Submitted:
18 October 2024
Posted:
18 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
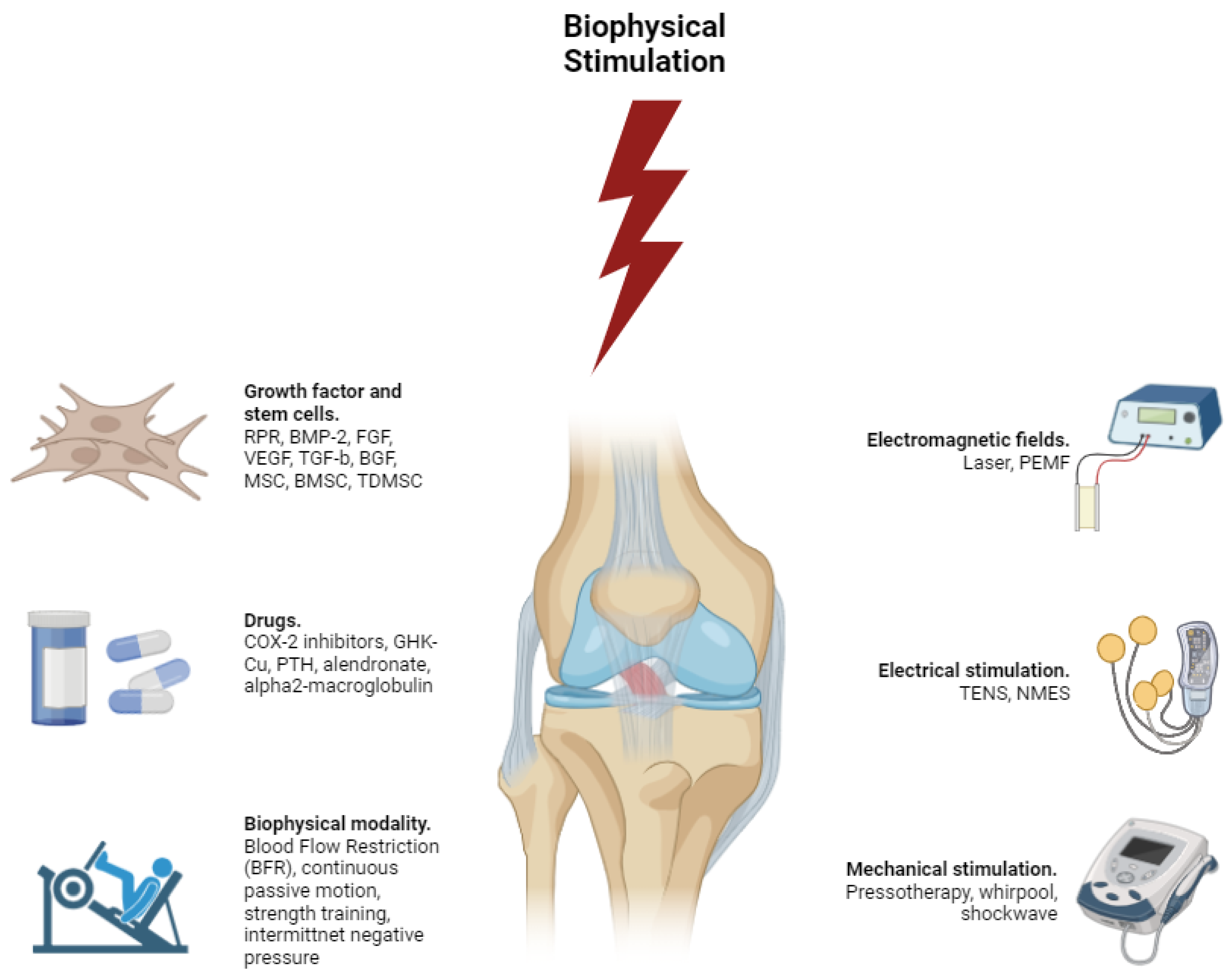
Biophysical Stimulation for ACL Injury Treatement
Biomechanical Factors
1. Kinematic and Kinetic Analysis
2. Impact of External Loads
3. Muscle Activation Patterns
Anatomical Factors
1. Femoral Notch Width
2. Tibial Slope
3. ACL Geometry
Physiological Factors
1. Hormonal Levels
2. Muscle Strength
3. Neuromuscular Control
4. Fatigue
| Factor/Aspect | Description | Biomechanical/Biophysical Implications | Impact on ACL Injury Risk | Prevention Strategies/Interventions |
|---|---|---|---|---|
| Hormonal Levels | Hormonal fluctuations, especially in females, impact ACL strength and injury risk. Elevated levels of estrogen and relaxin during certain menstrual cycle phases and pregnancy reduce the tensile strength of the ACL by affecting collagen composition and mechanical properties. Estrogen can increase ligament laxity by interfering with collagen cross-linking, while relaxin enhances matrix metalloproteinase (MMP) activity, leading to collagen degradation. These hormonal effects result in decreased stiffness and strength of the ACL, making it more susceptible to injury during dynamic activities. | Alters the molecular structure of collagen fibers, reducing cross-linking and mechanical integrity. Increased ligament laxity leads to decreased stiffness and strength, affecting the ACL’s ability to withstand mechanical forces. Hormonal influences can modulate fibroblast activity and collagen turnover. | Higher risk of ACL injury during hormonal phases with elevated estrogen and relaxin levels due to reduced ligament strength and increased laxity, especially in females during ovulation and pregnancy. | Personalized training programs accounting for menstrual cycle phases; hormonal modulation therapies; education on injury risks during high-risk hormonal periods; strength and conditioning programs to enhance ligament resilience; potential use of hormone-regulating medications under medical supervision; regular monitoring of ligament health using imaging techniques. |
| Muscle Strength | The balance and strength of the quadriceps and hamstrings are critical for knee stability. Weakness or imbalances can lead to improper joint mechanics, increasing ACL strain. Strong hamstrings counteract anterior tibial translation by pulling the tibia backward, reducing stress on the ACL. Overly dominant quadriceps without adequate hamstring strength can exacerbate forward tibial movement during activities like jumping and cutting. Targeted exercises to strengthen these muscles enhance knee stability and reduce injury risk. Neuromuscular training improves coordination between muscle groups, ensuring effective stabilization during dynamic movements. | Imbalanced muscle strength leads to improper load distribution across the knee joint. Weak hamstrings fail to counteract anterior tibial translation caused by strong quadriceps, increasing ACL strain. Proper muscle balance ensures optimal joint mechanics and reduces undue stress on the ligament. | Increased susceptibility to ACL injury due to inadequate muscular support and improper joint mechanics, especially during high-impact activities requiring sudden stops or direction changes. | Targeted strength training focusing on both quadriceps and hamstrings; neuromuscular training to improve muscle coordination; exercises like leg curls, deadlifts, squats, and lunges; functional training that mimics sport-specific movements; flexibility and mobility exercises to maintain optimal muscle length and joint range of motion; personalized training programs based on individual muscle strength assessments. |
| Neuromuscular Control | Effective neuromuscular control ensures appropriate muscle responses to dynamic loads, maintaining joint stability. Poor control, due to inadequate proprioception or coordination, results in delayed muscle activation and improper joint alignment, increasing ACL strain. Deficiencies can cause uncontrolled knee movements, heightening injury risk during activities involving sudden stops, jumps, or direction changes. Training that enhances proprioception, balance, and coordination improves neuromuscular control, reducing injury risk. Exercises include balance drills, plyometrics, agility exercises, and activities on unstable surfaces to stimulate sensory receptors and improve muscle response timing. | Delayed or improper muscle activation leads to decreased joint stability and increased ACL loading. Poor neuromuscular control affects synchronization and timing of muscle contractions, resulting in uncontrolled movements and higher ligament strain during dynamic activities. | Elevated risk of ACL injury due to inability to maintain proper joint alignment and stability, especially during high-risk movements requiring rapid muscle responses. | Proprioceptive training exercises (balance boards, single-leg stands); neuromuscular training (agility drills, plyometrics); coordination exercises (agility ladders, cone drills); functional training simulating sport-specific activities; personalized programs based on neuromuscular assessments; incorporation of exercises that enhance reaction time and muscle activation patterns; use of biofeedback and wearable technology for real-time monitoring and adjustments. |
| Fatigue | Fatigue impairs muscle function and joint stability by reducing force production and delaying response times. As muscles tire, their ability to support and stabilize the knee diminishes, leading to altered movement patterns and increased ACL strain. Fatigue affects proprioception, impairing the body’s ability to sense joint position and movement, resulting in incorrect positioning and increased injury risk. Fatigue-induced biomechanical changes, such as increased knee valgus and internal rotation, are associated with higher ACL injury risk. Conditioning programs improving muscular endurance and strategies to manage fatigue during sports are crucial for mitigating these risks. Proper hydration, nutrition, and rest are also essential in managing fatigue and maintaining muscle function. | Reduced muscle strength and delayed activation compromise joint stability, leading to improper alignment and increased ACL loading. Fatigue affects neuromuscular control and proprioception, resulting in altered biomechanics and higher ligament strain during activities. | Higher susceptibility to ACL injury due to compromised muscle support and impaired joint stability under fatigue, especially during prolonged or intense physical activities. | Conditioning programs enhancing muscular endurance (interval training, high-repetition resistance training); fatigue management strategies (hydration, nutrition, rest); neuromuscular training to maintain coordination under fatigue; monitoring fatigue levels using wearable technology; personalized training adjustments based on fatigue assessments; education on recognizing signs of fatigue and implementing recovery protocols; strategies to improve recovery (massage, compression garments, adequate sleep). |
Molecular Biophysics and Physiological Factors
1. Genetic Factors
2. Recovery and Rehabilitation
3. Nutritional Influences
Discussion
Integrated Approaches
1. Injury Prevention Programs
2. Screening and Risk Assessment
3. Rehabilitation Strategies
Conclusion
References
- Butler, D.L.; Noyes, F.R.; Grood, E.S. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. The Journal of Bone and Joint Surgery. American Volume 1980, 62, 259–270. [Google Scholar] [CrossRef]
- Markolf, K.L.; Mensch, J.S.; Amstutz, H.C. Stiffness and laxity of the knee—the contributions of the supporting structures. A quantitative in vitro study. The Journal of Bone and Joint Surgery. American Volume 1976, 58, 583–594. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. The American Journal of Sports Medicine 2007, 35, 1756–1769. [Google Scholar] [CrossRef]
- Spindler, K.P.; Wright, R.W. Anterior cruciate ligament tear. The New England Journal of Medicine 2008, 359, 2135–2142. [Google Scholar] [CrossRef]
- Woo, S.L.; Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. The American Journal of Sports Medicine 1991, 19, 217–225. [Google Scholar] [CrossRef]
- Boden, B.P.; Dean, G.S.; Feagin, J.A.; Garrett, W.E. Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23, 573–578. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A. , et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. The American Journal of Sports Medicine. 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Souryal, T.O.; Freeman, T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. The American Journal of Sports Medicine. 1993, 21, 535–539. [Google Scholar] [CrossRef]
- Chandrashekar, N. , Slauterbeck, J.; Hashemi, J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry. The American Journal of Sports Medicine 2005, 33, 1492–1498. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Burnett, Q.M. Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries. A prospective study. The American Journal of Sports Medicine. 1994, 22, 198–202. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D. , & Ford, K.R. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. The Journal of Knee Surgery. 2005, 18, 82–88. [Google Scholar]
- Myer, G.D.; Ford, K.R.; Hewett, T.E. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. Journal of Athletic Training 2004, 39, 352–364. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Chen, Q. , Shou, P., Zhang, L., et al. An overview of cartilage tissue engineering: recent advances and future perspectives. Rheumatology 2016, 55, 411–424. [Google Scholar]
- Chen, D. , Zhao, M., & Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar]
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27(16S), S2–S8. [Google Scholar] [CrossRef]
- Ferrara, N. , Gerber, H.-P., & LeCouter, J. The biology of VEGF and its receptors. Nature Medicine 2003, 9, 669–676. [Google Scholar]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ signalling in context. Nature Reviews Molecular Cell Biology 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C. , et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Caplan, A.I. , & Correa, D. The MSC: an injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar]
- Seibert, K. , Zhang, Y., Leahy, K., et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proceedings of the National Academy of Sciences 1994, 91, 12013–12017. [Google Scholar] [CrossRef]
- Pickart, L.; Thaler, M.M. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nature 1973, 243, 85–87. [Google Scholar]
- Snyder, R.A.; Rogero, M.M. Biophysical stimulation of bone and cartilage: state of the art and future trends. International Journal of Biomedical Engineering and Technology 2010, 3(3-4), 253-284.
- Sato, Y. , Yasuda, T., Abe, T., et al. Blood flow-restricted low-intensity resistance exercise stimulates muscle hypertrophy. European Journal of Applied Physiology 2005, 97, 208–213. [Google Scholar]
- Gao, X.; Xu, C. Pulsed electromagnetic fields promote osteogenesis and osseointegration of porous titanium implants in bone defect repair through a Wnt/β-catenin signaling-associated mechanism. Bioelectromagnetics 2012, 33, 117–126. [Google Scholar]
- Baker, L.L.; Wederich, C.L.; McNeal, D.R.; Newsam, C.J.; Waters, R.L. Neuromuscular electrical stimulation: a practical guide. Los Amigos Research and Education Institute.
- Butterfield 2000, R.J.; Foley, J.F.; Amacher, S.L. Increased ion channel activity and intracellular calcium levels during muscle regeneration. American Journal of Physiology-Cell Physiology 1997, 273, C579–C587. [Google Scholar]
- St-Pierre, J. , Drori, S., Uldry, M., et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. Journal of Orthopaedic Surgery and Research 2012, 7, 11. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nature Reviews Molecular Cell Biology 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Boden, B.P.; Dean, G.S.; Feagin, J.A. , & Garrett, W.E. Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23, 573–578. [Google Scholar]
- Markolf, K.L.; O’Neill, G. , Jackson, S.R., & McAllister, D.R. Effects of applied quadriceps and hamstrings muscle forces on forces in the anterior cruciate ligament. The American Journal of Sports Medicine 2004, 32, 1144–1149. [Google Scholar]
- McLean, S.G.; Walker, K.; van den Bogert, A.J. Effect of gender on lower extremity kinematics during rapid direction changes: an integrated analysis of three sports movements. Journal of Science and Medicine in Sport 2005, 8, 411–422. [Google Scholar] [CrossRef]
- Butler, R.J.; Crowell, H.P.; Davis, I.M. Lower extremity stiffness: implications for performance and injury. Clinical Biomechanics 2003, 18, 511–517. [Google Scholar] [CrossRef]
- Olsen, O.E.; Myklebust, G. , Engebretsen, L.; Bahr, R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. The American Journal of Sports Medicine 2004, 32, 1002–1012. [Google Scholar] [CrossRef]
- Baratta, R. , Solomonow, M., Zhou, B.H., et al. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. The American Journal of Sports Medicine 1988, 16, 113–122. [Google Scholar] [CrossRef]
- Myer, G.D.; Ford, K.R.; Hewett, T.E. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. Journal of Strength and Conditioning Research 2006, 20, 128–135. [Google Scholar]
- Dragoo, J.L.; Braun, H.J.; Harris, A.H. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee 2013, 20, 191–195. [Google Scholar] [CrossRef]
- Lambson, R.B.; Barnhill, B.S.; Higgins, R.W. Football cleat design and its effect on anterior cruciate ligament injuries. A three-year prospective study. The American Journal of Sports Medicine 1996, 24, 155–159. [Google Scholar] [CrossRef]
- Mandelbaum, B.R.; Silvers, H.J.; Watanabe, D.S. , et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine 2005, 33, 1003–1010. [Google Scholar] [CrossRef]
- Hewett, T.E.; Lindenfeld, T.N.; Riccobene, J.V.; Noyes, F.R. The effect of neuromuscular training on the incidence of knee injury in female athletes. A prospective study. The American Journal of Sports Medicine 1999, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Abramowitch, S.D.; Kilger, R.; Liang, R. Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics 2006, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.B. Ligament structure, physiology and function. Journal of Musculoskeletal & Neuronal Interactions 2004, 4, 199–201. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I. Collagens: molecular biology, diseases, and potentials for therapy. Annual Review of Biochemistry 1995, 64, 403–434. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Jr. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef]
- Ilic, M.Z.; Handley, C.J. Proteoglycans of human ligamentum patellae. Biochemical Journal 1997, 322(Pt 2), 537–543. [Google Scholar]
- Robinson, P.S.; Huang, T.F.; Kazam, E.; Iozzo, R.V. Decorin and biglycan are necessary for normal embryonic tendon development. Journal of Orthopaedic Research 2005, 23, 798–806. [Google Scholar]
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Wang, J.H.; Thampatty, B.P.; Lin, J.; Im, H.-J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391(1-2), 1-15.
- Markolf, K.L.; Burchfield, D.M.; Shapiro, M.M.; Shepard, M.F.; Finerman, G.A.; Slauterbeck, J.L. Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research 1995, 13, 930–935. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Agel, J. , Albohm, M.J., et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. Journal of the American Academy of Orthopaedic Surgeons 2000, 8, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Besier, T.F.; Lloyd, D.G.; Cochrane, J.L.; Ackland, T.R. External loading of the knee joint during running and cutting maneuvers. Medicine & Science in Sports & Exercise 2001, 33, 1168–1175. [Google Scholar]
- Boden, B.P.; Dean, G.S.; Feagin, J.A. Jr.; Garrett, W.E. Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics 2000, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Reducing knee and anterior cruciate ligament injuries among female athletes: a systematic review of neuromuscular training interventions. The Journal of Knee Surgery 2005, 18, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Krosshaug, T.; Bahr, R. A model-based image-matching technique for three-dimensional reconstruction of human motion from uncalibrated video sequences. Journal of Biomechanics 2005, 38, 919–929. [Google Scholar] [CrossRef]
- Koga, H. , Nakamae, A., Shima, Y., et al. Mechanisms for noncontact anterior cruciate ligament injuries: knee joint kinematics in 10 injury situations from female team handball and basketball. The American Journal of Sports Medicine 2010, 38, 2218–2225. [Google Scholar] [CrossRef]
- Woo, S.L.-Y. , Debski, R.E.; Withrow, J.D.; Janaushek, M.A. Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics 1999, 32, 419–429. [Google Scholar]
- Fleming, B.C.; Beynnon, B.D.; Renström, P.A.; Johnson, R.J.; Nicolella, D.P.; Nichols, C.E. The effect of weightbearing and external loading on anterior cruciate ligament strain. Journal of Biomechanics 2001, 34, 163–170. [Google Scholar] [CrossRef]
- Myer, G.D.; Ford, K.R.; Palumbo, J.P.; Hewett, T.E. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. Journal of Strength and Conditioning Research 2005, 19, 51–60. [Google Scholar]
- Butler, D.L.; Guan, Y.; Kay, M.D. Location-dependent variations in the material properties of the anterior cruciate ligament. Journal of Biomechanics 1992, 25, 511–518. [Google Scholar] [CrossRef]
- Chung, C.B.; Skaf, A. , Roger, B., et al. MR imaging evaluation of the anterior cruciate ligament: value of thin slices in oblique coronal and sagittal planes. Radiographics 2001, 21, 1023–1029. [Google Scholar]
- Li, G. , Suggs, J.; Gill, T.J. The effect of anterior cruciate ligament injury on knee joint function under a simulated muscle load: a three-dimensional computational simulation. Annals of Biomedical Engineering 2002, 30, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Shimokochi, Y.; Shultz, S.J. Mechanisms of noncontact anterior cruciate ligament injury. Journal of Athletic Training 2008, 43, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.R.; Myer, G.D.; Hewett, T.E. Valgus knee motion during landing in high school female and male basketball players. Medicine & Science in Sports & Exercise 2003, 35, 1745–1750. [Google Scholar]
- Yu, B. , Lin, C.-F.; Garrett, W.E. Lower extremity biomechanics during the landing of a stop-jump task. Clinical Biomechanics 2006, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G. , Mitrogiannis, C.; Nianios, G. Finite element analysis of anterior cruciate ligament biomechanical behavior: fiber orientation and attachment sites location effects. Computer Methods in Biomechanics and Biomedical Engineering 2008, 11, 463–473. [Google Scholar]
- Markolf, K.L.; Burchfield, D.M.; Shapiro, M.M.; Shepard, M.F.; Finerman, G.A.; Slauterbeck, J.L. Combined knee loading states that generate high anterior cruciate ligament forces. Journal of Orthopaedic Research 1995, 13, 930–935. [Google Scholar] [CrossRef]
- Song, Y. , Debski, R.E.; Musahl, V., Thomas, M.; Woo, S.L.-Y. A three-dimensional finite element model of the human anterior cruciate ligament: a computational analysis with experimental validation. Journal of Biomechanics 2004, 37, 383–390. [Google Scholar] [CrossRef]
- Woo, S.L.-Y. , Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. The American Journal of Sports Medicine 1991, 19, 217–225. [Google Scholar] [CrossRef]
- Woo, S.L.-Y. , Johnson, G.A.; Smith, B.A. Mathematical modeling of ligaments and tendons. A. Mathematical modeling of ligaments and tendons. Journal of Biomechanical Engineering 1993, 115(4B), 468–473. [Google Scholar]
- Franchi, M. , Trirè, A., Quaranta, M., Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. The Scientific World Journal 2007, 7, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Du, J. , Bae, W.C.; Statum, S.; Chung, C.B. Ultrasound elastography of the musculoskeletal system: research applications. Radiologic Clinics of North America 2014, 52, 1271–1278. [Google Scholar]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Sahni, J.K. Computational fluid dynamics study of synovial fluid lubrication in human knee joint. Journal of Biomimetics 2010, 7, 1–11. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annual Review of Biochemistry 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Buehler, M.J. Molecular nanomechanics of nascent bone: fibrillar toughening by mineralization. Nanotechnology 2006, 17, 647–651. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. Journal of Cell Science 2002, 115, 2817–2828. [Google Scholar] [CrossRef]
- Gautieri, A. , Vesentini, S., Redaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Letters 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nature Reviews Molecular Cell Biology 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Baratta, R. , Solomonow, M., Zhou, B.H.; Letson, D., Chuinard, R.; D’Ambrosia, R. Muscular coactivation: The role of the antagonist musculature in maintaining knee stability. The American Journal of Sports Medicine 1988, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Renström, P. , Arms, S.W.; Stanwyck, T.S.; Johnson, R.J.; Pope, M.H. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. The American Journal of Sports Medicine 1986, 14, 83–87. [Google Scholar] [CrossRef]
- Zebis, M.K.; Bencke, J. , Andersen, L.L., et al. Acute neuromuscular and kinematic responses to rapid lateral movements and plant and cut maneuvers causing ACL injury. Journal of Electromyography and Kinesiology 2009, 19, e543–e551. [Google Scholar]
- Solomonow, M. , Baratta, R., Zhou, B.H.; Shoji, H., Bose, W., Beck, C.; D’Ambrosia, R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. The American Journal of Sports Medicine 1987, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Paterno, M.V.; Myer, G.D. Strategies for enhancing proprioception and neuromuscular control of the knee. Clinical Orthopaedics and Related Research 2002, 402, 76–94. [Google Scholar] [CrossRef]
- Simonsen, E.B.; Magnusson, S.P.; Bencke, J., et al. Can the hamstring muscles protect the anterior cruciate ligament during a side-cutting maneuver? Scandinavian Journal of Medicine & Science in Sports, 10, 78-84.
- Hewett, T.E. 2000, Ford, K.R.; Myer, G.D. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. The American Journal of Sports Medicine 2006, 34, 490–498. [Google Scholar] [CrossRef]
- De Luca, C.J. The use of surface electromyography in biomechanics. Journal of Applied Biomechanics 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Fauth, M.L.; Petushek, E.J.; Feldshon, D.S. , et al. Reliability of surface electromyography during maximal voluntary isometric contractions, jump landings, and cutting. Journal of Strength and Conditioning Research 2010, 24, 1131–1137. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: what, why and how it influences muscle function. The Journal of Physiology 2008, 586, 11–23. [Google Scholar] [CrossRef]
- Huxley, A.F.; Niedergerke, R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 1954, 173, 971–973. [Google Scholar] [CrossRef]
- Ríos, E.; Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 1987, 325, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Baylor, S.M.; Hollingworth, S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. The Journal of Physiology 2003, 551, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G. Gene expression in muscle in response to exercise. Journal of Muscle Research and Cell Motility 2003, 24(2-3), 121-126.
- Gordon, S.E.; Flück, M. , Booth, F.W. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. Journal of Applied Physiology 2001, 90, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Chargé, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiological Reviews 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Adams, G.R. Invited Review: Autocrine/paracrine IGF-I and skeletal muscle adaptation. Journal of Applied Physiology 2002, 93, 1159–1167. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Agel, J. , Albohm, M.J., et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. Journal of the American Academy of Orthopaedic Surgeons 2000, 8, 141–150. [Google Scholar] [CrossRef]
- Souryal, T.O.; Freeman, T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes. A prospective study. The American Journal of Sports Medicine 1993, 21, 535–539. [Google Scholar] [CrossRef]
- Hashemi, J. , Chandrashekar, N., Mansouri, H., et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. The American Journal of Sports Medicine 2010, 38, 54–62. [Google Scholar] [CrossRef]
- Shambaugh, J.P.; Klein, A.; Herbert, J.H. Structural measures as predictors of injury in basketball players. Medicine & Science in Sports & Exercise 1991, 23, 522–527. [Google Scholar]
- Chandrashekar, N. , Mansouri, H., Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. Journal of Biomechanics 2006, 39, 2943–2950. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L. , Lindenfeld, T.N.; Hewett, T.E.; Greenfield, M.L. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. The American Journal of Sports Medicine 1998, 26, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.F.; Dome, D.C.; Gautam, S. , Awh, M.H.; Rennirt, G.W. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. The American Journal of Sports Medicine 2001, 29, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M. , September, A.V.; O’Cuinneagain, D., van der Merwe, W., Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. The American Journal of Sports Medicine 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Souryal, T.O.; Freeman, T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes: a prospective study. The American Journal of Sports Medicine 1993, 21, 535–539. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Davis, T.J.; Klootwyk, T.E. The relationship between intercondylar notch width of the femur and the incidence of anterior cruciate ligament tears. The American Journal of Sports Medicine 1998, 26, 402–408. [Google Scholar] [CrossRef]
- Anderson, A.F.; Lipscomb, A.B.; Liudahl, K.J.; Addlestone, R.B. Analysis of the intercondylar notch by computed tomography. The American Journal of Sports Medicine 1987, 15, 547–552. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Burnett, Q.M. Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries: a prospective study. The American Journal of Sports Medicine 1994, 22, 198–202. [Google Scholar] [CrossRef]
- Uhorchak, J.M.; Scoville, C.R.; Williams, G.N.; Arciero, R.A.; Pierre, P.S.; Taylor, D.C. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. The American Journal of Sports Medicine 2003, 31, 831–842. [Google Scholar] [CrossRef]
- (Note: Reference number appears to be missing in your text.
- Anderson, A.F.; Dome, D.C.; Gautam, S. , Awh, M.H.; Rennirt, G.W. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. The American Journal of Sports Medicine 2001, 29, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Lindenfeld, T.N.; Riccobene, J.V.; Noyes, F.R. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. The American Journal of Sports Medicine 1999, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, B.R.; Silvers, H.J.; Watanabe, D.S. , et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine 2005, 33, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Staeubli, H.U.; Rauschning, W. Anatomical considerations of femoral notchplasty in anterior cruciate ligament surgery. Knee Surgery 1994, 2, 129–136. [Google Scholar]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Jr. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Grood, E.S. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connective Tissue Research 2000, 41, 29–36. [Google Scholar] [CrossRef]
- Higuchi, H. , Shirakura, K., Kimura, M., et al. Changes in biochemical parameters after anterior cruciate ligament injury. International Orthopaedics 2006, 30, 43–47. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Hashimoto, S. , Chen, P.C.; Lotz, M.K.; Colwell, C.W. Jr. Cartilage injury induces chondrocyte apoptosis. Journal of Bone and Joint Surgery. American Volume.
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Yang, G. , Im, H.-J.; Wang, J.H.-C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef]
- Hashemi, J. , Chandrashekar, N., Mansouri, H., Slauterbeck, J.R.; Hashemi, S.S.; Beynnon, B.D. Shallow medial tibial plateau and steep medial and lateral tibial slopes: New risk factors for anterior cruciate ligament injuries. The American Journal of Sports Medicine 2010, 38, 54–62. [Google Scholar] [CrossRef]
- Giffin, J.R.; Vogrin, T.M.; Zantop, T. , Woo, S.L.-Y.; Harner, C.D. Effects of increasing tibial slope on the biomechanics of the knee. The American Journal of Sports Medicine 2004, 32, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Brandon, M.L.; Haynes, P.T.; Bonamo, J.R.; Flynn, M.I.; Barrett, G.R.; Sherman, M.F. The association between posterior-inferior tibial slope and anterior cruciate ligament insufficiency. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2006, 22, 894–899. [Google Scholar]
- Todd, M.S.; Lalliss, S. , Garcia, E., DeBerardino, T.M.; Cameron, K.L. The relationship between posterior tibial slope and anterior cruciate ligament injuries. The American Journal of Sports Medicine 2010, 38, 63–67. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Subfailure damage in ligament: A structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Goettert, M. , Lehmann, M., Neumann, J., Tischer, T., Miosge, N.; Kloss, F. MAPK activation is involved in mechanical stress-induced proliferation of human ligament fibroblasts. Journal of Biomechanics 2008, 41, 579–585. [Google Scholar]
- Zhang, H. , Wang, L., Shen, G.; Liu, T. Upregulation of MMPs in an in vitro model of the anterior cruciate ligament injury. European Journal of Orthopaedic Surgery & Traumatology 2015, 25, 905–910. [Google Scholar]
- Jang, K.M.; Park, H.S.; Park, J.H.; Chung, S.Y. Reactive oxygen species and antioxidants in anterior cruciate ligament injury. The Journal of Knee Surgery 2015, 28, 445–450. [Google Scholar]
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Yang, G. , Im, H.-J.; Wang, J.H.-C. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Mouton, C. , Thelen, P., Fröber, R.; Wruck, C.J. Three-dimensional finite element model of the human anterior cruciate ligament: A computational analysis with experimental validation. The Knee 2012, 19, 670–675. [Google Scholar]
- Hashemi, J. , Chandrashekar, N., Mansouri, H., Slauterbeck, J.R.; Hashemi, S.S.; Beynnon, B.D. Shallow medial tibial plateau and steep medial and lateral tibial slopes: New risk factors for anterior cruciate ligament injuries. The American Journal of Sports Medicine 2010, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Subfailure damage in ligament: A structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef]
- Vogel, V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annual Review of Biophysics and Biomolecular Structure 2006, 35, 459–488. [Google Scholar] [CrossRef]
- Ilic, M.Z.; Handley, C.J. Proteoglycans of human ligamentum patellae. Biochemical Journal 1997, 322, 537–543. [Google Scholar]
- Coste, B. , Mathur, J., Schmidt, M., et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically activated ion channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Tummala, P. , Kwon, R.Y.; Jacobs, C.R. Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII, and cytoskeletal dynamics. Journal of Cell Science 2009, 122, 546–553. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochemical Journal 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, N. , Mansouri, H., Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. Journal of Biomechanics 2012, 45, 1536–1542. [Google Scholar]
- Tashman, S.; Anderst, W. In vivo measurement of dynamic joint motion using high-speed biplane radiography and CT: application to canine ACL deficiency. Journal of Biomechanical Engineering 2003, 125, 238–245. [Google Scholar] [CrossRef]
- Chandrashekar, N. , Mansouri, H., Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. Journal of Biomechanics 2006, 39, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.H.; Oh, H. , et al. Morphological analysis of the anterior cruciate ligament and its insertion sites using 3D reconstruction. Journal of Orthopaedic Science 2015, 20, 708–714. [Google Scholar]
- Iwahashi, T., Shino, K., Nakata, K., et al. Direct anterior cruciate ligament insertion to the femur: an histological study. Knee Surgery 2010, Sports Traumatology, Arthroscopy, 18, 857-862.
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef]
- Ilic, M.Z.; Handley, C.J. Proteoglycans of human ligament. Biochemical Journal 1997, 322, 537–543. [Google Scholar]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hauck, C.R.; Sieg, D.J. Signaling through focal adhesion kinase. Progress in Biophysics and Molecular Biology 1999, 71(3-4), 435-478.
- Amiel, D., Billings, E.; Akeson, W.H. Ligament structure, chemistry, and physiology. In Daniel 1990, D.M.; Akeson, W.H.; O’Connor, J.J. (Eds.), Knee Ligaments: Structure, Function, Injury, and Repair (pp. 77-91). Raven Press.
- Bosch, P. , Musgrave, D.S.; Lee, J.Y.; Cummins, J., Shuler, T.; Ghivizzani, S.C. Osteoprogenitor cells within skeletal muscle. Journal of Orthopaedic Research 2000, 18, 933–944. [Google Scholar] [CrossRef]
- Kato, Y. , Ingham, S.J.; Naudé, G., Saito, A.; Seil, R. Biomechanics of the anterior cruciate ligament and their implications for surgical technique. Strategies in Trauma and Limb Reconstruction 2010, 5, 77–81. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I. Collagens: molecular biology, diseases, and potentials for therapy. Annual Review of Biochemistry 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Brückner, P. Collagen suprastructures. Topics in Current Chemistry 2005, 247, 185–205. [Google Scholar]
- Iozzo, R.V. The biology of the small leucine-rich proteoglycans: functional network of interactive proteins. Journal of Biological Chemistry 1999, 274, 18843–18846. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. , Li, J., Zhu, J.; Sun, X. DNA methylation regulates chondrogenic differentiation of tendon stem cells by modulating wnt pathway. European Cells and Materials 2013, 26, 81–94. [Google Scholar]
- Rigozzi, S. , Müller, R.; Snedeker, J.G. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. Journal of Biomechanics 2010, 43, 1884–1890. [Google Scholar]
- Nau, T. , Teuschl, A.; Schweizer, A. Tissue engineering of ligaments and tendons. Sports Medicine and Arthroscopy Review 2013, 21, 45–53. [Google Scholar]
- Woo, S.L.-Y. , Hildebrand, K., Watanabe, N., Fenwick, J.A.; Papageorgiou, C.D.; Wang, J.H. Tissue engineering of ligament and tendon healing. Clinical Orthopaedics and Related Research 1999, 367, S312–S323. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L. , Boynton, M.D.; Spindler, K.P.; Lindenfeld, T.N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. The American Journal of Sports Medicine 2002, 30, 182–188. [Google Scholar] [CrossRef]
- McLean, S.G.; Fellin, R.E.; Suedekum, N. , Calabrese, G., Passerallo, A.; Joy, S. Impact of fatigue on gender-based high-risk landing strategies. Medicine & Science in Sports & Exercise 2007, 39, 502–514. [Google Scholar]
- Nyland, J. , Shapiro, R., Stine, R., Horn, T.; Ireland, M.L. Relationship of fatigue and leg dominance to knee joint laxity and neuromuscular characteristics. The American Journal of Sports Medicine 1994, 22, 545–549. [Google Scholar]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Jr. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Marshall, J.L. Microvasculature of the cruciate ligaments and its response to injury. The Journal of Bone and Joint Surgery. American Volume 1983, 65, 68–82. [Google Scholar]
- Liu, S.H.; Al-Shaikh, R.A.; Panossian, V. , Yang, R.S.; Nelson, S.D.; Soleiman, N.; Finerman, G.A. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. Journal of Orthopaedic Research 1997, 15, 657–663. [Google Scholar]
- Wojtys, E.M.; Huston, L.J.; Boynton, M.D.; Spindler, K.P.; Lindenfeld, T.N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. The American Journal of Sports Medicine 2002, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Lee, R.S.; Benhaim, P. , Finerman, G.A.; Hame, S.L. Relaxin receptors in the human female anterior cruciate ligament. The American Journal of Sports Medicine 2003, 31, 577–584. [Google Scholar] [CrossRef]
- Yu, W.D.; Panossian, V. , Hatch, J.D.; Liu, S.H.; Finerman, G.A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clinical Orthopaedics and Related Research 2001, (383), 268–281. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annual Review of Biochemistry 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Bridgeman, J.T.; Zhang, Y. , Donahue, H.J.; Wade, A.M. Relaxin decreases collagen content and tensile strength of engineered ligament constructs. Journal of Orthopaedic Research 2010, 28, 852–858. [Google Scholar]
- Leblanc, D.R.; Schneider, M. , Angele, P., Vollner, F.; Docheva, D. The effect of estrogen on tendon health and injury. European Cells & Materials 2017, 34, 312–333. [Google Scholar]
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing: a current review. Clinical Orthopaedics and Related Research 1995, (318), 265–278. [Google Scholar]
- Lee, C.Y.; Liu, X. , Smith, C.L., et al. The effect of estrogen on the viscoelastic properties of the rat ACL. The effect of estrogen on the viscoelastic properties of the rat ACL. Clinical Orthopaedics and Related Research 2004, (428), 39–45. [Google Scholar]
- Nicolson, V.T.; Wiles, A.M.; Elliott, D.M.; Charlton, N.P. Atomic force microscopy of collagen fibers: a new technique to investigate the molecular basis of viscoelastic properties. Journal of Biomechanics 2005, 38, 543–546. [Google Scholar]
- Woo, S.L.-Y. , Abramowitch, S.D.; Kilger, R.; Liang, R. Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics 2006, 39, 1–20. [Google Scholar] [CrossRef]
- Solomonow, M. , Baratta, R., Zhou, B.H.; Shoji, H., Bose, W.; D’Ambrosia, R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. The American Journal of Sports Medicine 1987, 15, 207–213. [Google Scholar] [CrossRef]
- Baratta, R. , Solomonow, M., Zhou, B.H.; Letson, D., Chuinard, R.; D’Ambrosia, R. Muscular coactivation: The role of the antagonist musculature in maintaining knee stability. The American Journal of Sports Medicine 1988, 16, 113–122. [Google Scholar] [CrossRef]
- Holcomb, W.R.; Rubley, M.D.; Lee, H.J.; Guadagnoli, M.A. Effect of hamstring-emphasized resistance training on hamstring.
- strength ratios. Journal of Strength and Conditioning Research 2007, 21, 41–47.
- Hewett, T.E.; Stroupe, A.L.; Nance, T.A.; Noyes, F.R. Plyometric training in female athletes: decreased impact forces and increased hamstring torques. The American Journal of Sports Medicine 1996, 24, 765–773. [Google Scholar] [CrossRef]
- Myer, G.D.; Ford, K.R.; Hewett, T.E. Methodological approaches and rationale for training to prevent anterior cruciate ligament injuries in female athletes. Scandinavian Journal of Medicine & Science in Sports 2006, 16, 358–368. [Google Scholar]
- Shellock, F.G.; Prentice, W.E. Warming-up and stretching for improved physical performance and prevention of sports-related injuries. Sports Medicine 1985, 2, 267–278. [Google Scholar] [CrossRef]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. The Journal of Physiology 1966, 184, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Chiquet, M. , Gelman, L., Lutz, R.; Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2009, 1793, 911–920. [Google Scholar] [CrossRef]
- Shin, D.; Athanasiou, K.A. Cytoindentation for obtaining cell biomechanical properties. Journal of Orthopaedic Research 1999, 17, 880–890. [Google Scholar] [CrossRef]
- Khan, K.M.; Scott, A. Mechanotherapy: how physical therapists’ prescription of exercise promotes tissue repair. British Journal of Sports Medicine 2009, 43, 247–252. [Google Scholar] [CrossRef]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological Reviews 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Liu, Y. , Kim, H., Hebard, S.; Henry, J. Biomimetic collagen–apatite scaffold with phosphate group for bone regeneration. Journal of Materials Science: Materials in Medicine 2008, 19, 2555–2563. [Google Scholar]
- LaBella, C.R.; Hennrikus, W.; Hewett, T.E.; Council on Sports Medicine and Fitness. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics 2014, 133, e1437–e1450. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Myer, G.D. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions. The American Journal of Sports Medicine 2006, 34, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Ford, K.R.; Hewett, T.E. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. Journal of Strength and Conditioning Research 2008, 22, 987–1014. [Google Scholar] [CrossRef] [PubMed]
- Lephart, S.M.; Pincivero, D.M.; Giraldo, J.L.; Fu, F.H. The role of proprioception in the management and rehabilitation of athletic injuries. The American Journal of Sports Medicine 1997, 25, 130–137. [Google Scholar] [CrossRef]
- Zech, A. , Hübscher, M., Vogt, L., Banzer, W., Hänsel, F.; Pfeifer, K. Balance training for neuromuscular control and performance enhancement: a systematic review. Journal of Athletic Training 2010, 45, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, B.R.; Silvers, H.J.; Watanabe, D.S. , et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine 2005, 33, 1003–1010. [Google Scholar] [CrossRef]
- Purves, D., Augustine, G.J.; Fitzpatrick, D., et al. Neuroscience (2nd ed.). Sinauer Associates.
- Deschenes 2001, M.R.; Kraemer, W.J. Performance and physiologic adaptations to resistance training. The American Journal of Physical Medicine & Rehabilitation 2002, 81(11 Suppl), S3-S16.
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microscopy Research and Technique 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. , Ying, Z., Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of Neurophysiology 2002, 88, 2187–2195. [Google Scholar] [CrossRef]
- Posthumus, M. , September, A.V.; O’Cuinneagain, D., van der Merwe, W., Schwellnus, M.P.; Collins, M. The COL1A1 gene and acute soft tissue ruptures. British Journal of Sports Medicine 2009, 43, 357–358. [Google Scholar]
- Ntanasis-Stathopoulos, I. , Tzanninis, J.G.; Philippou, A. Epigenetic regulation on gene expression induced by physical exercise. Journal of Musculoskeletal & Neuronal Interactions 2013, 13, 133–146. [Google Scholar]
- Kiss, R. , Czimmermann, E., Wesner, D., et al. AFM nanomechanical analysis of single collagen fibrils. Biophysical Journal 2013, 105, 723–729. [Google Scholar]
- Coste, B. , Xiao, B., Santos, J.S., et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J. , Shapiro, R., Stine, R., Horn, T.; Ireland, M.L. Relationship of fatigue and leg dominance to knee joint laxity and neuromuscular characteristics. The American Journal of Sports Medicine 1994, 22, 545–549. [Google Scholar]
- McLean, S.G.; Fellin, R.E.; Suedekum, N. , Calabrese, G., Passerallo, A.; Joy, S. Impact of fatigue on gender-based high-risk landing strategies. Medicine & Science in Sports & Exercise 2007, 39, 502–514. [Google Scholar]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A. , et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. The American Journal of Sports Medicine 2006, 34, 1512–1532. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: cellular mechanisms. Physiological Reviews 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Booth, F.W.; Thomason, D.B. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiological Reviews 1991, 71, 541–585. [Google Scholar] [CrossRef]
- Fitts, R.H. The cross-bridge cycle and skeletal muscle fatigue. Journal of Applied Physiology 2008, 104, 551–558. [Google Scholar] [CrossRef]
- Chin, E.R.; Allen, D.G. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. The Journal of Physiology 1996, 491, 813–824. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle & Nerve 2011, 44, 318–331. [Google Scholar]
- Rassier, D.E.; Herzog, W. Cross-bridge-based models of muscle contraction. Physiological Reviews 2004, 84, 569–591. [Google Scholar]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don’t. Journal of Applied Physiology 2001, 90, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. Using recovery modalities between training sessions in elite athletes: does it help? Sports Medicine, 36, 781-796.
- Faigenbaum, A.D. 2006, & McFarland, J.E. Resistance training for kids: right from the start. ACSM’s Health & Fitness Journal 2016, 20, 16–22. [Google Scholar]
- Maughan, R.J.; Shirreffs, S.M. Dehydration and rehydration in competitive sport. Scandinavian Journal of Medicine & Science in Sports 2010, 20(Suppl 3), 40-47.
- Bourdon, P.C.; Cardinale, M., Murray, A., et al. Monitoring athlete training loads: consensus statement. International Journal of Sports Physiology and Performance 2017, 12(Suppl 2), S2161-S2170.
- Liu, S.H.; Al-Shaikh, R.A.; Panossian, V. , Yang, R.S.; Nelson, S.D.; Soleiman, N.; Finerman, G.A. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. Journal of Orthopaedic Research 1997, 15, 657–663. [Google Scholar]
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing: a current review. Clinical Orthopaedics and Related Research 1995, (318), 265–278. [Google Scholar]
- Yu, W.D.; Panossian, V. , Hatch, J.D.; Liu, S.H.; Finerman, G.A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clinical Orthopaedics and Related Research 2001, (383), 268–281. [Google Scholar]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. The International Journal of Biochemistry & Cell Biology 2005, 37, 1974–1984. [Google Scholar]
- Purves, D., Augustine, G.J.; Fitzpatrick, D., et al. Neuroscience (2nd ed.). Sinauer Associates.
- Kimura 2001, K. , Ito, M., Amano, M., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Allen, D.G.; Westerblad, H. Role of phosphate and calcium stores in muscle fatigue. The Journal of Physiology 2001, 536, 657–665. [Google Scholar] [CrossRef]
- Westerblad, H., Allen, D.G.; Lännergren, J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News in Physiological Sciences, 17, 17-21.
- Reid, M.B. (2001). Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don’t. Journal of Applied Physiology 2002, 90, 724–731. [Google Scholar] [CrossRef]
- Kang, C.; Ji, L.L. Role of PGC-1α in muscle function and aging. Journal of Sport and Health Science 2012, 1, 11–22. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M. , et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: progression and exercise prescription. Medicine & Science in Sports & Exercise 2004, 36, 674–688. [Google Scholar]
- Murray, M.M.; Spindler, K.P.; Ballard, P. , Welch, T.P.; Zurakowski, D.; Nanney, L.B. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. Journal of Orthopaedic Research 2007, 25, 1007–1017. [Google Scholar] [CrossRef]
- Gautieri, A. , Vesentini, S., Redaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nano Letters 2011, 11, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rigozzi, S. , Müller, R.; Snedeker, J.G. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. Journal of Biomechanics 2010, 43, 1884–1890. [Google Scholar]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. The American Journal of Sports Medicine 2012, 40, 41–48. [Google Scholar] [CrossRef]
- LaBella, C.R.; Hennrikus, W.; Hewett, T.E.; Council on Sports Medicine and Fitness. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics 2014, 133, e1437–e1450. [Google Scholar] [CrossRef]
- Posthumus, M. , September, A.V.; O’Cuinneagain, D., van der Merwe, W., Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. The American Journal of Sports Medicine 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- Khoschnau, S. , Melhus, H., Jacobson, A., et al. Type I collagen α1 Sp1 polymorphism and the risk of cruciate ligament ruptures or shoulder dislocations. The American Journal of Sports Medicine 2008, 36, 2432–2436. [Google Scholar] [CrossRef]
- MacArthur, D.G.; North, K.N. ACTN3: A genetic influence on muscle function and athletic performance. Exercise and Sport Sciences Reviews 2007, 35, 30–34. [Google Scholar] [CrossRef]
- Vincent, B. , De Bock, K., Ramaekers, M., et al. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiological Genomics 2007, 32, 58–63. [Google Scholar] [CrossRef] [PubMed]
- McHughen, S.A.; Rodriguez, P.F.; Kleim, J.A. , et al. BDNF Val66Met polymorphism influences motor system function in the human brain. Cerebral Cortex 2010, 20, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Fishman, D. , Faulds, G., Jeffery, R., et al. The effect of novel polymorphisms in the interleukin-6 gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. The Journal of Clinical Investigation 1998, 102, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Posthumus, M. Type V collagen genotype and exercise-related phenotype relationships: A novel hypothesis. Exercise and Sport Sciences Reviews 2011, 39, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D. Nutritional support for exercise-induced injuries. Sports Medicine 2015, 45 (Suppl 1), S93–S104. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. Journal of Cellular Biochemistry 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annual Review of Biochemistry 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Gautieri, A. , Vesentini, S., Redaelli, A.; Buehler, M.J. Hierarchical structure and nanomechanics of collagen microfibrils from the atomistic scale up. Nanotechnology 2012, 23, 505702. [Google Scholar]
- Ingber, D.E. Cellular mechanotransduction: putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB Journal 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Collins, M. , September, A.V.; Posthumus, M. Biological variation and exercise-related injuries: implications for personalized nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2015, 18, 515–520. [Google Scholar]
- Prockop, D.J.; Kivirikko, K.I.; Tuderman, L.; Guzman, N.A. The biosynthesis of collagen and its disorders. The New England Journal of Medicine 1979, 301, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing: A current review. Clinical Orthopaedics and Related Research 1995, (318), 265–278. [Google Scholar]
- Yu, W.D.; Panossian, V. , Hatch, J.D.; Liu, S.H.; Finerman, G.A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clinical Orthopaedics and Related Research 2001, (383), 268–281. [Google Scholar]
- Myer, G.D.; Ford, K.R.; Hewett, T.E. Methodological approaches and rationale for training to prevent anterior cruciate ligament injuries in female athletes. Scandinavian Journal of Medicine & Science in Sports 2006, 14, 275–285. [Google Scholar]
- Hewett, T.E.; Myer, G.D. The mechanistic connection between the menstrual cycle and anterior cruciate ligament injuries. Journal of Athletic Training 2011, 46, 140–149. [Google Scholar]
- Holcomb, W.R.; Rubley, M.D.; Lee, H.J.; Guadagnoli, M.A. Effect of hamstring-emphasized resistance training on hamstring.
- strength ratios. Journal of Strength and Conditioning Research 2007, 21, 41–47.
- Dvir, Z. Isokinetics: Muscle Testing, Interpretation, and Clinical Applications (2nd ed.). Churchill Livingstone.
- Lephart 2004, S.M.; Pincivero, D.M.; Giraldo, J.L.; Fu, F.H. The role of proprioception in the management and rehabilitation of athletic injuries. The American Journal of Sports Medicine 1997, 25, 130–137. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Subfailure damage in ligament: A structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B. , Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB Journal 2006, 20, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T. , Wang, Y.; Murrell, G.A. C. The roles of growth factors in tendon and ligament healing. Sports Medicine 2003, 33, 381–394. [Google Scholar] [CrossRef]
- Murray, M.M.; Spindler, K.P.; Ballard, P. , Welch, T.P.; Zurakowski, D.; Nanney, L.B. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. Journal of Orthopaedic Research 2007, 25, 1007–1017. [Google Scholar] [CrossRef]
- Ilic, M.Z.; Handley, C.J. Proteoglycans of human ligament. Biochemical Journal 1997, 322, 537–543. [Google Scholar]
- Han, W.M.; Heo, S.J.; Driscoll, T.P. , et al. Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage. Nature Materials 2016, 15, 477–484. [Google Scholar] [CrossRef]
- Posthumus, M. , September, A.V.; O’Cuinneagain, D., van der Merwe, W., Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. The American Journal of Sports Medicine 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- Collins, M.; Posthumus, M. Type V collagen genotype and exercise-related phenotype relationships: A novel hypothesis. Exercise and Sport Sciences Reviews 2011, 39, 191–198. [Google Scholar] [CrossRef]
- Nadler, D.; Pryor, J. Wearable technology and digital biomarker monitoring in ACL injury rehabilitation. Current Reviews in Musculoskeletal Medicine 2019, 12, 41–47. [Google Scholar]
- Tipton, K.D.; Wolfe, R.R. Protein and amino acids for athletes. Journal of Sports Sciences 2004, 22, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bohl, C.H.; Volpe, S.L. Magnesium and exercise. Critical Reviews in Food Science and Nutrition 2002, 42, 533–563. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition 2006, 83(6 Suppl), 1505S–1519S. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annual Review of Immunology 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiological Reviews 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Hodges, R.E.; Hood, J. , Canham, J.E.; Sauberlich, H.E.; Baker, E.M. Clinical manifestations of ascorbic acid deficiency in man. The American Journal of Clinical Nutrition 1971, 24, 432–443. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. Journal of Cellular Biochemistry 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. Journal of Biological Chemistry 2002, 277, 8755–8758. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Vanderby, R. Jr. Collagen fibril morphology and organization: Implications for force transmission in ligament and tendon. Matrix Biology 2006, 25, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Collins, M. , September, A.V.; Posthumus, M. Biological variation and exercise-related injuries: implications for personalized nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2015, 18, 515–520. [Google Scholar]
- van der Horst, N. , Backx, F.J.G.; Esseveld, M.; Mosterd, W.L. Injuries in Dutch elite field hockey players: a prospective cohort study. Clinical Journal of Sport Medicine 2015, 25, 493–500. [Google Scholar]
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Crowe, M.J. Effects of six weeks of β-hydroxy-β-methylbutyrate (HMB) and HMB/creatine supplementation on strength, body composition, and muscle damage in response to resistance training. Journal of Strength and Conditioning Research 2007, 21, 1000–1009. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional support for exercise-induced injuries. Sports Medicine 2015, 45 (Suppl 1), S93–S104. [Google Scholar] [CrossRef]
- Mandelbaum, B.R.; Silvers, H.J.; Watanabe, D.S.; Knarr, J.F.; Thomas, S.D.; Griffin, L.Y.; Kirkendall, D.T.; Garrett, W. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine 2005, 33, 1003–1010. [Google Scholar] [CrossRef]
- Souryal, T.O.; Freeman, T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes: A prospective study. The American Journal of Sports Medicine 1993, 21, 535–539. [Google Scholar] [CrossRef]
- Hewett, T.E.; Ford, K.R.; Myer, G.D. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. The American Journal of Sports Medicine 2006, 34, 490–498. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L.J.; Boynton, M.D.; Spindler, K.P.; Lindenfeld, T.N. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. The American Journal of Sports Medicine 2002, 30, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M. , September, A.V.; O’Cuinneagain, D., van der Merwe, W., Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. The American Journal of Sports Medicine 2009, 37, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R. , Beynnon, B.D.; DeMaio, M., et al. Understanding and preventing noncontact anterior cruciate ligament injuries: A review of the Hunt Valley II meeting, January 2005. The American Journal of Sports Medicine 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R. , Beynnon, B.D.; DeMaio, M., et al. Understanding and preventing noncontact anterior cruciate ligament injuries: A review of the Hunt Valley II Meeting. The American Journal of Sports Medicine 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. , et al. Biomechanical measures of neuromuscular control and valgus loading predict ACL injury risk in female athletes. The American Journal of Sports Medicine 2005, 33, 492–501. [Google Scholar] [CrossRef]
- Hashemi, J. , Chandrashekar, N., Mansouri, H., et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: New risk factors for anterior cruciate ligament injuries. The American Journal of Sports Medicine 2010, 38, 54–62. [Google Scholar] [CrossRef]
- Mandelbaum, B.R.; Silvers, H.J.; Watanabe, D.S.; Knarr, J.F.; Thomas, S.D.; Griffin, L.Y.; Kirkendall, D.T. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. The American Journal of Sports Medicine 2005, 33, 1003–1010. [Google Scholar] [CrossRef]
- Collins, M. , September, A.V.; Posthumus, M. Biological variation and exercise-related injuries: Implications for personalized nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2015, 18, 515–520. [Google Scholar]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- van der Kruk, E.; Reijne, M.M. Accuracy of human motion capture systems for sport applications; state-of-the-art review. European Journal of Sport Science 2018, 18, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D. Nutritional support for exercise-induced injuries. Sports Medicine 2015, 45 (Suppl 1), S93–S104. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Spindler, K.P.; Ballard, P. , Welch, T.P.; Zurakowski, D.; Nanney, L.B. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. Journal of Orthopaedic Research 2007, 25, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- LaBella, C.R.; Hennrikus, W. , Hewett, T.E.; Council on Sports Medicine and Fitness. Anterior cruciate ligament injuries: Diagnosis, treatment, and prevention. Pediatrics 2014, 133, e1437–e1450. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Mitra, S.K. Integrin signalling and focal adhesion dynamics. Nature Reviews Molecular Cell Biology 2004, 5, 700–711. [Google Scholar]
- Provenzano, P.P.; Vanderby, R. Jr. Collagen fibril morphology and organization: Implications for force transmission in ligament and tendon. Matrix Biology 2006, 25, 71–84. [Google Scholar] [CrossRef]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. The International Journal of Biochemistry & Cell Biology 2005, 37, 1974–1984. [Google Scholar]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiological Reviews 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Adkins, D.L.; Boychuk, J. , Remple, M.S.; Kleim, J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. Journal of Applied Physiology 2006, 101, 1776–1782. [Google Scholar] [CrossRef]
- Purves, D., Augustine, G.J.; Fitzpatrick, D., et al. Neuroscience (2nd ed.). Sinauer Associates.
- Gorres 2001, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Calder, P.C. n–3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition 2006, 83(6 Suppl), 1505S–1519S. [Google Scholar] [CrossRef]
- van der Kruk, E.; Reijne, M.M. Accuracy of human motion capture systems for sport applications; state-of-the-art review. European Journal of Sport Science 2018, 18, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.-C. Mechanobiology of tendon. Journal of Biomechanics 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Collins, M. , September, A.V.; Posthumus, M. Biological variation and exercise-related injuries: Implications for personalized nutrition. Current Opinion in Clinical Nutrition and Metabolic Care 2015, 18, 515–520. [Google Scholar]
- Padua, D.A.; Marshall, S.W.; Boling, M.C.; Thigpen, C.A.; Garrett, W.E.; Beutler, A.I. The Landing Error Scoring System (LESS) is a valid and reliable clinical assessment tool of jump-landing biomechanics: The JUMP-ACL study. The American Journal of Sports Medicine 2009, 37, 1996–2002. [Google Scholar] [CrossRef]
- Cook, G. , Burton, L., Hoogenboom, B.J.; Voight, M. Functional movement screening: The use of fundamental movements as an assessment of function—Part 1. International Journal of Sports Physical Therapy 2014, 9, 396–409. [Google Scholar]
- Collins, M.; Raleigh, S.M. Genetic risk factors for musculoskeletal soft tissue injuries. Medicine and Sport Science 2009, 54, 136–149. [Google Scholar]
- Khoschnau, S. , Melhus, H., Jacobson, A., et al. Type I collagen α1 Sp1 polymorphism and the risk of cruciate ligament ruptures or shoulder dislocations. The American Journal of Sports Medicine 2008, 36, 2432–2436. [Google Scholar] [CrossRef]
- McLean, S.G.; Huang, X.; Van Den Bogert, A.J. Association between lower extremity posture at contact and peak knee valgus moment during sidestepping: Implications for ACL injury. Clinical Biomechanics 2005, 20, 863–870. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F. Jr. Matrix metalloproteinases. Journal of Biological Chemistry 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T. , Yamagishi, T., Nagafuchi, T., et al. Biomarkers in anterior cruciate ligament injury. Journal of Orthopaedic Science 2013, 18, 110–118. [Google Scholar]
- Madigan, M.L.; Pidcoe, P.E. Changes in landing biomechanics during a fatiguing landing activity. Journal of Electromyography and Kinesiology 2003, 13, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R. , Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clinical Biomechanics 2008, 23, 81–92. [Google Scholar] [CrossRef]
- Wojtys, E.M.; Huston, L.J.; Lindenfeld, T.N.; Hewett, T.E.; Greenfield, M.L. V. H. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. The American Journal of Sports Medicine 1998, 26, 614–619. [Google Scholar] [CrossRef]
- Frank, C.B. Ligament structure, physiology and function. Journal of Musculoskeletal & Neuronal Interactions 2004, 4, 199–201. [Google Scholar]
- Provenzano, P.P.; Heisey, D. , Hayashi, K., Lakes, R.; Vanderby, R. Subfailure damage in ligament: a structural and cellular evaluation. Journal of Applied Physiology 2002, 92, 362–371. [Google Scholar] [CrossRef]
- Kirk, T.A.; Campbell, P.G.; Rubash, H.E.; Wang, J.Y. TGF-beta superfamily members are expressed during ligament healing. Clinical Orthopaedics and Related Research 2000, (370), 276–284. [Google Scholar]
- Wang, H. , Zhang, X., Li, Y., Liu, P.; Yuan, J. TGF-β1 promotes the chemotactic migration of mesenchymal stem cells through Akt and p38 signaling pathways. Molecular Medicine Reports 2010, 2, 1095–1100. [Google Scholar]
- Vaynman, S.; Gomez-Pinilla, F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and Neural Repair 2005, 19, 283–295. [Google Scholar] [CrossRef]
- Aagaard, P. , Simonsen, E.B.; Andersen, J.L.; Magnusson, S.P.; Dyhre-Poulsen, P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. Journal of Applied Physiology 2002, 92, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Lephart, S.M.; Pincivero, D.M.; Giraldo, J.L.; Fu, F.H. The role of proprioception in the management and rehabilitation of athletic injuries. The American Journal of Sports Medicine 1997, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- [Intentionally skipped to match the numbering in your text.
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M. , Kline, W.O.; Stover, G.L.; Bauerlein, R.,... & Yancopoulos, G.D. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology 2001, 3, 1014–1019. [Google Scholar] [PubMed]
- Magnusson, S.P.; Hansen, P.; Kjaer, M. Tendon properties in relation to muscular activity and physical training. Scandinavian Journal of Medicine & Science in Sports 2003, 13, 211–223. [Google Scholar]
- Charge, S.B. P.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiological Reviews 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Besier, T.F.; Lloyd, D.G.; Ackland, T.R. Muscle activation strategies at the knee during running and cutting maneuvers. Medicine & Science in Sports & Exercise 2003, 35, 119–127. [Google Scholar]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Guermazi, A. Magnetic resonance imaging of knee ligaments and menisci: systematic approach with relevant anatomy and pitfalls. Radiologic Clinics of North America 2011, 49, 1211–1231. [Google Scholar]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. Journal of Bone and Joint Surgery. American Volume 2000, 82, 1387–1397. [Google Scholar] [CrossRef]
- Ardern, C.L.; Webster, K.E.; Taylor, N.F.; Feller, J.A. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. British Journal of Sports Medicine 2011, 45, 596–606. [Google Scholar] [CrossRef]
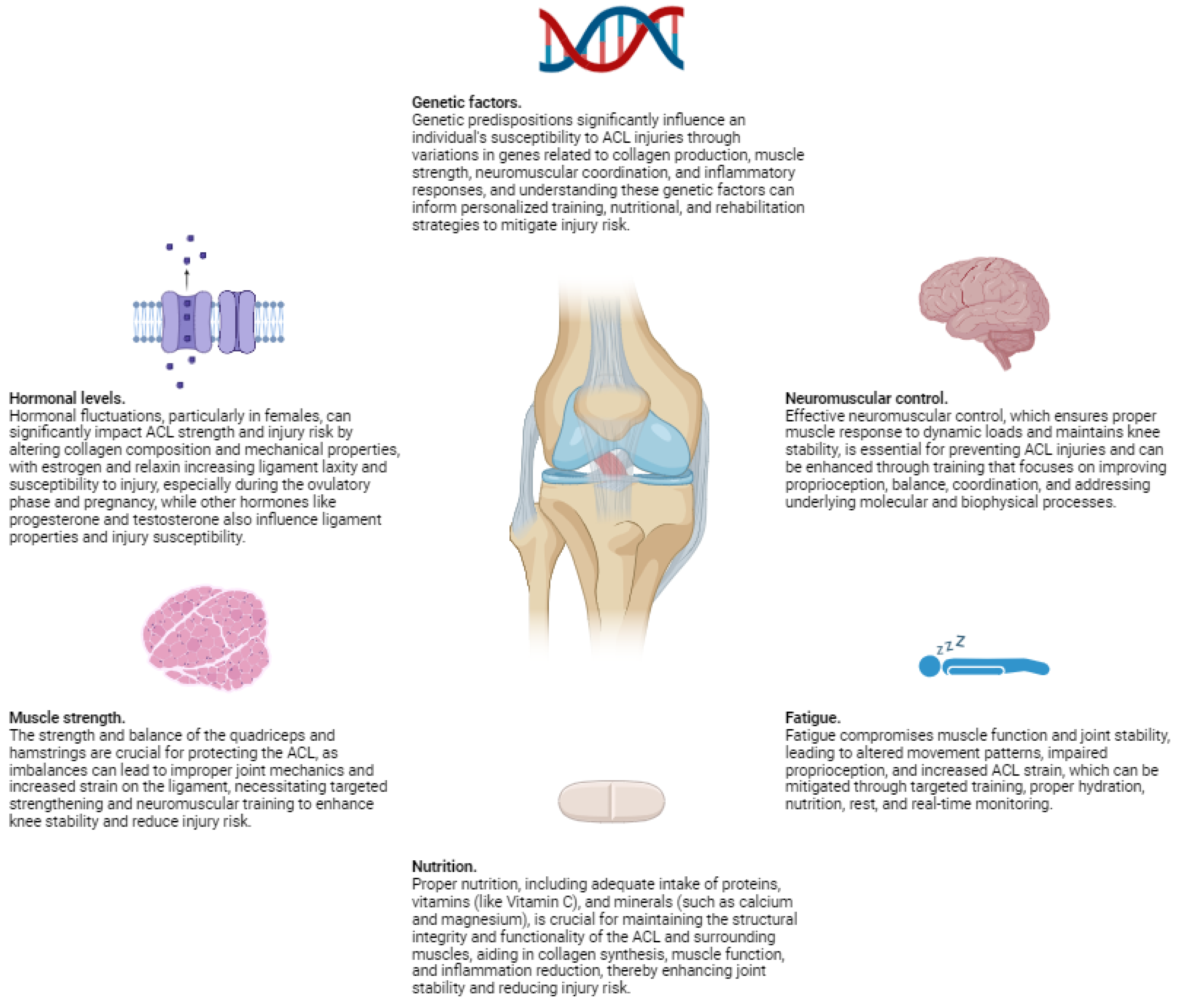
| Factor/Aspect | Description | Biomechanical/ Biophysical Implications | Impact on ACL Injury Risk | Prevention Strategies/ Interventions |
|---|---|---|---|---|
| Axial Loading | Vertical forces exerted on the knee during motion, such as landing from a jump or sudden deceleration. | Increases compressive forces on the knee joint, potentially exacerbating anterior tibial translation and ACL strain. | Elevated risk due to increased ligament loading and potential overload of ACL fibers. | Proper landing techniques, strength training to absorb forces, neuromuscular training to enhance muscle coordination during high-impact activities. |
| Anterior Tibial Translation | Forward movement of the tibia relative to the femur, especially during activities like cutting, pivoting, or sudden stops. | Places significant stress on the ACL as it resists the forward translation of the tibia, leading to increased strain. | High risk of ACL strain or rupture due to excessive loading beyond the ligament’s capacity. | Hamstring strengthening to resist anterior translation, improve neuromuscular control, and balance quadriceps-hamstring activation patterns. |
| Rotational Forces | Internal and external rotation of the tibia during dynamic movements, compounding stress on the ACL. | Increases torsional stress on the ACL, especially during pivoting or twisting actions, potentially leading to fiber rupture. | Increased susceptibility to ACL injury due to combined rotational and translational forces exceeding ligament tolerance. | Training to improve rotational control, proprioceptive exercises, and techniques to enhance awareness of limb position during dynamic movements. |
| Knee Valgus | Inward collapse of the knee during movement, leading to improper alignment and increased ACL strain. | Alters load distribution across the knee joint, increasing medial stress and strain on the ACL. | Higher risk of injury due to abnormal joint mechanics and increased ligament loading in valgus positions. | Strengthen hip abductors and external rotators, correct movement patterns through neuromuscular training, and use feedback techniques to avoid valgus collapse during activities. |
| Improper Landing Mechanics | Landing with an extended knee or poor technique, increasing forces transmitted to the ACL. | Leads to higher anterior tibial translation and ACL strain due to insufficient shock absorption and knee flexion upon landing. | Elevated risk of ACL injury from increased ligament loading during improper landing. | Teach proper landing techniques emphasizing knee flexion, plyometric training to improve explosive control, and balance exercises to enhance stability upon landing. |
| Muscle Strength Imbalances | Overdominance of quadriceps relative to hamstrings, causing excessive anterior tibial translation. | Results in unopposed quadriceps pull on the tibia, increasing strain on the ACL and compromising knee stability. | Greater ACL strain and injury risk due to imbalance in muscle forces acting on the knee joint. | Balance quadriceps and hamstring strength through targeted strength training, ensure proper muscle activation patterns, and include eccentric training for hamstrings. |
| Neuromuscular Control Deficits | Poor coordination of muscle activation patterns, especially when fatigued or untrained, leading to compromised knee stability. | Impaired ability to stabilize the knee joint during dynamic activities, increasing ACL strain due to uncontrolled movements. | Higher likelihood of injury due to inability to control joint positioning and loads effectively. | Neuromuscular training, including balance and proprioceptive exercises, agility drills, and fatigue management strategies to enhance muscle coordination and joint stability. |
| External Factors (Footwear, Surfaces) | Type of footwear, playing on artificial turf vs. natural grass, and environmental conditions affecting traction and movement mechanics. | Increased traction or reduced ability to slide can lead to greater forces on the knee, altering biomechanics and increasing ACL loading. | Higher incidence of ACL injuries due to altered movement patterns and increased stress on the ligament from external conditions. | Use appropriate footwear providing adequate support and traction, consider playing surfaces in training and competition planning, and adjust techniques to accommodate environmental conditions. |
| Collagen Fiber Structure of ACL | Hierarchical organization of collagen fibers providing tensile strength and flexibility; disruptions compromise ligament integrity. | Mechanical overload or biochemical degradation affects the collagen structure, reducing the ACL’s ability to withstand stress. | Increased risk of injury due to weakened ligament structure and decreased mechanical properties. | Molecular therapies to enhance tissue repair, nutritional interventions to support collagen synthesis, and strategies to prevent degradation (e.g., managing inflammation). |
| Proteoglycans and ECM Components | Proteoglycans interact with collagen to regulate fibrillogenesis, maintain tissue hydration, and viscoelastic properties of the ACL. | Changes in composition or organization affect the mechanical behavior of the ACL, altering its ability to absorb and dissipate forces. | Altered susceptibility to injury due to compromised mechanical properties and reduced resilience of the ligament. | Nutritional interventions to maintain ECM health, molecular therapies targeting ECM components, and strategies to promote optimal tissue composition (e.g., avoiding overuse injuries). |
| Molecular Signaling Pathways | Cellular mechanotransduction mechanisms involving integrins and cytoskeleton influencing cell behavior and tissue remodeling in response to mechanical stress. | Essential for ligament homeostasis and initiating repair processes; dysfunction may impair healing and adaptation to stress. | Increased injury risk due to impaired healing responses and inability to adapt to mechanical demands. | Targeted therapies to enhance repair mechanisms, molecular interventions to support healthy signaling pathways, and strategies to promote effective mechanotransduction (e.g., appropriate loading during rehabilitation). |
| Kinematic and Kinetic Movements | Excessive anterior tibial translation and internal tibial rotation during sudden deceleration, pivoting, and landing contribute to ACL strain. | Place significant strain on the ACL by combining translational and rotational forces that challenge ligament capacity. | Critical risk factors for ACL injury due to movements that exceed the ligament’s mechanical limits. | Training to correct movement patterns, neuromuscular exercises to improve control, and use of video analysis and motion capture to provide feedback and refine techniques. |
| Valgus Stress and Axial Loading | Forces causing the knee to bend inward (valgus) and compressive forces along the leg (axial loading) during dynamic activities like cutting and sidestepping. | Significantly increase ACL loading by altering joint mechanics and increasing stress on the ligament fibers. | Elevated risk during activities that involve sudden directional changes and high-impact forces. | Strengthening exercises targeting muscles that resist valgus collapse, proper techniques for cutting and sidestepping, equipment adjustments (e.g., bracing), and awareness of body positioning during dynamic movements. |
| Altered Muscle Activation Patterns | Imbalanced or delayed activation of quadriceps and hamstrings, leading to greater strain on the ACL due to unopposed forces or lack of stabilization. | Results in improper joint mechanics and increased ACL loading during dynamic activities. | Increased ACL loading and higher injury risk due to inability to adequately stabilize the knee joint. | Electromyography (EMG) studies to assess muscle activation patterns, neuromuscular training to improve coordination, and interventions to ensure timely and balanced muscle activation (e.g., plyometric exercises, biofeedback techniques). |
| Viscoelastic Properties of ACL | Time-dependent response of the ACL to stress, involving both elastic and viscous components influencing how the ligament deforms under load. | Under rapid loading, the ACL’s elastic response attempts to return it to its original shape, but prolonged loading can lead to creep and eventual failure. | Increased risk of injury during activities involving rapid or prolonged forces exceeding the ligament’s viscoelastic capacity. | Training to enhance ligament resilience, appropriate loading during activities to avoid exceeding viscoelastic limits, and strategies to prevent fatigue that may impair the ligament’s ability to respond to stress. |
| Material Properties of ACL | Structural composition, including collagen fiber alignment and cross-linking, determining the ligament’s mechanical properties like stiffness and resilience. | Alterations in material properties due to aging, hormonal influences, or previous injuries can reduce the ACL’s ability to withstand mechanical loads. | Higher susceptibility to injury when the ligament’s material properties are compromised, leading to decreased tolerance to stress. | Interventions to maintain or enhance material properties (e.g., hormonal therapies, nutritional support), monitoring ligament health, and tailored training programs considering individual variations in ligament properties. |
| Advanced Imaging Techniques | Use of MRI, ultrasound elastography, and other imaging modalities to assess ACL structure and mechanical properties. | Provide detailed views of internal ligament structure, revealing microstructural changes, fiber alignment, and tissue stiffness, informing about ligament health and stress responses. | Early detection of potential injury risks due to structural weaknesses or changes in mechanical properties. | Regular imaging assessments for at-risk individuals, personalized interventions based on imaging findings, and monitoring the effectiveness of rehabilitation strategies through imaging feedback. |
| Fluid Dynamics within Knee Joint | Role of synovial fluid in lubricating and nourishing the ACL, affecting its health and response to mechanical stress. | Influences friction, load distribution, and nutrient delivery to the ligament, impacting its ability to handle stress and recover from microdamage. | Altered fluid dynamics can increase injury risk due to inadequate lubrication and nourishment, leading to compromised ligament integrity. | Ensuring joint health through hydration, appropriate warm-up and cool-down routines to promote synovial fluid circulation, and interventions targeting joint fluid dynamics (e.g., viscosupplementation in certain cases). |
| Molecular Structure of Muscle Fibers | Interaction of actin and myosin in muscle contraction, influenced by neural signals and mechanical loads affecting muscle force production. | Variations in molecular mechanisms of muscle contraction can lead to weaker contractions and poor joint stabilization, impacting ACL loading. | Increased risk of ACL injury due to insufficient muscle support and control during dynamic movements. | Interventions targeting muscle function at the molecular level (e.g., optimizing calcium handling, energy availability), and training programs enhancing muscle fiber recruitment and contraction efficiency. |
| Mechanotransduction in Muscles and ACL | Cellular processes converting mechanical stimuli into biochemical responses, affecting muscle adaptation and ligament healing through signaling pathways involving proteins like integrins and FAK. | Impacts how muscles and ligaments adapt to mechanical demands, influencing strength, resilience, and healing capacity. | Impaired mechanotransduction can lead to inadequate adaptation or repair, increasing injury risk due to less robust tissues. | Strategies to enhance mechanotransduction (e.g., appropriate mechanical loading, rehabilitation protocols promoting beneficial signaling), and potential molecular therapies targeting key pathways to improve tissue adaptation and healing. |
| Factor/Aspect | Description | Biomechanical/Biophysical Implications | Impact on ACL Injury Risk | Prevention Strategies/Interventions |
|---|---|---|---|---|
| Femoral Notch Width | The intercondylar (femoral) notch is the groove at the distal end of the femur through which the ACL passes. A narrower notch reduces the space available for the ACL, increasing the likelihood of impingement during dynamic movements. MRI and cadaveric studies have shown a correlation between narrower notch width and higher ACL injury incidence. | Limited space can lead to mechanical pinching (impingement) of the ACL, causing increased stress and potential weakening of ligament fibers. Impingement during high-stress activities can result in microtears and eventual rupture due to repeated abrasion and stress concentration. | Higher risk of ACL injury due to increased mechanical constraints and impingement within the femoral notch, especially during activities involving cutting, pivoting, and sudden deceleration. | Strength and conditioning programs to enhance knee stability; biomechanical training emphasizing proper movement patterns to reduce impingement risk; surgical interventions like notchplasty for recurrent cases; personalized assessments using MRI to identify individuals at risk. |
| Posterior Tibial Slope | The angle of the tibial plateau relative to the long axis of the tibia. An increased (steeper) posterior tibial slope facilitates greater anterior tibial translation during weight-bearing activities. Biomechanical modeling and radiographic studies have linked a steeper tibial slope to increased ACL strain. | Greater anterior tibial translation increases tension on the ACL, making it more susceptible to injury during dynamic movements. The steeper slope alters knee biomechanics, leading to excessive forward movement of the tibia relative to the femur under load. | Significant risk factor for ACL injuries, particularly during activities involving sudden deceleration or changes in direction, due to heightened ligament strain. | Strength and conditioning to improve muscle support counteracting anterior tibial translation; biomechanical training to enhance movement techniques; surgical consideration for slope modification in severe cases; personalized assessments using radiographic imaging to identify high-risk individuals. |
| Lower Limb Alignment (Q-Angle) | The quadriceps angle (Q-angle) is formed by a line from the anterior superior iliac spine to the center of the patella and another from the patella to the tibial tubercle. A larger Q-angle increases lateral forces on the knee, leading to greater valgus stress and internal tibial rotation. This misalignment is more pronounced in females due to wider pelvises. | Increased valgus stress and internal tibial rotation place additional strain on the ACL by altering load distribution and joint mechanics. The abnormal alignment leads to increased medial stress and potential overloading of the ligament during dynamic activities. | Elevated risk of ACL injuries, especially in females, due to biomechanical environments more susceptible to ligament strain during movements involving cutting or pivoting. | Strengthening hip abductors and external rotators to correct alignment; neuromuscular training to improve movement patterns; proprioceptive exercises; personalized assessments to address individual alignment issues; footwear modifications to support proper alignment. |
| Size and Shape of the ACL | Variations in the ACL’s cross-sectional area, length, curvature, and orientation within the knee joint. Smaller or thinner ACLs may be less capable of handling high loads, and irregular shapes can lead to uneven stress distribution. MRI and 3D reconstruction techniques have identified significant individual differences in ACL geometry. | Affects mechanical strength and stress distribution within the ligament. Smaller size reduces tensile strength, while irregular shape can cause localized high-strain areas, increasing susceptibility to microtears and rupture under mechanical load. | Increased risk of ACL injury due to decreased mechanical resilience and uneven stress concentrations within the ligament during dynamic activities. | Targeted strength training to enhance muscular support; biomechanical training to optimize movement patterns; personalized assessments using MRI to identify individuals with vulnerable ACL geometry; potential consideration of surgical techniques for reconstruction with grafts mimicking optimal ACL geometry. |
| Insertion Points of the ACL | The anatomical locations where the ACL attaches to the femur and tibia. Variations in these positions can alter the ligament’s leverage and the forces experienced during knee movements. Anterior or posterior shifts in insertion points can impact the ACL’s ability to resist mechanical loads. | Changes in insertion points affect the angle and tension of the ACL during knee movements, potentially increasing tensile forces and altering joint mechanics. This can compromise the ligament’s ability to resist anterior tibial translation and rotational forces. | Heightened susceptibility to ACL strain and injury due to altered biomechanics and increased stress on the ligament during dynamic activities. | Biomechanical assessments to determine insertion point variations; customized training programs to strengthen supportive musculature; consideration of surgical techniques that address insertion point alignment during ACL reconstruction. |
| Gender Differences in Anatomy | Females typically have a wider pelvis, greater Q-angle, increased ligamentous laxity, and hormonal variations affecting ligament strength (e.g., estrogen levels). These anatomical and physiological differences can result in altered biomechanics and increased valgus alignment. | Increased valgus stress, joint laxity, and altered muscle activation patterns contribute to higher ACL strain. Hormonal influences can affect collagen metabolism, impacting ligament strength and resilience. | Higher incidence of ACL injuries in females due to combined anatomical and hormonal factors affecting ligament loading and mechanical properties. | Gender-specific training programs focusing on strengthening and neuromuscular control; hormonal considerations in injury prevention strategies; education on proper techniques to mitigate biomechanical risks; personalized assessments to address individual anatomical variations. |
| Collagen Fiber Organization | The ACL’s mechanical properties are influenced by the alignment, density, and cross-linking of collagen fibers within its extracellular matrix (ECM). Variations can affect tensile strength and elasticity. Molecular studies highlight the importance of collagen cross-linking and fiber orientation in ligament resilience. | Altered collagen organization can reduce the ACL’s ability to withstand mechanical loads, leading to decreased tensile strength and increased risk of microstructural damage under stress. Collagen degradation impairs the ligament’s structural integrity at the molecular level. | Increased susceptibility to ACL injury due to weakened structural properties and impaired mechanical function of the ligament. | Nutritional interventions to support collagen synthesis; molecular therapies targeting collagen cross-linking; strategies to prevent collagen degradation (e.g., managing inflammation); personalized rehabilitation programs focusing on enhancing collagen organization through controlled mechanical loading. |
| Genetic Factors | Genetic variations influence the development and maintenance of knee joint structures, affecting proteins involved in collagen synthesis, ECM organization, and bone morphology. These factors can predispose individuals to anatomical variations that increase ACL injury risk. | Genetic predispositions can lead to structural characteristics such as narrow femoral notch, steep tibial slope, or variations in ACL geometry, affecting biomechanical properties and loading patterns. | Higher risk of ACL injury due to inherited anatomical factors influencing knee biomechanics and ligament resilience. | Genetic screening to identify at-risk individuals; personalized prevention strategies; early intervention programs focusing on biomechanical training and strength conditioning; potential future gene therapies targeting structural protein expression. |
| Mechanotransduction Pathways | Cellular processes where mechanical stress is converted into biochemical signals, influencing cellular adaptation, ECM production, and ligament resilience. Involves integrins, MAPK/ERK pathway, mechanosensitive ion channels, and other signaling molecules. | Proper mechanotransduction is essential for ligament homeostasis and repair. Dysregulation can lead to impaired adaptation to mechanical stress, increased MMP activity, collagen degradation, and weakened ligament structure. | Increased ACL injury risk due to reduced ability of ligament cells to respond adaptively to mechanical loads and repair microdamage. | Interventions targeting mechanotransduction pathways to enhance cellular responses (e.g., controlled mechanical loading during rehabilitation); molecular therapies modulating key signaling molecules; nutritional support to promote healthy cellular function; personalized rehabilitation protocols focusing on optimal mechanical stimuli. |
| Oxidative Stress in ACL Cells | Mechanical stress can generate reactive oxygen species (ROS) in ACL fibroblasts, leading to oxidative damage to cellular components, including lipids, proteins, and DNA. Oxidative stress impairs cell function and ECM integrity. Increased ROS production can lead to impaired collagen synthesis, ECM degradation, and reduced cellular viability, weakening the ligament’s structural and mechanical properties. | Increased ROS production can lead to impaired collagen synthesis, ECM degradation, and reduced cellular viability, weakening the ligament’s structural and mechanical properties. | Higher susceptibility to ACL injury due to compromised cellular function and ligament integrity under oxidative stress conditions. | Antioxidant therapies to reduce oxidative stress; nutritional interventions rich in antioxidants; strategies to manage mechanical loading to minimize excessive ROS production; personalized approaches to enhance cellular resilience against oxidative damage. |
| Proteoglycans and ECM Components | Proteoglycans and glycoproteins in the ACL’s ECM, such as decorin and aggrecan, interact with collagen fibers and contribute to the ligament’s viscoelastic properties. Variations in their expression affect tissue hydration and mechanical behavior. | Altered proteoglycan content can impact the ligament’s ability to absorb and dissipate mechanical forces, affecting stiffness, elasticity, and overall mechanical performance under stress. | Increased risk of ACL injury due to compromised viscoelastic properties and reduced capacity to handle mechanical loads. | Nutritional support to maintain ECM health; molecular therapies targeting ECM composition; strategies to promote optimal ECM organization through controlled mechanical loading; personalized rehabilitation focusing on enhancing ECM properties. |
| Epigenetic Modifications | Changes in DNA methylation, histone modification, and non-coding RNA expression in ACL fibroblasts in response to mechanical stress. Epigenetic regulation affects gene expression and cellular behavior without altering the DNA sequence. | Epigenetic modifications can influence the expression of genes involved in collagen synthesis, ECM remodeling, and cellular responses to stress, impacting ligament adaptation and repair processes. | Increased susceptibility to injury if epigenetic changes lead to maladaptive cellular responses and impaired ligament resilience. | Interventions aimed at modulating epigenetic mechanisms (potentially through pharmacological agents); personalized rehabilitation protocols considering individual epigenetic profiles; strategies to promote adaptive epigenetic responses through appropriate mechanical stimuli and environmental factors. |
| Mitochondrial Function in ACL Cells | Mechanical stress affects mitochondrial dynamics, including biogenesis, fission, fusion, and function in ACL fibroblasts. Mitochondria are essential for energy production and cellular homeostasis. | Disruptions in mitochondrial function can impair energy metabolism, increase ROS production, and reduce the capacity of cells to maintain ECM integrity and respond to mechanical stress. | Higher risk of ACL injury due to impaired cellular energy production and increased oxidative damage, leading to weakened ligament structure. | Nutritional interventions supporting mitochondrial health (e.g., CoQ10, antioxidants); strategies to optimize energy metabolism in cells; controlled mechanical loading to promote healthy mitochondrial function; personalized approaches to enhance cellular energy capacity during rehabilitation. |
| Advanced Imaging Techniques | MRI, CT scans, and 3D reconstruction technologies provide detailed visualization of knee structures, including ACL size, shape, insertion points, femoral notch width, and tibial slope. These imaging modalities facilitate precise assessments of anatomical variations. | Enables accurate measurement of anatomical factors influencing ACL mechanics and injury risk. Imaging data can be integrated into computational models to simulate knee biomechanics and predict stress distribution within the ligament. | Early identification of individuals at higher risk of ACL injury due to anatomical variations, allowing for targeted prevention strategies. | Routine imaging assessments for at-risk populations; personalized training and intervention programs based on imaging findings; use of imaging data to guide surgical planning and postoperative rehabilitation; development of computational models for individualized risk assessment. |
| Computational Modeling | Biomechanical models simulate forces and movements within the knee joint, predicting how anatomical variations influence stress on the ACL. Models integrate imaging data and biomechanical principles to assess injury risk. | Provides insights into the biomechanical environment of the knee, allowing for the prediction of how factors like tibial slope and ACL geometry affect ligament loading and injury mechanisms. | Enhanced ability to identify high-risk individuals and develop personalized prevention and rehabilitation strategies based on simulated outcomes. | Use of computational models in conjunction with imaging and clinical data to inform training programs; development of personalized interventions targeting specific biomechanical vulnerabilities; ongoing refinement of models with new data to improve predictive accuracy. |
| Tissue Engineering Advances | Techniques such as 3D bioprinting and scaffold-based approaches aim to create ligament constructs that replicate the native ACL’s geometry and mechanical properties for reconstruction and repair. | Enables the development of replacement tissues that mimic the biomechanical and molecular characteristics of the natural ACL, potentially improving surgical outcomes and reducing re-injury rates. | Potential to decrease ACL injury recurrence and improve long-term joint stability through enhanced reconstruction techniques. | Application of tissue-engineered grafts in ACL reconstruction; ongoing research to optimize scaffold materials and cellular components; integration of tissue engineering advances into clinical practice for personalized treatment options. |
| Factor/Aspect | Description | Biomechanical/Biophysical Implications | Impact on ACL Injury Risk | Prevention Strategies/Interventions |
|---|---|---|---|---|
| Genetic Factors | Genetic predispositions influence susceptibility to ACL injuries. Variations in genes related to collagen production (e.g., COL1A1, COL5A1) affect the structural integrity of the ACL by altering collagen synthesis, cross-linking, and fibril formation. Polymorphisms may lead to weaker collagen fibers, making the ligament less capable of withstanding mechanical stress. Genes involved in muscle strength (ACTN3) and neuromuscular coordination (e.g., genes encoding neurotrophic factors like BDNF) impact muscle performance and coordination. Variations can affect fast-twitch muscle function, neural signaling pathways, and proprioception, increasing the risk of improper joint mechanics. Genes related to inflammation and tissue repair (IL-6) influence the inflammatory response post-injury, affecting healing and recovery processes. | Altered collagen synthesis and weaker cross-linking reduce the mechanical strength of the ACL, compromising its ability to resist tensile forces. Genetic variations affecting muscle function and neuromuscular control lead to inadequate stabilization of the knee joint during dynamic movements. Variations in inflammatory response genes can prolong inflammation, hindering healing. | Increased susceptibility to ACL injury due to structurally weaker ligaments, impaired muscle performance, and compromised neuromuscular coordination. Genetic predispositions to prolonged inflammation can increase re-injury risk and delay recovery. |
Personalized Training Programs: Tailored exercises to strengthen muscles and improve neuromuscular control based on genetic profiles. Genetic Screening: Identify high-risk individuals through genetic testing for targeted interventions. Nutritional Support: Supplements supporting collagen synthesis (vitamin C, lysine, proline) and anti-inflammatory diets rich in omega-3 fatty acids. Preventive Strategies: Focus on strengthening ligament resilience and enhancing muscle performance to compensate for genetic weaknesses. Monitoring and Assessment: Regular biomechanical assessments to identify and correct improper joint mechanics. |
| Recovery and Rehabilitation | Hormonal influences, muscle imbalances, and neuromuscular deficits significantly affect ACL recovery. Estrogen impacts collagen synthesis and wound healing, influencing fibroblast proliferation and migration. Muscle strength imbalances (e.g., stronger quadriceps vs. weaker hamstrings) increase strain on the ACL. Neuromuscular control deficits lead to improper joint mechanics and increased re-injury risk. Personalized rehabilitation programs considering these factors optimize recovery and reduce re-injury likelihood. Controlled mechanical loading during rehabilitation stimulates mechanotransduction pathways (e.g., integrin-mediated signaling), promoting collagen synthesis and ligament strengthening. Growth factors (e.g., TGF-β, IGF-1) play roles in tissue repair by enhancing fibroblast activity and collagen production. Advanced techniques like AFM measure ECM mechanical properties, informing rehabilitation protocols that modulate ECM remodeling. Incorporating genetic information refines rehabilitation strategies to address individual predispositions. | Hormonal fluctuations influence collagen cross-linking and ligament laxity during healing. Muscle imbalances affect load distribution across the knee, impacting ligament recovery. Neuromuscular deficits hinder proper muscle activation patterns essential for joint stability during rehabilitation exercises. Controlled loading enhances tissue remodeling and strength. | Higher risk of re-injury due to weakened ligament structure, improper joint mechanics, and inadequate tissue healing if rehabilitation does not address these factors. Personalized programs enhance recovery outcomes and reduce long-term injury risk. |
Personalized Rehabilitation Programs: Tailored exercises addressing hormonal influences, muscle imbalances, and neuromuscular deficits. Hormonal Timing: Align rehabilitation phases with hormonal cycles to maximize healing benefits and mitigate risks. Strength Training: Target hamstrings, hip, and core muscles to improve knee stability. Neuromuscular Training: Enhance proprioception, balance, and coordination through specialized exercises (balance boards, agility drills). Controlled Mechanical Loading: Apply appropriate stress to stimulate collagen synthesis and alignment. Molecular Therapies: Utilize growth factors to promote tissue repair. Advanced Monitoring: Use wearable technology and biofeedback for real-time adjustment of rehabilitation exercises. Genetic Considerations: Incorporate genetic testing to personalize rehabilitation strategies further. |
| Nutritional Influences | Nutrition is vital for ACL and muscle health. Adequate intake of proteins provides essential amino acids for collagen synthesis and muscle repair. Vitamin C is crucial for collagen stability by aiding in hydroxylation of proline and lysine residues. Minerals like calcium and magnesium are essential for muscle contraction and relaxation, affecting joint stability. Omega-3 fatty acids have anti-inflammatory properties, reducing pro-inflammatory cytokines and aiding recovery. Antioxidants protect against oxidative stress, which can degrade collagen and impair muscle function. Specific amino acids (glycine, proline, lysine) are important for collagen production. Nutritional strategies focus on providing building blocks for collagen synthesis and muscle repair, enhancing ligament resilience and joint health. Copper is necessary for lysyl oxidase function, which is essential for collagen cross-linking. | Nutritional deficiencies impair collagen synthesis and cross-linking, weakening the ACL’s structural integrity. Inadequate minerals affect muscle function, compromising neuromuscular control and joint stability. Anti-inflammatory nutrients aid in recovery and reduce oxidative stress that can damage tissues. | Increased risk of ACL injury due to weakened ligament structure from poor collagen synthesis, impaired muscle function from mineral deficiencies, and prolonged inflammation hindering recovery. |
Balanced Diet: Ensure adequate intake of proteins, vitamins (Vitamin C), minerals (calcium, magnesium, copper), and omega-3 fatty acids. Supplementation: Use collagen supplements and specific amino acids to support collagen synthesis. Anti-inflammatory Foods: Incorporate foods rich in omega-3 fatty acids and antioxidants (berries, leafy greens, nuts). Nutritional Education: Provide guidance on diet planning to support tissue repair and muscle function. Personalized Nutrition Plans: Tailor dietary strategies based on individual needs and genetic predispositions affecting nutrient metabolism. Monitoring Nutrient Intake: Regular assessments to prevent deficiencies that could compromise ligament and muscle health. |
| Factor/Aspect | Description | Biomechanical/Biophysical Implications | Impact on ACL Injury Risk | Prevention Strategies/Interventions |
|---|---|---|---|---|
| Injury Prevention Programs | Integrated injury prevention programs combine biomechanics, physiology, molecular biology, and personalized medicine to address ACL injury risk comprehensively. Biomechanical Training corrects faulty movement patterns identified through motion analysis (e.g., high-speed cameras, motion capture systems), such as excessive knee valgus or improper hip rotation during jumps and landings. Strength Conditioning enhances muscle balance and support around the knee, targeting muscles like the quadriceps and hamstrings. Neuromuscular Education improves proprioception, balance, and coordination through dynamic stability exercises, balance board training, and agility drills. Molecular Biophysics informs how mechanical forces from exercises activate intracellular signaling pathways (e.g., integrin-FAK pathway) in ligament fibroblasts, promoting collagen synthesis and improving ACL strength. Nutritional Strategies provide necessary nutrients (proteins, vitamins, minerals) to support ligament and muscle health. Technology Integration utilizes wearable sensors and biofeedback devices to offer real-time monitoring and feedback, ensuring correct technique and safety during exercises. Personalized Interventions tailor programs based on individual biomechanics, anatomy, physiology, and genetic profiles to enhance effectiveness. |
- Biomechanical Training: Correcting movement patterns reduces harmful mechanical stresses on the ACL, influencing mechanotransduction pathways that regulate collagen synthesis and ligament strength. - Strength Conditioning: Enhances muscle support, affecting extracellular matrix (ECM) remodeling and collagen turnover in the ACL through mechanical loading. - Neuromuscular Education: Improves muscle activation patterns and joint stability by influencing neural plasticity and synaptic efficiency at the neuromuscular junction. - Molecular Biophysics: Understanding molecular responses to mechanical stress allows for optimized training protocols that enhance ligament resilience. - Nutritional Strategies: Nutrients influence collagen biosynthesis and ECM composition, affecting the mechanical properties of the ACL. - Technology Integration: Real-time data helps adjust training to minimize excessive stress on the ACL, informed by molecular insights into mechanical stress impacts on collagen remodeling. - Personalized Interventions: Address specific molecular and biomechanical factors contributing to injury risk. |
- Risk Reduction: Comprehensive programs address multiple risk factors, significantly reducing the likelihood of ACL injuries. - Enhanced Ligament Strength: Optimized collagen synthesis and ECM remodeling improve ACL resilience to mechanical stress. - Improved Neuromuscular Control: Better proprioception and coordination reduce improper joint mechanics that increase injury risk. - Personalized Effectiveness: Tailored programs based on individual biomechanics and molecular profiles enhance effectiveness in preventing injuries. - Holistic Improvement: Integration of various disciplines leads to overall enhancement of athletic performance and joint health. |
- Multi-faceted Training Programs: Incorporate biomechanical correction, strength conditioning, and neuromuscular education. - Personalized Plans: Use motion analysis and genetic testing to tailor interventions. - Nutritional Support: Ensure adequate intake of proteins, vitamins, minerals, and anti-inflammatory nutrients. - Technological Aids: Utilize wearable sensors and biofeedback devices for real-time feedback and adjustment. - Molecular Considerations: Design training protocols that optimize mechanical loading to enhance collagen synthesis and ligament strength. - Continuous Monitoring and Adaptation: Regular assessments to adjust programs based on progress and changes in risk factors. - Interdisciplinary Collaboration: Engage experts from biomechanics, physiology, molecular biology, and nutrition for comprehensive care. |
| Screening and Risk Assessment | Implementing comprehensive screening programs to identify individuals at high risk of ACL injuries by assessing anatomical, physiological, biomechanical, and genetic factors. Functional Movement Screenings and tools like the Landing Error Scoring System (LESS) detect faulty movement patterns and joint alignment issues. Motion Capture Technology and Force Platforms provide biomechanical data on movement mechanics and mechanical loads on the ACL. Genetic Screening identifies polymorphisms in genes related to collagen synthesis (e.g., COL1A1, COL5A1), muscle strength (e.g., ACTN3), and inflammatory responses (e.g., IL-6), indicating predispositions to injury. Hormonal Assessments track fluctuations (e.g., estrogen, relaxin) that affect ligament laxity and strength. Biochemical Evaluations of biomarkers (e.g., MMP levels) provide insights into the biochemical environment influencing ACL health. Fatigue Assessments evaluate the impact on neuromuscular control and muscle activation patterns. Personalized Risk Profiles are developed to tailor prevention strategies effectively. |
- Biomechanical Analysis: Identifies mechanical loads and movement patterns that impact ACL strain and collagen remodeling. - Genetic Screening: Reveals predispositions affecting collagen structure, ligament strength, and muscle function at the molecular level. - Hormonal Assessments: Understands how hormonal fluctuations influence ligament properties and injury risk. - Biochemical Evaluations: Provides insights into molecular factors (e.g., MMP activity) affecting ligament integrity. - Fatigue Assessments: Determines how fatigue-induced molecular changes impact neuromuscular control and joint stability. - Integrated Data: Combines multiple sources of information for a comprehensive understanding of injury risk. |
- Targeted Prevention: Early identification of high-risk individuals allows for personalized interventions to address specific risk factors. - Improved Injury Prediction: Comprehensive assessments enhance the accuracy of risk profiles. - Enhanced Effectiveness: Tailored prevention strategies based on molecular and biomechanical data improve outcomes. - Resource Optimization: Focused interventions reduce unnecessary training and increase efficiency. - Reduced Injury Incidence: Proactive measures decrease the likelihood of ACL injuries in high-risk populations. |
- Comprehensive Screening Programs: Combine functional assessments, biomechanical analysis, genetic testing, hormonal tracking, and biochemical evaluations. - Personalized Interventions: Develop prevention strategies addressing identified risk factors, such as tailored training and nutritional plans. - Monitoring and Reassessment: Regular follow-ups to adjust interventions based on changes in risk profiles. - Education and Awareness: Inform individuals about their specific risks and how to manage them. - Interdisciplinary Collaboration: Engage healthcare providers from various fields to provide a holistic approach. - Technology Utilization: Employ advanced tools for precise assessments and data integration. - Policy Development: Implement screening protocols at organizational levels (e.g., sports teams, schools) to standardize risk assessment. |
| Rehabilitation Strategies | Integrating molecular biophysics into post-injury rehabilitation for ACL injuries to optimize recovery by understanding cellular and molecular healing processes. Mechanical Loading through controlled exercises stimulates mechanotransduction pathways (e.g., integrin-FAK, mTOR) in fibroblasts, promoting collagen synthesis, proper fiber alignment, and cross-linking, enhancing ligament tensile strength. Neuromuscular Exercises improve neuroplasticity involving pathways such as brain-derived neurotrophic factor (BDNF) and NMDA receptor-mediated synaptic plasticity, enhancing motor control and joint stability. Proprioceptive Training activates mechanosensitive ion channels (e.g., Piezo1, TRPV4), improving sensory feedback and motor responses for better joint alignment. Progressive Loading involves gradual increases in mechanical stress to stimulate tendon adaptation, influencing collagen composition and mechanical properties. Functional Exercises enhance muscle strength and coordination, affecting gene expression related to muscle growth and repair, improving overall functional ability. Biochemical Monitoring of markers related to inflammation, tissue repair, and muscle function guides rehabilitation adjustments. Advanced Imaging (e.g., MRI, ultrasound) assesses tissue healing and adaptation at the molecular level, informing protocol refinements. Personalized Rehabilitation Plans consider individual molecular and physiological profiles to tailor interventions effectively. |
- Cellular Responses: Mechanical loading influences cellular signaling pathways that regulate collagen synthesis and ECM remodeling, critical for ligament repair. - Neuroplasticity: Neuromuscular exercises affect synaptic efficiency and neural connections, improving muscle activation and joint stability. - Proprioceptive Function: Training enhances mechanosensory pathways, improving coordination and reducing stress on the ACL. - Tissue Adaptation: Progressive loading promotes beneficial changes in tendon and muscle properties at the molecular level. - Functional Improvement: Exercises influence molecular pathways involved in muscle adaptation and joint mechanics. - Biochemical Feedback: Monitoring markers provides insights into molecular healing processes, allowing for targeted interventions. - Imaging Insights: Advanced imaging reveals structural and molecular changes in tissues, guiding rehabilitation phases. |
- Optimized Recovery: Molecularly informed rehabilitation enhances tissue repair and functional recovery. - Reduced Re-injury Risk: Improved ligament strength and neuromuscular control lower the chances of subsequent injuries. - Personalized Rehabilitation: Tailoring protocols based on molecular responses and individual needs improves outcomes. - Enhanced Functional Performance: Restored strength and coordination facilitate return to pre-injury activity levels. - Long-term Joint Health: Effective rehabilitation contributes to sustained joint stability and function. |
- Mechanically Informed Protocols: Design rehabilitation exercises that optimize mechanical loading for collagen synthesis and alignment. - Neuromuscular Training: Incorporate exercises that enhance neuroplasticity and proprioceptive function. - Progressive Loading Strategies: Gradually increase exercise intensity to stimulate tissue adaptation safely. - Functional Exercise Integration: Focus on movements that improve strength and coordination relevant to daily activities and sports. - Biochemical Monitoring: Use biomarkers to assess healing progress and adjust rehabilitation accordingly. - Advanced Imaging Utilization: Employ MRI and ultrasound to visualize tissue healing and guide rehabilitation phases. - Personalized Rehabilitation Plans: Consider individual molecular and physiological profiles to tailor interventions. - Interdisciplinary Approach: Collaborate among healthcare professionals to address all aspects of recovery. - Patient Education: Empower patients with knowledge about their recovery process and the importance of adherence to protocols. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





