Submitted:
13 August 2024
Posted:
14 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
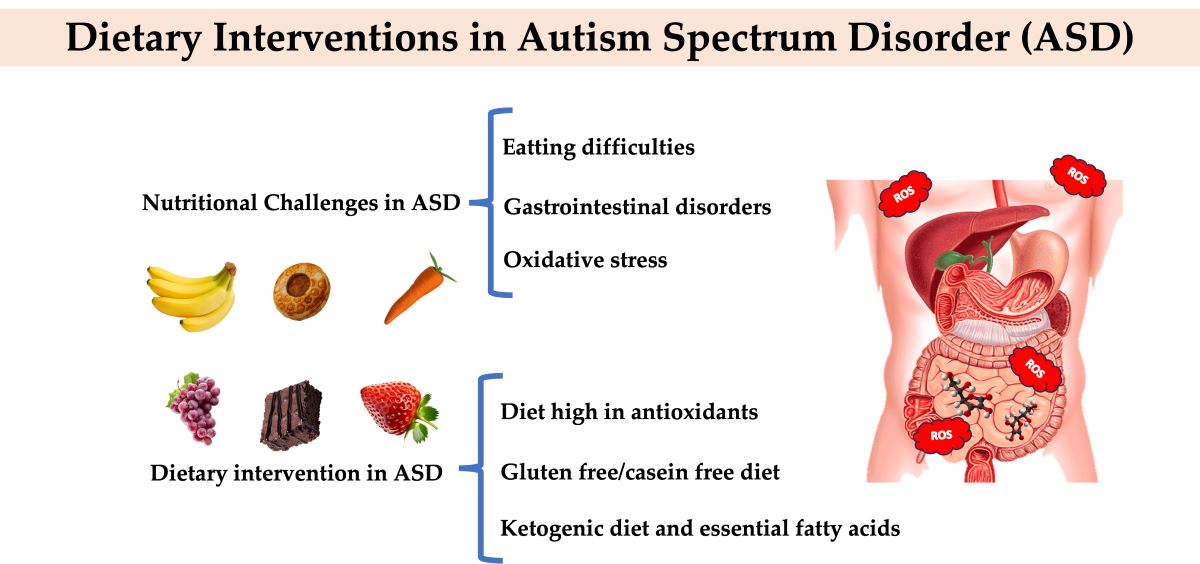
2. Nutritional Challenges in Autism Spectrum Disorder
2.1. Eating Difficulties
2.2. Gastrointestinal Disorders
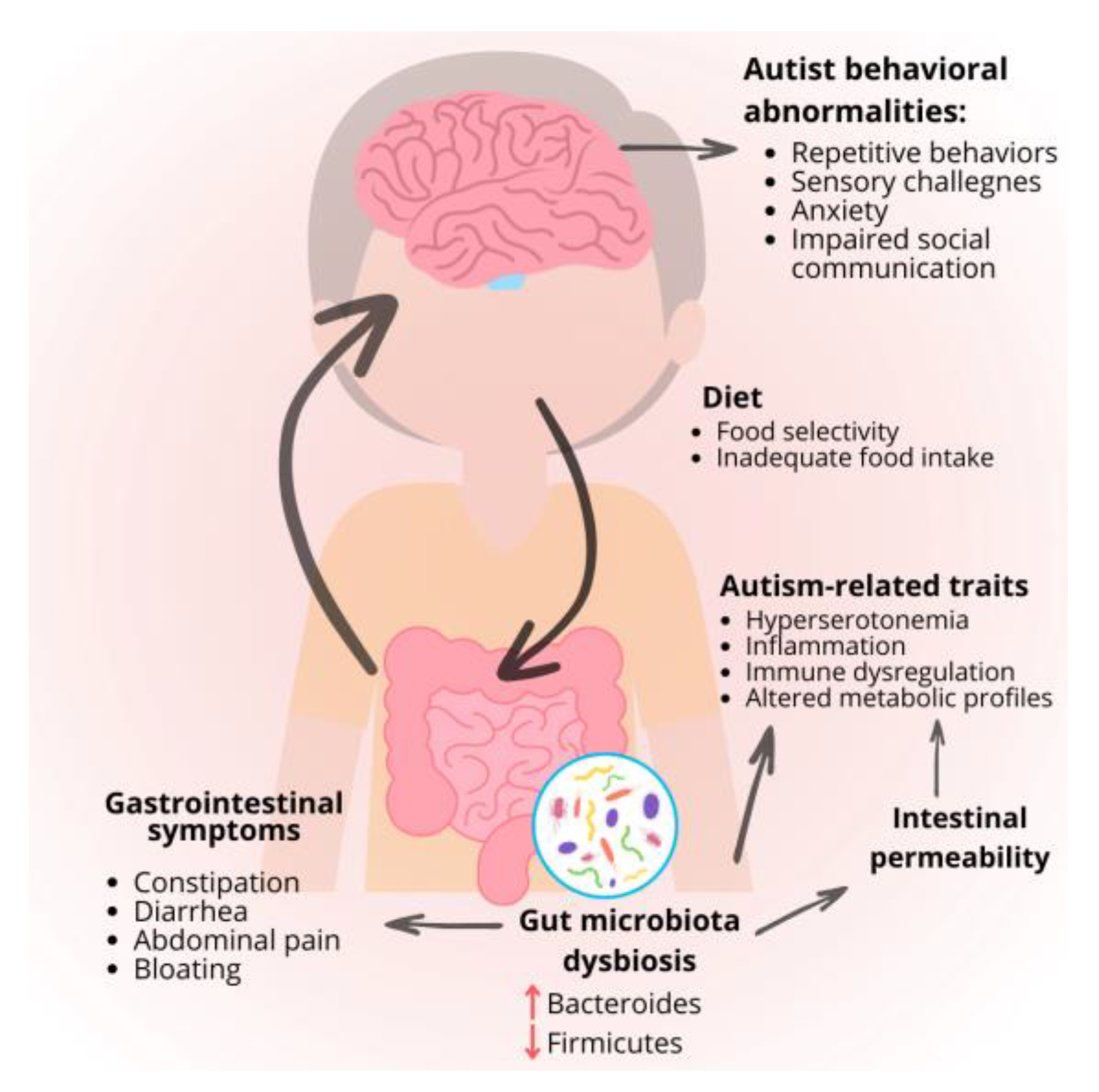
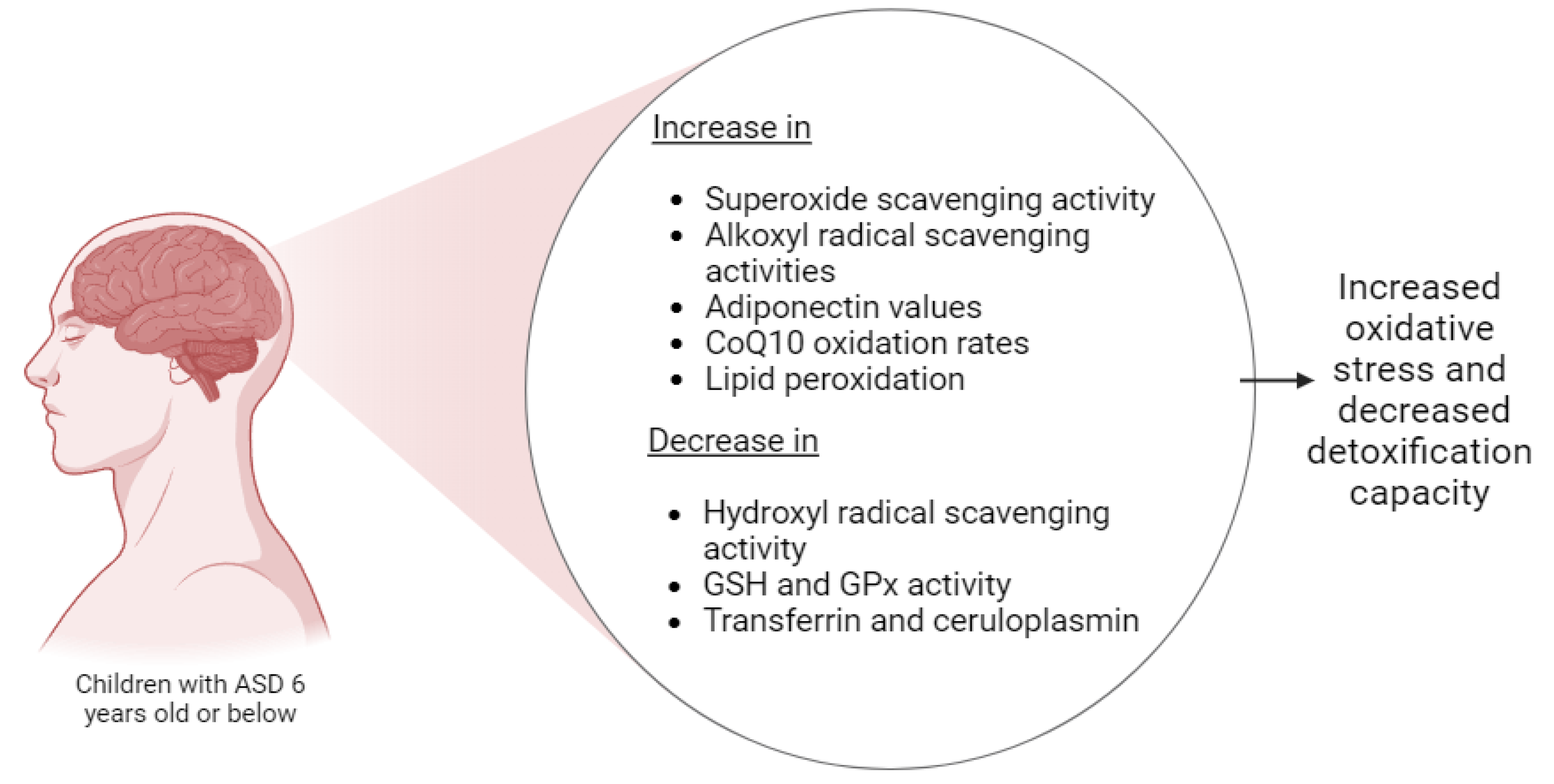
2.3. Oxidative Stress
3. Dietary Intervention in ASD
3.1. Diet High in Antioxidants
3.2. Gluten Free/Casein Free Diet
3.3. Ketogenic Diet and Essential Fatty Acids
4. Conclusions and Future Directions
Author Contributions
Conflicts of Interest
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. The Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.; Adams, J.B. Ratings of the effectiveness of 13 therapeutic diets for Autism Spectrum Disorder: Results of a national survey. Journal of Personalized Medicine 2023, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An overview of Autism Spectrum Disorder, heterogeneity and treatment options. Neuroscience Bulletin 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- APA DSM-5: Manual diagnóstico y estadístico de los trastornos mentales. 2014.
- Mehra, C.; Sil, A.; Hedderly, T.; Kyriakopoulos, M.; Lim, M.; Turnbull, J.; Happe, F.; Baird, G.; Absoud, M. Childhood disintegrative disorder and autism spectrum disorder: a systematic review. Developmental Medicine and Child Neurology 2019, 61, 523–534. [Google Scholar] [CrossRef] [PubMed]
- WHO Autismo. https://www.who.int/es/news-room/fact-sheets/detail/autism-spectrum-disorders (November),.
- CDC Data and statistics on Autism Spectrum Disorder. https://www.cdc.gov/autism/data-research/index.html.
- Guizar, D. En México, uno de cada 115 niños padece autismo. https://www.dgcs.unam.mx/boletin/bdboletin/2020_291.html (November),.
- Byrska, A.; Błażejczyk, I.; Faruga, A.; Potaczek, M.; Wilczyński, K.M.; Janas-Kozik, M. Patterns of food selectivity among children with Autism Spectrum Disorder. Journal of Clinical Medicine 2023, 12, 5469. [Google Scholar] [CrossRef] [PubMed]
- Molina-López, J.; Leiva-García, B.; Planells, E.; Planells, P. Food selectivity, nutritional inadequacies, and mealtime behavioral problems in children with autism spectrum disorder compared to neurotypical children. International Journal of Eating Disorders 2021, 54, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Baraskewich, J.; von Ranson, K.M.; McCrimmon, A.; McMorris, C.A. Feeding and eating problems in children and adolescents with autism: A scoping review. Autism: The International Journal of Research and Practice 2021, 25, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Robea, M.-A.; Luca, A.-C.; Ciobica, A. Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina (Kaunas, Lithuania) 2020, 56, 245. [Google Scholar] [CrossRef]
- Monteiro, M.A.; Santos, A.A.A.D.; Gomes, L.M.M.; Rito, R.V.V.F. Autism Spectrum Disorder: A systematic review about nutritional interventions. Revista paulista de pediatria : orgao oficial da Sociedade de Pediatria de Sao Paulo 2020, 38, e2018262–e2018262. [Google Scholar] [CrossRef]
- Chapman, R.; Botha, M. Neurodivergence-informed therapy. Developmental Medicine and Child Neurology 2023, 65, 310–317. [Google Scholar] [CrossRef]
- Tye, C.; Runicles, A.K.; Whitehouse, A.J.; Alvares, G.A. Characterizing the interplay between autism spectrum disorder and comorbid medical conditions: An integrative review. Frontiers in Psychiatry 2019, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Mejía, J.; Ramos-Jiménez, A.; Jiménez-Vega, F.; Campos-Vega, R.; González-Córdova, A.F.; Wall-Medrano, A. Alimentación funcional para corregir desórdenes gastrointestinales asociados a trastornos del espectro autista: una revisión sistemática. Nutrición Hospitalaria 2022, 39, 663–677. [Google Scholar]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of oxidative stress and antioxidants in autism. Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management 2020, 193–206. [Google Scholar]
- Karhu, E.; Zukerman, R.; Eshraghi, R.S.; Mittal, J.; Deth, R.C.; Castejon, A.M.; Trivedi, M.; Mittal, R.; Eshraghi, A.A. Nutritional interventions for autism spectrum disorder. Nutrition Reviews 2020, 78, 515–531. [Google Scholar] [CrossRef]
- Hopf, K.P.; Madren, E.; Santianni, K.A. Use and perceived effectiveness of complementary and alternative medicine to treat and manage the symptoms of autism in children: A survey of parents in a community population. Journal of Alternative and Complementary Medicine 2016, 22, 25–32. [Google Scholar] [CrossRef]
- Winburn, E.; Charlton, J.; McConachie, H.; McColl, E.; Parr, J.; O’Hare, A.; Baird, G.; Gringras, P.; Wilson, D.C.; Adamson, A.; Adams, S.; Le Couteur, A. Parents’ and child health professionals’ attitudes towards dietary interventions for children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders 2013, 44, 747–757. [Google Scholar] [CrossRef]
- Şenel, H.G. Parents’ views and experiences about complementary and alternative medicine treatments for their children with Autistic Spectrum Disorder. Journal of Autism and Developmental Disorders 2009, 40, 494–503. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Suganthy, N.; Kesika, P.; Chaiyasut, C. The role of microbiome, dietary supplements, and probiotics in Autism Spectrum Disorder. International Journal of Environmental Research and Public Health 2020, 17, 2647. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.N.; Kadar, M.; Fenech, M.; Hamzaid, N.H. Interrelation of food selectivity, oral sensory sensitivity, and nutrient intake in children with autism spectrum disorder: A scoping review. Research in Autism Spectrum Disorders 2022, 93, 101928. [Google Scholar] [CrossRef]
- Rodrigues, J.V.S.; Poli, M.C.F.; Petrilli, P.H.; Dornelles, R.C.M.; Turcio, K.H.; Theodoro, L.H. Food selectivity and neophobia in children with autism spectrum disorder and neurotypical development: a systematic review. Nutrition Reviews 2023, 81, 1034–1050. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Food selectivity in autism spectrum disorders. Journal of Child Neurology 2014, 29, 1554–1561. [Google Scholar] [CrossRef]
- Page, S.D.; Souders, M.C.; Kral, T.V.E.; Chao, A.M.; Pinto-Martin, J. Correlates of feeding difficulties among children with Autism Spectrum Disorder: A systematic review. Journal of Autism and Developmental Disorders 2022, 52, 255–274. [Google Scholar] [CrossRef]
- Gerhant, A.; Olajossy, M.; Olajossy-Hilkesberger, L. Neuroanatomical, genetic and neurochemical aspects of infantile autism. Psychiatria Polska 2013, 47, 1101–1111. [Google Scholar] [PubMed]
- Masgutova, S.; Masgutov, D. Reflex integration disorder as a ne w treatment paradigm for children with autism. Svetlana Masgutova Educational Institute for Neuro-Sensory-Motor and Reflex Integration, SMEI 2015, 171-180.
- Hubbard, K.L.; Anderson, S.E.; Curtin, C.; Must, A.; Bandini, L.G. A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. Journal of the Academy of Nutrition and Dietetics 2014, 114, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Kozioł-Kozakowska, A.; Piórecka, B.; Schlegel-Zawadzka, M. Prevalence of food neophobia in pre-school children from southern Poland and its association with eating habits, dietary intake and anthropometric parameters: a cross-sectional study. Public Health Nutrition 2018, 21, 1106–1114. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. Journal of the American Dietetic Association 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Bandini, L.G.; Curtin, C.; Phillips, S.; Anderson, S.E.; Maslin, M.; Must, A. Changes in food selectivity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders 2017, 47, 439–446. [Google Scholar] [CrossRef]
- Kral, T.V.; Souders, M.C.; Tompkins, V.H.; Remiker, A.M.; Eriksen, W.T.; Pinto-Martin, J.A. Child eating behaviors and caregiver feeding practices in children with autism spectrum disorders. Public Health Nursing 2015, 32, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Malhi, P.; Saini, S.; Bharti, B.; Attri, S.; Sankhyan, N. Sensory processing dysfunction and mealtime behavior problems in children with autism. Indian Pediatrics 2021, 58, 842–845. [Google Scholar] [CrossRef]
- Riccio, M.P.; Franco, C.; Negri, R.; Ferrentino, R.I.; Maresca, R.; D'alterio, E.; Greco, L.; Bravaccio, C. Is food refusal in autistic children related to TAS2R38 genotype? Autism Research 2018, 11, 531–538. [Google Scholar] [CrossRef]
- Nadon, G.; Feldman, D.E.; Dunn, W.; Gisel, E. Association of sensory processing and eating problems in children with autism spectrum disorders. Autism Research and Treatment 2011, 2011, 541926–541926. [Google Scholar] [CrossRef]
- Lafraire, J.; Rioux, C.; Giboreau, A.; Picard, D. Food rejections in children: Cognitive and social/environmental factors involved in food neophobia and picky/fussy eating behavior. Appetite 2016, 96, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.d.O.; Gomes, D.R.; Mattos, M.P. Factors associated with food neophobia in children: systematic review. Revista Paulista de Pediatria :Ogao Oficial da Sociedade de Pediatria de Sao Paulo 2021, 39, e2020089–e2020089. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary intake, nutrient status, and growth parameters in children with autism spectrum disorder and severe food selectivity: An electronic medical record review. Journal of the Academy of Nutrition and Dietetics 2018, 118, 1943–1950. [Google Scholar] [CrossRef]
- Huxham, L.; Marais, M.; van Niekerk, E. Idiosyncratic food preferences of children with autism spectrum disorder in England. South African Journal of Clinical Nutrition 2021, 34, 90–96. [Google Scholar] [CrossRef]
- Rashid, A.; Iftikhar, N.; Badar, S.A.; Masood, F.; Rehman, I. Factors influencing food selectivity and food preferences of children with autism spectrum disorder. Journal of Pharmaceutical Research International 2021, 33, 152–159. [Google Scholar] [CrossRef]
- Webber, A.; Robinson, C.; Gray, H.L. Diet quality in children with Autism Spectrum Disorder. Journal of Nutrition Education and Behavior 2018, 50, S125–S126. [Google Scholar] [CrossRef]
- Vissoker, R.; Latzer, Y.; Stolar, O.; Rabenbach, A.; Gal, E. Eating problems and patterns among toddlers and young boys with and without autism spectrum disorders. Research in Autism Spectrum Disorders 2019, 59, 1–9. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The influence of food intake specificity in children with autism on gut microbiota. International Journal of Molecular Sciences 2020, 21, 2797. [Google Scholar] [CrossRef]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Research: Official Journal of the International Society for Autism Research 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, S.J.; Kim, Y.; Park, J.; Kim, Y.-R.; Lee, S.-H.; Jung, S.J.; Cho, M.S.; Oh, J.E. Dietary behavior and food preferences according to age and the parents' nutrition education needs of children with autism spectrum disorder. Journal of the Korean Society of Food Culture 2020, 35, 241–255. [Google Scholar]
- Hsiao, E.Y. Gastrointestinal issues in autism spectrum disorder. Harvard Review of Psychiatry 2014, 22, 104–111. [Google Scholar] [CrossRef]
- Leader, G.; Abberton, C.; Cunningham, S.; Gilmartin, K.; Grudzien, M.; Higgins, E.; Joshi, L.; Whelan, S.; Mannion, A. Gastrointestinal symptoms in Autism Spectrum Disorder: A systematic review. Nutrients 2022, 14, 1471. [Google Scholar] [CrossRef]
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; Calderoni, S.; Muratori, F. Behavioral phenotype of ASD preschoolers with gastrointestinal symptoms or food selectivity. Journal of Autism and Developmental Disorders 2017, 47, 3574–3588. [Google Scholar] [CrossRef]
- Esposito, M.; Sloan, J.; Nappo, R.; Fadda, R.; Fotia, F.; Napoli, E.; Mazzone, L.; Valeri, G.; Vicari, S. Sensory processing, gastrointestinal symptoms and parental feeding practices in the explanation of food selectivity: Clustering children with and without autism. International Journal of Autism and Related Disabilities 2019, 2019, 1–12. [Google Scholar]
- Leader, G.; O’Reilly, M.; Gilroy, S.P.; Chen, J.L.; Ferrari, C.; Mannion, A. Comorbid feeding and gastrointestinal symptoms, challenging behavior, sensory issues, adaptive functioning and quality of life in children and adolescents with autism spectrum disorder. Developmental Neurorehabilitation 2021, 24, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: focus on gut microbiota. Expert Review of Gastroenterology and Hepatology 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PloS one 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Bjørklund, G.; Pivina, L.; Dadar, M.; Meguid, N.A.; Semenova, Y.; Anwar, M.; Chirumbolo, S. Gastrointestinal alterations in autism spectrum disorder: What do we know? Neuroscience & Biobehavioral Reviews 2020, 118, 111–120. [Google Scholar]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & Behavior 2015, 138, 179–187. [Google Scholar]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders 2014, 44, 1117–1127. [Google Scholar] [CrossRef]
- Liu, J.; Wan, G.-b.; Huang, M.-s.; Agyapong, G.; Zou, T.-l.; Zhang, X.-y.; Liu, Y.-W.; Song, Y.-q.; Tsai, Y.-C.; Kong, X.-j. Probiotic therapy for treating behavioral and gastrointestinal symptoms in autism spectrum disorder: a systematic review of clinical trials. Current Medical Science 2019, 39, 173–184. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutritional Neuroscience 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Nogay, N.H.; Nahikian-Nelms, M. Can we reduce autism-related gastrointestinal and behavior problems by gut microbiota based dietary modulation? A review. Nutritional Neuroscience 2021, 24, 327–338. [Google Scholar] [CrossRef]
- de Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; Iardino, P.; Marotta, R.; Tolone, C.; Fasano, A.; Pascotto, A.; Bravaccio, C. Antibodies against food antigens in patients with autistic spectrum disorders. BioMed Research International 2013, 2013, 729349–729349. [Google Scholar] [CrossRef] [PubMed]
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. Journal of Pediatric Gastroenterology and Nutrition 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs III, G.J.; Furuta, G.T.; Levy, J.; VandeWater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics 2010, 125, S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, W.; Yao, H.; Zheng, R.; Chen, W.; Zhang, W. Association between Autism Spectrum Disorder and Food Allergy: A Systematic Review and Meta-analysis. Autism Research 2021, 14, 220–230. [Google Scholar]
- Al-Beltagi, M. Autism medical comorbidities. World Journal of Clinical Pediatrics 2021, 10, 15. [Google Scholar] [PubMed]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative stress in autism spectrum disorder—current progress of mechanisms and biomarkers. Frontiers in Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Membrino, V.; Di Paolo, A.; Alia, S.; Papiri, G.; Vignini, A. The Role of Oxidative Stress in Autism Spectrum Disorder: A Narrative Literature Review. Oxygen 2023, 3, 34–44. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological efficacy of medicinal plant extracts in preventing oxidative damage. Oxidative Medicine and Cellular Longevity 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Docea, A.O.; Calina, D.; Tsarouhas, K.; Zamfira, L.-M.; Mitrut, R.; Sharifi-Rad, J.; Kovatsi, L.; Siokas, V.; Dardiotis, E. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. Journal of Clinical Medicine 2019, 8, 1295. [Google Scholar] [CrossRef] [PubMed]
- Pangrazzi, L.; Balasco, L.; Bozzi, Y. Oxidative stress and immune system dysfunction in autism spectrum disorders. International Journal of Molecular Sciences 2020, 21, 3293. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PloS one 2020, 15, e0233550–e0233550. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Iwata, K.; Miyachi, T.; Takagai, S.; Wakusawa, K.; Nara, T.; Tsuchiya, K.J.; Matsumoto, K.; Kurita, D.; Kameno, Y. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.L.; Cobb, J.; Agarwal, R.; Maddux, M.; Cooke, M.S. How robust is the evidence for a role of oxidative stress in autism spectrum disorders and intellectual disabilities? Journal of Autism and Developmental Disorders 2021, 51, 1428–1445. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R. Oxidative stress in autism spectrum disorder. Molecular Neurobiology 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V.; Brown, W.T.; Cohen, I. Oxidative stress in autism: Increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin-the antioxidant proteins. Life Sciences 2004, 75, 2539–2549. [Google Scholar] [CrossRef]
- Zoroglu, S.S.; Armutcu, F.; Ozen, S.; Gurel, A.; Sivasli, E.; Yetkin, O.; Meram, I. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. European Archives of Psychiatry and Clinical Neuroscience 2004, 254, 143–147. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.; Frye, R.; James, S. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Translational Psychiatry 2012, 2, e134–e134. [Google Scholar] [CrossRef] [PubMed]
- Söğüt, S.; Zoroğlu, S.S.; Özyurt, H.; Yılmaz, H.R.; Özuğurlu, F.; Sivaslı, E.; Yetkin, Ö.; Yanık, M.; Tutkun, H.; Savaş, H.A. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clinica Chimica Acta 2003, 331, 111–117. [Google Scholar] [CrossRef]
- Chauhan, A.; Gu, F.; Essa, M.M.; Wegiel, J.; Kaur, K.; Brown, W.T.; Chauhan, V. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. Journal of Neurochemistry 2011, 117, 209–220. [Google Scholar] [CrossRef]
- Al-Mosalem, O.; El-Ansary, A.; Attas, O.; Al-Ayadhi, L. Metabolic biomarkers related to energy metabolism in Saudi autistic children. Clinical Biochemistry 2009, 42, 949–957. [Google Scholar] [CrossRef]
- Aranburu, E.; Matias, S.; Simón, E.; Larretxi, I.; Martínez, O.; Bustamante, M.Á.; Fernández-Gil, M.D.P.; Miranda, J. Gluten and FODMAPs relationship with mental disorders: Systematic review. Nutrients 2021, 13, 1894. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Frontiers in Aging Neuroscience 2010, 2, 1224. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Frontiers in Physiology 2014, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Morakotsriwan, N.; Wattanathorn, J.; Kirisattayakul, W.; Chaisiwamongkol, K. Autistic-like behaviors, oxidative stress status, and histopathological changes in cerebellum of valproic acid rat model of autism are improved by the combined extract of purple rice and silkworm pupae. Oxidative Medicine and Cellular Longevity 2016, 2016, 3206561. [Google Scholar] [CrossRef]
- Hirai, T.; Usui, N.; Iwata, K.; Miyachi, T.; Tsuchiya, K.J.; Xie, M.-J.; Nakamura, K.; Tsujii, M.; Sugiyama, T.; Matsuzaki, H. Increased plasma lipoprotein lipase activity in males with autism spectrum disorder. Research in Autism Spectrum Disorders 2020, 77, 101630. [Google Scholar] [CrossRef]
- Hirayama, A.; Wakusawa, K.; Fujioka, T.; Iwata, K.; Usui, N.; Kurita, D.; Kameno, Y.; Wakuda, T.; Takagai, S.; Hirai, T.; Nara, T.; Ito, H.; Nagano, Y.; Oowada, S.; Tsujii, M.; Tsuchiya, K.J.; Matsuzaki, H. Simultaneous evaluation of antioxidative serum profiles facilitates the diagnostic screening of autism spectrum disorder in under-6-year-old children. Scientific Reports 2020, 10, 20602–20602. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. Journal of Allergy and Clinical Immunology 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Xu, G.; Snetselaar, L.G.; Jing, J.; Liu, B.; Strathearn, L.; Bao, W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Network Open 2018, 1, e180279–e180279. [Google Scholar] [CrossRef]
- Curieses Andrés, C.M.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant metabolism pathways in vitamins, polyphenols, and selenium: Parallels and divergences. International Journal of Molecular Sciences 2024, 25, 2600. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology 2020, 11, 694. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural compounds and products from an anti-aging perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Gangemi, S. Vitamin D in health and disease. Biomedicines 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Mohammadi, M.; Pezeshki, A.; Jafari, S.M. , Health benefits of beta-carotene. In Handbook of food bioactive ingredients: properties and applications, Springer: 2023; pp 1-26.
- Puebla-Duarte, A.L.; Santos-Sauceda, I.; Rodríguez-Félix, F.; Iturralde-García, R.D.; Fernández-Quiroz, D.; Pérez-Cabral, I.D.; Del-Toro-Sánchez, C.L. Active and Intelligent Packaging: A Review of the Possible Application of Cyclodextrins in Food Storage and Safety Indicators. Polymers 2023, 15, 4317. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J.; Chang, J.S.T.; Neidlinger, T.R. β-Cryptoxanthin- and α-carotene-rich foods have greater apparent bioavailability than β-carotene-rich foods in Western diets. British Journal of Nutrition 2011, 105, 212–219. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutrition Reviews 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Kaviarasan, K.; Pugalendi, K.V. Influence of flavonoid-rich fraction from Spermacoce hispida seed on PPAR-alpha gene expression, antioxidant redox status, protein metabolism and marker enzymes in high-fat-diet fed STZ diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology 2009, 20, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants (Basel, Switzerland) 2023, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Critical Reviews in Food Science and Nutrition 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: an overview. Journal of Nutritional Science 2016, 5, e47. [Google Scholar] [CrossRef]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Materials for Renewable and Sustainable Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food and Nutrition Research 2017, 61, 1361779–1361779. [Google Scholar] [CrossRef]
- Catarino, M.; Alves-Silva, J.; Pereira, O.; Cardoso, S. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Current Topics in Medicinal Chemistry 2014, 15, 105–119. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from nature: A phytochemistry and biological activity review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Deepika; Maurya, P. K. Health benefits of quercetin in age-related diseases. Molecules (Basel, Switzerland) 2022, 27, 2498. [Google Scholar] [CrossRef]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.-R.; Shahriar, A.; Ahmed, H.; Mosfeq-Ul-Hasan, M.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, M.F.R. Natural flavonols: actions, mechanisms, and potential therapeutic utility for various diseases. Beni-Suef University Journal of Basic and Applied Sciences 2023, 12, 47–47. [Google Scholar] [CrossRef]
- Brahmachari, G. Naturally occurring flavanones: An overview. Natural Product Communications 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules (Basel, Switzerland) 2019, 24, 1076. [Google Scholar] [CrossRef]
- Indika, N.-L.R.; Frye, R.E.; Rossignol, D.A.; Owens, S.C.; Senarathne, U.D.; Grabrucker, A.M.; Perera, R.; Engelen, M.P.K.J.; Deutz, N.E.P. The rationale for vitamin, mineral, and cofactor treatment in the precision medical care of Autism Spectrum Disorder. Journal of Personalized Medicine 2023, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Xiong, X.-Q.; Yang, T.; Cui, T.; Hou, N.-L.; Lai, X.; Liu, S.; Guo, M.; Liang, X.-H.; Cheng, Q.; Chen, J.; Li, T.-Y. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders - a pilot study. BMC Microbiology 2017, 17, 204–204. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Lei, Y.; Chen, J.; Li, T. Vitamin A and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutritional Neuroscience 2018, 22, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Bastos Maia, S.; Costa Caminha, M.d.F.; Lins da Silva, S.; Rolland Souza, A.S.; Carvalho Dos Santos, C.; Batista Filho, M. The prevalence of vitamin A deficiency and associated factors in pregnant women receiving prenatal care at a reference maternity hospital in northeastern Brazil. Nutrients 2018, 10, 1271. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Xiong, X.; Yang, T.; Hou, N.; Liang, X.; Chen, J.; Cheng, Q.; Li, T. Correlation between nutrition and symptoms: Nutritional survey of children with Autism Spectrum Disorder in Chongqing, China. Nutrients 2016, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L. ESPEN micronutrient guideline. Clinical Nutrition 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Robitaille, L.; Eintracht, S.; MacNamara, E.; Hoffer, L.J. Effects of vitamin C and vitamin D administration on mood and distress in acutely hospitalized patients. The American Journal of Clinical Nutrition 2013, 98, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Frontiers in Physiology 2015, 6, 397–397. [Google Scholar] [CrossRef]
- Krajcovicova-Kudlackova, M.; Valachovicova, M.; Mislanova, C.; Hudecova, Z.; Sustrova, M.; Ostatnikova, D. Plasma concentrations of selected antioxidants in autistic children and adolescents. Bratislavske Lekarske Listy 2009, 110, 247–250. [Google Scholar]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatrics 2011, 11, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Waly, M.I.; Al-Farsi, Y.; Saad, K.; Dadar, M.; Rahman, M.M.; Elhoufey, A.; Chirumbolo, S.; Jóźwik-Pruska, J.; Kałużna-Czaplińska, J. The role of vitamins in autism spectrum disorder: what do we know? Journal of Molecular Neuroscience 2019, 67, 373–387. [Google Scholar] [CrossRef]
- Meguid, N.A.; Bjørklund, G.; Gebril, O.H.; Doşa, M.D.; Anwar, M.; Elsaeid, A.; Gaber, A.; Chirumbolo, S. The role of zinc supplementation on the metallothionein system in children with autism spectrum disorder. Acta Neurologica Belgica 2019, 119, 577–583. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00889538 (July),.
- Önal, S.; Sachadyn-Król, M.; Kostecka, M. A review of the nutritional approach and the role of dietary components in children with autism spectrum disorders in light of the latest scientific research. Nutrients 2023, 15, 4852. [Google Scholar] [CrossRef]
- Serra, D.; Henriques, J.F.; Sousa, F.J.; Laranjo, M.; Resende, R.; Ferreira-Marques, M.; de Freitas, V.; Silva, G.; Peça, J.; Dinis, T.C. Attenuation of autism-like behaviors by an anthocyanin-rich extract from portuguese blueberries via microbiota–gut–brain axis modulation in a valproic acid mouse model. International Journal of Molecular Sciences 2022, 23, 9259. [Google Scholar] [CrossRef]
- Wang, H.; Hu, L.; Peng, L.; Du, J.; Lan, M.; Cheng, Y.; Ma, L.; Zhang, Y. Dual encapsulation of β-carotene by β-cyclodextrin and chitosan for 3D printing application. Food Chemistry 2022, 378, 132088. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Chen, F.; Wang, Y.; Wang, X.; Yang, X.; Zhang, C. Lycopene maintains mitochondrial homeostasis to counteract the enterotoxicity of deoxynivalenol. Antioxidants 2023, 12, 1958. [Google Scholar] [CrossRef]
- Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Dowlati Beirami, A.; Sharifi-Rad, J.; Sharopov, F.; Cho, W.C.; Martins, N. Avocado-soybean unsaponifiables: A panoply of potentialities to be exploited. Biomolecules 2020, 10, 130. [Google Scholar] [CrossRef]
- Reissmann, A. Gluten-free and casein-free diets in the management of autism spectrum disorder: A systematic literature review. Movement and Nutrition in Health and Disease 2020, 4. [Google Scholar]
- González-Domenech, P.J.; Diaz-Atienza, F.; Gutiérrez-Rojas, L.; Fernández-Soto, M.L.; González-Domenech, C.M. A narrative review about autism spectrum disorders and exclusion of gluten and casein from the diet. Nutrients 2022, 14, 1797. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Xu, X.; Cui, Y.; Han, H.; Hendren, R.L.; Zhao, L.; You, X. A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutrition Reviews 2022, 80, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism spectrum disorders and the gut microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Itokazu, N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with Autism Spectrum Disorder. Neuropsychobiology 2002, 46, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Buckley, J.A. Autism and Dietary Therapy. Journal of Child Neurology 2013, 28, 975–982. [Google Scholar] [CrossRef]
- Keller, A.; Rimestad, M.L.; Friis Rohde, J.; Holm Petersen, B.; Bruun Korfitsen, C.; Tarp, S.; Briciet Lauritsen, M.; Händel, M.N. The effect of a combined gluten- and casein-free diet on children and adolescents with autism spectrum disorders: A systematic review and meta-analysis. Nutrients 2021, 13, 470. [Google Scholar] [CrossRef]
- Saravia, L.; González-Zapata, L.I.; Rendo-Urteaga, T.; Ramos, J.; Collese, T.S.; Bove, I.; Delgado, C.; Tello, F.; Iglesia, I.; Gonçalves Sousa, E.D.; De Moraes, A.C.F.; Carvalho, H.B.; Moreno, L.A. Development of a food frequency questionnaire for assessing dietary intake in children and adolescents in South America. Obesity 2018, 26. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Haracopos, D.; Knivsberg, A.-M.; Reichelt, K.L.; Parlar, S.; Jacobsen, J.; Seim, A.; Pedersen, L.; Schondel, M.; Shattock, P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutritional Neuroscience 2010, 13, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Shankar, M.; Shuster, J.; Theriaque, D.; Burns, S.; Sherrill, L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. Journal of Autism and Developmental Disorders 2006, 36, 413–420. [Google Scholar] [CrossRef]
- Johnson, C.R.; Handen, B.L.; Zimmer, M.; Sacco, K.; Turner, K. Effects of gluten free / casein free diet in young children with autism: A pilot study. Journal of Developmental and Physical Disabilities 2010, 23, 213–225. [Google Scholar] [CrossRef]
- Navarro, F.; Pearson, D.A.; Fatheree, N.; Mansour, R.; Hashmi, S.S.; Rhoads, J.M. Are ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutritional Neuroscience 2015, 18, 177–185. [Google Scholar] [CrossRef]
- Pusponegoro, H.D.; Ismael, S.; Firmansyah, A.; Sastroasmoro, S.; Vandenplas, Y. Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatrica 2015, 104. [Google Scholar] [CrossRef]
- González-Domenech, P.J.; Díaz Atienza, F.; García Pablos, C.; Fernández Soto, M.L.; Martínez-Ortega, J.M.; Gutiérrez-Rojas, L. Influence of a combined gluten-free and casein-free diet on behavior disorders in children and adolescents diagnosed with autism spectrum disorder: a 12-month follow-up clinical trial. Journal of Autism and Developmental Disorders 2020, 50, 935–948. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Foley, J.; Cain, U.; Peck, R.; Morris, D.D.; Wang, H.; Smith, T. The gluten-free/casein-free diet: a double-blind challenge trial in children with autism. Journal of Autism and Developmental Disorders 2016, 46, 205–220. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-free diet in children with Autism Spectrum Disorders: A randomized, controlled, single-blinded trial. Journal of Autism and Developmental Disorders 2019, 50, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.; Rodgers, J.; Savery, D.; Shattock, P. A Gluten-free diet as an intervention for autism and associated spectrum disorders: Preliminary findings. Autism 1999, 3, 45–65. [Google Scholar] [CrossRef]
- Pennesi, C.M.; Klein, L.C. Effectiveness of the gluten-free, casein-free diet for children diagnosed with autism spectrum disorder: Based on parental report. Nutritional Neuroscience 2012, 15, 85–91. [Google Scholar] [CrossRef]
- Alamri, E.S. Efficacy of gluten- and casein-free diets on autism spectrum disorders in children. Saudi Medical Journal 2020, 41, 1041–1046. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic diet for epilepsy: an overview of systematic review and meta-analysis. European Journal of Clinical Nutrition 2022, 76, 1234–1244. [Google Scholar] [CrossRef]
- Mu, C.; Corley, M.J.; Lee, R.W.Y.; Wong, M.; Pang, A.; Arakaki, G.; Miyamoto, R.; Rho, J.M.; Mickiewicz, B.; Dowlatabadi, R.; Vogel, H.J.; Korchemagin, Y.; Shearer, J. Metabolic framework for the improvement of Autism Spectrum Disorders by a modified ketogenic diet: A pilot study. Journal of Proteome Research 2020, 19, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.Y.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; Lum-Jones, A.; Wong, M. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiology & Behavior 2018, 188, 205–211. [Google Scholar]
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.S.; dos Santos Riesgo, R. Effect of a ketogenic diet on autism spectrum disorder: A systematic review. Research in Autism Spectrum Disorders 2015, 20, 31–38. [Google Scholar] [CrossRef]
- Vera-González, A. , Pathophysiological mechanisms underlying the etiologies of seizures and epilepsy. In Epilepsy, Exon Publications: 2022.
- Liu, X.; Sun, X.; Sun, C.; Zou, M.; Chen, Y.; Huang, J.; Wu, L.; Chen, W.-X. Prevalence of epilepsy in autism spectrum disorders: A systematic review and meta-analysis. Autism 2021, 26, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, A.; Vlachonikolis, I.; Mihailidou, H.; Spilioti, M.; Skarpalezou, A.; Makaronas, N.; Prokopiou, A.; Christodoulou, P.; Liapi-Adamidou, G.; Helidonis, E.; Sbyrakis, S.; Smeitink, J. Application of a Ketogenic Diet in Children With Autistic Behavior: Pilot Study. Journal of Child Neurology 2003, 18, 113–118. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metabolic Brain Disease 2017, 32, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Sullivan, P.G.; Davis, L.; Kim, D.Y.; Rho, J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Edemann-Callesen, H.; Bernhardt, N.; Hlusicka, E.B.; Hintz, F.; Habelt, B.; Winter, R.; Neubert, I.; Pelz, M.; Filla, A.; Soto-Montenegro, M.L.; Winter, C.; Hadar, R. Supplement treatment with NAC and omega-3 polyunsaturated fatty acids during pregnancy partially prevents schizophrenia-related outcomes in the poly i:c rat model. Antioxidants 2023, 12, 1068. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.; Manohar, K.; Shariff, A.; Kinattingal, N.; Wani, S.U.D.; Alshehri, S.; Imam, M.T.; Shakeel, F.; Krishna, K.L. Omega-3 fatty acids supplementation in the treatment of depression: An observational study. Journal of Personalized Medicine 2023, 13, 224. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O'Keefe, J.H. The importance of marine omega-3s for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Amminger, G.P.; Berger, G.E.; Schäfer, M.R.; Klier, C.; Friedrich, M.H.; Feucht, M. Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biological Psychiatry 2007, 61, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.C.; Conlon, C.A.; Beck, K.L.; von Hurst, P.R. Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients 2017, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Çıray, O.; Wu, H.; Schneider-Thoma, J.; Bighelli, I.; Krause, M.; Rodolico, A.; Ceraso, A.; Deste, G.; Huhn, M.; Fraguas, D.; San José Cáceres, A.; Mavridis, D.; Charman, T.; Murphy, D.G.; Parellada, M.; Arango, C.; Leucht, S. Pharmacological and dietary-supplement treatments for autism spectrum disorder: a systematic review and network meta-analysis. Molecular autism 2022, 13, 10–10. [Google Scholar] [CrossRef] [PubMed]
| Food/characteristic studied | Population number (n) | Methodology | Important results | Reference |
|---|---|---|---|---|
| Fruits, Vegetables, Dairy/milk, Flours, Fats, Legumes, Meats, Sweets, Snacks | 105 | An "Aut-Eat-questionnaire" survey was administered to parents of 105 children with ASD and 95 neurotypical children of 137 items or foods regularly consumed by their children. | Neurotypical children eat a significantly greater variety of food groups than children with ASD. Toddlers with ASD ate more snacks than neurotypical toddlers; Older children with ASD ate significantly fewer snacks than neurotypical children of the same age. |
[43] |
| Fruits, Fruit juice, Vegetables, Starchy vegetables, Unrefined carbohydrates, Refined carbohydrates, Eggs, Raw meats, Processed meats, Meat alternative, Dairy | 325 | Online surveys of parents and caregivers of children (ages 3 to 16) neurodivergent | The level of food acceptance varies in response to different food attributes, such as color, presentation, temperature, and texture. The food attributes preferred are crunchy, smooth, moist, and soft foods, with refined carbohydrates being the most accepted group. | [40] |
| Grains, Fruit, Vegetables, Dairy, Fats, Sugars | 39 | 24-hour reminder with parents of 39 children ages 2 to 17 with ASD | Compared to the diet of neurotypical children, the diet of children with ASD has fewer grains and vegetables and greater amounts of sugar in their diet. | [42] |
| Rice, Refined carbohydrates, Fruits, Vegetables | 68 | 68 surveys of parents of children with ASD aged 2 to 11 years and older | Children with ASD prefer easy chew foods, such as rice and bread. They also prefer junk food and some fruits and vegetables. Because they have sensory problems, they do not like crunchy or crunchy food and show an aversion to trying new food. | [41] |
| Grains and starches, Vegetables and marine food, Fruits, Meats, Foods rich in proteins, Dairy, Fat and sweets, Snacks | 130 | Survey of 130 parents who may have more than one neurodivergent child. | Children with ASD like foods that are high in sugar and fat. Children eat snacks more than once a day. Parents of children are interested in developing more nutritious snacks that are high in protein and low in calories with a minimum of additives. | [46] |
| Attributes of food such as texture, taste, color, and temperature | 173 | Questionnaire to parents about their child with ASD to determine consumption of a variety of foods according to different attributes (color, texture, etc.) and preferences | The average group of children with ASD preferred foods with a certain appearance, disliked sticky foods, preferred crispy foods, sweet foods, and refuses foods with mixed ingredients. | [9] |
| Animal products, plant products, bakery products, fats, milk, fruits, vegetables, and others | 62 | 46 children with ASD and 16 non-ASD children were included in this study to find a correlation between ASD children's diet and gut microbiota. Parents participated in mealtime questionnaires. | Children with ASD showed lower values of docosahexaenoic acid, docosapentanoic acid, iron, copper, iodine, and vitamins K, B6, and C. | [44] |
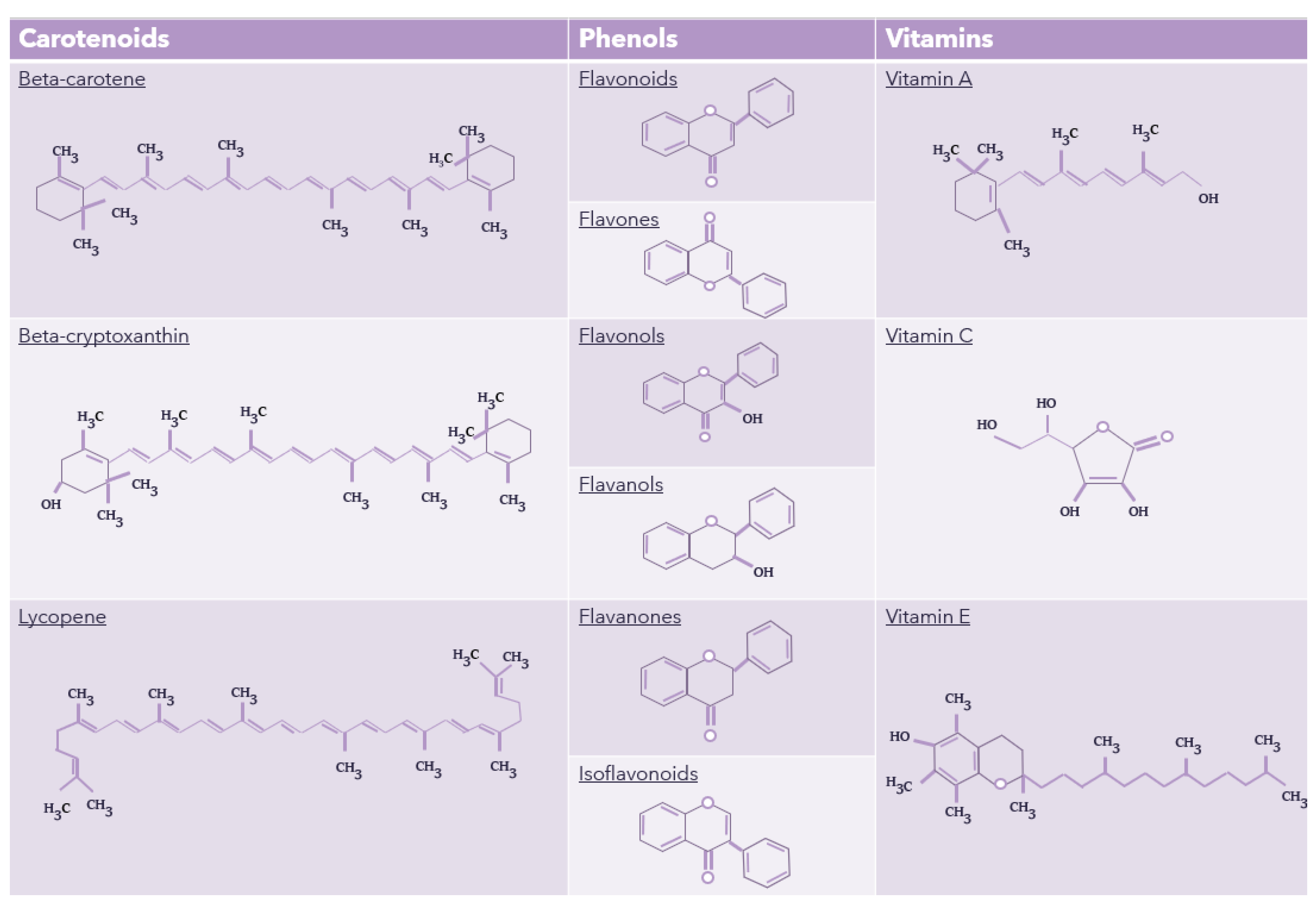
| Antioxidant | Nature (hydro/liposoluble) | Found in | Biological properties | References |
|---|---|---|---|---|
| Anthocyanins | Hydrosoluble | Berries, grapes, cherries, black beans, etc. | Reduce cellular lipid peroxidation. High ORAC value. Antithrombotic and antimicrobial activity. | [123] |
| Beta-carotene | Liposoluble | Carrots, sweet potatoes, apricots, mangos, etc. | Lower cardiovascular risk and eye disease. Antioxidant effects. | [124] |
| Beta-cryptoxanthin | Liposoluble | Tangerine, oranges, persimmon, papaya, paprika, carrot, etc. | Prevent progression of osteoarthritis in mice, cancer-preventive effects, anti-metabolic syndrome effects, etc. | [96] |
| Flavonoids | Hydrosoluble | Fruits, vegetables, fungi, etc. | Antioxidant, anti-inflammatory, anti-mutagenic effect. | [100] |
| Flavones | Hydrosoluble | Celery, red pepper, chamomile, mint, etc. | Anti-inflammatory, antimicrobial, and anticancer activities. | [103] |
| Flavanols | Hydrosoluble | Onion, tomatoes, kale, apples, grapes, etc. | Help reduce risk for cardiovascular diseases, anti-inflammatory, antioxidant, anticancer, antiviral, antimicrobial, etc. | [104] |
| Flavonols | Liposoluble | Cranberries, lettuce, broccoli, potatoes, tomatoes, etc. | Protect against oxidative stress, anti-inflammatory and antimicrobial effects, etc. | [105] |
| Flavanones | Hydrosoluble | Oranges, lemons, grapes, etc. | Free radical-scavenging properties, anti-inflammatory, blood lipid & cholesterol lowering properties, etc. | [107] |
| Isoflavonoids | Hydrosoluble | Soybeans and other legumes. | Antifungal, antibacterial, antiviral, and antioxidant activities. | [108] |
| Lycopene | Liposoluble | Tomato, watermelon, papaya, pink grapefruit, etc. | Help maintain mitochondrial homeostasis and intestinal health. Anti-inflammatory and antioxidant. | [125] |
| Vitamin A | Liposoluble | Carrots, etc. | Participant in cell reproduction and differentiation in the immune system. Play a role in vision. Contribute to reducing ASD symptoms, etc. | [109, 112] |
| Vitamin C | Hydrosoluble | Citrus fruit, strawberry, kiwi, broccoli, peppers, etc. | Neutralize oxidant agents, regulate the immune system, play a role in tissue growth and neurons. | [114, 116] |
| Vitamin E | Liposoluble | Nuts, broccoli, fish, vegetable oils, etc. | Inhibit lipid peroxidation, scavenges lipid peroxyl radicals, Immunity, Neuroprotective, Treating and preventing osteoarthritis | [69, 126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





