1. Introduction
Mould is small organism which occur on many types of surfaces and are widely disseminated through spores. The mould is important in biochemical cycle of elements, the movement and transformation of chemical elements and compounds between living organisms and it enable decomposition of materials and therefor is necessary in live. For human, they can cause several health problems, especially for immune deficiency ones [1–5]. The most dangerous mould for human health is species as Aspergillus spp. or Stachybotris spp. [6]. In buildings, they can deteriorate interior finishing and opened finishing for other deterioration agents. Both effect, deterioration of finishing and human health problem, can cause economic and social damage.
Because of the mentioned, knowledge of probability of occurrence of mould in building is very important. The mould requires for its life available nutrients and humidity, the same as a sufficient pH [7–8]. The amount of humidity required for germination and subsequent development of mycelium and dissemination of mould vary depends upon species [9]. The amount of humidity on finishing materials dependent on ratio of environmental humidity and temperature. Generally, the building materials in condition below 80% relative humidity (RH) do not support mould growth [10].
In order to assess the parameters that contribute to mould growth on wood, it is necessary to consider the effect of temperature and RH. To accurately simulate the conditions that occur in real building conditions where mould growth occurs, it is necessary to employ a dynamic prediction model of mould growth that includes changing conditions over time. Furthermore, it is crucial to consider the specific species of wood, the portion of the tree from which the samples were obtained, and the direction of the cut [11–14]. Furthermore, the species of wood, the part of the tree, and the method of cutting the sample are important information [15–16].
The more important for mould germination is moisture of substrate rather environment RH [17]. In general, the higher humidity is required for germination then for subsequence growth [18]. The minimum humidity requirements to growth are dependence on species, that divide to three groups. Hydrophilic fungi require relative humidity above 90 %, moderately xerophilic fungi begin to grow at RH 75 -79 % and slightly xerophilic fungi need RH in range 80 -89 % [19–21]. For example, Aspergillus spp., Penicillium spp. and Alternaria spp. begin germinated around 85 % RH [22–25]. These moisture range for mould growth were calculated from the experiments based on laboratory experiments on nutrients agar, where the nutrient condition is optimal [25–27]. For building materials, where nutrient availability is not optimal but rather limited, the requirement for available moisture is probably slightly higher then mentioned above [28–29]. Moisture requirements are also related to temperature; at lower temperatures, the mould requires more available humidity to germinate and grow [14, 30]. The growth of mould is also dependent on temperature, even when other conditions remain constant [14, 31]. A reduction in temperature from 20°C to 5°C has been observed to result in a fourfold reduction in the rate of mould growth. [14] The other requirements for mould grow are nutrients such as source of carbon, nitrogen and so others. Especially when glucose is available as a carbon source, germination is faster [32].
The majority of published results pertaining to mould growth are focused on pine wood [11, 15, 33 – 34]. Under constant conditions (approximately 90% relative humidity and 22°C, on pine sapwood), initial mould growth is observed around day 7, macroscopic mould growth is visible between days 10 and 22, which depends on surface preparation [8, 33]. The length of time required for germination is influenced by environmental conditions and the duration of unfavourable conditions. In cases where temperature and RH fluctuate, prolonging the duration of unfavourable conditions has been associated with an increase in the time required for germination to occur. A weekly RH cycle (90/60% RH, 22 °C) is only suitable for the germination and formation of a limited number of hyphae. A 12-hour cycle of fluctuating relative humidity (90/60 % RH, 22 °C) ultimately results in a change in germination time over a six-week period. Following germination, however, the fungal growth curve remained identical to that observed under constant conditions. A weekly temperature cycle (90% RH, 5/22°C) resulted in a prolongation of the germination period (approximately 49 days) and a slowing down of fungal regrowth [31].
The impact of fluctuating conditions on the growth of mould on wood are presented in this article examines. In alignment with the findings of the literature review, this study assesses mould growth from a multifaceted perspective, extending beyond the conventional parameters of temperature and humidity fluctuations. It considers the influence of wood species and wood type (pine and spruce, heartwood and sapwood), as well as the direction of the cut used to obtain samples (tangential, radial, and transverse).
2. Materials and Methods
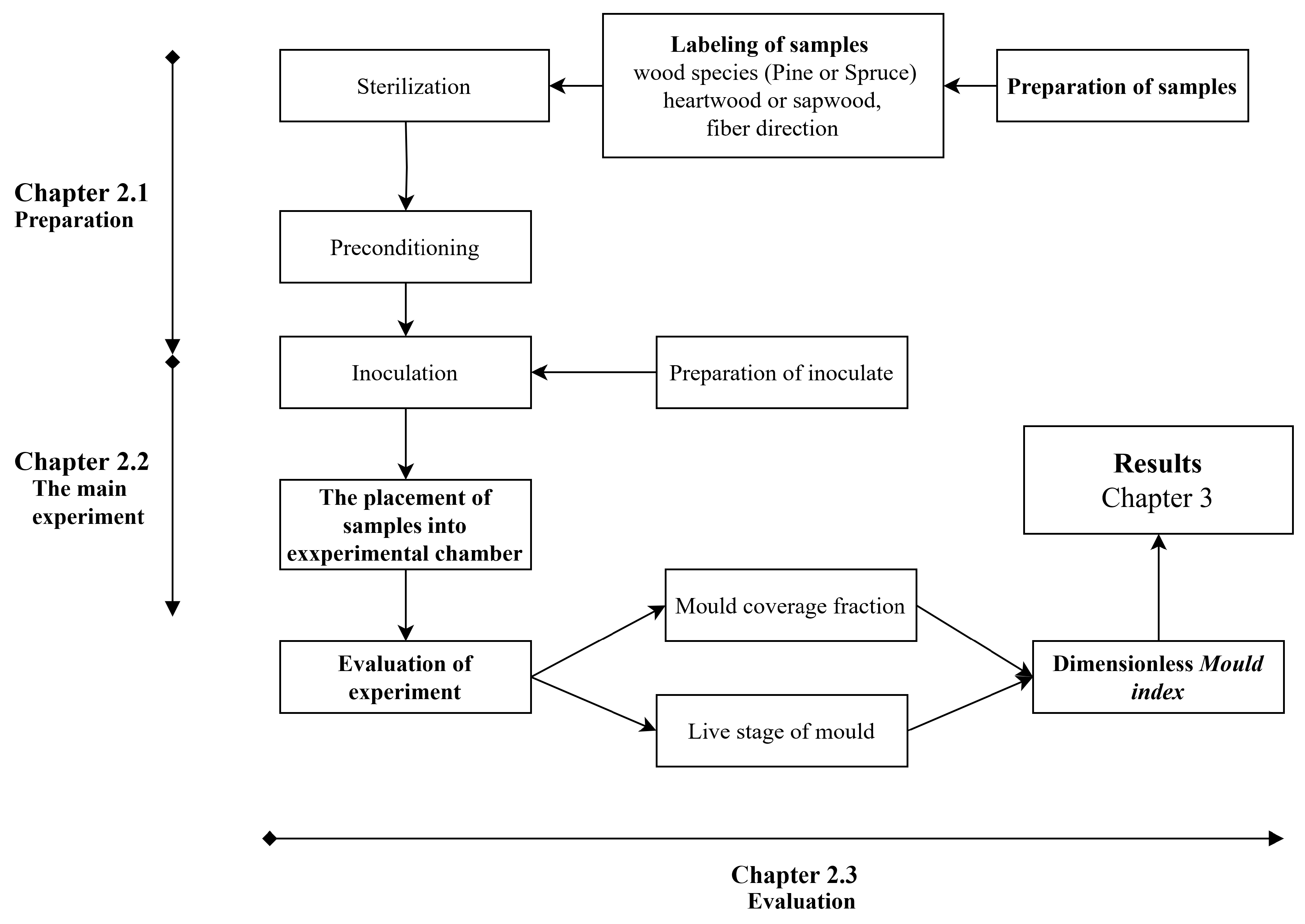
This paper presents the results of a laboratory experiment on pine and spruce samples of sapwood, which were made from heartwood with respect to fiber directions. The samples were exposed to varying humidity and temperature in 18/6-hour cycles. The temperature varied between 25°C and 15°C, while the humidity varied between 98% and 73%, with the higher condition present for 18 hours. Mould growth was observed weekly using a 10x magnification microscope and the naked eye. The live phase of the mould was also recorded, including germination, hyphae, sporulation, and decay. The more important stage was recorded by images. This research is notable for its use of samples with respect to the direction of wood fibers. The assessment of mould growth was conducted based on evaluations of mould coverage of samples and life phase of mould. The primary findings are presented in the form of a time-dependent relationship between the mould index and the mould growth. The mould index is described in detail, and typical images are provided for enhanced comprehension. The experiment was conducted in several steps, see
Figure 1. The initial step of the experiment, see Paragraph 2.1, involved sample preparation, which included their sterilization and humidity conditioning. Additionally, a spore suspension was prepared for inoculation. The second stage involved conducting the experiment within the experimental chamber described in [35]. The condition is described at Paragraph 2.2. The last step, see Paragraph 2.3 was the assessment of mould growth, which was based on the intermediate evaluations of the individual samples, notes, and photographic records.
2.1. The Initial Phase
2.1.1. Materials and Samples
The wood samples were prepared from spruce (Picea abies (L.) H. Karst.) and pine (Pinus sylvestris L.). The origin boards for preparing samples were four-meter-long boards which were obtained from a commercial lumberyard in 2017. The boards were free of defects, including blue stain mould, knots, or resin. The wood was dried at a temperature below 60 °C. The dry bulk density was 387-454 kg/m3 for spruce and 478-565 kg/m3 for pine. The variation in dry bulk density for the wood samples is expected to be lower due to the similar position on the trunk. The boards were stored under controlled environmental conditions (temperature 20-25°C, relative humidity 50-60%) before preparing the samples.
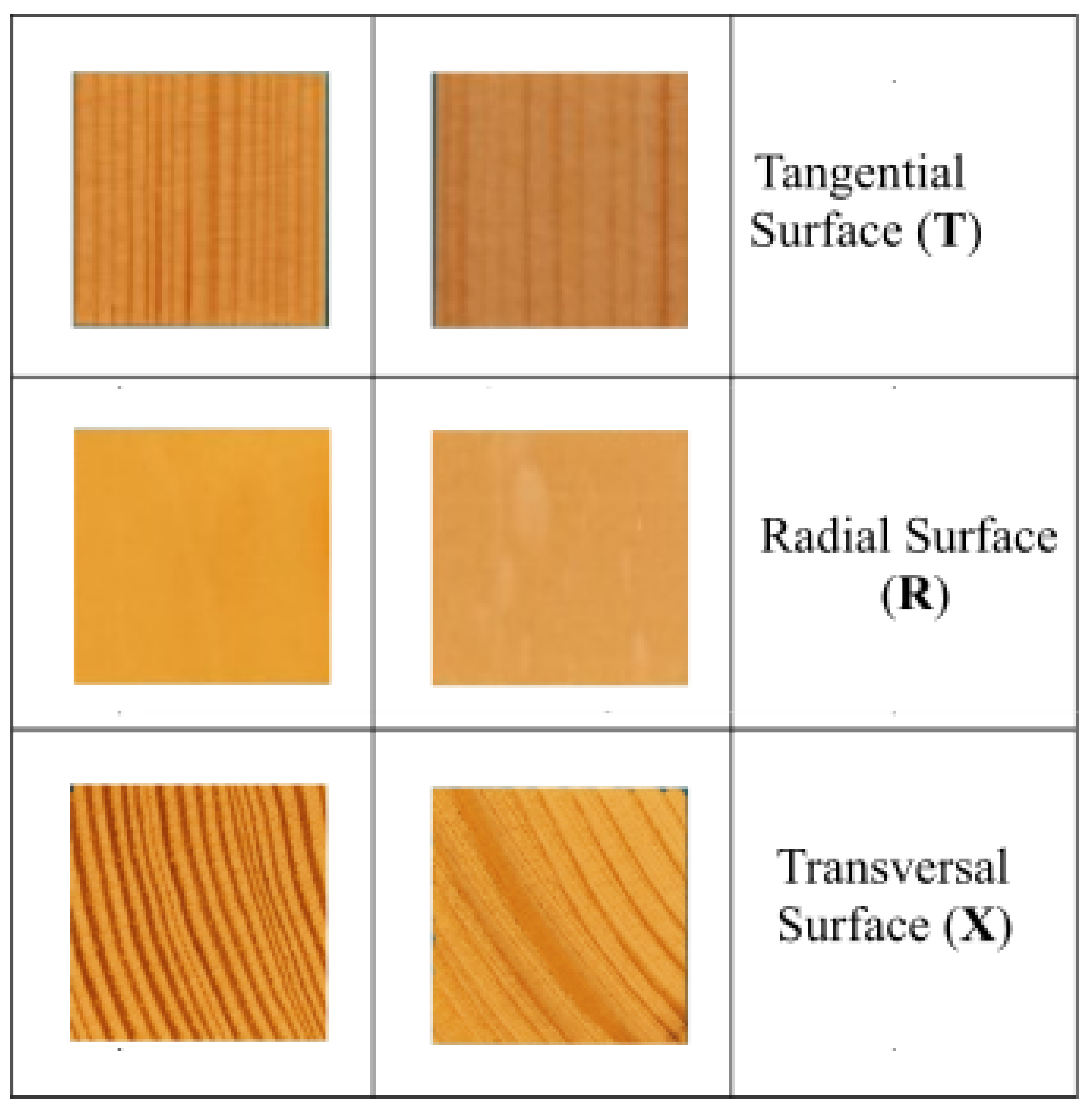
The samples were prepared from sapwood and heartwood portions and oriented according to the anatomical direction of wood fibers. Samples were created in 12 modifications, regarding anatomical species and fiber direction, see
Table 1 and illustrative images at
Figure 2. A particular type of samples were prepared in six repetitions.
The dimensions of the samples were 20 mm × 20 mm × 3 mm. Inoculation and mould growth observations were conducted on the main top surface of each specimen, specifically the 20 mm × 20 mm square.
The surface of the samples was treated with a planer (Makita 2012NB) in order to achieve uniform surface quality, with the exception of the transverse surface. The tangential surfaces of the specimens were composed exclusively of early wood. The samples were subjected to UV radiation for a period of 30 minutes on each side in order to sterilized. Prior to the commencement of the main experiment, the samples were conditioned to achieve equilibrium moisture content with RH of 86% at 22°C of the environmental conditions, see at Graph 1. The mentioned RH was established using saturated salt solutions (Na2SO4). This preconditioning was conducted for a period of seven days.
2.1.2. Preparation of the Inoculate
The experimental inoculum was prepared from mould species commonly found in the Czech Republic and known to occur in the wood built into the construction [12, 36–37]. The selected species were obtained from the Czech Collection of Microorganisms at the Faculty of Science, Masaryk University, Aspergillus niger van Tieghem (CCM 8189), Penicillium cf. brevicompactum Dierckx (CCM 8288) and Alternaria alternata (Fries) von Keissler (CCM F-397), their accession numbers of species are in the brackets.
Before inoculation, the moulds were reinoculated and spores collected as described in [1]. Each spore suspension was adjusted to a concentration of 1 × 106 CFU/ml (Colony formation units per millilitre) and the experimental spore suspension was prepared in a 1 : 1: 0.25 ratio of 1 : 1: 0.25 with the lowest level of Aspergillus sp. present.
2.2. Experimental Conditions during Main Experiment
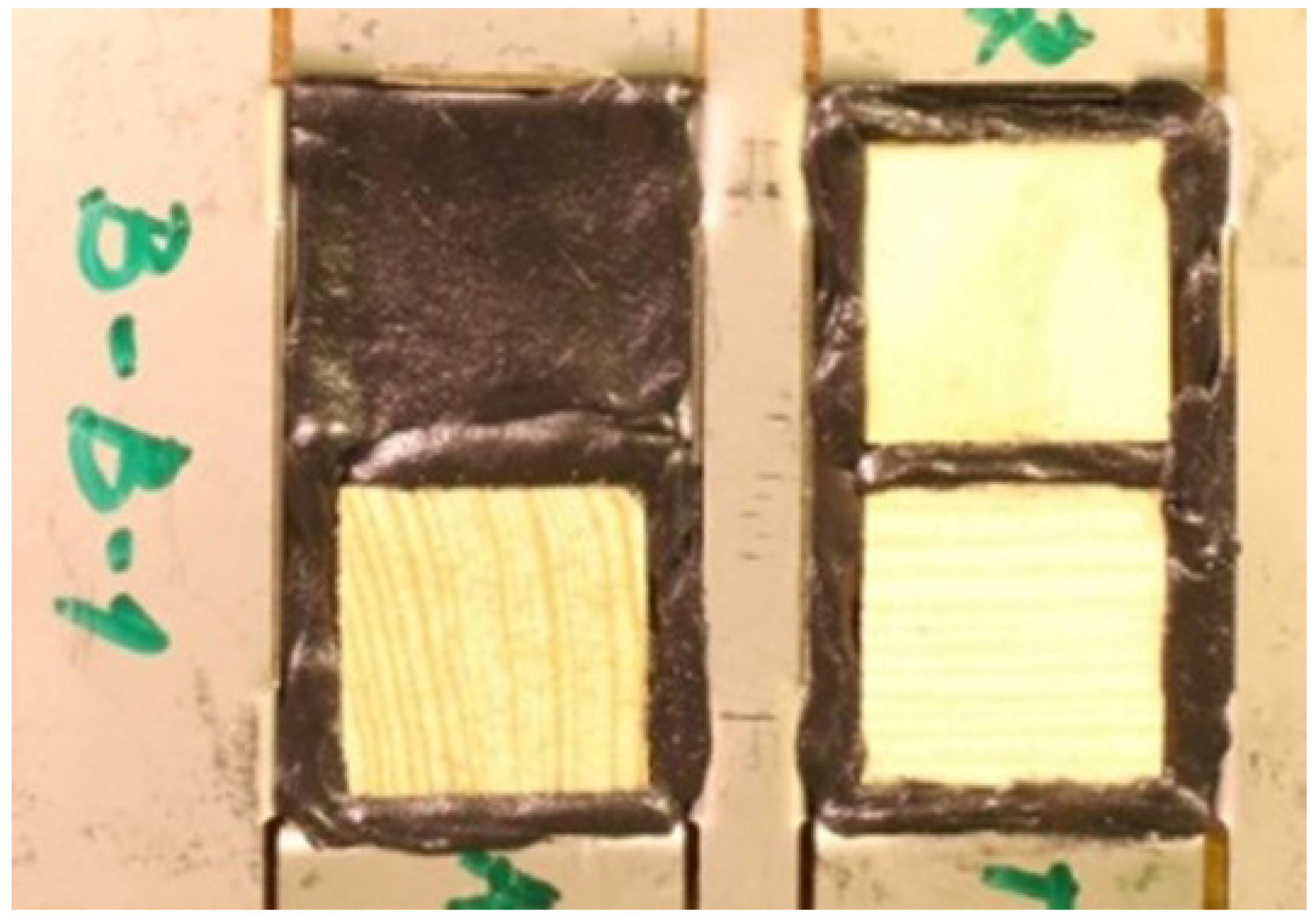
The samples were placed in an aluminium tray to limit the action of RH and T to only one side of the samples (1D transport), as illustrated in
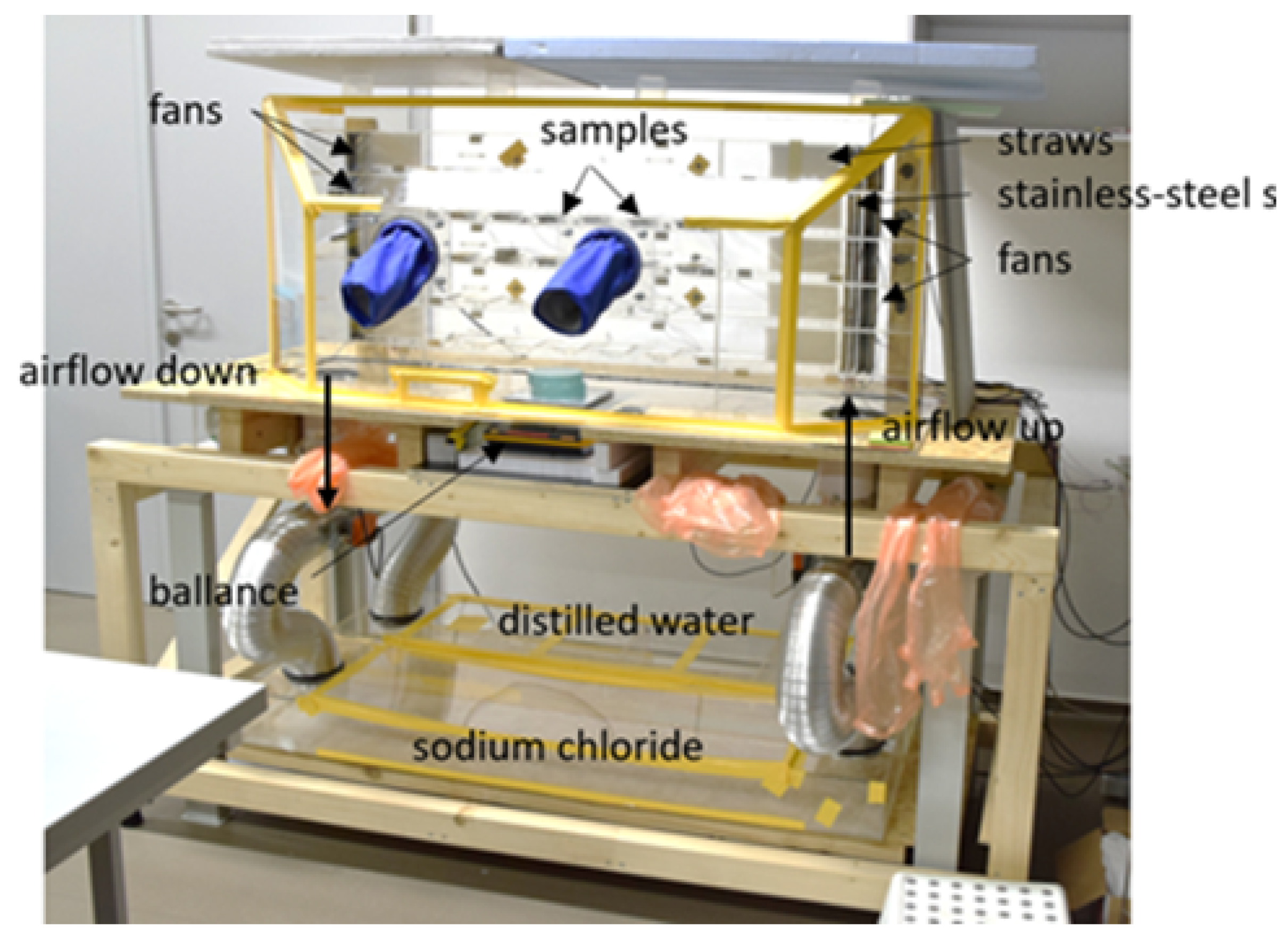
Figure 3. To prevent the transport of temperature and RH through these locations, bituminous sealant was versionied to the sides of the samples. Each tray always contained samples from one species of wood and samples with all directions (T, R, X). A total of six trays were prepared for each kind of wood, see Table 1. The trays were placed in the special box in rows from the top: pine sapwood, pine heartwood, spruce sapwood, and spruce heartwood. The special box (see
Figure 4) was used to create an environment with the desired relative humidity, as described in detail in Richter at al. 2023 [35]. Fans and settling chambers were used to maintain a constant laminar flow of treated air over the samples. The velocity of the airflow was measured at a height of 1.0 cm above the exposed surface of the sample and was found to be 0.3 m/s. The temperature and RH were monitored using COMET dataloggers, which exhibited an accuracy of ±0.3 °C and ±2.5% RH, respectively.
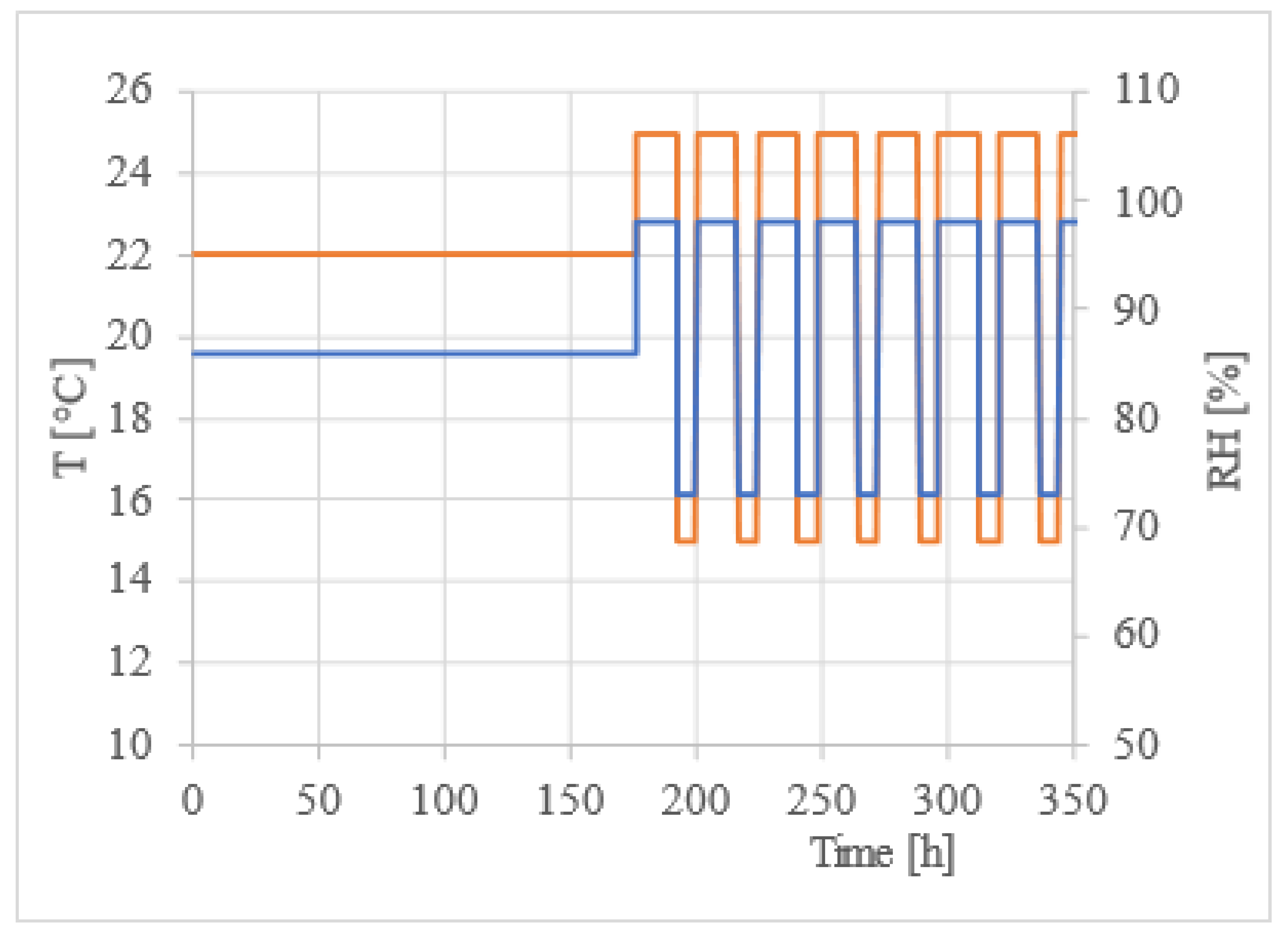
The experimental conditions were set up as follows: 98% RH at 25 °C for 16 hours and 73% RH at 15 °C for 8 hours, see
Figure 5. To maintain lower relative humidity, a saturated sodium chloride (NaCl) solution was employed, while distilled water was utilized to maintain higher relative humidity. The main experiment was conducted over a period of five months, the conditions are shown in the graph at
Figure 5.
2.3. Evaluation and Base of Assessments
Mould growth was observed using microscopic (BA 410E Epi microscope, Motic) and macroscopic methods. Microscopic images were taken with a Promicam Pro 3-3CP camera attached to the microscope and macroscopic images were taken by photographic camera. The observation had 40 – 100 x magnification. Observations were made every three days at the beginning of the experiment. During the middle phase of the experiment, the interval was extended to seven days, and at the end of the experiment it was extended to 14 days.
The experiment was carried out over five months. Both quantitative and qualitative indicators describing mould growth were recorded and used to determine the Mould Index (MI), see Table 3. The scale of MI was developed based on the work of Johansson [3] and subsequently augmented by the addition of the mould subindex (MSI), which provides a more accurate description. The MI and MIS are determined by the extent of visible coverage under 10x and 1x magnification, as well as the phase of mould growth.
The microscopic coverage of the surface was estimated through microscopic observations at 40× magnification, while the phase of mould growth was determined through observations at 100× magnification. Both approaches were utilized to ascertain MI or if it was possible the MIS was determinated. Macroscopic coverage, beginning at MI = 3, was gauged using photographic documentation at 40× and 1× magnifications. Additionally, the presence and condition (visibility) of hyphae and sporangia were documented.
The decision between MI 1 and 1.5 or between 3 and 3.5 is based on the rate of coverage. In general, MI Mould index 0 indicates no growth or germination, while MI 0.5 (germination) was not observed on wood samples. The MI 2 indicates microscopic visible mould growth, whilst the MI 3 indicates macroscopic visible Mould growth. Indexes 3 and 4 include macroscopic mould growth.
Table 2.
The definition of mould index and subsequent mould subindex, MI – mould index and SMI – mould subindex
Table 2.
The definition of mould index and subsequent mould subindex, MI – mould index and SMI – mould subindex
| MI [-] |
SMI [-] |
Magnification |
Description |
| |
|
40 x |
1x |
|
| 0 |
0 |
0 |
0 |
No growth, only spores |
| |
0.5 |
0.1 –1 |
|
Germination of spores |
| 1 |
1 |
1-5 |
1–5 |
Initial growth, first visible hyphae |
| |
1.5 |
5-10 |
|
Initial growth hyphae clearly visible |
| 2 |
2 |
10-30 |
|
Development of mycelium with first aerial hyphae, moulds are almost exclusively visible under a microscope |
| 3 |
3 |
30 –50 |
5–25 |
Development of mycelium with sporangium, Moulds start visible macroscopically |
| |
3.5 |
50 –70 |
25–50 |
Development of mycelium with sporangium, Moulds are visible macroscopically |
| 4 |
4 |
70 –90 |
50–70 |
Decay of mould or huge sporulation |
| |
|
90–100 |
70–100 |
|
Table 3.
Comparison of MI (mould growth rating scale) by several authors, Ryp – the mould index is used in this articles at [8], VTT – mould index defined at [14], Joh – mould index defined at [19], mould coverage of the sample was divided according to the method of observation, with the following divisions: 1x – macroscopically by the naked eye and 40x – microscopically with a magnification of 40x, MI – mould index, SMI – Mould subindex
Table 3.
Comparison of MI (mould growth rating scale) by several authors, Ryp – the mould index is used in this articles at [8], VTT – mould index defined at [14], Joh – mould index defined at [19], mould coverage of the sample was divided according to the method of observation, with the following divisions: 1x – macroscopically by the naked eye and 40x – microscopically with a magnification of 40x, MI – mould index, SMI – Mould subindex
| Magnification |
MI by VTT |
MI by Joh and Ryp |
SMI by Ryp |
| 40 x |
1x |
|
|
|
| 0 |
0 |
0 |
0 |
0 |
| 0.1–1 |
|
1 |
|
0.5 |
| 1-5 |
1–5 |
|
1 |
1 |
| 5-10 |
|
2 |
|
1.5 |
| 10-30 |
|
|
2 |
– |
| 30–50 |
5–25 |
3 |
3 |
3 |
| 50–70 |
25–50 |
4 |
|
3.5 |
| 70– 90 |
50–70 |
5 |
4 |
4 |
| 90–100 |
70–100 |
6 |
|
|
3. Results
Mould growth is evaluated as a time dependence of MI. The samples are limited in three groups. The first group consisted of heartwood samples, irrespective of the tree species. The second group consisted of pine sapwood samples, while the third group included spruce sapwood.
3.1. Pine and Spruce Heartwood
The results shown that heartwood is similar each other’s independence of kind wood and direction of fibbers on top site of samples. A total of 36 heartwood samples were analysed without regard to the botanical species or way the samples were cut. In most cases, mould growth was not observed on the heartwood samples. The mould growth observed on spruce sapwood samples was limited to two samples, with the maximum MI reaching 1.
3.2. Pine – Sapwood
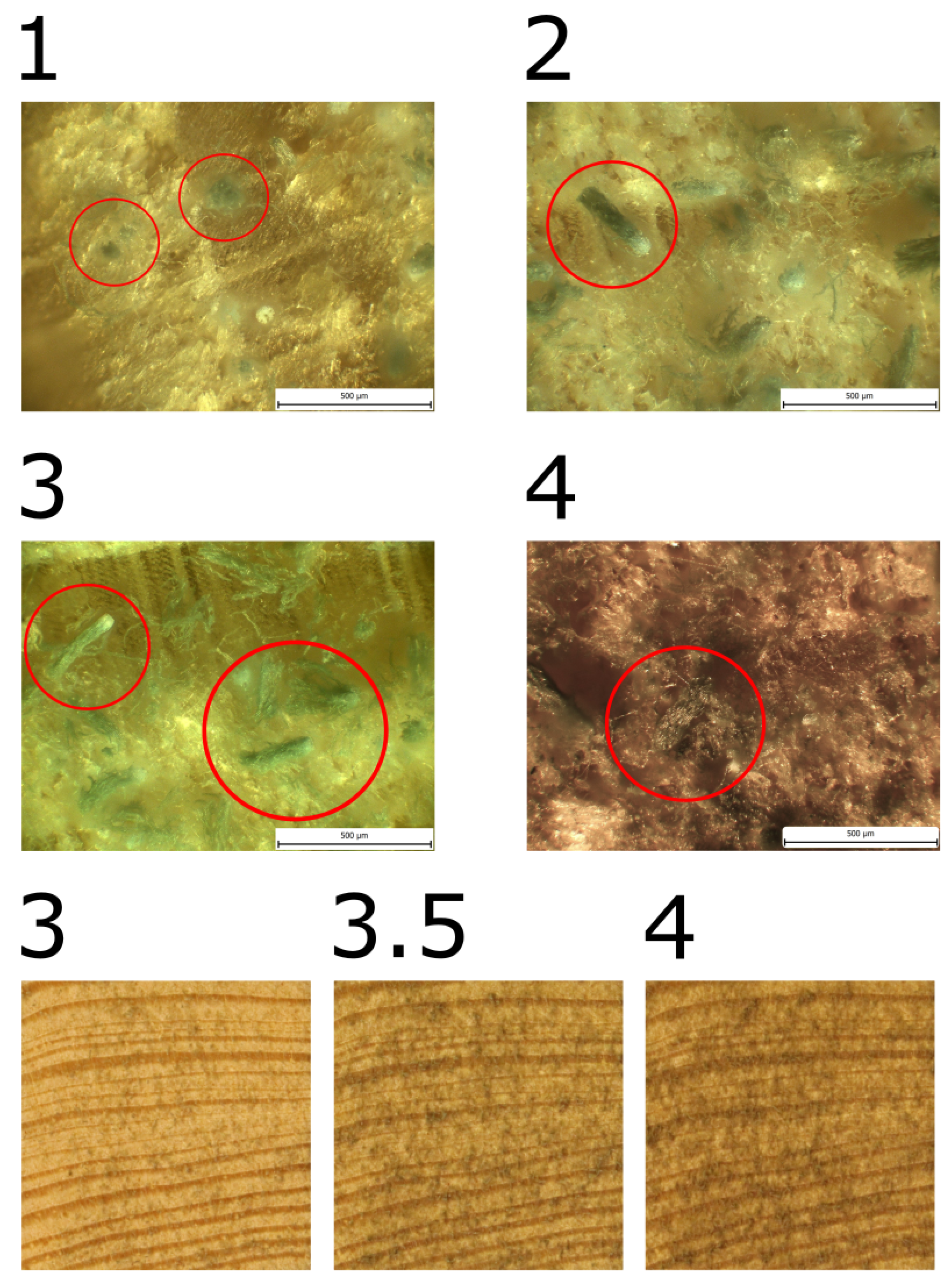
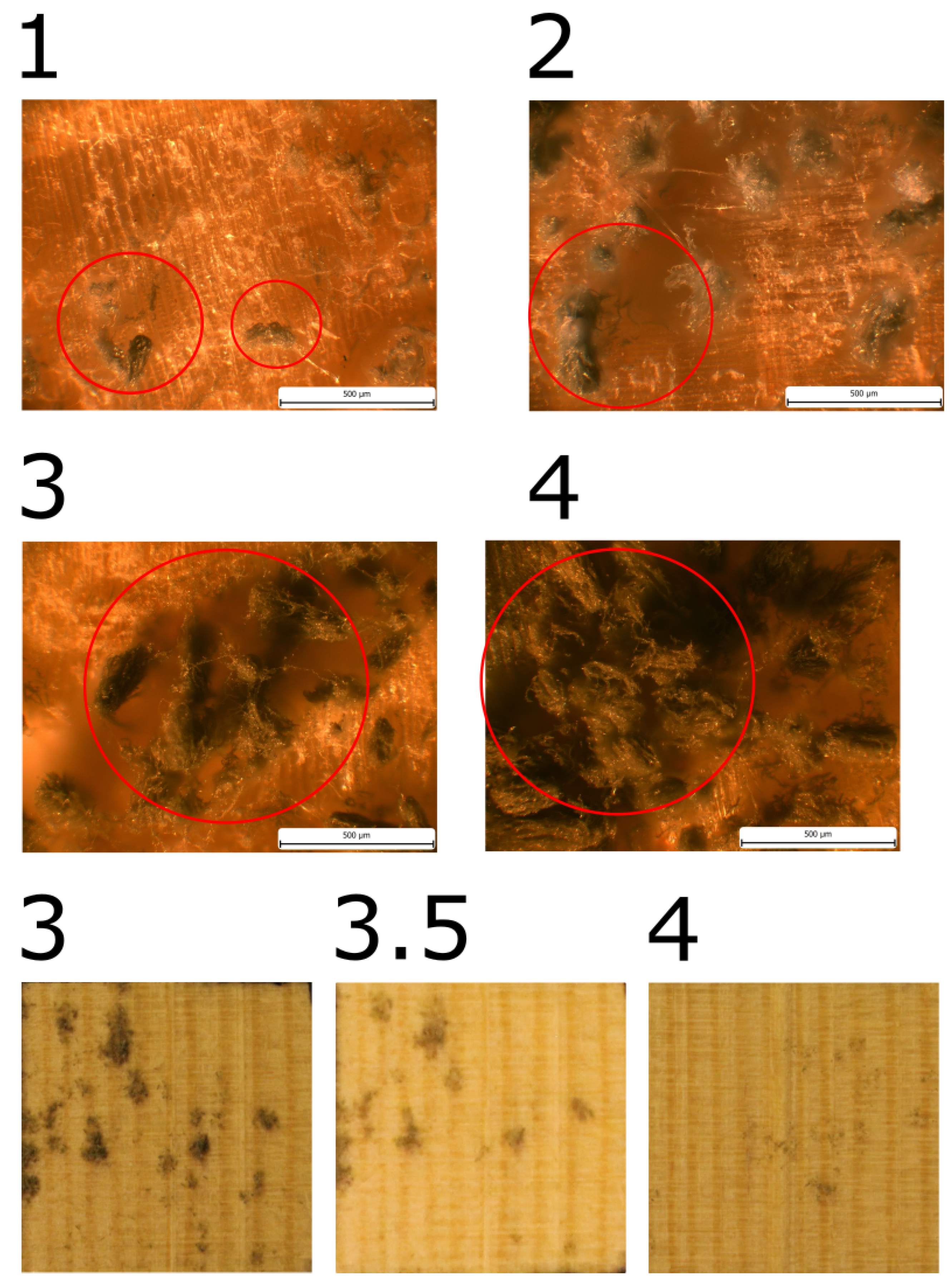
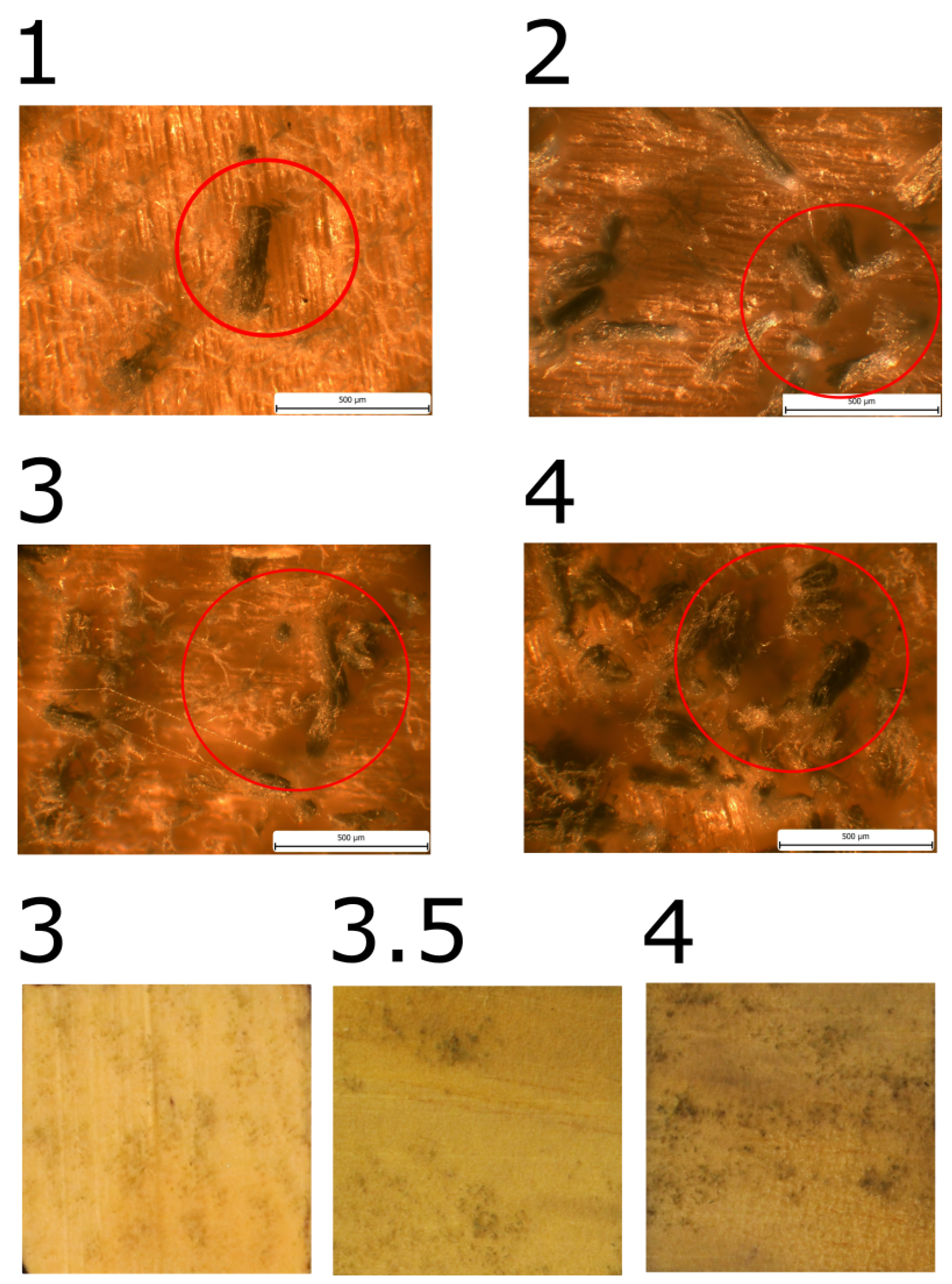
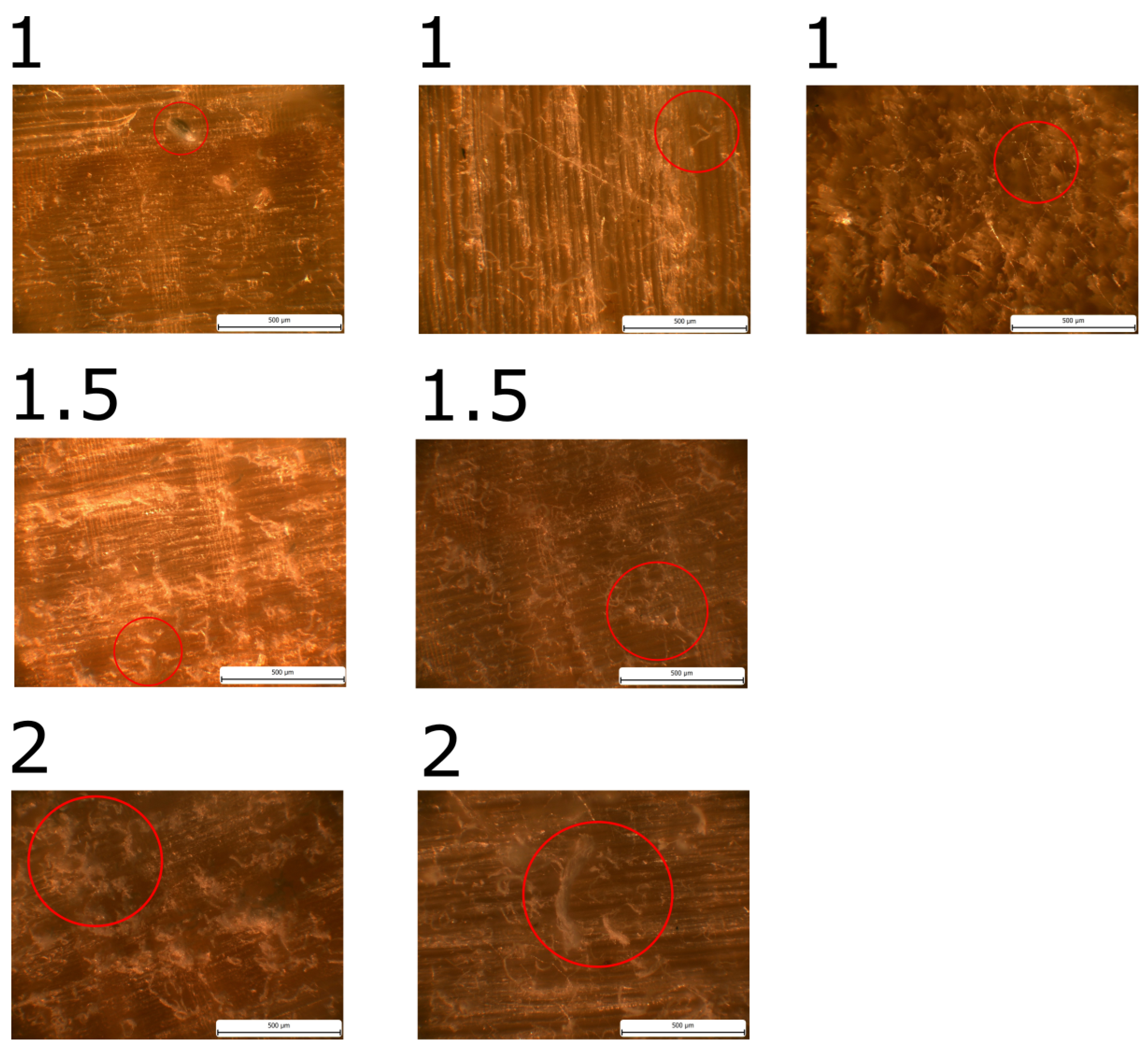
Mould growth was observed in all samples made of pine sapwood. When mould growth was evaluated in terms of wood fibber direction, a single group of three samples exhibited significantly different mould growth and was designated as PSRa, with the index "a" added to the designation.
The initial manifestation of growth in PSRa was observed on the 27th day, yet in general, the growth rate was notably slower than that observed in other samples from pine sapwood. Upon completion of the experiment, the mould coverage reached a maximum of MI = 4.
In the remaining samples, mould growth was comparable, with a maximum of MI = 3.5. The achievement of MI = 3 was noted in the designated row, designated as "P
SR." P
SX (101), P
SR (110), and P
ST (120). The highest mould index (MI) of 4 was observed exclusively in the P
SX samples. Table 4 provides a comprehensive account of the pertinent dates, while
Figure 6,
Figure 7, and
Figure 8 present illustrative images.
Table 4.
Result in range as a Mold index for pine sapwood
Table 4.
Result in range as a Mold index for pine sapwood
Samples
MI [-]/ Time [day] |
PST |
PSX |
PSR |
PSRa |
| 1 |
76.25 |
77.75 |
76 |
27 |
| 1.5 |
– |
– |
79 |
45.7 |
| 2 |
81.3 |
79.5 |
82 |
69 |
| 3 |
90.8 |
92.3 |
89.7 |
79 |
| 3.5 |
120 |
101.5 |
110 |
101 |
| 4 |
– |
103.3 |
– |
143 |
3.3. Spruce – Sapwood
Mould was observed only in S
ST and S
SR samples of the spruce sapwood samples, as shown in Table 6 and
Figure 9. It is worth noting that the growth was only observed microscopically. The growth was slower in the SR samples. No growth was observed in the SX direction samples until the end of the experiment.
Table 5.
Result in range as time of achievement of particular Mold index for spruce sapwood
Table 5.
Result in range as time of achievement of particular Mold index for spruce sapwood
Samples
MI [-]/ Time [day] |
SST |
SSX |
SSR |
| 1 |
70 |
– |
72 |
| 1.5 |
91 |
– |
98 |
| 2 |
110 |
– |
120 |
| 3 |
– |
– |
– |
| 3.5 |
– |
– |
– |
| 4 |
– |
– |
– |
4. Discussion
This study confirms that mould growth on the surface of heartwood has occurred in a negligible number of instances under fluctuating conditions (98/75% RH, 25/15°C in cycles of 16/8 h). The frequency of mould growth on heartwood was found to be only on two samples from 36 regardless of the wood species from which the samples were derived, whether pine or spruce. These results are probably because of low amount of nutrient for mould growth in heartwood part of tree [11, 19, 21, 38], mainly saccharides and source of basic elements as a nitrogen and phosphor. The mould growth on pine sapwood showed that the samples had different properties, because mould grown on four samples differently than rest 12 samples. The three samples were PSR and one PSX, which were more problematic samples for preparing with respect both to direction of fibers and differentiation of sapwood and heartwood. The group are labelled as a PRa and shown similar curve as [15] for pine sapwood at constant RH and fluctuation temperature (RH 90%, 22°C/ 5°C in weekly cycles). The difference was that the maximal MI was 4. The mould growth curve under fluctuation conditions (RH 97% at 25°C for 16 hour/ RH 73% at 15°C for 8 hour) for others pine sapwood samples (PST, PSX, PSR) have shift in 50 days for germination (MI = 1) compare of samples PSRa. Generally, pine sapwood samples have shift in germination and little slower regrowing then same samples made from same trunk are placed in constant condition (RH 95% at 22 °C, RH 87% at 22 °C) [8]. For motioned above it seems that the mould growth under fluctuating condition depends on duration of uncomfortable conditions for growth and amount of shift of conditions from optimal. When the condition is not far from optimal, the rate of growth depends on availability of nutrients. Mold growth is more affected by uncomfortable RH than by uncomfortable temperature. The temperature around 15°C and 73% RH for 8 hours (uncomfortable condition) makes germination slower by seven weeks but doesn’t affect the rate of regrowth. The spruce sapwood shown that there were more effects of fluctuation conditions compared to samples made from same trunk at constants conditions (87% RH and 95% RH at 23°C). Same as for pine sapwood the direction of fibers didn’t show difference. The germination had shift around 3 months and maximal MI was 2. It confirms that nutrient compose of spruce are not suitable for mould growth under fluctuations conditions and the composition of wood is important feather for rate of mould growth.
5. Conclusions
Mould growth in wood under fluctuating conditions (98-73% RH, 18/6 h) depends on the type of wood, such as botanical species (pine or spruce) or the type of wood (heartwood or sapwood). In pine sapwood, mould growth is almost independent of the direction of the fibre. The first microscopic signs of mould growth in pine were observed around day 76th and the first macroscopic signs around day 90th from the beginning of experiment. The spruce sapwood showed nutrient limit for mould growth. Mould growth was only visible microscopically and the first signs were seen between the 72nd and 80th day. The heartwood was not suitable for mould growth under fluctuating conditions. The evaluation of mould growth under fluctuating conditions depends on duration of uncomfortable conditions and for availability of nutrients.
Author Contributions
Conceptualization–P.R.; methodology–P.R.; formal analysis and investigation–P.R.; resources–Jan Richter.; data curation–P.R.; Z.R. writing—original draft preparation–P.R.; review and editing – P.R.; Z.R., visualization–P.R.; project administration–P.R.; funding acquisition–P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CZECH SCIENCE FOUNDATION (GAčR), grant number 20-12941S. The authors are grateful to laboratory technicians Ivana Loušová, Petra Schutová and Jan Richter for their contribution to the project.).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RH |
Relative humidity [-] |
| CFU / ml |
Colony-forming unit per millilitre |
| MI |
Mould index [-] |
| MSI |
Mould subindex [-] |
| spp. |
Linear plural of subspecies |
References
- Platt, S.D.; Martin, C.J.; Hunt, S.M.; Lewis, C.W. Damp housing, mould growth, and symptomatic health state. Br Med J 1989, 298, 1673–1678. [Google Scholar] [CrossRef]
- Lacey, J. Indoor aerobiology and health. Building mycology. 2006https://www.taylorfrancis.com/chapters/edit/10.4324/9780203974735-5/indoor-aerobiology-health-john-lacey.
- Baughman, A.V.; Arens, E.A. Indoor Humidity and Human Health-Part I: Literature Review of Health Effects of Humidity-Influenced Indoor Pollutants. Inddor Enviromental Quality. 1996, 102, 193–211. [Google Scholar]
- Burr, M.L.; Matthews, I.P.; Arthur, R.A.; Thorax, H.L.; Watson, H.L.; Gregory, C.J.; et al. Effects on patients with asthma of eradicating visible indoor mould: a randomised controlled trial. Thorax . 2007.
- Vereecken, E.; Roels, S. Review of mould prediction models and their influence on mould risk evaluation. Build Environ. 2012, 51, 296–310. [Google Scholar] [CrossRef]
- Brambilla, A.; Sangiorgio, A.; Brambilla, A. Mould growth in energy efficient buildings: Causes, health implications and strategies to mitigate the risk. 2020. [CrossRef]
- Adan, O.C.G. On the fungal defacement of interior finishes. 1994; Available from: https://research.tue.nl/en/publications/on-the-fungal-defacement-of-interior-finishes.
- Ryparová, P.; Kopecký, P.; Stanek, K.; Richter, J.; Tywoniak, J. Laboratory Investigations of Mold Growth on Transverse and Longitudinal Wood Surfaces. versionied Sciences 2023, 13, 228. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C. Characterization of Alternaria and Penicillium species from similar substrata based on growth at different temperature, pH and water activity. Syst version Microbiol. 2002, 25, 162–172. [Google Scholar] [CrossRef]
- Grant, C.; Hunter, C.A.; Flannigan, B.; Bravery, A.F. The moisture requirements of moulds isolated from domestic dwellings. International biodeterioration. 1989, 25, 259–284. [Google Scholar] [CrossRef]
- Arango, R.; Yang, V.; Lebow, S.; Lebow, P.; Wiemann, M.; Grejczyk, M.; et al. Variation in mold susceptibility among hardwood species under laboratory conditions. Int Biodeterior Biodegradation. 2020, 154, 105082. [Google Scholar] [CrossRef]
- Gobakken, L.R.; Lebow, P.K. Modelling mould growth on coated modified and unmodified wood substrates exposed outdoors. Wood Sci Technol. 2010, 44, 315–333. [Google Scholar] [CrossRef]
- Lie, S.K.; Vestol, G.I.; Hoibo, O.; Gobakken, L.R. Surface mould growth on wood: a comparison of laboratory screening tests and outdoor performance. European Journal of Wood and Wood Products. 2019, 77, 1137–1150. [Google Scholar] [CrossRef]
- Viitanen, H.; Toratti, T.; Makkonen, L.; Peuhkuri, R.; Ojanen, T.; Ruokolainen, L.; et al. Towards modelling of decay risk of wooden materials. European Journal of Wood and Wood Products. 2010, 68, 303–313. [Google Scholar] [CrossRef]
- Johansson, P.; Wamming, T.; Bok, G.; Edlund, M.L. Mould growth on kiln-dried and air-dried timber. European Journal of Wood and Wood Products 2013, 71, 473–481. [Google Scholar] [CrossRef]
- Viitanen, H.A. Modelling the time factor in the development of mould fungi–The effect of critical humidity and temperature conditions on pine and spruce sapwood. Holzforschung. 1997, 51, 6–14. [Google Scholar] [CrossRef]
- Armolik, N.; Dickson, J.G. Minimum humidity requirement for germination of conidia of fungi associated with storage of grain. Phytopathology. 1956, 46, 462–465. [Google Scholar]
- McGinnis, M.R. Indoor mould development and dispersal. Med Mycol. 2007, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Ekstrtobin, A.; Svensson, T.; Bok, G. Laboratory study to determine the critical moisture level for mould growth on building materials. Int Biodeterior Biodegradation 2012, 73, 23–32. [Google Scholar] [CrossRef]
- Lacey, J.; Hill, S.T.; Edwards, M.A. Micro-organisms in stored grains: their enumeration and significance. Tropical Stored Products Information. 1981, 39, 19–33. [Google Scholar]
- Huang, Y.; Chapman, B.; Wilson, M.; Hocking, A.D. Effect of agar concentration on the matric potential of glycerol agar media and the germination and growth of xerophilic and non-xerophilic fungi. Int J Food Microbiol. 2009, 133, 179–185. [Google Scholar] [CrossRef]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.C.; Van Dijck, P.W. On the safety of Aspergillus niger-review. version Microbiol Biotechnol. 2002, 59, 426–435. [Google Scholar]
- Abdel-Rahim, A.M.; Arbab, H.A. Factors affecting spore germination in Aspergillus niger. Mycopathologia 1985, 89, 75–79. [Google Scholar] [CrossRef]
- Marà n, S.; Sáenz, R.; Vinas, M. Ecological determinants for germination and growth of some Aspergillus and Penicillium spp. from maize grain. J version Microbiol. 1998, 84, 25–36. [Google Scholar] [CrossRef]
- Hocking, A.D.; Miscamble, B.F.; Pitt, J.I. Water relations of Alternaria alternata, Cladosporium cladosporioides, Cladosporium sphaerospermum, Curvularia lunata and Curvularia pallescens. Mycol Res. 1994, 98, 91–94. [Google Scholar] [CrossRef]
- van Laarhoven, K.A.; Huinink, H.P.; Segers, F.J.; Dijksterhuis, J.; Adan, O.C. Separate effects of moisture content and water activity on the hyphal extension of Penicillium rubens on porous media. Environ Microbiol. 2015, 17, 5089–5099. [Google Scholar] [CrossRef] [PubMed]
- Ruijten, P.; Huinink, H.P.; Adan, O.C.G. Penicillium rubens germination on desiccated and nutrient-depleted conditions depends on the water activity during sporogenesis. Fungal Biol. 2020, 124, 1058–1067. [Google Scholar] [CrossRef]
- Flannigan, B.; Samson, R.A.; Miller, J.D. Microorganisms in indoor air. Microorganisms in home and indoor work environments: Diversity, health impacts, investigation and control. 2001, 2, 17–31. [Google Scholar]
- Johansson, P. Determination of the critical moisture level for mould growth on building materials. 2014. [Google Scholar]
- Ayerst, G. The Effects of Moisture and Temperature on Growth and Spore Germination in some Fungi. Prod Res. 1969, 5, 127–141. [Google Scholar] [CrossRef]
- Johansson, P.; Bok, G.; Ekstr, T.A. The effect of cyclic moisture and temperature on mould growth onwood compared to steady state conditions. Build Environ. 2013, 65, 178–184. [Google Scholar] [CrossRef]
- Tribe, H.T.; Mabadeje, S.A. Growth of moulds on media prepared without organic nutrients. Transactions of the British Mycological Society. 1972, 58, 127–IN12. [Google Scholar] [CrossRef]
- Johansson, P.; MjÖrnell, K.; Arfvidsson, J. Examples of characteristics of wood that affect mould growth: a meta-analysis. European Journal of Wood and Wood Products. 2017, 75, 603–613. [Google Scholar] [CrossRef]
- Hukka, A.; Viitanen, H.A. A mathematical model of mould growth on wooden material. Wood Sci Technol. 1999, 33, 475–485. [Google Scholar] [CrossRef]
- Richter, J.; Stanek, K.; Kopecký, P.; Schutová, P.; Tywoniak, J. Dynamic moisture transport in spruce wood-Experiment in hygroscopic range under isothermal conditions. In: AIP Conference Proceedings.AIP Publishing; 2023. https://pubs.aip.org/aip/acp/article/2894/1/020016/2910842.
- Jang, Y.; Huh, N.; Lee, J.; Kim, G.H.; Kim, J.J. Phylogenetic analysis of major molds inhabiting woods and their discoloration characteristics. Part 2. Genus Penicillium. 2011, 65, 265–270. [Google Scholar]
- Ryparová, P.; Rácová, Z. Characterisation of Micoorganism from Individual Layers of the Building Envelope (ETICS) and Methods of their Sampling. Key Eng Mater [Internet]. 2016, 714, 196–200. [Google Scholar] [CrossRef]
- Venäläinen, M.; Harju, A.M.; Kainulainen, P.; Viitanen, H.; Nikulainen, H. Variation in the decay resistance and its relationship with other wood characteristics in old Scots pines. Ann For Sci. 2003, 60, 409–417. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).