1. Introduction
Myopia is a significant public health concern, affecting an increasing number of individuals worldwide [
1]. Approximately 30% to 50% of adults in USA and Europe have myopia [
2]. The prevalence of myopia is increasing everywhere and it is estimated that in 2050, 50% of the world population will be myopic [
3].
The Correction of Myopia Evaluation Trial (COMET) study defined progressive myopia for children aged 6 to 12 years as refractive error progressing more than -0.75 diopters (D) per year. However in clinical practice patients newly diagnosed with myopia have often the refractive error measurement available only at a single point, and previous data with well documented myopia progression over time are not present. In addition, a single cut-off of spherical equivalent to define progressive myopia for children aged 5-18 years could not be accurate [
4]. Previous studies indicated that the mean annual myopia progression rate in children was about half-a-diopter in Europe (-0.55D) and a slightly higher in Asia (-0.82D) [
5].
Vision-threatening complications are frequently correlated with high myopia, with an associated elevated risk of ocular diseases such as retinal detachment, glaucoma, and myopic maculopathy, which is one of the actual leading causes of low vision and blindness in developed countries [
1,
6,
7,
8].
Few prospective studies have shown the relationship between age at myopia onset and myopia severity [
9,
10,
11] but, since the high prevalence of myopia provides important public health and socio-economic problems [
12], and because the rising prevalence of myopia mainly occures in youngest population, it is growing the need to identify the more effective interventions for managing myopia progression. In this effort, a special importance could assume the evaluation of the effectiveness of each possible treatment as a function of the age [
1,
13,
14]. Because of myopia is usually detected in children before 10 years of age and the prevalence of myopia could record a fast progression after the age of six, and although the sight-threatening pathologies associated with myopia usually occur later in life, the therapeutic strategy to slow myopia increase needs to be delivered in childhood in the attempt to reduce a subsequent visual impairment [
15].
Several and different therapeutic options are actually considered for slowing myopia progression [
16]. Pharmacological treatment resulted relatively more effective than optical methods, as wearing contact lenses or spectacles [
17,
18]. Lower dose (0.01-0.1%) atropine is currently more prescribed because induces less associated side effects, such as photophobia and blurry vision, and some clinical trials indicated that 0.01% atropine eyedrops concentration achieves modest therapeutic effects but low myopic rebound and minimal side effects [
19,
20,
21,
22].
Theoretically the favorite treatment should be as less invasive as possible, making consequentially optical device the ideal alternative option [
16]. Optical interventions include a variety of spectacle and contact lens design. Spectacles should be the less invasive and most accessibile method for slowing myopia progression [
15].
Spectacle lenses with defocus technology specifically designed for myopia progression control have been recently introduced in the clinical practice because of their convenience, safety, and relatively low cost [
12,
14,
23]. One of these kind is the Defocus Incorporated Multiple Segments (DIMS) lens, that has emerged as a promising for myopia control [
16]. This dual-focus lens consists of a central optical zone for correcting distance refractive error, and an annular mid peripheral focal zone with multiple segments having a relative positive power of +3.50 D [
24]. This optical design that provides a myopic defocus in the peripheral retina while maintaining clear central vision, is hypothesized to modulate ocular growth and mitigate the elongation of the eyeball, a key factor in myopia progression. Several clinical studies, mainly focused on Asiatic population, have evaluated the effectiveness of defocus lens in slowing myopia progression, with encouraging results [
12,
15].
Several are the mechanisms underlying the efficacy of defocus lens in myopia control. Myopic defocus in the peripheral retina is believed to inhibit axial elongation by stimulating the release of retinal neurotransmitters associated with scleral remodeling. Moreover, defocus lens may influence visual feedback mechanisms, such as accommodation and vergence, which play a role in regulating ocular growth. By providing clear central vision and myopic defocus in the periphery, these lenses may help to maintain optical balance and signal retinal homeostasis.
Recently [
1,
3,
14], the potential synergistic effects of combining defocus lens with other myopia control strategies, such as atropine therapy or outdoor activities, have been explored. Combining defocus lenses with low-dose atropine has shown promising results in further reducing myopia progression compared to either intervention alone [
3]. Similarly, interesting findings have been reported regarding the beneficial effects of outdoor activities in conjunction with defocus lenses.
However, age-specific results for DIMS spectacles lens are still not widely available in the literature. The existing studies suggest that DIMS spectacles represent a promising intervention for myopia control in children and adolescents. Further research specifically examining the age-related effects of DIMS spectacles on myopia progression could provide valuable insights into the optimal use of this treatment across different age groups.
Aim of this study is to evaluate the effectiveness of DIMS spectacle lens in myopia progression control as a function of the age in European pediatric patients.
2. Methods
This was a non-randomized experimenter-masked retrospective controlled observational study of European children with documented progressive myopia. The inclusion criteria were patient between 6-16 years of age, progressive myopia with cycloplegic spherical equivalent from -0.50 to -4.00 D, refractive astigmatism less than 2.0 D, anisometropia under 1.0 D. The exclusion criteria were genetic syndromes suspected (e.g., Stickler, Marfan, etc.) and all systemic or eye diseases (such as glaucoma, congenital or juvenile cataract, retinal diseases, any form of strabismus). These criteria were used to select participants for the study and ensure that the sample population met specific requirements related to age, ethnicity, refractive error, and ocular health status. All participants underwent a full ophthalmological assessment including symptoms and history, orthoptic testing, refraction (with cycloplegic autorefraction) and dilated fundoscopy.
Participants who met the inclusion criteria and did not meet any of the exclusion criteria were eligible to participate in the study on myopia progression control intervention. Participants were allocated to receiving DIMS (Hoya® MiyoSmart®) spectacle lens, or wearing single vision spectacle lenses. All the pediatric patients considered in the treatment groups wore the DIMS spectacle lenses, while those components of the control groups wore ordinary single vision spectacle lenses. The period considered for enrolment lasted from the 1st of December 2020 to the 30th of June 2021.
Informed consent was obtained for each patient, and all investigations followed the guidelines required by the institution. The study adhered to the Tenets of the Declaration of Helsinki. (ClinicalTrials.gov: submitted).
To determine the age-specific myopia progression, individuals were further categorized as myopes who are at least 10 years or younger and those who are above 10. In the attempt to evaluate DIMS spectacle lens effectiveness as a function of the age, the results were stratified into four groups: patients that wore DIMS spectacle lenses oldest or youngest than 10 years (respectively, group A, 20 patients mean age 13.6±2.2 and group C, 20 patients mean age 9.0±1.2) and age-matched control groups (group B, 18 patients mean age 13.2±2.5 and group D, 22 patients mean age 8.5±0.9) that wore single vision spectacle lenses.
At baseline and at 36 month follow up, spherical equivalent refractive error (SE) under cycloplegia and axial length (AL) were measured for each patient. Myopia progression was calculated as the difference between mean SE and AL at 36 month visit and baseline. Cycloplegic refraction was measured with the PRK-5000 POTEK autorefractor (Potec, Korea), and the AL was calculated with the Nidek optical biometer AL-Scan (Nidek Co, LTD). Average of five measurements of autorefraction and biometer for each eye were obtained for analysis.
No treatment-related adverse event was reported.
An independent Student t test was used to compare myopia progression between DIMS spectacle lens wearer and control group patients, respectively for age >10 (groups A and B) and <10 (groups C and D). Linear regression analysis was calculated to evaluate SE and AL change as a function of patient age for each group. A P value less than 0.05 was considered significant.
3. Results
A total of 80 participants with 36 month follow up were included in the study. Of them, 38 were above 10 years (group A and B) and 42 at least 10 years or younger (group C and D). At baseline (
Table 1), no significant difference in mean age, SE and AL value among corresponding age groups were observed (p>0.05). The
Table 2 summurizes the results recorded at 36 month follow up. Mean SE and AL change significantly decreased (p<0.05) in group A (-0.3±0.4 D) and C (-0.4±0.5 D) respectively compared to group B (-1.2±0.9 D) and D (-1.7±0.7 D).
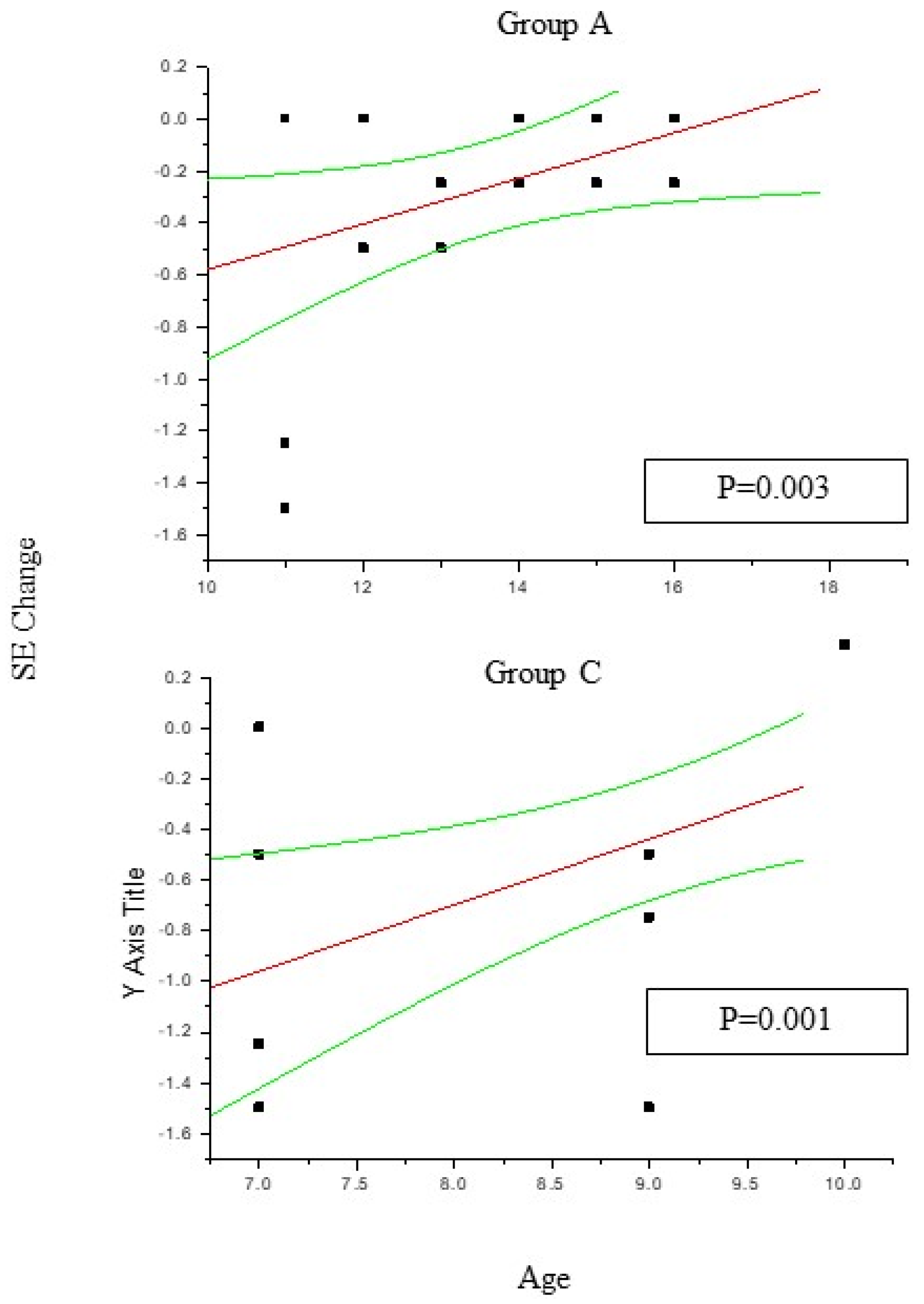
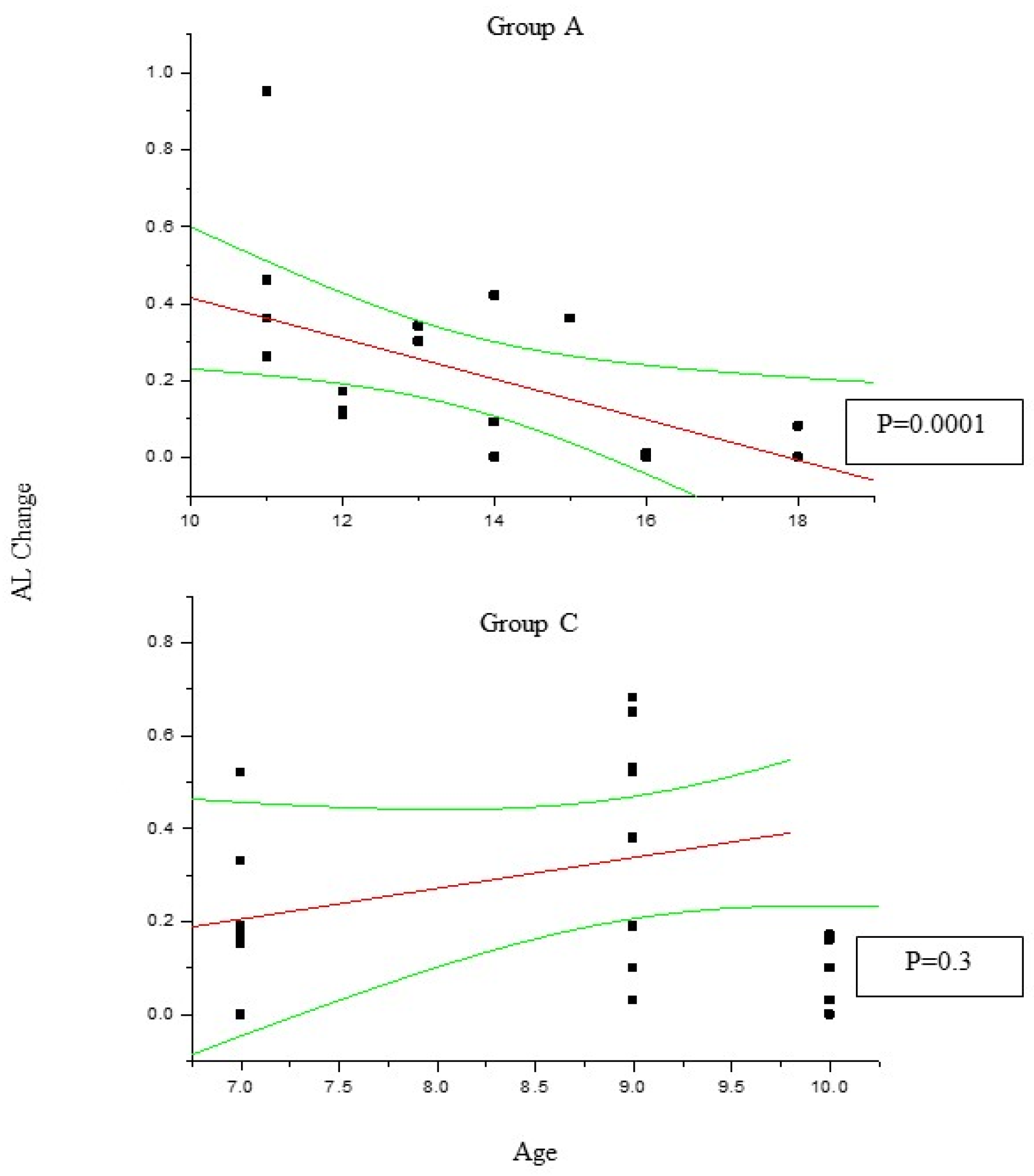
In group A and C (
Figure 1) the change in SE recorded at 36 month follow up results positively correlated with patient age (P<0.05), which means the increasing age was correlated to decreasing myopia progression. Differently, the change in AL (
Figure 2) recorded at 36 month follow up appears inversely correlated with age in group A (P<0.05) but not in group C (P>0.05), which means the increasing age was correlated to decreasing myopia progression only for DIMS spectacle lens wearer oldest than 10 years of age. Differently, control groups B and D did not show any significant linear correlation between SE and AL change and patients age (P>0.05).
4. Discussion
Myopia is a complex condition influenced by various factors such as genetics, environmental exposures, and lifestyle habits [
1,
13,
14]. Few papers reported epidemiologic data on European myopic children, particularly with respect to myopia progression.
Tricard et al. [
25] published a prospective study involving a nationwide cohort, in order to describe the progression of myopia in France using a cohort of individuals aged 4–17 years at baseline and followed for up to 6.5 years from 2013 to 2019. The authors recorded that factors associated with faster myopia progression were gender, with girls being more prone to progression than boys, higher myopia at baseline, and age between 7 and 12 years. Multivariate analysis showed that younger individuals aged 4–12 years, girls and individual with higher myopia at baseline were more likely to develop high myopia. The authors found that age is the most important factor determining the mean progression rate and the relative speed, but they observed that age is not a monotonic factor, with 7–9 year old myopes progressing faster and both younger and older children progressing more slowly. The slower progression in younger children, with very young onset of myopia could reflect a different aetiology. Ethnicity for sure represents an important factor for determinating a different rate of myopia progression, in fact Asian showed a faster progressing than European children [
26].
Tailored therapy could allow healthcare providers to customize treatment plans based on the specific needs and characteristics of each patient, optimizing the effectiveness of interventions [
1,
13,
14]. Several experimental studies [
27,
28,
29,
30,
31,
32] demonstrated that myopic eye growth could be inhibited inducing a myopic defocus using dual focus lens. The efficacy of DIMS lens in slowing myopia progression has been largely reported, but up to now few authors analysed the relationship between myopic progression (in terms of SE and AL change) and patient age.
Age-specific results for the efficacy of DIMS spectacles lens in slowing myopia progression are important for several reasons. Different age groups may respond differently to myopia control interventions. Age-specific results can help to identify the most effective age range for using DIMS spectacle lens, allowing for tailored treatment approaches based on age-related factors. Understanding how the efficacy of DIMS lens varies as a function of the age could also help to determine the optimal timing for initiating treatment. Age-specific results can guide healthcare providers for recommending the most appropriate age to start the use of DIMS spectacles in the attempt to achieve maximum effectiveness. Age-specific data can provide insights into the long-term outcomes of using DIMS spectacles for myopia control. By analyzing how myopia progression changes over time depending on the age, researchers and clinicians can better predict the sustained benefits of treatment. Age-specific results could finally inform the development of targeted treatment strategies for specific age groups. This can include adjusted treatment protocols, follow-up schedules, and intervention combinations based also on the age-related responses to DIMS spectacle lens.
Lam et al [
24] performed a 2-year double-masked randomized controlled trial in Chinese children aged 8-13 years, with myopia between -1.00 and -5.00 D and astigmatism ≤1.50 D and reported that age was the only significant parameter which affects the efficacy of DIMS spectacle lens in slowing myopia progression. The authors observed that the effect of myopia control with DIMS lens was greater in children oldest than 10 years of age. In fact the 80% of DIMS spectacle lens wearers that showed myopia progression were children aged 8-9 years. The authors observed that these findings could be related to different retinal profile or peripheral refraction among children. In case of high amount of peripheral hyperopia, the value of effective myopic defocus at the peripheral retina will be less, and thereby minimizing the treatment effect. Because youngest children presented a myopic peripheral refraction at baseline, and because the effectiveness of DIMS spectacle lens depends on the counterbalance between the hyperopic defocus of the eye and the myopic defocus achieved by the DIMS lens, when a baseline peripheral myopic refraction is combined with the myopic defocus induced by the lens, the total amount of myopic defocus at mid-periphery retina could be too much to provide a satisfactory control of myopia progression.
Long et al [
33] in a retrospective study compared Chinese myopic patients aged 6 to 15 years with SE refraction ranging between -0.50 and -8 D that wore DIMS and single-vision spectacle lenses, and, in accordance with Lam et al [
12], found that the chances of achieving myopia control with DIMS spectacle lens design were better in children aged 10 to 15 years than in children aged 6 to 9 years
To our knowledge, this is the first study that evaluates DIMS technology effectiveness as a function of the age in European pediatric patients. In fact Lam et al. [
12] as well as Long et al. [
33] limited their study to Chinese children, and no author up to now evaluated the DIMS spectacle lenses effectiveness in other ethnic populations.
Some authors evaluated the effectiveness of DIMS lens in the European population. Recently, Nucci et al [
3] compared the efficacy of DIMS spectacle lens, atropine eye drops, and a combination of DIMS and atropine in slowing the progression of myopia in European patients. These results showed that both DIMS lens and 0.01% atropine were individually effective in slowing myopia progression in this population. However the study did not provide detailed results based on different age groups, while the overall findings indicated that both DIMS spectacles and atropine were effective in reducing myopia progression in the population of European children and adolescents studied. The combination of atropine and DIMS was shown to be particularly effective in slowing myopia progression compared to either treatment alone.
A recent review of myopia control study reported that age does not affect the effectiveness of treatment options [
34]. However, as well as some authors [
12,
33] found that in Chinese pediatric population that had worn DIMS spectacle lens oldest children showed a slower myopia progression, while 8 year old patients showed more myopia progression and AL elongation, our findings confirm that also in European pediatric patients DIMS technology does not produces any adverse effect, and provides to slow myopia progression. Our data suggest that DIMS effectiveness evaluated as a function of the age significantly improves in patients aged > 10 years. In fact, while SE change resulted significantly correlated with patient age independently from the age, AL increase was inversely correlated only with patients oldest than 10 years of age.
These findings appear in accordance with several authors that already observed how the primary endpoints for judging efficacy in clinical trials of myopia control intervention should include change in axial length, more than in refractive error [
15].
In this study there were some limitations. The parents generally chose for their own child which kind of spectacles wearing, and, because of various reasons, we were not able to perform an every 6-month monitoring.
The relationship broadly reported between age of onset and severity of myopia in late childhood, makes very important to screen children at high risk of early onset myopia in order to start an early treatment. Genetics supported the significant association between parental myopia and onset of childhood myopia, and environmental exposures including education, near work, and outdoor activities have been identified as key factors in the development of myopia [
4]. Cross linked studies for investigating possible correlations between myopia onset, possible related risk factors and treatment option could give important future indications, then improving the therapeutic approach.
To recap, myopia frequently appears in childhood, with a peak incidence occurring between 8 and 10 years of age. There is major disparity in the prevalence of myopia in children according to ethnic origin. The progression of myopia has been analysed in various studies, and a younger age of myopia onset or longer duration of myopia progression are strong predictors of high myopia. Ethnicity is clearly an important factor in rate of progression with children of East Asian descent progressing faster than those of European ancestry [
25].
Although emerging treatments for myopia are promising and some have been incorporated into clinical practice, identifying populations who require and benefit from intervention remains the most important step for optimizing clinical behavior .
In conclusion, age-specific results for the efficacy of DIMS spectacle lens in myopia control are crucial for optimizing treatment outcomes, tailoring interventions to specific age groups, and guiding clinical decision making in the management of myopia in children and adolescents, because an early management seems crucial to mitigate long-term consequences on ocular health.
Further prospective studies need to validate these early findings, however our retrospective study suggests the effectiveness of DIMS spectacle lens in slowing myopia progression and axial elongation among European children with progressive myopia, mainly if oldest than 10 years of age.
Author Contributions
Conceptualization, L.B.. and S.P.; Methodology, L.B..; Validation, G.I. and P.V.; Formal Analysis, G.I. Investigation, S.P. and PV:; Data Curation, L.B. and M.F.; Writing – Original Draft Preparation, S.P..; Writing – Review & Editing, L.B and GI.
Funding/Acknowledgement
This work was supported also by the Italian Ministry of Health with "Current Research funds”.
References
- Dhakal R, Verkicharla PK, Li D, Mavi S, Kernohan A, Li T, Walline JJ. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev. 2023 Feb 16;2(2):CD014758.
- Dolgin E. The myopia boom. Nature 2015;519(7543):276-278.
- Nucci P, Lembo A, Schiavetti I, Shah R, Edgar DF, Evans BJW. A comparison of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) spectacles, atropine, and combined DIMS/atropine. PLoS ONE 18(2): e0281816. [CrossRef]
- Chen YX, Liao CM, Tan Z, He MG. Who needs myopia control? Int J Ophthalmol, 2021;14:1297-1301.
- Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith 3rd EL, Holden BA. Myopia progression rates in urban children wearing single-vision spectacles. Optometry and Vision Science 2012;89:27–32.
- Tideman, JW, Snabel MCC, Tedja MS, Van Rijn GA et al. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA. Ophthalmol. 2016;134:1355–1363.
- Tang Y, Wang X, Huang W, Wang J, Gao Y, Lui Y, Lu Y. Prevalence and causes of visual impairment in a Chinese adult population: The Taizhou Eye Study. Ophthalmology 2015;122:1480–1488.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Progress in Retinal and Eye Research 2012;31(6):622-630.
- Chua SYL, Sabanayagam C, Cheung YB, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt 2016;36:388–394. [CrossRef]
- Jensen H. Myopia in teenagers. An eight-year follow-up study on myopia progression and risk factors. Acta Ophthalmol Scand 1995;73:389–393.
- Saw S-M, Tong L, Chua W-H, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci 2005;46:51. [CrossRef]
- Lam CSY, Tang WC, Zhang HY, Lee PH, Tse DYY, Qi H, Vlasak N, To CH. Long-term myopia control effect and safety in children wearing DIMS spectacle lenses for 6 years. Scientific Reports (2023);13:5475-5485. [CrossRef]
- Eppenberger LS, Grzybowski A, Schmetterer L, Ang M. Myopia Control: Are We Ready for an Evidence Based Approach? Ophthalmol Ther. 2024 Jun;13(6):1453-1477.
- Zhang XJ, Zaabaar E, French AN, Tang FY, Kam KW, Tham CC, Chen LJ, Pang CP, Yam JC. Advances in myopia control strategies for children. Br J Ophthalmol. 2024 May 22:bjo-2023-323887. [CrossRef]
- Lawrenson JG, Shah R, Huntjens B, Downie LE, Virgili G, Dhakal R, Verkicharla PK, Li D, Mavi S, Kernohan A, Li T, Walline JJ. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2023, Issue 2. Art. No.: CD014758.
- Lam CSY, Tang WC, Tse DYY, Tang YY, To CH. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomized clinical trial. British Journal of Ophthalmology 2019;98(1):40-45.
- Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 2016;123:697–708.
- Smith MJ, Walline JJ. Controlling myopia progression in children and adolescents. Adolesc Health Med Ther 2015;6:133–140. [CrossRef]
- Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012;119:347–354.
- Clark TY, Clark RA. Atropine 0.01% Eyedrops Significantly Reduce the Progression of Childhood Myopia. J Ocul Pharmacol Ther 2015;31:541–545. [CrossRef]
- Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019;126:113–124.
- Chia A, Lu QS, Tan D. (ATOM-2) five-year clinical trial on Atropine for the treatment of Myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology 2016;123: 391–399.
- Cheng, D., Woo, G. C., Drobe, B., & Schmid, K. L. (2014). Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: Three-year results of a randomized clinical trial. JAMA Ophthalmology, 132(3), 258-264.
- Lam CSY. et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2020;104: 363–368.
- Tricard D, Marillet S, Ingrand P, Bullimore MA, Bourne RRA, Leveziel N. Progression of myopia in children and teenagers: a nationwide longitudinal study. Br J Ophthalmol 2022;106:1104–1109. [CrossRef]
- Donovan L, Sankaridurg P, Ho A, et al. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci 2012;89:27–32. [CrossRef]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron 2004;43:447–468. [CrossRef]
- Liu Y, Wildsoet C. The effect of two-zone concentric bifocal spectacle lenses on refractive error development and eye growth in young chicks. Invest Ophthalmol Vis Sci 2011;52:1078–1086. [CrossRef]
- Tse DY, Lam CS, Guggenheim JA, et al. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci 2007;48:5352–5359. [CrossRef]
- McFadden SA, Tse DY, Bowrey HE, et al. Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian eye. Invest Ophthalmol Vis Sci 2014;55:908–917. [CrossRef]
- Benavente-Perez A, Nour A, Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Invest Ophthalmol Vis Sci 2012;53:6479–6487. [CrossRef]
- Arumugam B, Hung L-F, To C-H, et al. The effects of simultaneous dual focus lenses on refractive development in infant monkeys. Invest Ophthalmol Vis Sci 2014;55:7423–7432. [CrossRef]
- Long W, Chen K, Yu S, Liang M, Zheng B, Zeng J, Cui D. One-year Efficacy of the Defocus Incorporated Multiple Segment Lens in Chinese Myopic Children. Optom Vis Sci 2023;100:111–116. [CrossRef]
- Brennan NA, et al. Efficacy in myopia control. Prog. Retin. Eye. Res. 2021;83:100923. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).