Submitted:
10 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and methods
2.1. Reagents and Chemicals
2.2. Cyclocarya paliurus Samples
2.3. Electronic Tongue Analysis for C. paliurus Sample
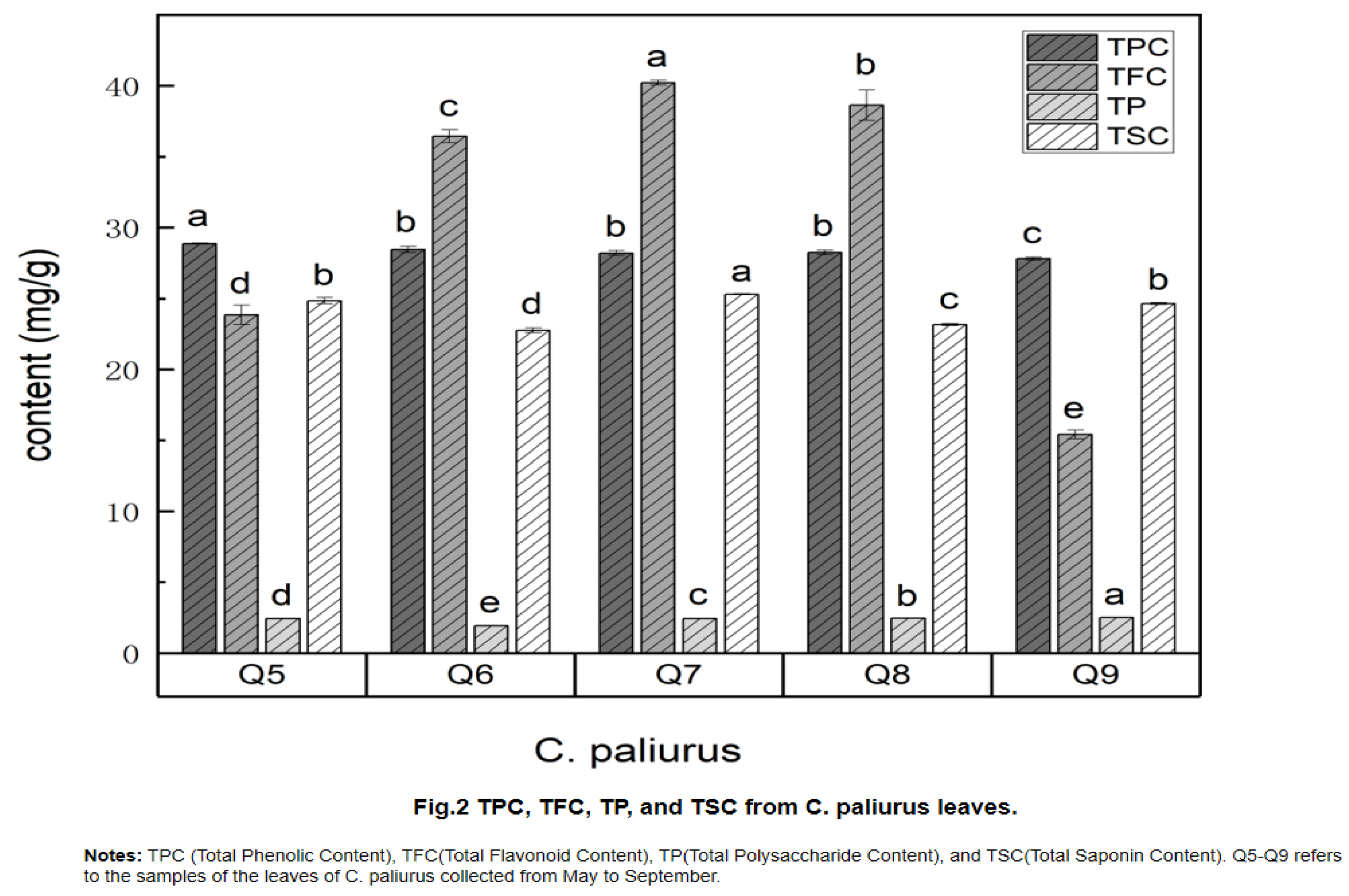
2.4. Determination of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), Total Polysaccharide Content (TP) and Total Saponin Content (TSC)
2.5. Nontargeted Metabolomics Analysis by UHPLC-MS/MS
2.5.1. Metabolite Extraction
2.5.2. UHPLC-MS/MS Analysis
2.5.3. Metabolome Data Preprocessing
2.6. Computational Study of Sweet/Bitter Compound Binding to T1R2/T1R3 and T1R4/T1R14 Receptors
2.7. Statistical Analysis
Results & Discussion
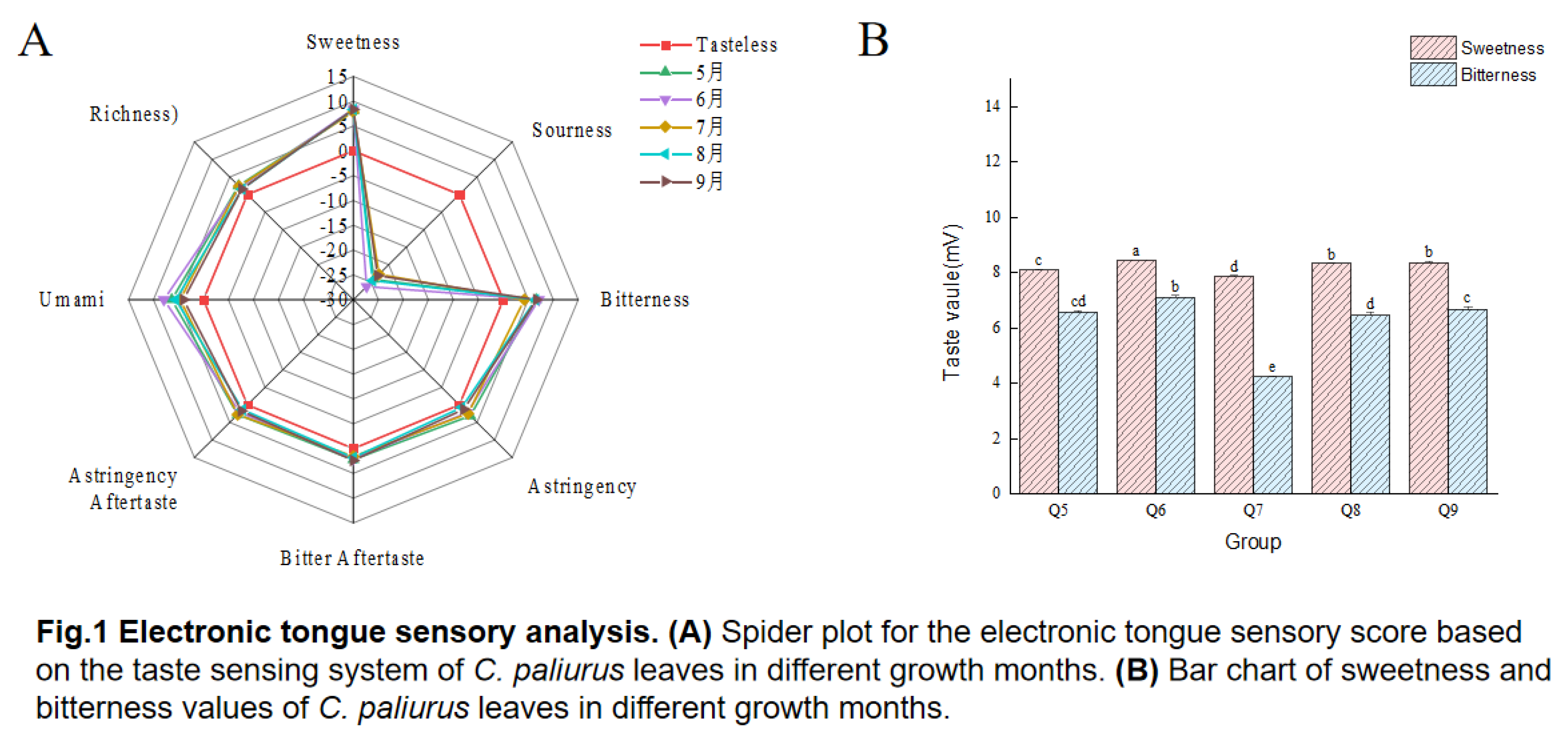
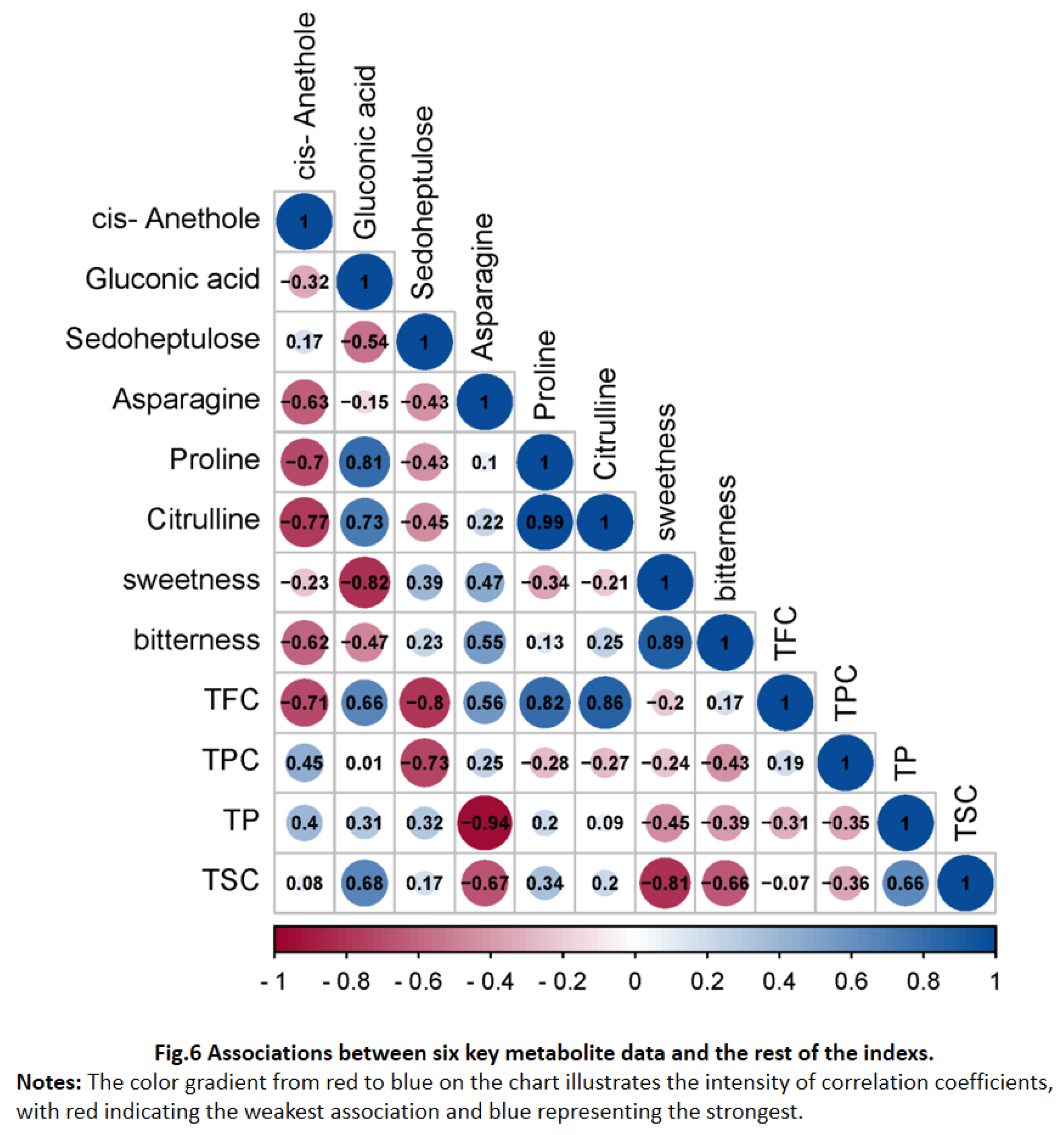
3.1. Electronic Tongue Analysis and Correlation Analysis
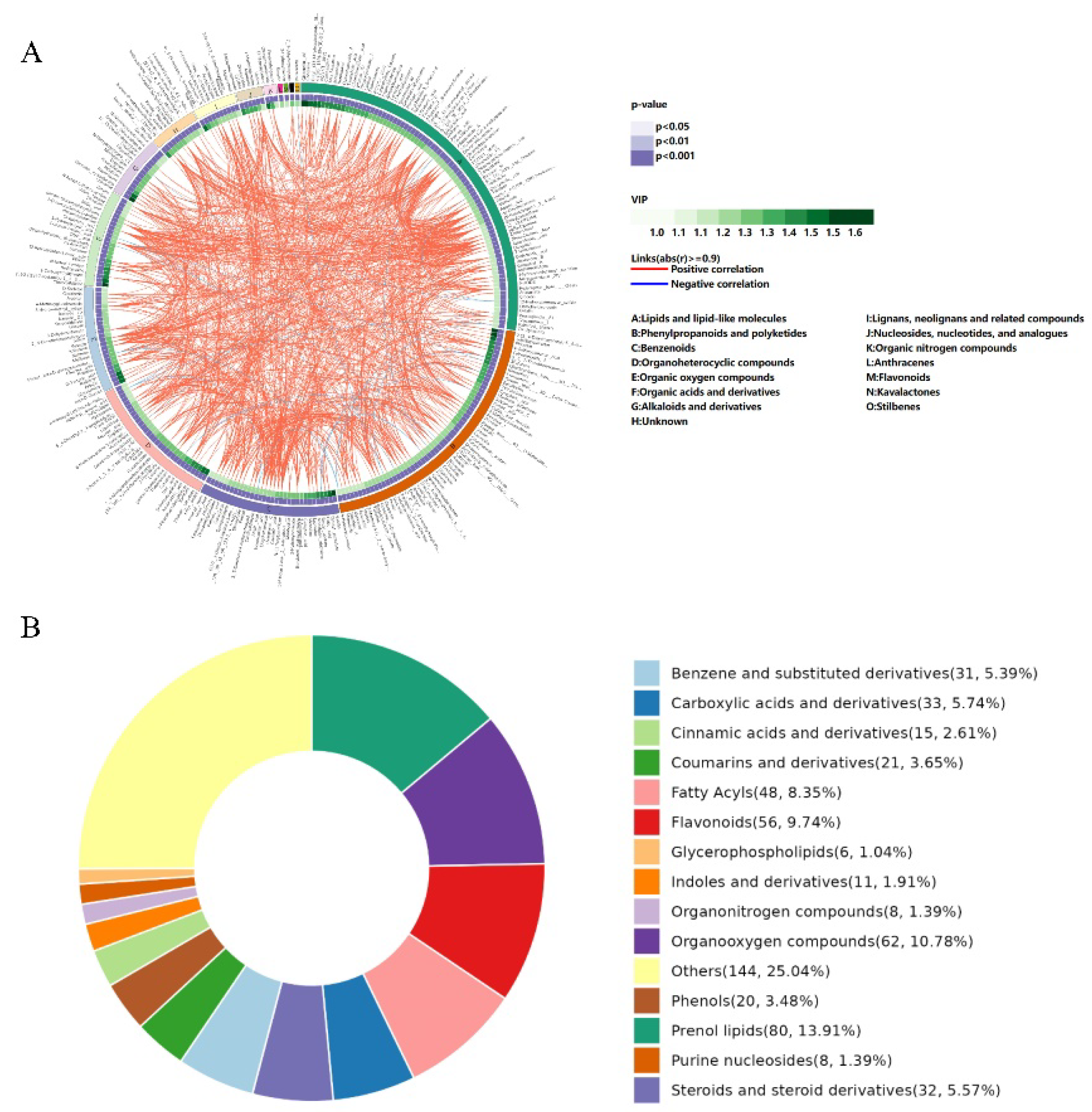
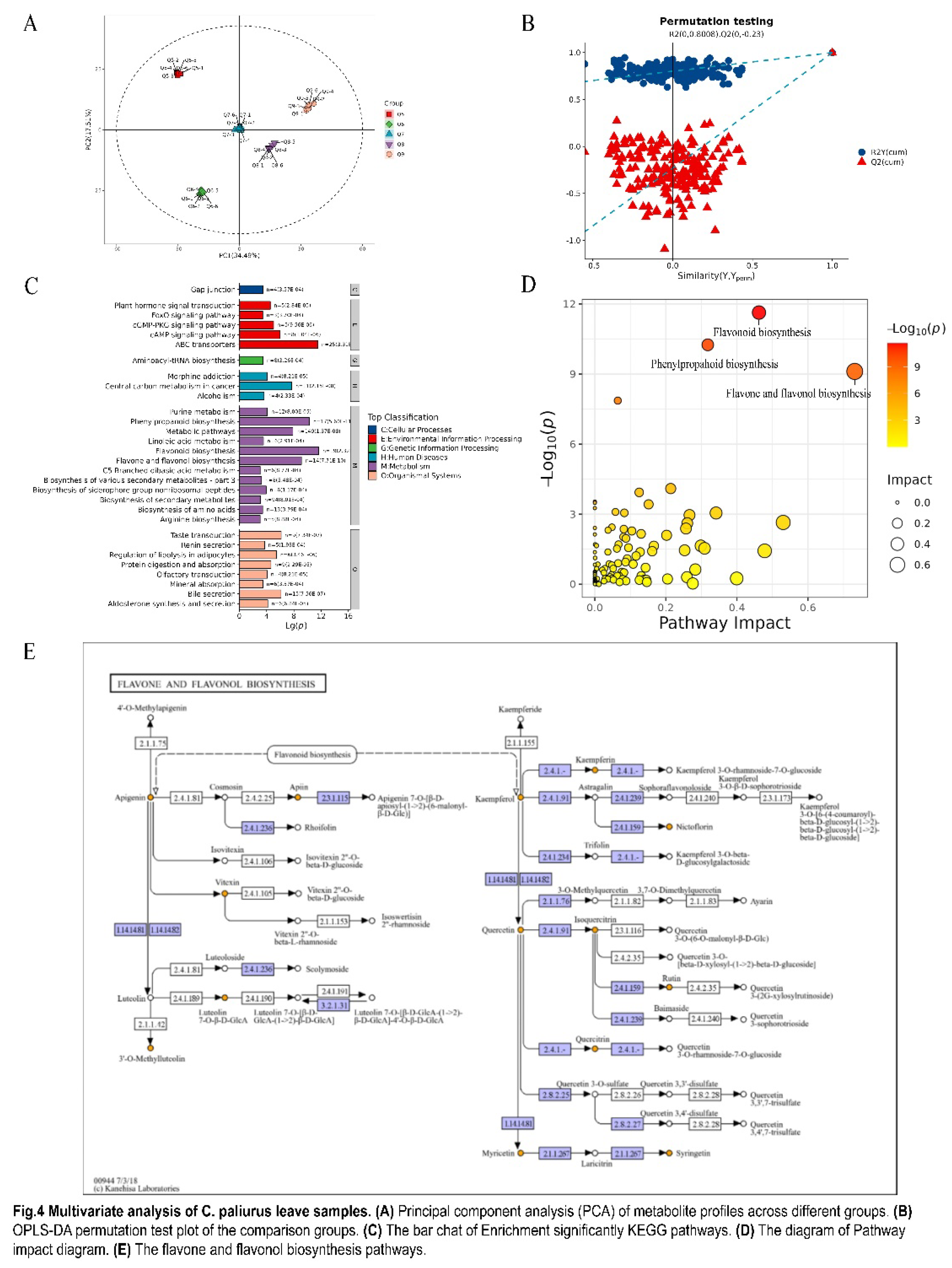
3.3. Nontargeted Metabolomics Analysis of C. paliurus Leaves
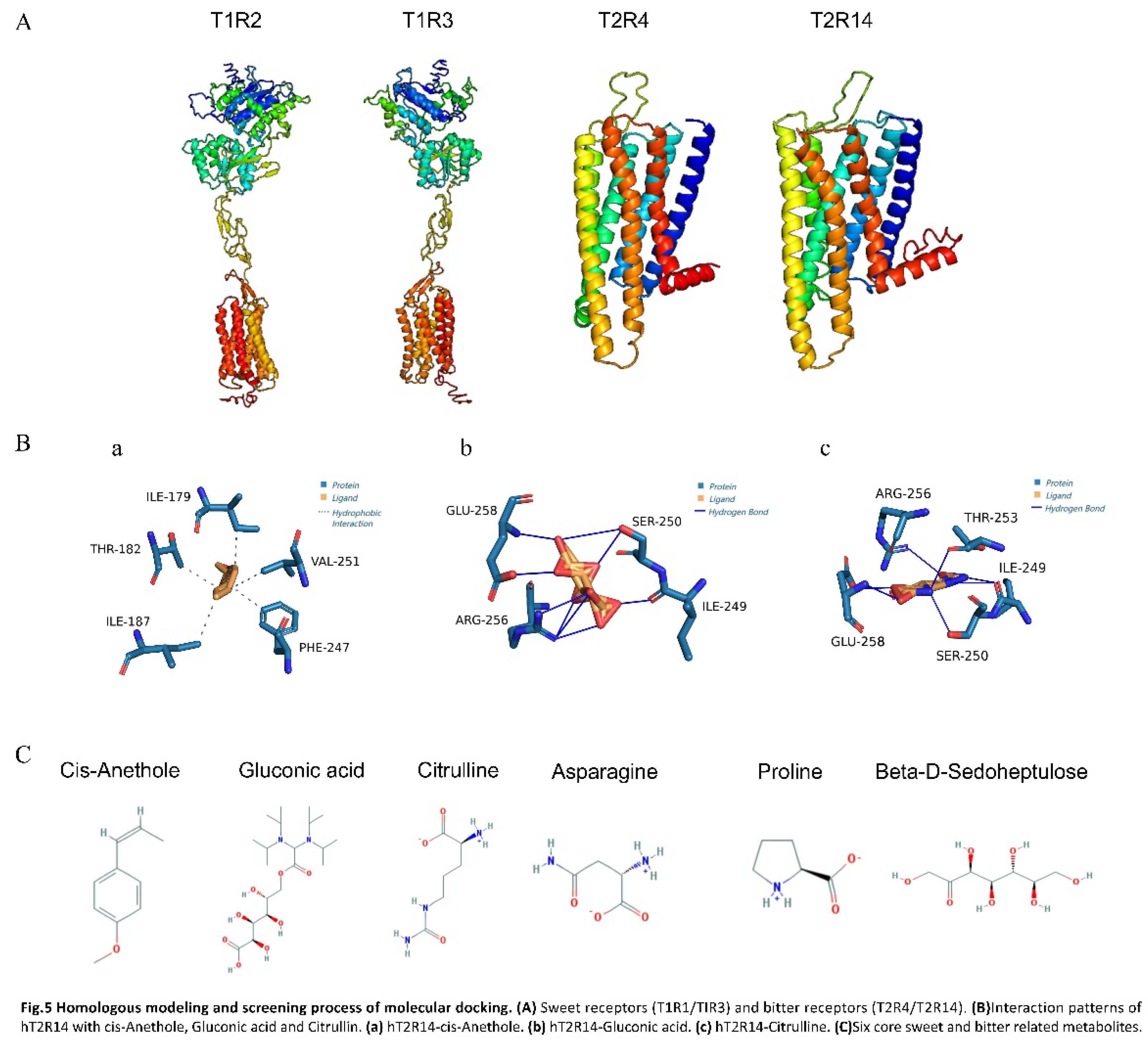
3.4. Molecular Docking of Candidate Sweet/Bitter Substances with the T1R1/ T1R3、T2R4/T2R14 Receptors
4. Conclusion
CRediT authorship contribution statement
Acknowledgments
Declaration of Competing Interest
References
- Shu, R. G.; Xu, C. R.; Li, L. N.; Yu, Z. L. Cyclocariosides II and III: two secodammarane triterpenoid saponins from Cyclocarya paliurus. Planta Med.. 1995, 61, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Sun, D.; Shang, X.; Fu, X.; Yang, W. Variation in radial growth and wood density of Cyclocarya paliurus across its natural distribution. NEW FORESTS. 2020, 51, 453–467. [Google Scholar] [CrossRef]
- Xie, J.; Xie, M.; Nie, S.; Shen, M.; Wang, Y.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Q.; Lu, J.; Li, J.; Liu, W.; Yang, J.; Li, J.; Xiao, K. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 2010, 119, 1491–1496. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, T.; Zhong, R.; Lin, Z.; Jiang, C.; Ouyang, S.; Zhao, M.; Che, C.; Zhang, J.; Yin, Z. Antihyperlipidaemic effect of triterpenic acid-enriched fraction from Cyclocarya paliurus leaves in hyperlipidaemic rats. Pharm Biol. 2017, 55, 712–721. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Shen, M.; Nie, S.; Gong, B.; Li, H.; Zhao, Q.; Li, W.; Xie, M. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Functional Hydrocolloids: A Key to Human HealthFood Hydrocolloids. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K. Fatigue-alleviating effect of polysaccharides from Cyclocarya paliurus (Batal) Iljinskaja in mice. African Journal of Microbiology Research. 2012, 6, 5243–5248. [Google Scholar]
- Rong, L. J. Q. Research Progress on the Effects of Cyclocarya Paliurus on Decreasing Blood Glucose and lts Application Prospect in the Development of Auxiliary Hypoglycemic Substitute Tea. Journal of Tea Communication. 2021, 48, 606–611. [Google Scholar]
- Shen, Y.; Peng, Y.; Zhu, X.; Li, H.; Zhang, L.; Kong, F.; Wang, J.; Yu, D. The phytochemicals and health benefits of Cyclocarya paliurus (Batalin) Iljinskaja. Front Nutr. 2023, 10, 1158158. [Google Scholar] [CrossRef]
- Zhang, S.; Nie, L.; Zhao, W.; Cui, Q.; Wang, J.; Duan, Y.; Ge, C. Metabolomic analysis of the occurrence of bitter fruits on grafted oriental melon plants. PLoS ONE. 2019, 14, e0223707. [Google Scholar] [CrossRef]
- Kim, S. S.; Kwak, H. S.; Kim, M. J. The effect of various salinity levels on metabolomic profiles, antioxidant capacities and sensory attributes of doenjang, a fermented soybean paste. Food Chem. 2020, 328, 127176. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Yamamoto, S.; Nakajima, A.; Kodama, Y.; Imamura, M.; Sato, T.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Metabolic Profiling Approach To Explore Compounds Related to the Umami Intensity of Soy Sauce. Journal of Agricultural and Food ChemistryJournal of Agricultural and Food ChemistryJ. Agric. Food Chem.. 2014, 62, 7317–7322. [Google Scholar] [CrossRef]
- Sumner, L. W.; Mendes, P.; Dixon, R. A. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Plant MetabolomicsPhytochemistry. 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Zeki, Ö. C.; Eylem, C. C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC–MS and LC–MS for untargeted metabolomics profiling. J Pharm Biomed Anal. 2020, 190, 113509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Tian, X.; Wang, H.; Chen, L.; Yuan, H.; Peng, C.; Wang, W. Distinct Changes in Metabolic Profile and Sensory Quality with Different Varieties of Chrysanthemum (Juhua) Tea Measured by LC-MS-Based Untargeted Metabolomics and Electronic Tongue. Foods. 2024, 13, 1080. [Google Scholar] [CrossRef] [PubMed]
- Fritz, F.; Preissner, R.; Banerjee, P. VirtualTaste: a web server for the prediction of organoleptic properties of chemical compounds. Nucleic Acids Res.. 2021, 49, W679–W684. [Google Scholar] [CrossRef]
- Dagan-Wiener, A.; Di Pizio, A.; Nissim, I.; Bahia, M. S.; Dubovski, N.; Margulis, E.; Niv, M. Y. BitterDB: taste ligands and receptors database in 2019. Nucleic Acids Res.. 2019, 47, D1179–D1185. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, L.; Zhao, W.; Wu, S.; Ding, L.; Zheng, F.; Liu, J.; Li, J. Identification of novel umami peptides from myosin via homology modeling and molecular docking. Food Chem. 2021, 344, 128728. [Google Scholar] [CrossRef]
- Acevedo, W.; Ramírez-Sarmiento, C. A.; Agosin, E. Identifying the interactions between natural, non-caloric sweeteners and the human sweet receptor by molecular docking. Food Chem. 2018, 264, 164–171. [Google Scholar] [CrossRef]
- Hutasingh, N.; Chuntakaruk, H.; Tubtimrattana, A.; Ketngamkum, Y.; Pewlong, P.; Phaonakrop, N.; Roytrakul, S.; Rungrotmongkol, T.; Paemanee, A.; Tansrisawad, N.; et al. Metabolite profiling and identification of novel umami compounds in the chaya leaves of two species using multiplatform metabolomics. Food Chem. 2023, 404, 134564. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.; Di Pizio, A.; Cozzini, P. Sweet, umami and bitter taste receptors: State of the art of in silico molecular modeling approaches. Trends Food Sci Technol. 2020, 96, 21–29. [Google Scholar] [CrossRef]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T. Human psychometric and taste receptor responses to steviol glycosides. J. Agric. Food Chem.. 2012, 60, 6782–6793. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses. 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, H.; Guo, X. Memristive Synapses and Neurons for Bioinspired Computing. Advanced Electronic MaterialsAdvanced Electronic MaterialsAdvanced Electronic Materials. 2019, 5, 1900287. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Ren, W. C.; Su, Y.; Li, J.; Du, Y. Q.; Wang, Q. H.; Kuang, H. X. Rapid determination and origin identification of total polysaccharides contents in Schisandra chinensis by near-infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2022, 264, 120327. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: biosynthesis, in vitro production, and pharmacological relevance. Protoplasma. 2019, 25, 1463–1486. [Google Scholar] [CrossRef] [PubMed]
- Volkamer, A.; Kuhn, D.; Grombacher, T.; Rippmann, F.; Rarey, M. Combining Global and Local Measures for Structure-Based Druggability Predictions. Journal of Chemical Information and ModelingJournal of Chemical Information and ModelingJ. Chem. Inf. Model.. 2012, 52, 360–372. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A. F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J Chem Inf Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Gharibzahedi, S.; Barba, F. J.; Zhou, J.; Wang, M.; Altintas, Z. Electronic Sensor Technologies in Monitoring Quality of Tea: A Review. Biosensors (Basel). 2022, 12, 356. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, F.; Tang, P.; Huang, D.; Li, Q.; Lin, M. Widely Targeted Metabolomics Analysis of the Changes to Key Non-volatile Taste Components in Stropharia rugosoannulata Under Different Drying Methods. Front Nutr. 2022, 9, 884400. [Google Scholar] [CrossRef]
- Osakabe, N.; Shimizu, T.; Fujii, Y.; Fushimi, T.; Calabrese, V. Sensory Nutrition and Bitterness and Astringency of Polyphenols. Biomolecules. 2024, 14, 234. [Google Scholar] [CrossRef]
- Wei, L. The effect of cyclocarya flavonoids extrat on the liver insulin resistance of db/db mices and the Pi3K/AKT /FOXO1 pathway. Beijing University Of Chinese Medicine. 2021. [CrossRef]
- Gong, X.; Ji, M.; Xu, J.; Zhang, C.; Li, M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit Rev Food Sci Nutr. 2020, 60, 2303–2326. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Su, X.; Li, F.; She, Z.; He, X.; Lin, Y. A Norsesquiterpene Lactone and a Benzoic Acid Derivative from the Leaves of Cyclocarya paliurus and their Glucosidase and Glycogen Phosphorylase Inhibiting Activities. Planta Med.. 2008, 74, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, Y.; Yi, X.; Wang, X. A study on the structure and contents of flavonoids in the leaves of Cyclocarya paliurus (Batal.) Iljinsk. Fenxi Huaxue. 2004, 32, 1053–1056. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. 2 - Polyphenols: Absorption, bioavailability, and metabolomics. 2018, 45-67. [CrossRef]
- Fraser, K.; Lane, G. A.; Otter, D. E.; Hemar, Y.; Quek, S.; Harrison, S. J.; Rasmussen, S. Analysis of metabolic markers of tea origin by UHPLC and high resolution mass spectrometry. Tea – from bushes to mugs: composition, stability and health aspectsFood Research International. 2013, 53, 827–835. [Google Scholar] [CrossRef]
- Nakaya, A.; Katayama, T.; Itoh, M.; Hiranuka, K.; Kawashima, S.; Moriya, Y.; Okuda, S.; Tanaka, M.; Tokimatsu, T.; Yamanishi, Y.; et al. KEGG OC: a large-scale automatic construction of taxonomy-based ortholog clusters. Nucleic Acids Res.. 2013, 41, D353–D357. [Google Scholar] [CrossRef]
- Xi, H.; Xu, W.; He, F.; Liu, Z.; Wang, Y.; Xie, J. Spatial metabolome of biosynthesis and metabolism in Cyclocarya paliurus leaves. Food Chem. 2024, 443, 138519. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E. W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Reports Methods. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Singla, R.; Jaitak, V. Synthesis of rebaudioside A from stevioside and their interaction model with hTAS2R4 bitter taste receptor. Phytochemistry. 2016, 125, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Servant, G.; Kenakin, T.; Zhang, L.; Williams, M.; Servant, N. The function and allosteric control of the human sweet taste receptor. Advances in pharmacology (San Diego, Calif.). 2020, 88, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Joseph Naguib, M.; Moustafa Kamel, A.; Thabet Negmeldin, A.; Elshafeey, A. H.; Elsayed, I. Molecular docking and statistical optimization of taurocholate-stabilized galactose anchored bilosomes for the enhancement of sofosbuvir absorption and hepatic relative targeting efficiency. Drug Deliv. 2020, 27, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Keast, R. S. J.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr.. 2017, 118, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shaik, F. A.; Myal, Y.; Chelikani, P. Chemosensory bitter taste receptors T2R4 and T2R14 activation attenuates proliferation and migration of breast cancer cells. Mol. Cell. Biochem.. 2020, 465, 199–214. [Google Scholar] [CrossRef]
- Xu, Q.; Singh, N.; Hong, H.; Yan, X.; Yu, W.; Jiang, X.; Chelikani, P.; Wu, J. Hen protein-derived peptides as the blockers of human bitter taste receptors T2R4, T2R7 and T2R14. Food Chem. 2019, 283, 621–627. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Zhao, W.; Li, J.; Shuian, D.; Liu, J. Identification of Oncorhynchus mykiss nebulin-derived peptides as bitter taste receptor TAS2R14 blockers by in silico screening and molecular docking. Food Chem. 2021, 368. [Google Scholar] [CrossRef]
| Taste Characteristic | Samples of C. paliurus | ||||
| Q5 | Q6 | Q7 | Q8 | Q9 | |
| Sweetness | 8.09±0.02c | 8.45±0.00a | 7.86±0.04d | 8.33±0.01b | 8.36±0.04b |
| Sourness | -24.2±0.15c | -26.25±0.06e | -22.77±0.23a | -24.55±0.11d | -23.12±0.13b |
| Bitterness | 6.57±0.05cd | 7.12±0.06b | 4.23±0.04e | 6.46±0.1d | 6.67±0.10c |
| Astringency | 3.1±0.23a | 2.21±0.24c | 2.46±0.16bc | 0.67±0.31e | 1.24±0.24d |
| Bitter Aftertaste | 2.26±0.06c | 2.09±0.05d | 1.59±0.04f | 1.85±0.05e | 2.4±0.08b |
| Astringency Aftertaste | 2.76±0.15a | 2.13±0.11b | 2.74±0.16a | 1.39±0.09d | 1.7±0.11c |
| Umami | 6.35±0.19b | 7.9±0.17a | 4.65±0.21d | 5.55±0.19c | 3.98±0.20e |
| Richness | 2.48±0.23b | 2.08±0.14c | 2.33±0.12bc | 1.5±0.07d | 1.56±0.19d |
| parameters | R2X(cum) | R2Y(cum) | Q2(cum) | RMSEE | pre | ort | pR2Y |
| Total | 0.576 | 0.999 | 0.997 | 0.0238 | 1 | 2 | 0.005 |
| Metabolite name | hT1R2 | hT1R3 | hT1R4 | hT1R14 | As | Ab |
| Category 1 | ||||||
| Ganoderic Acid B | -13.0 | -11.9 | -12.3 | -11.7 | -24.9 | -24.0 |
| Deoxycholic acid | -10.9 | -10.8 | -11.7 | -10.7 | -21.7 | -22.4 |
| Indole-3-acetyl-valine | -9.2 | -8.9 | -8.6 | -7.9 | -18.1 | -16.5 |
| Category 2 | ||||||
| Ginsenoside Rf | -10.1 | -12.7 | -10.6 | -10.4 | -22.8 | -21.0 |
| FA 18:3;O | -6.8 | -8.7 | -7.9 | -7.0 | -15.5 | -14.9 |
| cis-Anethole | -5.8 | -5.6 | -5.8 | -5.6 | -11.4 | -11.4 |
| Icariside F2 | -10.1 | -9.7 | -8.4 | -9.0 | -19.8 | -17.4 |
| Forsythoside E | -10.3 | -10.7 | -8.0 | -8.7 | -21.0 | -16.7 |
| Gluconic acid | -5.7 | -5.6 | -5.0 | -5.1 | -11.3 | -10.1 |
| Olivil 4'-O-Glucoside | -9.2 | -10.6 | -9.2 | -8.7 | -19.8 | -17.9 |
| Category 3 | ||||||
| Centaurein | -10.5 | -10.0 | -8.4 | -9.1 | -20.5 | -17.5 |
| Iridin | -9.2 | -10.0 | -8.8 | -8.0 | -19.2 | -16.8 |
| Syringetin | -9.9 | -9.6 | -8.3 | -8.3 | -19.5 | -16.6 |
| Dihydromelilotoside | -8.6 | -8.5 | -7.8 | -7.1 | -17.1 | -14.9 |
| Lamiide | -10.3 | -10.1 | -8.6 | -8.0 | -20.4 | -16.6 |
| Category 4 | ||||||
| Sedoheptulose | -7.0 | -6.8 | -5.8 | -6.6 | -13.8 | -12.4 |
| Hematoxylin | -10.5 | -9.3 | -9.4 | -8.9 | -19.8 | -18.3 |
| Asparagine | -5.1 | -5.4 | -4.6 | -5.2 | -10.5 | -9.8 |
| Proline | -5.2 | -5.3 | -5.0 | -5.4 | -10.5 | -10.4 |
| Citrulline | -5.7 | -5.8 | -5.1 | -5.7 | -11.5 | -10.8 |
| Gaultherin | -9.6 | -9.0 | -7.8 | -8.1 | -18.6 | -15.9 |
| Apiopaeonoside | -9.7 | -9.3 | -8.5 | -8.2 | -19.0 | -16.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





