1. Introduction
The genus
Xyleborus is a group of ambrosia beetles belonging to the subfamily
Scolytinae within the family
Curculionidae. These beetles are known for their unique symbiotic relationships with fungi, which they cultivate within the galleries they bore into wood. This mutualistic association is a defining characteristic of ambrosia beetles, distinguishing them from other bark beetles that primarily feed on the phloem of trees. The name "ambrosia" refers to the fungal "gardens" these beetles create and tend, which serve as their primary food source. This fascinating interaction has significant ecological and economic implications, making
Xyleborus an important subject of study in both forestry and agriculture [
1,
2].
The study of
Xyleborus dates back to the early 19th century when entomologists first began to document the diversity and behavior of bark and ambrosia beetles [
3]. Over the decades, extensive research has been conducted to understand their taxonomy, life cycles, and the intricate relationships they maintain with their fungal symbionts. Early work focused primarily on identifying species and describing their morphological traits, but advancements in molecular biology and genetics have since revolutionized our understanding of these beetles. The ability to sequence DNA and analyze genetic material has provided deeper insights into the evolutionary relationships among
Xyleborus species and their symbiotic fungi [
4,
5,
6].
The study of
Xyleborus beetles is of paramount importance due to their significant ecological and economic impacts. These beetles are notorious for their ability to infest a wide range of host plants, including economically valuable timber and fruit trees [
1,
2,
3,
4]. Their burrowing activity can cause substantial damage, leading to reduced wood quality, decreased crop yields, and even tree mortality. This not only results in economic losses for forestry and agricultural industries but also affects ecosystem stability and biodiversity [
7]. The symbiotic relationship between
Xyleborus beetles and their fungal partners adds another layer of complexity. The fungi cultivated by these beetles can be pathogenic to plants, exacerbating the damage caused by the beetles themselves [
6]. Understanding the dynamics of this relationship is crucial for developing effective control measures.
Despite their destructive potential,
Xyleborus beetles also play important roles in their natural ecosystems. As decomposers, they contribute to the breakdown of dead and dying wood, facilitating nutrient cycling and promoting forest health. Their galleries provide habitats for other organisms, including fungi, bacteria, and other invertebrates. This intricate web of interactions underscores the importance of a balanced perspective when studying
Xyleborus beetles. While they can be pests in managed environments, they also fulfill essential ecological functions in natural settings [
8,
9,
10,
11].
The economic impact of
Xyleborus beetles is profound, particularly in regions where forestry and agriculture are major economic drivers. In the forestry sector, infestations can lead to significant losses in timber quality and quantity [
12,
13]. Beetle-infested wood often suffers from staining and structural damage, reducing its market value. In extreme cases, large-scale infestations can necessitate costly management interventions, including tree removal and replacement. In agriculture,
Xyleborus beetles pose a threat to fruit trees and other crops [
10]. For example, infestations in avocado and mango plantations can lead to reduced yields and increased production costs [
14,
15]. The global trade in these commodities means that infestations can have far-reaching economic consequences, affecting not only local producers but also international markets.
Effective management of
Xyleborus beetles requires a multifaceted approach. Preventive measures, such as monitoring and early detection, are critical for managing infestations before they become unmanageable [
16,
17,
18]. Chemical treatments, while effective in some cases, must be used judiciously to minimize environmental impact and the development of resistance [
19,
20,
21,
22,
23,
24]. Biological control methods, including the use of natural predators and entomopathogenic fungi, offer promising alternatives to chemical treatments [
25]. Integrated Pest Management (IPM) strategies, which combine multiple control methods, are increasingly being recognized as the most effective approach for sustainable management [
26].
Recent advances in molecular genetics have opened new avenues for research on
Xyleborus beetles. Techniques such as DNA barcoding and genome sequencing are providing detailed insights into the genetic diversity and population structure of these beetles [
4,
27,
28]. Behavioral studies are shedding light on their mating and dispersal strategies, while innovative control methods, such as pheromone-based trapping systems, are being developed [
29]. Despite these advances, significant challenges remain. The complexity of
Xyleborus biology and their interactions with symbiotic fungi means that there is still much to learn. Future research needs to focus on understanding these interactions in greater detail, as well as developing more sustainable and ecologically sound management practices.
This review aims to provide a comprehensive synthesis of the current knowledge on Xyleborus beetles, with a focus on their biology, ecological roles, and the challenges they pose to forestry and agriculture. The objectives are to describe the biology and ecology of Xyleborus beetles, evaluate their economic and ecological impact, critically assess current control and management strategies, highlight recent advances in Xyleborus research, and identify future research needs. By synthesizing current knowledge and identifying future research needs, this review aims to provide a comprehensive resource for researchers, practitioners, and policymakers. Effective management of Xyleborus beetles will not only mitigate economic losses but also contribute to the health and stability of forest ecosystems.
2. Biology and Ecology of Xyleborus
2.1. Life Cycle and Reproduction
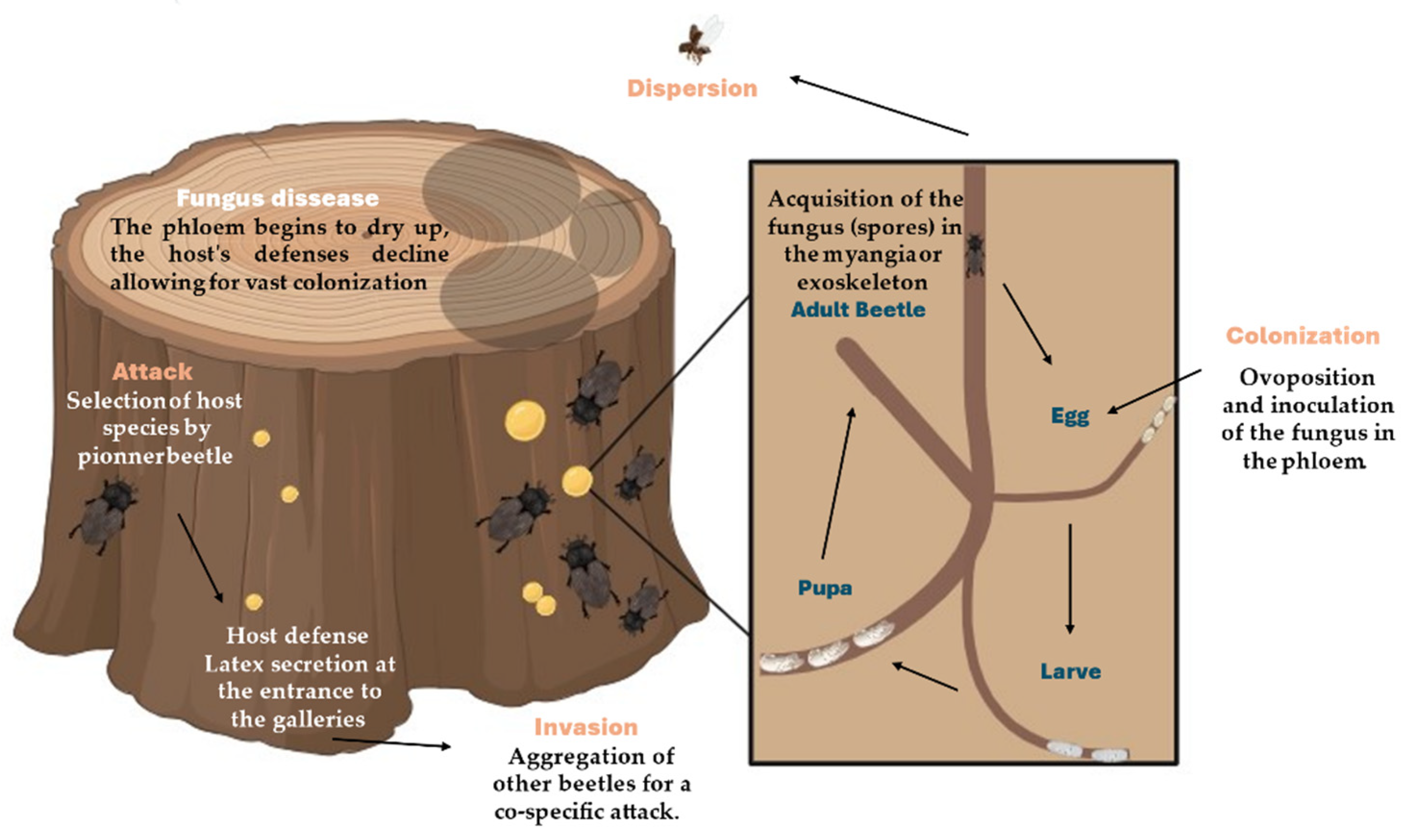
The life cycle and reproduction of
Xyleborus beetles are complex processes that involve the construction of galleries, fungal inoculation, larval development, and adult dispersal (
Figure 1). Their reproductive strategy is characterized by inbreeding and a close symbiotic relationship with fungi, which are critical for their survival and success. Understanding these processes is essential for managing
Xyleborus populations and mitigating their impact on forest ecosystems.
2.1.1. Egg Laying and Gallery Construction
The life cycle of
Xyleborus beetles begins when a female selects a suitable host tree and starts to bore into the wood to construct a gallery system. These galleries are intricately designed tunnels that serve as both a breeding site and a nursery for the fungal symbionts. The female beetle uses her mandibles to chew through the wood, creating a network of tunnels. She lays her eggs in these galleries, which are strategically placed to optimize the growth of both the beetle larvae and the fungal garden [
1,
3].
2.1.2. Fungal Inoculation
A critical aspect of the gallery construction is the inoculation of the tunnels with fungal spores. Female
Xyleborus beetles carry these spores in specialized structures called mycangia, which are located near their mandibles or in other parts of their bodies, depending on the species. As the female excavates the galleries, she releases these spores into the wood. The spores quickly germinate and proliferate within the moist, nutrient-rich environment of the galleries, forming a dense fungal mat [
30,
31].
Larvae grow by feeding on symbiotic fungi seeded by their mother, but during the pupal stage, the contents of their gut are emptied. Then, the naïve adults acquire their own symbionts through feeding [
32].
It has been observed that the fungal communities in the mycangia may be influenced by host trees, environments, and other factors. However, these associations are still not well understood [
31]. A study conducted during the life cycle of
X. affinis suggests that the yeasts and bacteria contained in the insect's microbiome are closely involved in the cultivation of filamentous fungi such as
Raffaelea [
33].
2.1.3. Larval Development
The eggs laid by the female beetle hatch into larvae, which are entirely dependent on the fungi for nourishment. The larvae feed on the growing fungal mycelium, which provides them with all the necessary nutrients for their development. This relationship is highly specialized, as the fungi break down complex wood polymers into simpler compounds that are easier for the larvae to digest. The larvae undergo several molts as they grow, eventually reaching the pupal stage within the safety of the gallery system [
1,
2,
34].
2.1.4. Pupal Stage and Emergence
After completing their larval development, the beetle larvae enter the pupal stage. During this stage, they undergo metamorphosis, transforming into adult beetles. The pupal stage is a period of significant physiological and morphological changes, preparing the beetles for their life outside the galleries. Once metamorphosis is complete, the new adult beetles emerge from the pupal cases and continue to reside in the galleries until they are ready to disperse [
1,
2,
34].
2.1.5. Reproductive Strategy and Inbreeding
One of the most notable features of
Xyleborus beetles' reproductive strategy is their reliance on inbreeding. The sex ratio within the galleries is heavily skewed towards females, with males being produced in much smaller numbers. Males are typically haploid and smaller than females. This skewed sex ratio facilitates brother-sister mating within the confined space of the galleries. The inbreeding strategy ensures that the fungal symbionts are passed on to the next generation without the need for the beetles to find new fungal partners, thus maintaining the stability of the mutualistic relationship [
2,
35].
2.1.6. Dispersal and Colony Establishment
After mating, the fertilized females leave the natal gallery to establish new colonies. They exit the galleries and disperse to find new host trees, carrying fungal spores in their mycangia. This dispersal phase is critical for the expansion of
Xyleborus populations and the spread of their symbiotic fungi. Upon finding a suitable host, the females begin the cycle anew by boring into the wood and constructing fresh galleries [
2,
35].
To locate a new host,
Xyleborus utilizes both chemical and visual cues, though the exact way they use these signals remains unclear. It is believed that beetles find wider silhouettes easier to spot, and larger stem diameters enhance the likelihood of
X. glabratus detecting and landing on them [
36].
2.1.7. Generational Overlap and Population Dynamics
Xyleborus beetles often exhibit overlapping generations within a single gallery system. This means that different life stages (eggs, larvae, pupae, and adults) can coexist within the same gallery. Most Xyleborus species like X. dispar, X. affinis, and X. glabratus reproduce by arrhenotokous parthenogenesis, where males are rare, and females can reproduce without fertilization. In this system, females produce unfertilized eggs that develop into males, while fertilized eggs develop into females. This allows them to maintain high populations even in the absence of males, contributing to their success as pests.
The population dynamics of
Xyleborus beetles are thus closely linked to the health and availability of their host trees, as well as the successful cultivation of their fungal symbionts [
13,
37,
38,
39].
2.1.8. Ecological and Evolutionary Implications
The reproductive strategies and life cycle of
Xyleborus beetles have significant ecological and evolutionary implications. The reliance on inbreeding and the tight association with fungal symbionts have led to highly specialized and co-evolved relationships. These beetles have evolved to exploit a niche that is relatively inaccessible to other insects, giving them a competitive advantage. However, this specialization also makes them vulnerable to disruptions in their environment, such as the loss of suitable host trees or changes in the composition of their fungal partners [
2,
38,
39].
2.2. Habitat and Distribution
The habitat and distribution of Xyleborus beetles are shaped by a combination of environmental factors, host tree availability, and their symbiotic relationships with fungi. Their ability to adapt to various environments and colonize a wide range of tree species has facilitated their global distribution and, in some cases, their status as invasive pests. Effective management requires a thorough understanding of their ecological requirements and the factors that influence their distribution and impact on forest ecosystems.
2.2.1. Global Distribution
Xyleborus beetles are distributed globally, with species occurring in a wide range of climatic zones, from tropical rainforests to temperate woodlands. They are particularly diverse and abundant in tropical and subtropical regions, where the warm and humid conditions favor the growth of their fungal symbionts and the decomposition of wood [
55]. This global distribution is facilitated by their ability to colonize a variety of woody plants, making them highly adaptable to different environments [
1,
2,
38].
Table 1.
Distribution and fungal association of economically important Xyleborus.
Table 1.
Distribution and fungal association of economically important Xyleborus.
Xyleborus
species |
Fungus-associated |
Affected plants |
Distribution |
| Xyleborus affinis |
Fusarium oxysporum |
Archidendron clypearia (Jack) Benth.[40,41] |
Global |
|
Ceratocystis fimbriata
|
Manguifera indica [2,42] |
|
Raffaelea lauricola
|
Persea americana [43]
Persea borbonia [44]
Persea palustris [44]
Sassafras albidum [44]
Lindera benzoin [44]
Cinnamomum camphora [44] |
| Xyleborus dispar |
Ophiostoma novo-ulmi
|
Dutch elm disease [45] |
Northern Europe |
| Xyleborus glabratus |
Raffaelea lauricola
|
Persea americana
Persea borbonia
Persea palustris
Sassafras albidum
Lindera benzoin
Cinnamomum camphora
|
Asia, North america, [8,46,47] |
Xyleborus
bispinatus
|
Unknown
|
Ficus carica L. [48] |
Italy |
|
Raffaelea lauricola
|
Persea americana |
Mexico [41,49] |
| Florida, USA [8,37] |
Xyleborus
volvulus
|
Raffaelea lauricola
|
Persea americana [8,39] |
From United States to South America. It is also found in Africa and Asia. |
Xyleborus
perforans
|
Fusarium parceramosum |
Pinnus spp. [50] |
America, Europe and Australis |
| Fusarium aff. solani |
| Ophiostoma ips |
| Raffaelea deltoideospora |
| Sporothrix pseudoabietina |
Xyleborus
ferrogineus
|
Ceratocystis cacaofunesta |
Theobroma cacao L. [51] |
Latin America and Africa |
2.2.2. Habitat Preferences
The habitat preferences of
Xyleborus beetles are primarily influenced by the availability of suitable host trees and the presence of their symbiotic fungi. These beetles typically inhabit forested areas, including both natural forests and managed plantations. They prefer environments where there is an abundance of dead or dying trees, as these provide the ideal conditions for gallery construction and fungal growth [
6,
13,
53]. However, some species are also capable of infesting healthy trees, particularly when environmental conditions are favorable or when they encounter weakened or stressed trees [
6,
54].
Xyleborus beetles exhibit a preference for certain types of wood, depending on the species. For example,
X. glabratus, known as the redbay ambrosia beetle, primarily infests trees in the
Lauraceae family, such as redbay and avocado [
1,
2,
34]. Other species may have a broader host range, colonizing various hardwood and softwood species. This host specificity is often linked to the particular fungal symbionts that the beetles carry, which are adapted to thrive in the wood of specific tree species [
54].
2.2.3. Invasive Species and Range Expansion
Several
Xyleborus species have become invasive outside their native ranges, causing significant ecological and economic damage. These invasions are often facilitated by human activities, such as the global trade of timber and ornamental plants, which can inadvertently transport beetles and their fungal symbionts to new areas [
31]. Once established in a new environment, invasive
Xyleborus species can spread rapidly, infesting local tree populations and outcompeting native bark and ambrosia beetles.
One notable example is
X. glabratus, which was introduced to the southeastern United States and has since spread throughout the region, causing widespread mortality in redbay and avocado trees due to laurel wilt disease, a condition caused by its fungal symbiont
Raffaelea lauricola [
55]. Similarly,
X. similis has been reported in various countries outside its native range, affecting a wide range of tree species and contributing to significant ecological disruptions [
31].
Another example is X. perforans, which has associated fungi such as Fusarium parceramosum, Fusarium aff. solani, Ophiostoma ips, Raffaelea deltoideospora, and Sporothrix pseudoabietina. These fungi affect Pinus spp. trees, causing significant economic and ecological impact [
56].
2.2.4. Ecological Niches and Microhabitats
Within their habitats,
Xyleborus beetles occupy specific ecological niches and microhabitats. They are primarily found within the wood of their host trees, where they construct their galleries and cultivate their fungal gardens. The microhabitat within the wood provides a stable environment with controlled humidity and temperature, which is essential for the growth of their fungal symbionts [
2,
6,
10].
These beetles are also influenced by the physical and chemical properties of the wood they infest. Factors such as wood moisture content, density, and chemical composition can affect the suitability of a tree as a host. For example, trees that are stressed or weakened by environmental factors, such as drought or disease, are often more susceptible to
Xyleborus infestation [
1,
2]. This susceptibility is partly due to the reduced defenses of the stressed trees, making it easier for the beetles to penetrate the wood and establish their galleries.
Some studies have shown that the attraction of
X. glabratus to its host trees is based on a complex set of chemical signals. Eucalyptol and α-copaene are critical components in the long-range attraction and boring behavior of the beetle, while the volatiles emitted by the symbiotic fungus
R. lauricola also play a crucial role, especially in the later stages of tree infection. These findings can be useful for developing management and control strategies for this invasive pest [
57,
58].
2.2.5. Conservation and Management Considerations
Understanding the habitat preferences and distribution patterns of Xyleborus beetles is essential for developing effective conservation and management strategies, like
early detection, sanitation, prophylactic fungicide treatments, ambrosia beetle control, and trenching. Regular scouting and helicopter surveys help identify infected trees. Immediate removal and destruction of infected trees, including chipping and burning, prevent disease spread. Prophylactic treatments with propiconazole protect groves, while bark-directed insecticide applications control beetle populations [
59].
2.3. Diet and Feeding Habits
The diet and feeding habits of Xyleborus beetles are centered around their symbiotic relationship with fungi. This mutualism is critical for their development and survival, influencing their host tree selection and ecological impact. Understanding these feeding behaviors is essential for managing Xyleborus populations and mitigating their effects on forests and agriculture.
2.3.1. Fungal Cultivation
Xyleborus beetles are unique among bark beetles due to their reliance on symbiotic fungi as their primary food source. Unlike other beetles that consume the phloem or wood of trees directly,
Xyleborus species cultivate fungal gardens within the galleries they excavate in the wood. These fungi belong to genera such as
Raffaelea,
Ambrosiella, and
Fusarium, which are carried in specialized structures called mycangia located on the beetles bodies 31,39]. The species composition of fungi found in the mycangia, as well as their abundance, are influenced by location, host trees, environments, and other factors [
54]. The fungi break down complex wood polymers into simpler compounds, providing a nutrient-rich food source for both the larvae and adult beetles [
33].
2.3.2. Fungal Spore Inoculation
Upon entering a new host tree, female
Xyleborus beetles inoculate their galleries with fungal spores. These spores quickly germinate and proliferate within the galleries, forming a dense fungal mat that serves as the primary food source for the beetles and their offspring. The relationship between the beetles and their fungal symbionts is highly specific, with each beetle species typically associated with particular fungal species that are best suited to the wood of their host trees [
33,
39]. This mutualistic relationship is crucial for the survival and growth of
Xyleborus populations.
2.3.3. Larval Feeding
The larvae of
Xyleborus beetles are entirely dependent on the fungal mycelium for nourishment. As the larvae feed on the fungi, they ingest the nutrients necessary for their development. The fungi provide a balanced diet that includes proteins, lipids, and carbohydrates, which are essential for larval growth and development. The larvae pass through several instar stages, molting as they grow larger. The availability and quality of the fungal food source directly influence the growth rate and survival of the larvae [
1,
2,
39].
2.3.4. Adult Feeding
Adult
Xyleborus beetles also feed on the cultivated fungi. The fungi serve as a food source not only during the developmental stages but also for adult beetles that remain in the galleries. This continuous access to a reliable food source allows adult beetles to focus on reproduction and gallery expansion without the need to forage outside. The fungi sustain the beetles throughout their life cycle, reinforcing the importance of the fungal symbiosis in the beetles' biology [
1,
2,
39].
2.3.5. Host Tree Selection
The choice of host tree by Xyleborus beetles is influenced by the suitability of the wood for fungal growth. Trees that are stressed, weakened, or recently dead are more likely to be infested by these beetles, as they provide the ideal conditions for fungal cultivation. Factors such as wood moisture content, chemical composition, and the presence of secondary metabolites can affect the growth of the symbiotic fungi and, consequently, the suitability of the tree as a host.
In an experiment conducted by Martini et al., it was observed how the volatile compounds generated by Persea palustris, inoculated with
R. lauricola, play an important role in the attraction and repulsion of
X. glabratus to the plant. In three days after infection,
X. glabratus is significantly repelled by infected leaf odors due to an increase in methyl salicylate, a known behavioral repellent. However, at 10 and 20 day, the beetles are more attracted to the infected leaf odors compared to non-infected plants. This increased attraction is associated with a rise in sesquiterpenes and aldehydes in the leaf volatiles. Additionally, compounds such as eucalyptol, cubeb and α-copaene, which are known attractants, are present in higher amounts in infected leaves during these later stages. This changes in volatile profiles are linked to the activation of the salicylic acid pathway by the fungal infection, which initially increases methyl salicylate release and later alters other volatile emissions, thereby impacting
X. glabratus behavior [
57,
58,
59].
3. Economic and Ecological Impact
Xyleborus beetles have significant economic and ecological impacts, particularly the redbay ambrosia beetle,
X. glabratus, is significant due to their role as vectors of plant pathogenic fungi, leading to severe damage in various tree species and agricultural crops. Their role as decomposers contributes to nutrient cycling and forest health, but their ability to infest healthy trees and introduce pathogenic fungi can lead to substantial economic losses and ecological disruptions. Effective management strategies are essential to mitigate these impacts and protect both economic resources and ecosystem health [
60,
61].
3.1. Economic Impact
Xyleborus beetles have a profound economic impact, particularly in regions where forestry and agriculture are major economic drivers. In the forestry sector, these beetles cause significant losses by infesting timber, which reduces both its quantity and quality. Infested wood often suffers from structural damage and staining due to the fungal symbionts introduced by the beetles. This staining, commonly referred to as "ambrosia stain," can significantly reduce the market value of timber, even if the structural integrity remains intact. Additionally, severe infestations can lead to tree mortality, necessitating costly management interventions such as tree removal and replacement [
1,
2,
18].
In the agricultural sector,
Xyleborus beetles pose a major threat to fruit trees and other crops. For example, the redbay ambrosia beetle,
X. glabratus is a significant pest in avocado orchards in the southeastern United States. This beetle transmits the fungus
R. lauricola, which causes laurel wilt, a disease that has led to extensive tree mortality in commercial avocado plantations. The economic losses associated with laurel wilt include reduced yields, increased management costs, and the loss of marketable fruit, which can have far-reaching impacts on the profitability of avocado production [
34,
37].
The economic impact of
Xyleborus beetles, particularly through laurel wilt disease affecting the avocado industry, is substantial. Annual potential sales losses in southern Florida's avocado industry could reach up to
$30 million, with 75% and 50% crop reductions leading to
$22.5 million and
$15 million in losses, respectively. Property values of avocado groves could plummet by about
$326 million if all bearing trees are destroyed. Additionally, increased management and control costs, including prophylactic treatments and intensified monitoring, could raise annual expenses by approximately
$4.5 million. Overall, the Florida avocado industry faces an estimated annual economic impact of around
$100 million due to this disease [
62,
63].
Other crops impacted by ambrosia beetles and their associated fungi include citrus, grapevine, cacao, coffee, macadamia, peach, and tea. These beetles, by shifting from dead or declining trees to healthy ones, pose a significant threat to these crops, leading to decreased yields and increased management costs [
18].
For example,
X. ferrugineus is a species capable of infesting wood pieces stored in fields and sawmills, as well as piles of freshly cut and moist wood. It causes the death of apparently healthy trees by introducing fungi that cause vascular wilts, such as
Ceratocystis fimbriata, which can lead to the death of cacao trees [
64,
65]. Similarly,
X. affinis infests tropical woods and exhibits similar behavioral characteristics and effects, impacting cacao and mango crops and trees within the laurel family [
65,
66].
The global trade in timber and ornamental plants has facilitated the spread of
Xyleborus beetles to new regions, where they can become invasive pests. Invasive
Xyleborus species, such as
X. glabratus and
X. similis, have established populations outside their native ranges, causing substantial economic damage to local forestry and agriculture industries. The introduction of these beetles often leads to increased management costs, including monitoring, quarantine measures, and chemical treatments to control their spread [
67,
68].
3.2. Ecological Impact
Invasive species of Xyleborus have the capacity to profoundly alter the ecosystems they invade by competing with native species, modifying community structures, and spreading dangerous pathogens. Therefore, it is crucial to understand and manage these impacts to protect the biodiversity and integrity of affected ecosystems.
Invasive species of the genus
Xyleborus have demonstrated significant impacts on the ecosystems they colonize. Scientific studies have documented how these species disperse intercontinentally, negatively affecting local communities. These invasions can alter the composition of native communities and compete with native species for essential resources, resulting in decreased biodiversity and changes in ecosystem structure [
31].
Additionally, a study about the potential invasion of exotic ambrosia beetles, such as
Xyleborus glabratus, in various ecosystems used ecological niche models to predict their impacts. This study indicate that these beetles can establish themselves in a variety of habitats, spreading pathogens like the fungus
R. lauricola, which causes the disease known as "laurel wilt." This disease severely affects trees in the Lauraceae family, posing a significant ecological risk to these ecosystems [
68].
Moreover, the fungi introduced by
Xyleborus beetles can also affect the microbial communities within the infested trees. The fungal symbionts can outcompete native fungi and other microorganisms, leading to shifts in the microbial diversity of the wood. These changes can affect the decomposition processes and nutrient cycling within the forest ecosystem, further impacting the overall health and biodiversity of the forest [
7].
3.4. Ecosystem Services
The activities of
Xyleborus beetles can have both positive and negative impacts on ecosystem services. On the positive side, their role in the decomposition of dead wood contributes to nutrient cycling and soil formation, essential processes for maintaining forest productivity and health. Their burrowing activities create microhabitats that support a diverse array of organisms, enhancing ecosystem resilience. These services include carbon sequestration, air and water purification, climate regulation, and the provision of habitat for wildlife [
7,
8].
Moreover, the loss of these services due to disruptions caused by invasive beetles can have significant implications for both the environment and human well-being. The decomposition process facilitated by these beetles helps in nutrient cycling and soil formation, maintaining the productivity and health of forests [
9]. Additionally, the creation of microhabitats by their burrowing activities supports biodiversity and ecosystem resilience, contributing to essential services like carbon sequestration and climate regulation [
10].
Understanding and managing the impact of Xyleborus beetles is crucial to preserving these vital ecosystem functions and mitigating the broader environmental and societal consequences of their activities.
4. Control and Management Strategies
4.1. Preventive Measures
Preventive measures methods focus on minimizing the conditions favorable for
X. glabratus establishment and spread. Removing and properly disposing of infested plant material is crucial. Sanitation practices, such as chipping and burning infected wood, help reduce beetle populations and prevent disease spread. Additionally, maintaining tree health through proper irrigation and fertilization can make trees less susceptible to beetle attacks. Practices such as removing infested trees and destroying them promptly can significantly reduce the spread of laurel wilt. [
48,
70,
71].
Understanding the host selection behavior of
X. glabratus is crucial for developing effective management strategies. Studies have shown that
X. glabratus uses the diameter of the host tree's stem as a visual cue to select its host. Beetles are more likely to infest trees with larger diameters, indicating that tree size is an important factor in host selection [
72,
73]. With the knowledge of these preferences it’s possible the development of management strategies that target high-risk trees.
Effective management of
X. glabratus requires diligent monitoring and early detection. Continuous surveillance programs are essential in areas with established populations and regions at risk of infestation. Trap-based monitoring systems using lure-baited traps can detect beetle presence before the onset of laurel wilt symptoms in host trees [
60].
Various types of lures have been tested, including those based on manuka oil, phoebe oil, cubeb oil, and α-copaene, derived from different botanical sources. Manuka oil, extracted from the New Zealand tea tree (
Leptospermum scoparium), and phoebe oil, derived from Brazilian walnut (
Phoebe porosa), have been effective in attracting beetles. Cubeb oil, derived from the cubeb pepper plant (
Piper cubeba), and α-copaene, a sesquiterpene found in various plants, are particularly potent attractants. Cubeb oil and α-copaene traps have demonstrated higher capture rates of
X. glabratus, making them favorable for monitoring programs [
75,
76].
Additionally, studies have been conducted using volatile substances from different sources. Volatile compounds from the leaves of redbay (
Persea borbonia) have been found to attract
X. glabratus, highlighting the role of host plant volatiles in beetle behavior [
77,
78]. Research has also shown that volatile organic compounds produced by the laurel wilt pathogen,
R. lauricola, can attract
X. glabratus, suggesting a symbiotic relationship between the beetle and the fungus [
4]. Regular monitoring using these lures allows for timely intervention and the implementation of control measures to prevent further spread.
Experiments comparing the attraction of
X. glabratus to avocado wood and litchi wood demonstrated that the beetle is more attracted to litchi wood. This increased attraction is attributed to the higher content of α-copaene in litchi wood compared to avocado wood. This finding underscores the importance of α-copaene in the beetle's host selection process and provides insights for developing more effective lures [
75].
Studies have also explored the use of eucalyptol as a lure. Field trials demonstrated that traps baited with eucalyptol captured significant numbers of
X. glabratus, underscoring the compound's attractiveness to the beetle. [
73] This is particularly relevant for avocado trees, as variations in eucalyptol content among different avocado cultivars may influence their susceptibility to the beetle. Some varieties of avocado might have higher eucalyptol levels, making them more attractive to
X. glabratus and thereby more susceptible to infestation and subsequent laurel wilt infection [
72].
4.2. Chemical Control
Chemical control involves the use of insecticides and fungicides to protect high-value trees from
X. glabratus and laurel wilt. Preventive treatments with systemic insecticides like bifenthrin and permethrin have shown effectiveness in reducing beetle populations and preventing new infestations [
79]. Fungicide treatments, particularly with propiconazole, have demonstrated efficacy in protecting trees from laurel wilt when applied through root flare injections. However, these treatments require regular reapplication and may not be feasible on a large scale due to cost and potential environmental impacts. [
77]
Recent studies have been focused on evaluating of possibles repellents for
X. glabratus. Cloonan et. al. (2023) compared the performance of Piperitone to that of the known repellent verbenone to assess its potential as a cost-effective alternative. Piperitone showed significant repellent effects against
X. glabratus, comparable to verbenone, but at potentially lower costs. The study suggests that piperitone could be an effective tool for integrated pest management (IPM) programs aimed at controlling
X. glabratus and managing laurel wilt [
81]. Our research group is also working on chemical control strategies to mitigate the risks posed by
Xyleborus species, specifically through the development of chiral nitroguanidine-based compounds. These compounds have shown promise in targeting these beetles, aiming to enhance the efficacy of pest control methods while minimizing environmental impact [
20,
23,
69,
82].
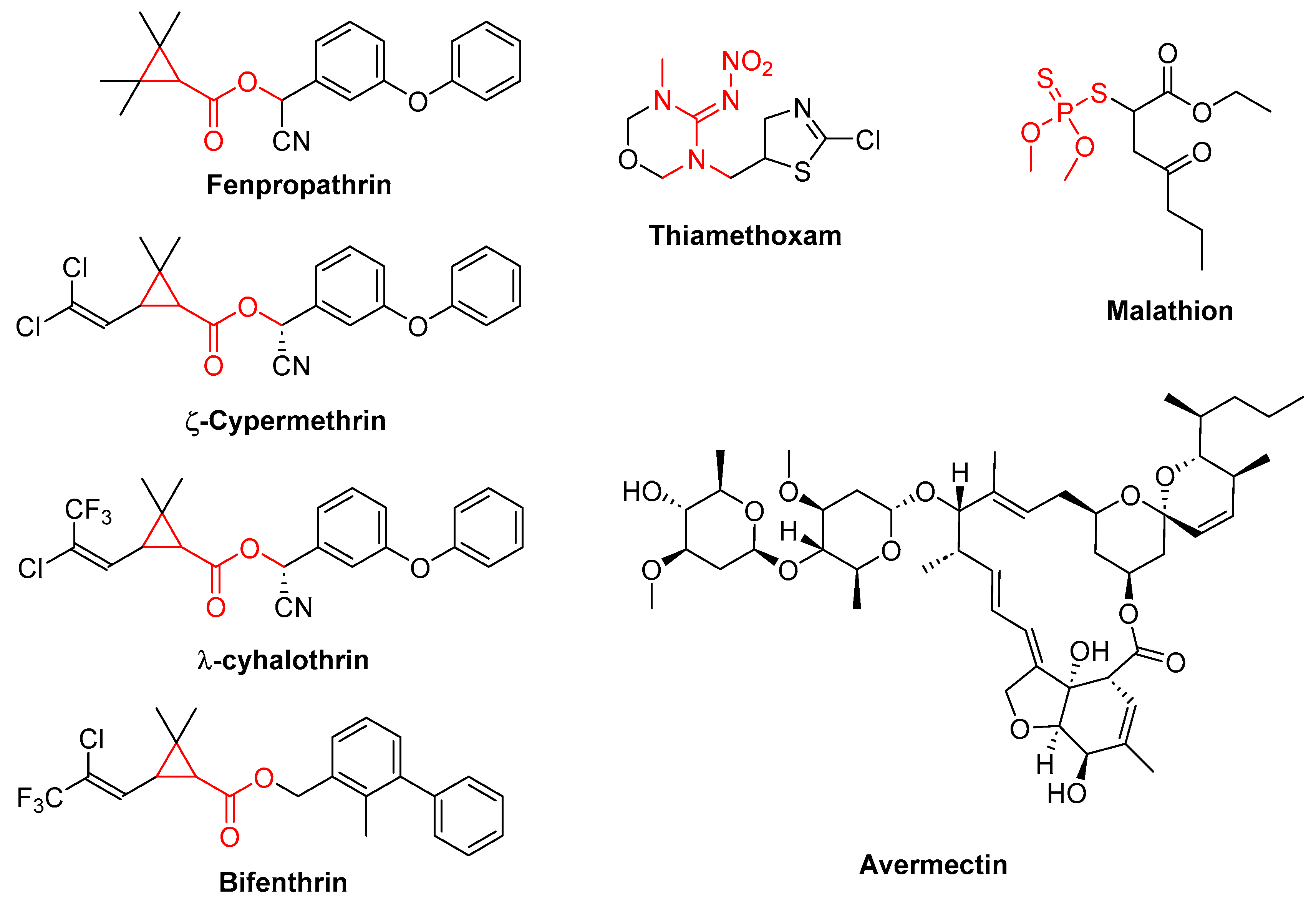
Due to most of the beetle population is found within infected trees, insecticides have shown little effectiveness in controlling the pest. However, it is important to apply these insecticides around the trunks of infected trees to prevent their movement. Malathion, Danitol® (fenpropathrin), and Epi-merk® (abamectin) are the most commonly used insecticides [
83].
There are also adjuvants like NuFilm® that improve the efficacy of the insecticides [
83]. Additionally, there are studies on insecticides that have been evaluated in combinations, such as zeta-cypermethrin + bifenthrin and lambda-cyhalothrin + thiamethoxam, provides useful tools for integrated pest management strategies (
Figure 2). [
79] However, the variable effectiveness of other insecticides highlights the need for further research and the development of more robust control measures.
Biological control methods are being explored to manage
X. glabratus populations. Entomopathogenic fungi (EPF) such as
Beauveria bassiana and
Isaria fumosorosea, have shown promise in laboratory and field trials by causing significant mortality in beetle populations [
70]. Research is ongoing to optimize delivery methods and assess the long-term effectiveness of these biological agents in various environmental conditions. Additionally, biological control agents, such as parasitoids and predators, are being investigated for their potential to reduce beetle populations naturally [
81].
Research has shown that several strains of
B. bassiana infect more than 12 species of bark beetles, indicating its broad-spectrum potential. Moreover, field studies have demonstrated that
B. bassiana and
Metarhizium brunneum can significantly reduce beetle populations, suggesting that these fungi could be integrated into pest management programs for
X. glabratus [
67]
In addition to these studies, research on
X. affinis, a closely related species, has provided valuable insights into the effectiveness of
B. bassiana as a biological control agent. This study highlighted that
B. bassiana not only caused significant mortality in
X. affinis but also exhibited potential for horizontal transmission among beetle populations. The findings suggest that similar strategies could be effective against
X. glabratus, offering a broader application of EPF in managing ambrosia beetles [
67].
A study by Carrillo et al. (2015) highlighted the role of predators and parasitoids associated with Scolytinae in Persea species in both Florida (USA) and Taiwan. Predators identified included species from the families
Laemophloeidae,
Staphylinidae,
Zopheridae, and
Monotomidae, while parasitoids from the families
Braconidae,
Eulophidae,
Pteromalidae,
Encyrtidae,
Eupelmidae, and
Bethylidae were also found. Notably, in the context of
X. glabratus,
Bethylidae,
Braconidae,
Encyrtidae (possibly Closterocerus sp.), and
Scelionidae were observed emerging from infested wood [
70].
The development of host resistance through breeding programs is a critical long-term strategy. Efforts are underway to identify and propagate tree genotypes that exhibit resistance to laurel wilt. Preliminary studies have identified redbay and swamp bay clones with tolerance to the disease, and these clones are being evaluated in field trials [
71]. Conservation and propagation of resistant germplasm are essential components of integrated management strategies. Resistant varieties of avocado and other lauraceous hosts are being developed to provide long-term solutions for managing laurel wilt [
18].
4.4. Integrated Pest Management (IPM)
An Integrated Pest Management (IPM) approach combines multiple control methods to manage
X. glabratus and laurel wilt effectively. IPM strategies include monitoring and scouting, sanitation, chemical and biological control, and the development of resistant host plants. Public education and outreach are also vital to prevent the spread of the beetle and disease through human activities, such as the movement of infested firewood [
76]. IPM emphasizes the integration of these methods to achieve sustainable and effective management while minimizing environmental impact.
These beneficial effects of applying IPM strategies have been observed in other pests caused by ambrosia beetles.
Trypodendron lineatum (striped ambrosia beetle), is a significant pest in British Columbia's forest industry. The IPM program for ambrosia beetles in British Columbia began in 1981, with the cornerstone being the use of semiochemical-based mass trapping, particularly for
T. lineatum [
77]. This program was developed as an alternative to the previous reliance on chemical insecticides, such as DDT and lindane, which were phased out due to environmental concerns. Despite the initial costs of setting up and maintaining these traps, the long-term savings and reduction in pest damage have proven the IPM strategy to be cost-effective [
78]. Additionally, the environmental benefits of reducing chemical pesticide use have added value to this approach.
In Sri Lanka, tea plants (
Camellia sinensis) were affected by
Xyleborus fornicatus, and various IPM techniques such as biological control, cultural practices, mechanical control, and the judicious use of chemical pesticides were employe for the management of
X. fornicatus. [
79]
Selection and planting of tea cultivars that are either resistant or tolerant to
X. fornicatus is a primary strategy for control of
X. fornicatus, also, proper pruning techniques and sanitation measures are critical in reducing the wood rot that can occur due to fractures and wounds induced by
X. fornicatus. This includes removing affected wood and applying wound dressings to large prune cuts to prevent further damage [
80].
5. Recent Research and Advances
Recent years have witnessed significant advancements in the study of
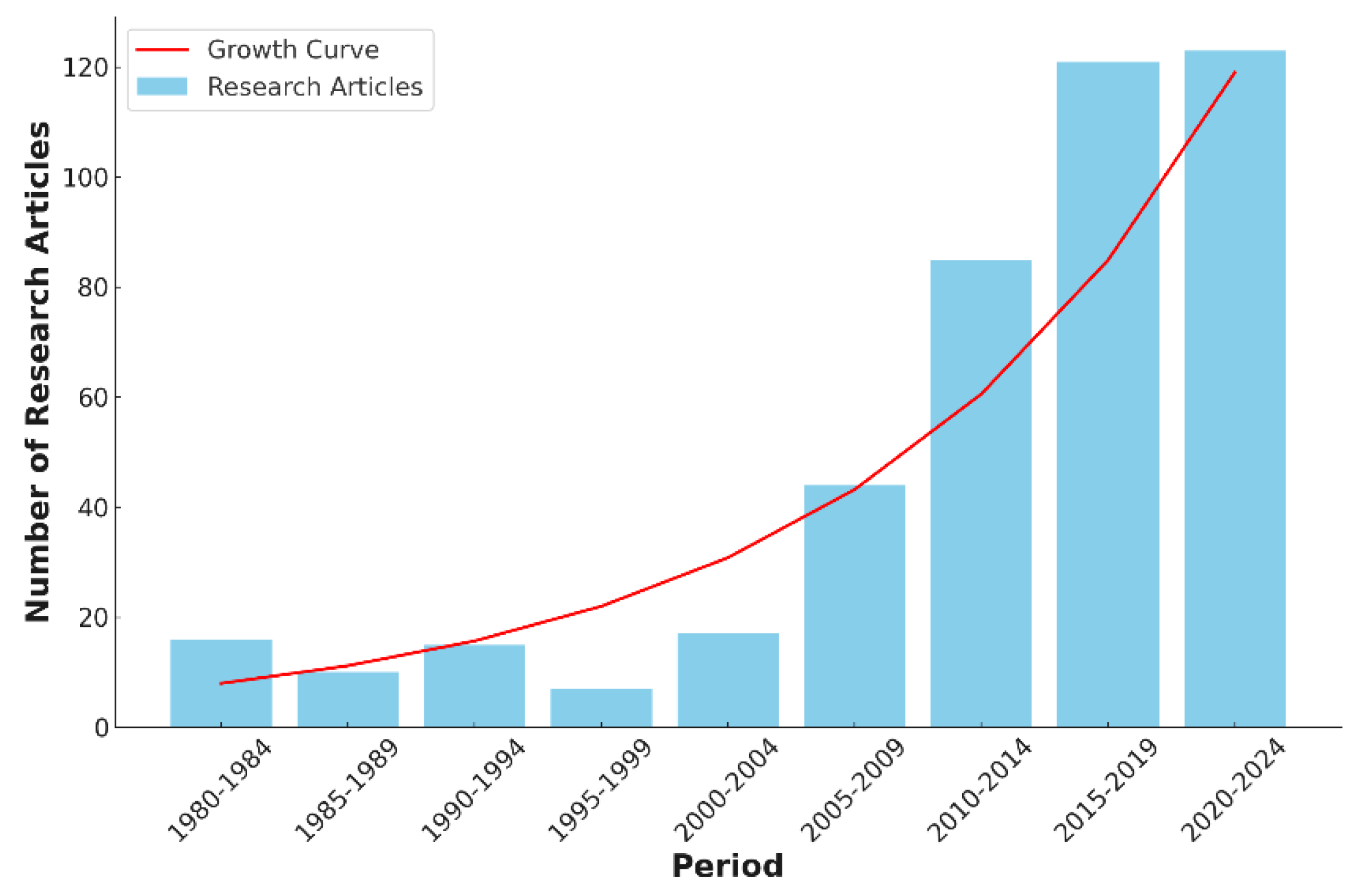
Xyleborus beetles, with an increasing number of research articles being published annually. This trend reflects the growing recognition of the ecological and economic impact of these beetles and the urgent need for effective management strategies.
Figure 3 illustrates the surge in research activities over different periods, highlighting the accelerated pace of scientific inquiry into the biology, behavior, and control of
Xyleborus beetles. The exponential growth in publications underscores the importance of continuous research to address the challenges posed by these pests and to develop sustainable solutions for their management.
5.1. Molecular and genetic studies
The genetic variability in different populations of X. glabratus in various Asian countries was studied using COI (mitochondrial DNA) and CAD (nuclear DNA) markers. Several types of COI and CAD were identified, indicating genetic diversity within the beetles' native range. Phylogenetic studies using these same COI and CAD data revealed significant genetic divergence, leading to the identification of two new species of Xyleborus (X. insidiosus and X. mysticulus). Complementing these findings with morphological data of the newly discovered species aids in the identification and differentiation of these species from existing ones [[4].
In addition, one of the first pieces of evidence of sexual dimorphism in the size and shape of mandibles in adult
X. affinis species found that temperature influences phenotypic variation during the growth stage, as well as the expression of sexual dimorphism in the mandibles. This variation in the mandibles could have important morphofunctional consequences in different ecological activities, such as feeding, highlighting the damage caused by this species [
89].
For X. principalis, sequences of various genes were analyzed, of which the mitochondrial cytochrome oxidase I (COI) gene and the large subunit of ribosomal RNA (28S) showed variations. COI exhibited the greatest variations with a maximum divergence of 14.2% among individuals of X. principalis. However, with the nuclear genes, these variations were smaller, with only one substitution noted in the 28S gene. Regarding morphology, no geographic pattern was found, nor in the genetic data. Thus, X. principalis is a monophyletic species in all the analyses conducted in this study, but without a consistent pattern within the species [[5].
5.2. Behavioral Studies
X. affinis is one of the ambrosia beetle species potentially considered harmful. However, observations indicate that tunnel excavation, once the species is established in the trunk, is an exclusive task of the females, who extend the gallery where they live. The larvae, females, and adult males graze and feed on fungi that cover the gallery walls. In a blocking function, the females remain inactive to use their bodies to protect the tunnels. Activities related to nest care include grooming the nest and individuals, cultivating fungi, and caring for the brood. Cannibalism is an observed behavior where they feed on larvae, pupae, or dead adults to maintain hygiene in the nest [
52].
In the galleries of
X. bispinatus, eggs are laid, and the larvae feed on fungi cultivated by the females on the gallery walls. These same females are responsible for constructing the main and secondary tunnels. The length of these tunnels is related to the number of adults, suggesting that gallery extension is vital for the amount of available food. Laying eggs at the farthest ends of the galleries may represent an adaptation for optimal use of space and food resources. The founding females seem to keep the main gallery clear to allow it to be used as a corridor to manage ambrosia gardens, care for the eggs, and larvae. In their symbiotic relationship with fungi,
Raffaelea arxii and
Raffaelea subfusca are the most prevalent and are cultivated in the galleries, as they are essential for the nutrition of larvae and adults
[90].
Ambrosia beetles, such as
X. affinis, engage in symbiotic mutualisms with fungi for nutritional purposes. The practice of fungal farming involves selecting beneficial fungi as the main food source, protecting these fungi from pathogens, and providing the necessary nutrients for their growth. Bacteria play an important role in the agricultural practices of ambrosia beetles, helping to defend fungal crops, fix atmospheric nitrogen, degrade plant biomass, and plant defenses. The metabolic capabilities of bacteria and yeasts are crucial for supporting the beetle-fungus agricultural symbiosis, especially in the early stages of gallery development. Bacteria and fungi provide essential nutrients that the beetles need for their development, significantly influencing their behavior and reproductive success [
33].
6. Discussion
The increasing prevalence and impact of
Xyleborus beetles necessitates a comprehensive understanding of their biology, ecology, and management strategies. Our review highlights several key findings that inform effective management approaches.
Xyleborus beetles exhibit a unique life cycle heavily dependent on their symbiotic relationship with fungi. This mutualistic association is crucial for their survival and reproductive success, enabling them to colonize a wide range of host trees. Their reproductive strategy, characterized by inbreeding and female-biased offspring, ensures the continuity of fungal symbionts across generations and facilitates rapid population growth and dispersal. Habitat preferences and global distribution patterns are shaped by the availability of suitable host trees and environmental conditions conducive to fungal growth. Invasive species such as
X. glabratus and
X. similis demonstrate the beetles' ability to adapt and spread in new environments, often leading to significant ecological and economic damage. [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39]
The economic impact of
Xyleborus beetles is profound, particularly in forestry and agriculture. Infestations result in substantial losses in timber quality and quantity, as well as reduced yields in fruit crops such as avocados.[
7,
8,
9,
10] Ecologically, the beetles' ability to introduce pathogenic fungi to host trees can cause severe disruptions to forest ecosystems, reducing biodiversity and altering community structures.[
31,
68] The spread of diseases like laurel wilt underscores the broader environmental implications of
Xyleborus infestations. Effective management of these beetles requires an integrated approach that combines preventive measures, chemical control, biological control, and IPM strategies.[
70,
71,
72,
73,
74,
75,
76,
77,
78,
79] Recent advances in molecular genetics and behavioral studies have provided new insights into the beetles' biology and potential control methods. Monitoring and early detection are critical components of successful management programs, with lure-baited traps and pheromone-based trapping systems showing promise in detecting and controlling beetle populations. Biological control methods, including the use of entomopathogenic fungi and natural predators, offer sustainable alternatives to chemical treatments. However, further research is needed to optimize these methods and assess their long-term effectiveness.[
4,
5,
89]
Current challenges in managing Xyleborus beetles are multifaceted. The intricate relationship between Xyleborus beetles and their fungal symbionts presents significant challenges for management. Disrupting this mutualism without adversely affecting other components of the ecosystem is a complex task that requires a nuanced understanding of their interactions. Managing invasive Xyleborus species in non-native regions poses additional challenges. The rapid spread and establishment of these beetles necessitate stringent quarantine measures and continuous monitoring to prevent further invasions. Balancing the economic benefits of forestry and agriculture with the need to protect ecosystems from beetle infestations is a critical concern. Sustainable management practices must be developed to minimize the environmental impact of control measures while ensuring economic viability.
Future research needs to focus on several areas to enhance our ability to manage Xyleborus beetles effectively. Further research into the genetic diversity and population structure of Xyleborus beetles is essential for developing targeted management strategies. Molecular tools such as DNA barcoding and genome sequencing can provide valuable insights into beetle-fungal interactions and inform the development of biocontrol agents. Understanding the behavioral ecology of Xyleborus beetles, including their host selection mechanisms and mating behaviors, is crucial for improving detection and control methods. Studies on the role of volatile organic compounds in beetle attraction and repulsion can aid in the development of more effective lures and repellents. The integration of multiple control methods into cohesive IPM strategies requires further research and validation. The effectiveness of combining chemical, biological, and cultural control measures should be evaluated in various environmental conditions to develop robust and adaptable IPM programs. Conservation of resistant tree genotypes and the development of breeding programs for disease-resistant varieties are essential for long-term management. Identifying and propagating resistant germplasm will provide sustainable solutions to mitigate the impact of Xyleborus beetles on forestry and agriculture.
7. Conclusions
The pervasive impact of Xyleborus beetles, especially as vectors of plant pathogenic fungi, highlights the critical need for integrated management strategies that combine preventive measures, chemical and biological controls, and robust Integrated Pest Management (IPM) programs. These beetles cause significant economic losses in forestry and agriculture and disrupt ecological balance by spreading invasive pathogens. Advances in molecular genetics and the understanding of beetle-fungal symbiosis offer promising avenues for targeted and sustainable control methods, such as the use of key volatile compounds and biological agents like entomopathogenic fungi. However, the complexity of Xyleborus biology requires ongoing research and collaboration among scientists, policymakers, and industry stakeholders. Future efforts should focus on developing resistant tree varieties, refining IPM strategies, and ensuring continuous monitoring to mitigate the beetles' devastating effects while preserving forest ecosystems and agricultural productivity.
Supplementary Materials
NA
Author Contributions
Conceptualization, T.J.P. and J.L.O.R.; methodology, T.J.P. and J.L.O.R.; software, S.H.R.B. and T.J.P.; validation, S.H.R.B., T.J.P., and J.L.O.R; formal analysis, H.S.R.B., K.I.V.H., T.J.P.; investigation, H.S.R.B., K.I.V.H., and R.V.R.; resources, T.J.P. and J.L.O.R; data curation, T.J.P. and J.L.O.R.; writing—original draft preparation, H.S.R.B., K.I.V.H., R.V.R., and T.J.P.; writing—review and editing, T.J.P. and J.L.O.R.; visualization, T.J.P. and J.L.O.R.; supervision, T.J.P. and J.L.O.R.; project administration, T.J.P. and J.L.O.R.; funding acquisition, T.J.P. and J.L.O.R. All authors have read and agreed to the published version of the manuscript.
Funding
“This research was funded by CONAHCYT, The Master Scholarship of K.I.V.H. [CVU:1301333)], Ph.D. Scholarship of S.H.R.B. [CVU:1138449] and Postdoctoral Fellowship of T.J.P. [MOD.ORD.10/2023-I1200/331/2023]” and “This work was published thanks to the support of Consejo Veracruzano de Investigación Científica y Desarrollo Tecnológico - COVEICYDET”, .
Data Availability Statement
NA
Acknowledgments
The authors thank CONAHCYT for Scholarships and economical support to the INECOL.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring north of Mexico with an illustrated key. Annals of the Entomological Society of America 2006, 99, 1034-1056. [CrossRef]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Annual Review of Entomology 2017, 62, 285-303. [CrossRef]
- Schmidberger, J. (1836). Beiträge zur Obstbaumzucht und zur Naturgeschichte der den Obstbäumen schädlichen Insekten (Vol. 4). Haslinger.
- Cognato, A.I.; Smith, S.M.; Li, Y.; Pham, T.H.; Hulcr, J. Genetic Variability Among Xyleborus glabratus Populations Native to Southeast Asia (Coleoptera: Curculionidae: Scolytinae: Xyleborini) and the Description of Two Related Species. Journal of Economic Entomology 2019, 112, 725-735. [CrossRef]
- Jordal, B.; Tischer, M. Genetic and taxonomic assessment of the widespread Afrotropical ambrosia beetle Xyleborus principalis (Coleoptera, Scolytinae). International Journal of Tropical Insect Science 2020, 40, 707-715. [CrossRef]
- Osborn, R.K.; Castro, J.; Duong, T.A.; Hulcr, J.; Li, Y.; Martínez, M.; Cognato, A.I. Symbiotic fungi associated with Xyleborine ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) and the imperative of global collaboration. Annals of the Entomological Society of America 2023, 116, 51-71. [CrossRef]
- Dreaden, T.J.; Davis, J.M.; de Beer, Z.W.; Ploetz, R.C.; Soltis, P.S.; Wingfield, M.J.; Smith, J.A. Phylogeny of ambrosia beetle symbionts in the genus Raffaelea. Fungal Biology 2014, 118, 970-978. [CrossRef]
- Saucedo-Carabez, J.R.; Ploetz, R.C.; Konkol, J.L.; Carrillo, D.; Gazis, R. Partnerships Between Ambrosia Beetles and Fungi: Lineage-Specific Promiscuity Among Vectors of the Laurel Wilt Pathogen, Raffaelea lauricola. Microbial Ecology 2018, 76, 925-940. [CrossRef]
- Gugliuzzo, A.; Biedermann, P.H.W.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Haddi, K.; Hulcr, J.; Jactel, H.; Kajimura, H.; Kamata, N.; Meurisse, N.; Li, Y.; Oliver, J.B.; Ranger, C.M.; Rassati, D.; Stelinski, L.L.; Sutherland, R.; Tropea Garzia, G.; Wright, M.G.; Biondi, A. Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. Journal of Pest Science 2021, 94, 615-637. [CrossRef]
- Cloonan, K.R.; Montgomery, W.S.; Narvaez, T.I.; Carrillo, D.; Kendra, P.E. Community of Bark and Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in Agricultural and Forest Ecosystems with Laurel Wilt. Insects 2022, 13, 971. [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.J.; Svoboda, M.; Viiri, H.; Seidl, R. Bark Beetle Outbreaks in Europe: State of Knowledge and Ways Forward for Management. Current Forestry Reports 2021, 7, 138-165. [CrossRef]
- Spence, D.J.; Smith, J.A.; Ploetz, R.; Hulcr, J.; Stelinski, L.L. Effect of Chipping on Emergence of the Redbay Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae) and Recovery of the Laurel Wilt Pathogen From Infested Wood Chips. Journal of Economic Entomology 2013, 106, 2093-2100. [CrossRef]
- Biedermann, P.H.W.; Klepzig, K.D.; Taborsky, M. Fungus Cultivation by Ambrosia Beetles: Behavior and Laboratory Breeding Success in Three Xyleborine Species. Environmental Entomology 2009, 38, 1096-1105. [CrossRef]
- Ward, S.F.; Riggins, J.J. Drivers of Invasion by Laurel Wilt of Redbay and Sassafras in the Southeastern US. Landscape Ecology 2023, 38, 567-581. [CrossRef]
- Souza, A.G.C.; Maffia, L.A.; Murta, H.M.; Alves, Y.H.; Pereira, R.M.; Picanço, M.C. First Report on the Association Between Ceratocystis fimbriata, an Agent of Mango Wilt, Xyleborus affinis, and the Sawdust Produced During Beetle Colonization in Brazil. Plant Disease 2013, 97, 1116. [CrossRef]
- Olatinwo, R.O.; Fraedrich, S.W.; Mayfield, A.E. III. Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management. Forests 2021, 12, 181. [CrossRef]
- Hughes, M.A.; Inch, S.A.; Ploetz, R.C.; Er, H.L.; van Bruggen, A.H.C.; Smith, J.A. Responses of Swamp Bay, Persea palustris, and Avocado, Persea americana, to Various Concentrations of the Laurel Wilt Pathogen, Raffaelea lauricola. Forest Pathology 2014.
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E. III; Hanula, J.L.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J.; Carrillo, D.; Rabaglia, R.; Eickwort, J.; Pernas, T. Recovery Plan for Laurel Wilt on Redbay and Other Forest Species Caused by Raffaelea lauricola and Disseminated by Xyleborus glabratus. Plant Health Progress 2015. [CrossRef]
- Carrillo, D.; Crane, J.H.; Peña, J.E. Potential of Contact Insecticides to Control Xyleborus glabratus (Coleoptera: Curculionidae), a Vector of Laurel Wilt Disease in Avocados. Journal of Economic Entomology 2013, 106, 2286-2295. [CrossRef]
- Bonilla-Landa, I.; Cuapio-Muñoz, U.; Luna-Hernández, A.; Reyes-Luna, A.; Rodríguez-Hernández, A.; Ibarra-Juárez, A.; Suarez-Mendez, G.; Barrera-Méndez, F.; Caram-Salas, N.; Enríquez-Medrano, J.F.; Díaz de León, R.E.; Olivares-Romero, J.L. L-Proline as a Valuable Scaffold for the Synthesis of Novel Enantiopure Neonicotinoids Analogs. Journal of Agricultural and Food Chemistry 2021, 69, 1455-1465. [CrossRef]
- Reyes-Luna, A.; Yáñez-Barrientos, E.; Alba-Mares, X.N.; Olivares-Romero, J.L.; Alonso-Castro, Á.J.; Cruz, D.C.; Villegas Gómez, C. Metabolomic Approaches in Assessing the Insecticidal Activity of the Extracts from Argemone ochroleuca Sweet (Papaveraceae) Against Three Diverse Crop Pests of Economic Importance. Chemistry & Biodiversity 2024, 21, e202301279. [CrossRef]
- Guerrero-Analco, J.A.; Murrieta-León, D.L.; Licona-Velazquez, S.; Monribot-Villanueva, J.L.; Ibarra-Juárez, L.A.; Hernández-Cervantes, G.; Ramírez-Vázquez, M.; Carmona-Hernández, O.; Lozada-García, J.A.; Pucheta-Fiscal, E.; León-Wilchez, Y.Y.; Martínez-Viveros, J.A.; Valencia-Mejía, E. Antifungal and Insecticidal Activities of Selected Plant Species from Cloud Forest of Veracruz, Mexico: A Contribution to the Search of Novel Control Agents against Ambrosia Pest Complexes. Chemistry & Biodiversity 2023, 20, e202300274. [CrossRef]
- Rodríguez-Hernández, A.; Bonilla-Landa, I.; Vidal-Limon, A.; Ibarra-Juárez, A.; Barrera-Méndez, F.; Enríquez Medrano, F.J.; Díaz de León, R.E.; Olivares-Romero, J.L. Synthesis, Insecticidal Activity, and Ensembled Docking of Nitroguanidines Bearing S- and R-Proline. Pest Management Science 2023, 79, 1912-1921. [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; Montesinos-Matías, R.; Ek-Ramos, M.J.; Garza-López, P.M.; Arredondo-Bernal, H.C. Selection of Beauveria bassiana (Hypocreales: Cordycipitaceae) Strains to Control Xyleborus affinis (Curculionidae: Scolytinae) Females. PeerJ 2020, 8, e9472. [CrossRef]
- Castillo-Esparza, J.F.; Mora-Velasco, K.A.; Rosas-Saito, G.H.; Rodríguez-Haas, B.; Sánchez-Rangel, D.; Ibarra-Juárez, L.A.; Ortiz-Castro, R. Microorganisms Associated with the Ambrosial Beetle Xyleborus affinis with Plant Growth-Promotion Activity in Arabidopsis Seedlings and Antifungal Activity Against Phytopathogenic Fungus Fusarium sp. INECOL_BM-06. Microbial Ecology 2023, 85, 1396-1411. [CrossRef]
- Rather, B.A.; Mir, M.M.; Iqbal, U.; Mir, S.A. Integrated Pest Management of Stone Fruits. In Mir, M.M.; Iqbal, U.; Mir, S.A. (Eds.), Production Technology of Stone Fruits. Springer Nature Singapore Pte Ltd. 2021, pp. 397-421. [CrossRef]
- Hamilton, J.L.; Workman, J.N.; Nairn, C.J.; Fraedrich, S.W.; Villari, C. Rapid Detection of Raffaelea lauricola Directly from Host Plant and Beetle Vector Tissues Using Loop-Mediated Isothermal Amplification. Plant Disease 2020, 104, 3151-3158. [CrossRef]
- Biswas, C.; Hartley, S.E.; Taylor, G.; Hanley, M.E. Genomic Comparisons of the Laurel Wilt Pathogen, Raffaelea lauricola, and Related Tree Pathogens Highlight an Arsenal of Pathogenicity Related Genes. Fungal Genetics and Biology 2019, 125, 84-92. [CrossRef]
- Ward, S.F.; Riggins, J.J. Warm Temperatures and Host Tree Abundance Explain Variation in Directional Spread by Laurel Wilt. Biological Invasions 2023. [CrossRef]
- Saucedo, J.R.; Ploetz, R.C.; Konkol, J.L.; Ángel, M.; Mantilla, J.; Menocal, O.; Carrillo, D. Nutritional symbionts of a putative vector Xyleborus bispinatus of the laurel wilt pathogen of avocado Raffaelea lauricola. Symbiosis 2018, 75, 29–38. [CrossRef]
- Campbell, A.S.; Ploetz, R.C.; Dreaden, T.J.; Kendra, P.E. Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 2016, 108, 884-894. [CrossRef]
- Six, D.L. Ecological and evolutionary determinants of bark beetles-fungus symbioses. Insects 2012, 3, 339–366. [CrossRef]
- Ibarra-Juarez, L.A.; Burton, M.A.J.; Biedermann, P.H.W.; Cruz, L.; Desgarennes, D.; Ibarra-Laclette, E.; Latorre, A.; Alonso-Sánchez, A.; Villafan, E.; Hanako-Rosas, G.; López, L.; Vázquez-Rosas-Landa, M.; Carrion, G.; Carrillo, D.; Moya, A.; Lamelas, A. Evidence for succession and putative metabolic roles of fungi and bacteria in the farming mutualism of the ambrosia beetle Xyleborus affinis. mSystems 2020, 5, e00541-20. [CrossRef]
- Mayfield, A.E. III; Thomas, M.C. The redbay ambrosia beetle Xyleborus glabratus Eichhoff (Scolytinae: Curculionidae). Pest Alert Florida Department of Agriculture and Consumer Services Division of Forestry. Retrieved from http://www.doacs.state.fl.us/pi/enpp/pathology/laurel_wilt_disease.html.
- Biedermann, P.H.W.; Vega, F.E. Ecology and evolution of insect–fungus mutualisms. Annual Review of Entomology 2020, 65, 431-455. [CrossRef]
- Mayfield, A.E. III; Brownie, C. The redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) uses stem silhouette diameter as a visual host-finding cue. Environmental Entomology 2013, 42, 743-750. [CrossRef]
- Menocal, O.; Cruz, L.F.; Kendra, P.E.; Crane, J.H.; Ploetz, R.C.; Carrillo, D. Rearing Xyleborus volvulus (Coleoptera: Curculionidae) on media containing sawdust from avocado or silkbay with or without Raffaelea lauricola (Ophiostomatales: Ophiostomataceae). Environmental Entomology 2017, 46, 1275-1283. [CrossRef]
- Rabaglia, R.J.; Smith, S.L.; Rugman-Jones, P.; Digirolomo, M.F.; Ewing, C.; Eskalen, A. Establishment of a non-native xyleborine ambrosia beetle Xyleborus monographus (Fabricius) (Coleoptera: Curculionidae: Scolytinae) new to North America in California. Zootaxa 2020, 4786, 269-276. [CrossRef]
- Cruz, L.F.; Menocal, O.; Mantilla, J.; Ibarra-Juarez, L.A.; Carrillo, D. Xyleborus volvulus (Coleoptera: Curculionidae): Biology and fungal associates. Appl. Environ. Microbiol. 2019, 85(19), e01190-19. [CrossRef]
- Yin, M.-L.; Chen, H.-X.; He, Y.-Z.; Gao, X.; Huang, S.; Wang, J. First report of Fusarium oxysporum causing Fusarium dieback on Archidendron clypearia in China. Plant Dis. 2020, 104, 2730. [CrossRef]
- Batra, L.R. Ambrosia fungi: A taxonomic revision, and nutritional studies of some species. Mycologia 1967, 59, 976-1017. [CrossRef]
- Vega, F.E.; Hofstetter, R.W. The Bark Beetles: Biology and Ecology of Native and Invasive Species. Elsevier: 1st Edition, 2015, 640 pages. [CrossRef]
- Mayers, C.G.; Harrington, T.C.; Biedermann, P.H.W.; Six, D.L. Patterns of coevolution between bark beetles and symbionts: A review of interactions and implications for beetle-fungus ecology. Microb. Ecol. 2020, 80, 161-176. [CrossRef]
- Mendel, Z.; Protasov, A.; González-Audino, P.; Schlyter, F.; Vega, F.E.; Biedermann, P.H.W. Global invasion of ambrosia beetles: Can cryptic species bring cryptic threats? Ann. Rev. Entomol. 2023, 68, 241-261. [CrossRef]
- Jürisoo, L.; Süda, I.; Agan, A.; Drenkhan, R. Vectors of Dutch Elm Disease in Northern Europe. Insects 2021, 12, 393. [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008, 92, 215-224. [CrossRef]
- Menocal, O.; Cruz, L.F.; Kendra, P.E.; Berto, M.; Carrillo, D. Flexibility in the ambrosia symbiosis of Xyleborus bispinatus. Front. Microbiol. 2023, 14, 1110474. [CrossRef]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys 2018, 768, 19-68. [CrossRef]
- Gohli, J.; Selvarajah, T.; Kirkendall, L.R.; Jordal, B.H. Globally distributed Xyleborus species reveal recurrent intercontinental dispersal in a landscape of ancient worldwide distributions. BMC Evol. Biol. 2016, 16, 37. http://doi.org/10.1186/s12862-016-0610-7.
- Paladines-Rezabala, A.; Moreira-Morrillo, A.A.; Mieles, A.E.; Garcés-Fiallos, F.R. Avances en la comprensión de la interacción entre Ceratocystis cacaofunesta y Xyleborus ferrugineus (Coleoptera: Curculionidae: Scolytinae) en árboles de cacao. Scientia Agropecuaria 2022, 13, 43-52. [CrossRef]
- Faccoli, M.; Campo, G.; Perrotta, G.; Rassati, D. Two newly introduced tropical bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) damaging figs (Ficus carica) in southern Italy. Zootaxa 2016, 4138, 189-194. [CrossRef]
- Biedermann, P.H.W. Cooperative breeding in the ambrosia beetle Xyleborus affinis and management of its fungal symbionts. Frontiers in Ecology and Evolution 2020, 8, 518954. [CrossRef]
- Bateman, C.; Kendra, P.E.; Rabaglia, R.; Hulcr, J. Fungal symbionts in three exotic ambrosia beetles Xylosandrus amputatus Xyleborinus andrewesi and Dryoxylon onoharaense (Coleoptera: Curculionidae: Scolytinae: Xyleborini) in Florida. Symbiosis 2015, 66, 141-148. [CrossRef]
- Hulcr, J.; Black, A.; Prior, K.; Chen, C.-Y.; Li, H.-F. Studies of ambrosia beetles (Coleoptera: Curculionidae) in their native ranges help predict invasion impact. Florida Entomologist 2017, 100, 257-261. [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J. Laurel wilt: a new and devastating disease of redbay caused by a fungal symbiont of the exotic redbay ambrosia beetle. Newsletter of the Michigan Entomological Society 2007, 52(1-2), 14-15.
- Mahony, Z.I.; Scarlett, K.; Carnegie, A.J.; Trollip, C.; Laurence, M.; Guest, D.I. Fungi Associated with the Ambrosia Beetle Xyleborus perforans (Coleoptera: Curculionidae: Scolytinae) on Drought-Stressed Pinus in New South Wales Australia. Australasian Plant Pathology 2024, 53, 51-62. [CrossRef]
- Martini, X.; Hughes, M.A.; Killiny, N.; George, J.; Lapointe, S.L.; Smith, J.A.; Stelinski, L.L. The Fungus Raffaelea lauricola Modifies Behavior of Its Symbiont and Vector the Redbay Ambrosia Beetle (Xyleborus glabratus) by Altering Host Plant Volatile Production. Journal of Chemical Ecology 2017, 43, 477-493. [CrossRef]
- Kuhns, E.H.; Martini, X.; Tribuiani, Y.; Coy, M.; Gibbard, C.; Peña, J.; Hulcr, J.; Stelinski, L.L. Eucalyptol is an Attractant of the Redbay Ambrosia Beetle Xyleborus glabratus. Journal of Chemical Ecology 2014, 40, 355-362. [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Efficacy of α-Copaene Cubeb and Eucalyptol Lures for Detection of Redbay Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae). Journal of Economic Entomology 2016, 109, 2428-2435. [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Disease 2008, 92, 215-224. [CrossRef]
- Harrington, T.; Fraedrich, S.; Aghayeva, D. Raffaelea lauricola a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404.
- Evans, E.A.; Crane, J.; Hodges, A.; Osborne, J.L. Potential Economic Impact of Laurel Wilt Disease on the Florida Avocado Industry. HortTechnology 2010, 20, 234-238.
- Laurel wilt of avocado: management of an unusual and lethal disease. Available online: https://portal.nifa.usda.gov/web/crisprojectpages/1007583-laurel-wilt-of-avocado-management-of-an-unusual-and-lethal-disease.html (accessed on 09 08 2024).
- Reverchon, F.; Contreras-Ramos, S.M.; Eskalen, A.; Guerrero-Analco, J.A.; Quiñones-Aguilar, E.E.; Rios-Velasco, C.; Velázquez-Fernández, J.B. Microbial biocontrol strategies for ambrosia beetles and their associated phytopathogenic fungi. Frontiers in Sustainable Food Systems 2021, 5, 737977. [CrossRef]
- Paladines-Rezabala, A.; Moreira-Morrillo, A.A.; Mieles, A.E.; Garcés-Fiallos, F.R. Advances in understanding of the interaction between Ceratocystis cacaofunesta and Xyleborus ferrugineus (Coleoptera: Curculionidae: Scolytinae) on cocoa trees. Scientia Agropecuaria 2022, 13, 43-52. [CrossRef]
- Romero, P.; Ibarra-Juárez, L.A.; Carrillo, D.; Guerrero-Analco, J.A.; Kendra, P.E.; Kiel-Martínez, A.L.; Guillén, L. Electroantennographic Responses of Wild and Laboratory-Reared Females of Xyleborus affinis Eichhoff and Xyleborus ferrugineus (Fabricius) (Coleoptera: Curculionidae: Scolytinae) to Ethanol and Bark Volatiles of Three Host-Plant Species. Insects 2022, 13, 655. [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; García-Ortiz, N.; Muñiz-Paredes, F.; Sánchez-Rangel, J.C.; Montesinos-Matías, R. Biocontrol of Xyleborus affinis (Curculionidae: Scolitinae) Females and Progeny by Beauveria bassiana (Hypocreales: Cordycipitaceae) in a Sawdust Artificial Diet Model. Insects 2023, 14, 477. [CrossRef]
- Gohli, J.; Selvarajah, T.; Kirkendall, L.R.; Jordal, B.H. Globally distributed Xyleborus species reveal recurrent intercontinental dispersal in a landscape of ancient worldwide distributions. BMC Evolutionary Biology 2016, 16, 37. [CrossRef]
- Luna-Hernández, S.A.; Bonilla-Landa, I.; Reyes-Luna, A.; Rodríguez-Hernández, A.; Cuapio-Muñoz, U.; Ibarra-Juárez, L.A.; Suarez-Mendez, G.; Barrera-Méndez, F.; Pérez-Landa, I.D.; Enríquez-Medrano, F.J.; Díaz de León-Gómez, R.E.; Olivares-Romero, J.L. Synthesis and Insecticidal Evaluation of Chiral Neonicotinoids Analogs: The Laurel Wilt Case. Molecules 2021, 26, 4225. [CrossRef]
- Carrillo, D.; Cruz, L.F.; Kendra, P.E.; Montgomery, W.S.; Monterroso, A.; De Grave, C.; Cooperband, M.F.; Peña, J.E. Distribution, Pest Status and Fungal Associates of Redbay Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae) in Miami-Dade County, Florida. Journal of Economic Entomology 2015, 108, 998-1006. [CrossRef]
- Crane, J.H.; Balerdi, C.F.; Maguire, I. Management of laurel wilt disease, caused by Raffaelea lauricola, on avocado. Acta Horticulturae 2013, 1005, 149-156. [CrossRef]
- Hughes, M.A.; Brar, G.; Mayfield, A.E. Synthesis of volatile organic compounds by the laurel wilt pathogen, Raffaelea lauricola, and their potential for use in detection and control. Phytopathology 2015, 105, 624-633. [CrossRef]
- Hulcr, J.; Mann, R.; Stelinski, L.L. The scent of a partner: Ambrosia beetles are attracted to volatiles from their fungal symbionts. Journal of Chemical Ecology 2011, 37, 1374-1377. [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Epsky, N.D. Analyzing volatile emissions from manuka oil and phoebe oil to identify host attractants for the redbay ambrosia beetle, Xyleborus glabratus. Phytochemistry 2012, 78, 42-52. [CrossRef]
- Kendra, P.E.; Niogret, J.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Temporal analysis of sesquiterpene emissions from manuka and phoebe oil lures and efficacy for attraction of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Journal of Economic Entomology 2014, 107, 481-487. [CrossRef]
- Kendra, P.E.; Owens, D.; Montgomery, W.S.; Narvaez, T.; Schnell, E.Q.; Tabanca, N.; Epsky, N.D. α-Copaene, a precursor to spiroketals in volatile emissions of the redbay ambrosia beetle, Xyleborus glabratus. PLOS ONE 2016, 11, e0150533. [CrossRef]
- MacConnell, J.; Borden, J.H.; Gries, R.; Pierce, H.D.; Chong, L.J.; Stokkink, R. Potential for Nonhost Volatiles as Repellents in Integrated Pest Management of Ambrosia Beetles. Integrated Pest Management Reviews 2001, 6, 221-236. [CrossRef]
- Borden, J.H.; Stokkink, E. Semiochemical-based integrated pest management of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in British Columbia's forest industry: implemented in 1982 and still running. The Canadian Entomologist 2020, 1-12. [CrossRef]
- Sivapalan, P. An Integrated Management Strategy to Minimize the Economic Damage to Mature Tea, Caused by the Shot-Hole Borer Beetle (Xyleborus fornicatus Eichh.). Journal of Tea Science 1985, 54, 4-10.
- Walgama, R.S. Ecology and Integrated Pest Management of Xyleborus fornicatus (Coleoptera: Scolytidae) in Sri Lanka. Journal of Integrated Pest Management (IPM) 2012, 3, 1-9. [CrossRef]
- Mayfield, A.E.; Peña, J.E.; Crane, J.H.; Smith, J.A.; Branch, C.L.; Ottoson, E.D.; Hughes, M. Suitability of Florida avocado cultivars to attack by the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and infection by its symbiotic fungus Raffaelea lauricola. Florida Entomologist 2013, 96, 1595-1606. [CrossRef]
- Niogret, J.; Kendra, P.E.; Epsky, N.D.; Heath, R.R. Comparative analysis of terpenoid emissions from Florida host trees of the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Journal of Chemical Ecology 2011, 37, 931-941. [CrossRef]
- Peña, J.E.; Duncan, R.E.; Crane, J.H. Chemical control of the redbay ambrosia beetle, Xyleborus glabratus Eichhoff, and its fungal symbiont causing laurel wilt in avocados. Proceedings of the Florida State Horticultural Society 2011, 124, 187-192.
- Ploetz, R.C.; Pérez-Martínez, J.M.; Smith, J.A.; Hughes, M.; Dreaden, T.; Inch, S.A.; Fu, Y.; Konkol, J. Responses of avocado to laurel wilt, caused by Raffaelea lauricola. Plant Pathology 2011, 60, 614-623. [CrossRef]
- Cloonan, K.R.; Montgomery, W.S.; Narvaez, T.I.; Kendra, P.E. A New Repellent for Redbay Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae), Primary Vector of the Mycopathogen That Causes Laurel Wilt. Plants 2023, 12, 2406.
- Pawar, T.J.; Olivares-Romero, J.L.; Delgado-Alvarado, E.; Martinez-Castillo, J.; Alvarado-Silva, A.; Vallejo-Montesinos, J.; Jimenez-Halla, J.O. Synthesis and Insecticidal Activity of Nitroguanidines Featuring R- and S-Proline. 2023 IEEE International Conference on Engineering Veracruz (ICEV), Boca del Río, Veracruz, Mexico, 2023, 1–5. [CrossRef]
- Peña, J.E.; Weihman, S.W.; McLean, S.; Cave, R.D.; Carrillo, D.; Duncan, R.E.; Evans, G.; Krauth, S.; Thomas, M.C.; Lu, S.S.; Kendra, P.E.; Roda, A.L. Predators and Parasitoids Associated with Scolytinae in Persea Species (Laurales: Lauraceae) and Other Lauraceae in Florida and Taiwan. Fla. Entomol. 2015, 98, 903-910.
- Crane, J.; Ploetz, R.; Carrillo, D.; Evans, E.; Wasielewski, J.; Pybas, D. Current Management Recommendations for Laurel Wilt of Avocados. Proc. Fla. State Hort. Soc. 2016, 129, 4-10.
- Ospina-Garcés, S.M.; Ibarra-Juarez, L.A.; Escobar, F.; Lira-Noriega, A. Growth temperature effect on mandibles ontogeny and sexual dimorphism in the ambrosia beetle Xyleborus affinis (Curculionidae: Scolytinae). Arthropod Struct. Dev. 2021, 61, 101029. [CrossRef]
- Cruz, L.F.; Rocio, S.A.; Duran, L.G.; Menocal, O.; Garcia-Avila, C.D.J.; Carrillo, D. Developmental biology of Xyleborus bispinatus (Coleoptera: Curculionidae) reared on an artificial medium and fungal cultivation of symbiotic fungi in the beetle's galleries. Fungal Ecol. 2018, 35, 116-126. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).