Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Extraction of Lipidic Pigments
2.2. Characterization
2.3. DSSC Assembly and Characterization
3. Results
3.1. Visible Absorbance Spectroscopy
3.2. Electrochemical Evaluation of the Extracts
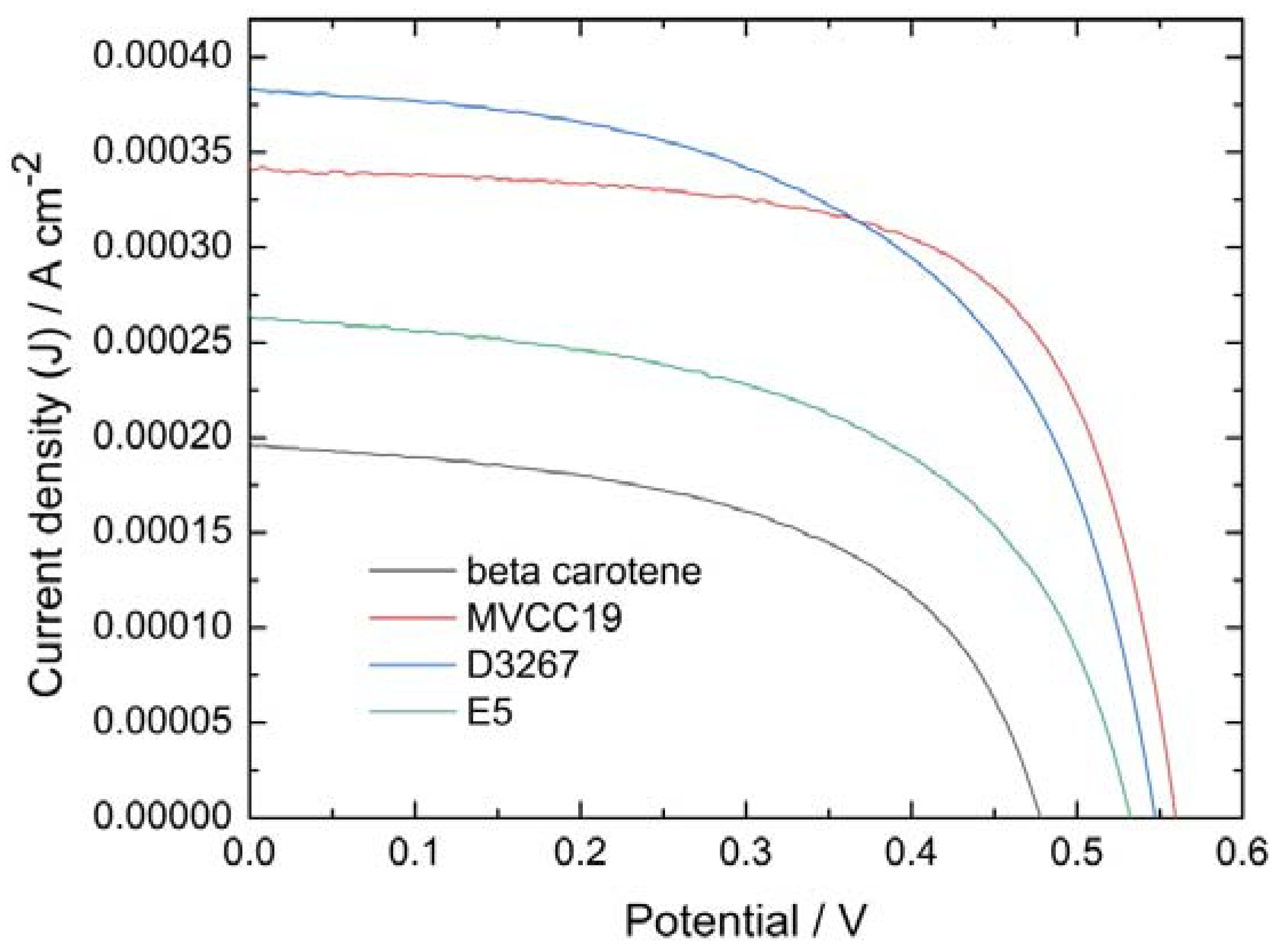
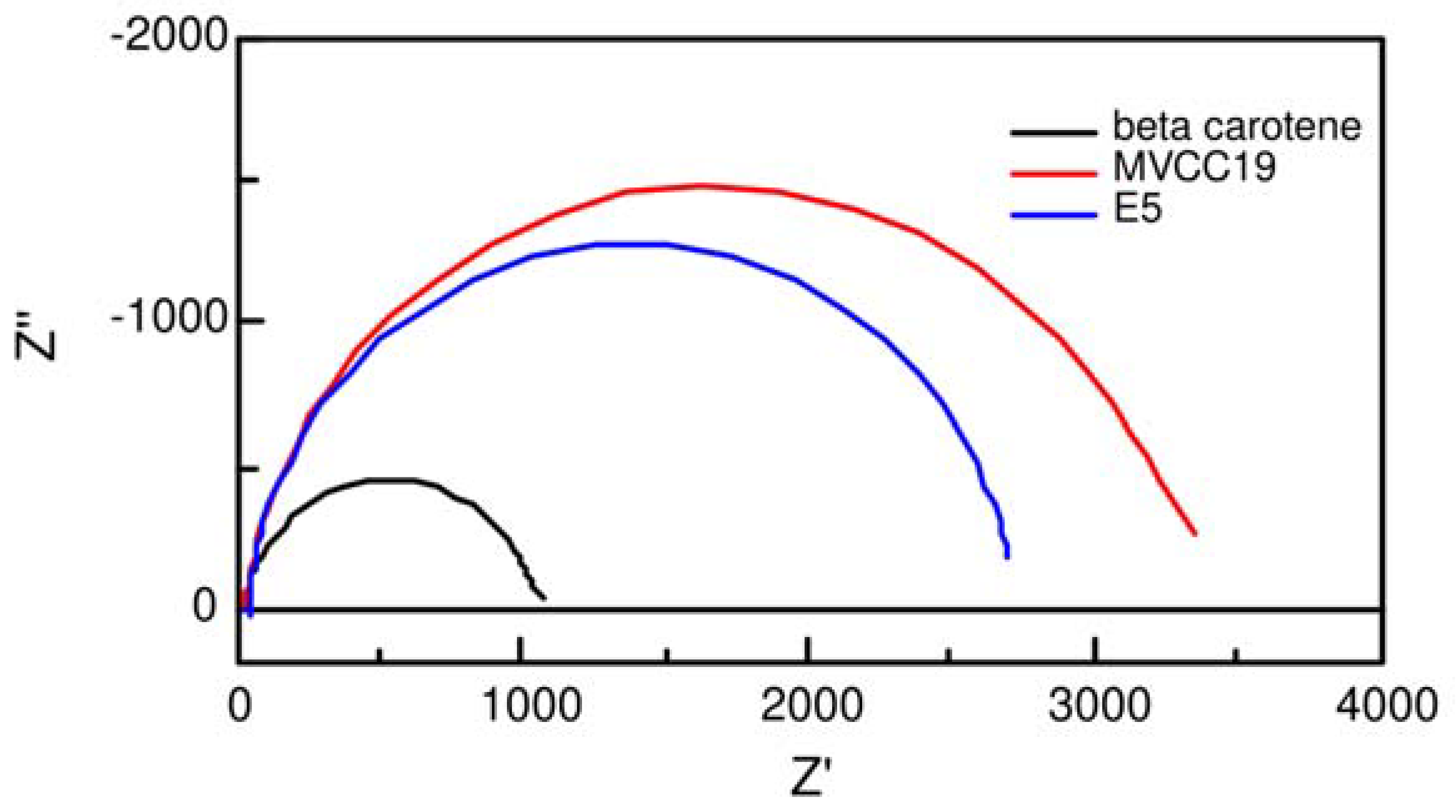
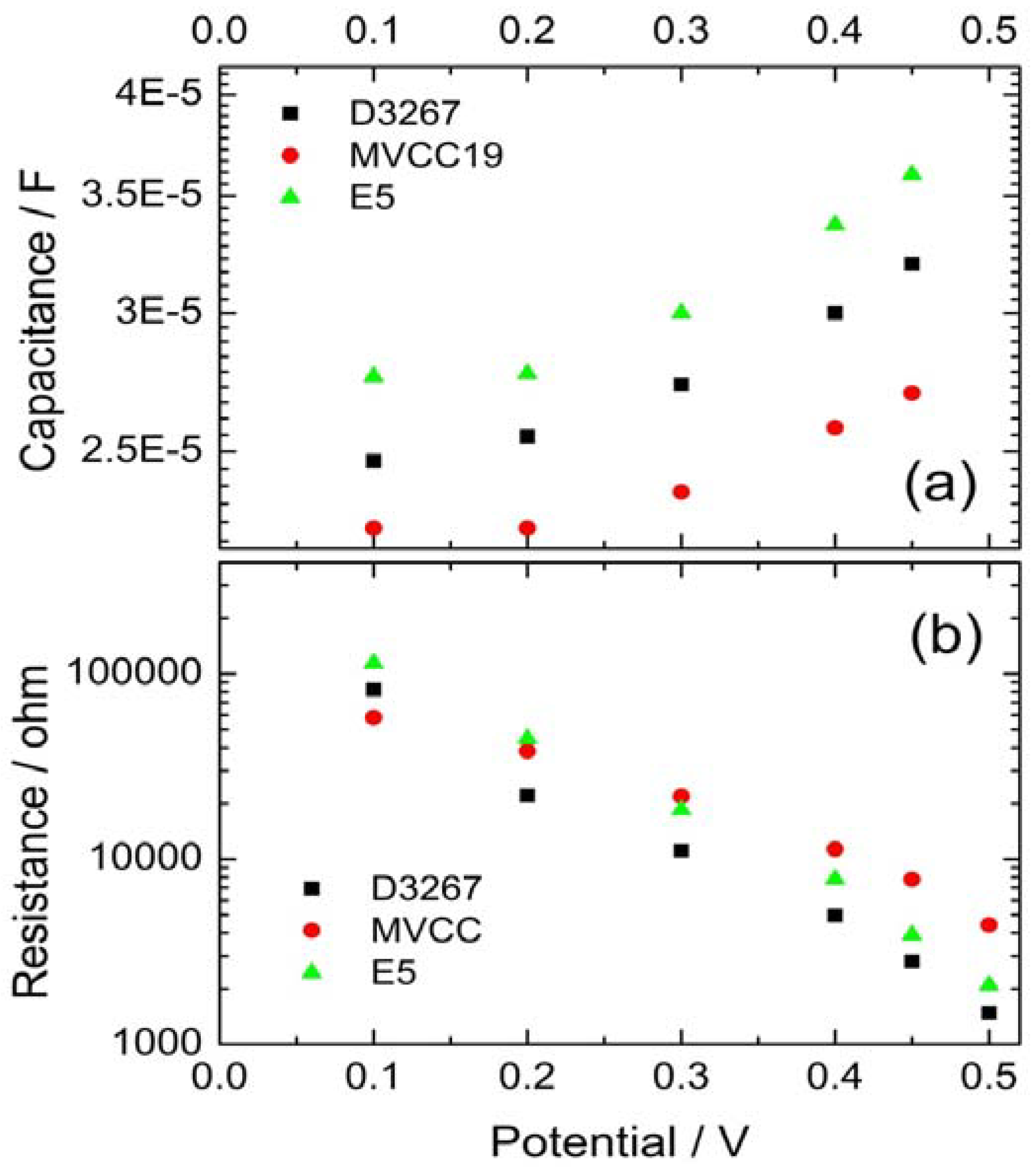
3.3. Electrochemical Evaluation of the DSSC
3.4. Thermodynamic Considerations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O'Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Y.; Baryshnikov, G.; Luo, J.; Wang, X.; Ågren, H.; Li, C.; Xie, Y. Efficient Dye-Sensitized Solar Cells Based on a New Class of Doubly Concerted Companion Dyes. ACS Appl. Mater. Interfaces 2022, 14, 33274–33284. [Google Scholar] [CrossRef]
- Bisquert, J.; Cahen, D.; Hodes, G.; Rühle, S.; Zaban, A. Physical Chemical Principles of Photovoltaic Conversion with Nanoparticulate, Mesoporous Dye-Sensitized Solar Cells. J. Phys. Chem. B 2004, 108, 8106–8118. [Google Scholar] [CrossRef]
- Zhang, D.; Stojanovic, M.; Ren, Y.; Cao, Y.; Eickemeyer, F.T.; Socie, E.; Vlachopoulos, N.; Moser, J-E. ; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A molecular photosensitizer achieves a Voc of 1.24 V enabling highly efficient and stable dye-sensitized solar cells with copper(II/I)-based electrolyte. Nat. Commun. 2021, 12, 1777. [Google Scholar] [CrossRef]
- Khan, M.; Iqbal, M.A.; Malik, M.; Hashmi, S.U.; Bakhsh, S.; Sohail, M.; Qamar, M.T.; Al-Bahrani, M.; Capangpangan, R.Y.; Alguno, A.C.; Choi, J.R. Improving the efficiency of dye-sensitized solar cells based on rare-earth metal modified bismuth ferrites. Sci Rep 2023, 13, 3123. [Google Scholar] [CrossRef]
- Boschloo, G. Improving the Performance of Dye-Sensitized Solar Cells. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sil, M.C.; Chen, L.S.; Lai, C.W.; Lee, Y.H.; Chang, C.C.; Chen, C.M. Enhancement of power conversion efficiency of dye-sensitized solar cells for indoor applications by using a highly responsive organic dye and tailoring the thickness of photoactive layer. J. Power Sources 2020, 479, 229095. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid preadsorption raises efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Solar –IEA – International Energy Agency https://www.iea.org › renewables › solar-pv. Accessed on June 21st.
- Pouras, H.H.; Barenji, R.V.; Khojastehnezhad, V.M. Solar energy status in the world: A comprehensive review. Energy Reports 2023, 10, 3474–3493. [Google Scholar] [CrossRef]
- International Renewable Energy Agency IRENA. Renewable Power: Sharply falling generation costs. Accessed on June 21st. https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2017/Nov/%20IRENA_Sharply_falling_costs_2017.pdf.
- Victoria, M.; Haegel, N.; Peters, I.M.; Sinton, R.; Jäger-Waldau, A.; del Cañizo, C.; Breyer, C.; Stocks, M.; Blakers, A.; Kaizuka, I.; Komoto, K.; Smets, A. Solar photovoltaics is ready to power a sustainable future. Joule 2021, 5, 1041–1056. [Google Scholar] [CrossRef]
- Cannavale, A.; Martellotta, F.; Fiorito, F.; Ayr, U. The Challenge for Building Integration of Highly Transparent Photovoltaics and Photoelectrochromic Devices. Energies 2020, 13, 1929. [Google Scholar] [CrossRef]
- Muñoz-Garcia, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; Freitag, M. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, A.; Bhandari, S.; Selvaraj, P.; Sundaram, S.; Mallick, T.K. Color Comfort Evaluation of Dye-Sensitized Solar Cell (DSSC) Based Building-Integrated Photovoltaic (BIPV) Glazing after 2 Years of Ambient Exposure. J. Phys. Chem. C 2019, 123, 23834–23837. [Google Scholar] [CrossRef]
- Lu, L.; Ya’acob, M.E.; Anuar, M.S.; Chen, G.; Othman, M.H.; Noor Iskandar, A.; Roslan, N. Thermal analysis of a portable DSSC mini greenhouse for botanical drugs cultivation. Energy Rep. 2020, 6, 238–253. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Ursu, D.; Vajda, M.; Miclau, M. Highly efficient dye-sensitized solar cells for wavelength-selective greenhouse: A promising agrivoltaic system. Int. J. Energy Res. 2022, 46, 18550–18561. [Google Scholar] [CrossRef]
- Cerdá, M.F. Dyes from the Southern Lands: An Alternative or a Dream? Solar 2022, 2, 519–539. [Google Scholar] [CrossRef]
- Montagni, T.; Rodríguez Chialanza, M.; Cerdá, M.F. Blueberries as a Source of Energy: Physical Chemistry Characterization of Their Anthocyanins as Dye-Sensitized Solar Cells’ Sensitizers. Solar 2023, 3, 283–297. [Google Scholar] [CrossRef]
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3495. [Google Scholar] [CrossRef]
- Calogero, G.; Yum, J.H.; Sinopoli, A.; Di Marco,G. ; Grätzel, M; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Solar Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Ndiaye, A.; Dioum, A.; Oprea, C.I.; Dumbrava, A.; Lungu, J.; Georgescu, A.; Moscalu, F.; Gîr¸tu, M.A.; Beye, A.C.; Youm, I. A Combined Experimental and Computational Study of Chrysanthemin as a Pigment for Dye-Sensitized Solar Cells. Molecules 2021, 26, 225. [Google Scholar] [CrossRef]
- Aslan, F. New natural dyes extracted by ultrasonic and soxhlet method: Effect on dye-sensitized solar cell photovoltaic performance. Opt Quant Electron 2024, 56, 645. [Google Scholar] [CrossRef]
- Narayan, M.R. Review: Dye sensitized solar cells based on natural photosensitizers. Renew. Sustain. Energy Rev. 2012, 16, 208–215. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.; Gao, Y.; Ma, T. Dye-sensitized solar cells using 20 natural dyes as sensitizers. J. Photochem. Photobiol. A Chem. 2011, 219, 188–194. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Mohamad, A.B.; Ludin, N.A.; Kadhum, A.A.H.; Sopian, K. Dye-sensitised solar cells: Development, structure, operation principles, electron kinetics, characterisation, synthesis materials and natural photosensitisers. Renew. Sustain. Energy Rev 2016, 65, 183–213. [Google Scholar] [CrossRef]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sustain. Energy Rev 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Shukor, N.I.A.; Chan, K.Y.; Thien, G.S.H.; Yeoh, M.E.; Low, P.L.; Devaraj, N.K.; Ng, Z.N.; Yap, B.K. A Green Approach to Natural Dyes in Dye-Sensitized Solar Cells. Sensors 2023, 23, 8412. [Google Scholar] [CrossRef] [PubMed]
- da Conceição, L.R.B.; da Cunha, H.O.; Leite, A.M.B.; Suresh Babu, R.; Raja, S.; Ribeiro, C.; de Barros, A.L.F. Evaluation of Solar Conversion Efficiency in Dye-sensitized Solar Cells Using Natural Dyes Extracted from Alpinia purpurata and Alstroemeria Flower Petals as Novel Photosensitizers. Colorants 2023, 2, 618–631. [Google Scholar] [CrossRef]
- Ren, Y.; Flores-Díaz, N.; Zhang, D.; Cao, Y.; Decoppet, J.D.; Fish, G.C.; Moser, E.; Zakeeruddin, S.M.; Wang, P.; Hagfeldt, A.; Grätzel, M. Blue Photosensitizer with Copper(II/I) Redox Mediator for Efficient and Stable Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2020, 2004804. [Google Scholar] [CrossRef]
- Cai, L:; Moehl, T. ; Moon, S.J.; Decoppet, J.D.; Humphry-Baker, R.; Xue, Z.; Bin, L.; Zakeeruddin, S.M.; Grätzel, M. 4,9-Dihydro-4,4,9,9-tetrahexyl-s-indaceno [1,2-b:5,6-b′]dithiophene as a π-Spacer of Donor−π–Acceptor Dye and Its Photovoltaic Performance with Liquid and Solid-State Dye-Sensitized Solar Cells. Org. Lett. 2014, 16, 106–109. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy. Sol. Energy Mater. Sol. Cells, 2005, 87, 117–131. [Google Scholar] [CrossRef]
- Hardin, B.; Snaith, H; McGehee, M. The renaissance of dye-sensitized solar cells. Nature Photon 2012, 6, 162–169. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Nigam, K.D.P.; Cerdán-Pasarán, A.; Ornelas-Soto, A. Alternative sources of natural pigments for dye-sensitized solar cells: Algae, cyanobacteria, bacteria, archaea and fungi. J. Biotechnol. 2021, 332, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, A; Mukherjee, T. Application of carotenoids in sustainable energy and green electronics. Mater. Adv., 2022, 3, 1341–1358. [Google Scholar] [CrossRef]
- Alhorani, S.; Kumar, S.; Genwa, M.; Meena, P.L. Performance of dye-sensitized solar cells extracted dye from wood apple leaves. J. Phys. Comm. 2022, 6, 085012. [Google Scholar] [CrossRef]
- Adedokun, O.; Adedeji, O.L.; Bello, I.T.; Awodele, M.K.; Awodugba, A.O. Fruit peels pigment extracts as a photosensitizer in ZnO-based Dye-Sensitized Solar Cells. Chem. Phys. Impact 2021, 3, 100039. [Google Scholar] [CrossRef]
- Orona-Navar, A.; Aguilar-Hernández, I.; Cerdán-Pasarán, A.; López-Luke, T.; Rodríguez-Delgado, M.; Cárdenas-Chávez, D.L.; Cepeda-Pérez, E.; Ornelas-Soto, N. Astaxanthin from Haematococcus pluvialis as a natural photosensitizer for dye-sensitized solar cell. Algal Research 2017, 26, 15–24. [Google Scholar] [CrossRef]
- Rowan, K.S. Photosynthetic pigments of algae. Cambridge University Press, Cambridge, UK, 1989.
- Takaichi, S.; Mochimaru, M. Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides. Cell Mol Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Whitton, B.A. Ecology of cyanobacteria II: their diversity in space and time. Dordrecht, Springer Netherlands, 2012.
- Bonilla, S.; Cremella, B.; Acuña, V.; Haakonsson, S. Pigment assemblages in subtropical bloom-forming cyanobacteria strains. J. Plankton Res. 2023, 45, 746–750. [Google Scholar] [CrossRef]
- Vézina, S.; Vincent, W. Arctic cyanobacteria and limnological properties of their environment: Bylot Island, Northwest Territories, Canada (73°N, 80°W). Polar Biol 1997, 17, 523–534. [Google Scholar] [CrossRef]
- Żbik, P.; Kłodawska, K.; Malec, P. The effect of solvent on the optical properties of myxoxanthophyll from Synechocystis sp. PCC6803. J. Mol. Liq. 2023, 375, 121367. [Google Scholar] [CrossRef]
- Allen, M.M.; Stanier, R.Y. Selective isolation of blue-green algae from water and soil. J. Gen. Microbiol 1968, 51, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, S.; Das, M.P.; Seshiah, C.; Rebecca, L.J. Extraction and purification of carotenoids from vegetables. JOCPR 2014, 6, 594–598. [Google Scholar]
- de Bon, M.; Rodríguez Chialanza, M.; Cerdá, M.F. Fucoxanthin from the Antarctic Himantothallus grandifollius as a sensitizer in DSSC. J. Iranian Chem. Soc. 2022, 19, 3627–3636. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Focsan, A.L.; Bowman, M.K.; Kispert, L.D. Free Radical Formation in Novel Carotenoid Metal Ion Complexes of Astaxanthin. J. Phys. Chem. B 2010, 114, 16968–16977. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, E.L.; Nyman, M.C.; Shelley, J.T.; Mattson, N.S. Spectral properties and stability of selected carotenoid and chlorophyll compounds in different solvent systems. Food Chem. Adv. 2023, 2, 100178. [Google Scholar] [CrossRef]
- Hertzberg, S.; Liaaen-Jensen, S. The constitution of aphanizophyll. Phytochem. 1971, 10, 3251–3252. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M.; Maoka, T.; Katoh, H. Myxol and 4-Ketomyxol 2′-Fucosides, not Rhamnosides, from Anabaena sp. PCC 7120 and Nostoc punctiforme PCC 73102, and Proposal for the Biosynthetic Pathway of Carotenoids. Plant Cell Physiol. 2005, 46, 497–504. [Google Scholar] [CrossRef]
- Takaichi, S.; Maoka, T.; Mochimaru, M. Unique Carotenoids in the Terrestrial Cyanobacterium Nostoc commune NIES-24: 2-Hydroxymyxol 20-Fucoside, Nostoxanthin and Canthaxanthin. Curr Microbiol 2009, 59, 413–419. [Google Scholar] [CrossRef]
- Foppen, F.H. Tables for the identification of carotenoid pigments. Chromatographic reviews 1971, 14, 133–298. [Google Scholar] [CrossRef]
- Hertzberg, S.; Jensen, S.L. The carotenoids of blue-green algae II: the carotenoids of Aphanizomenon flos-aquae. Phytochemistry 1966, 5, 565–570. [Google Scholar] [CrossRef]
- Hertzberg, S.; Jensen, S.L. The structure of myxoxanthophyll. Phytochem. 1969, 8, 1259–1280. [Google Scholar] [CrossRef]
- Srivastava, A.; Thapa, S.; Chakdar, H.; Babele, P. K.; Shukla, P. Cyanobacterial myxoxanthophylls: biotechnological interventions and biological implications. Crit. Rev. Biotechnol. 2022, 44, 63–77. [Google Scholar] [CrossRef]
- Mohamed, H.E.; van de Meene, A.M.L.; Roberson, R.W.; Vermaas, W.F.J. Myxoxanthophyll Is Required for Normal Cell Wall Structure and Thylakoid Organization in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 2005, 187, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Y.; Kispert, L.D. Electrochemical properties of natural carotenoids. J. Electroanal. Chem. 2000, 488, 140–150. [Google Scholar] [CrossRef]
- Hapiot, P.; Kispert, L.D.; Konovalov, V.V.; Saveant, J.M. Single Two-Electron Transfers vs Successive One-Electron Transfers in Polyconjugated Systems Illustrated by the Electrochemical Oxidation and Reduction of Carotenoids. J. Am. Chem. Soc. 2001, 123, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Cizmek, L.; Komorsky-Lovric, S. Study of Electrochemical Behaviour of Carotenoids in Aqueous Media. Electroanalysis 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Ishikita, H.; Loll, B.; Biesiadka, J.; Saenger, W.; Knapp, E.W. Redox potentials of chlorophylls in the photosystem II reaction center. Biochem 2005, 44, 4118–4124. [Google Scholar] [CrossRef]
- Enciso, P.; Cerdá, M.F. Solar cells based on the use of photosensitizers obtained from Antarctic red algae. Cold Regions Sci. Technol. 2016, 126, 51–54. [Google Scholar] [CrossRef]
- Edge, R.; Land, E.J.; McGarvey, D.; Mulroy, L.; Truscott, G. Relative One-Electron Reduction Potentials of Carotenoid Radical Cations and the Interactions of Carotenoids with the Vitamin E Radical Cation. J. Am. Chem. Soc. 1998, 120, 4087–4090. [Google Scholar] [CrossRef]
- Shono, T.; Matsumura, Y.; Nakagawa, Y. Electroorganic chemistry. XII. Anodic oxidation of enol esters. J. Am. Chem. Soc 1974, 96, 3532–3536. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Ligia Focsan, A.L.; Bowman, M.K.; Kispert, L.D. Free Radical Formation in Novel Carotenoid Metal Ion Complexes of Astaxanthin. Phys. Chem. B 2010, 114, 16968–16977. [Google Scholar] [CrossRef] [PubMed]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.M.; Bisquert, J. Properties of chromophores determining recombination at TiO2-dye-electrolyte interface. Langmuir 2013, 29, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Yuan, S.; Ren, X.; Zhao, Y.; Wang, Z.; Zhang, M.; Li, D.; Shi, L. Effects of acetyl acetone-typed co-adsorbents on the interface charge recombination in dye-sensitized solar cell photoanodes. Electrochimica Acta 2015, 154, 190–196. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; Losciale, P.; Mishra, V.K.; Misra, A.N.; Nebauer, S.G; Shelonzek, H.; Rusinowski, S.; Bąba, W. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Yañuk, J.G.; Cabrerizo, F.M.; Dellatorre, F.G.; Cerdá, M.F. Photosensitizing role of R-phycoerythrin red protein and β-carboline alkaloids in Dye sensitized solar cell. Electrochemical and spectroscopic characterization. Energy Rep. 2020, 6, 25–36. [Google Scholar] [CrossRef]
- Enciso, P.; Decoppet, J.D.; Grätzel, M.; Wörner, M.; Cabrerizo, F.M.; Cerdá, M.F. A cockspur for the DSS cells: Erythrina crista-galli sensitizers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 176, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J. Theory of the impedance of electron diffusion and recombination in a thin layer. J. Phys. Chem. B 2002, 106, 325–333. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Heo, N.; Jun, Y.; Park, J. Dye molecules in electrolytes: new approach for suppression of dye-desorption in dye-sensitized solar cells. Sci Rep 2013, 3, 1712–1718. [Google Scholar] [CrossRef]
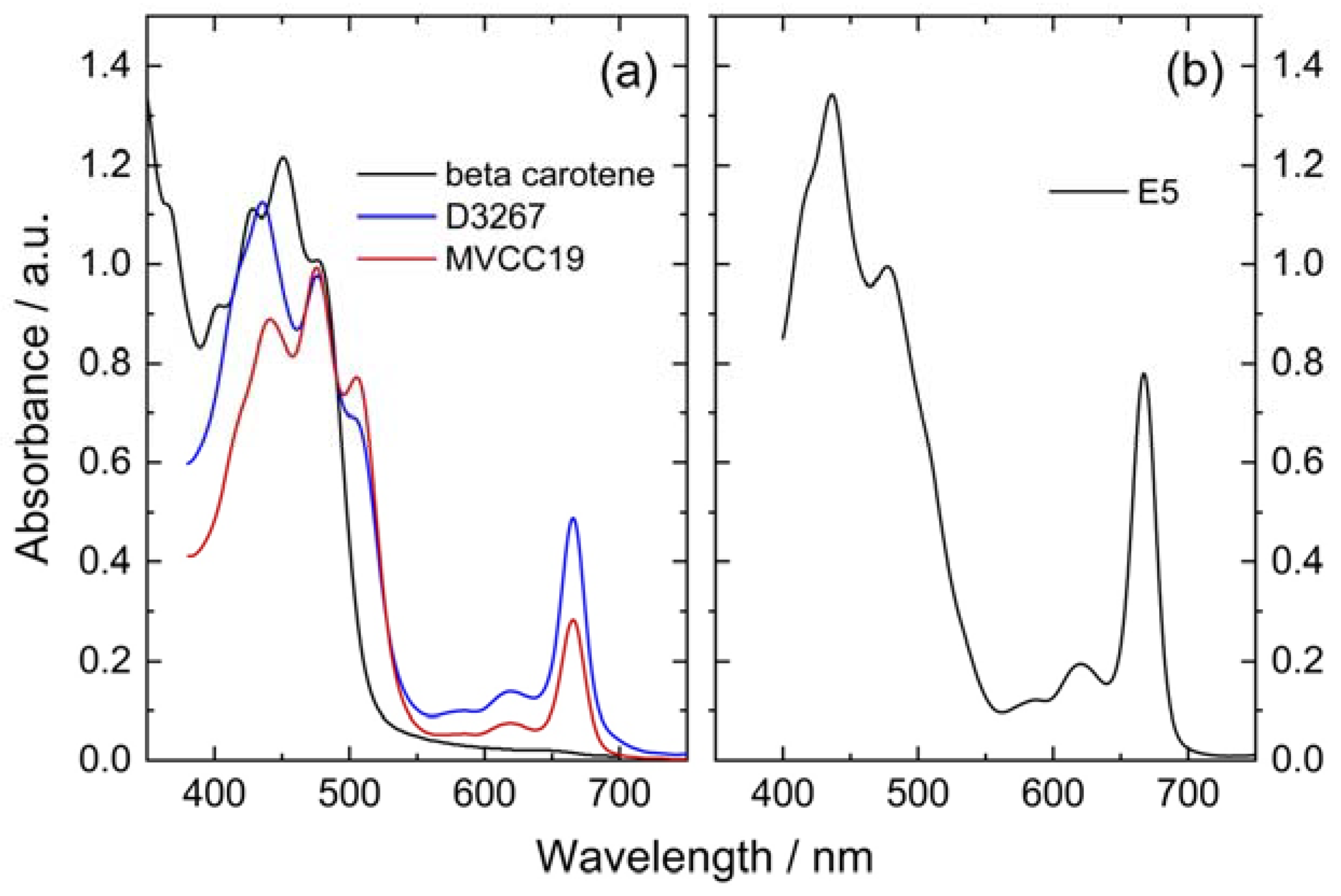

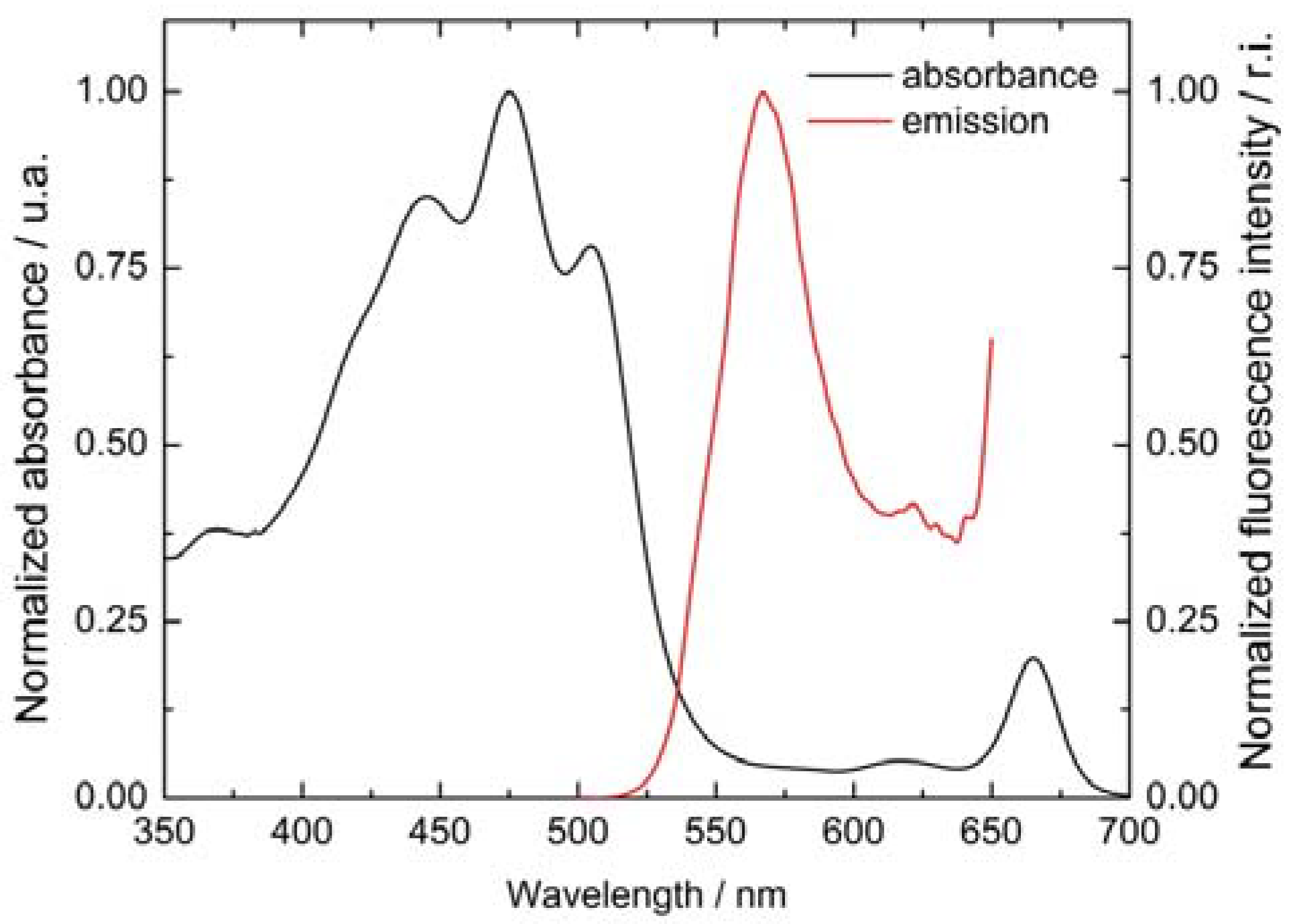
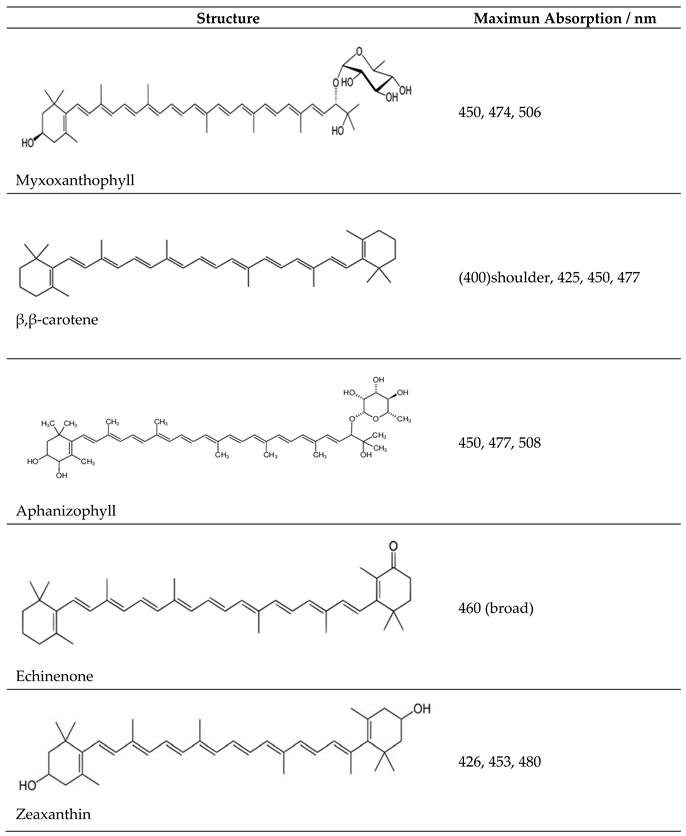
| Dye | Anodic / V | assignation | Cathodic / V |
|---|---|---|---|
| MVCC19 |
0.70 (0.72 and 0.84) | -C=C- (β,β-carotene) | - 0.78 |
| 0.90 - 1.10 | CHL | ||
| 1.25 (1.36 and 1.42) | -OH and -O- | ||
| D3267 |
0.95 | CHL | - 1.0 |
| 1.3 | -OH | ||
| E5 | 0.70 | -C=C- (β,β-carotene) | - 0.75 |
| 0.95 | CHL | ||
| 1.27 (1.30 and 1.40) | -OH and -O- | ||
| β,β-carotene | 0.75 | -C=C- | - 1.05 |
| Property/units | MVCC19 | D3267 | E5 | β,β-carotene |
|---|---|---|---|---|
| Jsc / Acm-2 | 3.4E-4 | 3.8E-4 | 2.6E-4 | 1.9E-4 |
| Voc / V | 0.56 | 0.54 | 0.50 | 0.48 |
| FF | 0.67 | 0.57 | 0.53 | 0.51 |
| η / % | 0.127 | 0.120 | 0.078 | 0.048 |
| MVCC19 | D3267 | E5 | β,β -carotene | |
|---|---|---|---|---|
| Γrec = Rct x Cµ / s | 0.018-0.03 | 0.012-0.02 | 0.0064 | 0.0060 |
| Γt = Rt x Cµ / s | 0.0010 | 0.0016 | 0.0023 | 0.0018 |
| Rce / ohm | 2 | 6 | 22 | 190 |
| Dye | Wavelength / nm | E0-0 / eV | ΔG° / eV |
|---|---|---|---|
| MVCC19 | 536 | 2.40 | -0.39 |
| D3267 | 517 | 2.31 | -0.72 |
| E5 | 614 | 2.02 | -0.22 |
| β,β-carotene | 432 | 2.87 | -0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





