Proprioceptors and Their Roles in Motor Control.
Proprioceptors within the anterior cruciate ligament (ACL) play a fundamental role in the body’s ability to execute and control precise movements, particularly those involving the knee joint. These specialized sensory receptors are essential for maintaining balance, posture, and the overall stability of the joint, providing real-time feedback to the central nervous system (CNS) about the mechanical state of the knee. The ACL, a critical ligament in the knee, is densely populated with different types of proprioceptors—Ruffini endings, Pacinian corpuscles, Golgi tendon organs, and free nerve endings—each of which has a distinct function in monitoring various aspects of the joint’s position and movement (
Table 1).
Ruffini endings are sensitive to sustained pressure and stretch within the ligament, providing continuous feedback about the degree of tension in the ACL as the knee moves through different positions. These receptors are particularly important for detecting changes in joint angle, such as when the knee bends or straightens, and they help the brain maintain a constant awareness of the knee’s orientation in space. This information is crucial for the smooth execution of movements that require precise control, such as walking on uneven surfaces or performing athletic maneuvers that involve quick changes in direction. By responding to slow, sustained mechanical changes, Ruffini endings help to ensure that the knee remains stable even during prolonged activities or in postures where the joint is under consistent stress.
Pacinian corpuscles, in contrast, are highly sensitive to rapid changes in pressure and vibration, making them essential for detecting quick, dynamic movements of the knee joint. These receptors are particularly active during activities that involve sudden impacts or shifts in position, such as jumping, sprinting, or landing from a height. The rapid adaptation of Pacinian corpuscles allows them to respond almost instantaneously to these changes, providing the CNS with critical information that helps the body stabilize the knee joint in real-time. This fast response is vital for preventing injuries during high-speed or high-impact activities, where the knee is subjected to significant forces that could destabilize the joint.
Golgi tendon organs, located at the junctions where muscles and tendons meet the ACL, play a protective role by monitoring the tension within the ligament. These proprioceptors are activated when the tension in the ACL reaches a certain threshold, typically during activities that involve heavy lifting or intense physical exertion. When Golgi tendon organs detect excessive force, they trigger a reflex that inhibits muscle contraction, thereby reducing the load on the ligament and preventing potential damage. This mechanism is crucial for protecting the ACL from overstretching or tearing, particularly during activities that place a high demand on the knee joint, such as weightlifting or sudden deceleration.
Free nerve endings, while less specialized than the other types of proprioceptors, are critical for detecting a wide range of stimuli, including pain, temperature, and mechanical stress. These receptors provide the body with essential protective feedback, alerting the CNS to conditions that could potentially harm the knee joint. When free nerve endings detect harmful stimuli, they initiate pain responses or other protective reflexes that encourage the body to modify its movements or reduce the intensity of activity to prevent injury. For example, if the knee is subjected to excessive strain or if there is a risk of ligament damage, the activation of free nerve endings may result in a sensation of pain that prompts the individual to stop the activity or change posture, thereby protecting the joint from further harm.
The mechanisms by which these proprioceptors operate are complex and involve intricate interactions between mechanical stimuli, nerve signal transmission, and CNS processing. When these sensory receptors detect changes in the knee joint, they convert mechanical stimuli into electrical signals that are transmitted along nerve fibers to the spinal cord and brain. Once in the CNS, these signals are integrated with other sensory inputs and processed to produce appropriate motor responses. This processing allows the CNS to make rapid adjustments to muscle activity around the knee, ensuring that the joint remains stable and aligned during movement.
Together, these proprioceptors form a sophisticated system that continuously monitors the knee’s mechanical state, allowing for the precise control of movement and ensuring that the joint functions optimally during a wide range of activities. Whether the body is performing a simple task like standing still, or a complex activity like running or jumping, the real-time feedback provided by the proprioceptors within the ACL is essential for maintaining balance, coordinating movements, and protecting the knee joint from injury. Their contribution to the neurophysiological processes that govern movement highlights the critical role of the ACL not only as a structural component of the knee but also as a key player in the body’s sensory-motor integration system.
Ruffini Endings: Slow-Adapting Mechanoreceptors
Ruffini endings are highly specialized mechanoreceptors embedded deep within the dense, fibrous matrix of the anterior cruciate ligament (ACL), a key ligament that provides essential stability to the knee joint. These receptors are integral to the body’s proprioceptive system, which is responsible for the intricate sense of limb position and movement. Their primary function is to detect mechanical changes within the ligament, such as continuous stretch or pressure, and to relay this information to the central nervous system (CNS). The unique structure and function of Ruffini endings, particularly their slow-adapting nature, make them exceptionally well-suited for monitoring sustained mechanical stimuli that the ACL experiences during various physical activities. This ability to maintain a persistent response over extended periods is critical for activities that require constant monitoring of joint stability, such as standing, walking, or engaging in dynamic sports.
When the knee moves—whether it flexes, extends, or rotates—the ACL undergoes mechanical deformation. This deformation can involve stretching, compressing, or twisting of the ligament depending on the nature of the movement and the forces involved. Ruffini endings, which are embedded within the ligament’s structure, are highly sensitive to these mechanical changes. These receptors are composed of specialized nerve endings intertwined with collagen fibers within the ligament, providing them with the ability to detect even slight changes in tension or stretch. As the ligament stretches or compresses, the Ruffini endings themselves undergo conformational changes—essentially altering their shape in response to the mechanical forces applied to them. This structural change is the initial step in a complex molecular process that converts mechanical stimuli into neural signals.
The conformational change in Ruffini endings triggers the opening of mechanically gated ion channels in the membranes of these sensory receptors. These ion channels are proteins embedded within the cell membrane that respond to mechanical forces by changing their shape, thereby allowing ions to pass through. Specifically, when Ruffini endings detect a stretch or pressure, these ion channels, such as Piezo channels or other mechanosensitive ion channels, open up to allow the influx of positively charged ions, primarily sodium (Na+) and calcium (Ca2+), into the receptor cells. This influx of ions alters the electrical charge across the cell membrane, generating what is known as a receptor potential, an electrical signal that is directly proportional to the degree of mechanical deformation experienced by the ligament.
The receptor potential generated within Ruffini endings is a graded response, meaning its amplitude is directly related to the intensity of the mechanical stimulus. The stronger and more sustained the stretch or pressure on the ACL, the more significant the receptor potential generated by the Ruffini endings. This receptor potential serves as the precursor to the action potentials, which are the all-or-nothing electrical impulses that carry this information to the CNS. The conversion of the receptor potential into action potentials occurs at the axon hillock of the neuron, where the depolarization of the membrane reaches a threshold that triggers the opening of voltage-gated sodium channels. This results in a rapid influx of sodium ions, leading to the generation of action potentials.
The action potentials are then transmitted through the peripheral nervous system via myelinated nerve fibers, which are coated with a fatty substance called myelin. Myelin acts as an insulating layer, allowing the electrical impulses to travel quickly along the nerve fibers through a process called saltatory conduction, where the action potentials “jump” between the nodes of Ranvier, the gaps in the myelin sheath. This rapid transmission ensures that the sensory information reaches the CNS, particularly the brain and spinal cord, with minimal delay.
Once these action potentials reach the CNS, they are processed by various regions, including the primary somatosensory cortex, which plays a critical role in interpreting proprioceptive signals. The frequency and pattern of these action potentials encode critical information about the knee joint’s position, the extent of its movement, and the forces acting upon it. The CNS integrates this information with other sensory inputs, such as visual and vestibular cues, to form a comprehensive understanding of the body’s overall state. This integration allows the brain to maintain an accurate and constantly updated representation of the knee’s orientation in space, which is essential for coordinating movements and maintaining balance, particularly in complex or unstable environments.
The continuous feedback provided by Ruffini endings plays a critical role in the precise control of muscle activity around the knee joint. For example, during activities such as walking on uneven terrain, the knee must continuously adjust to variations in surface height and stability. The real-time input from Ruffini endings informs the brain of any changes in the knee’s position or the forces acting on it, enabling the CNS to adjust muscle contractions accordingly. This fine-tuning of muscle activity ensures that the knee remains stable, preventing it from collapsing or moving improperly, which could lead to injury.
In addition to their role in normal movement, Ruffini endings are crucial in situations where the knee joint is under stress or at risk of injury. For example, if an individual is about to lose balance, or if the knee experiences an unexpected twist or force, the Ruffini endings quickly detect the change in mechanical stress. The resulting neural signals prompt the CNS to initiate reflexive muscle contractions that counteract the destabilizing forces, helping to restore balance and prevent potential damage to the joint. This reflexive action is part of the body’s protective mechanisms, which rely heavily on the continuous feedback from proprioceptors like Ruffini endings to respond quickly and effectively to challenges in joint stability.
On a molecular level, the ability of Ruffini endings to respond to sustained stimuli without fatigue is due to the specific properties of their ion channels and the associated intracellular signaling pathways. The slow adaptation of these receptors allows them to maintain sensitivity to prolonged stimuli, ensuring that they can provide continuous feedback even during extended periods of physical activity. This capability is essential for long-term joint health, as it allows the body to adapt to sustained physical demands and helps prevent overuse injuries.
Moreover, Ruffini endings contribute to long-term joint health by enabling the body to adapt to sustained physical demands. During activities that involve prolonged periods of standing, walking, or other forms of exertion, Ruffini endings provide ongoing feedback that helps the CNS manage muscle fatigue and maintain joint integrity. By monitoring and responding to gradual changes in joint position and mechanical stress, these receptors help prevent overuse injuries and ensure that the knee joint remains functional and resilient over time.
In summary, Ruffini endings are essential components of the ACL’s proprioceptive system, providing continuous and detailed feedback about the mechanical state of the knee joint. Their slow-adapting nature allows them to monitor sustained mechanical stimuli, ensuring that the brain receives accurate and timely information needed to maintain joint stability and control movement. The complex process by which Ruffini endings detect mechanical changes, generate electrical signals, and relay this information to the CNS is fundamental to the body’s ability to perform a wide range of activities, from simple postural adjustments to complex athletic maneuvers. Through their ongoing contribution to the neurophysiological processes that govern movement, Ruffini endings play a vital role in protecting the knee joint, enabling coordinated and responsive movement, and ensuring long-term joint health.
Pacinian Corpuscles: Rapidly Adapting Receptors for Dynamic Movements
Pacinian corpuscles are highly specialized mechanoreceptors located within the anterior cruciate ligament (ACL) and other areas of the body, known for their ability to detect rapid changes in mechanical stimuli, such as pressure, vibration, and dynamic movement. On a molecular level, the operation of these receptors is a finely tuned process involving the precise interaction of mechanical forces with cellular components, which ultimately results in the generation and transmission of electrical signals to the central nervous system (CNS). Understanding the molecular mechanisms underlying the function of Pacinian corpuscles provides deep insights into how these receptors contribute to proprioception and the body’s ability to respond to dynamic forces.
The structure of Pacinian corpuscles is key to their sensitivity and rapid response capabilities. These receptors are encapsulated by multiple concentric layers of connective tissue, which are composed of flattened, lamellar cells separated by fluid-filled spaces. This unique “onion-like” architecture plays a crucial role in the receptor’s ability to filter and amplify mechanical forces. When a mechanical stimulus, such as a sudden pressure change or vibration, is applied to the knee, these concentric layers compress and transmit the force towards the core of the receptor, where the nerve ending is located. The mechanical energy from the stimulus is efficiently funneled through these layers, focusing the force onto the nerve terminal and thereby enhancing the receptor’s sensitivity.
At the molecular level, the deformation of the nerve ending triggers a cascade of events within the Pacinian corpuscle. The nerve terminal is rich in specialized ion channels that are mechanically gated, meaning they open in response to physical deformation of the cell membrane. These channels are primarily composed of proteins that are sensitive to the stretching or compression of the membrane. One of the key ion channels involved is the Piezo1 channel, a large transmembrane protein that directly converts mechanical forces into electrochemical signals. When the Pacinian corpuscle is compressed, the mechanical force induces a conformational change in the Piezo1 channel, causing it to open.
Once the Piezo1 channel opens, it allows a rapid influx of sodium (Na+) and calcium (Ca2+) ions into the nerve ending. The entry of these positively charged ions depolarizes the membrane potential of the nerve cell, creating a receptor potential. The receptor potential is an electrical signal that is graded; its amplitude varies depending on the intensity of the mechanical stimulus. The stronger the deformation of the Pacinian corpuscle, the greater the number of ion channels that open, and the larger the receptor potential generated. This graded response allows the Pacinian corpuscle to encode information about the magnitude of the mechanical stimulus.
The receptor potential must be converted into action potentials for the signal to be transmitted to the CNS. Action potentials are all-or-nothing electrical impulses that are initiated when the receptor potential reaches a certain threshold. This conversion occurs at the axon hillock, a specialized region of the neuron where the density of voltage-gated sodium channels is highest. If the receptor potential is strong enough, it will trigger the opening of these voltage-gated sodium channels, leading to a rapid influx of sodium ions that depolarizes the membrane further and initiates an action potential.
The action potentials generated by Pacinian corpuscles are transmitted along the nerve fibers to the CNS. The nerve fibers associated with Pacinian corpuscles are typically myelinated, meaning they are wrapped in a fatty layer of myelin produced by Schwann cells. Myelination greatly increases the speed at which action potentials travel along the nerve fiber by allowing the electrical signal to “jump” between the nodes of Ranvier, which are small gaps in the myelin sheath. This process, known as saltatory conduction, enables the rapid transmission of signals from the Pacinian corpuscle to the CNS, ensuring that sensory information about mechanical changes in the knee is relayed almost instantaneously.
Once the action potentials reach the CNS, they are processed by various regions of the brain and spinal cord. The primary somatosensory cortex, for instance, receives and interprets these signals, integrating them with other sensory inputs to form a coherent picture of the body’s position and movement. This processing allows the brain to make real-time adjustments to muscle activity, stabilizing the knee joint and coordinating complex motor responses.
On a broader scale, the molecular mechanisms that govern the function of Pacinian corpuscles are essential for the body’s ability to respond to dynamic forces and protect the knee joint from injury. For example, during high-impact activities such as jumping, sprinting, or rapidly changing direction, the rapid detection of mechanical stimuli by Pacinian corpuscles allows the CNS to quickly activate reflexive responses that stabilize the knee and absorb shocks. The swift feedback provided by these receptors helps prevent excessive strain on the ACL and other knee structures, reducing the risk of ligament tears or other injuries.
Furthermore, the ability of Pacinian corpuscles to detect subtle vibrations and changes in pressure also plays a role in enhancing proprioception, particularly in situations where fine motor control is required. For instance, in athletes performing at a high level, the precise feedback from Pacinian corpuscles allows for the execution of complex movements with a high degree of accuracy and coordination. This capability is essential not only for athletic performance but also for everyday activities that require balance, agility, and quick reflexes.
In summary, the molecular mechanisms underlying the function of Pacinian corpuscles involve a complex interplay between mechanical forces, ion channel dynamics, and rapid signal transmission. These processes enable these specialized receptors to detect and respond to changes in pressure and vibration with remarkable speed and precision. By converting mechanical stimuli into electrical signals that are transmitted to the CNS, Pacinian corpuscles play a critical role in maintaining knee stability, protecting the joint from injury, and enabling the body to perform complex and dynamic movements with confidence and control.
Golgi Tendon Organs: Tension Sensors and Protective Mechanisms
Golgi tendon organs (GTOs) are highly specialized proprioceptive receptors located at the critical junctures where muscles attach to tendons, including those that connect to key ligaments like the anterior cruciate ligament (ACL). These receptors are fundamental to the body’s proprioceptive system, which is responsible for sensing and regulating the tension within tendons during muscle contraction. On a molecular level, GTOs are intricately designed to detect even the slightest changes in mechanical force, ensuring that the body can prevent excessive tension that could lead to damage of the tendon, ligament, or surrounding structures.
GTOs are embedded within the collagen fibers of the tendon, and their structure is uniquely suited to their function. The sensory nerve endings of GTOs are entwined around the collagen fibrils, which are the primary structural components of tendons. These collagen fibrils are composed of triple-helical molecules that provide strength and flexibility, allowing the tendon to withstand the forces generated during muscle contraction. When a muscle contracts, it pulls on the tendon, which in turn stretches the collagen fibrils. This stretching of the collagen fibrils applies pressure to the intertwined nerve endings of the GTO, initiating the sensory transduction process.
At the molecular level, the deformation of the collagen fibrils and the associated nerve endings triggers a series of events that convert mechanical stimuli into electrochemical signals. The primary players in this process are mechanosensitive ion channels embedded in the membrane of the sensory neurons. These ion channels are proteins that can detect changes in mechanical force and respond by altering their conformation, opening to allow the flow of ions across the cell membrane.
One key type of mechanosensitive ion channel involved in the functioning of GTOs is the Piezo channel. Piezo channels are large, transmembrane proteins that are directly gated by mechanical forces such as stretch or pressure. When the tension within the tendon increases due to muscle contraction, the resulting deformation of the collagen fibrils causes the Piezo channels in the GTO nerve endings to open. This opening allows an influx of positively charged ions, primarily sodium (Na+) and calcium (Ca2+), into the sensory nerve cells. The entry of these ions depolarizes the cell membrane, generating a receptor potential—a type of graded electrical signal that is directly proportional to the degree of mechanical force applied to the tendon.
The receptor potential generated by the activation of Piezo channels is a crucial step in the sensory transduction process. The magnitude of the receptor potential depends on the intensity of the mechanical stimulus: the greater the tension in the tendon, the more Piezo channels open, and the larger the receptor potential becomes. If this receptor potential reaches a certain threshold, it triggers the opening of voltage-gated sodium channels located at the axon hillock of the sensory neuron. The rapid influx of sodium ions through these channels initiates an action potential, which is an all-or-nothing electrical impulse that propagates along the sensory nerve fiber.
The action potentials generated by GTOs are then transmitted along myelinated nerve fibers to the central nervous system (CNS). Myelination is critical for the rapid conduction of these signals, as it allows the action potentials to travel quickly along the nerve fibers through a process called saltatory conduction. In saltatory conduction, the action potential “jumps” from one node of Ranvier (gaps in the myelin sheath) to the next, significantly speeding up the transmission of sensory information to the CNS.
Once the action potentials reach the CNS, they synapse with neurons in the spinal cord, specifically with inhibitory interneurons. These interneurons play a pivotal role in the process known as autogenic inhibition, a protective reflex mechanism that regulates muscle force output. When the inhibitory interneurons are activated by the signals from GTOs, they release neurotransmitters such as gamma-aminobutyric acid (GABA) or glycine, which bind to receptors on the alpha motor neurons that control the contracting muscle. The binding of these neurotransmitters to their receptors causes hyperpolarization of the motor neurons, reducing their activity and effectively decreasing the force of the muscle contraction.
This reduction in muscle force output is crucial for preventing excessive tension within the tendon, which could otherwise lead to overstretching or tearing of the tendon, ligament, or surrounding structures like the ACL. The autogenic inhibition reflex acts as a feedback loop that protects the musculoskeletal system from the damaging effects of excessive force. For example, during activities such as heavy lifting or sprinting, where the muscles generate significant force, GTOs continuously monitor the tension levels within the tendons. If the force becomes too great, the GTOs quickly activate this protective reflex, reducing muscle contraction and preventing potential injury.
Beyond their immediate protective role, GTOs are also involved in the fine-tuning of muscle activity during both voluntary and reflexive movements. By providing the CNS with real-time information about tendon tension, GTOs help modulate motor outputs, ensuring that movements are smooth, coordinated, and adapted to the physical demands placed on the body. This fine-tuning is particularly important during activities that require precise control of muscle force, such as maintaining posture, performing delicate tasks, or executing complex athletic maneuvers.
On a molecular level, the sensitivity and rapid response of GTOs are supported by various intracellular signaling pathways that regulate the activity of mechanosensitive ion channels and the generation of receptor potentials. These pathways may involve the activation of secondary messengers such as cyclic adenosine monophosphate (cAMP) or calcium-dependent protein kinases, which modulate the sensitivity of the ion channels to mechanical stimuli. Additionally, the expression of specific proteins that anchor the mechanosensitive ion channels to the cytoskeleton of the nerve cell ensures that the channels are optimally positioned to detect changes in tendon tension.
Furthermore, GTOs play a role in the adaptation of the neuromuscular system to prolonged or repeated physical activity. During training or repetitive movements, the sensitivity of GTOs can be modulated, allowing the nervous system to adjust to increased or sustained levels of muscle tension. This adaptation helps improve muscle efficiency and coordination, enhancing overall performance and reducing the risk of injury over time.
In summary, Golgi tendon organs are critical proprioceptors that operate through a complex molecular mechanism to monitor and regulate tendon tension during muscle contraction. The activation of mechanosensitive ion channels, such as Piezo channels, in response to mechanical deformation leads to the generation of receptor potentials, which are then converted into action potentials that are transmitted to the CNS. Through the process of autogenic inhibition, GTOs protect the musculoskeletal system from the damaging effects of excessive force, while also contributing to the fine-tuning of muscle activity. Their role in maintaining joint integrity, especially in high-stress activities, underscores the importance of proprioception in ensuring the safety, efficiency, and adaptability of the body’s movements.
Free Nerve Endings: Generalist Receptors for Pain and Stress Detection
Free nerve endings, which are the most widespread type of sensory receptors found within the anterior cruciate ligament (ACL) and other joint structures, are critical components of the body’s sensory system. These receptors are highly versatile and can detect a broad range of stimuli, including mechanical stress, chemical changes, temperature fluctuations, and noxious (painful) stimuli. Unlike more specialized proprioceptors, free nerve endings are relatively simple in structure but incredibly sophisticated in their functional capabilities. On a molecular level, these receptors are equipped with various ion channels, receptors, and signaling molecules that enable them to respond to diverse environmental changes within the tissue.
At the core of the functionality of free nerve endings is their ability to transduce mechanical and chemical stimuli into electrical signals, a process known as sensory transduction. The molecular mechanisms involved in this process begin with the detection of changes in the tissue environment, such as mechanical stress or chemical signals released during tissue damage or inflammation.
When the knee experiences mechanical stress, such as excessive force, overextension, or physical trauma, the tissue surrounding the ACL is deformed. This deformation can stretch, compress, or even tear the extracellular matrix, a complex network of proteins and other molecules that provides structural support to tissues. Free nerve endings embedded within this matrix are directly affected by these mechanical changes.
The mechanical deformation of the tissue is sensed by mechanosensitive ion channels located in the membranes of the free nerve endings. These ion channels, such as the Piezo channels (particularly Piezo1 and Piezo2), are highly sensitive to changes in membrane tension. Piezo channels are large, trimeric proteins that span the cell membrane and respond to mechanical force by undergoing conformational changes. When the membrane is stretched or compressed, the Piezo channels open, allowing cations, primarily sodium (Na+) and calcium (Ca2+), to flow into the cell. This influx of ions leads to depolarization of the nerve ending, generating a receptor potential. The greater the mechanical force, the more Piezo channels open, leading to a stronger receptor potential.
In addition to mechanosensation, free nerve endings are highly responsive to chemical signals in their environment, a capability that is crucial for detecting tissue damage and initiating pain responses. When tissue is damaged, cells release a variety of inflammatory mediators, including prostaglandins, bradykinin, histamine, and ATP. These molecules play a key role in sensitizing and activating free nerve endings.
Prostaglandins, which are lipid-derived mediators, bind to G-protein-coupled receptors (GPCRs) on the surface of free nerve endings. This binding activates intracellular signaling pathways involving cyclic AMP (cAMP) and protein kinase A (PKA). The activation of these pathways can lead to the phosphorylation of ion channels, such as transient receptor potential (TRP) channels, making them more sensitive to stimuli. TRP channels, including TRPV1 (which is also known as the capsaicin receptor), are crucial for detecting changes in temperature and chemical irritants. For instance, TRPV1 is activated by heat, acidic conditions (low pH), and vanilloid compounds like capsaicin (the active component in chili peppers).
When bradykinin binds to its receptor (the B2 receptor), it activates phospholipase C (PLC), which leads to the production of inositol triphosphate (IP3) and diacylglycerol (DAG). These second messengers contribute to the release of calcium from intracellular stores and the activation of protein kinase C (PKC), which further sensitizes the nerve ending by modulating the activity of various ion channels. This sensitization enhances the responsiveness of free nerve endings to subsequent stimuli, a phenomenon known as hyperalgesia, which can lead to increased pain perception in response to normally non-painful stimuli.
The combined effects of mechanical and chemical stimuli result in the depolarization of the membrane potential of the free nerve endings, leading to the generation of receptor potentials. When these receptor potentials reach a certain threshold, they trigger the opening of voltage-gated sodium channels, particularly the Nav1.7, Nav1.8, and Nav1.9 subtypes, which are heavily expressed in nociceptive neurons. The rapid influx of sodium ions through these channels generates an action potential, which is an all-or-nothing electrical impulse that propagates along the sensory nerve fibers toward the central nervous system (CNS).
The action potentials travel along the axons of sensory neurons, which are part of the peripheral nervous system (PNS), and reach the dorsal horn of the spinal cord. Here, the sensory neurons synapse with second-order neurons, transmitting the signal to higher brain centers, including the thalamus and cerebral cortex, where it is interpreted as pain. The neurotransmitters involved in this synaptic transmission include glutamate and substance P, both of which are critical for conveying pain signals.
The pain signals generated by free nerve endings serve not only as a warning system to prevent further injury but also as a trigger for protective reflexes. For example, when free nerve endings in the knee detect a sudden mechanical stress or an inflammatory response, the resulting action potentials can initiate a spinal reflex arc. This reflex involves the direct activation of motor neurons in the spinal cord, leading to the contraction of muscles surrounding the knee joint. This reflexive contraction helps stabilize the joint, preventing excessive movement that could exacerbate the injury.
Moreover, the pain perception mediated by free nerve endings plays a crucial role in modifying behavior. The intense pain resulting from significant mechanical stress or tissue damage compels individuals to alter their activities—such as stopping movement, reducing load on the affected joint, or seeking medical attention—thereby preventing further damage and promoting healing.
In cases of repeated or prolonged injury, the signaling pathways in free nerve endings can undergo changes that lead to long-term sensitization. This can result in chronic pain conditions where the pain persists even after the initial injury has healed. Molecular changes, such as upregulation of ion channels and receptors involved in nociception, can enhance the excitability of sensory neurons, making them more likely to generate action potentials in response to minimal stimuli.
Additionally, neuroplastic changes in the CNS, such as central sensitization, can occur. This involves the increased responsiveness of neurons in the spinal cord and brain to sensory input, contributing to the maintenance of chronic pain states. Understanding the molecular mechanisms underlying the activation and sensitization of free nerve endings is crucial for developing therapeutic strategies to manage acute and chronic pain, particularly in conditions involving joint damage or inflammatory diseases like arthritis.
In summary, free nerve endings are highly versatile sensory receptors within the ACL and other joint structures, capable of detecting a wide range of stimuli through complex molecular mechanisms. They are integral to the body’s ability to respond to mechanical stress, chemical changes, and temperature fluctuations, playing a crucial role in pain perception and protective reflexes. The molecular processes involved, from the activation of mechanosensitive and chemosensitive ion channels to the generation and propagation of action potentials, underscore the importance of free nerve endings in maintaining joint integrity and overall musculoskeletal health. Through their role in detecting and responding to potentially harmful stimuli, free nerve endings are essential for protecting the body from acute injuries and for contributing to the management of chronic pain conditions.
Integrated Feedback System: Ensuring Optimal Knee Function
The various proprioceptors embedded within the anterior cruciate ligament (ACL)—Ruffini endings, Pacinian corpuscles, Golgi tendon organs (GTOs), and free nerve endings—work together to form a complex and highly responsive feedback system that is crucial for the proper functioning of the knee joint. Each of these proprioceptors operates at a molecular level, employing a range of specialized ion channels, receptors, and signaling pathways to detect and respond to mechanical changes, tension, and potential threats to the joint. This intricate network ensures that the knee joint remains stable, balanced, and capable of adapting to the varied demands placed upon it during both everyday activities and intense physical exertion.
Ruffini endings are slow-adapting mechanoreceptors that are particularly sensitive to continuous pressure and stretch within the ACL. These receptors are embedded in the fibrous matrix of the ligament, where they are ideally positioned to detect changes in the joint’s angle and tension. On a molecular level, Ruffini endings are equipped with mechanosensitive ion channels, such as Piezo1 and Piezo2, which are directly activated by mechanical deformation of the surrounding collagen fibers. When the knee joint moves, these collagen fibers stretch or compress, causing the Ruffini endings to deform. This deformation triggers a conformational change in the mechanosensitive ion channels, leading to their opening.
The opening of Piezo channels allows positively charged ions, such as sodium (Na+) and calcium (Ca2+), to flow into the sensory nerve endings. This influx of ions depolarizes the cell membrane, generating a receptor potential that is directly proportional to the degree of mechanical deformation. The receptor potential is a graded response, meaning its amplitude reflects the intensity of the stimulus. If the receptor potential reaches a certain threshold, it triggers the opening of voltage-gated sodium channels, initiating action potentials that propagate along the sensory nerve fibers to the central nervous system (CNS).
The continuous feedback provided by Ruffini endings is crucial for maintaining knee stability. The action potentials generated by these receptors are transmitted to the CNS, where they are integrated with other sensory inputs to provide real-time information about the knee’s position. This allows the brain to make fine adjustments to muscle activity, ensuring that the joint remains stable during both static and dynamic activities.
Pacinian corpuscles are rapidly adapting mechanoreceptors that are highly specialized for detecting quick, transient changes in pressure and vibration. These receptors are encapsulated by concentric layers of connective tissue, which mechanically amplify the forces acting on the nerve ending. At the core of the Pacinian corpuscle, mechanosensitive ion channels—such as Piezo1, Piezo2, and possibly other types like TREK-1 or TRPC channels—are responsible for detecting these mechanical changes.
When the knee undergoes a rapid movement, such as during a jump or a sudden change in direction, the concentric layers of the Pacinian corpuscle compress, transmitting the force to the nerve ending. This mechanical compression leads to the activation of mechanosensitive ion channels, which open in response to the increased membrane tension. The rapid influx of sodium and calcium ions into the nerve ending generates a strong receptor potential, which is quickly converted into a series of action potentials. These action potentials are transmitted along myelinated nerve fibers to the CNS at high speeds, ensuring that the brain receives immediate feedback about the rapid changes in knee position.
The speed and sensitivity of Pacinian corpuscles are critical for enabling quick reflexes that protect the knee from injury. The CNS uses the information provided by these receptors to make rapid adjustments in muscle activity, helping to stabilize the knee joint and absorb shocks during high-impact activities.
Golgi tendon organs (GTOs) are specialized proprioceptors located at the junctions between muscles and tendons, including those connected to the ACL. These receptors are uniquely designed to monitor the tension generated within tendons during muscle contraction. At the molecular level, GTOs contain mechanosensitive ion channels, similar to those found in Ruffini endings and Pacinian corpuscles, which are activated by the stretching of collagen fibers within the tendon.
When muscle contraction generates tension within the tendon, the collagen fibers deform, compressing the sensory nerve endings within the GTO. This mechanical deformation activates mechanosensitive ion channels, leading to the opening of these channels and the influx of sodium and calcium ions. The resulting receptor potential generates action potentials that are transmitted to the CNS.
In the spinal cord, the action potentials from GTOs synapse with inhibitory interneurons, which release neurotransmitters such as gamma-aminobutyric acid (GABA) or glycine. These neurotransmitters bind to receptors on the alpha motor neurons that control the contracting muscle, causing hyperpolarization of the motor neurons. This reduces their activity, leading to a decrease in muscle contraction force. This process, known as autogenic inhibition, serves as a protective mechanism that prevents excessive force from damaging the ACL and surrounding structures.
Free nerve endings are the most widespread type of sensory receptors within the ACL and other joint structures. These receptors are versatile and capable of detecting a broad range of stimuli, including mechanical stress, chemical changes, temperature fluctuations, and noxious stimuli. On a molecular level, free nerve endings are equipped with a variety of ion channels and receptors that allow them to respond to these diverse stimuli.
For mechanical stress, free nerve endings utilize mechanosensitive ion channels, such as Piezo channels, to detect changes in membrane tension. For chemical stimuli, free nerve endings are equipped with receptors for inflammatory mediators, such as prostaglandin receptors (EP1-4) and bradykinin receptors (B1 and B2). When these inflammatory mediators bind to their respective receptors, they activate intracellular signaling pathways that lead to the opening of ion channels, such as transient receptor potential (TRP) channels, including TRPV1, which is sensitive to heat and acidity.
The activation of these channels results in the influx of sodium and calcium ions, generating a receptor potential that, if strong enough, triggers action potentials. These action potentials are transmitted to the CNS, where they are interpreted as pain or discomfort. The pain signals serve as an immediate alert, prompting the individual to alter their behavior to avoid further injury. Additionally, the activation of free nerve endings can trigger reflexive protective actions, such as muscle contractions that stabilize the joint.
The signals generated by these various proprioceptors are transmitted to the CNS, where they are integrated and processed to form a comprehensive picture of the knee’s current state. This integration involves the convergence of sensory inputs from different types of proprioceptors, each contributing unique information about the mechanical and chemical environment of the knee joint.
For example, during a rapid change in direction, the CNS receives immediate signals from Pacinian corpuscles about the shift in knee position, while Ruffini endings provide continuous feedback about joint angle and tension. Simultaneously, GTOs monitor the tension within the tendons, ensuring that muscle contractions do not generate excessive force, and free nerve endings detect any harmful stress or tissue damage, initiating pain signals if necessary.
This integrated feedback system allows the brain to regulate muscle activity in real-time, ensuring that the knee remains stable, balanced, and capable of adapting to varying demands. The CNS can quickly adjust muscle contractions to stabilize the joint, prevent injury, and optimize performance. Whether during routine activities like walking or during more complex movements such as those required in sports, the feedback from these proprioceptors enables the body to perform movements with precision and safety.
In summary, the proprioceptors within the ACL—Ruffini endings, Pacinian corpuscles, Golgi tendon organs, and free nerve endings—form a highly sophisticated and responsive feedback system that operates at a molecular level to ensure the proper functioning of the knee joint. Through the detection of mechanical changes, tension, and potential threats, these proprioceptors continuously monitor and adjust the mechanical state of the knee, playing a crucial role in maintaining overall joint health and function. Their coordinated activity enables both everyday movements and peak athletic performance, providing the body with the necessary information to protect the knee joint from injury and to perform complex, dynamic movements with confidence and precision.
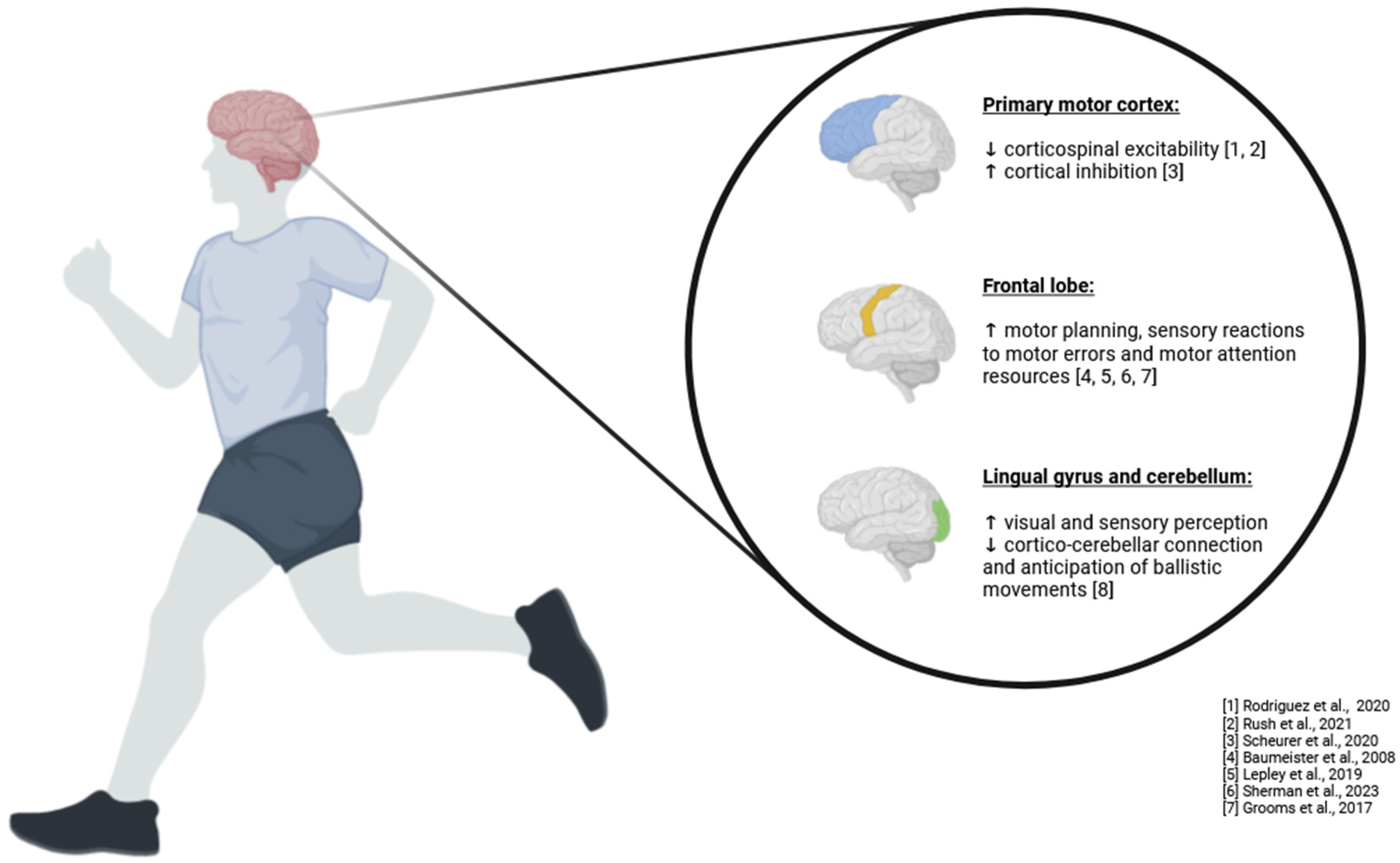
Brain Structures Involved in ACL Neurophysiology
The neurophysiological processes associated with the anterior cruciate ligament (ACL) are deeply complex and involve an intricate network of brain structures that work in tandem to process and integrate sensory information collected by proprioceptors embedded within the ligament. These proprioceptors constantly monitor and relay critical data concerning the position, movement, and mechanical stress experienced by the knee joint. This sensory information is then rapidly transmitted to the central nervous system (CNS), where it is meticulously processed by various specialized brain regions responsible for motor control and coordination.
Each of these brain regions plays a distinct yet interconnected role in transforming raw sensory data into actionable motor responses. For instance, the primary somatosensory cortex in the parietal lobe is central to interpreting proprioceptive input, creating a detailed, moment-by-moment map of the knee joint’s orientation and dynamics. This information is crucial not only for conscious awareness of limb position but also for informing subsequent motor planning and execution, which occurs in other parts of the brain, such as the primary motor cortex.
Moreover, the integration of proprioceptive feedback with other sensory inputs—most notably visual information from the eyes and vestibular data from the inner ear—enhances the brain’s ability to maintain balance, execute precise, coordinated movements, and quickly adapt to changes in the environment. Visual input allows the brain to contextualize proprioceptive data within the broader visual landscape, ensuring that movements are appropriate for the surroundings. Meanwhile, the vestibular system, which detects changes in head position and motion, provides crucial information for maintaining equilibrium and posture. The convergence of these sensory streams within the CNS creates a comprehensive and dynamic picture of the body’s orientation in space, which is essential for executing even the simplest of movements, such as walking or standing upright.
This complex and highly coordinated processing allows the body to respond appropriately to a wide variety of physical demands, from routine activities like walking and climbing stairs to more dynamic actions such as running, jumping, or changing direction during sports. For example, when an individual encounters an unexpected obstacle or shifts their body weight, the brain relies on the integrated feedback from the ACL and other sensory inputs to rapidly adjust muscle activation patterns around the knee, ensuring that the joint remains stable and aligned.
Furthermore, this network’s ability to adapt to changing conditions is vital for maintaining the stability and functionality of the knee joint over time. Whether it’s reacting to sudden changes in terrain, compensating for fatigue during prolonged activity, or recovering from an injury, the brain’s ability to process and integrate sensory information from the ACL and other sources is fundamental to sustaining joint health and preventing injury. The ongoing dialogue between the ACL’s proprioceptors and the brain not only supports the immediate demands of movement but also plays a role in longer-term motor learning and adaptation, allowing the body to fine-tune its responses based on past experiences and evolving physical conditions.
In summary, the neurophysiological processes related to the ACL involve a highly sophisticated and integrated network of brain structures that process proprioceptive input alongside other sensory data. This comprehensive processing enables the body to maintain balance, execute precise movements, and adapt to environmental changes, ensuring the knee joint’s stability and functionality across a wide range of physical activities. This system’s adaptability and precision highlight the critical role of the ACL in both immediate motor control and long-term joint health, underscoring the importance of proprioceptive feedback in the overall functioning of the musculoskeletal system (
Figure 1).
Primary Somatosensory Cortex: Central Hub for Sensory Integration
The primary somatosensory cortex, situated within the parietal lobe of the brain, serves as an essential processing hub for the vast array of sensory information that originates from every part of the body. This region is especially crucial for interpreting the highly specialized feedback from proprioceptors embedded in the anterior cruciate ligament (ACL), among other key structures. Far from being a passive receiver of sensory signals, the primary somatosensory cortex actively engages in the complex task of interpreting and integrating these inputs, enabling the brain to form a coherent and detailed understanding of the body’s physical state. This region processes information related to the position, movement, and tension of various joints and muscles, which is vital not only for conscious awareness of the body’s posture but also for the subconscious motor control that governs smooth and coordinated movement (
Table 2).
When proprioceptive data from the ACL reaches the primary somatosensory cortex, it undergoes a sophisticated processing sequence where it is integrated with sensory inputs from other regions of the body. These inputs include tactile sensations from the skin, pressure data from muscles, and balance-related signals from the vestibular system. This intricate integration allows the brain to construct an exceptionally accurate and dynamic representation of the knee joint in real-time. The primary somatosensory cortex acts as a highly detailed and continuously updated sensory map, which provides the brain with essential information about the knee joint’s current state. This includes its precise position, the forces acting upon it, and the trajectory of its movements. This sensory map is crucial for enabling the brain to plan, initiate, and refine movements with exceptional precision, ensuring that physical actions are both coordinated and effective.
The role of the primary somatosensory cortex extends far beyond simply perceiving the position of the knee; it is deeply involved in coordinating complex motor activities that require the synchronization of multiple joints and muscle groups. For example, during activities such as running, jumping, or navigating uneven terrain, the brain relies heavily on the proprioceptive feedback processed by the primary somatosensory cortex to ensure that the knee joint remains stable and properly aligned. This capability is particularly critical in dynamic situations where the body must rapidly adapt to changing conditions, such as a sudden shift in body weight or the presence of an unexpected obstacle. The primary somatosensory cortex’s ability to rapidly integrate and interpret this sensory information allows for quick and precise adjustments in muscle tension and joint positioning, which are essential for preventing falls, missteps, and potential injuries.
In high-pressure situations, such as when an athlete is sprinting or a dancer is executing a complex turn, the primary somatosensory cortex plays a critical role in ensuring that these movements are executed with both accuracy and fluidity. The proprioceptive feedback from the ACL, processed and interpreted by the primary somatosensory cortex, provides the brain with the detailed and real-time information needed to control these movements precisely. For instance, when an athlete makes a sudden pivot during a game, the proprioceptive signals from the ACL inform the brain of the knee’s exact position and movement. This information allows for the fine-tuning of muscle contractions, ensuring that the movement is completed smoothly and without compromising stability. Even minor errors in movement execution can lead to a loss of balance or injury, particularly in activities that demand exact control over joint angles and muscle forces. The continuous and accurate feedback provided by the primary somatosensory cortex is therefore critical for maintaining balance and preventing injury during these high-stakes activities.
Beyond its role in immediate motor control, the primary somatosensory cortex also plays a crucial role in protecting the knee joint from injury. By maintaining an accurate and up-to-date understanding of the knee’s position and movement, the cortex helps prevent excessive strain on the ACL and other joint structures. This protective function is particularly important during high-stress activities, such as intense athletic events, where the risk of ligament tears, sprains, or other injuries is significantly elevated. The ability to dynamically adjust motor commands based on the latest sensory information ensures that the knee joint remains stable, even under challenging conditions, thereby preserving the integrity and function of the joint over time.
Moreover, the primary somatosensory cortex contributes to long-term musculoskeletal health by continuously optimizing movement patterns based on proprioceptive feedback. This process involves the refinement of motor skills through practice and experience, allowing for more efficient and less injury-prone movements. Over time, the integration of sensory feedback into motor planning not only enhances immediate physical performance but also reduces wear and tear on joints, ligaments, and muscles, contributing to overall physical well-being. This adaptive capability is vital for the development of advanced motor skills and the ability to maintain physical health throughout various stages of life.
The role of the primary somatosensory cortex extends into the realm of motor learning, where it supports the continuous improvement of movement efficiency and accuracy. As individuals practice and refine their motor skills, the primary somatosensory cortex helps to encode these movements into long-term motor memory, allowing for the more effortless execution of complex tasks over time. This process is fundamental for mastering activities that require high levels of precision and coordination, such as playing a musical instrument, performing in sports, or engaging in intricate dance routines.
In essence, the primary somatosensory cortex is a cornerstone of the body’s sensory-motor integration system, playing a vital role in maintaining the stability, functionality, and health of the knee joint. Its ability to process and integrate proprioceptive feedback from the ACL is fundamental to the brain’s capacity to coordinate movements, maintain balance, and adapt to a wide range of physical demands. Whether during routine activities like walking or more complex tasks like athletic performance, the primary somatosensory cortex ensures that the body can move with precision, safety, and efficiency. This sophisticated processing underscores the importance of the primary somatosensory cortex in overall motor control, highlighting its crucial role in both immediate physical actions and long-term musculoskeletal health. Through its continuous and dynamic processing of sensory information, the primary somatosensory cortex not only supports daily activities but also plays a central role in the development and refinement of advanced motor skills, ensuring that the body can perform with confidence, control, and resilience in all types of physical endeavors.
Primary Motor Cortex: Orchestrating Voluntary Movement
The primary motor cortex, located in the precentral gyrus of the frontal lobe, is one of the most vital regions of the brain when it comes to controlling voluntary movements. This region is not only responsible for generating the neural signals that initiate movement, but it also plays a critical role in coordinating the precise timing and force of muscle contractions needed to execute those movements with accuracy. The primary motor cortex functions as a sophisticated control center, where the brain’s intentions are translated into specific motor commands that guide the body’s muscles in performing complex tasks. Its involvement in motor control is extensive, covering everything from simple, everyday actions like walking and grasping objects, to highly skilled movements required in sports, dance, and other forms of physical performance.
A key aspect of the primary motor cortex’s functionality is its reliance on proprioceptive information, particularly from structures like the anterior cruciate ligament (ACL), to fine-tune the motor commands that it generates. The ACL is a critical ligament in the knee that provides stability to the joint, especially during dynamic activities that involve sudden changes in direction or rapid acceleration and deceleration. Embedded within the ACL are proprioceptors—specialized sensory receptors that detect changes in the position and tension of the knee joint. These proprioceptors continuously send detailed feedback to the brain about the mechanical state of the knee, including its angle, movement, and the forces acting upon it.
This sensory feedback is relayed to the primary motor cortex through a network of neural pathways that include the primary somatosensory cortex and other brain regions such as the cerebellum and basal ganglia. The primary somatosensory cortex, which is located adjacent to the primary motor cortex, processes and interprets this proprioceptive information, creating a detailed map of the body’s current position in space. This map is constantly updated as the body moves, ensuring that the brain has an accurate and up-to-date understanding of the joint’s orientation and movement dynamics.
The primary motor cortex uses this proprioceptive feedback to adjust its motor output, fine-tuning the activation of muscles around the knee to ensure that the joint remains stable and properly aligned during various physical activities. This fine-tuning is particularly important during dynamic movements such as running, jumping, or pivoting, where the knee joint is subjected to high forces and rapid shifts in position. For example, when a basketball player makes a quick crossover move, the primary motor cortex must rapidly process the proprioceptive feedback from the ACL to coordinate the contraction of the quadriceps, hamstrings, and calf muscles, ensuring that the knee remains stable and that the movement is executed smoothly and effectively.
The primary motor cortex’s role in motor control extends beyond merely responding to immediate sensory inputs; it is also deeply involved in the planning and initiation of complex, goal-directed movements. These movements often require the coordination of multiple muscle groups across different parts of the body, and the primary motor cortex must integrate sensory feedback from the entire musculoskeletal system to achieve this coordination. For instance, in a soccer player preparing to take a shot on goal, the primary motor cortex must not only generate the command to kick the ball but also synchronize the movements of the legs, torso, and arms to maintain balance and control throughout the action. This requires precise timing and force in each muscle contraction, which the primary motor cortex regulates based on real-time feedback from proprioceptors and other sensory receptors.
Moreover, the primary motor cortex is crucial in adapting motor commands based on experience and learning. As an individual practices a particular movement or activity, such as a gymnast perfecting a routine or a musician learning a new piece, the motor cortex refines its neural pathways to optimize the efficiency and accuracy of those movements. This process of motor learning involves the repeated integration of sensory feedback with motor output, allowing the brain to adjust and improve the coordination and execution of movements over time. The primary motor cortex’s ability to adapt and refine motor commands is essential for developing and mastering new skills, as well as for maintaining and improving performance in activities that require high levels of precision and control.
In addition to its role in voluntary movement and motor learning, the primary motor cortex also plays a protective role in maintaining joint integrity and preventing injury. By continuously monitoring the state of the knee joint and adjusting motor outputs accordingly, the primary motor cortex helps to prevent excessive strain on the ACL and other joint structures. This is particularly important in high-stress activities where the risk of injury is elevated, such as in contact sports or intense physical labor. The ability of the primary motor cortex to rapidly adjust muscle contractions in response to proprioceptive feedback ensures that the knee joint remains stable, even under conditions of extreme physical demand, thereby reducing the likelihood of ligament tears, sprains, or other injuries.
Furthermore, the primary motor cortex is involved in the broader network of brain regions that work together to control movement. It interacts closely with the premotor cortex and supplementary motor area, which are involved in the planning and preparation of movements, as well as with the basal ganglia and cerebellum, which help regulate movement initiation, coordination, and balance. This collaborative network allows the brain to manage the complexity of motor control, from the initiation of a simple movement to the execution of highly coordinated, multi-joint actions.
In summary, the primary motor cortex is a central player in the brain’s motor control system, responsible for generating, fine-tuning, and coordinating the motor commands that drive voluntary movement. Its ability to integrate proprioceptive feedback from the ACL and other sensory inputs ensures that movements are precise, effective, and safe, particularly during dynamic activities that place high demands on the knee joint. The primary motor cortex’s role in motor learning and adaptation further highlights its importance in the development and refinement of motor skills, enabling individuals to perform complex tasks with greater efficiency and control. Through its intricate connections with other brain regions and its continuous adjustment of motor outputs, the primary motor cortex plays a critical role in maintaining the body’s functional integrity and movement capabilities across a wide range of physical activities.
Cerebellum: Fine-Tuning and Coordinating Movements
The cerebellum, a critical structure located at the posterior part of the brain beneath the cerebral cortex and just above the brainstem, plays an indispensable role in the coordination, regulation, and refinement of motor activities. Despite its relatively small size compared to other brain regions, the cerebellum houses an extraordinarily dense network of neurons—nearly half of the brain’s total—dedicated to ensuring that our movements are not only smooth and coordinated but also precise and appropriately timed. The cerebellum’s functions are deeply interconnected with the body’s sensory systems, particularly through the extensive proprioceptive inputs it receives from structures like the anterior cruciate ligament (ACL) and other components of the musculoskeletal system. These inputs provide the cerebellum with detailed, real-time information about the position, movement, and mechanical forces acting on the joints, muscles, and tendons throughout the body.
At a molecular level, the cerebellum processes this sensory information through a highly specialized network of cells, including Purkinje cells, granule cells, and climbing fibers, among others. Purkinje cells, which are large neurons with elaborate dendritic trees, are the primary output neurons of the cerebellar cortex. These cells receive inputs from two main types of fibers: mossy fibers, which convey sensory information from the body via the spinal cord and brainstem, and climbing fibers, which originate from the inferior olivary nucleus and carry error signals related to motor performance. The integration of these inputs within the cerebellar cortex allows the cerebellum to fine-tune motor commands, making real-time adjustments that are crucial for maintaining balance, coordination, and smooth execution of movements.
The cerebellum’s ability to integrate and process proprioceptive input is fundamental to tasks that require a high degree of accuracy and coordination. For example, when an individual is balancing on one leg, the cerebellum continuously receives and processes sensory information from the ACL, the muscles, and the joints of the lower limb. This information includes details about the angle of the knee, the tension in the ligaments, the position of the foot, and the distribution of weight. The cerebellum uses this input to make rapid, precise adjustments to muscle activity, ensuring that the body remains stable and upright. This process involves the dynamic regulation of muscle tone, the timing of contractions, and the coordination of multiple muscle groups to counteract any destabilizing forces.
Similarly, when walking on uneven surfaces, the cerebellum plays a critical role in adapting motor commands to the changing terrain. As the foot encounters different textures, slopes, or obstacles, the proprioceptive feedback from the ACL and other joint structures is rapidly processed by the cerebellum. This allows for immediate adjustments in gait, stride length, and foot placement, ensuring that the individual maintains balance and avoids tripping or falling. The cerebellum’s role in these adjustments is so finely tuned that most of these corrections occur automatically, without conscious effort, allowing the individual to navigate challenging environments with confidence and ease.
In the context of more complex athletic maneuvers, the cerebellum’s contributions become even more evident. For instance, consider a soccer player executing a powerful kick or a gymnast performing a series of flips and twists. In these scenarios, the cerebellum is responsible for coordinating the activation of multiple muscle groups across the body, ensuring that each movement is executed with precise timing and force. The cerebellum processes the proprioceptive information from the ACL and other parts of the body to synchronize the movements of the legs, hips, torso, and arms, creating a fluid and cohesive action. This coordination is critical not only for the success of the movement but also for minimizing the risk of injury, as the cerebellum helps ensure that the joints are properly aligned and that excessive strain is avoided.
Beyond its role in real-time motor coordination, the cerebellum is also deeply involved in motor learning, the process by which the brain acquires, refines, and retains new motor skills. This aspect of cerebellar function is particularly important when it comes to adapting to changes in movement patterns, such as those necessitated by an ACL injury or following surgical intervention. After such an injury, the proprioceptive input from the knee joint may be altered, either due to changes in the ligament’s tension and elasticity or due to modifications in joint mechanics resulting from surgery. The cerebellum’s capacity for plasticity—its ability to reorganize and form new neural connections—allows it to recalibrate motor commands based on the new proprioceptive feedback it receives.
During the rehabilitation process, the cerebellum is actively engaged in helping the individual relearn how to move efficiently and safely. For example, as a patient begins physical therapy following an ACL reconstruction, the cerebellum continuously processes the altered sensory input from the knee and adjusts motor outputs to compensate for any deficits in stability or proprioception. Over time, through repetitive practice and motor learning, the cerebellum refines these adjustments, leading to improvements in balance, coordination, and overall motor function. This adaptability is crucial for the recovery of functional movement and for the patient’s ability to return to their pre-injury level of activity.
Furthermore, the cerebellum’s involvement in motor learning extends to its ability to store and refine motor memories. As an individual repeatedly practices a specific movement or skill, such as a tennis serve or a dance routine, the cerebellum optimizes the motor commands by reducing unnecessary movements and enhancing the efficiency of the desired action. This process, known as motor memory consolidation, allows the movement to become more automatic and less dependent on conscious control. The cerebellum’s role in this process is essential for the development of expertise in any motor activity, whether it is a complex athletic performance or a simple daily task.
In addition to its well-established roles in motor control and learning, emerging research suggests that the cerebellum may also contribute to cognitive processes and emotional regulation, further highlighting its importance in overall brain function. While traditionally viewed as a structure primarily concerned with motor activity, the cerebellum’s extensive connections to other parts of the brain, including the prefrontal cortex and limbic system, suggest that it may also play a role in tasks such as problem-solving, attention, and emotional processing. This broader view of cerebellar function underscores the complexity and versatility of this brain region, as it contributes to both the physical and cognitive aspects of human behavior.
In summary, the cerebellum is a central brain structure that plays an essential role in coordinating, regulating, and refining motor activity. Its ability to process and integrate proprioceptive input from the ACL and other parts of the body is critical for maintaining balance, executing precise movements, and ensuring that actions are smooth, coordinated, and well-timed. The cerebellum’s involvement in motor learning enables the body to adapt to changes in movement patterns following an ACL injury or surgical intervention, making it vital for rehabilitation and the recovery of functional movement. Beyond its contributions to motor control, the cerebellum’s role in cognitive and emotional processes highlights its importance in the overall functioning of the brain. This intricate and adaptable system allows individuals to perform a wide range of physical activities with confidence, precision, and grace, ensuring that the body can meet the demands of both everyday life and peak athletic performance.
Basal Ganglia: Regulating and Modulating Movement
The basal ganglia, a collection of deep brain nuclei including the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra, are integral to the regulation of voluntary movement, motor planning, and the initiation of precise and purposeful actions. These structures are not only involved in the execution of movement but also play a central role in modulating motor signals to ensure that movements are fluid, well-timed, and efficient. The basal ganglia operate as a critical processing center, receiving a multitude of inputs from various regions of the brain, particularly the somatosensory cortex, which provides detailed proprioceptive feedback from the anterior cruciate ligament (ACL) and other parts of the body. This feedback is crucial for the basal ganglia to assess the current state of the body, including the position and tension of joints and muscles, which is essential for maintaining stability and coordination during movement.
At the core of the basal ganglia’s function is their ability to act as a gatekeeper for motor commands. This process involves the delicate balance between facilitating desired movements and suppressing unwanted or competing motor activities. The basal ganglia achieve this by influencing the activity of the primary motor cortex and other motor-related areas, such as the supplementary motor area (SMA) and premotor cortex. The interaction between these brain regions is mediated through complex neural circuits that involve excitatory and inhibitory pathways. One key pathway is the direct pathway, which facilitates the initiation of voluntary movements by reducing inhibition of the thalamus, thereby allowing it to activate motor areas of the cortex. Conversely, the indirect pathway increases inhibition of the thalamus, which suppresses competing or potentially disruptive motor programs. This dual pathway system allows the basal ganglia to fine-tune motor output, ensuring that only the most appropriate and efficient movements are executed.
The basal ganglia’s ability to manage the selection and suppression of motor programs is particularly important in dynamic and rapidly changing environments. For instance, when an athlete is engaged in a fast-paced game and needs to make a quick directional change, the basal ganglia play a crucial role in coordinating this action. The decision to change direction is initiated in higher brain centers, such as the prefrontal cortex, which sends signals to the basal ganglia. The basal ganglia then integrate this decision with sensory information from the somatosensory cortex, which includes proprioceptive data from the ACL about the knee joint’s position, movement, and tension. This integration enables the basal ganglia to modulate motor output by facilitating the activation of specific muscles needed to execute the directional change, while simultaneously inhibiting other muscles that might interfere with the movement. This precise control is essential for ensuring that the movement is smooth and efficient, preventing any unnecessary or conflicting actions that could lead to a loss of balance or injury.
Moreover, the basal ganglia are deeply involved in the timing of movements, which is critical for coordinating complex motor tasks that require precise sequencing of muscle contractions. For example, in activities such as playing a musical instrument, performing a dance routine, or executing a complex athletic maneuver, the timing of each movement must be carefully regulated to achieve the desired outcome. The basal ganglia contribute to this process by ensuring that the initiation and progression of each movement are timed correctly, allowing for a seamless transition between different phases of the task. This temporal coordination is achieved through the integration of inputs from various sensory and cognitive sources, allowing the basal ganglia to anticipate the timing of future movements based on the current state of the body and the demands of the task.
In addition to their role in real-time motor control, the basal ganglia are also crucial for motor learning and the reinforcement of motor skills. Motor learning involves the process of acquiring new motor behaviors through practice and experience, and the basal ganglia are central to this process. The substantia nigra, one of the nuclei within the basal ganglia, plays a key role in motor learning through its production of dopamine, a neurotransmitter that is critical for signaling reward and motivation. When an individual successfully performs a motor task, such as hitting a target or completing a movement sequence, dopamine is released, reinforcing the neural circuits involved in that action. Over time, this reinforcement strengthens the connections within these circuits, making the movement more automatic and efficient. This process, known as reinforcement learning, allows the basal ganglia to contribute to the refinement of motor skills, enabling individuals to improve their performance through repetition and practice.
The basal ganglia’s involvement in motor learning is particularly important in the context of recovery from injury or adaptation to new physical challenges. For instance, after an ACL injury or surgery, the proprioceptive feedback from the knee joint may be altered, requiring the individual to relearn how to control the joint effectively. The basal ganglia play a crucial role in this rehabilitation process by helping to recalibrate motor commands based on the new sensory input. Through repeated practice and motor learning, the basal ganglia facilitate the adaptation of movement patterns, allowing the individual to regain stability, coordination, and functional movement.
Beyond their role in motor control and learning, the basal ganglia also have broader implications for cognitive and emotional functions. Recent research has revealed that the basal ganglia are involved in various non-motor processes, including decision-making, habit formation, and emotional regulation. These functions are mediated through the basal ganglia’s extensive connections with other brain regions, such as the prefrontal cortex and the limbic system. For example, the basal ganglia’s role in decision-making involves evaluating the potential outcomes of different actions and selecting the most appropriate course of action based on past experiences and current goals. Similarly, in the context of habit formation, the basal ganglia help to automate repetitive behaviors, allowing individuals to perform routine tasks with minimal conscious effort.
In clinical settings, the importance of the basal ganglia is highlighted by the impact of their dysfunction on movement and behavior. Disorders such as Parkinson’s disease and Huntington’s disease, which involve degeneration of specific parts of the basal ganglia, lead to severe motor impairments and cognitive disturbances. In Parkinson’s disease, the loss of dopaminergic neurons in the substantia nigra results in decreased facilitation of movement, leading to symptoms such as bradykinesia (slowness of movement), rigidity, and tremors. These symptoms underscore the basal ganglia’s critical role in enabling smooth and coordinated movement.
In conclusion, the basal ganglia are a complex and highly interconnected network of nuclei that play a central role in the regulation of voluntary movement, motor planning, and the initiation of smooth, efficient, and well-timed actions. By integrating sensory and cognitive inputs, particularly proprioceptive feedback from the ACL and other parts of the body, the basal ganglia modulate motor responses to ensure that movements are precise, purposeful, and appropriate for the given context. Their ability to manage the selection and suppression of motor programs is essential for performing complex motor tasks, particularly in dynamic and rapidly changing environments. Additionally, the basal ganglia are crucial for motor learning and the reinforcement of motor skills, allowing individuals to refine their movements through practice and experience. Through their intricate and multifaceted functions, the basal ganglia are vital for maintaining the fluidity, accuracy, and effectiveness of voluntary movements, as well as for supporting cognitive and emotional processes that influence behavior and decision-making.
Supplementary Motor Area (SMA) and Premotor Cortex: Planning and Preparing Movements
The supplementary motor area (SMA) and the premotor cortex are two critical regions of the brain that play a fundamental role in the planning, preparation, and execution of complex movements, particularly those that require the coordination of multiple joints and muscle groups. Located in the frontal lobe, these regions are intricately connected with various sensory and motor areas of the brain, making them essential hubs for integrating sensory inputs and generating motor outputs that are precise, coordinated, and adaptive to the demands of the environment. Their function goes beyond simple movement initiation; they are deeply involved in the high-level orchestration of motor activities, ensuring that every phase of a movement is meticulously timed and aligned with the body’s overall motor strategy.
The SMA is particularly important for the initiation and planning of movements that are internally generated, meaning those that are deliberate and premeditated rather than reactions to external stimuli. This region is involved in the sequencing of movements, helping to organize the order in which different muscle groups are activated. For example, when a gymnast prepares to perform a complex routine, the SMA is responsible for planning the intricate sequence of movements, such as the timing of a flip, the rotation of the torso, and the precise landing of the feet. This planning process involves the integration of proprioceptive feedback from the anterior cruciate ligament (ACL) and other proprioceptors, which provide real-time information about the position and tension of joints and muscles. The SMA uses this information to refine the motor plan, ensuring that each movement is executed with the correct timing and force, thereby contributing to the fluidity and precision of the routine.
In addition to its role in movement planning, the SMA is also involved in motor learning and the development of motor skills. As individuals practice and refine their movements, the SMA helps to encode these motor patterns into long-term memory, allowing for the more effortless execution of these movements in the future. This process of motor learning is crucial for mastering complex skills that require a high degree of coordination, such as playing a musical instrument, performing athletic feats, or executing intricate dance moves. Over time, the SMA fine-tunes these motor patterns based on feedback from successful and unsuccessful attempts, leading to improved performance and the ability to execute movements with greater ease and accuracy.
The premotor cortex, on the other hand, is more engaged in the preparation and planning of movements in response to external cues. This region is responsible for integrating sensory information from the environment with proprioceptive feedback from the body to generate motor plans that are adaptive to the specific demands of the task at hand. For example, when a tennis player prepares to return a fast serve, the premotor cortex processes visual information about the trajectory and speed of the ball, while also considering proprioceptive feedback from the ACL and other joints about the body’s current position and readiness to move. The premotor cortex then generates a motor plan that involves the coordinated activation of the leg, hip, and arm muscles to position the body correctly and execute the return stroke with precision and power.
The premotor cortex is also crucial for anticipatory postural adjustments, which are necessary to maintain balance and stability during movement. Before a movement is even initiated, the premotor cortex prepares the body by adjusting muscle tone and joint angles to ensure that the body remains stable throughout the movement. This is particularly important for activities that involve rapid or unpredictable changes in posture, such as navigating uneven terrain, jumping, or quickly changing direction during a sporting event. By integrating proprioceptive feedback with sensory data from the environment, the premotor cortex ensures that the body is optimally prepared to execute the movement while maintaining balance and preventing falls or injuries.
The coordination between the SMA and premotor cortex is essential for the successful execution of complex motor tasks. These regions work together to ensure that motor commands are not only accurately timed but also precisely sequenced and adapted to the specific context of the movement. This coordination is particularly critical for activities that involve the lower limbs, such as running, jumping, or climbing, where the integration of movements across the knee, hip, and ankle joints is necessary to achieve the desired movement outcome. For instance, when running, the SMA and premotor cortex collaborate to synchronize the rhythmic flexion and extension of the legs, the stabilization of the hips, and the propulsion of the body forward, all while adjusting to changes in terrain and maintaining balance.
By processing proprioceptive information from the ACL and other sensory inputs, the SMA and premotor cortex help to ensure that the timing and sequencing of muscle activations are appropriately coordinated. This allows for smooth and efficient movement, reducing the likelihood of errors and minimizing the risk of injury. The ability of these brain regions to dynamically adjust motor plans based on real-time feedback is crucial for maintaining joint health and optimizing physical performance, particularly during high-stress activities that demand both strength and precision.
In the broader context of motor control, the SMA and premotor cortex are integral components of a sophisticated network of brain structures that work together to process sensory information, generate motor commands, and refine movements in real-time. The primary somatosensory cortex, for instance, plays a key role in integrating proprioceptive feedback with other sensory inputs to create a detailed representation of the body’s position in space. This information is then used by the primary motor cortex to generate and fine-tune motor commands, ensuring that movements are executed with precision. The cerebellum further adjusts and refines these movements, enhancing coordination and accuracy, while the basal ganglia regulate movement initiation and suppression to achieve smooth and efficient actions.
Finally, the SMA and premotor cortex are responsible for the high-level planning and preparation of complex movements, integrating proprioceptive feedback with motor plans to ensure that movements are executed with precise timing and coordination. Together, these brain structures form a dynamic and interconnected system that enables the body to maintain balance, execute precise movements, and adapt to changes in the environment. This network ensures the stability and functionality of the knee joint during a wide range of physical activities, from routine tasks like walking to more complex actions like athletic performance.
The continuous and dynamic processing of sensory information by the SMA, premotor cortex, and other motor-related brain regions not only supports immediate motor actions but also plays a critical role in the long-term development and refinement of motor skills. This process is essential for maintaining overall musculoskeletal health and ensuring that the body can perform with confidence, control, and resilience in all types of physical endeavors. Through their intricate interplay, these brain regions enable the body to move efficiently and effectively, preserving joint integrity and enhancing physical performance across a lifetime.
Comprehensive Rehabilitation Approaches
When the anterior cruciate ligament (ACL) is injured, the disruption of its intricate neurophysiological network has profound and extensive consequences that ripple throughout the entire motor control system. This disruption is not confined to the knee joint itself; it has wide-ranging effects on various brain structures and neural pathways that are crucial for maintaining balance, coordination, and precise movement. The immediate loss of mechanoreceptors within the damaged ligament—such as Ruffini endings, Pacinian corpuscles, Golgi tendon organs, and free nerve endings—results in a significant reduction in proprioceptive input to the central nervous system (CNS). These mechanoreceptors play a critical role in continuously monitoring the mechanical state of the knee joint, providing the brain with essential information about joint position, movement, and the forces acting on the joint. Without this input, the brain’s ability to accurately perceive and respond to the position and movement of the knee is severely compromised.
The reduction in proprioceptive feedback from the ACL has immediate and detrimental effects on the primary somatosensory cortex, which is responsible for processing and integrating sensory information from the body to create a detailed map of the body’s physical state. This map is essential for both conscious awareness of body posture and the subconscious control of movement. When the sensory input from the ACL is diminished or lost, the primary somatosensory cortex struggles to maintain an accurate and dynamic representation of the knee’s position and movement. This degraded proprioceptive map leads to a distorted perception of the knee’s spatial orientation, resulting in impaired motor planning and execution. The brain’s ability to coordinate and fine-tune movements is compromised, leading to less precise and less efficient motor outputs.
This impairment is particularly concerning when it comes to the primary motor cortex, which relies on the information processed by the somatosensory cortex to generate and adjust motor commands. The primary motor cortex plays a crucial role in initiating and controlling voluntary movements, and its ability to do so accurately depends heavily on the quality of the sensory input it receives. When this input is compromised, the motor commands generated by the primary motor cortex become less precise, leading to movements that are awkward, uncoordinated, and potentially unsafe. For instance, the lack of accurate proprioceptive feedback from the ACL may cause an individual to misjudge the position of their knee during a movement, leading to improper foot placement, altered gait mechanics, or an inability to maintain balance during dynamic activities. These deficits not only impair performance but also increase the risk of further injury, as the knee joint may be subjected to abnormal forces or stresses that it is not equipped to handle.
The cerebellum, another key player in the motor control system, is also adversely affected by the loss of proprioceptive input from the ACL. The cerebellum is responsible for refining and coordinating movements, ensuring that they are smooth, precise, and well-timed. It constantly monitors and adjusts motor commands based on sensory feedback, allowing for real-time corrections that enhance movement accuracy and fluidity. However, when the proprioceptive input from the ACL is disrupted, the cerebellum’s ability to perform these functions is significantly diminished. The lack of reliable sensory feedback makes it difficult for the cerebellum to detect and correct errors in movement, leading to a decline in coordination and motor control. As a result, tasks that require precise timing and coordination—such as balancing on one leg, adjusting to uneven surfaces, or performing complex athletic maneuvers—become much more challenging. The cerebellum’s reduced capacity to refine and coordinate movements may manifest as unsteady, jerky, or imprecise movements, which not only compromise performance but also heighten the risk of injury due to poor movement control.
The basal ganglia, which are involved in regulating movement initiation and suppression, also suffer from the loss of proprioceptive feedback. The basal ganglia play a critical role in selecting and filtering motor programs, ensuring that the most appropriate and efficient motor actions are carried out while suppressing unnecessary or competing movements. When the proprioceptive input from the ACL is compromised, the basal ganglia’s ability to modulate motor responses is impaired. This can lead to the persistence of abnormal movement patterns, as the brain struggles to suppress maladaptive motor programs that develop in response to the altered sensory environment. These abnormal patterns often involve compensatory movements, where the body attempts to redistribute load or stabilize the knee by engaging other muscles and joints. While these compensatory strategies may provide temporary stability, they can place excessive strain on other parts of the body, such as the hips, ankles, or lower back, leading to secondary injuries or chronic pain conditions.
In response to the altered sensory input from the injured ACL, the brain undergoes a process known as neuroplasticity, where it reorganizes and adapts its neural pathways to accommodate the new sensory environment. Neuroplasticity is a fundamental mechanism that allows the brain to recover from injury and adapt to changes in the body, but in the context of an ACL injury, this reorganization can have both positive and negative consequences. Functional MRI (fMRI) and electroencephalography (EEG) studies have shown that individuals with ACL injuries often exhibit increased activity in brain regions associated with visual and vestibular processing. This shift in neural activity suggests that the brain compensates for the loss of proprioceptive input by relying more heavily on visual and vestibular cues to maintain balance, posture, and motor control.
While this compensatory shift helps individuals maintain some level of function in the absence of reliable proprioceptive feedback, it also comes with significant drawbacks. The increased reliance on visual and vestibular inputs places a greater cognitive load on the brain, as it must consciously process information that would otherwise be managed automatically by the proprioceptive system. This heightened cognitive demand can lead to slower and less efficient motor responses, particularly in situations that require rapid adjustments or reflexive actions. For example, during a sudden change in direction or an unexpected perturbation, the lack of immediate proprioceptive feedback from the ACL can result in delayed or inappropriate motor responses. This delay in response time increases the risk of re-injury, as the body may not be able to react quickly enough to protect the knee joint from further damage.
Moreover, the neuroplastic changes that occur in response to an ACL injury are not always beneficial in the long term. The brain’s increased dependence on visual and vestibular inputs may lead to persistent deficits in proprioception, even after the ACL has been surgically repaired and rehabilitated. These persistent proprioceptive deficits can manifest as ongoing difficulties with balance, coordination, and neuromuscular control, which may affect an individual’s ability to return to pre-injury levels of physical activity. The altered sensory processing and motor patterns that develop post-injury can become ingrained, making it challenging to fully restore normal movement mechanics. This can result in a cycle of injury and re-injury, as the brain continues to rely on compensatory strategies that are less efficient and more prone to error than the original proprioceptive mechanisms.
In addition to the impact on physical performance and injury risk, the cognitive load associated with compensatory reliance on visual and vestibular inputs can also affect mental performance. The need to consciously monitor and adjust movements can lead to increased mental fatigue, reduced focus, and impaired decision-making, particularly during high-intensity activities that require both physical and cognitive exertion. This can further compromise performance and increase the likelihood of errors that could lead to injury.
The disruption of the neurophysiological network following an ACL injury highlights the profound interconnectedness of the sensory and motor systems in the body. The loss of proprioceptive input from the ACL has a cascading effect on various brain structures and neural pathways, leading to widespread impairments in motor control, coordination, and balance. While the brain’s ability to adapt through neuroplasticity provides some compensation for these deficits, it also introduces new challenges that can complicate recovery and long-term rehabilitation. Understanding these mechanisms is crucial for developing effective treatment and rehabilitation strategies that address both the physical and neurological aspects of recovery, ultimately aiming to restore full functionality and reduce the risk of future injuries. This holistic approach to rehabilitation must consider not only the repair of the ACL itself but also the retraining of the brain and body to reintegrate proprioceptive feedback into the motor control system, ensuring that the individual can regain confidence, control, and efficiency in their movements.
Molecular Responses to ACL Injury
At the molecular level, an ACL injury initiates a complex and highly coordinated cascade of biological events designed to repair and remodel the damaged ligament tissue. This process begins almost immediately after the injury occurs, triggering an acute inflammatory response that is critical for clearing damaged cells and initiating tissue repair. This initial phase is characterized by the release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). These cytokines are secreted by a variety of immune cells, including macrophages, neutrophils, and mast cells, which are rapidly recruited to the site of injury. These immune cells are attracted by chemokines and other signaling molecules released by the damaged tissue.
The cytokines IL-1, TNF-α, and IL-6 play central roles in the inflammatory response by promoting the expression of adhesion molecules on endothelial cells, which facilitates the extravasation of immune cells from the bloodstream into the injured tissue. Once in the tissue, these immune cells release additional pro-inflammatory mediators, including more cytokines, chemokines, and reactive oxygen species (ROS). This inflammatory milieu is essential for breaking down and removing necrotic tissue, a process that is mediated in part by the upregulation of matrix metalloproteinases (MMPs), enzymes that degrade extracellular matrix (ECM) components such as collagen, elastin, and proteoglycans.
The activation of MMPs following an ACL injury is largely regulated by the nuclear factor-kappa B (NF-κB) signaling pathway. NF-κB is a critical transcription factor that is normally kept inactive in the cytoplasm through its association with an inhibitory protein called IκB. In response to inflammatory signals such as IL-1 and TNF-α, IκB is phosphorylated by the IκB kinase (IKK) complex, leading to its ubiquitination and subsequent degradation by the proteasome. This degradation frees NF-κB to translocate to the nucleus, where it binds to specific DNA sequences known as κB sites in the promoter regions of target genes. These target genes include those encoding MMPs, cytokines, and other inflammatory mediators.
While the activation of MMPs is necessary for clearing damaged ECM components and making way for new tissue formation, excessive MMP activity can be detrimental. Overactive MMPs can degrade healthy tissue as well, leading to the breakdown of cartilage and other joint structures. This excessive degradation is a key factor in the development of post-traumatic osteoarthritis (PTOA), a chronic condition characterized by the progressive loss of articular cartilage, subchondral bone remodeling, and joint inflammation. In PTOA, the balance between ECM degradation and synthesis is disrupted, leading to joint instability, pain, and functional impairment.
Another critical player in the molecular response to ACL injury is transforming growth factor-beta (TGF-β), a cytokine that has profound effects on cell behavior and ECM production. TGF-β exists in a latent form that is activated by mechanical stress, proteolytic cleavage, or interaction with integrins. Upon activation, TGF-β binds to its receptors on the surface of target cells, initiating a signaling cascade that involves the phosphorylation of receptor-regulated SMAD proteins (R-SMADs). These R-SMADs form a complex with SMAD4, which then translocates to the nucleus to regulate the transcription of genes involved in ECM production, fibrosis, and tissue remodeling.
In the context of ACL injury, TGF-β plays a dual role. On one hand, it promotes the synthesis of new ECM components, such as type I and type III collagen, fibronectin, and proteoglycans, which are necessary for repairing the damaged ligament. On the other hand, TGF-β is a potent inducer of fibrosis, leading to the accumulation of scar tissue within the joint. While fibrosis is a natural part of the healing process, excessive fibrosis can result in the formation of stiff, non-elastic tissue that impairs the normal function of the knee joint. The fibrotic tissue may limit the range of motion, increase joint stiffness, and compromise the overall stability of the knee, making it more prone to future injuries.
TGF-β also interacts with other signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K)/Akt pathway, which further influence cellular responses such as proliferation, differentiation, and survival. The crosstalk between TGF-β signaling and these other pathways adds additional layers of complexity to the regulation of tissue repair and fibrosis, underscoring the need for precise control over TGF-β activity during the healing process.
In addition to the roles of cytokines and growth factors, neurotrophic factors such as brain-derived neurotrophic factor (BDNF) are also critically involved in the response to ACL injury, particularly in the context of neuromuscular recovery and proprioception. BDNF is a member of the neurotrophin family, which also includes nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). These neurotrophins are crucial for the survival, differentiation, and maintenance of neurons, as well as for synaptic plasticity, which is essential for learning and memory.
BDNF is synthesized as a precursor protein, proBDNF, which is cleaved to produce mature BDNF. This mature BDNF then binds to the tropomyosin receptor kinase B (TrkB) receptor on the surface of neurons, leading to the activation of several downstream signaling pathways, including the PI3K/Akt pathway, the MAPK/ERK pathway, and the phospholipase C-gamma (PLCγ) pathway. These pathways promote neuronal survival, growth, and synaptic plasticity, which are critical for the recovery of proprioceptive function following ACL injury.
Following ACL injury, the expression of BDNF is upregulated in response to both mechanical stress and inflammatory signals. BDNF plays a key role in supporting the survival and regeneration of proprioceptive nerve endings that are essential for detecting joint position, movement, and tension. The re-establishment of proprioceptive feedback is crucial for restoring neuromuscular control, maintaining balance, and preventing re-injury. BDNF also enhances synaptic plasticity in the central nervous system, potentially facilitating the neuroplastic changes necessary for adapting to altered sensory input following injury. This suggests that BDNF is a vital molecular link between the physical repair of the ligament and the recovery of the neurophysiological processes that underlie movement coordination and stability.
The molecular biology of ACL injury and repair is further complicated by the involvement of additional signaling molecules and pathways, such as insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), and hypoxia-inducible factors (HIFs). IGF-1, for example, promotes cell proliferation, differentiation, and ECM production, while VEGF is crucial for angiogenesis, the formation of new blood vessels that supply oxygen and nutrients to the healing tissue. HIFs, on the other hand, are activated in response to the hypoxic conditions that often occur in injured tissues, where they promote the expression of genes involved in angiogenesis, energy metabolism, and cell survival.
The interplay between these various signaling molecules and pathways highlights the complexity of the body’s response to ACL injury. Each of these factors must be carefully regulated to ensure that the repair process proceeds efficiently without leading to excessive inflammation, fibrosis, or other complications. Dysregulation of these molecular pathways can result in suboptimal healing, chronic pain, and long-term deficits in joint function, underscoring the importance of developing targeted therapeutic strategies that modulate these pathways in a controlled manner.
Therapeutic approaches that target specific molecular pathways involved in ACL injury and repair hold significant potential for improving outcomes. For example, inhibitors of specific MMPs could be used to prevent excessive ECM degradation, while modulators of TGF-β signaling could be employed to balance tissue regeneration with the prevention of fibrosis. Similarly, therapies that enhance BDNF signaling or other neurotrophic factors may support the recovery of proprioceptive function and improve neuromuscular control following ACL reconstruction. As research in this area continues to advance, it is likely that new molecular targets and therapeutic strategies will be identified, leading to more effective treatments for ACL injuries and better long-term outcomes for patients.
Interaction Between Neurophysiology and Molecular Biology
The complex interplay between neurophysiological mechanisms and molecular biology is intricately woven into the pathophysiology of ACL injury, with far-reaching implications for both the immediate response to injury and the long-term processes involved in recovery. At the molecular level, an ACL injury initiates a highly sophisticated and coordinated cascade of biochemical events that serve dual purposes: directing the repair of the damaged ligament tissue and simultaneously influencing neural processes within the joint and the central nervous system. These molecular events not only dictate the pace and quality of tissue healing but also play a critical role in modulating pain perception, proprioception, and motor control, all of which are crucial for effective rehabilitation and long-term joint health (
Table 3).
When the ACL is injured, the body responds almost immediately by activating a series of inflammatory and immune responses designed to mitigate damage and initiate the healing process. This acute inflammatory response is characterized by the rapid release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). These cytokines are secreted by immune cells like macrophages, neutrophils, and mast cells that are swiftly recruited to the injury site. Their release triggers a complex signaling cascade that includes the activation of key molecular pathways such as the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. These pathways are critical for the upregulation of genes involved in inflammation, immune response, and tissue repair, including those encoding for matrix metalloproteinases (MMPs), enzymes that break down the extracellular matrix (ECM) to allow for tissue remodeling.
The inflammatory response, while necessary for initiating repair, also has profound effects on the neural processes within the joint. The pro-inflammatory cytokines released during this phase sensitize nociceptors—the sensory neurons responsible for detecting pain—by increasing the expression of ion channels such as transient receptor potential vanilloid 1 (TRPV1) and acid-sensing ion channels (ASICs). This sensitization leads to heightened pain perception, known as hyperalgesia, which can significantly alter movement patterns as the body instinctively tries to avoid pain. These altered movement patterns can disrupt the normal proprioceptive feedback mechanisms that provide the brain with real-time information about joint position and movement, leading to impaired motor control and stability.
Proprioception, which is critical for maintaining joint stability and coordinating complex movements, is heavily reliant on the accurate transmission of sensory signals from mechanoreceptors within the ACL and surrounding joint structures. When these signals are disrupted by pain or inflammation, the resulting deficits in proprioception can exacerbate the mechanical stress on the injured tissue. This increased stress can perpetuate the inflammatory response, creating a vicious cycle that hinders the healing process and complicates rehabilitation efforts. The interplay between these molecular and neurophysiological factors underscores the complexity of ACL injury recovery, where each aspect of the body’s response is interconnected with others, making the overall outcome dependent on the delicate balance of these processes.
As the initial inflammatory phase subsides, the body shifts into a reparative phase, where the focus is on rebuilding the damaged ligament tissue. This phase is marked by the activity of growth factors such as transforming growth factor-beta (TGF-β), which plays a key role in promoting the synthesis of new extracellular matrix components like collagen and fibronectin. TGF-β also regulates the proliferation and differentiation of fibroblasts, the cells responsible for producing the ECM. However, TGF-β has a dual role; while it promotes tissue repair, it also induces fibrosis, leading to the formation of scar tissue. Excessive fibrosis can impair the flexibility and functionality of the knee joint, leading to stiffness and a reduced range of motion, which are common challenges in ACL recovery.
Concurrently, neuroplasticity—the brain’s ability to reorganize itself in response to injury—is activated to compensate for the altered sensory input from the injured joint. Neuroplastic changes are facilitated by neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which supports the survival, growth, and differentiation of neurons, including those involved in proprioception. BDNF binds to its receptor, tropomyosin receptor kinase B (TrkB), initiating signaling cascades that promote synaptic plasticity and the regeneration of proprioceptive nerve endings. This neuroplasticity is crucial for restoring neuromuscular control and adapting motor patterns to the changes in joint mechanics caused by the injury and subsequent repair processes.
The ongoing interaction between these molecular and neurophysiological mechanisms determines the effectiveness of rehabilitation and the long-term health of the knee joint. For instance, if inflammation is not adequately controlled, it can lead to chronic pain and persistent proprioceptive deficits, which may result in maladaptive movement patterns that increase the risk of re-injury. Conversely, successful modulation of these molecular pathways—through targeted therapies that reduce inflammation, enhance neuroplasticity, and promote balanced tissue remodeling—can lead to more effective recovery and better long-term outcomes.
In summary, the complex interplay between neurophysiological mechanisms and molecular biology in the context of ACL injury is deeply embedded in the pathophysiology of the condition and plays a pivotal role in shaping the processes involved in recovery. The molecular events triggered by the injury not only direct the physical repair of the ligament but also profoundly influence neural processes that are critical for pain management, proprioception, and motor control. Understanding and modulating these molecular and neurophysiological interactions is key to developing more effective therapeutic strategies that can improve rehabilitation outcomes, prevent re-injury, and ensure long-term joint health.
Inflammatory Response and Molecular Signaling
When the ACL is injured, the body’s immediate response is to trigger an acute inflammatory reaction, a vital first step in initiating the healing process. This response is orchestrated by the release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). These cytokines are produced and released by various immune cells, including macrophages, neutrophils, and mast cells, which are rapidly recruited to the site of injury through chemotactic signals. Upon arrival, these immune cells release the cytokines into the local environment, where they engage with specific receptors on the surface of various target cells, including fibroblasts, endothelial cells, and other immune cells. The binding of these cytokines to their respective receptors triggers a cascade of intracellular signaling pathways, most notably the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, which are pivotal in propagating and sustaining the inflammatory response.
The NF-κB pathway is a critical mediator of inflammation and plays a central role in regulating the immune response following ACL injury. Under normal conditions, NF-κB is sequestered in the cytoplasm by an inhibitory protein called IκB. However, when pro-inflammatory cytokines such as IL-1 and TNF-α bind to their receptors, they activate upstream kinases like IκB kinase (IKK). This kinase phosphorylates IκB, marking it for ubiquitination and subsequent degradation by the proteasome. The degradation of IκB frees NF-κB, allowing it to translocate into the nucleus. Once in the nucleus, NF-κB binds to specific DNA sequences known as κB sites within the promoter regions of various target genes. These genes include those encoding additional pro-inflammatory cytokines, chemokines, adhesion molecules, and matrix metalloproteinases (MMPs), which collectively amplify the inflammatory response and recruit more immune cells to the injury site.
The upregulation of MMPs is particularly significant in the context of ACL injury. MMPs are a family of zinc-dependent proteolytic enzymes that are essential for the remodeling of the extracellular matrix (ECM), a complex network of proteins and polysaccharides that provide structural support to tissues. In the damaged ACL, MMPs such as MMP-1 (collagenase), MMP-3 (stromelysin), and MMP-9 (gelatinase) are significantly upregulated in response to NF-κB activation. These enzymes degrade various components of the ECM, including collagen, elastin, and proteoglycans, which are critical for the structural integrity of the ligament and surrounding tissues. The controlled degradation of these ECM components by MMPs is necessary for removing damaged tissue, clearing space for new tissue formation, and allowing for the migration of repair cells such as fibroblasts and mesenchymal stem cells to the injury site.
However, the activity of MMPs must be tightly regulated to prevent excessive ECM degradation, which can have detrimental effects on joint health. If MMP activity is unchecked, it can lead to the breakdown of not only the damaged ECM but also the surrounding healthy tissue, weakening the overall structure of the joint. This excessive degradation disrupts the balance between ECM breakdown and synthesis, a balance that is crucial for maintaining the integrity of the joint. When this balance is lost, as can occur in the aftermath of an ACL injury, it can lead to the development of post-traumatic osteoarthritis (PTOA). PTOA is characterized by the progressive degradation of articular cartilage, subchondral bone sclerosis, and chronic inflammation, ultimately leading to joint instability, pain, and loss of function.
The MAPK pathway, another key signaling pathway activated by pro-inflammatory cytokines, also contributes to the regulation of MMP expression and the broader inflammatory response. MAPKs, which include the extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinases (JNKs), and p38 MAPKs, are activated by a variety of extracellular stimuli, including cytokines and stress signals. Upon activation, MAPKs phosphorylate a range of target proteins, including transcription factors such as AP-1, which cooperate with NF-κB to enhance the transcription of genes involved in inflammation and ECM remodeling. The MAPK pathway also plays a role in the proliferation, differentiation, and apoptosis of cells involved in the repair process, further highlighting its importance in the overall response to ACL injury.
In addition to their role in ECM remodeling, MMPs and other enzymes regulated by NF-κB and MAPK pathways also influence the inflammatory milieu by modulating the activity of cytokines and growth factors. For instance, MMPs can cleave and activate latent TGF-β (transforming growth factor-beta), a cytokine that plays a dual role in tissue repair by promoting ECM synthesis and fibrosis. While TGF-β is essential for the healing process, its overactivation can lead to excessive fibrosis, resulting in the formation of scar tissue that impairs the flexibility and function of the knee joint.
The regulation of ECM degradation and synthesis is further complicated by the involvement of tissue inhibitors of metalloproteinases (TIMPs), which are natural inhibitors of MMPs. TIMPs bind to MMPs and inhibit their proteolytic activity, thus preventing excessive ECM breakdown. The balance between MMPs and TIMPs is crucial for ensuring proper tissue remodeling; a disruption in this balance can lead to either excessive tissue degradation or the accumulation of fibrotic tissue, both of which are detrimental to joint health.
Overall, the molecular response to ACL injury is a highly coordinated process that involves the interplay of numerous signaling pathways, cytokines, and enzymes. The careful regulation of these molecular processes is essential for ensuring effective tissue repair while minimizing the risk of chronic inflammation, excessive fibrosis, and joint degeneration. Understanding these molecular mechanisms provides valuable insights into potential therapeutic targets for enhancing ACL recovery and preventing long-term complications such as PTOA. For example, strategies that modulate NF-κB or MAPK signaling, control MMP activity, or balance the actions of TIMPs could be used to optimize the healing process, promote proper ECM remodeling, and preserve joint function following an ACL injury.
Impact on Nociception and Pain Pathways
The inflammatory cytokines released in response to ACL injury have profound effects not only on the cellular and molecular environment within the joint but also on the neural circuits that govern pain perception and motor control. Among the most significant impacts of these cytokines is their ability to sensitize nociceptors—the specialized sensory neurons responsible for detecting painful stimuli. Key pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) play crucial roles in modulating the activity of nociceptors by influencing the expression and functionality of various ion channels on these neurons, particularly transient receptor potential vanilloid 1 (TRPV1) channels and acid-sensing ion channels (ASICs).
TRPV1 is a non-selective cation channel that is highly sensitive to temperature changes, particularly to heat, and to chemical stimuli such as capsaicin, the active component in chili peppers. In the context of inflammation, TRPV1 channels are activated by protons (H+ ions) and other endogenous ligands released during tissue injury, leading to the sensation of pain. The activation of TRPV1 channels by pro-inflammatory cytokines like IL-1 and TNF-α enhances their sensitivity to heat and acidic conditions, which are characteristic of inflamed tissues. This sensitization is mediated through several intracellular signaling pathways, including the phospholipase C (PLC) pathway and the protein kinase C (PKC) pathway, both of which are upregulated in response to inflammatory mediators. The phosphorylation of TRPV1 by PKC, for example, lowers the activation threshold of these channels, meaning that they become more easily triggered by stimuli that would not normally cause pain.
Similarly, ASICs, which are also non-selective cation channels, are activated by extracellular acidosis, a condition often found in inflamed and injured tissues due to the accumulation of lactic acid and other metabolic byproducts. ASICs contribute to the sensation of pain by depolarizing nociceptors in response to acidic pH levels. Inflammatory cytokines such as IL-1 and TNF-α enhance the expression of ASICs on nociceptors, thereby increasing the neurons’ responsiveness to acidic environments. The increased expression and activity of ASICs contribute to the heightened pain sensitivity observed in inflammatory conditions, a phenomenon known as hyperalgesia.
The combined sensitization of TRPV1 and ASICs on nociceptors leads to a state of hyperalgesia, where the nociceptors exhibit an exaggerated response to both noxious and non-noxious stimuli. This hyperactive state means that even minor movements or normal mechanical pressures that would typically be painless can trigger intense pain. The experience of this pain can significantly alter an individual’s movement patterns as they unconsciously adopt strategies to minimize discomfort. For instance, individuals might avoid placing weight on the affected limb, alter their gait to reduce joint loading, or limit the range of motion to prevent aggravating the pain. While these compensatory movements may provide temporary relief, they can lead to secondary issues.
The altered movement patterns associated with hyperalgesia can place abnormal stress on the joint and surrounding tissues. For example, by favoring one leg over the other or by adopting an unnatural gait, the individual may inadvertently increase the load on other joints, muscles, and ligaments, potentially leading to overuse injuries or strain in these areas. Moreover, these compensatory strategies can disrupt the normal biomechanics of the knee, leading to inefficient movement patterns that strain the injured ACL and surrounding structures. This increased stress can further damage the already compromised tissues, exacerbating the initial injury and perpetuating the inflammatory response.
The ongoing inflammation driven by continued cytokine release and the mechanical stress from altered movement patterns create a vicious cycle, where pain, inflammation, and abnormal joint loading continuously reinforce each other. This feedback loop not only complicates the healing process but also increases the risk of developing chronic pain conditions and long-term joint dysfunction. As the inflammatory response persists, it may lead to structural changes in the joint, such as fibrosis, cartilage degradation, and the development of post-traumatic osteoarthritis (PTOA). These changes can further impair joint function and contribute to the persistence of pain, making it more difficult for the individual to fully recover and return to normal activities.
At the molecular level, this feedback loop is driven by the sustained activation of signaling pathways such as the NF-κB and MAPK pathways, which continue to promote the expression of pro-inflammatory cytokines and the upregulation of ion channels like TRPV1 and ASICs. The prolonged activation of these pathways can also lead to the expression of genes involved in chronic pain states, such as those encoding for calcitonin gene-related peptide (CGRP) and substance P, which are neuropeptides that further sensitize nociceptors and promote neurogenic inflammation.
Moreover, the neuroplastic changes that occur in response to chronic pain and inflammation can lead to central sensitization, a condition in which the central nervous system (CNS) becomes hypersensitive to sensory inputs. Central sensitization is characterized by an amplification of pain signals within the spinal cord and brain, leading to persistent pain even in the absence of ongoing tissue damage. This phenomenon is mediated by several molecular mechanisms, including the increased expression of NMDA (N-methyl-D-aspartate) receptors, alterations in GABAergic (gamma-aminobutyric acid) inhibitory pathways, and the upregulation of neurotrophic factors like brain-derived neurotrophic factor (BDNF), which can facilitate the persistence of pain by enhancing synaptic plasticity in pain pathways.
In conclusion, the inflammatory cytokines released in response to ACL injury have significant molecular impacts on nociceptors by sensitizing key ion channels such as TRPV1 and ASICs, leading to hyperalgesia and altered movement patterns. These changes can perpetuate a cycle of pain and inflammation that complicates recovery and increases the risk of chronic pain and joint dysfunction. Understanding the molecular basis of these interactions provides crucial insights into potential therapeutic strategies aimed at breaking this feedback loop, such as targeting specific ion channels, modulating inflammatory signaling pathways, and addressing central sensitization to improve pain management and support more effective rehabilitation following ACL injury.
Proprioception and Neuromuscular Control
The disruption of normal movement due to pain and inflammation in the aftermath of an ACL injury not only impairs the immediate physical function of the knee joint but also has profound effects on the body’s proprioceptive system. Proprioception, the body’s ability to sense the position, movement, and load of its joints and muscles, is critical for coordinating motor responses, maintaining joint stability, and ensuring smooth, efficient movements. This complex sensory feedback system is primarily mediated by specialized mechanoreceptors located in the ACL and surrounding joint structures, including Ruffini endings, Pacinian corpuscles, and Golgi tendon organs. These mechanoreceptors are responsible for detecting changes in joint angles, muscle tension, and the dynamics of movement, and they relay this information to the central nervous system (CNS), where it is integrated and processed to guide motor output.
At the molecular level, these mechanoreceptors are highly specialized sensory neurons equipped with a variety of ion channels, receptors, and signaling molecules that allow them to respond to mechanical stimuli. Ruffini endings, for instance, are sensitive to sustained pressure and stretch within the joint capsule and ligaments, and they contribute to the detection of joint angle and the direction of movement. Pacinian corpuscles are rapidly adapting receptors that detect high-frequency vibrations and sudden changes in joint position, making them crucial for sensing dynamic movements. Golgi tendon organs, which are located at the junctions of muscles and tendons, monitor the tension within tendons and provide feedback about the force of muscle contractions.
These mechanoreceptors rely on mechanically gated ion channels, such as Piezo channels, to transduce mechanical forces into electrical signals. When the ACL is injured, or when inflammation and pain disrupt normal movement, the mechanical environment of the joint changes dramatically. The altered joint mechanics can impair the normal functioning of these mechanoreceptors by affecting the opening and closing of these ion channels, leading to a disruption in the proprioceptive signals sent to the CNS. For example, the Piezo1 and Piezo2 channels, which are critical for mechanotransduction in proprioceptive neurons, may be less effectively activated due to changes in joint pressure, tension, and movement patterns. This can lead to a decrease in the accuracy and reliability of proprioceptive feedback.
The disruption of proprioceptive feedback has significant consequences for neuromuscular control. Proprioception is integral to the body’s ability to execute coordinated movements and maintain joint stability, particularly during complex activities such as walking, running, or jumping. When proprioceptive input is diminished or becomes unreliable due to an ACL injury or compensatory movement patterns, the CNS receives distorted or incomplete information about the position and movement of the knee joint. This can lead to inappropriate motor responses, such as the activation of the wrong muscle groups or the generation of insufficient or excessive muscle force. Consequently, the knee joint may become improperly aligned during movement, leading to abnormal loading patterns.
Abnormal joint loading exacerbates the mechanical stress on the injured tissue, further perpetuating the inflammatory response. The ongoing inflammation not only delays the healing process by disrupting the normal tissue repair mechanisms but also contributes to the degradation of the extracellular matrix (ECM) within the joint. This degradation is mediated by enzymes such as matrix metalloproteinases (MMPs), which are upregulated in response to inflammatory cytokines. The breakdown of ECM components such as collagen and proteoglycans weakens the structural integrity of the ACL and surrounding tissues, making the joint more susceptible to further injury.
In addition to the direct effects on joint stability, impaired proprioception can hinder the effective reorganization of motor patterns during rehabilitation. Neuroplasticity—the brain’s ability to adapt and reorganize itself in response to injury—is a critical component of recovery from ACL injury. Effective rehabilitation relies on the CNS’s ability to integrate proprioceptive feedback with visual, vestibular, and other sensory inputs to recalibrate motor patterns and restore normal movement. However, when proprioceptive feedback is compromised, the brain may struggle to accurately map the body’s movements and position in space. This can lead to the persistence of maladaptive movement patterns, which not only impair functional recovery but also increase the risk of re-injury.
On a molecular level, the impaired proprioceptive feedback and the resulting deficits in neuromuscular control are linked to alterations in synaptic plasticity within the CNS. Synaptic plasticity, the ability of synapses to strengthen or weaken over time in response to activity, is essential for learning and memory, including motor learning. Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) play a key role in supporting synaptic plasticity by promoting the growth and differentiation of neurons and the formation of new synaptic connections. However, when proprioceptive signals are distorted or absent, the neural circuits involved in motor control may not receive the appropriate stimuli needed to drive neuroplastic changes. This can result in a failure to fully adapt to the altered mechanical environment of the knee, leading to incomplete or ineffective motor learning.
Furthermore, the lack of accurate proprioceptive input can lead to central sensitization, a condition where the CNS becomes hyper-responsive to sensory stimuli. Central sensitization is characterized by increased excitability of neurons in the spinal cord and brain, leading to amplified pain responses and a heightened perception of discomfort even in the absence of significant tissue damage. This phenomenon is driven by molecular changes such as the upregulation of NMDA (N-methyl-D-aspartate) receptors and alterations in GABAergic (gamma-aminobutyric acid) inhibitory pathways. Central sensitization can exacerbate the pain experienced by individuals with ACL injuries, further complicating rehabilitation and prolonging recovery.
The interaction between impaired proprioception, altered motor control, and central sensitization underscores the complexity of ACL injury recovery and highlights the need for comprehensive rehabilitation strategies that address both the sensory and motor aspects of the injury. Therapeutic approaches that aim to restore proprioceptive function—such as proprioceptive training exercises, neuromuscular re-education, and the use of biofeedback—are essential for recalibrating the CNS and improving joint stability. Additionally, interventions that target the molecular pathways involved in synaptic plasticity and central sensitization, such as BDNF signaling or NMDA receptor modulation, could enhance the effectiveness of rehabilitation and reduce the risk of re-injury.
In summary, the disruption of proprioceptive feedback following an ACL injury has profound molecular and physiological consequences that extend beyond the immediate effects on joint stability. The impairment of mechanoreceptor function and the resulting deficits in neuromuscular control can lead to abnormal joint loading, perpetuate inflammation, and hinder the reorganization of motor patterns necessary for recovery. Understanding the molecular biology underlying these processes is crucial for developing targeted therapies that can optimize proprioceptive function, enhance neuroplasticity, and support effective rehabilitation following ACL injury.
Neuroplasticity and Recovery
One of the most critical aspects of recovery from ACL injury is the process of neuroplasticity, the brain’s remarkable ability to reorganize itself by forming new neural connections in response to changes in sensory input or injury. Neuroplasticity is a dynamic process that enables the central nervous system (CNS) to adapt to the altered sensory input following an injury, which is essential for the restoration of proprioceptive function and motor control. This adaptive process involves not only the reorganization of existing neural circuits but also the formation of new synaptic connections, which are crucial for recalibrating motor patterns and restoring joint stability.
At the molecular level, neuroplasticity is supported by a family of proteins known as neurotrophic factors, which are critical for the survival, growth, and differentiation of neurons. Among these, brain-derived neurotrophic factor (BDNF) is particularly important in the context of ACL injury recovery. BDNF is a member of the neurotrophin family, which also includes nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). These neurotrophins play essential roles in the development and maintenance of the nervous system, and their expression is tightly regulated in response to physiological and pathological stimuli.
Following an ACL injury, the expression of BDNF is upregulated in response to mechanical stress, inflammatory signals, and changes in neural activity. This upregulation is part of the body’s natural response to injury, aimed at promoting the survival and regeneration of damaged neurons, particularly those involved in proprioception and motor control. BDNF exerts its effects by binding to its high-affinity receptor, tropomyosin receptor kinase B (TrkB), which is expressed on the surface of neurons and other cell types. The binding of BDNF to TrkB activates a cascade of intracellular signaling pathways that are crucial for neuroplasticity and neural repair.
One of the key signaling pathways activated by the BDNF-TrkB interaction is the phosphoinositide 3-kinase (PI3K)/Akt pathway. This pathway plays a pivotal role in promoting cell survival by inhibiting apoptotic processes and supporting the growth and differentiation of neurons. Upon activation, PI3K catalyzes the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a lipid molecule that recruits and activates protein kinase B (Akt). Activated Akt then phosphorylates a variety of downstream targets that promote cell survival and growth, including the inhibition of pro-apoptotic proteins such as Bad and the activation of mTOR (mechanistic target of rapamycin), which stimulates protein synthesis and cell growth. In the context of ACL injury, the PI3K/Akt pathway supports the survival of proprioceptive neurons that may be at risk due to altered mechanical loading and inflammatory damage, thereby preserving the neural circuits necessary for proprioception.
Another critical pathway activated by BDNF is the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. The MAPK/ERK pathway is involved in regulating gene expression, synaptic plasticity, and cellular differentiation. Upon BDNF binding to TrkB, the receptor undergoes autophosphorylation, which creates docking sites for adapter proteins such as Shc, leading to the activation of the MAPK/ERK cascade. This cascade culminates in the phosphorylation and activation of ERK, which then translocates to the nucleus, where it regulates the transcription of genes involved in neuronal survival, growth, and plasticity. In the context of neuroplasticity following ACL injury, the MAPK/ERK pathway contributes to the formation of new synaptic connections and the strengthening of existing ones, which are essential for recalibrating motor patterns and restoring normal movement.
The phospholipase C-gamma (PLCγ) pathway is another important signaling cascade activated by BDNF-TrkB interaction. PLCγ is an enzyme that hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into two secondary messengers: inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to receptors on the endoplasmic reticulum, leading to the release of calcium ions (Ca2+) into the cytoplasm, while DAG activates protein kinase C (PKC). The increase in intracellular Ca2+ and the activation of PKC are crucial for various cellular processes, including the regulation of synaptic plasticity. In neurons, these signals contribute to the modulation of synaptic strength and the growth of dendritic spines, the small protrusions on neurons where synapses are formed. By enhancing synaptic plasticity, the PLCγ pathway supports the regeneration of nerve endings and the re-establishment of functional neural circuits involved in proprioception and motor control.
Beyond the direct effects on neurons, BDNF and its downstream signaling pathways also influence the microenvironment within the injured joint, which is critical for successful recovery. For instance, BDNF has been shown to modulate the activity of glial cells, including astrocytes and microglia, which play essential roles in maintaining homeostasis in the CNS and supporting neural repair. Activated astrocytes, in response to BDNF signaling, can release neuroprotective factors and support the re-establishment of the blood-brain barrier, which may be compromised following injury. Microglia, the resident immune cells of the CNS, can be influenced by BDNF to adopt a more anti-inflammatory and neuroprotective phenotype, reducing the chronic inflammation that can otherwise impair neuroplasticity and prolong recovery.
Additionally, BDNF-TrkB signaling has been implicated in the regulation of neurotransmitter systems that are critical for motor control. For example, BDNF can enhance the function of glutamatergic synapses, which are essential for excitatory signaling in the brain. By modulating the release and uptake of glutamate, BDNF can influence synaptic plasticity and the strength of neural circuits involved in movement and proprioception. Moreover, BDNF has been shown to interact with the GABAergic system, which provides inhibitory control over neural activity. By fine-tuning the balance between excitatory and inhibitory neurotransmission, BDNF helps to maintain the stability and flexibility of neural networks during the recovery process.
The interplay between these molecular pathways underscores the importance of BDNF in supporting the neuroplastic changes required for the recovery of proprioceptive function and motor control following ACL injury. However, the regulation of BDNF expression and signaling is complex and can be influenced by various factors, including genetic predispositions, the extent of the injury, and environmental stimuli such as physical activity and rehabilitation exercises. For instance, physical exercise has been shown to increase BDNF levels, suggesting that rehabilitation programs that incorporate specific types of exercise may enhance the neuroplasticity and overall recovery process by boosting BDNF signaling.
In conclusion, the recovery from ACL injury is deeply dependent on the process of neuroplasticity, which allows the CNS to adapt to altered sensory input and restore motor control. Neuroplastic changes are driven by neurotrophic factors like BDNF, which, through its interaction with TrkB receptors, activates critical signaling pathways such as PI3K/Akt, MAPK/ERK, and PLCγ. These pathways promote the survival and regeneration of proprioceptive neurons, enhance synaptic plasticity, and support the reorganization of neural circuits necessary for proprioception and motor function. Understanding the molecular biology underlying these processes provides valuable insights into potential therapeutic strategies that could optimize recovery and improve outcomes for individuals recovering from ACL injuries.
Cross-Talk Between Inflammatory and Neurotrophic Pathways
The effectiveness of neuroplasticity during recovery from ACL injury is intricately tied to the delicate balance between pro-inflammatory and neurotrophic signals, which together orchestrate the processes of tissue repair, neural adaptation, and overall functional recovery. Neuroplasticity, the brain’s ability to reorganize itself by forming new neural connections, is critical for restoring proprioceptive function and motor control after injury. However, the success of these neuroplastic changes is highly dependent on the modulation of the inflammatory response and the integrity of the extracellular matrix (ECM), both of which can significantly influence the healing environment.
Chronic inflammation is one of the major challenges in achieving effective neuroplasticity during ACL recovery. Prolonged activation of the nuclear factor-kappa B (NF-κB) pathway, a central regulator of inflammation, can lead to the sustained production of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). While these cytokines are essential for initiating the inflammatory response and recruiting immune cells to the site of injury, their prolonged activity can have detrimental effects on the nervous system. One of the critical consequences of chronic inflammation is the production of neurotoxic molecules such as nitric oxide (NO) and reactive oxygen species (ROS).
NO, a small diffusible molecule produced by nitric oxide synthases (NOS), plays a dual role in the body. At physiological levels, NO is involved in various signaling processes, including vasodilation and neurotransmission. However, during chronic inflammation, inducible NOS (iNOS) is upregulated, leading to the excessive production of NO. This excessive NO can react with superoxide anions (O2-) to form peroxynitrite (ONOO-), a potent oxidant that can cause significant damage to cellular components, including lipids, proteins, and DNA. Peroxynitrite and other ROS can induce oxidative stress, which disrupts the redox balance within cells and leads to the activation of apoptotic pathways. In neurons, oxidative stress can cause damage to mitochondria, impairing energy production and leading to cell death. Moreover, oxidative damage to synaptic proteins and receptors can impair synaptic plasticity, counteracting the beneficial effects of neurotrophic factors like brain-derived neurotrophic factor (BDNF).
BDNF is crucial for promoting neuronal survival, synaptic plasticity, and the regeneration of neural circuits during recovery. It exerts its effects by binding to the tropomyosin receptor kinase B (TrkB) receptor, triggering downstream signaling pathways such as phosphoinositide 3-kinase (PI3K)/Akt and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), which are involved in promoting cell survival and synaptic growth. However, in the presence of chronic inflammation and oxidative stress, the signaling efficacy of BDNF can be compromised. For instance, ROS can alter the structure and function of TrkB receptors or their associated signaling molecules, reducing the neuroprotective and neuroplastic effects of BDNF. Furthermore, oxidative modifications of transcription factors involved in the BDNF signaling pathway can impair the expression of genes essential for neuroplasticity, thus hindering the recovery process.
The extracellular matrix (ECM) is another critical factor that modulates the balance between inflammation and neuroplasticity. The ECM is a complex network of proteins and polysaccharides that provides structural support to tissues and plays a key role in cell signaling. It serves as a reservoir for various growth factors, including transforming growth factor-beta (TGF-β) and BDNF, which are sequestered within the matrix and released upon tissue injury or remodeling. Following ACL injury, enzymes such as matrix metalloproteinases (MMPs) are upregulated in response to inflammatory cytokines and mechanical stress. MMPs degrade ECM components like collagen and proteoglycans, facilitating tissue remodeling and the release of growth factors that are essential for tissue repair and neuroplasticity.
The controlled degradation of the ECM by MMPs is necessary for the proper release of growth factors like BDNF, which can then bind to their receptors on neurons and support neuroplastic changes. However, if MMP activity is excessive or unregulated, it can lead to the over-degradation of the ECM, resulting in the loss of structural integrity within the joint and impaired signaling. This excessive ECM breakdown can reduce the availability of growth factors necessary for healing and neuroplasticity, thus disrupting the balance needed for effective recovery. Additionally, the degradation products of the ECM, such as fragmented collagen and proteoglycans, can themselves have pro-inflammatory effects, further perpetuating the cycle of chronic inflammation and tissue damage.
Moreover, the ECM plays a significant role in mechanotransduction—the process by which cells sense and respond to mechanical stimuli. Mechanoreceptors in the ECM, such as integrins, are linked to the cytoskeleton of cells and can transmit mechanical signals from the ECM to the intracellular signaling machinery. These signals can influence cellular behaviors such as proliferation, differentiation, and apoptosis, all of which are crucial for tissue repair and regeneration. In the context of neuroplasticity, the mechanical properties of the ECM can influence the growth and orientation of neural processes, thereby affecting the reorganization of neural circuits necessary for proprioceptive recovery. A disrupted ECM can impair these mechanotransduction pathways, leading to suboptimal neuroplastic outcomes.
The interplay between the ECM and inflammatory signals also affects the expression and activation of TGF-β, a multifunctional cytokine that has complex roles in tissue repair, fibrosis, and inflammation. TGF-β is stored in a latent form in the ECM and is activated by mechanical stress, proteolytic enzymes like MMPs, or interactions with integrins. While TGF-β is essential for promoting ECM synthesis and wound healing, its overactivation can lead to excessive fibrosis, resulting in the formation of scar tissue that impairs joint flexibility and function. In the nervous system, TGF-β signaling has been shown to interact with neurotrophic pathways, influencing neuroplasticity and neural repair. However, chronic inflammation and excessive ECM degradation can disrupt the normal regulation of TGF-β, leading to imbalances that impair both tissue repair and neuroplasticity.
Given the complex molecular interactions between inflammation, oxidative stress, ECM remodeling, and neuroplasticity, controlling the inflammatory response is crucial for facilitating effective rehabilitation after ACL injury. Strategies that target the NF-κB pathway to reduce chronic inflammation, while preserving the necessary inflammatory response for tissue repair, could help mitigate the production of neurotoxic factors like ROS and NO. Additionally, therapies aimed at protecting or restoring ECM integrity, such as the use of MMP inhibitors or ECM-mimicking scaffolds, could enhance the availability of growth factors like BDNF and support the mechanotransduction processes necessary for effective neuroplasticity.
In conclusion, the effectiveness of neuroplasticity during recovery from ACL injury is deeply influenced by the balance between pro-inflammatory and neurotrophic signals, as well as the integrity of the ECM. Chronic inflammation and oxidative stress can undermine neuroplastic processes by damaging neurons and synapses, while excessive ECM degradation can disrupt the release of growth factors and impair mechanotransduction. Understanding these molecular interactions is essential for developing therapeutic strategies that optimize the healing environment, enhance neuroplasticity, and improve functional outcomes following ACL injury.
Potential Therapeutic Approaches
The intricate molecular biology of ACL injury and recovery offers a wealth of opportunities for developing targeted therapeutic strategies that not only enhance tissue healing but also prevent long-term complications and optimize functional outcomes. These strategies involve both pharmacological and non-pharmacological approaches, each designed to address the multifaceted challenges posed by ACL injuries by targeting specific molecular pathways and supporting the body’s natural healing processes.
Pharmacological interventions hold significant potential in modulating the key molecular pathways involved in ACL recovery. One such intervention is the use of Matrix Metalloproteinase (MMP) inhibitors. MMPs play a crucial role in the remodeling of the extracellular matrix (ECM), which is vital for clearing damaged tissue and facilitating new tissue formation. However, excessive MMP activity can lead to over-degradation of the ECM, compromising the structural integrity of the joint and increasing the risk of post-traumatic osteoarthritis (PTOA). By selectively inhibiting specific MMPs, it is possible to limit this excessive degradation, thereby preserving the ECM’s structure and function. This not only protects the joint from further damage but also ensures that the ECM can continue to serve as a reservoir for growth factors such as BDNF and TGF-β, which are essential for supporting neuroplasticity and tissue repair. Proper regulation of MMP activity can thus create a more favorable environment for both tissue regeneration and the re-establishment of neural pathways essential for proprioception and motor control.
Another critical target for pharmacological intervention is the NF-κB signaling pathway, a key regulator of the inflammatory response. Prolonged activation of NF-κB can lead to chronic inflammation, resulting in the production of neurotoxic factors such as nitric oxide (NO) and reactive oxygen species (ROS). These molecules can cause oxidative stress, damaging neurons and synapses, and thereby impairing synaptic plasticity, which is crucial for neuroplasticity and effective rehabilitation. Inhibitors of the NF-κB pathway can help control the inflammatory response, reducing the production of these harmful molecules, and preventing further tissue damage. Moreover, by mitigating oxidative stress, these inhibitors can enhance the efficacy of neurotrophic factors like BDNF, whose signaling pathways can be compromised by the presence of ROS. This dual action of controlling inflammation while promoting neuroplasticity could significantly improve the outcomes of ACL recovery by ensuring that the nervous system remains responsive to rehabilitation efforts.
Enhancing BDNF signaling or increasing its levels represents another promising pharmacological approach. BDNF is a critical neurotrophic factor that supports the survival, growth, and differentiation of neurons, particularly those involved in proprioception and motor control. By enhancing BDNF expression or mimicking its activity, it is possible to promote the brain’s ability to reorganize itself in response to the altered sensory input following ACL injury. This could involve the use of BDNF analogs, TrkB agonists (which activate the BDNF receptor), or small molecules that upregulate BDNF production. Enhancing BDNF signaling not only supports the recovery of proprioceptive function but also accelerates the reorganization of motor patterns, reducing the risk of re-injury and improving overall functional outcomes. This approach could be particularly beneficial when combined with physical therapies that provide the mechanical stimuli necessary to maximize the effects of increased BDNF levels on neuroplasticity.
Non-pharmacological approaches are equally critical in the recovery process. Physical therapy is foundational to ACL rehabilitation, playing a crucial role in restoring motor function, reducing pain, and preventing re-injury. The mechanical stimuli provided by physical therapy are essential for driving neuroplastic changes in the central nervous system (CNS). These stimuli help recalibrate proprioceptive feedback mechanisms, restore normal movement patterns, and strengthen the muscles surrounding the knee joint. Physical therapy also enhances the effectiveness of pharmacological interventions by providing the physical challenges necessary for the brain to adapt and reorganize, thereby reinforcing the molecular changes induced by treatments such as BDNF enhancers or MMP inhibitors.
Proprioceptive training specifically targets the sensory feedback systems that are often impaired following an ACL injury. Exercises that challenge balance, coordination, and joint position sense are designed to retrain the nervous system to accurately perceive and respond to joint movements. This type of training can help restore the proprioceptive input that is critical for coordinating muscle activity and maintaining joint stability. By improving proprioceptive function, this training reduces the likelihood of abnormal joint loading and subsequent injury, supporting long-term joint health. Moreover, proprioceptive training can complement molecular therapies by providing the sensory experiences necessary to maximize neuroplastic changes, particularly in the context of BDNF signaling. This synergy between sensory training and molecular therapy can significantly enhance the recovery process, leading to more robust and lasting improvements in joint function.
Neuromuscular re-education is another essential non-pharmacological approach. This type of therapy focuses on retraining the neuromuscular system to respond appropriately to proprioceptive inputs. It is particularly important for correcting maladaptive movement patterns that may have developed as a result of pain, inflammation, or altered proprioception. By improving the timing and coordination of muscle contractions, neuromuscular re-education can help restore normal joint alignment and function, reducing the risk of further injury. This re-education process also helps reinforce the neural circuits involved in motor control, making it easier for the CNS to adapt to the changes brought about by pharmacological treatments, such as those targeting BDNF or the NF-κB pathway. By integrating neuromuscular re-education into the rehabilitation process, patients can achieve a higher level of functional recovery, ensuring that their movements are both efficient and safe.
The integration of pharmacological and non-pharmacological therapies offers a comprehensive approach to ACL injury recovery. Pharmacological interventions can provide the molecular support needed to modulate inflammation, protect neural structures, and enhance neuroplasticity, while non-pharmacological therapies offer the mechanical and sensory stimuli necessary to drive these molecular changes. For instance, a patient undergoing treatment with BDNF enhancers might engage in proprioceptive training and physical therapy to maximize the effects of increased BDNF levels on neuroplasticity and motor function. Similarly, patients receiving NF-κB inhibitors could benefit from neuromuscular re-education to ensure that the reduction in inflammation translates into improved movement patterns and joint stability. This holistic approach, which combines the strengths of both pharmacological and non-pharmacological strategies, is likely to yield the best outcomes, improving the quality of life for individuals recovering from ACL injuries and reducing the long-term impact of these injuries on joint health and overall mobility.
In conclusion, the molecular biology of ACL injury and recovery is characterized by a highly intricate interplay between various molecular components, including pro-inflammatory cytokines, neurotrophic factors, and extracellular matrix (ECM) elements. These components do not operate in isolation; rather, they form a dynamic and interconnected network that profoundly influences the pathophysiology of the injury and the subsequent healing processes. The pro-inflammatory cytokines, such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), play a pivotal role in initiating the body’s inflammatory response, which is essential for clearing damaged tissue and preventing infection. However, if this inflammatory response is not properly regulated, it can lead to chronic inflammation, which can be detrimental to tissue repair and overall joint health.
Neurotrophic factors, most notably brain-derived neurotrophic factor (BDNF), are critical for promoting the survival, growth, and differentiation of neurons, particularly those involved in proprioception and motor control. BDNF and other neurotrophic factors help support neuroplasticity, the process by which the central nervous system (CNS) reorganizes itself to adapt to the altered sensory input following an ACL injury. This reorganization is essential for restoring proprioceptive function and motor control, which are often compromised after such an injury. However, the effectiveness of these neuroplastic changes is highly dependent on the balance between neurotrophic support and the potentially damaging effects of chronic inflammation. Excessive inflammation, driven by sustained activation of the NF-κB pathway, can lead to the production of neurotoxic molecules such as reactive oxygen species (ROS) and nitric oxide (NO), which can damage neurons and synapses, thereby impairing synaptic plasticity and hindering the recovery process.
The ECM also plays a crucial role in the recovery process, serving not only as a structural scaffold for tissue repair but also as a reservoir for growth factors like BDNF and transforming growth factor-beta (TGF-β). These growth factors are sequestered within the ECM and released in response to mechanical stress and tissue remodeling, providing the necessary signals for tissue regeneration and neuroplasticity. However, excessive degradation of the ECM, often driven by overactive matrix metalloproteinases (MMPs), can disrupt this balance, leading to the loss of structural integrity within the joint and impaired signaling that is crucial for effective healing. This degradation can also result in the release of ECM degradation products, which can have pro-inflammatory effects, further perpetuating the cycle of chronic inflammation and tissue damage.
The balance between these molecular signals—pro-inflammatory cytokines, neurotrophic factors, and ECM components—is critical for determining the overall outcome of recovery. When this balance is optimal, it supports effective tissue repair, the reorganization of neural circuits, and the restoration of normal joint function. Conversely, an imbalance, such as excessive inflammation or uncontrolled ECM degradation, can lead to poor healing outcomes, including chronic pain, joint instability, and an increased risk of re-injury.
By targeting specific molecular pathways, there is significant potential to enhance the recovery process. For instance, inhibitors of the NF-κB pathway could be used to reduce chronic inflammation and its neurotoxic effects, while MMP inhibitors could help preserve ECM integrity and support proper tissue remodeling. Enhancing BDNF signaling could promote neuroplasticity and improve proprioceptive recovery, thereby reducing the likelihood of re-injury and enhancing long-term joint health.
This integrated approach, which considers both the molecular and neurophysiological aspects of ACL injury, holds great promise for developing more effective therapeutic strategies. Such strategies would not only address the immediate needs of joint repair but also support the neuromuscular recovery that is critical for long-term function and mobility. By understanding and modulating the complex molecular interactions involved in ACL injury and recovery, it may be possible to create comprehensive treatment plans that enhance healing, prevent complications, and ultimately improve the quality of life for individuals recovering from these injuries. This holistic approach, which combines targeted molecular therapies with supportive physical rehabilitation, represents a promising frontier in the treatment of ACL injuries, offering hope for more efficient and complete recoveries in the future.
Implications for Treatment and Rehabilitation
The insights gleaned from understanding the neurophysiological and molecular biology of ACL injury and recovery offer significant potential for the development of advanced, targeted treatment strategies that go far beyond traditional rehabilitation methods. While traditional approaches focus primarily on restoring joint range of motion, strength, and proprioception, they often do not fully address the underlying molecular and neurophysiological deficits that can hinder complete recovery. These deficits include disrupted proprioceptive feedback, altered motor control, and chronic inflammation, all of which can significantly impact the healing process if not adequately managed. Addressing these issues at the molecular and neurophysiological levels is crucial for optimizing recovery and reducing the likelihood of re-injury.
At the molecular level, ACL injury triggers an intricate cascade of inflammatory responses, which are necessary for initiating tissue repair but can become detrimental if sustained over time. The release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) activates the nuclear factor-kappa B (NF-κB) pathway, a key regulator of inflammation. While the NF-κB pathway is crucial for initiating the healing process, its prolonged activation can lead to chronic inflammation, resulting in the overproduction of reactive oxygen species (ROS) and nitric oxide (NO). These molecules induce oxidative stress, which can damage cellular components, including lipids, proteins, and DNA, impairing the function and survival of neurons and other cells critical for tissue repair and neuroplasticity.
Oxidative stress poses a significant challenge during ACL recovery because it disrupts the balance between cell survival and apoptosis (programmed cell death). ROS can activate apoptosis through the mitochondrial pathway by inducing the release of cytochrome c from mitochondria, which in turn activates caspases—enzymes that dismantle the cell. In neurons, oxidative stress can damage synaptic structures, impairing synaptic plasticity, a process essential for neuroplasticity and the reorganization of neural circuits following injury. Disrupted synaptic plasticity can impede the recovery of proprioceptive function and motor control, increasing the risk of long-term deficits and re-injury.
To counteract the negative effects of chronic inflammation and oxidative stress, targeted pharmacological interventions are being explored. NF-κB inhibitors offer a promising therapeutic approach by reducing the chronic activation of inflammatory pathways, thereby decreasing the production of ROS and NO. By protecting neurons and other critical cells from oxidative damage, NF-κB inhibitors can enhance the effectiveness of neurotrophic factors like brain-derived neurotrophic factor (BDNF), which play a pivotal role in neuroplasticity.
BDNF is essential for the survival, growth, and differentiation of neurons, particularly those involved in proprioception and motor control. BDNF binds to its receptor, tropomyosin receptor kinase B (TrkB), activating downstream signaling pathways such as the phosphoinositide 3-kinase (PI3K)/Akt pathway, the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, and the phospholipase C-gamma (PLCγ) pathway. These pathways promote cell survival, synaptic plasticity, and dendritic spine growth, which are crucial for forming and strengthening synaptic connections. Enhancing BDNF signaling during ACL recovery can support the reorganization of neural circuits necessary for proprioceptive recovery and motor control, potentially accelerating neuroplasticity and improving long-term functional outcomes.
The extracellular matrix (ECM) also plays a crucial role in the molecular response to ACL injury. The ECM provides structural support to tissues and serves as a reservoir for growth factors such as BDNF and transforming growth factor-beta (TGF-β), which are sequestered within the matrix and released in response to mechanical stress or tissue remodeling. Matrix metalloproteinases (MMPs), enzymes that degrade ECM components like collagen and proteoglycans, facilitate tissue remodeling and the controlled release of these growth factors. However, excessive MMP activity can lead to over-degradation of the ECM, compromising joint integrity and impairing the signaling processes necessary for effective tissue repair and neuroplasticity. Targeting MMPs with specific inhibitors can help preserve ECM integrity, ensuring that the ECM continues to provide structural support and controlled release of growth factors essential for healing and recovery.
Beyond pharmacological interventions, non-pharmacological approaches play a vital role in optimizing recovery by providing the necessary mechanical and sensory stimuli to drive molecular changes that are essential for effective healing. These approaches not only complement traditional rehabilitation techniques but also target specific biological pathways that are often overlooked in conventional therapies, thereby accelerating recovery and improving functional outcomes.
One of the most promising non-pharmacological techniques is Blood Flow Restriction (BFR) training, which has gained considerable attention for its ability to enhance muscle hypertrophy and strength even with low-load resistance training. This is particularly advantageous during the early stages of rehabilitation when high-load exercises may be contraindicated due to the vulnerability of the healing tissue. BFR training involves the application of a tourniquet or cuff to restrict venous blood flow from the working muscles while maintaining arterial inflow. This creates a hypoxic (low oxygen) environment within the muscle, which mimics the effects of high-intensity exercise.
The hypoxia induced by BFR training triggers a cascade of molecular responses, most notably the stabilization of hypoxia-inducible factors (HIFs). HIFs are transcription factors that play a crucial role in cellular adaptation to low oxygen levels. Under normal oxygen conditions, HIFs are rapidly degraded by the ubiquitin-proteasome pathway through the action of prolyl hydroxylase enzymes, which hydroxylate specific proline residues on HIF-1α, marking it for degradation. However, under hypoxic conditions, the activity of prolyl hydroxylases is inhibited, leading to the accumulation of HIF-1α. The stabilized HIF-1α translocates to the nucleus, where it dimerizes with HIF-1β and binds to hypoxia-responsive elements (HREs) on DNA, initiating the transcription of various target genes involved in angiogenesis, metabolism, and cell survival.
One of the critical pathways activated by HIFs during BFR training is the mammalian target of rapamycin (mTOR) pathway, a central regulator of cell growth, proliferation, and protein synthesis. The activation of mTOR is a key step in promoting muscle hypertrophy. mTOR exists in two distinct complexes, mTORC1 and mTORC2, with mTORC1 being primarily responsible for regulating protein synthesis. In response to hypoxia and other growth signals, mTORC1 is activated, leading to the phosphorylation of downstream targets such as S6 kinase (S6K) and the eukaryotic initiation factor 4E-binding protein (4E-BP1). These events enhance the translation of mRNAs encoding ribosomal proteins and other components necessary for protein synthesis, thereby increasing muscle protein synthesis and promoting muscle fiber growth.
In parallel, hypoxia and BFR training stimulate the upregulation of insulin-like growth factor-1 (IGF-1), a potent anabolic hormone that also activates the PI3K/Akt/mTOR pathway. IGF-1 binds to its receptor, IGF-1R, on the muscle cell surface, activating the PI3K/Akt signaling cascade. Akt phosphorylates and inhibits the activity of the tuberous sclerosis complex (TSC1/2), a negative regulator of mTORC1, thereby further enhancing mTORC1 activity and promoting muscle growth. Additionally, Akt inhibits the activity of the forkhead box O (FOXO) transcription factors, which are involved in the transcription of genes associated with muscle atrophy. By suppressing FOXO, IGF-1 reduces muscle protein degradation, creating a favorable environment for muscle hypertrophy.
The effects of BFR training extend beyond muscle hypertrophy. The hypoxic conditions also induce the expression of vascular endothelial growth factor (VEGF), a key regulator of angiogenesis. VEGF is a target gene of HIF-1α and plays a crucial role in promoting the formation of new blood vessels. Increased VEGF expression enhances capillary density in the muscle, improving oxygen and nutrient delivery, which is essential for supporting the increased metabolic demands of hypertrophied muscle tissue and facilitating overall tissue repair.
Moreover, the metabolic stress generated during BFR training stimulates the production of myokines, which are cytokines or peptides released by muscle fibers in response to contraction. One such myokine, interleukin-6 (IL-6), is produced in greater amounts during BFR training. IL-6 has both pro-inflammatory and anti-inflammatory effects, depending on the context. In the setting of BFR, IL-6 acts as an anti-inflammatory agent and plays a role in metabolic regulation and the activation of satellite cells, which are essential for muscle repair and growth. Satellite cells, once activated, proliferate and differentiate into mature muscle fibers, contributing to muscle hypertrophy and the repair of damaged muscle tissue.
In addition to its effects on muscle and vascular adaptation, BFR training influences the nervous system by modulating neuroplasticity. The increased metabolic demand and muscle activation during BFR create a stimulus that can enhance neuromuscular adaptations, including improved proprioception and motor control. At the molecular level, the repeated activation of muscle spindles and Golgi tendon organs during BFR training may enhance the sensitivity of these proprioceptive sensors, which are critical for detecting changes in muscle length and tension. This heightened sensitivity, combined with the central nervous system’s response to the altered afferent feedback, can lead to neuroplastic changes in the brain and spinal cord, ultimately improving coordination and reducing the risk of re-injury.
The ability of BFR training to modulate these molecular and neurophysiological pathways demonstrates its potential as a powerful tool in rehabilitation. By leveraging the body’s natural responses to hypoxia and mechanical stress, BFR training not only enhances muscle hypertrophy and strength but also supports vascularization, tissue repair, and neuroplasticity. When integrated into a comprehensive rehabilitation program, BFR training can optimize recovery by targeting the molecular mechanisms that underlie muscle and joint health, accelerating healing, and improving long-term functional outcomes.
Eccentric training is a specialized form of exercise that focuses on the controlled lengthening of muscles under tension, and it is increasingly recognized for its powerful effects on muscle and tendon adaptation. At the core of its effectiveness is the ability to induce specific molecular responses that enhance tendon resilience, improve muscle coordination, and support overall joint stability. The molecular biology underlying these adaptations reveals a complex interplay of signaling pathways, growth factors, and cellular processes that work together to strengthen the musculoskeletal system, particularly in response to the unique demands of eccentric contractions.
During eccentric contractions, muscles experience mechanical strain as they elongate while generating force. This strain triggers a range of molecular signaling pathways that are critical for muscle growth, repair, and adaptation. One of the primary pathways activated by eccentric exercise is the mammalian target of rapamycin (mTOR) pathway, a central regulator of cell growth, protein synthesis, and hypertrophy. Eccentric contractions generate mechanical tension and microdamage within muscle fibers, which activates mechanoreceptors on the cell membrane, such as integrins and focal adhesion kinase (FAK). These receptors transduce mechanical signals into biochemical responses, leading to the activation of mTORC1, a complex that controls the initiation of protein synthesis.
When mTORC1 is activated, it phosphorylates key downstream targets, including p70S6 kinase (S6K) and 4E-binding protein 1 (4E-BP1). S6K enhances the translation of mRNAs that encode ribosomal proteins and other components necessary for protein synthesis, thereby promoting muscle hypertrophy. 4E-BP1, when phosphorylated, releases eukaryotic initiation factor 4E (eIF4E), which is essential for the initiation of cap-dependent translation, further boosting the synthesis of proteins required for muscle growth and repair. This anabolic response is crucial for the recovery and strengthening of muscle tissue following the mechanical stress of eccentric exercise, making it a highly effective approach for increasing muscle mass and functional strength.
In addition to mTOR, eccentric contractions also activate the AMP-activated protein kinase (AMPK) pathway, which acts as an energy sensor within cells. AMPK is activated in response to increased energy demands and metabolic stress, both of which are heightened during eccentric exercise due to the intense mechanical load on the muscles. Once activated, AMPK enhances the uptake and utilization of glucose and fatty acids, optimizing the energy production processes within the muscle. Moreover, AMPK promotes mitochondrial biogenesis by activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a master regulator of mitochondrial function. Increased mitochondrial density and function enhance the muscle’s oxidative capacity, improving endurance and resistance to fatigue during prolonged or repeated bouts of eccentric exercise.
The benefits of eccentric training extend beyond the muscle fibers themselves, significantly impacting the extracellular matrix (ECM) and tendons. The ECM is a dynamic network of proteins, including collagen, elastin, and proteoglycans, that provides structural support to tissues and mediates cell signaling. Eccentric contractions stimulate the synthesis of ECM components, particularly collagen, through the activation of growth factors such as transforming growth factor-beta (TGF-β) and insulin-like growth factor-1 (IGF-1). These growth factors are critical for the remodeling and strengthening of tendons.
TGF-β plays a key role in regulating the deposition of ECM proteins by promoting the differentiation of fibroblasts into myofibroblasts, which are specialized cells that produce collagen and other matrix components. This process is mediated by the Smad signaling pathway, which is activated when TGF-β binds to its receptors on the cell surface. The Smad proteins translocate to the nucleus, where they regulate the transcription of genes involved in collagen synthesis and ECM remodeling. The increased production of collagen and other ECM proteins enhances the tensile strength of tendons, making them more resilient to mechanical stress.
IGF-1, which is upregulated during eccentric exercise, further supports ECM and tendon adaptation by stimulating the proliferation and differentiation of satellite cells. These muscle stem cells are activated in response to muscle damage and contribute to muscle repair and hypertrophy by fusing with existing muscle fibers. IGF-1 also enhances collagen synthesis within tendons by activating the PI3K/Akt pathway, which promotes protein synthesis and inhibits protein degradation. This dual role of IGF-1 in both muscle and tendon adaptation underscores its importance in the overall recovery and strengthening process following eccentric training.
Moreover, the mechanical loading during eccentric exercise induces mechanotransduction, a process by which mechanical stimuli are converted into biochemical signals that regulate cellular responses. In tendons, mechanotransduction promotes the alignment of collagen fibers, which is crucial for their ability to withstand tensile forces. Properly aligned collagen fibers ensure that tendons can effectively transmit forces between muscles and bones, thereby stabilizing joints and enhancing movement efficiency. This is particularly important for the knee joint, where strong and well-aligned tendons, such as the quadriceps and patellar tendons, play a critical role in maintaining joint stability and preventing injuries such as anterior cruciate ligament (ACL) tears.
Eccentric training also induces significant changes in neuromuscular coordination and proprioception, both of which are essential for joint stability and injury prevention. The controlled and repetitive nature of eccentric contractions requires precise neural control, which enhances the communication between the nervous system and muscles. This improved neuromuscular coordination leads to more efficient muscle activation patterns, reducing the risk of injury during dynamic activities. Additionally, the heightened demand on proprioceptive feedback mechanisms during eccentric exercise enhances the sensitivity of muscle spindles and Golgi tendon organs, which are critical for detecting changes in muscle length and tension.
These sensory adaptations contribute to improved proprioception, allowing individuals to better sense and respond to potentially harmful joint positions or movements. Enhanced proprioception is vital for preventing re-injury, particularly in sports or activities that involve rapid changes in direction, speed, or load. The neuroplastic changes induced by eccentric training further strengthen the neural circuits involved in motor control, leading to more effective movement patterns and reduced injury risk.
In summary, eccentric training offers a multifaceted approach to muscle and tendon conditioning by activating key molecular pathways such as mTOR and AMPK, promoting the synthesis of collagen and other ECM components, and enhancing neuromuscular coordination. These molecular and cellular adaptations lead to stronger, more resilient tendons, improved muscle function, and better joint stability, which are particularly important for preventing re-injury during dynamic activities. By integrating eccentric training into a comprehensive rehabilitation or strength training program, individuals can achieve significant improvements in muscle strength, tendon health, and overall movement efficiency, ultimately enhancing athletic performance and long-term musculoskeletal health.
Isometric training, which involves muscle contractions without joint movement, plays a crucial role in the early stages of anterior cruciate ligament (ACL) rehabilitation. This type of exercise is particularly beneficial because it allows patients to engage and strengthen muscle groups surrounding the knee while minimizing the risk of further injury to the healing ligament. The controlled environment provided by isometric exercises is ideal for maintaining muscle function, preventing atrophy, and restoring neuromuscular coordination, all without imposing excessive strain on the joint. The underlying molecular biology and neurophysiology of these processes reveal a sophisticated interplay of signaling pathways, cellular mechanisms, and neural adaptations that contribute to recovery.
During isometric contractions, muscles generate force without changing length, maintaining a constant joint angle throughout the exercise. This approach is especially advantageous in the initial phase of ACL rehabilitation, where the primary objective is to preserve muscle mass and strength while avoiding movement that could stress the healing ACL. By preventing joint motion, isometric exercises reduce shear forces and mechanical stress on the ACL, which is vital for proper healing and protection of the injured tissue.
At the molecular level, isometric contractions initiate several critical signaling pathways that contribute to muscle maintenance, hypertrophy, and neuromuscular re-education. One of the most prominent pathways activated by isometric training is the mammalian target of rapamycin (mTOR) pathway, a central regulator of protein synthesis and muscle growth. Although isometric exercises do not involve dynamic movement, the mechanical tension generated during these contractions is sufficient to activate mechanosensitive receptors on the muscle cell membrane, such as integrins and focal adhesion kinase (FAK). These receptors convert mechanical stimuli into biochemical signals, leading to the activation of mTORC1, a key component of the mTOR pathway.
Once activated, mTORC1 phosphorylates downstream targets, including p70S6 kinase (S6K) and 4E-binding protein 1 (4E-BP1), which are essential for the initiation of protein synthesis. S6K enhances the translation of mRNAs that encode ribosomal proteins and other components necessary for muscle protein synthesis, thus supporting muscle hypertrophy and preventing atrophy during periods of limited activity. The preservation and growth of muscle mass are crucial during ACL rehabilitation, as they help maintain the strength and stability of the knee joint, facilitating a smoother transition to more dynamic exercises as the patient progresses.
Another important pathway engaged by isometric contractions is the AMP-activated protein kinase (AMPK) pathway, which is activated in response to the energetic demands of sustained muscle contraction. AMPK acts as a metabolic sensor, regulating cellular energy homeostasis by promoting glucose uptake, fatty acid oxidation, and mitochondrial biogenesis. During isometric exercise, the activation of AMPK ensures that muscle cells efficiently produce and utilize energy, supporting prolonged muscle contraction and reducing the risk of muscle fatigue. Additionally, AMPK activation plays a role in maintaining mitochondrial function, which is vital for overall muscle health and endurance, particularly in a rehabilitation context where muscle preservation is key.
Neuromuscular re-education is another critical component of isometric training, particularly following an ACL injury. The period of immobilization or altered movement patterns that often follows such an injury can disrupt the neuromuscular pathways that control movement and proprioception. Isometric exercises help re-establish these pathways by encouraging the brain and muscles to work together to produce force without movement. This process involves the activation of motor units, which consist of a motor neuron and the muscle fibers it innervates. Repeated isometric contractions help reinforce the synaptic connections between the nervous system and the muscle, leading to improved neuromuscular coordination and control.
Proprioception, the body’s ability to sense its position and movement in space, is often compromised after an ACL injury due to damage to the sensory receptors in and around the knee. Isometric training helps maintain and enhance proprioceptive feedback by engaging muscle spindles and Golgi tendon organs, the primary sensory receptors involved in detecting changes in muscle length and tension. These receptors provide critical information to the central nervous system about the position and movement of the knee joint, which is essential for maintaining balance, coordination, and joint stability. By keeping these proprioceptive pathways active, isometric exercises contribute to the prevention of re-injury and support the recovery of functional movement patterns.
At a deeper level, the neurophysiological impact of isometric training on motor cortex plasticity and spinal reflexes is significant. The sustained activation of motor units during isometric contractions can lead to changes in the cortical representation of the muscles involved, a phenomenon known as cortical reorganization. This neuroplasticity is crucial for regaining motor control and coordination, as it reflects the brain’s ability to adapt to new patterns of muscle activation. Enhanced cortical representation improves the precision of muscle control, which is vital for the complex movements required in later stages of rehabilitation.
In addition to its effects on muscle and nerve function, isometric training positively influences tendon and ligament health at the molecular level. The static load applied during isometric contractions has been shown to stimulate the production of collagen, the primary structural protein in tendons and ligaments. This increase in collagen synthesis is mediated by growth factors such as insulin-like growth factor-1 (IGF-1), which is upregulated in response to mechanical load and plays a critical role in tissue repair and regeneration. The localized increase in collagen production strengthens the tendons and ligaments surrounding the knee, providing additional support to the healing ACL and enhancing joint stability.
Moreover, isometric training can modulate inflammatory responses in the injured area, which is particularly beneficial during the early stages of recovery. While inflammation is a natural part of the healing process, excessive or prolonged inflammation can hinder recovery and contribute to pain and further tissue damage. Isometric exercises have been shown to reduce inflammation by decreasing the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), and by promoting the release of anti-inflammatory cytokines. This anti-inflammatory effect not only alleviates pain and swelling but also creates a more conducive environment for tissue healing and muscle recovery.
Isometric training also plays a role in the regulation of oxidative stress, a condition characterized by an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify them. During injury and subsequent recovery, ROS can cause damage to cells and tissues, including those in the muscle and joint. Isometric exercises help mitigate oxidative stress by promoting the activation of endogenous antioxidant pathways, such as the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Nrf2 is a transcription factor that regulates the expression of antioxidant proteins that protect against oxidative damage. By enhancing the body’s antioxidant defenses, isometric training helps protect muscle and joint tissues from the deleterious effects of ROS, facilitating a more effective recovery.
In summary, isometric training offers a multifaceted approach to ACL rehabilitation, engaging both molecular and neurophysiological mechanisms that support muscle maintenance, neuromuscular re-education, and joint stability. By activating key signaling pathways such as mTOR and AMPK, isometric exercises promote muscle hypertrophy, prevent atrophy, and maintain metabolic health. Additionally, the engagement of proprioceptive and neuromuscular pathways helps restore motor control and coordination, while the enhancement of collagen production supports tendon and ligament integrity. The anti-inflammatory and antioxidant effects of isometric training further contribute to a favorable recovery environment, reducing pain and protecting tissues from damage. As a foundational component of a comprehensive rehabilitation program, isometric exercises play a critical role in optimizing outcomes for patients recovering from ACL injuries, laying the groundwork for a successful return to full function.
Percutaneous Electrolysis Therapy (EPTE) stands at the forefront of modern therapeutic interventions, offering a sophisticated means of enhancing the body’s intrinsic ability to repair and regenerate damaged tissues. By intertwining the disciplines of molecular biology, neurophysiology, and clinical therapeutics, EPTE provides a multifaceted approach to healing that addresses both the underlying biological processes and the symptomatic manifestations of tissue injury.
At the molecular level, EPTE’s application of a low-intensity electrical current to the site of injury initiates a series of precise biochemical responses that are critical for effective tissue regeneration. One of the key molecular pathways activated by EPTE involves the stimulation of growth factors such as fibroblast growth factor (FGF) and transforming growth factor-beta (TGF-β). FGF is crucial not only for the proliferation and differentiation of fibroblasts—the cells responsible for collagen synthesis and extracellular matrix production—but also for angiogenesis, the process by which new blood vessels are formed. This is particularly important in the context of tissue repair, as enhanced blood flow ensures a steady supply of oxygen and nutrients to the healing tissue, thereby accelerating the regeneration process.
TGF-β, another growth factor upregulated by EPTE, plays a dual role in tissue repair. It is heavily involved in regulating the synthesis of extracellular matrix components, such as collagen, which provide the structural framework necessary for tissue integrity. Moreover, TGF-β modulates the activity of various cell types involved in the repair process, including immune cells, which are critical for clearing debris and orchestrating the repair response. By influencing these molecular pathways, EPTE not only speeds up the healing process but also enhances the quality of the repaired tissue, reducing the likelihood of scarring and fibrosis, which can compromise function.
In addition to its effects on growth factors, EPTE modulates the inflammatory response—a critical component of tissue healing. The inflammatory phase of healing, while essential, can become dysregulated in chronic conditions, leading to prolonged pain, swelling, and delayed repair. EPTE helps to rebalance this process by reducing the production of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which are often elevated in chronic inflammation. Concurrently, EPTE promotes the expression of anti-inflammatory mediators such as interleukin-10 (IL-10), which helps to resolve inflammation and restore tissue homeostasis. This fine-tuning of the inflammatory response not only alleviates pain but also prevents the chronic inflammatory states that can lead to degenerative changes in tissues over time.
From a neurophysiological perspective, the benefits of EPTE extend beyond the cellular level to encompass the modulation of pain pathways and nervous system function. The electrical current applied during EPTE can alter the activity of peripheral nerves by influencing ion channels, such as sodium and calcium channels, which are crucial for nerve conduction. This modulation can decrease the excitability of nociceptors, the nerve fibers responsible for detecting and transmitting pain signals, thereby reducing the perception of pain at the injury site. Additionally, EPTE can induce neuroplastic changes within the central nervous system, particularly in the spinal cord and brain, where it may reduce the amplification of pain signals—a phenomenon known as central sensitization, which is often implicated in chronic pain conditions.
The neurophysiological effects of EPTE also include improvements in the autonomic regulation of blood flow and tissue oxygenation. By enhancing the autonomic nervous system’s control over vascular tone, EPTE can increase blood perfusion to the injured area, ensuring that the tissue receives adequate oxygen and nutrients to support the repair process. This increased perfusion not only accelerates healing but also helps to clear metabolic byproducts and inflammatory mediators from the injury site, further reducing pain and swelling.
EPTE’s influence on the nervous system also has important implications for rehabilitation. By reducing pain and improving tissue function, EPTE enables patients to engage more fully in physical therapy and other rehabilitative exercises. This active participation is crucial for restoring strength, flexibility, and function to the injured tissue, thereby reducing the risk of re-injury and promoting long-term recovery.
In summary, Percutaneous Electrolysis Therapy (EPTE) is a highly sophisticated therapeutic modality that integrates the principles of molecular biology and neurophysiology to provide a comprehensive approach to tissue repair and pain management. By stimulating key molecular pathways involved in tissue regeneration, modulating the inflammatory response, and influencing the nervous system’s control of pain and blood flow, EPTE offers a powerful tool for enhancing the body’s natural healing processes. This makes EPTE not only effective in treating acute injuries but also invaluable in managing chronic conditions, reducing pain, and improving the overall effectiveness of rehabilitation efforts. As such, EPTE represents a significant advancement in the field of regenerative medicine, offering patients a more efficient and holistic pathway to recovery.
Compression therapy, delivered through devices such as compression boots, is a highly effective therapeutic strategy that harnesses principles of molecular biology to enhance circulation, reduce edema, and ultimately promote tissue healing. The application of controlled mechanical pressure to the body not only improves blood and lymphatic flow but also triggers a cascade of molecular events that are crucial for efficient tissue repair and recovery.
One of the primary molecular effects of compression therapy is its ability to improve lymphatic drainage. The lymphatic system is responsible for the removal of excess fluid, proteins, and waste products from tissues, and it plays a key role in immune surveillance and the regulation of inflammation. By enhancing lymphatic flow, compression therapy helps to clear out inflammatory cytokines, cell debris, and other potentially harmful substances from the site of injury. This process reduces the extent of edema (tissue swelling), which is important because excessive edema can disrupt cellular function and hinder the delivery of nutrients and oxygen to the healing tissue.
Increased blood flow, another significant outcome of compression therapy, is critical for supplying oxygen and essential nutrients to damaged tissues. On a molecular level, the improved circulation facilitated by compression therapy ensures that cells involved in tissue repair, such as fibroblasts, receive an adequate supply of the amino acids, glucose, and lipids necessary for energy production and the synthesis of key structural proteins. One of the most important proteins in this context is collagen, the primary component of the extracellular matrix that provides tensile strength and structural integrity to tissues.
The synthesis of collagen is a complex, tightly regulated process that involves the transcription of collagen genes, the translation of collagen mRNA into precursor proteins, and the subsequent post-translational modifications that allow collagen molecules to form a stable, functional extracellular matrix. Compression therapy supports this process by ensuring that fibroblasts are well-nourished and metabolically active, enabling them to produce collagen efficiently. Moreover, the mechanical stimuli provided by compression therapy can directly influence the gene expression profiles of cells within the tissue, upregulating the expression of collagen genes and other genes involved in tissue repair and regeneration.
In addition to its effects on collagen synthesis, compression therapy influences the activity of growth factors, which are essential signaling molecules that regulate cellular processes such as proliferation, differentiation, and migration. For instance, the increased blood flow induced by compression therapy enhances the local concentration of growth factors like vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). VEGF plays a pivotal role in angiogenesis, the formation of new blood vessels, which is critical for re-establishing adequate blood supply to healing tissues. This ensures that the regenerating tissue is well-oxygenated and can receive the necessary nutrients for sustained repair.
Fibroblast growth factor (FGF), on the other hand, is involved in the proliferation and differentiation of fibroblasts and other cells involved in tissue repair. By promoting FGF activity, compression therapy supports the expansion of the cell populations needed to rebuild and restore tissue structure. Additionally, FGF has been shown to promote the synthesis of extracellular matrix components, further enhancing the structural repair of injured tissues.
The modulation of inflammatory pathways is another important molecular effect of compression therapy. Inflammation is a natural and necessary response to injury, but chronic or excessive inflammation can impede healing and lead to further tissue damage. Compression therapy helps to modulate the inflammatory response by reducing the levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1), while promoting the production of anti-inflammatory cytokines like interleukin-10 (IL-10). This shift in the balance of cytokines fosters a more controlled and efficient inflammatory response, reducing pain and preventing the development of chronic inflammatory conditions.
Furthermore, compression therapy can influence the mechanotransduction pathways within cells—molecular mechanisms by which cells convert mechanical stimuli into biochemical signals. When tissues are subjected to the mechanical pressure of compression, cells within those tissues experience changes in their cytoskeleton and cell membrane tension. These mechanical changes can activate signaling pathways such as the mitogen-activated protein kinase (MAPK) pathway and the focal adhesion kinase (FAK) pathway, both of which play roles in regulating cell proliferation, survival, and migration. By activating these pathways, compression therapy can promote cell survival and enhance the regenerative capacity of the tissue.
In summary, compression therapy is far more than just a mechanical intervention; it is a powerful tool that influences molecular biology to promote tissue healing and recovery. Through its effects on lymphatic drainage, blood flow, collagen synthesis, growth factor activation, inflammatory modulation, and mechanotransduction, compression therapy supports a comprehensive and multifaceted approach to tissue repair. This integration of molecular biology into compression therapy not only accelerates recovery but also ensures that the healing process is more efficient and effective, making it an invaluable modality in both acute and chronic therapeutic settings.
Incorporating advanced virtual reality (VR) training into anterior cruciate ligament (ACL) rehabilitation introduces a highly innovative approach that leverages neurophysiological principles to optimize recovery. This cutting-edge technique not only enhances the physical aspects of rehabilitation but also profoundly impacts the brain and nervous system, promoting a deeper and more effective level of healing through the manipulation of neuroplasticity, sensory integration, and motor learning.
From a neurophysiological standpoint, VR training represents a sophisticated tool for re-engaging and rewiring the brain’s neural networks that are disrupted following an ACL injury. The ACL injury does not just damage the knee structurally; it also disrupts the communication pathways between the knee and the central nervous system. This disruption leads to deficits in proprioception—the body’s ability to sense joint position and movement—which are critical for maintaining balance, coordination, and motor control. The neurophysiological changes following an ACL injury involve alterations in the brain’s motor cortex, cerebellum, and sensory pathways, all of which are responsible for processing information about body position and movement.
VR training provides a unique platform to stimulate and restore these neural circuits through its immersive, multisensory environment. By engaging the brain in a controlled yet dynamic setting, VR exercises activate sensory and motor areas of the brain simultaneously, reinforcing the connections between these regions. For instance, when a patient performs a movement in a VR environment, visual and proprioceptive feedback is immediately processed by the brain, which then adjusts motor commands in real-time. This continuous loop of sensory input and motor output enhances synaptic plasticity, the process by which connections between neurons are strengthened based on experience and practice. As patients repeatedly engage in these VR-enhanced exercises, the brain’s neural circuits become more efficient, leading to more accurate and refined motor control.
One of the key neurophysiological benefits of VR training is its ability to enhance sensory integration—the brain’s capacity to process and integrate information from different sensory modalities. After an ACL injury, the brain’s ability to integrate proprioceptive feedback from the knee with visual and vestibular information may be impaired, leading to challenges in maintaining balance and executing coordinated movements. VR training helps to restore and improve sensory integration by providing a rich, immersive environment where visual, auditory, and proprioceptive cues are aligned in a way that mimics real-world conditions. For example, when a patient navigates through a VR scenario that requires balancing on uneven surfaces, the brain is forced to integrate visual information about the environment with proprioceptive feedback from the muscles and joints. This multisensory stimulation is crucial for recalibrating the brain’s sensory processing capabilities, ensuring that the nervous system can effectively coordinate movement in both virtual and real-world scenarios.
Moreover, VR training has a profound impact on motor learning—the process by which the nervous system acquires, refines, and retains new movement patterns. Motor learning is essential for recovering from an ACL injury, as patients must relearn how to move their knee and leg in ways that are both safe and efficient. The repetitive and progressive nature of VR exercises enhances motor learning by providing continuous practice in a controlled environment, where the difficulty of tasks can be adjusted to match the patient’s current abilities. This is critical in promoting the gradual adaptation of neural circuits responsible for movement control, such as those in the motor cortex and basal ganglia, which are involved in the planning, execution, and refinement of movements.
Furthermore, VR’s ability to simulate real-life scenarios and unexpected challenges plays a vital role in neurophysiological adaptation. The brain’s prefrontal cortex, which is involved in decision-making and adapting to new situations, is highly engaged during VR training as patients are required to react to changing environments or stimuli. This engagement promotes cognitive-motor integration, a process where cognitive functions such as attention, decision-making, and planning are closely linked with motor execution. By practicing in these simulated environments, patients not only improve their physical coordination but also develop the cognitive strategies necessary to respond to unpredictable situations, such as those encountered in sports or daily activities. This dual enhancement of cognitive and motor functions is particularly important in reducing the risk of re-injury, as it equips patients with the skills needed to make quick, accurate decisions under pressure.
The immersive nature of VR also contributes to neurophysiological benefits through its impact on patient engagement and motivation. The brain’s reward systems, particularly those involving the release of dopamine, are activated when patients are engaged in enjoyable and challenging activities. VR training, with its interactive and game-like elements, stimulates these reward pathways, increasing patient motivation and adherence to the rehabilitation program. This heightened engagement leads to more frequent and sustained practice of movement patterns, which is essential for reinforcing the neural changes necessary for recovery. Over time, the repetitive practice of these movements in a highly engaging environment results in the consolidation of motor memories, making the newly learned skills more robust and resistant to forgetting.
In conclusion, the integration of advanced VR training into ACL rehabilitation provides a powerful neurophysiological boost to the recovery process. By enhancing sensory integration, promoting motor learning, stimulating neuroplasticity, and engaging cognitive-motor circuits, VR training offers a comprehensive approach to rehabilitation that not only accelerates physical recovery but also optimizes the brain’s ability to control and coordinate movement. This makes VR an invaluable tool in the rehabilitation of ACL injuries, providing patients with the neural and physical foundation needed for a successful return to full function.
Stroboscopic glasses, as an advanced tool in ACL rehabilitation, offer a profound opportunity to engage the neurophysiological mechanisms that are crucial for restoring balance, coordination, and motor control after injury. The neurophysiological impact of stroboscopic training extends far beyond the surface-level benefits of improving proprioception; it deeply engages the brain’s sensory processing systems, motor planning circuits, and adaptive neuroplasticity processes, making it a powerful adjunct to traditional rehabilitation methods.
At the core of neurophysiology is the brain’s ability to process and integrate sensory information to generate appropriate motor responses. Typically, the brain relies heavily on visual input to inform and guide movement. However, this reliance can sometimes overshadow the contribution of other sensory modalities, such as proprioception (the body’s ability to sense its position and movement in space) and the vestibular system (which contributes to balance and spatial orientation). Following an ACL injury, the proprioceptive pathways—responsible for detecting and transmitting information about the position and movement of the knee joint—can be significantly impaired. This disruption can lead to compromised motor control, increased instability, and a higher risk of re-injury.
Stroboscopic glasses create a unique neurophysiological challenge by intermittently depriving the visual system of continuous information. This visual disruption forces the brain to recalibrate its reliance on other sensory inputs, particularly proprioceptive and vestibular signals, which are less affected by the injury but often underutilized in comparison to visual cues. The intermittent nature of stroboscopic glasses—flashing between clear and obscured vision—requires the brain to constantly adapt to changes in sensory input. This adaptation process stimulates the brain’s neuroplasticity, encouraging the strengthening and reorganization of neural circuits responsible for proprioception and balance.
Neuroplasticity, the brain’s ability to reorganize itself by forming new neural connections, is a fundamental aspect of recovery in ACL rehabilitation. When visual information is intermittently blocked by stroboscopic glasses, the brain must quickly adjust by enhancing its processing of proprioceptive and vestibular inputs. This increased reliance on these non-visual sensory modalities drives the brain to create and strengthen new neural pathways, effectively reorganizing its sensory processing network to compensate for the lack of visual data. This reorganization is not just a temporary adjustment; repeated exposure to stroboscopic training can lead to lasting changes in the brain’s architecture, making the proprioceptive and vestibular systems more dominant and efficient in guiding movement.
The neurophysiological effects of stroboscopic training also extend to the brain’s motor planning and execution circuits. The prefrontal cortex, which is involved in decision-making, anticipatory planning, and the execution of complex movements, plays a critical role during stroboscopic training. When visual input is intermittently unavailable, the brain must rely more heavily on its internal representations of movement, known as motor schemas or motor programs. These motor programs are stored in the brain and are responsible for the execution of learned movement patterns. By training under conditions where visual feedback is unreliable or absent, stroboscopic glasses encourage the brain to refine these motor programs, making them more robust and less dependent on external cues.
The cerebellum, a key structure involved in the fine-tuning of motor movements, is also heavily engaged during stroboscopic training. The cerebellum receives input from proprioceptive sensors in the muscles and joints, as well as from the vestibular system, and uses this information to adjust and correct movements in real-time. When visual input is disrupted, the cerebellum must work harder to ensure that movements remain accurate and coordinated. This increased demand on the cerebellum can lead to enhanced synaptic efficiency within its circuits, improving the brain’s overall ability to regulate and fine-tune motor actions.
Moreover, the vestibular system, which provides critical information about head movement and spatial orientation relative to gravity, is further activated during stroboscopic training. The absence of reliable visual information forces the brain to rely more on vestibular input to maintain balance and posture. This increased reliance enhances the integration of vestibular signals with proprioceptive feedback, leading to improved postural control and balance, which are essential for the prevention of future injuries.
Stroboscopic training also has significant implications for the brain’s sensory-motor integration, the process by which sensory information is translated into motor actions. The parietal lobe, which integrates sensory information to coordinate movement, becomes more active as it works to combine proprioceptive and vestibular inputs in the absence of consistent visual cues. This heightened activity promotes the development of more efficient sensory-motor pathways, allowing for smoother and more coordinated movements. The strengthening of these pathways is particularly important in ACL rehabilitation, where precise and coordinated movements are necessary to protect the knee joint from further injury.
In addition to these neurophysiological changes, stroboscopic training can influence the brain’s attentional networks. The intermittent visual deprivation caused by the glasses forces the brain to focus more intensely on the available sensory information, sharpening attention and enhancing the brain’s ability to filter and prioritize relevant stimuli. This heightened focus can lead to improved reaction times and more accurate motor responses, which are critical in sports and other activities that require quick, reflexive actions.
In summary, stroboscopic glasses offer a powerful neurophysiological tool in ACL rehabilitation by challenging the brain to adapt and reorganize its sensory processing and motor control systems. Through the forced reliance on proprioceptive and vestibular inputs, stroboscopic training promotes neuroplastic changes that enhance balance, coordination, and motor planning. By integrating this innovative approach with traditional physical therapy, patients can achieve a more comprehensive and resilient recovery, reducing the risk of re-injury and improving overall functional outcomes.
The integration of these advanced therapeutic techniques—neuromodulation, blood flow restriction (BFR) training, eccentric and isometric exercises, percutaneous electrolysis therapy (EPTE), compression therapy, virtual reality (VR) training, and stroboscopic glasses—represents a significant leap forward in the field of ACL rehabilitation. By combining these modalities, clinicians can address the complex interplay between the mechanical, neural, molecular, and cellular processes involved in recovery, offering a comprehensive and multifaceted approach that targets every aspect of healing and functional restoration.
Neuromodulation techniques such as transcranial magnetic stimulation (TMS) and transcutaneous electrical nerve stimulation (TENS) are central to enhancing neuroplasticity during ACL rehabilitation. These techniques work by applying electrical or magnetic stimuli to specific brain regions, thereby facilitating the reorganization of neural circuits that have been disrupted by injury. TMS, for example, can enhance the excitability of motor cortex neurons, promoting the recovery of motor functions and improving coordination between the brain and the injured limb. TENS, on the other hand, can modulate pain pathways by stimulating peripheral nerves, reducing pain perception, and allowing patients to engage more fully in rehabilitation exercises. On a molecular level, these neuromodulation techniques may upregulate neurotrophic factors like brain-derived neurotrophic factor (BDNF), which plays a crucial role in supporting the growth and survival of neurons, further enhancing the brain’s capacity for adaptation and recovery.
Blood flow restriction (BFR) training, along with eccentric and isometric exercises, specifically targets the molecular pathways involved in muscle hypertrophy, tendon resilience, and neuromuscular coordination. BFR training involves the application of a tourniquet or cuff to restrict blood flow to the muscles during low-intensity exercise. This restriction leads to a hypoxic environment that triggers a cascade of molecular responses, including the activation of mTOR signaling pathways, which are essential for muscle protein synthesis and growth. Additionally, BFR training induces the production of hypoxia-inducible factors (HIFs), which can promote angiogenesis and improve vascularization in the healing tissues. Eccentric and isometric exercises complement BFR by targeting the tendons and muscles around the knee, promoting collagen synthesis and enhancing the tensile strength of the tissues. These exercises also stimulate the mechanotransduction pathways within cells, leading to the upregulation of anabolic processes that are critical for rebuilding and strengthening the injured joint.
Percutaneous electrolysis therapy (EPTE) and compression therapy contribute significantly to the tissue healing process by modulating inflammatory responses and enhancing cellular repair mechanisms. EPTE applies a low-intensity electrical current directly to the injured tissue, stimulating the production of growth factors such as transforming growth factor-beta (TGF-β) and fibroblast growth factor (FGF). These growth factors are vital for collagen production, angiogenesis, and the overall remodeling of the extracellular matrix, ensuring that the repaired tissue is both strong and functional. Compression therapy, meanwhile, improves lymphatic drainage and increases blood circulation, which are crucial for reducing edema and delivering oxygen and nutrients to the healing tissues. The mechanical pressure from compression therapy can also enhance the mechanotransduction signaling pathways in cells, further supporting tissue repair and reducing the risk of chronic inflammation.
Virtual reality (VR) training and stroboscopic glasses introduce innovative ways to enhance proprioceptive and motor control recovery by leveraging advanced technology to simulate real-world challenges in a controlled setting. VR training immerses patients in a dynamic environment where they can practice complex movements and respond to virtual stimuli, promoting the refinement of motor programs and improving neurocognitive function. The multisensory feedback provided by VR stimulates the brain’s sensory integration centers, enhancing the coordination between visual, proprioceptive, and vestibular inputs. This not only improves balance and coordination but also strengthens the neural circuits involved in motor planning and execution.
Stroboscopic glasses, by intermittently blocking visual input, force the brain to rely more heavily on proprioceptive and vestibular feedback, driving neuroplastic changes that enhance the brain’s ability to process and integrate these sensory modalities. This sensory deprivation can lead to the upregulation of sensory processing genes and the strengthening of synaptic connections within the proprioceptive pathways, resulting in more accurate and reliable movement patterns. Together, these technologies challenge the brain’s adaptive capabilities, promoting the reorganization of neural circuits that are essential for maintaining stability and preventing future injuries.
By combining these pharmacological and non-pharmacological strategies into a cohesive rehabilitation program, clinicians can address both the mechanical and neural aspects of recovery while targeting the molecular and cellular processes that underpin healing. This holistic approach not only improves the immediate outcomes of ACL recovery but also reduces the risk of long-term complications, such as chronic pain and re-injury, ultimately leading to better long-term joint health and overall mobility. For example, the enhanced neuroplasticity facilitated by neuromodulation and sensory training techniques ensures that the brain can effectively compensate for any deficits in motor control, while the molecular benefits of BFR, eccentric, and isometric training strengthen the structural integrity of the muscles and tendons surrounding the knee.
In summary, the advanced understanding of the neurophysiological and molecular mechanisms involved in ACL injury and recovery has opened new possibilities for therapeutic intervention. By integrating the latest advances in molecular biology with cutting-edge rehabilitation techniques like VR training and stroboscopic glasses, it is possible to create a comprehensive and individualized rehabilitation program that enhances healing, optimizes neuroplasticity, and supports long-term functional recovery. This integrated approach holds great promise for improving the quality of life for individuals recovering from ACL injuries, ensuring a more complete and effective recovery, and minimizing the risk of future injuries. As research continues to advance, these combined modalities may become the standard of care in ACL rehabilitation, offering a blueprint for the recovery of other musculoskeletal injuries as well.