Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Drugs and Compounds of Covid-Box
2.2. Parasites
2.3. Antiproliferative Assays against Tachyzoite Stage
2.4. Cytotoxicity Assay in NHDF Cell
2.5. Druggability Analysis of Drugs and Compounds
2.6 Transmission Electron Microscopy (TEM)
2.7. Statistical Analysis
3. Results
3.1. Drugs of Covid-Box Showed High Activity and Selectivity against T. gondii Tachyzoites
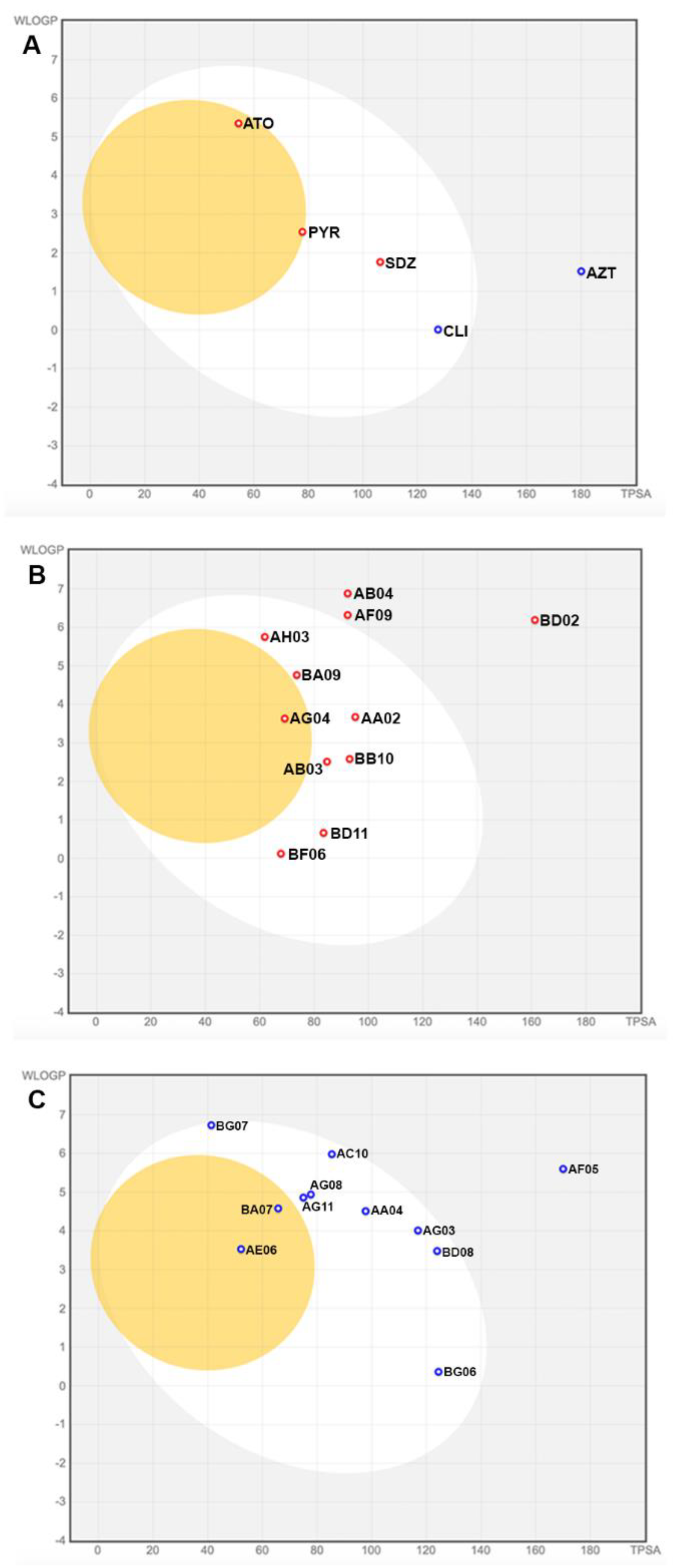
3.2. Covid-Box Drugs Show Potential Oral Druggability
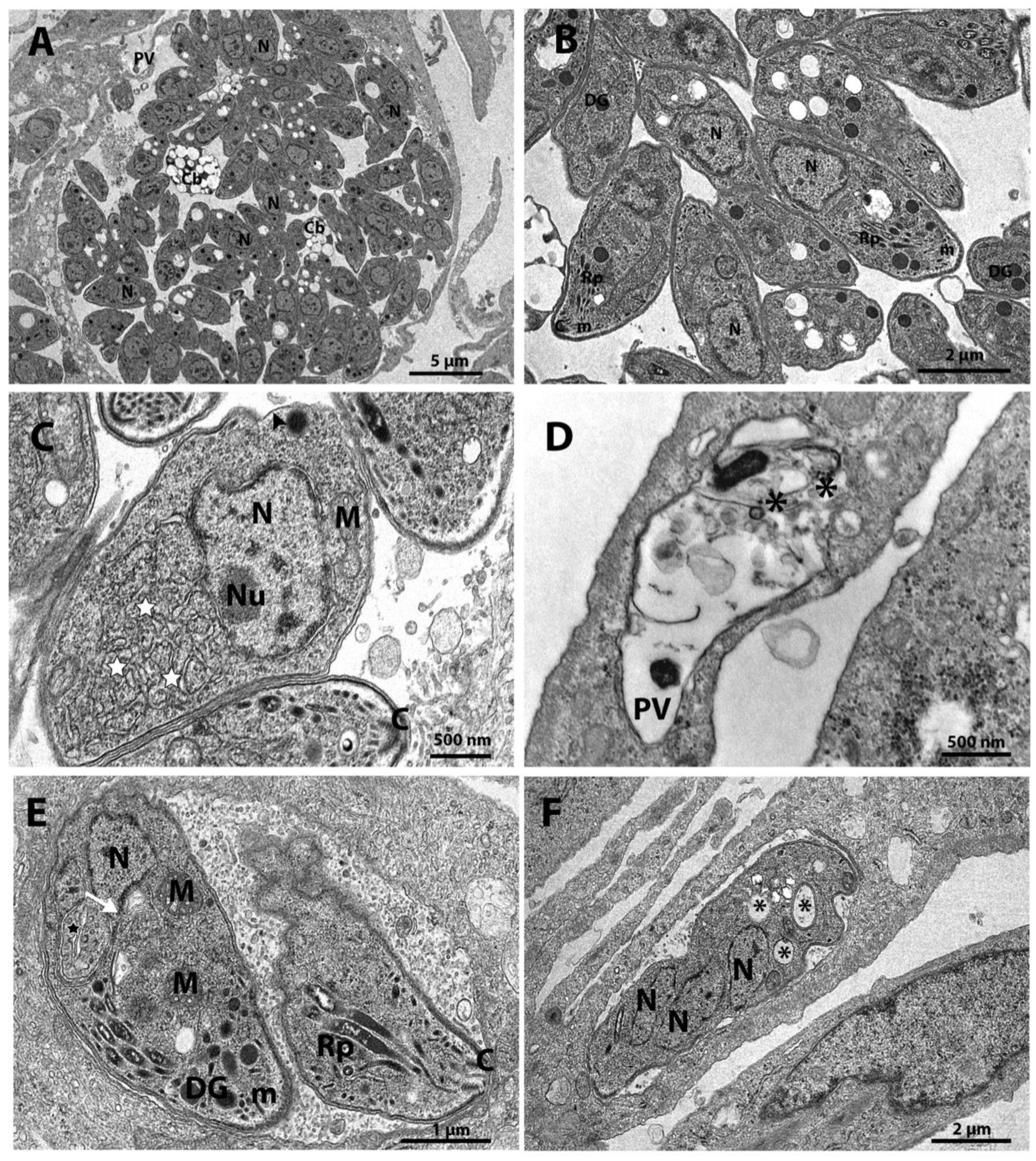
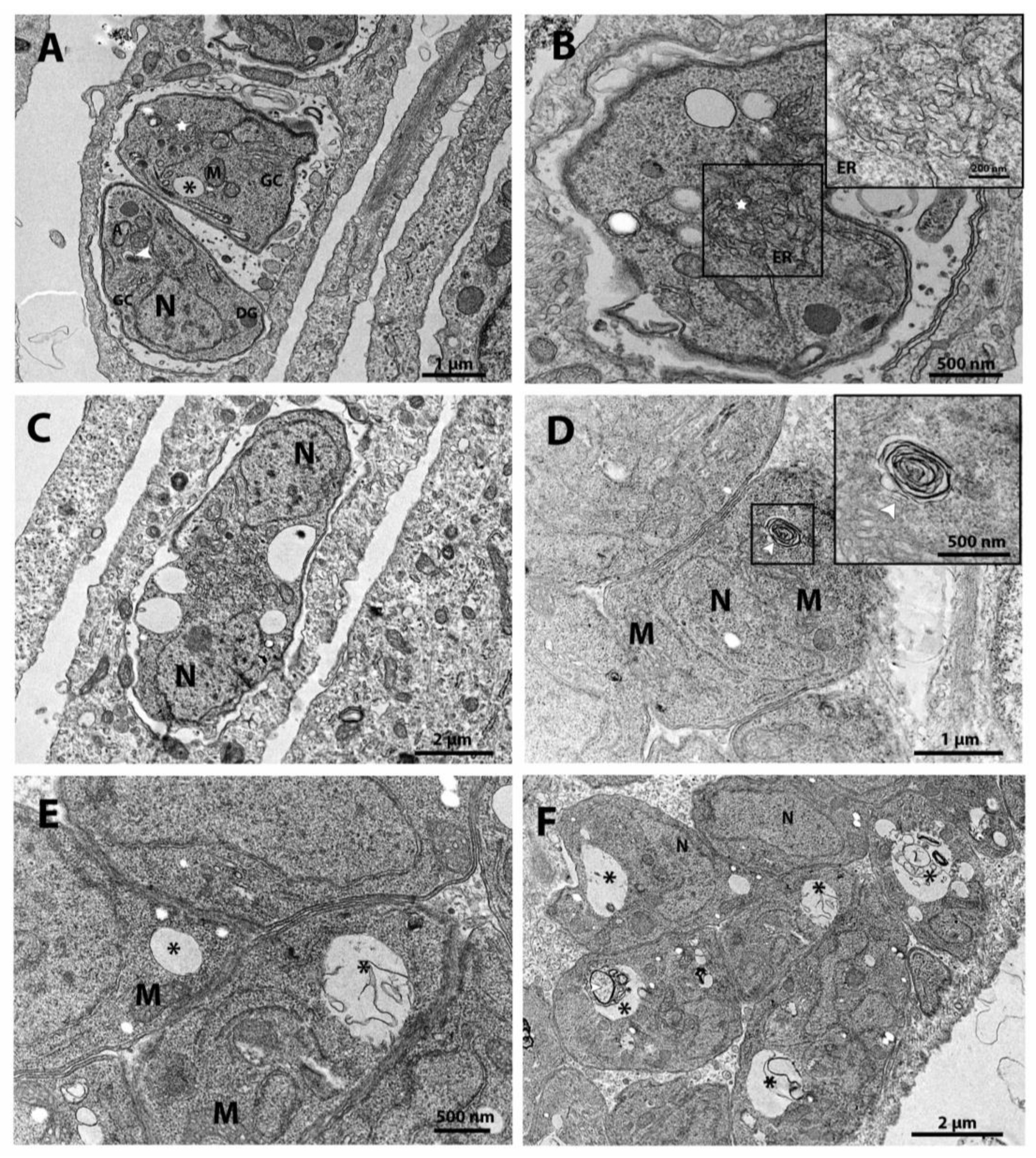
3.3. Analysis of the Effect on Ultrastructure Induced by Drugs and Compounds of Covid-Box by Transmission Electron Microscopy (TEM)
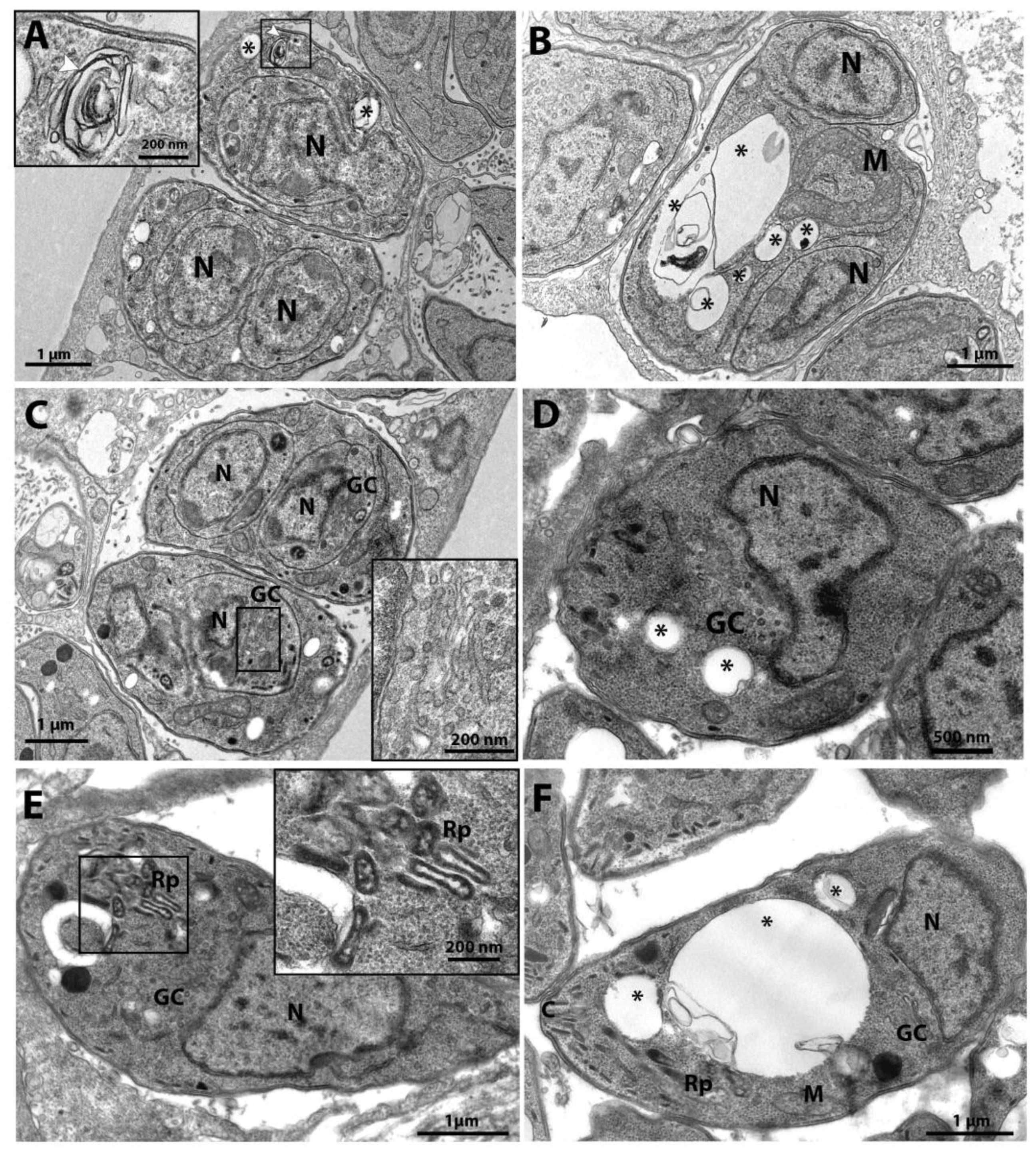
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Youda, E.L.; Sime, P.S.; Nansseu, J.R. Global, Regional, and Country Seroprevalence of Toxoplasma gondii in Pregnant Women: A Systematic Review, Modelling and Meta-Analysis. Sci Rep 2020, 10, 12102. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin Microbiol Rev 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Zangerle, R.; Allerberger, F.; Pohl, P.; Fritsch, P.; Dierich, M.P. High Risk of Developing Toxoplasmic Encephalitis in AIDS Patients Seropositive to Toxoplasma Gondii. Med Microbiol Immunol 1991, 180. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Santos, D.V.; Machado Azevedo, D.O.; Campos, W.R.; Oréfice, F.; Queiroz-Andrade, G.M.; Carellos, É.V.M.; Castro Romanelli, R.M.; Januário, J.N.; Resende, L.M.; Martins-Filho, O.A. Congenital Toxoplasmosis in Southeastern Brazil: Results of Early Ophthalmologic Examination of a Large Cohort of Neonates. Ophthalmology 2009, 116, 2199–2205. [Google Scholar] [CrossRef]
- Gilbert, R.E. Is Ocular Toxoplasmosis Caused by Prenatal or Postnatal Infection? British Journal of Ophthalmology 2000, 84, 224–226. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Freeman, K.; Lago, E.G.; Bahia-Oliveira, L.M.G.; Tan, H.K.; Wallon, M.; Buffolano, W.; Stanford, M.R.; Petersen, E. ; for The European Multicentre Study on Congenital Toxoplasmosis (EMSCOT) Ocular Sequelae of Congenital Toxoplasmosis in Brazil Compared with Europe. PLoS Negl Trop Dis 2008, 2, e277. [Google Scholar] [CrossRef]
- Jones, J.L.; Lopez, A.; Wilson, M.; Schulkin, J.; Gibbs, R. Congenital Toxoplasmosis: A Review. Obstetrical and Gynecological Survey.
- Diesel, A.A.; Zachia, S.D.A.; Müller, A.L.L.; Perez, A.V.; Uberti, F.A.D.F.; Magalhães, J.A.D.A. Follow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil. Rev Bras Ginecol Obstet 2019, 41, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin Microbiol Rev 2018, 31, e00057–17. [Google Scholar] [CrossRef]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob Agents Chemother 2015, 59, 7161–7169. [Google Scholar] [CrossRef]
- Silva, L.A.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Vítor, R.W.A. Genetic Polymorphisms and Phenotypic Profiles of Sulfadiazine-Resistant and Sensitive Toxoplasma gondii Isolates Obtained from Newborns with Congenital Toxoplasmosis in Minas Gerais, Brazil. PLoS ONE 2017, 12, e0170689. [Google Scholar] [CrossRef]
- Silva, L.A.; Fernandes, M.D.; Machado, A.S.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Almeida Vitor, R.W. Efficacy of Sulfadiazine and Pyrimetamine for Treatment of Experimental Toxoplasmosis with Strains Obtained from Human Cases of Congenital Disease in Brazil. Experimental Parasitology 2019, 202, 7–14. [Google Scholar] [CrossRef] [PubMed]
- De Lima Bessa, G.; Vitor, R.W.D.A.; Lobo, L.M.S.; Rêgo, W.M.F.; De Souza, G.C.A.; Lopes, R.E.N.; Costa, J.G.L.; Martins-Duarte, E.S. In Vitro and in Vivo Susceptibility to Sulfadiazine and Pyrimethamine of Toxoplasma gondii Strains Isolated from Brazilian Free Wild Birds. Sci Rep 2023, 13, 7359. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. Journal of Pharmacy and Pharmacology 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Boyom, F.F.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Spangenberg, T.; Mfopa, A.N.; Kouipou, R.M.T.; Mbouna, C.J.; Donfack, V.F.D.; Zollo, P.H.A. Repurposing the Open Access Malaria Box To Discover Potent Inhibitors of Toxoplasma gondii and Entamoeba Histolytica. Antimicrob Agents Chemother 2014, 58, 5848–5854. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Belekar, M.A.; Shukla, A.; Tong, J.X.; Sinha, A.; Chu, T.T.T.; Kulkarni, A.S.; Preiser, P.R.; Reddy, D.S.; Tan, K.S.W.; et al. Targeted Phenotypic Screening in Plasmodium Falciparum and Toxoplasma gondii Reveals Novel Modes of Action of Medicines for Malaria Venture Malaria Box Molecules. mSphere 2018, 3, e00534–17. [Google Scholar] [CrossRef]
- Varberg, J.M.; LaFavers, K.A.; Arrizabalaga, G.; Sullivan, W.J. Characterization of Plasmodium Atg3-Atg8 Interaction Inhibitors Identifies Novel Alternative Mechanisms of Action in Toxoplasma gondii. Antimicrob Agents Chemother 2018, 62, e01489–17. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.-H.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villena, I. Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob Agents Chemother 2018, 62, e01640–17. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.B.; Burrows, J.N.; Goldberg, D.E.; Sibley, L.D. Evaluation of Current and Emerging Antimalarial Medicines for Inhibition of Toxoplasma gondii Growth in Vitro. ACS Infect. Dis. 2018, 4, 1264–1274. [Google Scholar] [CrossRef]
- dos Santos, M.; Oliveira Costa, A.L.; Vaz, G.H. de S.; de Souza, G.C.A.; Vitor, R.W. de A.; Martins-Duarte, É.S. Medicines for Malaria Venture Pandemic Box In Vitro Screening Identifies Compounds Highly Active against the Tachyzoite Stage of Toxoplasma gondii. Tropical Medicine and Infectious Disease 2023, 8. [Google Scholar] [CrossRef]
- Dos Santos, B.R.; Ramos, A.B.D.S.B.; De Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimão, J.Q. Anti- Toxoplasma gondii Screening of MMV Pandemic Response Box and Evaluation of RWJ-67657 Efficacy in Chronically Infected Mice. Parasitology 2023, 150, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Cajazeiro, D.C.; Toledo, P.P.M.; De Sousa, N.F.; Scotti, M.T.; Reimão, J.Q. Drug Repurposing Based on Protozoan Proteome: In Vitro Evaluation of In Silico Screened Compounds against Toxoplasma gondii. Pharmaceutics 2022, 14, 1634. [Google Scholar] [CrossRef]
- Dos Santos, B.R.; Ramos, A.B.D.S.B.; De Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimão, J.Q. Repurposing the Medicines for Malaria Venture’s COVID Box to Discover Potent Inhibitors of Toxoplasma gondii, and in Vivo Efficacy Evaluation of Almitrine Bismesylate (MMV1804175) in Chronically Infected Mice. PLoS ONE 2023, 18, e0288335. [Google Scholar] [CrossRef] [PubMed]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-Carboxymethoxyphenyl)-2-(4,5-Dimethylthiazolyl)-3-(4-Sulfophenyl)Tetrazolium, Inner Salt (MTS) and Related Analogs of 3-(4,5-Dimethylthiazolyl)-2,5-Diphenyltetrazolium Bromide (MTT) Reducing to Purple Water-Soluble Formazans As Cell-Viability Indicators. Bioorganic & Medicinal Chemistry Letters 1991, 1, 611–614. [Google Scholar] [CrossRef]
- Martins-Duarte, E.S.; Portes, J. de A.; da Silva, R.B.; Pires, H.S.; Garden, S.J.; de Souza, W. In Vitro Activity of N-Phenyl-1,10-Phenanthroline-2-Amines against Tachyzoites and Bradyzoites of Toxoplasma gondii. Bioorganic & Medicinal Chemistry 2021, 50, 116467. [Google Scholar] [CrossRef]
- Martins-Duarte, E.S.; Dubar, F.; Lawton, P.; França Da Silva, C.; Soeiro, M.D.N.; De Souza, W.; Biot, C.; Vommaro, R.C. Ciprofloxacin Derivatives Affect Parasite Cell Division and Increase the Survival of Mice Infected with Toxoplasma gondii. PLoS ONE 2015, 10, e0125705. [Google Scholar] [CrossRef]
- Martins-Duarte, É.D.S.; De Souza, W.; Vommaro, R.C. Itraconazole Affects Toxoplasma gondii Endodyogeny:. FEMS Microbiology Letters 2008, 282, 290–298. [Google Scholar] [CrossRef]
- Martins-Duarte, É.S.; Lemgruber, L.; De Souza, W.; Vommaro, R.C. Toxoplasma Gondii: Fluconazole and Itraconazole Activity against Toxoplasmosis in a Murine Model. Experimental Parasitology 2010, 124, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Comte, R.; Pechère, J.C. In Vitro and in Vivo Effects of Doxycycline on Toxoplasma gondii. Antimicrob Agents Chemother 1990, 34, 775–780. [Google Scholar] [CrossRef]
- McCabe, R.E.; Luft, B.J.; Remington, J.S. THE EFFECTS OF VYCLOSPORINE ON TOXOPLASMA GONDII IN VIVO AN IN VITRO: Transplantation 1986, 41, 611–615. [CrossRef]
- Dittmar, A.J.; Drozda, A.A.; Blader, I.J. Drug Repurposing Screening Identifies Novel Compounds That Effectively Inhibit Toxoplasma gondii Growth. mSphere 2016, 1, e00042–15. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Atolani, O.; Awakan, O.J.; Olaolu, D.; Nwonuma, C.O.; Alejolowo, O.; Otohinoyi, D.A.; Rotimi, D.; Owolabi, A.; Batiha, G.E.-S. In Vitro Screening to Identify Anti-Toxoplasma Compounds and In Silico Modeling for Bioactivities and Toxicity. 2019.
- Zhang, J.L.; Si, H.F.; Shang, X.F.; Zhang, X.K.; Li, B.; Zhou, X.Z.; Zhang, J.Y. New Life for an Old Drug: In Vitro and in Vivo Effects of the Anthelmintic Drug Niclosamide against Toxoplasma gondii RH Strain. International Journal for Parasitology: Drugs and Drug Resistance 2019, 9, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gurnett, A.M.; Dulski, P.M.; Darkin-Rattray, S.J.; Carrington, M.J.; Schmatz, D.M. Selective Labeling of Intracellular Parasite Proteins by Using Ricin. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 2388–2392. [Google Scholar] [CrossRef]
- Bilgin, M.; Yildirim, T.; Hokelek, M. In Vitro Effects ofIvermectin and Sulphadiazine on Toxoplasma gondii. Balkan Med J 2013, 30, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.-F.; Wang, M.-Y.; Ai, J.-P.; Shen, Q.-K.; Guo, H.-Y.; Jin, C.-M.; Chen, F.-E.; Quan, Z.-S.; Jin, L.; Zhang, C. Synthesis and Evaluation of Mycophenolic Acid Derivatives as Potential Anti-Toxoplasma gondii Agents. Med Chem Res 2021, 30, 2228–2239. [Google Scholar] [CrossRef]
- Castro-Elizalde, K.N.; Hernández-Contreras, P.; Ramírez-Flores, C.J.; González-Pozos, S.; Gómez De León, C.T.; Mondragón-Castelán, M.; Mondragón-Flores, R. Mycophenolic Acid Induces Differentiation of Toxoplasma gondii RH Strain Tachyzoites into Bradyzoites and Formation of Cyst-like Structure in Vitro. Parasitol Res 2018, 117, 547–563. [Google Scholar] [CrossRef]
- Egawa, Y.; Oshima, S.; Umezawa, S. Studies on Cycloheximide-Related Compounds. I Esters of Cycloheximide and Their Antitoxoplasmic Activity 1965.
- Beckers, C.J.; Roos, D.S.; Donald, R.G.; Luft, B.J.; Schwab, J.C.; Cao, Y.; Joiner, K.A. Inhibition of Cytoplasmic and Organellar Protein Synthesis in Toxoplasma gondii. Implications for the Target of Macrolide Antibiotics. J. Clin. Invest. 1995, 95, 367–376. [Google Scholar] [CrossRef]
- Fichera, M.E.; Bhopale, M.K.; Roos, D.S. In Vitro Assays Elucidate Peculiar Kinetics of Clindamycin Action against Toxoplasma gondii. Antimicrob Agents Chemother 1995, 39, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci Rep 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Advanced Drug Delivery Reviews 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A. The Effect of Cytochrome P450 Metabolism on Drug Response, Interactions, and Adverse Effects. Cytochrome P 2007, 76. [Google Scholar]
- Sanchez-Covarrubias, L.; Slosky, L.; Thompson, B.; Davis, T.; Ronaldson, P. Transporters at CNS Barrier Sites: Obstacles or Opportunities for Drug Delivery? CPD 2014, 20, 1422–1449. [Google Scholar] [CrossRef] [PubMed]
- Abdrakhmanov, A.; Gogvadze, V.; Zhivotovsky, B. To Eat or to Die: Deciphering Selective Forms of Autophagy. Trends in Biochemical Sciences 2020, 45, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Pedra-Rezende, Y.; Macedo, I.S.; Midlej, V.; Mariante, R.M.; Menna-Barreto, R.F.S. Different Drugs, Same End: Ultrastructural Hallmarks of Autophagy in Pathogenic Protozoa. Front. Microbiol. 2022, 13, 856686. [Google Scholar] [CrossRef] [PubMed]
- Pichler, H.; Gaigg, B.; Hrastnik, C.; Achleitner, G.; Kohlwein, S.D.; Zellnig, G.; Perktold, A.; Daum, G. A Subfraction of the Yeast Endoplasmic Reticulum Associates with the Plasma Membrane and Has a High Capacity to Synthesize Lipids. European Journal of Biochemistry 2001, 268, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Osborn, C.D.; Holloway, F.A. Can Commonly Used Antibiotics Disrupt Formation of New Memories? Bull. Psychon. Soc. 1984, 22, 356–358. [Google Scholar] [CrossRef]
- Macias-Silva, M.; Vazquez-Victorio, G.; Hernandez-Damian, J. Anisomycin Is a Multifunctional Drug: More than Just a Tool to Inhibit Protein Synthesis. curr chem biol 2010, 4, 124–132. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Serricchio, M.; Striepen, B.; Bütikofer, P. Lipid Synthesis in Protozoan Parasites: A Comparison between Kinetoplastids and Apicomplexans. Progress in Lipid Research 2013, 52, 488–512. [Google Scholar] [CrossRef]
- Martins-Duarte, É.S.; Carias, M.; Vommaro, R.; Surolia, N.; De Souza, W. Apicoplast Fatty Acid Synthesis Is Essential for Pellicle Formation at the End of Cytokinesis in Toxoplasma gondii. Journal of Cell Science 2016, 129, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Martins-Duarte, E.S.; Jones, S.M.; Gilbert, I.H.; Atella, G.C.; De Souza, W.; Vommaro, R.C. Thiolactomycin Analogues as Potential Anti-Toxoplasma gondii Agents. Parasitology International 2009, 58, 411–415. [Google Scholar] [CrossRef]
- Kisselev, A.F.; Callard, A.; Goldberg, A.L. Importance of the Different Proteolytic Sites of the Proteasome and the Efficacy of Inhibitors Varies with the Protein Substrate. Journal of Biological Chemistry 2006, 281, 8582–8590. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Huber, R.; Moroder, L. The Persisting Challenge of Selective and Specific Proteasome Inhibition. Journal of Peptide Science 2009, 15, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Bhat, G.; Guruprasad, K. Analysis of Bortezomib Inhibitor Docked within the Catalytic Subunits of the Plasmodium falciparum 20S Proteasome. SpringerPlus 2013, 2, 566. [Google Scholar] [CrossRef] [PubMed]
- Kreidenweiss, A.; Kremsner, P.G.; Mordmüller, B. Comprehensive Study of Proteasome Inhibitors against Plasmodium falciparum Laboratory Strains and Field Isolates from Gabon. Malar J 2008, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Mayol, G.F.; Revuelta, M.V.; Salusso, A.; Touz, M.C.; Rópolo, A.S. Evidence of Nuclear Transport Mechanisms in the Protozoan Parasite Giardia lamblia. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2020, 1867, 118566. [Google Scholar] [CrossRef]
- Gupta, S.; Vohra, S.; Sethi, K.; Gupta, S.; Bera, B.C.; Kumar, S.; Kumar, R. In Vitro Anti-Trypanosomal Effect of Ivermectin on Trypanosoma evansi by Targeting Multiple Metabolic Pathways. Trop Anim Health Prod 2022, 54, 240. [Google Scholar] [CrossRef] [PubMed]
- Fraccaroli, L.; Ruiz, M.D.; Perdomo, V.G.; Clausi, A.N.; Balcazar, D.E.; Larocca, L.; Carrillo, C. Broadening the Spectrum of Ivermectin: Its Effect on Trypanosoma cruzi and Related Trypanosomatids. Front. Cell. Infect. Microbiol. 2022, 12, 885268. [Google Scholar] [CrossRef]
- Reis, T.A.R.; Oliveira-da-Silva, J.A.; Tavares, G.S.V.; Mendonça, D.V.C.; Freitas, C.S.; Costa, R.R.; Lage, D.P.; Martins, V.T.; Machado, A.S.; Ramos, F.F.; et al. Ivermectin Presents Effective and Selective Antileishmanial Activity in Vitro and in Vivo against Leishmania infantum and Is Therapeutic against Visceral Leishmaniasis. Experimental Parasitology 2021, 221, 108059. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Chen, C.; Wang, X.; Qu, F.; Wang, H.; Yang, K.; Wang, Q.; Zhao, N.; Meng, J.; et al. Ivermectin Induces Autophagy-Mediated Cell Death through the AKT/mTOR Signaling Pathway in Glioma Cells. Bioscience Reports 2019, 39, BSR20192489. [Google Scholar] [CrossRef]
- Sintchak, M.D.; Nimmesgern, E. The Structure of Inosine 5′-Monophosphate Dehydrogenase and the Design of Novel Inhibitors. Immunopharmacology 2000, 47, 163–184. [Google Scholar] [CrossRef]
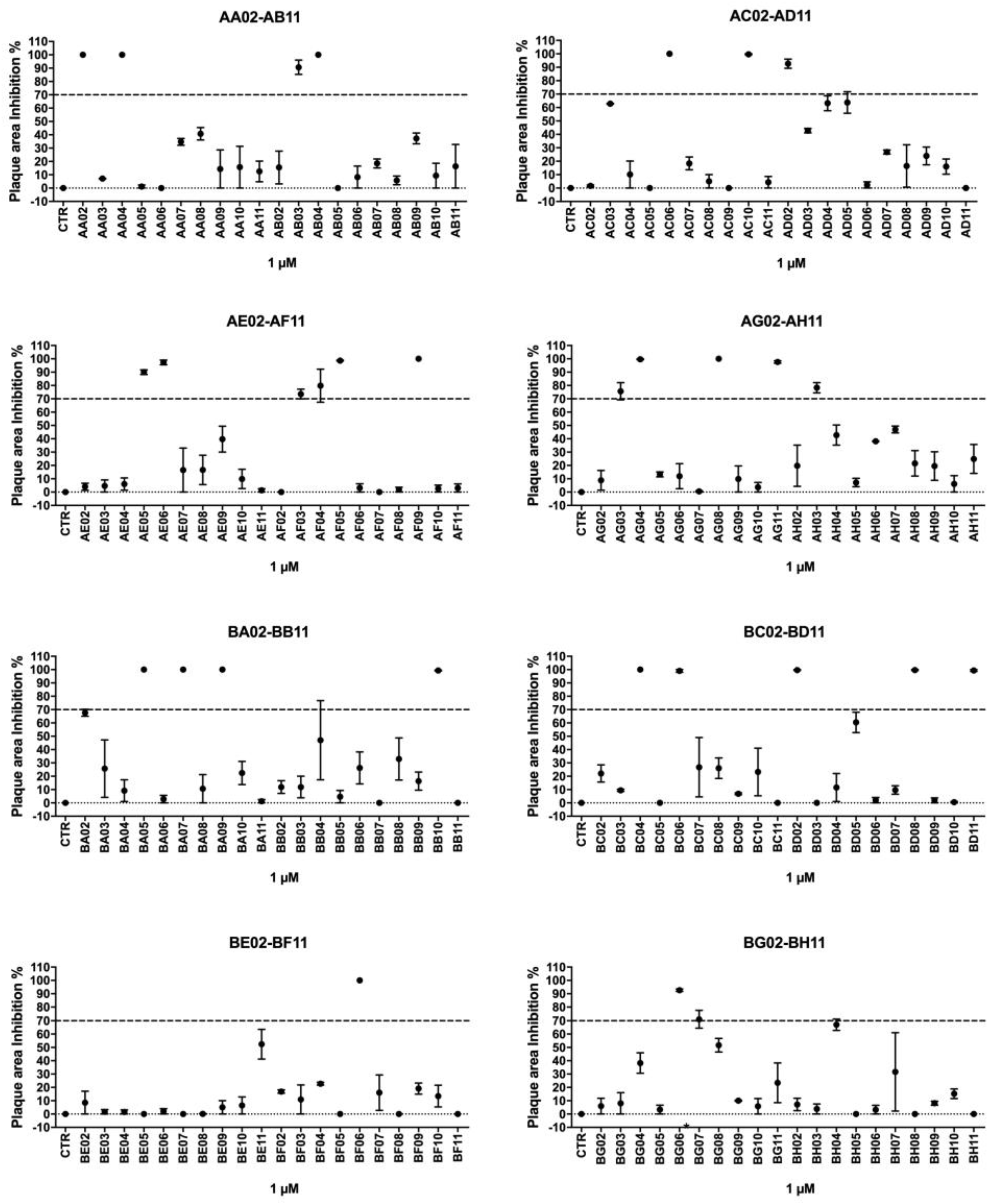
| Plate Position | MMV ID | Trivial Name |
Disease Area | Statusa | IC50 (μM) in tachyzoites of RH strain | Cytotoxicity against NHDF | Anti-T. gondii activityc | |
|---|---|---|---|---|---|---|---|---|
| CC50 (μM) | SIb | |||||||
| AA02 | MMV003461 | Niclosamide | Antiparasitic | Approved | 0.36 ± 0.02 | 3.05 ± 0.08 | 8 | Yes [24,34] |
| AA04 | MMV1804190 | Bemcentinib | Immune agent | Phase II | 0.15 ± 0.03 |
0.97 ± 0.15 | 6 | Yes [24] |
| AB03 | MMV1804187 | Apilimode | Antitumor agent | Phase II | 0.22 ± 0.06 |
2.12 ± 0.12 | 10 | New |
| AB04 | MMV1804185 | Regorafenibe | Antitumor Agent | Approved | 0.25 ± 0.04 |
3.03 ± 0.01 | 12 | Yes [24] |
| AC10 | MMV690777 | LY2228820 | Antitumor agent | Phase II, discontinued | 0.04 ± 0.00 |
nd | nd | Yes [24] |
| AD02 | MMV002832 | Digoxin | Antiarrhythmic | Approved | 0.03 ± 0.01 |
0.34 ± 0.32 | 11 | Yes [23] |
| AE06 | MMV688731 | Emetine | Antiparasitic | Approved | 0.05 ± 0.01 | 1.02 ± 0.19 | 20 | Yes [35] |
| AF05 | MMV672931 | Ivermectin | Antiparasitic | Approved | 0.21 ± 0.01 |
nd | nd | Yes [36] |
| AF09 | MMV010306 | Sorafenib | Antitumor agent | Approved | 0.56 ± 0.03 |
nd | nd | Yes [24] |
| AG03 | MMV1804194 | Manidipine | Antihypertensive | Phase III | 0.74 ± 0.09 |
nd | nd | Yes [24] |
| AG04 | MMV1804175 | Almitrine | Respiratory systemAgent | Approved* | 0.33 ± 0.04 |
nd | n.d | Yes [23,24] |
| AG08 | MMV010288 | Midostaurin | Antitumor agent | Approved | 0.08 ± 0.00 |
0.24 ± 0.02 | 3 | New |
| AG11 | MMV1804174 | Abemaciclib | Antitumor agent | Approved | 0.09 ± 0.01 |
# | # | Yes [24] |
| AH03 | MMV003277 | Tetrandrine | Antitumor Agent | Preclinical | 0.35 ± 0.07 |
4.77 ± 8.25 | 14 | Yes [24] |
| BA07 | MMV1580167 | Ponatinib | Antitumor agent | Approved | 0.33 ± 0.03 |
4.84 ± 0.89 | 15 | Yes [33] |
| BA09 | MMV007474 | Berbamine | Antitumor Agent | Preclinical | 0.31 ± 0.03 |
4.61 ± 7.52 | 15 | Yes [24] |
| BB10 | MMV003219 | Mycophenolic acid | Immunosuppressant | Approved | 0.07 ± 0.01 |
23.81 ± 31.97 | 340 | Yes [37,38] |
| BD02 | MMV1804312 | Salinomycin | Anti-microbial agent | Approved | 0.07 ± 0.01 |
1.14 ± 0.17 | 16 | New |
| BD08 | MMV1804359 | Merimepodib | Antiviral agent | Phase II | 0.48 ± 0.08 |
nd | nd | Yes [24] |
| BD11 | MMV000031 | Cycloheximide | Agricultural agent – Fungicid |
Research | 0.02 ± 0.00 |
6.08 ± 4.63 | 304 | Yes [39,40] |
| BF06 | MMV1634116 | (-) -Anisomycin | Anti-infective agent | Approved | 0.02 ± 0.00 |
0.25 ± 0.13 | 13 | Yes [41] |
| BG06 | MMV009415 | Bortezomib | Antitumor agent | Approved | 0.03 ± 0.00 |
0.72 ± 0.24 | 24 | Yes [23,33] |
| BG07 | MMV002137 | Pimozide | Antipsychotic | Approved | 0.64 ± 0.15 |
nd | nd | Yes [23,32] |
| Identification | LogPa | H-bond donors |
H-bond acceptors |
MWb | n◦ violations Lipinski | TPSA (Å2)c |
n◦ rotations |
n◦ violations Veber | |
| PYR | Pyrimethamine | 2.84 | 2 | 4 | 248.72 | 0 | 77.83 | 2 | 0 |
| SDZ | Sulfadiazine | 0.86 | 3 | 5 | 250.28 | 0 | 98.57 | 3 | 0 |
| CLI | Clindamycin | 0.39 | 4 | 7 | 424.99 | 0 | 102.25 | 7 | 1 |
| AZT | Azithromycin | 2.50 | 5 | 14 | 749.00 | 2 | 198.54 | 7 | 1 |
| ATO | Atovaquone | 5.34 | 1 | 3 | 366.84 | 1 | 54.70 | 2 | 0 |
| AA02 | Niclosamide | 3.85 | 2 | 4 | 327.12 | 0 | 128.62 | 3 | 0 |
| AA04 | Bemcentinib | 4.88 | 2 | 8 | 506.66 | 2 | 92.35 | 4 | 0 |
| AB03 | Apilimode | 3.08 | 1 | 8 | 418.50 | 0 | 84.77 | 8 | 0 |
| AB04 | Regorafenibe | 4.39 | 3 | 7 | 482.82 | 0 | 92.35 | 5 | 0 |
| AC10 | LY2228820 | 5.51 | 2 | 5 | 420.54 | 1 | 85.41 | 5 | 0 |
| AD02 | Digoxin | 2.22 | 6 | 14 | 780.95 | 3 | 203.06 | 7 | 1 |
| AE06 | Emetine | 3.04 | 1 | 6 | 480.65 | 0 | 52.20 | 7 | 0 |
| AF05 | Ivermectin | 4.37 | 3 | 14 | 875.11 | 2 | 170.06 | 8 | 1 |
| AF09 | Sorafenib | 4.10 | 3 | 7 | 464.83 | 0 | 92.35 | 9 | 0 |
| AG03 | Manidipine | 4.04 | 1 | 8 | 610.71 | 1 | 116.94 | 12 | 1 |
| AG04 | Almitrine | 4.34 | 2 | 6 | 477.55 | 0 | 69.21 | 10 | 0 |
| AG08 | Midostaurin | 4.05 | 1 | 4 | 570.65 | 1 | 77.73 | 4 | 0 |
| AG11 | Abemaciclib | 4.04 | 1 | 8 | 506.59 | 1 | 75.00 | 7 | 0 |
| AH03 | Tetrandrine | 5.49 | 0 | 8 | 622.75 | 2 | 61.86 | 4 | 0 |
| BA07 | Ponatinib | 4.30 | 1 | 8 | 532.56 | 1 | 65.77 | 6 | 0 |
| BA09 | Berbamine | 5.13 | 1 | 8 | 608.72 | 2 | 72.86 | 3 | 0 |
| BB10 | Mycophenolic acid | 2.72 | 2 | 6 | 320.34 | 0 | 93.07 | 6 | 0 |
| BD02 | Salinomycin | 4.98 | 4 | 11 | 751.01 | 2 | 161.21 | 12 | 2 |
| BD08 | Merimepodib | 2.36 | 3 | 7 | 452.46 | 0 | 123.96 | 11 | 1 |
| BD11 | Cycloheximide | 1.23 | 2 | 4 | 281.35 | 0 | 83.47 | 3 | 0 |
| BF06 | (-) -Anisomycin | 1.00 | 2 | 5 | 265.30 | 0 | 67.79 | 5 | 0 |
| BG06 | Bortezomib | 0.22 | 4 | 6 | 384.24 | 0 | 124.44 | 11 | 1 |
| BG07 | Pimozide | 5.67 | 1 | 5 | 461.55 | 1 | 41.29 | 7 | 0 |
| Identification | Caco-2 Permeabilitya | Intestinal Absorption (human) |
Fraction Unbound (human) |
VDss (Human)b |
CNS permeabilityc | BBB permeabilityd | |
|---|---|---|---|---|---|---|---|
| PYR | Pyrimethamine | 0.927 | 92.74% | 0.311 | -0.307 | -2.203 | -0.166 |
| SDZ | Sulfadiazine | 0.702 | 73.92% | 0.28 | 0.182 | -2.87 | -0.672 |
| CLI | Clindamycin | 0.063 | 53.28% | 0.747 | -0.206 | -3.63 | -0.943 |
| AZT | Azithromycin | -0.211 | 45.81% | 0.719 | -0.214 | -4.12 | -1.494 |
| ATO | Atovaquone | 1.483 | 91.41% | 0 | 0.329 | -1.418 | 0.401 |
| AA02 | Niclosamide | 0.886 | 89.48% | 0 | -0.022 | -1.972 | -0.626 |
| AA04 | Bemcentinib | 1.654 | 91.04% | 0.152 | 0.799 | -2.097 | -0,953 |
| AB03 | Apilimode | 1.388 | 96.75% | 0.044 | -0.173 | -2.901 | -1.047 |
| AB04 | Regorafenibe | 0.454 | 93.43% | 0 | -0.277 | -2.031 | -1.573 |
| AC10 | LY2228820 | 1.321 | 84.39% | 0.367 | 0.066 | -1.674 | -1.378 |
| AD02 | Digoxin | 0.381 | 78.23% | 0.287 | 0.085 | -4.19 | -1.927 |
| AE06 | Emetine | 1.333 | 95.40% | 0.155 | 1.632 | -2.041 | 0.045 |
| AF05 | Ivermectin | 0.602 | 89.45% | 0.126 | 0.587 | -3.438 | -2.000 |
| AF09 | Sorafenib | 0.907 | 84.99% | 0 | -0.105 | -1.995 | -1.675 |
| AG03 | Manidipine | 0.928 | 93.59% | 0.086 | 0.683 | -2.339 | -0.998 |
| AG04 | Almitrine | 1.392 | 87.59% | 0.136 | 1.189 | -2.727 | -0.945 |
| AG08 | Midostaurin | 0.985 | 98.28% | 0.286 | -1.663 | -1.764 | 0.039 |
| AG11 | Abemaciclib | 1.341 | 89.68% | 0.097 | 0.614 | -2.981 | -1.665 |
| AH03 | Tetrandrine | 0.737 | 92.83% | 0.371 | -0.808 | -2.576 | 0.074 |
| BA07 | Ponatinib | 1.000 | 91.44% | 0.040 | 0.565 | -1.862 | 0.278 |
| BA09 | Berbamine | 1.143 | 92.79% | 0.399 | -0.999 | -2.608 | -0.936 |
| BB10 | Mycophenolic acid | 0.244 | 62.24% | 0.217 | -0.651 | -2.908 | -0.159 |
| BD02 | Salinomycin | -0.140 | 58.65% | 0.220 | 0.372 | -3.068 | -1.754 |
| BD08 | Merimepodib | 1.024 | 93.76% | 0 | -0.122 | -3.32 | -1.647 |
| BD11 | Cycloheximide | 0.467 | 69.78% | 0.51 | -0.042 | -2.996 | -0.162 |
| BF06 | (-) -Anisomycin | 0.244 | 80.65% | 0.574 | 0.134 | -2.932 | -0.301 |
| BG06 | Bortezomib | 0.292 | 54.25% | 0.235 | -0.736 | -4.102 | -1.397 |
| BG07 | Pimozide | 0.399 | 85.97% | 0.137 | 0.071 | -1.314 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





