: A guide for integrated nutrient management. Rome: Food and Agriculture Organization of the United Nations, 2006. Disponível em: http://www.fao.org. Acesso em: 2 jun. 2023.
- ZACHARIAS, C. M. Estudo da toxicidade de metais pesados em solos agrícolas. 2013. Dissertação (Mestrado) – Universidade Federal de Viçosa, Viçosa, 2013.
- SAMADDER, S. R.; PRABHAKAR, R.; KHAN, D.; KISHAN, D.; CHAUHAN, M. S. Analysis of the contaminants released from municipal solid waste landfill site: A case study. Science of The Total Environment, v. 580, p. 593-601, 2017. ISSN 0048-9697. [CrossRef]
- EDOKPAYI, Joshua N.; DUROWOJU, Olatunde S.; ODIYO, John O. Assessment of heavy metals in landfill leachate: A case study of Thohoyandou landfill, Limpopo Province, South Africa. In: SALEH, Hosam El-Din M.; AGLAN, Refaat F. (Eds.). Heavy Metals. Cham: Springer, 2018. p. 123-145. Submitted on: 04 Oct. 2017. Revised on: 15 Jan. 2018. Published on: 30 Mar. 2018.
- BRASIL. Conselho Nacional de Meio Ambiente. Resolução nº 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento. CONAMA: Brasília, 2005.
- Maiti, S. K., Hazra, T., Debsarkar, A., & Dutta, A. (2016). Leachate characterization and identifcation of dominant pollutants using leachate pollution index for an uncontrolled landfll site. Global Journal of Environmental Science and Management, 2, 177–186. [CrossRef]
- Hussain et al., 2024 M.S. Hussain, G. Gupta, R. Mishra, N. Patel, S. Gupta, S.I. Alzarea, I. Kazmi, P. Kumbhar, J. Disouza, H. Dureja, N. Kukreti, S.K. Singh, K. Dua Unlocking the secrets: volatile organic compounds (VOCs) and their devastating effects on lung cancer. Pathol. Res. Pract., 155157 (2024).
- Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182. [CrossRef]
- RIGUETTI, Priscilla Fracalossi; CARDOSO, Cláudia Andréa Lima; CAVALHEIRO, Alberto Adriano; LENZI, Ervim; FIORUCCI, Antonio Rogério; SILVA, Margarete Soares da. Manganês, zinco, cádmio, chumbo, mercúrio e crômio no chorume de aterro sanitário em Dourados, MS, Brasil. Revista Ambiente & Água, Taubaté, v. 10, n. 1, p. 101-111, jan./mar. 2015.
- OLIVEIRA, D. L.; SANTANA, G. P. Influência do aterro municipal de Manaus sobre as águas superficiais da circunvizinhança: um enfoque ao estudo de metais pesados. Caminhos de Geografia, Uberlândia, v. 11, n. 34, p. 75-83, 2010.
- LINDAMULLA, L.M.L.K.B; JEGATHEESAN, V.; JINADASA, K.B.S.N.; NANAYAKKARA, K.G.N.; OTHMAN, Z. ; Integrated mathematical model to simulate the performance of membrane bioreactor. Chemosphere, n. 284, p. 131-319, 2021. [CrossRef]
- HUSSEIN, Munirah; YONEDA, Kenichi; MOHD-ZAKI, Zuhaida; AMIR, Amnorzahira; OTHMAN, Nor Ázizi. Heavy metals in leachate, impacted soils and natural soils of different landfills in Malaysia: An alarming threat. Chemosphere, v. 267, p. 128874, 2021. ISSN 0045-6535. [CrossRef]
- WONG, A. H. H.; CHIN, W. S. M. A case study of long-term CCA preservative leaching from treated hardwood poles in a humid tropical condition. Section 5 Sustainability and Environment. 2016.
- FU, Jie; ZHAO, Changpo; LUO, Yupeng; LIU, Chunsheng; KYZAS, George Z.; LUO, Yin; ZHAO, Dongye; AN, Shuqing; ZHU, Hailiang. Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. Journal of Hazardous Materials, v. 270, p. 102-109, 2014. ISSN 0304-3894.
- ALVES, R. I. S.; TONANI, K. A. A.; NIKAIDO, M.; CARDOSO, O. O.; TREVILATO, T. M. B.; SEGURAMUÑOZ, S. I. Avaliação das concentrações de metais pesados em águas superficiais e sedimentos do Córrego Monte Alegre e afluentes, Ribeirão Preto, SP, Brasil. Ambi-Agua, Taubaté, v. 5, n. 3, p. 122-132, 2010.
- V. Bhardwaj, D.S. Singh, A.K. Singh. Environmental repercussions of cane-sugar industries on the Chhoti Gandak river basin, Ganga Plain, India. Environ. Monit. Assess., 171 (2010), pp. 321-344.
- Kuajara O, Sanches JCD, Ballestrin RA, Teixeira EC. Environmental monitoring of the North Porto Alegre landfill, Brazil. Water Environ Res 1997; 69:1170-7.
- Oliveira FSJ, Jucá FTJ. Acúmulo de metais pesados e capacidade de impermeabilização do solo imediatamente abaixo de uma célula de um aterro de resíduos sólidos. Eng Sanit Ambient 2004; 9:211-7.
- CELERE, M. S.; OLIVEIRA, A. S.; TREVILATO, T. M. B.; SEGURA-MUNHÕZ, S. I. Metais presentes no chorume coletado no aterro sanitário de Ribeirão Preto, São Paulo, Brasil e sua relevância para saúde pública. Caderno de Saúde Pública, v. 23, n. 4, p. 939-947, 2007.
- CORT, E. P. D.; ALBERTI, V.; ROTTA, M.; BECEGATO, V.; MACHADO, W. C. P.; ONOFRE, S. B. Níveis de metais pesados presentes no chorume produzido em aterros sanitários da região sudoeste do Paraná. Geoambiente, Jataí, n. 11, p. 103-116, 2008.
- CINTRA, Ilka Soares. Estudo da influência da recirculação de chorume Cdu e chorume inoculado na aceleração do processo de digestão anaeróbia de resíduos sólidos urbanos. 2003. 352 p. Dissertação (Mestrado em Engenharia Ambiental) – Universidade Federal do Ceará, Fortaleza, 2003.

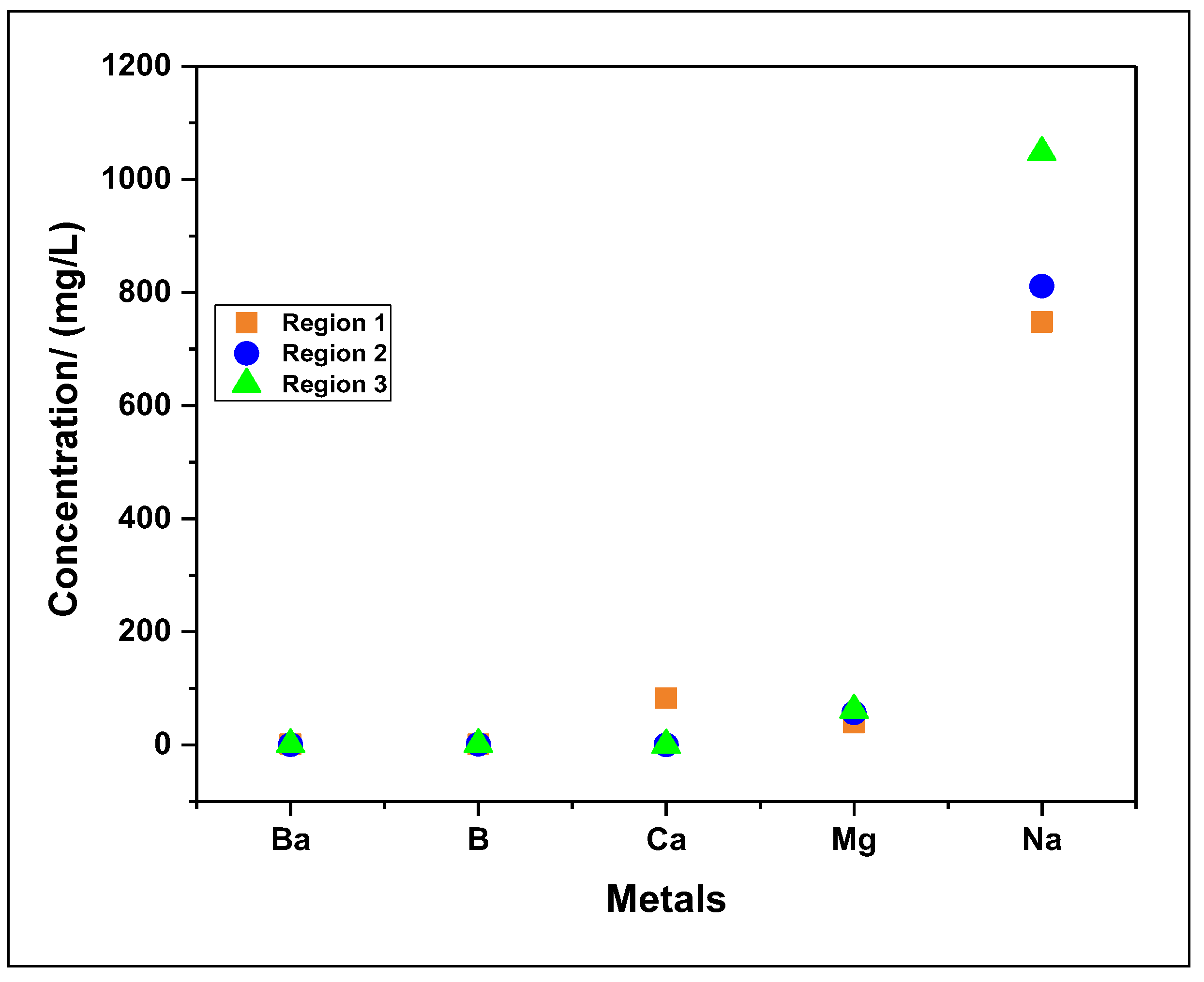
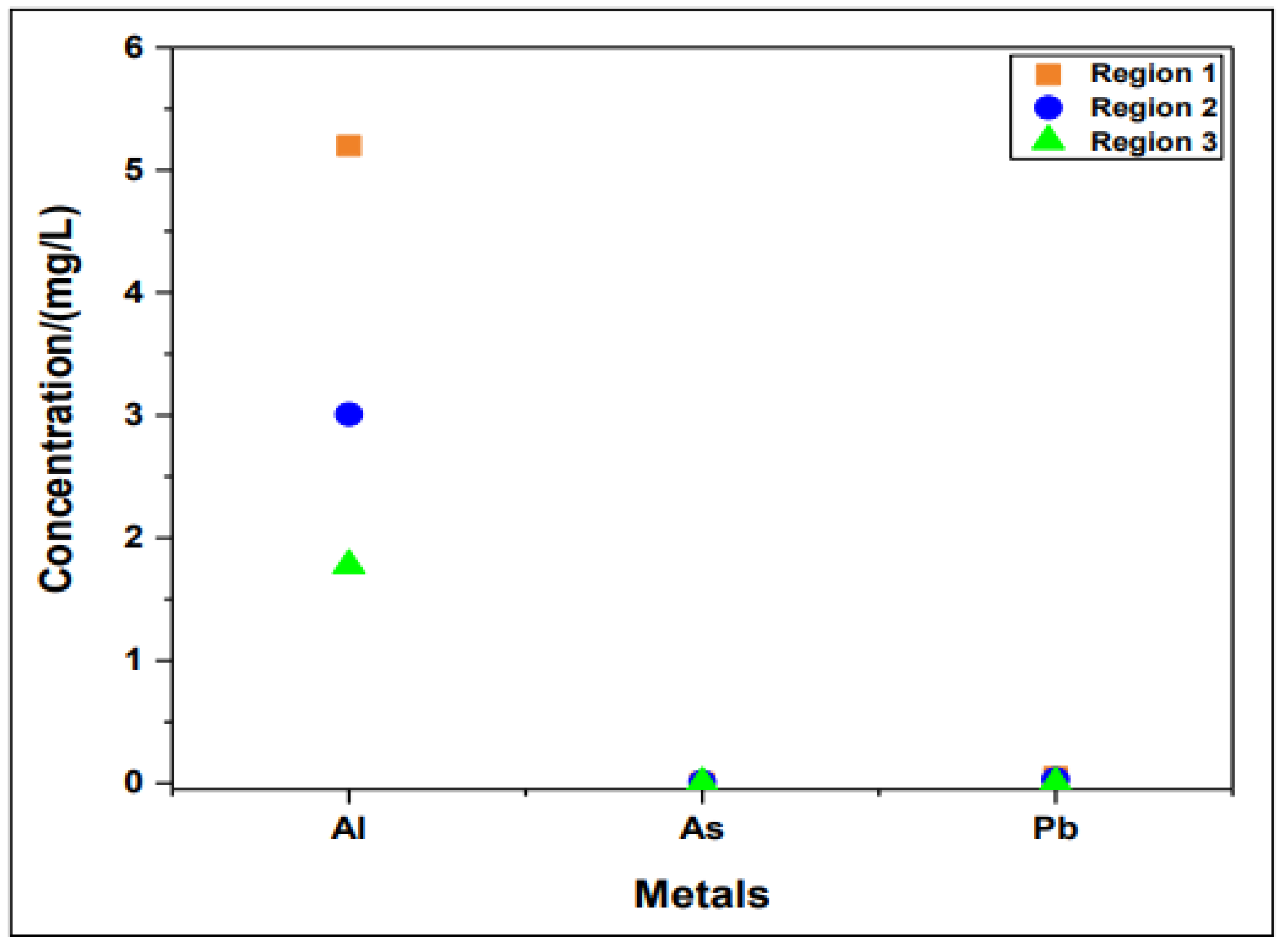
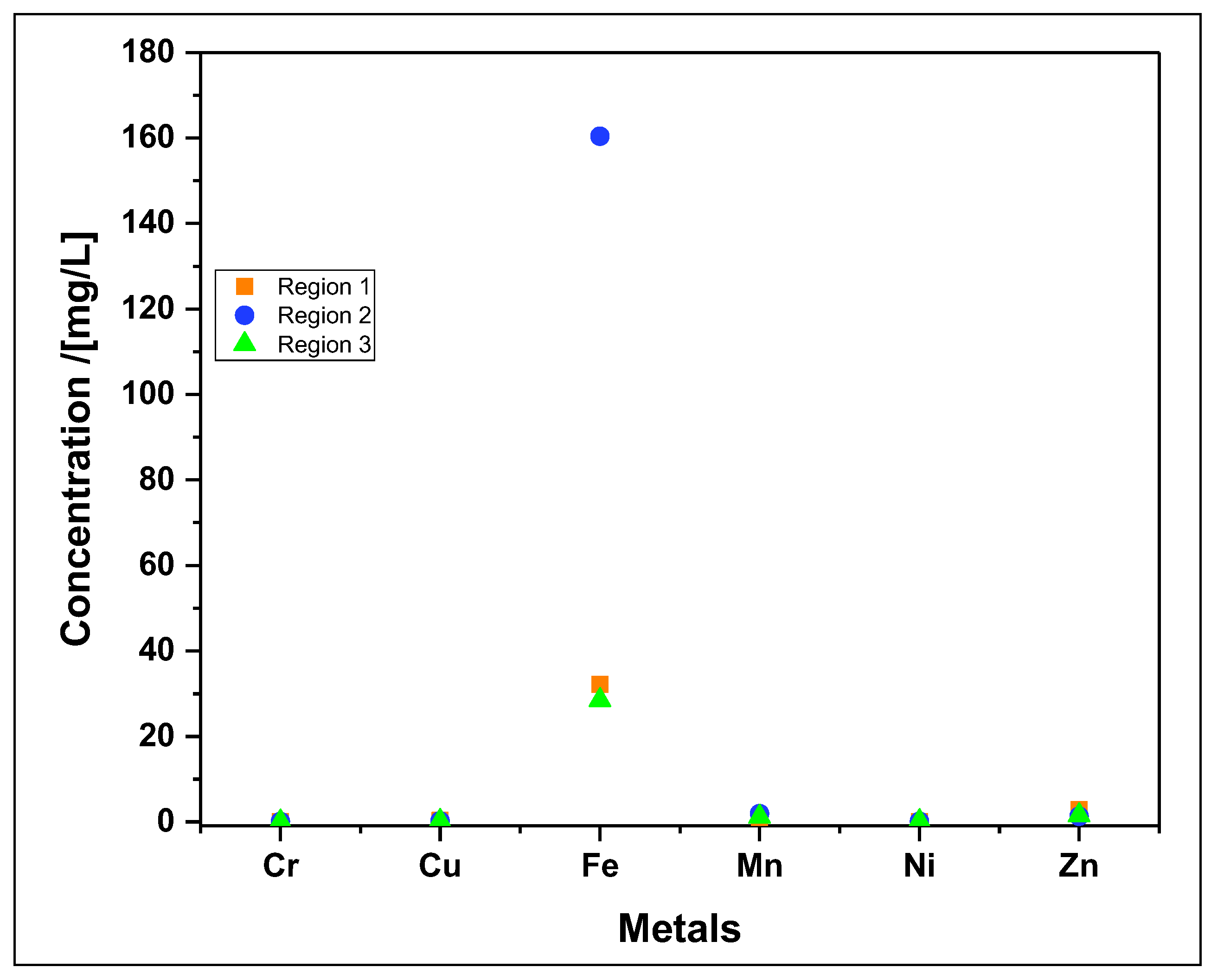
| Heavy metals (mg/L) |
Regions | ||
|---|---|---|---|
| R1 | R2 | R3 | |
| Al As |
5,198 0,002 |
3,007 0,00875 |
1,775 <0,002* |
| Ba | 1,33 | 0,44 | 0,95 |
| B | 1,19 | 1,30 | 1,16 |
| Pb | 0,05 | 0,03 | <0,002* |
| Cu | 0,35 | 0,22 | 0,23 |
| Cr | 0,060 | 0,075 | 0,055 |
| Fe | 32,2 | 160,4 | 28,5 |
| Mn | 0,265 | 1,910 | 1,205 |
| Ni | 0,06 | 0,14 | 0,15 |
| Na | 748 | 811,3 | 1047,8 |
| Zn | 2,85 | 1,30 | 1,55 |
| Ca | 82,9 | <0,02* | <0,02* |
| Mg | 39,83 | 56,82 | 61,92 |
| Metal Analysis (mg/L) (Region 1) CONAMA | |||
| Metals | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | yes | no | yes |
| Ba | yes | no | no |
| B | yes | no | no |
| Pb | yes | no | no |
| Cu | yes | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Mg | no | - | - |
| Metal Analysis (mg/L) (Region 2) CONAMA | |||
| Metals | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | yes | yes | yes |
| Ba | yes | yes | yes |
| B | yes | no | no |
| Pb | yes | no | no |
| Cu | yes | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Ca | - | - | - |
| Mg | no | - | - |
| Metal Analysis (mg/L) (Region 3) CONAMA | |||
| Metais | 430/2011 | 357/2005 | 396/2008 |
| Al | - | no | no |
| As | - | - | - |
| Ba | yes | no | no |
| B | yes | no | no |
| Pb | - | - | - |
| Cu | no | no | yes |
| Cr | yes | no | - |
| Fe | no | no | no |
| Mn | no | no | no |
| Ni | yes | no | no |
| Na | - | - | no |
| Zn | yes | no | yes |
| Mg | no | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





