1. Introduction
Vascular calcifications (VCs) are common complication of CKD mineral and bone disorders (CKD-MBD). In CKD patients, the prevalence of aortic calcification is higher than in the general population and VCs were found to be associated with a higher prevalence of bone fractures (BFs) [
1]. The pathogenesis of VC in CKD patients is multifactorial, with calcium and phosphate overload and secondary hyperparathyroidism (HPT) among the most relevant etiological factors [
2]. Calcification inhibitors are also important determinants of VCs [
3]. A vitamin K-dependent protein (VKDP), such as Matrix Gla protein (MGP) is characterized not only by 5 sites of carboxylation (c-MGP) but also by 3 serine residues (p-c MGP, through the enzyme casein kinase) [
4,
5]. These last seem to regulate protein secretion into the extracellular environment [
5]. The main function of MGP is associated with the carboxylate form, which makes it a strong inhibitor of vascular calcifications probably due to its interaction with Bone Morphogenetic Protein 2 (BMP-2: a powerful osteoinductive protein), which transforms vascular smooth muscle cells (VSMC) into osteoblasts-like cells [
6]. c-MGP binds calcification crystals in blood vessels forming vesicles and apoptotic bodies preventing both calcium phosphate precipitation and the trans-differentiation of VSMC into an osteogenic phenotype [
5]. Another remarkable VKDP is Bone Gla Protein (BGP) or Osteoalcin (Oc) which is mainly secreted by osteoblasts and in small quantities even by chondrocytes [
7,
8]. It has three carboxylated sites making it active in its main bone mineralization action allowing the interaction between the calcium-binding Gla residues and the calcium ions of hydroxyapatite forming hydroxyapatite crystals. Indeed, Oc also acts as an inhibitor of bone mineralization regulating this way the mineral maturation [
8].
The current paradigm for reducing the risk of vascular calcification (VC) development and progression revolves around the reduction of parathyroid hormone (PTH), calcium, and phosphorus. In particular, the recent Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend targeting PTH levels to a range between 2-9 times the upper normal limit [
2]. In this scenario, Etelcalcetide emerges as a novel synthetic peptide that activates the calcium-sensing receptor and reduces PTH, calcium, and phosphorus levels in clinical trials [
9].
The main aim of the study will be to assess whether Etelcalcetide, compared to Vitamin D or Vitamin D analog treatments, increases the levels of Vitamin K-Dependent Proteins (VKDPs) such as Bone Gla-Protein (BGP) and Matrix Gla-Protein (MGP) at 3, 9, and 18 months from baseline, resulting in proper bone mineralization and inhibition of vascular calcification. The secondary aims include evaluating, at 18 months, whether treatment with Etelcalcetide decreases the progression of vascular calcifications (in the aorta and iliac arteries) and vertebral fractures.
2. Methods
2.1. Study Design
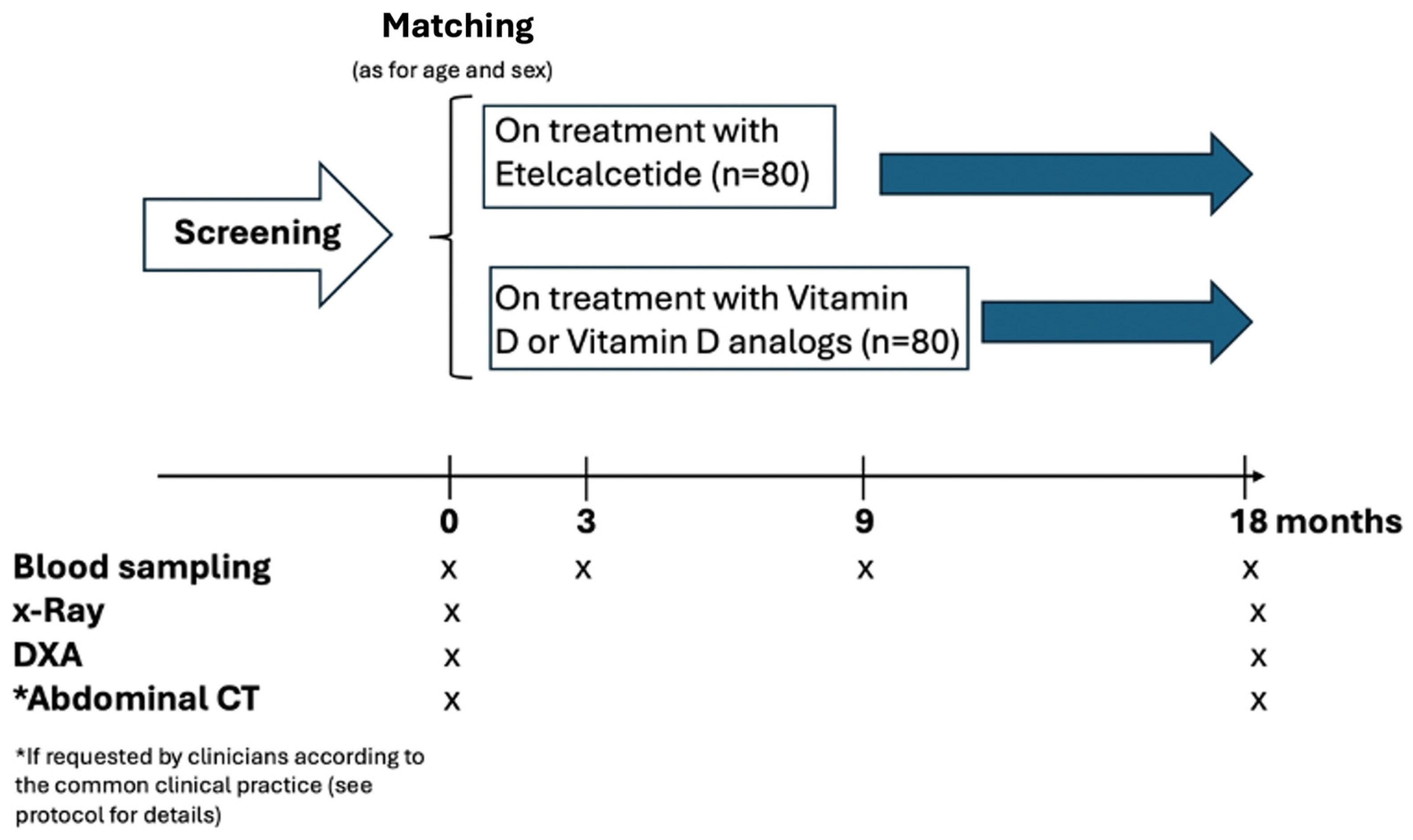
This is a national, multi-center, comparative effectiveness, observational, longitudinal study with no predefined interference on drug dosing by the investigators. Nineteen Italian centers will be involved. In each center, 14 patients will be screened for eligibility and about 10-12 patients are scheduled to be enrolled. The study will enroll 160 hemodialysis patients (80 patients treated with Etelcalcetide and 80 age and sex matched patients treated with Calcitriol or vitamin D analogs) (see
Table 1).
Given the observational nature of the study, the investigators will not recommend target goals of Etelcalcetide treatment. The treating nephrologists will tailor their target dose of Etelcalcetide on individual-level patient basis in order to achieve the KDIGO PTH target levels. In the Etelcalcetide-treated group, the addition of calcitriol is allowed when required by current clinical practice (for correction of hypocalcemia). Blood samples for the measurement of specific biomarkers will be collected in concomitance with the routine blood sampling scheduled in each center. At baseline, all relevant demographic, clinical and biochemical data will be collected in compliance with the privacy laws of the European Union. The study design is illustrated in
Figure 1.
2.2. Ethical Considerations
Written informed consent will be obtained from each participants. The study is registered in clinicaltrials.gov: NCT 06352957. The study will be conducted according to Helsinki declaration.
3. Study Objectives and Aims
3.1. Main Outcome Measures
The main outcome measure is the comparison of VKDPs levels between patients treated with Etelcalcetide and those treated with vitamin D or vitamin D analogues. The longitudinal changes of the following VKDPs (measured at baseline and after 3, 9, and 18 months) will be compared between the two groups: total MGP, dephosphorylated-undercarboxylated MGP (dp-ucMGP), total BGP, and undercarboxylated BGP (ucBGP).
3.2. Other Outcome Measures
The following bone vascular markers levels will be considered: Calcium, Phosphate, Magnesium, ALP, PTH, 25(OH)D, Procollagen I Intact N-Terminal or P1NP, C-terminal telopeptide or CTX, Tartrate-resistant acid phosphatase 5b or TRAP 5bC-Terminal to Intact, Bone-specific alkaline phosphatase (BSAP), Fibroblast Growth Factor 23 or cFGF23 and iFGF23, Klotho and soluble α-Klotho, Sclerostin and Bioactive Sclerostin, DKK1, Fetuin A, Zincand Irisin.
The analysis will assess whether etelcalcetide will affect the levels of the above mentioned biomarkers over time.
- 2.
Evaluation of serum calcification propensity T50 test [
10,
11].
- 3.
Changes from baseline of following anemia markers levels: hemoglobin (Hb), hematocrit (Ht), plates (PLTS), reticulocytes, Iron, Ferritin, Transferrin, transferrin saturation. To test whether baseline, 3, 9 and 18-months of treatment with Etelcalcetide improves anemia status (e.g.: reducing EPO and/or iron doses).
- 4.
Changes from baseline of following dialysis routine biomarkers: Albumin, KT/V, Aluminium, C-reactive Protein (CRP), Cholesterol, Triglycerides, Cholesterol HDL, Cholesterol LDL.
The analysis will assess whether etelcalcetide will affect the levels of the above mentioned biomarkers over time.
- 5.
Changes from baseline prevalence VCs (Aorta and Iliac arteries) by lateral Dorsal Lumbar spine x-Ray [Time Frame: 18 months].
To test whether 18-months of treatment with Etelcalcetide reduces VCs progression.
- 6.
Changes from baseline prevalence Vertebral Fractures (VFs, quantitative vertebral morphometry using dedicated software) by lateral Dorsal Lumbar spine x-Ray [Time Frame: 18 months].
To test whther 18-months of treatment with Etelcalcetide reduces the VFs progression.
- 7.
Changes from baseline Total Hip, Femoral neck Bone Mass Density (BMD) by Dual-energy X-ray absorptiometry (DEXA) including Trabecular Bone Score where it will be available (TBS).To test if 18-months of treatment with Etelcalcetide improves BMD and TBS.
- 8.
To assess the relationship of bone vascular biomarkers on clinical outcomes: VFs and VCs.
- 9.
To compare a novel quantitative computer-assisted scoring method for vascular calcifications with a three-dimensional assessment from CT data [
12,
13].
- 10.
To evaluate the effect of Etelcalcetide on cardiovascular events and all-cause mortality.
- 11.
To evaluate the safety of Etelcalcetide and drug interactions.
3.3. Inclusion Criteria
Patient has provided informed consent.
Patient is 18 years of age or older of both sex.
Patients receiving maintenance HD three times per week (Kt/V >1.2).
Parathyroid hormone concentrations >500 ng/l at screening, or if parathyroidectomy is planned or expected, Ca >8.3 mg/dL.
-
Will be considered in the exposed group:
patients who have started Etelcalcetide within 1-month before the study enrolment.
patients naïve to intravenous calcimimetics use;
patients who have suspended oral calcimimetics from at least 1-month;
patients who are not responder or not compliant to the treatment with calcitriol;
In the unexposed group, patients on treatment with calcitriol or vitamin D analogs and who are age (± 2 years) and sex comparable (matching) to those in the exposed group will be considered.
Native vitamin D can be used in both groups and should be administered to target a 25(OH)D level > 30 ng/ml.
Dialysate calcium concentration must be stable for at least 4 weeks prior to screening laboratory assessments.
Patient must have severe HPT as defined by two laboratory screening pre-dialysis serum PTH values > 500 pg/ml, measured on two consecutive lab checks prior to entering the study. PTH levels should be standardized as reported elsewhere [
14].
Total alkaline phosphatase greater than the normal range, or even within the normal range but if greater than the tertile of the reference range for the assay.
Patients will be eligible only if they will show at least a /moderate Aorta VCs [
15] and/or Iliac arteries VCs and at least a mild VF [
16,
17].
3.4. Exclusion Criteria
Previous treatment with oral calcimimetics (cinacalcet) must have been suspended for at least 30 days. Recent start of calcimimetics (Etelcalcetide) is acceptable, but patients are excluded if treatment lasts for more than 1 month.
Patients who received a bisphosphonate, denosumab or teriparatide during the 12 months prior to screening.
Patients who underwent parathyroidectomy in the 6 months before the start of the study or if scheduled soon.
Scheduled kidney transplant during the study period or anticipated living donor evaluation within three months of recruitment.
Patients with unstable medical condition based on medical history, physical examination, and routine laboratory tests, or otherwise deemed unstable in the judgment of the Investigator.
Having metabolic bone diseases not related to the kidney (i.e., Pagets, Osteogenesis Imperfecta).
With severe untreated hyperthyroidism.
With malignancy within the last 3 years (except non-melanoma skin cancers or cervical carcinoma in situ).
Patients pregnant or nursing.
Patients with Long QT Syndrome.
Patients who are unlikely to be available to complete all protocol-required study visits or procedures, and/or to comply with all required study procedures to the best of the patient’s and Investigator’s knowledge.
4. Statistical Considerations
4.1. Sample Size
One hundred and sixty patients on hemodialysis are needed to address the study hypothesis. The sample size was established on pragmatic ground by considering the statistical models that will be applied to address the study hypothesis [i.e. the linear mixed models (LMM) analysis or generalized estimating equations (GEE)]. In this case, a total of 640 observations over the time period will be collected in 160 patients, thus ensuring an adequate power to adjust for major potential confounders.
4.2. Data Analysis
Normally distributed variables will be summarized as mean and standard deviation, non-normally distributed variables as median and interquartile range, categorical data as absolute numbers and percentages. At baseline, the between groups comparisons will be performed by independent T-Test, Mann Whitney U Test, or Chi Square Test, as appropriate. The between groups comparisons of repeated measurements of biomarkers (as continuous variables) over time will be performed by Linear Mixed Models (LMM) or Generalized Estimating Equations (GEE). No imputation method of missing values will be applied. In these analyses, the effect of the exposure to Etelcalcetide in treated patients versus those untreated will be preliminary investigated by including the group variable, the time and the interaction term between the group variable and time. The effect of potential confounders on the effectiveness of Etelcalcetide on the study outcomes will be further investigated by adjusting for all potential confounders by multiple regression models. A sensitivity analysis by applying the propensity score approach will be also performed. The primary analysis will be conducted considering the patients as belonging to the treatment arm they had at the beginning of observation. A secondary analysis will also be performed taking into account any cross-over. Survival analyses will be performed using Kaplan-Meier and Cox regression methods. All statistical analyses will be performed by using a standard statistical package (STATA 16 for Windows, USA).
5. Laboratories and Instrumental Data
The following laboratory testing will be performed:
as part of current clinical practice, the following routine biomarkers such as Calcium, Phosphate, Magnesium, ALP, PTH, 25(OH)D, Hb, Ht, PLTS, reticulocytes, Fe, Ferritin, Transferrin and transferrin saturation will be measured in each participating center.
as specific bone vascular markers (that will be measured in a centralized laboratory) we will consider total MGP and BGP, dp-ucMGP, ucBGP, Procollagen I Intact N-Terminal or P1NP, C-terminal telopeptide or CTX, Tartrate-resistant acid phosphatase 5b or TRAP 5bC-Terminal to Intact, Bone-specific alkaline phosphatase (BSAP), Fibroblast Growth Factor 23 or cFGF23 and iFGF23, Klotho and soluble α-Klotho, Sclerostin and Bioactive Sclerostin, DKK1, Fetuin A and Zinc.
5.1. Instrumental Data
5.1.1. X-ray
According to the current indications of good clinical practice and guidelines, at baseline the patients will perform a lateral Dorsal Lumbar spine x-Ray using a dedicated software to evaluate vascular calcifications (VCs: Aorta and Iliac arteries) [
12,
13,
14,
15] and VFs, where vertebral fractures will be identified if the height of the vertebral body is reduced by at least 20% or 4 mm, according to Genant et al. [
16]. Furthermore, a lateral Dorsal Lumbar spine x-Ray will be performed at the end of follow-up at 18 months. Both VCs and VFs will be conducted independently by two physicians who are blinded to the patient’s clinical characteristics.
5.1.2. DXA
According to the current indications of good clinical practice and guidelines [
2], at baseline measurements of Total Hip, Femoral neck Bone Mineral Density (BMD) will be performed by Dual-energy X-ray absorptiometry (DXA), including Trabecular Bone Score (TBS) when available. Furthermore, DXA and TBS will be performed at the end of follow-up at 18 months.
5.1.3. Abdominal CT
If the physician, in case of presence moderate/severe Aorta VCs associated to co-morbidity with resulting higher risk of cardiovascular and mortality events, will prescribe Abdominal CT these findings will be included in the study as data reflecting the common clinical practice. Also, a subset of patients with confirmed Aorta Calcifications by x-Ray will be CT scanned at the lumbar spine. According to [
12] calcification will be considered to be present if an area of ≥1 mm
2 displayed a density of ≥130 Hounsfield units. AAC score will be calculated from the takeoff of the renal artery to the bifurcation of the aorta into the common iliac arteries. The cross section of the abdominal aorta on each slice will be divided radially into 12 segments. The abdominal aortic calcification index (ACI) will be calculated as follows: ACI = (total score for calcification in all slices)/12 × 1/(number of slices) × 100 (%) . Semi-quantitative measurement of AAC on lateral X-rays will be conducted independently by two physicians who are blinded to the patient’s clinical characteristics. The general description of the study (included the types of clinical examinations and blood sampling over time) is given in
Figure 1.
5. Preliminary results
In a previous paper by us [
7] we measured BGP and MGP levels, and checked for the presence of vertebral fractures and vascular calcifications using lateral Dorsal Lumbar spine x-ray through dedicated software to evaluate VFs identified by a height vertebral reduction of at least 20% or 4 mm, according to Genant et al. [
16,
25], and in the same x-ray we evaluated vascular calcifications (VCs: Aorta and Iliac arteries) [
25] in 387 hemodialysis patients enrolled in the VIKI study. Total BGP levels were found to be twice as high in patients on calcimimetics compared to those untreated with this drugs class (290 vs. 158.5 mcg/L, p < 0.0001) whereas total MGP levels resulted to be 19% higher in patients on calcimimetics (21.5 vs. 18.1 mcg/L, p = 0.04) than in those without. Median total BGP levels were 29% lower in patients with one or more vertebral fractures (151 vs. 213 mcg/L, p = 0.0091) and 36% lower in patients with vascular calcifications (164 vs. 262.1 mcg/L, p = 0.0003). In non-survivors, median BGP and MGP levels were lower, but only the difference in MGP levels reached statistical significance (152 vs. 191 mcg/L, p = 0.20 and 15.0 vs. 19.7 mcg/L, p = 0.02, respectively). In another paper by our group [
25] we assessed the prevalence of vitamin K deficiency and the relationship between vitamin K status, vertebral fractures, and vascular calcification in the same patients’ cohort. Overall, we found a relatively high proportion of patients with deficiencies in MK7 (35.4%), vitamin K1 (23.5%), and MK4 (14.5%). Of note, vitamin K1 deficiency was the strongest predictor of vertebral fractures (odds ratio [OR], 2.94; 95% confidence interval [CI], 1.38-6.26). MK4 deficiency was a predictor of aortic calcification (OR, 2.82; 95% CI, 1.14-7.01), whereas MK5 deficiency appeared to protect against it (OR, 0.38; 95% CI, 0.15-0.95). MK7 deficiency was a predictor of iliac calcification (OR, 1.64; 95% CI, 1.03-2.60). The presence of vertebral fractures was also a predictor of vascular calcifications (OR, 1.76; 95% CI, 1.00-3.08).
6. Discussion
In addition to its known suppressive effect on PTH, Etelcalcetide has demonstrated additional benefits in recent studies, both experimental and human. In rats, it has been shown to preserve cortical bone structure and bone strength by lowering PTH in subtotal Nx rats with established SHPT [
18,
19]. In humans, it has been found to improve areal bone mineral density and trabecular quality in the central skeleton, always by lowering bone turnover without affecting bone material properties, thereby reducing the risk of fractures [
20,
21].
Ca
2+-sensing receptors (CaSRs) are broadly expressed in the vascular system, including perivascular neurons, vascular endothelial cells (VECs) and vascular smooth muscle cells (VSMCs). Prolonged serum calcium is thought to reduce the sensitivity and expression of CaSR in arteries, thereby increasing intra-vascular Ca
2+ concentrations through a negative feedback loop. Excess intravascular calcium gradually deposits in VSMCs and may in part lead to transformation of VSMCs into osteoblast-like cells, leading to formation of vascular calcification [
22]. In experimental studies, overexpression of the CaSR reduced the deposition of calcium in Human Aortic Smooth Muscle Cells (HASMCs) [
22]. In uremic rats with secondary HPT treated with calcimimetics (R-568 or AMG 641) compared to untreated, Rodriguez et al reported that VCs were prevented and mortality was reduced in excess of what was expected from a reduction in calcium and phosphorus alone [
23]. They also have ascribed to calcimimetics action a protective role in the expression of proteins that prevent the development of VC, in particular highlighting an increase of the MGP [
23,
24]. To confirm these findings, our group conducted a secondary analysis of the Vitamin K Italian (VIKI) study to assess the prevalence of vitamin K deficiency in hemodialysis patients [
25] and to evaluate associations between drug consumption and VKDP levels in 387 hemodialyzed patients [
7]. A previous study by us reported in patients managed with compared to without calcimimetics that total BGP levels were twice as high (290 vs. 158.5 mcg/L, p<0.0001) and that total MGP levels were 19 % higher (21.5 vs. 18.1 mcg/L, p = 0.04) [
7,
25]. Furthermore, Yu et al showed in uremic rats treated with Etelcalcetide lower Aorta calcium content than in paricalcitol-treated [
26].
Furthermore, recent studies have also shown that Irisin, the hormone-like myokine crucial for muscle-bone communication [
27], was significantly lower in hemodialysis patients compared with control subjects. Most importantly, within the group of hemodialysis patients, Irisin was significantly lower in those with VC compared to patients with no calcification [
28].
6.1. Statistical and Methodological Issues
The study protocol rests on a solid longitudinal design contemplating repeated measurements of specific biomarkers over time. When dealing with an observational study of effectiveness and with LMM/GEE (see Statistical Analysis) having repeated measurements over time as dependent variables, the estimation of the sample size mainly depends on the number of potential confounders to be included into the models rather than on a predefined effect of the drug. In this case, a total of 640 observations over the time period are expected to be collected [i.e., 80 patients x 4 longitudinal visits including baseline (n=320 observations) in the exposed group and 80 patients x 4 longitudinal visits including baseline (n=320 observations) in the unexposed group]. This high number of observations will allow to adjust the effect of Etelcalcetide versus Calcitriol or vitamin D analogs on study endpoints for both baseline and longitudinal potential confounders thus obtaining robust and precise effect estimates. Furthermore, linear mixed models and generalised estimating equations analyses are robust methods for dealing with various patterns of missingness, an issue of particular relevance in studies of comparative effectiveness. Furhermore, since this isn't an interventional study, the observational nature of the protocol will be garanteed by inviting treating nephrologists to tailor the appropriate dosage for each patient individually, aiming to reach the KDIGO PTH target level as in every day clinical practice. In the group receiving Etelcalcetide, calcitriol may be also added as needed according to standard clinical approach to address hypocalcemia.
6.2. Dissemination Plan
The target audience to disseminate the results of ETERNITY-ITA study includes healthcare professionals (nephrologists, endocrinologists, and general practitioners), patients suffering from chronic kidney disease and related conditions, pharmaceutical companies involved in the production of etelcalcetide and calcitriol, and policy makers and regulatory bodies involved in healthcare decision-making. The dissemination channels includes scientific journals, national and international meetings, and online platforms such as research gate, linkedin, and professional forums.
6.3. Limitations
As an observational study of comparative effectiveness, ETERNITY-ITA can provide valuable insights into how treatments perform in real-world settings. However, the observational design comes with limitations that can affect the reliability and validity of findings, such as those due to confounding. However, such problems will be minimized by collecting data and adjusting for major potential confounders that may interfere with the pathway between treatments and outcome variables. Notwithstanding such an approach aimed at controlling for confounding variables, it is not possible to completely exclude that there may be unmeasured confounders that remain unaccounted for, leading to residual confounding and potentially biased estimates of treatment effects.
7. Conclusions
The clinical remarkable significance of this study is to provide first-time data on the ability of etelcalcetide in real life to prevent through the increased VKDPs levels, alone and or in association with other vascular-bone markers, from the skeletal fragility and vascular calcifications in hemodialysis patients with the resulting decrease of the morbidity and mortality.
Author Contributions
All authors have contributed, read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the ETERNITY-ITA Study Investigators, who will provide patient clinical care and collect clinical data. They are the following: Abaterusso Cataldo, Favaro Enrico (Castelfranco Veneto), Bosco Manuela, Barbecar Dorina (Gorizia-Monfalcone), Dattolo Pietro, Tsalouchos Aris (Firenze), Dugo Mauro, Gresa Baboci (Mestre, Venezia), Gallieni Maurizio, Cosmai Laura, Re Sartò Giulia (Sacco, Milano), Gambaro Giovanni, Ortalda Vittorio, Andreola Stefano (Verona Borgo Trento), Gesualdo Loreto, Di Palma Anna Maria, Cafiero Cesira, Ferrara Raffaella (Bari), La Manna Gaetano, Cianciolo Giuseppe, Zappulo Fulvia (Bologna), Lentini Paolo Luca, Fuso Valeria, Benedetti Claudia (Bassano del Grappa), Mallamaci Francesca, Panuccio Vincenzo (Reggio Calabria), Mancini Walter, Fabi Liana (Pordenone), Nordio Maurizio, Puggia Riccarda Maria (Treviso), Pani Antonello, Caria Stefania (Cagliari), Pinna Antonio Maria, Mereu Cristina, Patrizia Vatieri (Oristano), Santirosi Paola, Ciabattini Francesca (Foligno), Santoro Domenico, Gembillo Guido, Zirino Fortunata (Messina), Vezzoli Giuseppe, Quartagno Rita, Bologna Arianna, De Rosa Liliana (San Raffaele Milano), Zanella Monica, Mariotto Alice, Marchionna Nicola, Sgarabotto Luca (Vicenza).
Conflict of Interest
The authors declare no conflict of interest.
Statement of Ethics
The authors are committed to obtaining informed consent from all participants involved in ETERNITY-ITA. Prior to their participation, participants will be provided with clear and comprehensible information regarding the nature of the study, its objectives, potential risks and benefits, and their rights as participants. The authors will ensure that participants have the opportunity to ask questions and make informed decisions about their participation, without any form of coercion or undue influence. The data collection will strictly adhere to protocols for data anonymization and encryption to safeguard the identity of participants. All data collected by RED-CAP will be securely stored and accessible only to authorized members of the research team. Any identifiable information will be removed or coded to prevent identification of individual participants. Any potential risks will be disclosed to participants during the informed consent process, and steps will be taken to minimize these risks to the greatest extent possible. The study complies with all relevant regulations, guidelines, and ethical standards governing research involving human participants. The authors will obtain necessary approvals from institutional review boards or ethics committees prior to the commencement of the study and will adhere to all applicable laws and regulations regarding the collection, use, and dissemination of data, and obtain necessary permissions for any secondary use of data. Written informed consent will be obtained for each subject. The trial is registered at clinicaltrials.gov: NCT 06352957.
References
- Rodrıguez-Garcıa M, Gomez-Alonso C, Naves-Dıaz M, Diaz-Lopez JB,Diaz-Corte C, Cannata-Andıa JB; Asturias Study Group. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24:239–246.
- Ketteler M, Block GA, Evenepoel P et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017 Jul;92(1):26-36. [CrossRef]
- Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181.
- Fusaro M, Gallieni M, Porta C et al (2020) Vitamin K effects in human health: new insights beyond bone and cardiovascular health. J Nephrol 33:239–249.
- Fusaro M, Cianciolo G, Brandi ML, Ferrari S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La Manna G, Gallieni M, Vettor R, Aghi A, Gasperoni L, Giannini S, Sella S, M Cheung A. Vitamin K and Osteoporosis. Nutrients. 2020 Nov 25;12(12):3625. PMID: 33255760; PMCID: PMC7760385.). [CrossRef]
- Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 2002, 277: 4388-94.
- Fusaro M, Giannini S, Gallieni M, et al. Calcimimetic and vitamin D analog use inhemodialyzed patients is associated with increased levels of vitamin K dependent proteins. Endocrine. 2016; 51(2):333-341.
- Fusaro M, Mereu MC, Aghi A, Iervasi G, Gallieni M. Vitamin K and bone. Clin Cases Miner Bone Metab. 2017 May-Aug;14(2):200-206. Epub 2017 Oct 25. PMID: 29263734; PMCID: PMC5726210. [CrossRef]
- Russo D, Tripepi R, Malberti F et al. Etelcalcetide in Patients on Hemodialysis with Severe Secondary Hyperparathyroidism. Multicenter Study in Real Life. J Clin Med. 2019 Jul 20;8(7):1066. [CrossRef]
- Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, Jahnen-Dechent W. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol. 2012 Oct;23(10):1744-52. Epub 2012 Sep 6. PMID: 22956818; PMCID: PMC3458464. [CrossRef]
- Nakatani, S.; Mori, K.; Sonoda, M.; Nishide, K.; Uedono, H.; Tsuda, A.; Emoto, M.; Shoji, T. Association between Serum Zinc and Calcification Propensity (T50) in Patients with Type 2 Diabetes Mellitus and In Vitro Effect of Exogenous Zinc on T50. Biomedicines 2020, 8, 337. [CrossRef]
- Takayama Y, Yasuda Y, Suzuki S, Shibata Y, Tatami Y, Shibata K, Niwa M, Sawai A, Morimoto R, Kato S, Ishii H, Maruyama S, Murohara T. Relationship between abdominal aortic and coronary artery calcification as detected by computed tomography in chronic kidney disease patients. Heart Vessels. 2016 Jul;31(7):1030-7.
- Fusaro M, Schileo E, Crimi G, Aghi A, Bazzocchi A, Barbanti Brodano G, Girolami M, Sella S, Politi C, Ferrari S, Gasperini C, Tripepi G, Taddei F. A Novel Quantitative Computer-Assisted Score Can Improve Repeatability in the Estimate of Vascular Calcifications at the Abdominal Aorta. Nutrients. 2022 Oct 13;14(20):4276. PMID: 36296959; PMCID: PMC9607651. [CrossRef]
- Jean-Claude P Souberbielle, Hubert Roth, Denis P Fouque. Parathyroid hormone measurement in CKD. Kidney Int 2010 Jan;77(2):93-100. Epub 2009 Oct. [CrossRef]
- Witteman JC, Grobbee DE, Valkenburg HA et al. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet. 1994 Feb 26;343(8896):504-7. [CrossRef]
- Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D,Cummings SR. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–96.
- Guglielmi G, Diacinti D, van Kuijk C, Aparisi F, Krestan C, Adams JE, Link TM. Vertebral morphometry: current methods and recent advances. Eur Radiol. 2008;18:1484–96.
- Xiaodong Li, Longchuan Yu, Frank Asuncion et al. Etelcalcetide (AMG 416), a peptide agonist of the calcium-sensing receptor, preserved cortical bone structure and bone strength in subtotal nephrectomized rats with established secondary hyperparathyroidism. Bone. 2017 Dec:105:163-172. Epub 2017 Sep 1. [CrossRef]
- Igarashi S, Kasukawa Y, Nozaka K et al. Teriparatide and etelcalcetide improve bone, fibrosis, and fat parameters in chronic kidney disease model rats. Osteoporos Sarcopenia 2023 Dec;9(4):121-130. Epub 2023 Dec 21. [CrossRef]
- Khairallah P, Cherasard J, Sung J, et al. Changes in Bone Quality after Treatment with Etelcalcetide. Clin J Am Soc Nephrol. 2023; 18:1456-1465.
- Wakamatsu T, Yamamoto S, Matsuo K, Taniguchi M, Hamano T, Fukagawa M, Kazama JJ. Effectiveness of calcimimetics on fractures in dialysis patients with secondary hyperparathyroidism: meta-analysis of randomized trials. J Bone Miner Metab. 2024 Mar 27. Epub ahead of print. PMID: 38536478. [CrossRef]
- Guo Y, Yang X, He J, Liu J, Yang S, Dong H. Important roles of the Ca2+-sensing receptor in vascular health and disease. Life Sci. 2018 Sep 15;209:217-227. Epub 2018 Aug 8. [CrossRef]
- M. Rodriguez, E. Aguilera-Tejero, F.J. Mendoza et al., Effects of calcimimetics on extraskeletal calcifications in chronic kidney disease. KidneyInt. 74(Suppl 111), S50–S54 (2008). [CrossRef]
- Lopez I, Aguilera-Tejero E, Mendoza FJ et al. Calcimimetic R-568 decreases extraosseous calcifications in uremic rats treated with calcitriol. J AmSocNephrol 2006; 17: 795–804.
- Fusaro M, Noale M, Viola V, et al. Vitamin K, Vertebral Fractures, Vascular Calcifications, and Mortality: VItamin K Italian (VIKI) Dialysis Study. J Bone Miner Res 2012;27(11):1-8.
- Yu L, Tomlinson JE, Alexander ST el. Etelcalcetide, A Novel Calcimimetic, Prevents Vascular Calcification in A Rat Model of Renal Insufficiency with Secondary Hyperparathyroidism. Calcif Tissue Int. 2017 Dec;101(6):641-653.
- Colaianni G, Cuscito C, Mongelli T, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015 Sep 29;112(39):12157-62. Epub 2015 Sep 15. Erratum in: Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):E5763. PMID: 26374841; PMCID: PMC4593131. [CrossRef]
- He L, He W-Y, A L.-T, et al: Lower Serum Irisin Levels Are Associated with Increased Vascular Calcification in Hemodialysis Patients. Kidney Blood Press Res 2018;43:287-295. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).