Submitted:
12 August 2024
Posted:
12 August 2024
You are already at the latest version
Abstract
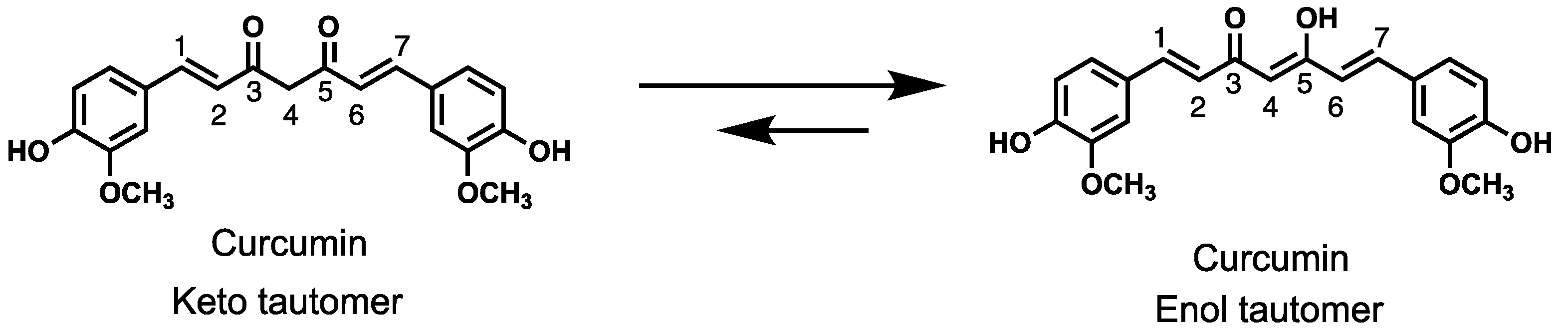
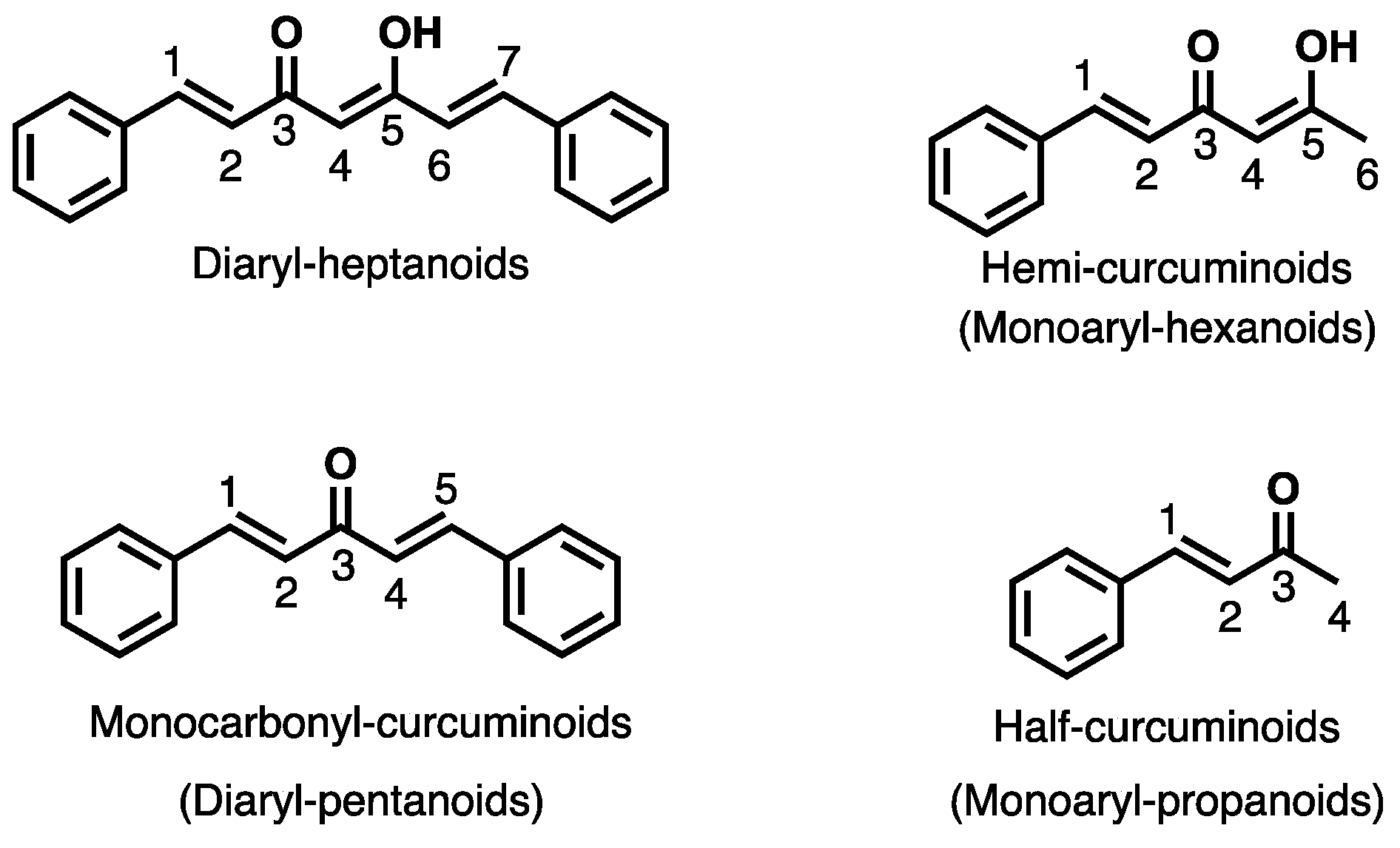
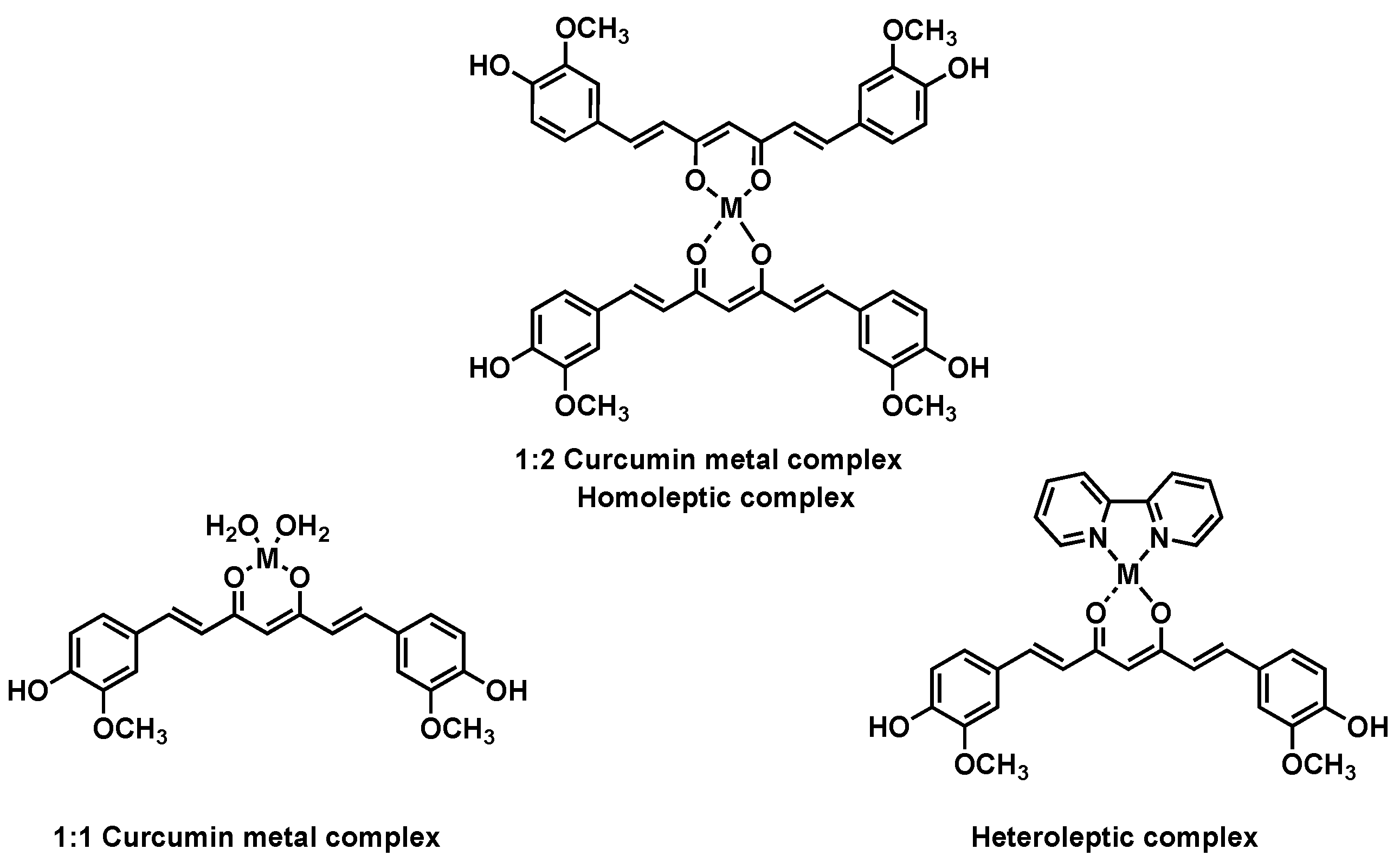
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Du, Z.; Xue, G.; Chen, Q.; Lu, Y.; Zheng, X.; Conney, A.H.; Zhang, K. Synthesis and Biological Evaluation of Unsymmetrical Curcumin Analogues as Tyrosinase Inhibitors. Molecules 2013, 18, 3948–3961. [CrossRef]
- Saladini, M.; Lazzari, S.; Pignedoli, F.; Rosa, R.; Spagnolo, F.; Ferrari, E. New Synthetic Glucosyl-Curcuminoids, and Their 1H and 13C NMR Characterization, from Curcuma Longa L. Plant Foods for Human Nutrition 2009, 64, 224–229. [CrossRef]
- Torres-Rodríguez, E.; Arias-Cedeño, Q.; Almeida-Saavedra, M.; Michalik-Michalik, M.; Vogel-Vogel, C. Study of the Keto-Enolic Equilibrium in Structures of Synthetic Curcuminoids by Means of RMN and X Rays Diffraction. Revista Cubana de Química 2013, XXV, 206.
- Haritakun, W.; Changtam, C. Cytotoxic Activity of Curcuminoids and Curcuminoid Analogues Against Human Oral Cancer KB Cells. SDU Res. J 2016, 9, 141–158.
- Deepthi, T. V.; Venugopalan, P. Synthesis, DNA-Binding, and Cytotoxic Studies on Three Copper(II) Complexes of Unsymmetrical Synthetic Analogues of Curcumin. J Coord Chem 2016, 69, 3403–3416. [CrossRef]
- Singh, R.; Tønnesen, H.H.; Vogensen, S.B.; Loftsson, T.; Másson, M. Studies of Curcumin and Curcuminoids. XXXVI. The Stoichiometry and Complexation Constants of Cyclodextrin Complexes as Determined by the Phase-Solubility Method and UV-Vis Titration. J Incl Phenom Macrocycl Chem 2010, 66, 335–348. [CrossRef]
- Cornago, P.; Cabildo, P.; Sanz, D.; Claramunt, R.M.; Torralba, M.C.; Torres, M.R.; Elguero, J. Structures of Hemi-Curcuminoids in the Solid State and in Solution. European J Org Chem 2013, 6043–6054. [CrossRef]
- Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules 2015, 20, 249–292.
- Dong, L.; Zheng, S.; Zhang, Y.; Jiang, X.; Wu, J.; Zhang, X.; Shan, X.; Liang, D.; Ying, S.; Feng, J.; et al. Design, Synthesis, and Evaluation of Semi-Conservative Mono-Carbonyl Analogs of Curcumin as Anti-Inflammatory Agents against Lipopolysaccharide-Induced Acute Lung Injury. Medchemcomm 2015, 6, 1544–1553. [CrossRef]
- Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Chávez, M.I.; Toscano, R.A.; Cassani, J.; Enríquez, R.G. Expected and Unexpected Products in Half Curcuminoid Synthesis: Crystal Structures of but-3-En-2-Ones and 3-Methylcyclohex-2-Enones. Crystals (Basel) 2021, 11. [CrossRef]
- Masuda, T.; Matsumura, H.; Oyama, Y.; Takeda, Y.; Jitoe, A.; Kida, A.; Hidaka, K. Synthesis of (+/-)-Cassumunins A and B, New Curcuminoid Antioxidants Having Protective Activity of the Living Cell against Oxidative Damage. J Nat Prod 1998, 61, 609–613.
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal Complexes of Curcumin - Synthetic Strategies, Structures and Medicinal Applications. Chem Soc Rev 2015, 44, 4986–5002. [CrossRef]
- Prasad, S.; Dubourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int J Mol Sci 2021, 22. [CrossRef]
- Mittal, A.; Nagpal, M.; Vashistha, V.K.; Arora, R.; Issar, U. Recent Advances in the Antioxidant Activity of Metal-Curcumin Complexes: A Combined Computational and Experimental Review. Free Radic Res 2024, 58, 11–26. [CrossRef]
- Figueroa-Depaz, Y.; Pérez-Villanueva, J.; Soria-Arteche, O.; Martínez-Otero, D.; Gómez-Vidales, V.; Ortiz-Frade, L.; Ruiz-Azuara, L. Casiopeinas of Third Generations: Synthesis, Characterization, Cytotoxic Activity and Structure–Activity Relationships of Mixed Chelate Compounds with Bioactive Secondary Ligands. Molecules 2022, 27. [CrossRef]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Grapin, M.; Fierascu, R.C.; Raut, I.; Constantin, M. Functionalized Palygorskite as a Delivery Platforms for Bioactive Asymmetric Beta-Diketone Dyes. Crystals (Basel) 2024, 14, 659. [CrossRef]
- Zimnitskiy, N.S.; Korotaev, V.Y.; Barkov, A.Y.; Kochnev, I.A.; Sosnovskikh, V.Y. Hemicurcuminoids (1-Styryl-1,3-Diketones) - Valuable Multi-Faceted Building Blocks for Organic Synthesis. New Journal of Chemistry 2023, 47, 5110–5149.
- Cheng, Y.J.; Li, C.W.; Kuo, C.L.; Shih, T.L.; Chen, J.J. Improved Synthesis of Asymmetric Curcuminoids and Their Assessment as Antioxidants. Molecules 2022, 27. [CrossRef]
- Maywald, M.; Rink, L. Zinc Deficiency and Zinc Supplementation in Allergic Diseases. Biomolecules 2024, 14, 863. [CrossRef]
- Bharti, A.; Bharati, P.; Chaudhari, U.K.; Singh, A.; Kushawaha, S.K.; Singh, N.K.; Bharty, M.K. Syntheses, Crystal Structures and Photoluminescent Properties of New Homoleptic and Heteroleptic Zinc(II) Dithiocarbamato Complexes. Polyhedron 2015, 85, 712–719. [CrossRef]
- Meza-Morales, W.; Mirian Estévez-Carmona, M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-Level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24. [CrossRef]
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Arenaza-Corona, A.; Ramírez-Apan, M.T.; Toscano, R.A.; Poveda-Jaramillo, J.C.; Enríquez, R.G. Three New Coordination Geometries of Homoleptic Zn Complexes of Curcuminoids and Their High Antiproliferative Potential. RSC Adv 2023, 13, 8577–8585. [CrossRef]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Meza-Morales, W.; Escobedo-Martínez, C.; Soriano-García, M.; Enríquez, R.G. Crystal Structure, Synthesis and Biological Activity of Ether and Ester Trans-Ferulic Acid Derivatives. Int J Org Chem (Irvine) 2018, 8, 359–377. [CrossRef]
- Mestrelab Research. MNova Software. Available Online Https://Mestrelab.Com/Download/Mnova/ (Accesed on March, 10, 2024).
- Obregón-Mendoza, M.A.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Estévez-Carmona, M.M.; Enríquez, R.G. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules 2023, 28. [CrossRef]
- Arenaza-Corona, A.; Obregón-Mendoza, M.A.; Meza-Morales, W.; Ramírez-Apan, M.T.; Nieto-Camacho, A.; Toscano, R.A.; Pérez-González, L.L.; Sánchez-Obregón, R.; Enríquez, R.G. The Homoleptic Curcumin–Copper Single Crystal (ML2): A Long Awaited Breakthrough in the Field of Curcumin Metal Complexes. Molecules 2023, 28. [CrossRef]
- Rigaku Oxford Diffraction: Yarnton, U. CrysAlisPRO Software System 2024.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr A 2008, 64, 112–122. [CrossRef]
- Sheldrick, G.M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr A 2015, 71, 3–8. [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J Appl Crystallogr 2020, 53, 226–235. [CrossRef]
- Spek, A.L. Single-Crystal Structure Validation with the Program PLATON. J. Appl. Cryst 2003, 36, 7–13.
- Coffin, A.; Ready, J.M. Selective Synthesis of (+)-Dysoline. Org Lett 2019, 21, 648–651. [CrossRef]
- Zawadiak, J.; Mrzyczek, M. Correlation of Substituted Aromatic β-Diketones’ Characteristic Protons Chemical Shifts with Hammett Substituent Constants. Magnetic Resonance in Chemistry 2013, 51, 689–694. [CrossRef]
- Li, W.; Wang, S.; Feng, J.; Xiao, Y.; Xue, X.; Zhang, H.; Wang, Y.; Liang, X. Structure Elucidation and NMR Assignments for Curcuminoids from the Rhizomes of Curcuma Longa. Magnetic Resonance in Chemistry 2009, 47, 902–908. [CrossRef]
- Jȩdrzkiewicz, D.; Marszałek-Harych, A.; Ejfler, J. Serendipitous Synthesis Found in the Nuances of Homoleptic Zinc Complex Formation. Inorg Chem 2018, 57, 8169–8180. [CrossRef]
- Rachwalski, M. Special Issue: Asymmetry and Symmetry in Organic Chemistry. Symmetry (Basel) 2023, 15.
- Wei, X.; Yang, Y.; Ge, J.; Lin, X.; Liu, D.; Wang, S.; Zhang, J.; Zhou, G.; Li, S. Synthesis, Characterization, DNA/BSA Interactions and in Vitro Cytotoxicity Study of Palladium(II) Complexes of Hispolon Derivatives. J Inorg Biochem 2020, 202. [CrossRef]
- Caruso, F.; Subbaraju, G. V.; Ramani, M. V.; Gariboldi, M.; Marras, E.; Kloer, C.; Sulovari, A.; Kaur, S.; Rossi, M. Synthesis, X-Ray Diffraction and Anti-Proliferative Biological Activity of Hispolon Derivatives and Their (H6-p-Cymene)(Hispolonato)Ruthenium[II] Chloride Complexes. Inorganica Chim Acta 2022, 542. [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr B Struct Sci Cryst Eng Mater 2016, 72, 171–179. [CrossRef]
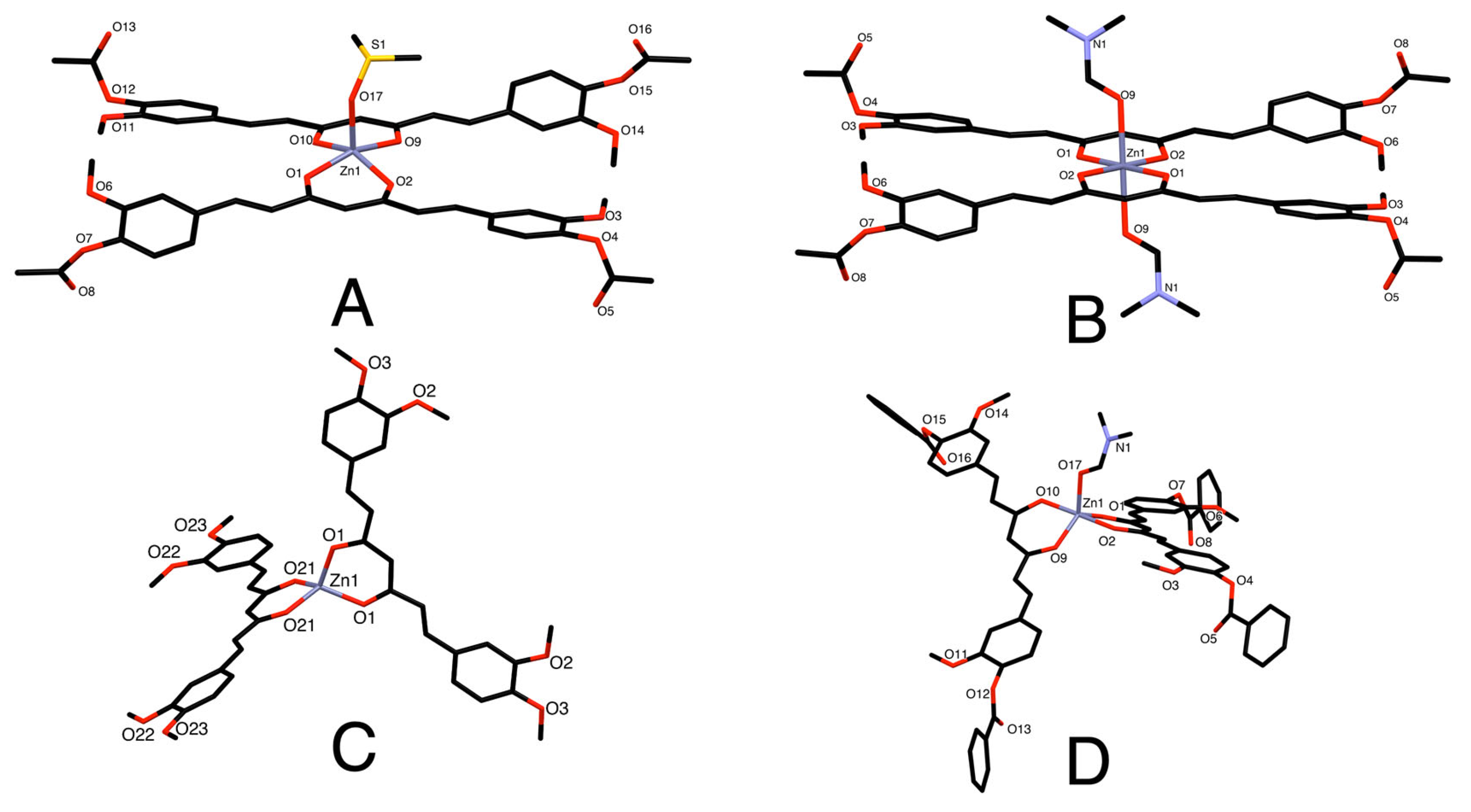
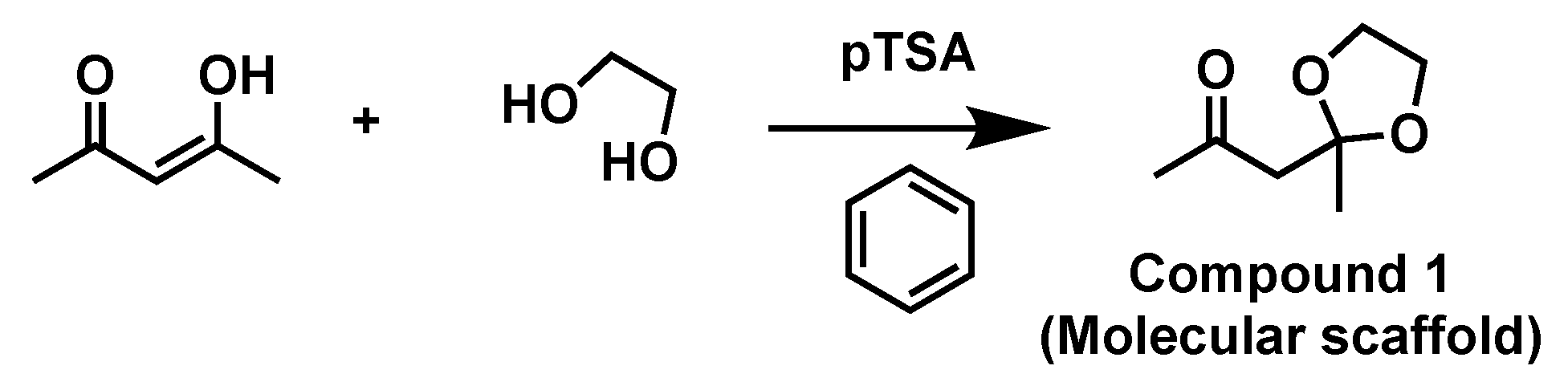
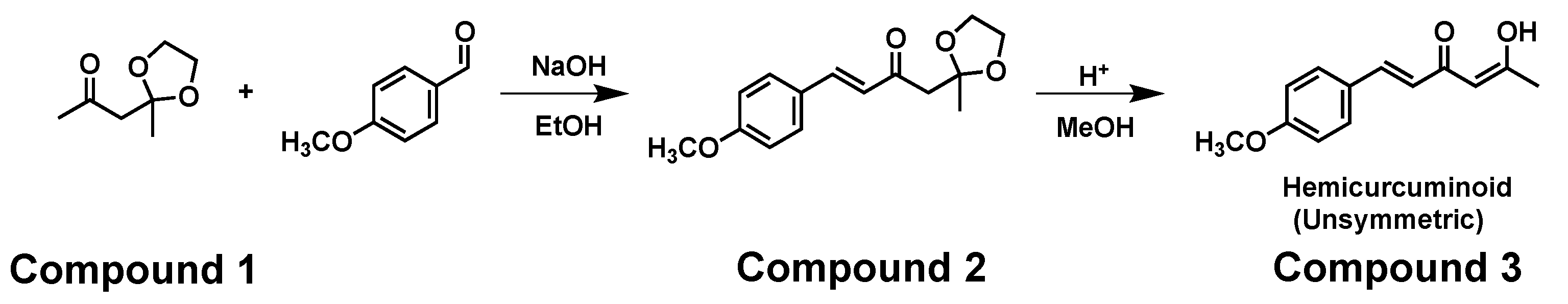
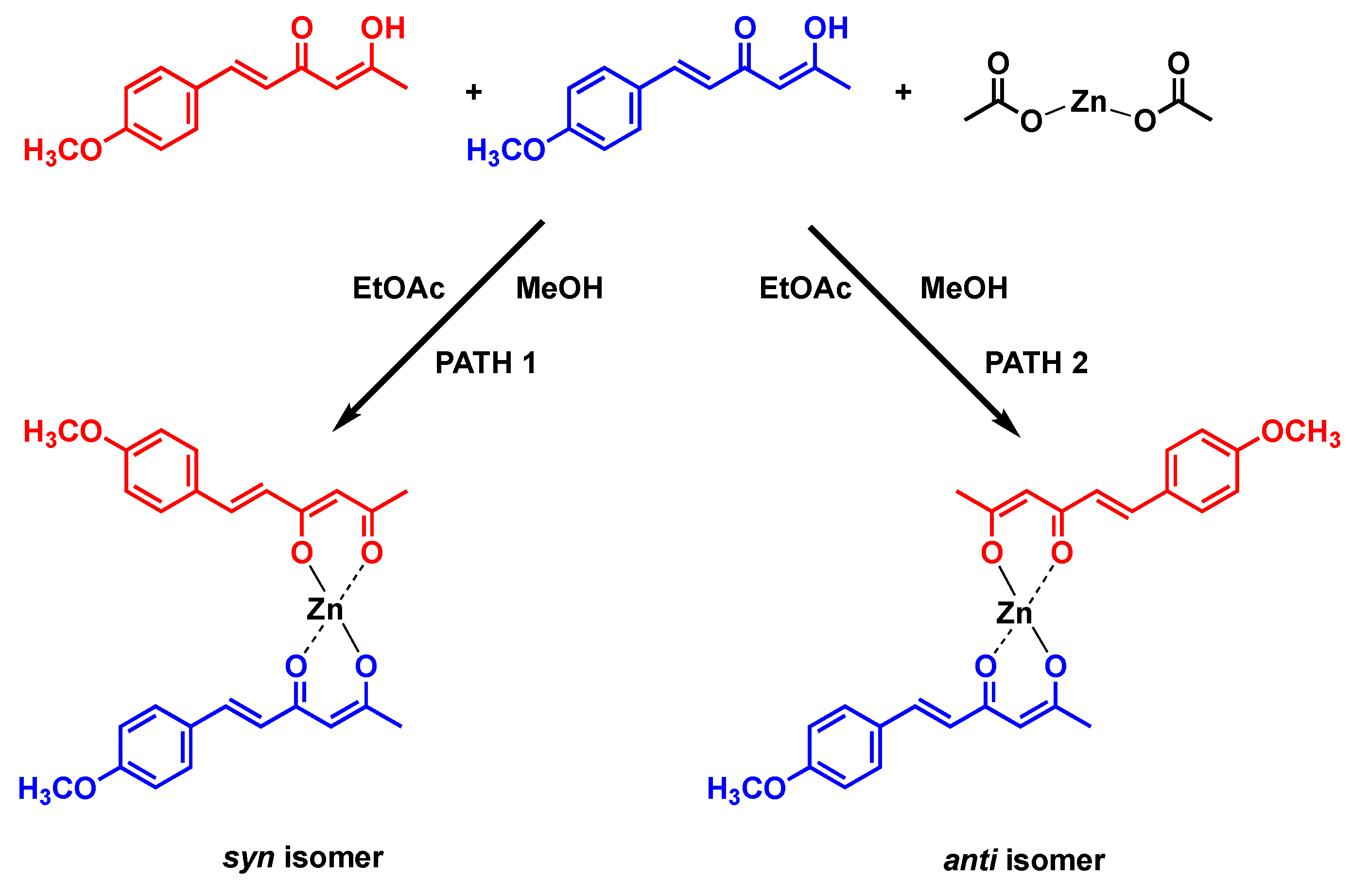
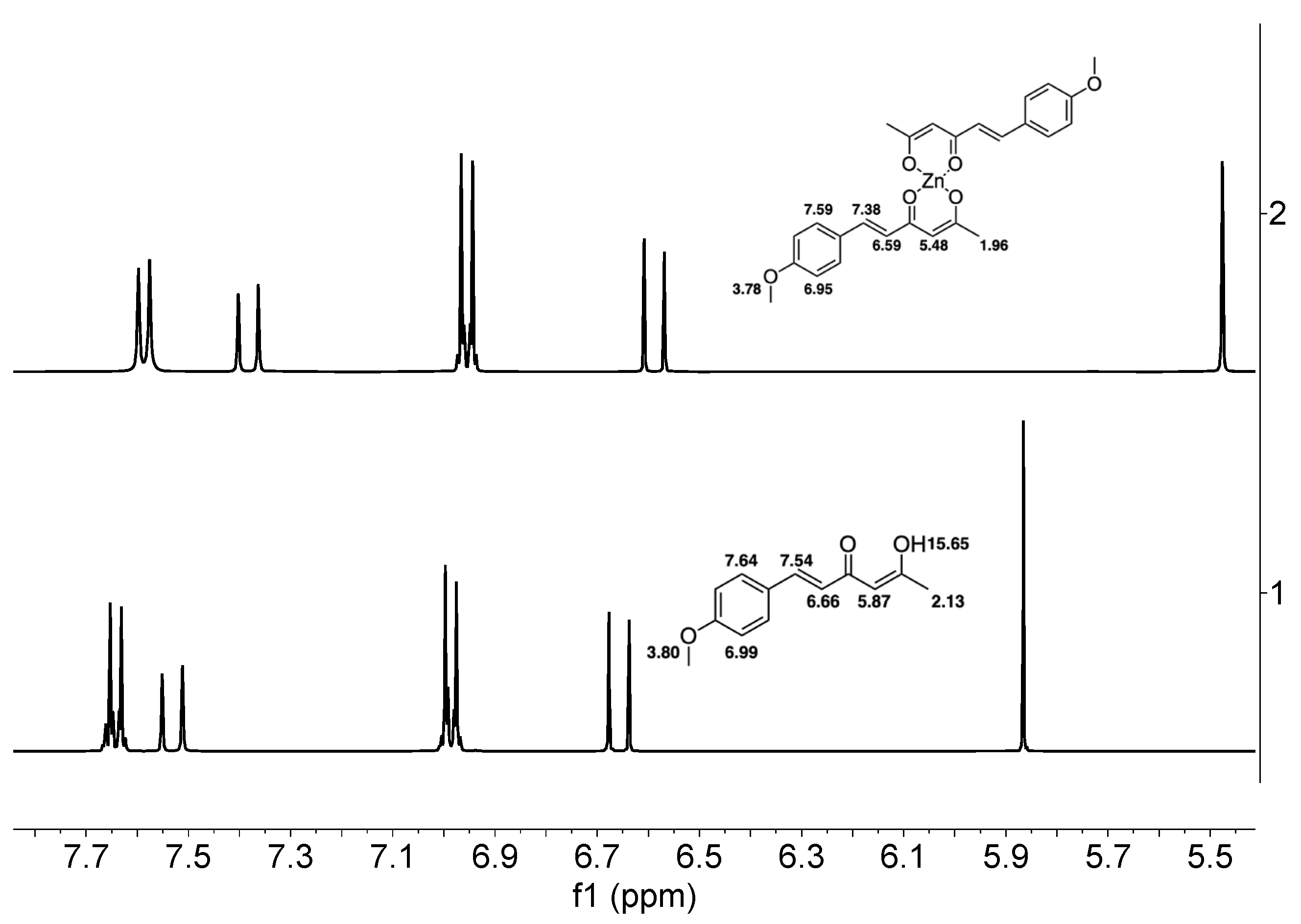
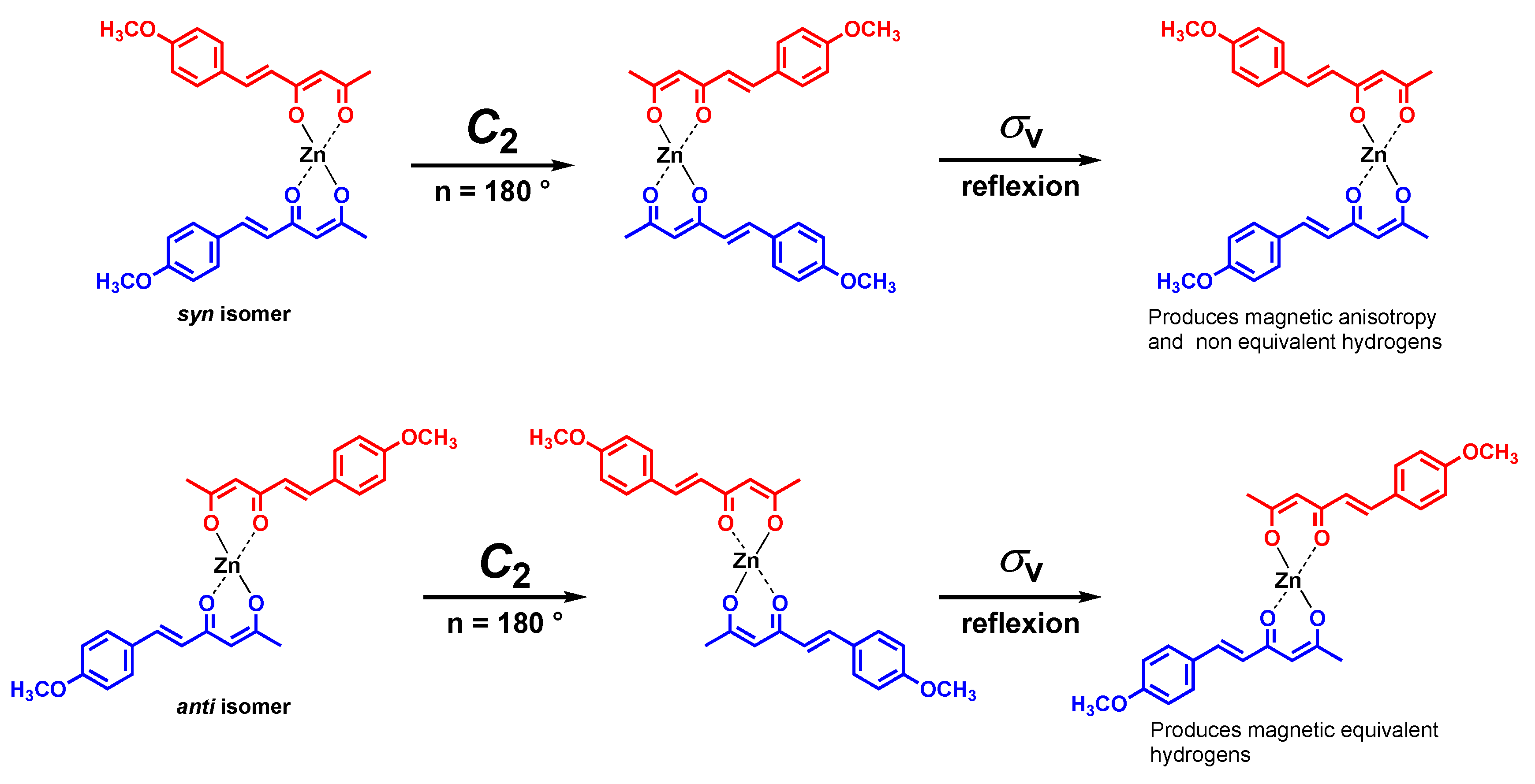
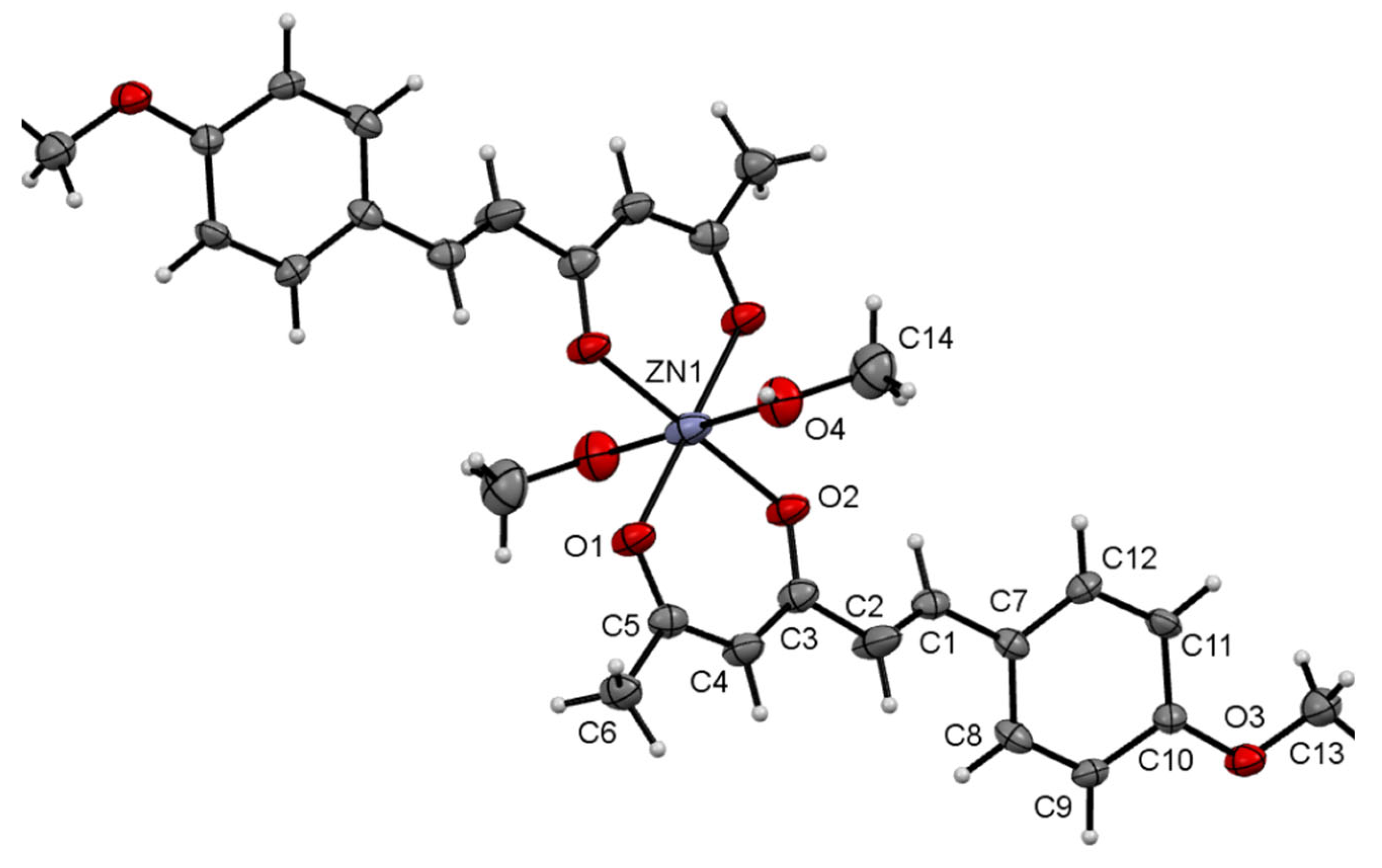
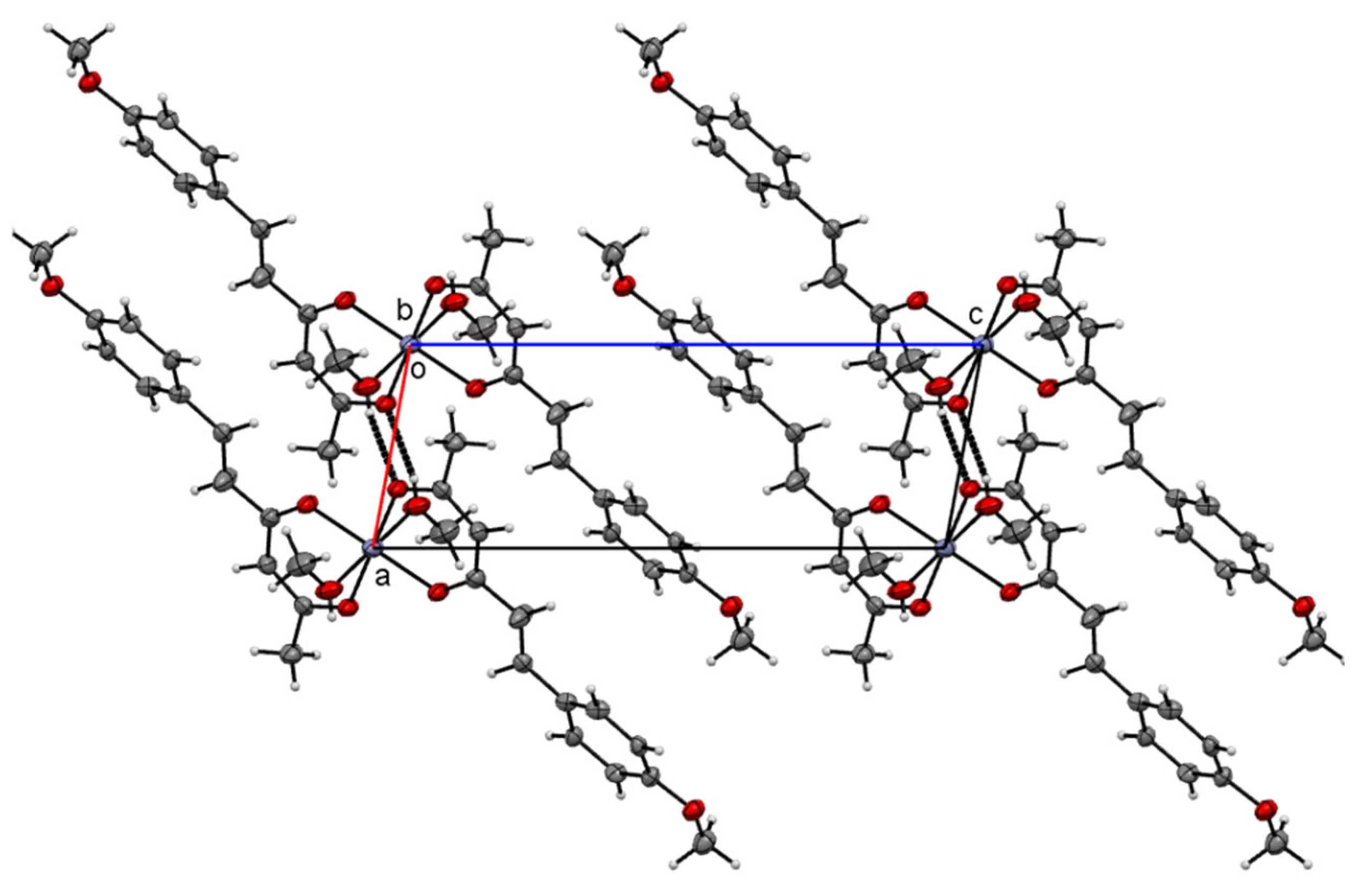
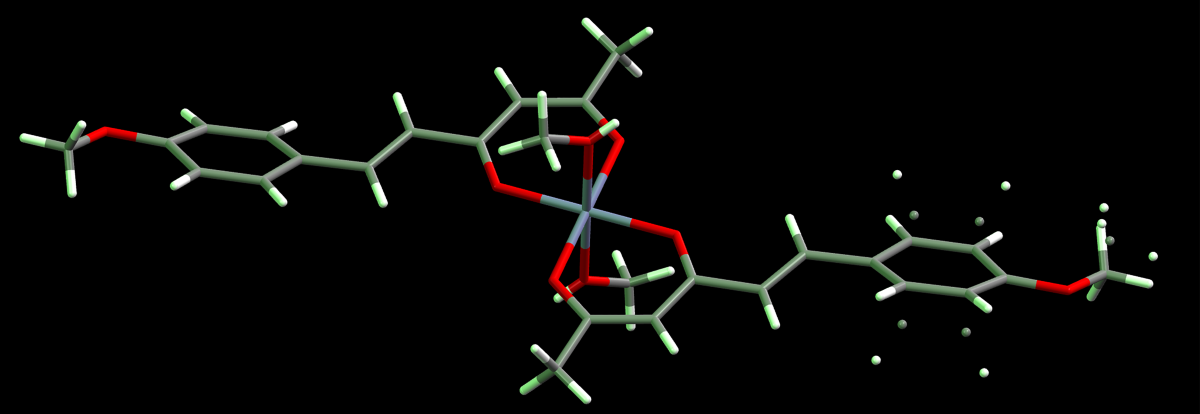
| Hydrogen | Compound 3, δ = ppm 1 | Compound 4, δ = ppm1 |
|---|---|---|
| -CH3 | 2.13 (singlet) | 1.96 (singlet) |
| =C-H (α) | 6.66 (doublet) | 6.59 (doublet) |
| =C-H (β) | 7.54 (doublet) | 7.38 (doublet) |
| -CH (Methine) | 5.87 (singlet) | 5.48 (singlet) |
| Ar-H (AA´) | 6.99 (multiplet) | 6.95 (multiplet) |
| Ar-H (BB´) | 7.64 (multiplet) | 7.59 (multiplet) |
| -O-CH3 | 3.80 (singlet) | 3.78 (singlet) |
| -OH (enol) | 15.65 (broad) | - |
| -OOC-CH3 (acetate) | - | 2.08 (singlet) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





