Submitted:
12 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Insights from Qudruple Therapy Clinical Trials
3.1.1. Daratumumab-Containing Induction Therapy Trials
-
Perseus Trial (Phase III) - Dara-VRd vs VRd:This landmark trial enrolled 709 patients to evaluate subcutaneous daratumumab in combination with bortezomib, lenalidomide, and dexamethasone (Dara-VRd) during the induction/consolidation phase and Dara-R for maintenance versus, VRd for induction/consolidation followed by lenalidomide alone for maintenance. The primary endpoint was PFS and at a median follow-up of 47.5 months, the PFS rates were significantly improved in the Dara-VRd group compared to the VRd group (84.3% vs 67.7%, p=<0.001). The ORR was not reported in the study, but the CR rate was higher in the Dara-VRd group than in the VRd group (87.9% vs 70.1%, p=<0.001). The benefit was noted across all subgroups, including high risk patients. The most common grade 3 and 4 treatment-emergent adverse events (TEAEs) for the Dara-VRd arm vs VRd alone were neutropenia, thrombocytopenia, and diarrhea. Serious TEAEs leading to treatment discontinuations were noted to be higher in the Dara-VRd group versus the VRd group (57% vs 49.9%, respectively) [15,16].
-
Griffin Trial (Phase II) - Dara-VRd vs VRd:In this study, 207 patients were randomized to receive either Dara-VRd or VRd therapy for four cycles of induction, followed by ASCT, then two consolidation cycles, and finally maintenance therapy for up to two years with Dara-R vs R alone. Stringent Complete Response (sCR) post ASCT consolidation therapy was the primary endpoint with a median follow up of 13.5 months and it showed better sCR rates with Dara-VRd vs VRd (42.4% vs 32%, p=0.068). The final analysis after a median follow-up of 49.6 months showed the PFS was higher in the Dara-VRd group compared to the VRd alone group (87.2% vs 70%, p=.032). There is a trend toward improved PFS Dara-VRd vs VRd (hazard ratio (HR) [95% CI]) in patients > or = 65 years (0.29 [0.06-1.48]), with HRCAs (0.38 [0.14-1.01]), and with gain/amp (1q21) (0.42 [0.14-1.27]). The ORR was measured at the 13.5-month follow-up and was higher in the Dara-VRd than the VRd alone groups (99% vs 91.8% p=<0.016). Most common grade 3 and 4 adverse events in both arms included neutropenia, lymphopenia, and thrombocytopenia. Treatment discontinuation due to TEAEs occurred in fewer patients in the Dara-VRd group than the VRd alone group (15.2% vs 20.6%, respectively) [17,18,19,20,21].
-
Cassiopeia Trial (Phase III) - Dara-VTd vs VTd:This two-part study compared daratumumab, bortezomib, thalidomide, and dexamethasone (Dara-VTd) versus VTd alone for the induction and consolidation cycles for 1085 patients who received ASCT, following this treatment. In part two, patients were randomly assigned to receive daratumumab maintenance every 8 weeks for up to 2 years vs observation. In part one, the primary endpoint was sCR 100 days after transplantation and showed that in the Dara-VTd group had higher sCR than the VTd group (29% vs 20%, p=<0.001), median PFS was not achieved. The primary end point for part 2 was PFS based on the second randomization and showed significant benefit in the VTd plus daratumumab group when compared to the VTd with only observation; no significant difference in PFS was seen in the original group receiving daratumumab for induction. The ORR of 92.6% was noted in the Dara-VTd group compared to the 89.9% VTd group. Subgroup analysis of 15.5% patients with high risk cytogenic abnormality analyzed sCR as a primary endpoint. sCR rates were higher within Dara-VTd vs VTd group (28.9% vs 20.3%, respectively), (95% [1.21-2.12]). During part 2 of the study, the rate of grade 3 or 4 adverse events was 28% in the daratumumab group vs 24% in the observation group. Serious adverse events occurred in 23% vs 19% of patients, respectively [22,23,24].
-
Master Trial (Phase II) - Dara-KRd:This study assessed the combination of daratumumab, carfilzomib, lenalidomide, and dexamethasone (Dara-KRd) in 123 MM patients. The next generation sequencing (NGS) based minimal residual disease (MRD) (<10-5) status was used for the assessment of treatment cessation based on 2 consecutive negative MRD results. MRD was evaluated at the end of induction, post-ASCT, and every 4 cycles of consolidation. The primary endpoint was achieving MRD negativity at any point during the study with 38% reaching MRD post induction, 65% reaching MRD post-AHCT and 80% reaching MRD-directed consolidation (78%, 82% and 79% of patients with 0, 1 and 2+ HRCA, respectively). Two-year PFS was 87% for MRD 10-5 cohort (91%, 97% and 58% for patients with 0, 1 and 2+ HRCA, respectively) compared to 81% for MRD 10-6 cohort. The ORR was 95% at 8 months in the study. The study reported that all 123 participants experienced at least one treatment emergent adverse event, 74% experienced grade 3-5 adverse events. Two patients discontinued carfilzomib due to AE and two patients discontinued lenalidomide due to AE. There were three treatment-related deaths reported [25,26].
-
LYRA Trial (Phase II): - Dara-Vcd:The study evaluated a combination treatment regimen consisting of daratumumab, bortezomib, cyclophosphamide, and dexamethasone (Dara-Vcd) in 87 patients with NDMM and 14 patients with relapsed MM with a specific focus on achieving MRD negativity and understanding the regimen’s safety profile. The primary endpoint was Very Good Partial Response or better (VGPR+) following 4 cycles of induction therapy. In the NDMM cohort the rate of CR+VGPR after 4 cycles of induction was 44.2% and the ORR was 79.1%. At the end of the induction therapy, CR+VGPR was 55.8% and the ORR was 81.4%. Median PFS was not reached among those with standard risk and was 33.1 months among those with high risk, while the 36-months PFS was 87.5%for standard risk patients vs 45.2% for high risk patients . All participants experienced at least one TEAEs. The most common AE included fatigue and neutropenia, which were the most frequent grade 3-4 TEAEs. Infusion reactions occurred in 54% of patients, primarily during the first dose, and were predominantly mild, with only 2% being grade 3. Three treatment related deaths were reported in the study [27,28,29].
3.1.2. Isatuximab-Containing Induction Therapy Trials
-
GMMG-HD7 (Phase III) - Isa-VRd vs. VRd:The trial involved a cohort of 660 patients divided into the isatuximab, bortezomib, lenalidomide, and dexamethasone (Isa-VRd) arm versus VRd arm for induction therapy. The primary endpoint was MRD negativity assessed by next generation flow (NGF) after induction and resulted in 50.1% vs 35.6%, p<0.001, respectively. The subgroup analysis evaluated patients with the high-risk cytogenetics (45.2% of patients) and the ultra-high-risk cytogenetics (14.1% of patients). In patients with high-risk cytogenetics, MRD negativity resulted in 50.4% in Isa-VRd arm vs 37.4% VRd arm (95% CI, 1.04-2.79). In patients with ultra-high-risk cytogenetics, MRD negativity resulted in 56.3% vs 44.1% with Isa-VRd arm vs VRd arm, respectively (95% CI, 0.67-3.99). Neither PFS nor ORR were secondary endpoints in this study so were not reported. At least one grade 3 or higher AEs were reported in 63% of Isa-VRd group vs 61% of VRd group. Five treatment-related deaths were reported: 1 death in the Isa-VRd vs 4 deaths in the control group [30,31,32,33].
3.1.3. Carfilzomib-Containing Induction Therapy Trials
-
Myeloma XI+ Trial (Phase III) - KRdc vs. Rdc/Tdc:This study compared carfilzomib, lenalidomide, dexamethasone, and cyclophosphamide (KRdc) against a regimen of dexamethasone and cyclophosphamide with either lenalidomide or thalidomide (Rdc/Tdc) in 1056 patients. Patients underwent genetic profiling and were stratified into no hit (standard risk), single hit (high risk) or double hit (ultra high risk). The primary endpoints in the study were PFS and OS. After a median follow-up of 34.5 months, the KRdc group showed significantly improved PFS compared to those receiving the control regimens (Rdc/Tdc). Specifically, KRdc patients were more likely to achieve at least VGPR by the end of induction therapy, with an 82.3% response rate compared to 58.9% in the control group. Additionally, the study noted that a higher percentage of KRdc patients achieved MRD negativity. Stratifying patients by genetic profile, lenalidomide maintenance had the biggest effect on PFS in patients with single hit (HR 0.38; 95% CI, 0.25-0.58; p < .0001), followed by patients patients with no hit (HR, 0.46; 95% CI, 0.33-0.64; p < .0001) and those with double hit (HR, 0.55; 95% CI, 0.34 - 0.90; P = 0.17). The incidence of serious AEs was higher in the KRdc group (69.5%) compared to the control groups (55.3%) [34,35].
3.1.4. Elotuzumab-Containing Induction Therapy Trials
-
SWOG-1211 Trial (Phase II) - Elo-VRd vs. VRd:This study compared the incorporation of elotuzumab into the bortezomib, lenalidomide, and dexamethasone (Elo-VRd) induction regimen for 100 high-risk multiple myeloma patients followed by attenuated maintenance dose with the same regimen. The primary endpoint analyzed was PFS at the time of disease progression and found that the PFS of the Elo-VRd group was 65% versus 60% in the VRd group, this finding was not found to be significant. The median time to follow-up was 53 months. The ORR between the two groups were 83% and 88% for the Elo-VRd and VRd groups, respectively; this was also not significant (p=0.29). There were no significant differences in grade 3–5 infections between the Elo-VRd and VRd groups. However, there was 1 treatment-related death reported in the Elo-VRd group [36].
-
EVOLUTION (Phase II) - VdcR, VRd, Vdc and Vdc-mod:This multiphased trial examined the tolerability of bortezomib, cyclophosphamide, lenalidomide, and dexamethasone (VdcR) and to study the combination concurrently with VRd and Vdc.The primary objective was to determine the combined rate of CR plus VGPR for the VdcR, VRd, and Vdc regimens. Secondary objectives included safety and tolerability, ORR, time to response, time to progression (TTP), PFS, and OS. The 140 patients were enrolled, including 7 in the VdcR arm treated at MTD in phase 1. The median follow-up was 20 months (range: 0-30); 20, 20, 22 and 15 months, respectively, for the VdcR, VRd, Vdc, and Vdc-mod arms. Overall, 36%, 41%, and 23% of patients, respectively, had ISS stage I, II, or III disease. High-risk MM was present in 17% of patients as determined by cytogenetics.After 4 cycles, 80%, 73%, 63%, and 82% of patients in the VdcR, VRd, VdC, and VdC-mod arms had a confirmed response including VGPR or better in 33%, 32%, 13%, and 41%, respectively. Across all treatment cycles, the ORR was 88%, 85%, 75%, and 100% for the VdcR, VRd, Vdc, and Vdc-mod arms including VGPR or better in 58%, 51%, 41%, and 53%, respectively. The median time-to-best response was 105, 91, 118, and 85 days in the VdcR, VRd, Vdc, and Vdc-mod arms, respectively. At least one grade ≥ 3 AE was seen in ∼ 80% of patients in each arm. AEs leading to discontinuation were seen in 21%, 19%, 12%, and 6% in the VdcR, VRd, Vdc, and Vdc-mod arms, respectively [37].
3.2. Insights from Triple Therapy Clinical Trials
3.2.1. Summary of Individual Triple Therapy Trials
- PETHEMA/GEM2012 (Phase III) - VRd: This trial of 458 patients with NDMM underwent six-cycles of bortezomib, lenalidomide, and dexamethasone (VRd), followed by ASCT, then conditioned with busulfan and melphalan versus melphalan and then consolidation with 2 cycles of VRd. The primary endpoint was PFS with a median follow-up time of 22.4-months, the median PFS was not reached in either arm. The ORR was not analyzed in this study, but the VGPR was noted to be improved with subsequent cycles from 55.6% by cycle 3 to 70.4% after induction (cycle 6). The 34.8 % of total population had high risk cytogenetics. The 81.5% of high risk cytogenetic patients achieved partial response or better. The grade >=3 adverse events during the induction phase was 3.9% and 3.1% had >= 1 TEAE leading to discontinuation of induction, in addition, 2% of patients died during the induction phase [39].
-
ENDURANCE (Phase III) - VRd vs. KRd:In this study, 1087 patients with NDMM were randomized into two groups that both received induction with lenalidomide and dexamethasone and one group each receiving either bortezomib or carfilzomib (VRd vs KRd). The primary endpoint was PFS with a median follow-up of 9.4 months. The median PFS in the VRd group was 37 months and 28 months in the KRd group. The ORR was 84% of VRd and 87% of KRd patients (p<0.01). The 11% of patients were identified to have high risk cytogenetic features with MRD negativity reported 21% for the VRd cohort and 29% for the KRd cohort (p=0.30). Serious AE were recorded as 22% vs 45% in the KRd and VRd groups, respectively. In the VRd group there were <1% treatment-related deaths and in the KRd group there were 2% [40].
-
Reeder Trial (Phase II) - Vcd:This study of 63 patients evaluated bortezomib, cyclophosphamide, and dexamethasone (Vcd) in bortezomib with dexamethasone (Cohort 1 with standard dosing) vs high dose bortezomib with 2 cycles of high dose dexamethasone followed by 2 cycles of low dose dexamethasone (Cohort 2 with modified dosing). The primary endpoint was the response following completion of the 4 cycles which was 89% for all patients. The PFS at five years was 42%. And the median follow-up was 12.4 months. In cohort 1 the ORR was 96% vs 92% in cohort 2. The 38% of total patients were considered high risk. The high risk cohort in comparison to standard risk cohort demonstrated lower 5 year PFS (33% vs 48%) and lower OS (54% vs 81%). Grade 3 AEs were 48% in cohort 1 and 37% in cohort 2 while grade 4 AEs were 12% in cohort 1 and 3% in cohort 2 [41,42,43,44].
-
NCT02405364 (Phase II) - KRd:This study of 48 patients investigated a triple therapy regimen that consisted of carfilzomib, lenalidomide, and dexamethasone (KRd) in NDMM patients. The primary endpoint was sCR at the completion of consolidation which was reached by 61.9% of patients and was 56.5% (p=0.0172). The median follow-up was 60.5 months with a 5-year PFS of 45.1%. The 20% of patients were considered high risk with 78% of them achieved at least CR at completion of consolidation and undetectable MRD. Serious AEs were reported in 8.7% of patients, but no treatment-related deaths occurred. At least 1 TEAE was noted in 97.8% of patients [45].
-
FORTE Trial (Phase II) - KRd vs. KCd:This study enrolled 477 patients and was divided into three cohorts: KRd plus ASCT, KRd for 12 cycles, and KCd plus ASCT. The 356 patients who were eligible for maintenance treatment were subsequently randomized into two cohorts: KR vs. R monotherapy. The median follow-up from the first randomization was 50.9 months, and from the second randomization, it was 37.3 months. The primary endpoints were VGPR, which was achieved by 70% of patients receiving KRd compared to 53% of patients receiving KCd, and PFS. The 4-year PFS in patients with MRD negativity was 87% in the KRd-ASCT cohort, 92% in the KRd12 cohort, and 76% in the KCd-ASCT cohort. The 3-year PFS from the second randomization was 75% in the KR cohort compared to 65% in the R monotherapy cohort. One year MRD negativity between patients with zero HRCA and one HRCA was 35% vs 41% respectively. Risk of progression or death in patients with one HRCA vs zero HRCA was higher (HR 1.68; 95% CI, 1.01-2.80, p=0.048). TRAEs occurred in 11% of the KRd-ASCT group, 19% of the KRd12 group, and 11% of the KCd-ASCT group [46,47,48,49].
-
DETERMINATION Trial (Phase III) - VRd vs VRd+ASCT:This study consisted of 722 patients who were randomly assigned to receive 3 cycles of bortezomib, lenaliodmide, and dexamethasone (VRd), stem cell mobilization, and then 5 more VRd cycles (Arm A) or melphalan + ASCT and 2 VRd cycles (Arm B). Both arms received R maintenance until progression or intolerance. The primary endpoint was PFS. Patients were randomly assigned to Arms A and B, respectively; 14% and 13% had ISS stage III MM, and 18% each had high-risk cytogenetics [t(4;14), t(14;16), del17p]. In the respective arms, 291 and 290 pts received R maintenance for a median duration of 36 and 41 months. After median follow-up of 76 months, median PFS was 46.2 vs. 67.6 months in Arm A vs. B (HR 1.53; 95% CI, 1.23–1.91; p < .0001). With 90 vs. 88 patients having died in Arm A vs. B, a 4 years OS was 84% (95% CI, 80–88%) vs. 85% (95% CI, 81–88%); HR 1.10 (95% CI, 0.81–1.47; p = .274). Grade ≥ 3 related adverse events were less common in Arm A vs. B (78% vs. 94%; hematologic: 61% vs. 90%, p < .0001); 10% vs. 11% had secondary malignancies (ALL, 7 vs. 3 patients, p = .22; AML/MDS, 0 vs. 10 patients, p = .002) [50,51].
-
IFM 2009 Trial (Phase III) - VRd vs VRd+ASCT:This study randomly assigned 70 patients with MM to receive induction therapy with three cycles of bortezomib, lenalidomide, and dexamethasone (VRd) and then consolidation therapy with either five additional cycles of VRd (350 patients) or high-dose melphalan plus stem-cell transplantation followed by two additional cycles of VRd (350 patients). Patients in both groups received maintenance therapy with lenalidomide for 1 year. The primary end point was PFS, which was significantly longer in the group that underwent transplantation than in the group that received VRd alone (50 months vs. 36 months; adjusted HR for disease progression or death, 0.65; p<0.001). OS at 4 years did not differ significantly between the transplantation group and the VRd-alone group (81% and 82%, respectively). The rate of grade 3 or 4 neutropenia was significantly higher in the transplantation group than in the VRd-alone group (92% vs. 47%), as were the rates of grade 3 or 4 gastrointestinal disorders (28% vs. 7%) and infections (20% vs. 9%) [52].
-
SWOG SO777 Trial (Phase III) - VRd vs Rd:This trial randomly assigned 525 patients to receive either an initial treatment of bortezomib, lenalidomide, and dexamethasone (VRd group) or lenalidomide and dexamethasone alone (Rd group). The VRd regimen was given as eight 21-day cycles. The Rd regimen was given as six 28-day cycles. The primary endpoint was PFS. Median PFS was significantly improved in the VRd group (43 months vs 30 months in the Rd group; stratified HR 0.712, 96% CI, 0.56–0.906; one-sided p= 0.0018). Adverse events of grade 3 or higher were reported in 82% in the VRd group and 75% in the Rd group; 23% and 10% patients discontinued induction treatment because of adverse events, respectively [53].
-
GIMEMA-MMY-3006 Trial (Phase III) - VTd vs Td:This was a trial of 480 patients with a median follow-up of 124.1 months and showed a 10-year PFS of 34% (95%, Cl 28-41) for VTd compared with a 17% with Td (Cl 13-23); for the Td group hazard ratio 0.62 (95% Cl 0.50-0.77; p<0.0001). The 60% (95%, Cl 54-67) of patients in the VTd group were alive at 10 years vs 46% (Cl 40-54) of patients in the Td group (HR 0.68; 95%, Cl 0.51-0.90; p=0.0068). Incorporation of VTd into double autologous HSCT resulted in clinically meaningful improvements in long-term PFS and OS [54,55].
-
Carthadex Trial (Phase II) - KTd:The trial investigated 111 patients with dose escalation of carfilzomib in the KTd regimen and showed a median PFS of 58 months with a median follow-up of 58.7 months. The 8 cycles of KTd resulted in slightly higher percentages of CR and VGPR as compared to 4 KTd, with almost all patients achieving at least a PR. However, more cardiac events and premature discontinuation of treatment were observed. Moreover, response percentages after HDM/ASCT as well as after consolidation were comparable between the 2 groups and more importantly, also PFS and OS were not different [56].
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ongoing Clinical Trial | Treatment Regimen | Purpose of the Trial |
| EUCTR2018-002089-37-GR 2018 [61] | Dara-Vcd vs VTd | PFS at 36 months |
| EUCTR2018-002992-16-GR 2018 [62] | Dara-VRd vs VRd | PFS |
| EUCTR2019-004844-32-GR 2020(IsKia Trial) [63] | Isa-KRd vs KRd | MRD negativity |
| NCT03896737 2019 [64] | Dara-Vcd vs VTd | PFS |
References
- American Cancer Society; Cancer Statistics Center. (2024, March 31). Https://Cancerstatisticscenter.Cancer.Org/#!/.
- International Myeloma Working Group (IMWG) criteria for the diagnosis of multiple myeloma. (n.d.). International Myeloma Foundation. https://www.myeloma.org/international-myeloma-working-group-imwg-criteria-diagnosis-multiple-myeloma.
- 23rd Congress of the European Hematology Association Stockholm, Sweden, June 14-17, 2018. HemaSphere. 2018;2(S1):1-1113. [CrossRef]
- The 46th Annual Meeting of the European Society for Blood and Marrow Transplantation: Physicians Oral Session (O010-O173). Bone Marrow Transplantation. 2020;55(S1):22-174. [CrossRef]
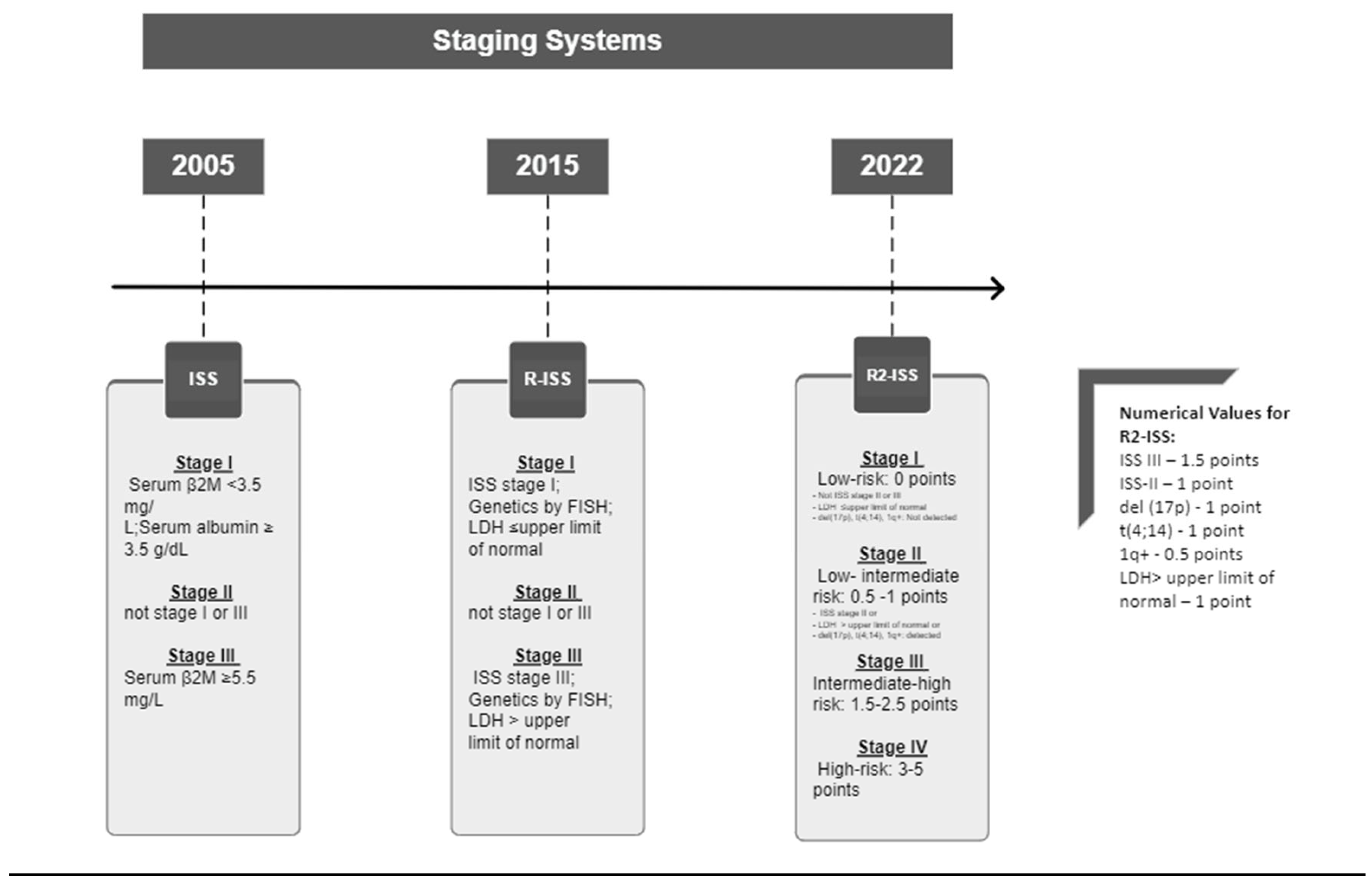
- Mattia D'Agostino et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. JCO 40, 3406-3418(2022). [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1445. Accessed [March 31, 2024].
- Rajkumar, S. V. (2016). Updated Diagnostic Criteria and Staging System for Multiple Myeloma. American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology. Annual Meeting, 35, e418-23. [CrossRef]
- Rajkumar, S. V. (2022). Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. American Journal of Hematology, 97(8), 1086–1107. [CrossRef]
- Rajkumar, S. V., Dimopoulos, M. A., Palumbo, A., Blade, J., Merlini, G., Mateos, M.-V., Kumar, S., Hillengass, J., Kastritis, E., Richardson, P., Landgren, O., Paiva, B., Dispenzieri, A., Weiss, B., LeLeu, X., Zweegman, S., Lonial, S., Rosinol, L., Zamagni, E., … Miguel, J. F. S. (2014). International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet. Oncology, 15(12), e538-48. [CrossRef]
- Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, Tuazon S, Gopal AK, Libby EN. Diagnosis and Management of Multiple Myeloma: A Review. JAMA. 2022 Feb 1;327(5):464-477. PMID: 35103762. [CrossRef]
- Boussi LS, Avigan ZM, Rosenblatt J. Immunotherapy for the treatment of multiple myeloma. Front Immunol. 2022 Oct 28;13:1027385. PMID: 36389674; PMCID: PMC9649817. [CrossRef]
- Kegyes D, Constantinescu C, Vrancken L, Rasche L, Gregoire C, Tigu B, Gulei D, Dima D, Tanase A, Einsele H, Ciurea S, Tomuleasa C, Caers J. Patient selection for CAR T or BiTE therapy in multiple myeloma: Which treatment for each patient? J Hematol Oncol. 2022 Jun 7;15(1):78. PMID: 35672793; PMCID: PMC9171942. [CrossRef]
- Rejeski K, Jain MD, Smith EL. Mechanisms of Resistance and Treatment of Relapse after CAR T-cell Therapy for Large B-cell Lymphoma and Multiple Myeloma. Transplant Cell Ther. 2023 Jul;29(7):418-428. Epub 2023 Apr 17. PMID: 37076102; PMCID: PMC10330792. [CrossRef]
- International Myeloma Foundation. Multiple Myeloma Drugs. International Myeloma Foundation. Available at: https://www.myeloma.org/multiple-myeloma-drugs. Accessed [March 27, 2024].
- Sonneveld, P., Dimopoulos, M. A., Boccadoro, M., Quach, H., Ho, P. J., Beksaç, M., Hulin, C., Antonioli, E., Leleu, X., Mangiacavalli, S., Perrot, A., Cavo, M., Belotti, A., Broijl, A., Gay, F., Mina, R., Nijhof, I. S., Van De Donk, N. W., Katodritou, E., . . . Moreau, P. (2023). Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. The New England Journal of Medicine. [CrossRef]
- Sonneveld, P., Dimopoulos, M. A., Boccadoro, M., Quach, H., Ho, P. J., Beksaç, M., Hulin, C., Antonioli, E., Leleu, X., Mangiacavalli, S., Perrot, A., Cavo, M., Belotti, A., Broijl, A., Gay, F., Mina, R., Nijhof, I. S., Van De Donk, N. W., Katodritou, E., . . . Moreau, P. (2023). Phase 3 Randomized Study of Daratumumab (DARA) + Bortezomib, Lenalidomide, and Dexamethasone (VRd) Versus Vrd Alone in Patients (Pts) with Newly Diagnosed Multiple Myeloma (NDMM) Who Are Eligible for Autologous Stem Cell Transplantation (ASCT): Primary Results of the Perseus Trial. Blood, 142(Supplement 2), LBA-1. [CrossRef]
- Sborov, D. W., Baljevic, M., Reeves, B., Laubach, J., Efebera, Y. A., Rodriguez, C., Costa, L. J., Chari, A., Silbermann, R., Holstein, S. A., Anderson Jr., L. D., Kaufman, J. L., Shah, N., Pei, H., Patel, S., Cortoos, A., Bartlett, J. B., Vermeulen, J., Lin, T. S., … Richardson, P. G. (2022). Daratumumab plus lenalidomide, bortezomib and dexamethasone in newly diagnosed multiple myeloma: Analysis of vascular thrombotic events in the GRIFFIN study. Br J Haematol, 199(3), 355–365. [CrossRef]
- Voorhees, P. M., Kaufman, J. L., Laubach, J. P., Sborov, D. W., Reeves, B., Rodriguez, C., Chari, A., Silbermann, R., Costa, L. J., Anderson, L. D., Nathwani, N., Shah, N., Efebera, Y. A., Holstein, S. A., Costello, C., Jakubowiak, A., Wildes, T., Orlowski, R. Z., Shain, K. H., … Richardson, P. G. (2020). Daratumumab, Lenalidomide, Bortezomib, & Dexamethasone for Transplant-eligible Newly Diagnosed Multiple Myeloma: GRIFFIN. Blood, (Voorhees) Levine Cancer Institute, Atrium Health, Charlotte, North Carolina, United States(Kaufman) Emory University Winship Cancer Institute, Atlanta, GA, United States(Laubach) Dana Farber Cancer Institute, Boston, MA, United States(Sborov) University. [CrossRef]
- Voorhees, P. M., Kaufman, J. L., Laubach, J., Sborov, D. W., Reeves, B., Rodriguez, C., Chari, A., Silbermann, R., Costa, L. J., Anderson, L. D., Nathwani, N., Shah, N., Efebera, Y. A., Costello, C., Jakubowiak, A., Wildes, T. M., Orlowski, R. Z., Shain, K. H., Cowan, A. J., … Richardson, P. G. (2020). Depth of response to daratumumab, lenalidomide, bortezomib, and dexamethasone improves over time in patients with transplant-eligible newly diagnosed multiple myeloma: Griffin study update. Bone Marrow Transplantation, 55((Voorhees) Levine Cancer Institute, Atrium Health, Charlotte, NC, United States(Kaufman) Emory University, Atlanta, GA, United States(Laubach, Richardson) Dana-Farber Cancer Institute, Boston, MA, United States(Sborov) University of Utah, Salt Lake City,), 127–128. [CrossRef]
- Voorhees, P. M., Rodriguez, C., Reeves, B., Nathwani, N., Costa, L. J., Lutska, Y., Bobba, P., Hoehn, D., Pei, H., Ukropec, J., Qi, M., Lin, T. S., & Richardson, P. G. (2021). Daratumumab plus RVd for newly diagnosed multiple myeloma: final analysis of the safety run-in cohort of GRIFFIN. Blood Advances, 5(4), 1092–1096. [CrossRef]
- Chari, A., Kaufman, J.L., Laubach, J. et al. Daratumumab in transplant-eligible patients with newly diagnosed multiple myeloma: final analysis of clinically relevant subgroups in GRIFFIN. Blood Cancer J. 14, 107 (2024). [CrossRef]
- Touzeau, C., Moreau, P., Perrot, A., Hulin, C., Dib, M., Tiab, M., Caillot, D., Facon, T., Leleu, X., N.W.C.J., V. D. D., Broijl, A., Zweegman, S., M.-D., L., Delforge, M., Pei, L., Vanquickelberghe, V., C, D. B., Kampfenkel, T., Vermeulen, J., & Sonneveld, P. (2020). Daratumumab + bortezomib, thalidomide, and dexamethasone (D-VTd) in transplant-eligible newly diagnosed multiple myeloma (TE NDMM): Baseline SLiM-CRAB based subgroup analysis of CASSIOPEIA. Journal of Clinical Oncology, 38(15). [CrossRef]
- Moreau, P., & Sonneveld, P. (2021). Daratumumab (DARA) maintenance or observation (OBS) after treatment with bortezomib, thalidomide and dexamethasone (VTd) with or without DARA and autologous stem cell transplant (ASCT) in patients (pts) with newly diagnosed multiple myeloma (NDMM): CASSIOPEIA Part 2. Journal of Clinical Oncology, 39(15_suppl), 8004. [CrossRef]
- Sonneveld P, Attal M, Perrot A, et al. Daratumumab plus bortezomib, thalidomide, and dexamethasone (D-VTD) in transplant-eligible Newly Diagnosed Multiple myeloma (NDMM): Subgroup Analysis of High-risk Patients (PTS) in CASSIOPEIA. Clinical Lymphoma Myeloma & Leukemia. 2019;19(10):e2-e3. [CrossRef]
- Costa, L. J., Chhabra, S., Medvedova, E., Dholaria, B. R., Schmidt, T. M., Godby, K. N., Silbermann, R., Dhakal, B., Bal, S., Giri, S., D’Souza, A., Hall, A., Hardwick, P., Omel, J., Cornell, R. F., Hari, P., & Callander, N. S. (2022). Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone With Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J Clin Oncol, 40(25), 2901–2912. [CrossRef]
- Luciano J. Costa et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone With Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. JCO 40, 2901-2912(2022). [CrossRef]
- Rifkin, R., Melear, J., Faber, E., Bensinger, W., Burke, J., Narang, M., Stevens, D., Gray, K., Lutska, Y., Bobba, P., Qi, K., Qi, M., Lin, T., & Yimer, H. (2021). Daratumumab plus cyclophosphamide, bortezomib, and dexamethasone induction therapy in multiple myeloma followed by daratumumab maintenance: End-of-study results from lyra. HemaSphere, 5(SUPPL 2), 453–454. [CrossRef]
- Yimer, H., Melear, J., Faber, E., Bensinger, W. I., Burke, J. M., Narang, M., Stevens, D., Gunawardena, S., Lutska, Y., Qi, K., Ukropec, J., Qi, M., Lin, T. S., & Rifkin, R. M. (2019). Daratumumab, bortezomib, cyclophosphamide and dexamethasone in newly diagnosed and relapsed multiple myeloma: LYRA study. Br J Haematol, 185(3), 492–502. [CrossRef]
- Habte Yimer, Jason Melear, Edward Faber, William I. Bensinger, John M. Burke, Mohit Narang, Don Stevens, Kathleen S. Gray, Yana Lutska, Padma Bobba, Keqin Qi, Daniela Hoehn, Ming Qi, Thomas S. Lin & Robert M. Rifkin (2022) Daratumumab, cyclophosphamide, bortezomib, and dexamethasone for multiple myeloma: final results of the LYRA study, Leukemia & Lymphoma, 63:10, 2383-2392, . [CrossRef]
- Goldschmidt, H., Mai, E. K., Bertsch, U., Fenk, R., Nievergall, E., Tichy, D., Besemer, B., Dürig, J., Schroers, R., von Metzler, I., & et al. (2022). Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. The Lancet. Haematology, 9(11), e810-e821-. [CrossRef]
- Subgroup Analysis of Phase 3 Trial GMMG-HD7 Evaluating Isa-RVd in Patients with High-Risk Cytogenetics. Oncology Practice Management. https://oncpracticemanagement.com/web-exclusive-articles/2883:subgroup-analysis-of-phase-3-trial-gmmg-hd7-evaluating-isa-rvd-in-patients-with-high-risk-cytogenetics.
- Goldschmidt H, Mai EK, Nievergall E, et al. Addition of isatuximab to lenalidomide, bortezomib and dexamethasone as induction therapy for newly-diagnosed, transplant-eligible multiple myeloma patients: the phase III GMMG-HD7 trial. Blood. 2021;138(suppl 1):463.
- Mai EK, Bertsch U, Fenk R, et al. Isatuximab, lenalidomide, bortezomib and dexamethasone as induction therapy for newly-diagnosed multiple myeloma patients with high-risk cytogenetics: a subgroup analysis from the GMMG-HD7 trial. Presented at: European Hematology Association Congress; June 9-17, 2022; Vienna, Austria.
- Jackson, G. H., Pawlyn, C., Cairns, D. A., M, de T. R., Hockaday, A., Collett, C., Jones, J. R., Kishore, B., Garg, M., Williams, C. D., Karunanithi, K., Lindsay, J., Rocci, A., Snowden, J. A., Jenner, M. W., Cook, G., Russell, N. H., Drayson, M. T., Gregory, W. M., … Morgan, G. J. (2021). Carfilzomib, lenalidomide, dexamethasone, and cyclophosphamide (KRdc) as induction therapy for transplant-eligible, newly diagnosed multiple myeloma patients (Myeloma XI+): Interim analysis of an open-label randomised controlled trial. PLoS Medicine, 18(1), e1003454-. [CrossRef]
- Aikaterini Panopoulou, David A. Cairns, Amy Holroyd, Isabel Nichols, Nikita Cray, Charlotte Pawlyn, Gordon Cook, Mark Drayson, Kevin Boyd, Faith E. Davies, Matthew Jenner, Gareth J. Morgan, Roger Owen, Richard Houlston, Graham Jackson, Martin F. Kaiser; Optimizing the value of lenalidomide maintenance by extended genetic profiling: an analysis of 556 patients in the Myeloma XI trial. Blood 2023; 141 (14): 1666–1674. [CrossRef]
- Usmani, S. Z., Hoering, A., Ailawadhi, S., Sexton, R., Lipe, B., Hita, S. F., Valent, J., Rosenzweig, M., Zonder, J. A., Dhodapkar, M., Callander, N., Zimmerman, T., Voorhees, P. M., Durie, B., Rajkumar, S. V., Richardson, P. G., Orlowski, R. Z., & SWOG1211 Trial Investigators. (2021). Bortezomib, lenalidomide, and dexamethasone with or without elotuzumab in patients with untreated, high-risk multiple myeloma (SWOG-1211): primary analysis of a randomised, phase 2 trial. The Lancet. Haematology, 8(1), e45–e54. [CrossRef]
- 55 Shaji Kumar, Ian Flinn, Paul G. Richardson, Parameswaran Hari, Natalie Callander, Stephen J. Noga, A. Keith Stewart, Francesco Turturro, Robert Rifkin, Jeffrey Wolf, Jose Estevam, George Mulligan, Hongliang Shi, Iain J. Webb, S. Vincent Rajkumar; Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012; 119 (19): 4375–4382. [CrossRef]
- Okazuka, K., Ishida, T., Nashimoto, J., Uto, Y., Sato, K., Miyazaki, K., Ogura, M., Yoshiki, Y., Abe, Y., Tsukada, N., & Suzuki, K. (2020). The efficacy and safety of modified bortezomib-lenalidomide-dexamethasone in transplant-eligible patients with newly diagnosed multiple myeloma. European Journal of Haematology, 104(2), 110–115. [CrossRef]
- Rosiñol L, Oriol A, Ríos R, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134(16):1337-1345. [CrossRef]
- Kumar, S., Jacobus, S., Cohen, A. D., Weiss, M., Callander, N. S., Singh, A. K., Parker, T. L., Menter, A., Yang, X., Parsons, B. M., Kumar, P., Kapoor, P., Rosenberg, A. S., Zonder, J. A., Faber, E. A., Lonial, S., Anderson, K. C., Richardson, P. G., Orłowski, R. Z., . . . Rajkumar, S. V. (2020). Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. The Lancet Oncology, 21(10), 1317–1330. [CrossRef]
- Reeder, C. B., Reece, D. E., Kukreti, V., Mikhael, J., Chen, C. I., Trudel, S., Laumann, K., Hentz, J., Piza, G., Fonseca, R., Bergsagel, P. L., Leis, J. F., Tiedemann, R. E., Spong, J., Mayo, A., & Stewart, K. (2013). Long-term survival with cybord induction therapy in newly diagnosed multiple myeloma. Blood, 122(21). http://bloodjournal.hematologylibrary.org/content/122/21/3192.abstract?sid=e4ac49ee-db15-44bc-92f1-7212f0461b12.
- Reeder, C. B., Reece, D. E., Kukreti, V., Mikhael, J. R., Chen, C., Trudel, S., Laumann, K., Hentz, J., Pirooz, N., Piza, J., Zepeda, V. J. J., Fonseca, R., Bergsagel, P. L., Leis, J., Tiedemann, R. E., & Stewart, A. K. (2009). A phase II trial comparison of once versus twice weekly bortezomib in CYBORD chemotherapy for newly diagnosed myeloma: identical high response rates and less toxicity. Blood, 114(22), 616.
- Craig B. Reeder, Donna E. Reece, Vishal Kukreti, Joseph Mikhael, Christine I. Chen, Suzanne Trudel, Kristina Laumann, Joseph Hentz, Giovani Piza, Rafael Fonseca, P. Leif Bergsagel, Jose F Leis, Rodger E. Tiedemann, FRCPA, Jacy Spong, Angela Mayo, Keith Stewart; Long-Term Survival With Cybord Induction Therapy In Newly Diagnosed Multiple Myeloma. Blood 2013; 122 (21): 3192. [CrossRef]
- Muranushi H, Kanda J, Kobayashi M, et al. Bortezomib-cyclophosphamide-dexamethasone induction/consolidation and bortezomib maintenance for transplant-eligible newly diagnosed multiple myeloma: phase 2 multicenter trial. Hematology (Luxembourg Print). 2022;27(1):239-248. [CrossRef]
- Roussel M, Lauwers-Cancès V, Wuillème S, et al. Up-front carfilzomib, lenalidomide, and dexamethasone with transplant for patients with multiple myeloma: the IFM KRd final results. Blood. 2021;138(2):113-121. [CrossRef]
- Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomized, open-label, phase 2 trial. Lancet Oncol. 2021;22(12):1705-1720. [CrossRef]
- Mina, R., Musto, P., Rota-Scalabrini, D., Paris, L., Gamberi, B., Palmas, A., Aquino, S., Paolo de Fabritiis, Giuliani, N., Luca De Rosa, Alessandro Gozzetti, Cellini, C., Luca Bertamini, Capra, A., Oddolo, D., Iolanda Donatella Vincelli, Ronconi, S., Pavone, V., Pescosta, N., & Cea, M. (2023). Carfilzomib induction, consolidation, and maintenance with or without autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma: pre-planned cytogenetic subgroup analysis of the randomised, phase 2 FORTE trial. Lancet Oncology/Lancet. Oncology, 24(1), 64–76. [CrossRef]
- ClinicalTrials.gov. (n.d.). Clinicaltrials.gov. Retrieved July 23, 2024, from https://clinicaltrials.gov/study/NCT01816971.
- Mina R, Musto P, Rota-Scalabrini D, et al. Carfilzomib induction, consolidation and maintenance with or without autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma: pre-planned cytogenetic subgroup analysis of the randomised phase 2 FORTE trial. Lancet Oncol. 2023 Jan;24(1):64-76. Epub 2022 Dec 14. Erratum in: Lancet Oncol. 2023 Feb;24(2):e72. PMID: 36528035. [CrossRef]
- Richardson PG, Jacobus SJ, Weller E, et al. Lenalidomide, bortezomib, and dexamethasone (RVd) ± autologous stem cell transplantation (ASCT) and R maintenance to progression for newly diagnosed multiple myeloma (NDMM): The phase 3 DETERMINATION trial. Journal of Clinical Oncology. 2022;40(17_suppl):LBA4. [CrossRef]
- Richardson PG, Jacobus SJ, Weller EA, et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. New England Journal of Medicine/the New England Journal of Medicine. 2022;387(2):132-147. [CrossRef]
- Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. New England Journal of Medicine/the New England Journal of Medicine. 2017;376(14):1311-1320. [CrossRef]
- Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. [CrossRef]
- Tacchetti, P., Pantani, L., Patriarca, F., Petrucci, M. T., Zamagni, E., Dozza, L., Galli, M., Di Raimondo, F., Crippa, C., Boccadoro, M., Barbato, S., Tosi, P., Narni, F., Montefusco, V., Testoni, N., Spadano, A., Terragna, C., Pescosta, N., Marzocchi, G., … Musolino, C. (2020). Bortezomib, thalidomide, and dexamethasone followed by double autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GIMEMA-MMY-3006): long-term follow-up analysis of a randomised phase 3, open-label study. The Lancet Haematology, 7(12), e861–e873. [CrossRef]
- Tacchetti, P., Patriarca, F., Petrucci, M. T., Galli, M., Pantani, L., Dozza, L., Di Raimondo, F., Boccadoro, M., Offidani, M., Montefusco, V., Pescosta, N., Ledda, A., Caravita, T., Cascavilla, N., Gamberi, B., Stefani, P. M., Terragna, C., Marzocchi, G., Ferrara, F., … Cavo, M. (2018). A triplet bortezomib-and immunomodulator-based therapy before and after double ASCT improves overall survival of newly diagnosed mm patients: final analysis of phase 3 gimema-MMY-3006 study. HemaSphere, 2, 4. [CrossRef]
- Wester, R., B, V. D. H., Asselbergs, E., Zweegman, S., Kersten, M. J., Vellenga, E., M, V. M. K., O, D. W., Minnema, M., Lonergan, S., Palumbo, A., Lokhorst, H., Broijl, A., & Sonneveld, P. (2019). Phase II study of carfilzomib, thalidomide, and low-dose dexamethasone as induction and consolidation in newly diagnosed, transplant eligible patients with multiple myeloma; the Carthadex trial. Haematologica, 104(11), 2265–2273. [CrossRef]
- April 12, 2024 Meeting of the Oncologic Drugs Advisory Committee Meeting Announcement - 04/12/2024. (2024, July 16). FDA. https://www.fda.gov/advisory-committees/advisory-committee-calendar/april-12-2024-meeting-oncologic-drugs-advisory-committee-meeting-announcement-04122024.
- https://www.cancer.gov/research/participate/clinical-trials-search/v?loc=0&tid=S1803&rl=2&id=NCI-2018-02465&pn=1&ni=10. (2016, June 23). Www.cancer.gov. https://www.cancer.gov/research/participate/clinical-trials-search/v?loc=0&tid=S1803&rl=2&id=NCI-2018-02465&pn=1&ni=10.
- Manier, S., Ingegnere, T., Escure, G., Prodhomme, C., Nudel, M., Mitra, S., & Facon, T. (2022). Current state and next-generation CAR-T cells in multiple myeloma. Blood Reviews, 54, 100929. [CrossRef]
- Yakoub-Agha, I., & Einsele, H. (2022). Multiple Myeloma.
- Euctr, G. R. (2018a). a multicenter, open label, randomized phase ii study comparing daratumumab combined with bortezomib-cyclophosphamide-dexamethasone (dara-vcd) versus the association of bortezomib-thalidomide-dexamethasone (vtd) as pre transplant induction and post transplant consolidation, both followed by a maintenance phase with ixazomib alone or in combination with daratumumab, in newly diagnosed multiple myeloma (mm) young patients eligible for autologous stem cell transplantation. Https://Trialsearch.Who.Int/Trial2.Aspx?TrialID=EUCTR2018-002089-37-GR.
- Euctr, G. R. (2018b). A Study of combination of Daratumumab, VELCADE (bortezomib), Lenalidomide, and Dexamethasone (D-VRd) compared to VELCADE, Lenalidomide, and Dexamethasone (VRd) in participants with Previously Untreated Multiple Myeloma. Https://Trialsearch.Who.Int/Trial2.Aspx?TrialID=EUCTR2018-002992-16-GR. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01948076/full.
- Euctr, G. R. (2020). A Clinical trial to compare the combination of Isatuximab-Carfilzomib-Lenalidomide-Dexamethasone versus the combination of Carfilzomib-Lenalidomide-Dexamethasone in newly diagnosed multiple myeloma patients eligible for autologous stem cell transplantation (IsKia TRIAL). Https://Trialsearch.Who.Int/Trial2.Aspx?TrialID=EUCTR2019-004844-32-GR. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02169552/full.
- Daratumumab-bortezomib-dexamethasone (Dara-VCd) vs Bortezomib-Thalidomide-Dexamethasone (VTd) Then Maintenance With Ixazomib (IXA) or IXA-Dara - Full Text View - ClinicalTrials.gov. https://classic.clinicaltrials.gov/ct2/show/NCT03896737.
- Olga Lytvynova, Jenna Jwayyed, Maha Hameed, Ali Baloch, Rohan Prasad, Ahmed Salman, Abdul Rafae, Debduti Mukhopadhyay, Vasanthan Kumarasamy, Faiz Anwer; Evidence-Based Recommendations for Induction Treatment of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients; A Scoping Review. Blood 2023; 142 (Supplement 1): 6590. [CrossRef]
| Clinical Trail | Number of Paticipants & Treatkment Regimen | Efficacy | Median Follow Up (months) |
| Perseus, Phase III | N=709 | 48 months PFS: 84.3% vs 67.7% | 47.5 |
| Dara-VRd vs VRd | CR: 87.9% vs 70.1% MRD negative 75.5% vs 47.5% |
||
| Griffin, Phase II | N=207 | 48 months PFS: 87.2% vs 70% | 49.6 |
| Dara-VRd vs VRd | 48 months CR: 83% vs 60% 22.1 months sCR: 63% vs 45.4% MRD negative: 64% vs 30% |
||
| Cassiopeia, Phase III | N=1085 | Median PFS: NR vs 46.7 months | 35.4 |
| Dara-VTd vs VTd | CR: 38.9% vs 26% sCR: 28.9% vs 20.3% |
||
| VGPR: 83.4% vs 78% | |||
| MRD negative: 63.7% vs 43.5% | |||
| Master, Phase II | N=123 Dara-KRd |
2 years PFS: None HRCA: 91% 1HRCA: 97% 2+HRCA:58% Overall: 87% MRD negative: None HRCA: 78% 1HRCA: 82% 2+HRCA:79% Overall: 80% |
25.1 |
| Lyra, Phase II | N=87 Dara-Vcd |
Median PFS: NR 36 months PFS: 69.3% in TE NDMM vs 72.6% TI NDMM 1 year PFS 87% CR: 49% vs 30% sCR: 23.1% vs 17% VGPR: 82% vs 70% |
35.7 |
| Clinical Trial | Treatment Regimen | Most Common Adverse Events: Grade 3/4 |
| Perseus, Phase III | Dara-VRd vs VRd | Neutropenia 62.1% vs 51 Thrombocytopenia 29.1% vs 17.3% Diarrhea 10.5% vs7.8% |
| Griffin, Phase II | Dara-VRd vs VRd | Neutropenia 37.0% vs 29.6% Lymphopenia 25.9% vs 11.1% |
| Cassiopeia, Phase III | Dara-VTd vs VTd | Neutropenia 27.6% vs 14.7% Lymphopenia 17% vs 9.7% Stomatitis 12.7% vs 16.4% Thrombocytopenia 11% vs 7.4% |
| Master, Phase II | Dara-KRd | Pneumonia 6% VTE 3% |
| Lyra, Phase II | Dara-Vcd | Neutropenia 12.8% Fatigue 7% Leukopenia 5.8% Diarrhea 4.7% Pneumonia 3.5% |
| Clinical Trail | Number of Paticipants & Treatkment Regimen | Efficacy | Median Follow Up (months) |
| GMMG-HD7, Phase III | N=660 | CR: 24% vs 22% | 4.1 |
|
Isa-VRd vs VRd |
nCR: 41% vs 37% VGPR: 77% vs 61% PR or better: 90% vs 84% MRD negative: 50% vs 36% |
||
| Myeloma XI+ , Phase III | N=1056 KRdc vs Rdc/Tdc |
At 100 days post ASCT: CR: 31% vs 24% nCR: 38.6% vs 31.7% VGPR: 22.3% vs 23.7% MRD negative: 75.2% vs 50%Median PFS: NR vs 36.2 months 3 years PFS: 64.5% vs 50.3% |
34.5 |
| SWOG-1211, Phase II | N=100 Elo-VRd vs VRd |
CR: 2.1% vs 6% VGPR: 21.3% vs 20% PR: 59.6% vs 62% uPR: 4.3% vs 6% SD: 12.8% vs 6% ORR: 83% vs 88% Median PFS: 31.47 vs 33.64 months |
53 |
| Evolution, Phase II | N= 140 VdcR, VRd, Vdc and Vdc-mod |
CR: 25% vs 10% vs 22% vs 47% sCR: 15% vs 17% vs 9% vs 29% VGPR: 58% vs 51% vs 41% vs 53% ORR: 88% vs 85% vs 75% 100% |
20 |
| Clinical Trial | Treatment Regimen | Most Common Adverse Events: Grade 3/4 |
| GMMG-HD7, Phase III | Isa-VRd vs VRd | Blood and Lymphatic system disorder 26% vs 17% Neutropenia 23% vs 7% Lymphopenia 15% vs 20% Infection 12% vs 10% |
| Myeloma XI+, Phase III | KRdc vs Rdc/Tdc | Neutropenia 16.4% vs 22.2%/12.4% Infections 16.1% vs 11.5%/11.7% Anemia 10.2% vs 5.8%/4.7% |
| SWOG-1211, Phase II | Elo-VRd vs VRd | Nervous system disorder 21% vs 12% Blood and Lymphatic 15% vs 17% Infections 17% vs 8% Vascular disorders 13% vs 16% |
| Evolution, Phase II | VdcR, VRd, Vdc and Vdc-mod | Neutropenia 44% vs 10% vs 30% vs 24% Neuropathy 13% vs 17% vs 9% vs 18% Fatigue 17% vs 7% vs 3% vs 0% |
| Clinical Trail | Number of Paticipants & Treatkment Regimen | Efficacy | Median Follow Up (months) |
| PETHEMA/ GEM2012 | N=458 VTd |
PFS: NR CR: Post induction: 33.4% Post ASCT: 44.1% Post consolidation: 50% MRD positive: Post induction: 57.6% Post-ASCT: 36.5% Post consolidation: 34.3% |
24.2 |
| Endurance | N=1087 | PFS Median: 34.4 vs 34.6 months | 9.4 |
| VRd vs KRd | ORR: 84% vs 87% VGPR 65% vs 74% PR 84% vs 87% CR 15% vs 18% MRD Negative 7% vs 10% |
||
| Reeder Trial | N=63 Vcd vs modified Vcd |
Overall PFS: 42% ORR: 88% vs 93% VGPR: 61% vs 60% CR/nCR: 39% vs 40% |
12.4 |
| VRd Lite [38] | N=48 Modified VRd |
ORR: 83% CR: 25% After ASCT: OR: 100% VGPR: 74% |
NA |
| NCT02405364 | N=46 KRd |
Median PFS: 56.4 months sCR: 56.5% VGPR: 92.9% ORR: 97.7% CR: Post induction: 23% Post-ASCT: 41.5% Post-consolidation: 64.5% |
60.5 |
| Gimema-MMY-3006 | N=480 VTd vs Td |
Median PFS: 60 vs 41 months 10 years PFS: 34% vs 17% CR: 27% vs 15% OS at 10 years: 60% vs 46% |
124.1 |
| Carthadex | N=111 KTd |
Median PFS: 58 months ORR: 90% VGPR: 68% Median OS: 83 months 5-year OS: 76% CR: Post induction: 18% Post Melphalan: 31% Post consolidation: 63% |
58.7 |
| FORTE | N=477 KRd vs Kcd |
sCR: 46% vs 32% MRD negativity: 62% vs 43% 4-year PFS : 69% vs 51% 4-year OS: 86% vs 76% |
50.9 |
| Determination | N=722 RVd vs RVd+ASCT |
Median PFS: 46.2 vs 67.6 months CR: 52 vs. 62% VGPR: 79% vs 83% PR: 94% vs 96% MRD negativity: 37.3% vs 52.1% 4 years OS: 84% vs 85% |
76 |
| IFM 2009 | N=70 RVd vs RVd+ASCT |
CR: 48% vs 59% VGPR: 29% vs 29% PR: 20% vs 11% MRD: 65% vs 79% |
44 |
| SWOG S0777 | N=525 VRd vs Rd |
Median PFS: 43 vs 30 months OR: 82% vs 72% VGPR: 27.8% vs 23.4% CR: 16% vs 8% Median OS: 75 vs 64 months |
55 |
| Clinical Trial | Treatment Regimen | Most Common Adverse Events: Grade 3/4 |
| PETHEMA/ GEM2012 | VRd | Neutropenia 12.9% Infection 9.2% Thrombocytopenia 6.3% |
| Endurance | VRd vs KRd | Peripheral neuropathy 8% vs <1% Fatigue 6% vs 6% Hyperglycemia 4% vs 6% Diarrhea 5% vs 3% |
| VRd Lite | Modified VRd | Neutropenia 19%, Lymphocytopenia 8% Infection 6% |
| NCT02405364 | KRd | Lymphopenia 65.2% Neutropenia 34.8% Thrombocytopenia 19.6% |
| Gimema-MMY-3006 | VTd vs Td | NA |
| Carthadex | KTd | Hematologic toxicity 10% Infection 11% Dermatologic 9% Vascular disorders 9% Respiratory disorder 8% |
| FORTE | KRd vs Kcd | Neutropenia: 13% vs 11% Dermatological toxicity: 6% vs 1% Hepatic toxicity: 8% vs 0% |
| Determination | RVd+ASCT vs RVd | Any hematologic event: 60.5% vs 89.9% Gastrointestinal disorder: 7.8% vs 18.6% Infections: 9.5% vs 18.4% |
| IFM 2009 | RVd vs RVd+ASCT | Any hematologic event: 63.7% vs 94.9% Infections: 8.9% vs 20.3% Gastrointestinal disorder: 6.9% vs 27.7% |
| SWOG S0777 | VRd vs Rd | Anemia: 13% vs 16% Fatigue: 16% vs 14% Neuropathy: 23% vs 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





