1. Introduction
Beef quality is defined by several traits that influence the eating experience and desirability of the meat. Key traits include palatability factors such as tenderness, juiciness, and flavor which directly impact consumer satisfaction (Esmailizadeh et al., 2011). Tenderness refers to the ease of chewing and breaking down the meat, while juiciness is the moisture released during mastication (Purslow et al., 2012). Flavor encompasses the combined sensations of taste and aroma that make the meat appealing (Mottram, 1998). Visual characteristics like meat color, fat color, and marbling also play a crucial role in perceived quality (Killinger et al., 2004). The bright, desirable lean color, white fat color, and intramuscular fat distribution (marbling) enhance the appearance and contribute to flavor and juiciness (Hocquette et al., 2010). Other traits like water-holding capacity, pH, and intramuscular fat content further influence overall quality, shelf life, and sensory properties (Pethick et al., 2011; Warner et al., 2010). This complex array of beef quality traits is shaped by the relationships between genetic factors, breed influences, nutrition, management practices, and post-harvest handling procedures (Liu et al., 2022). These factors make beef quality a challenging target for traditional breeding strategies. However, the availability of the high-quality bovine genome assembly coupled with the advent of high-throughput sequencing technologies has paved the way for the integration of omics technologies, encompassing genomics, transcriptomics, proteomics, and metabolomics, for unraveling the complex mechanisms underlying beef quality.
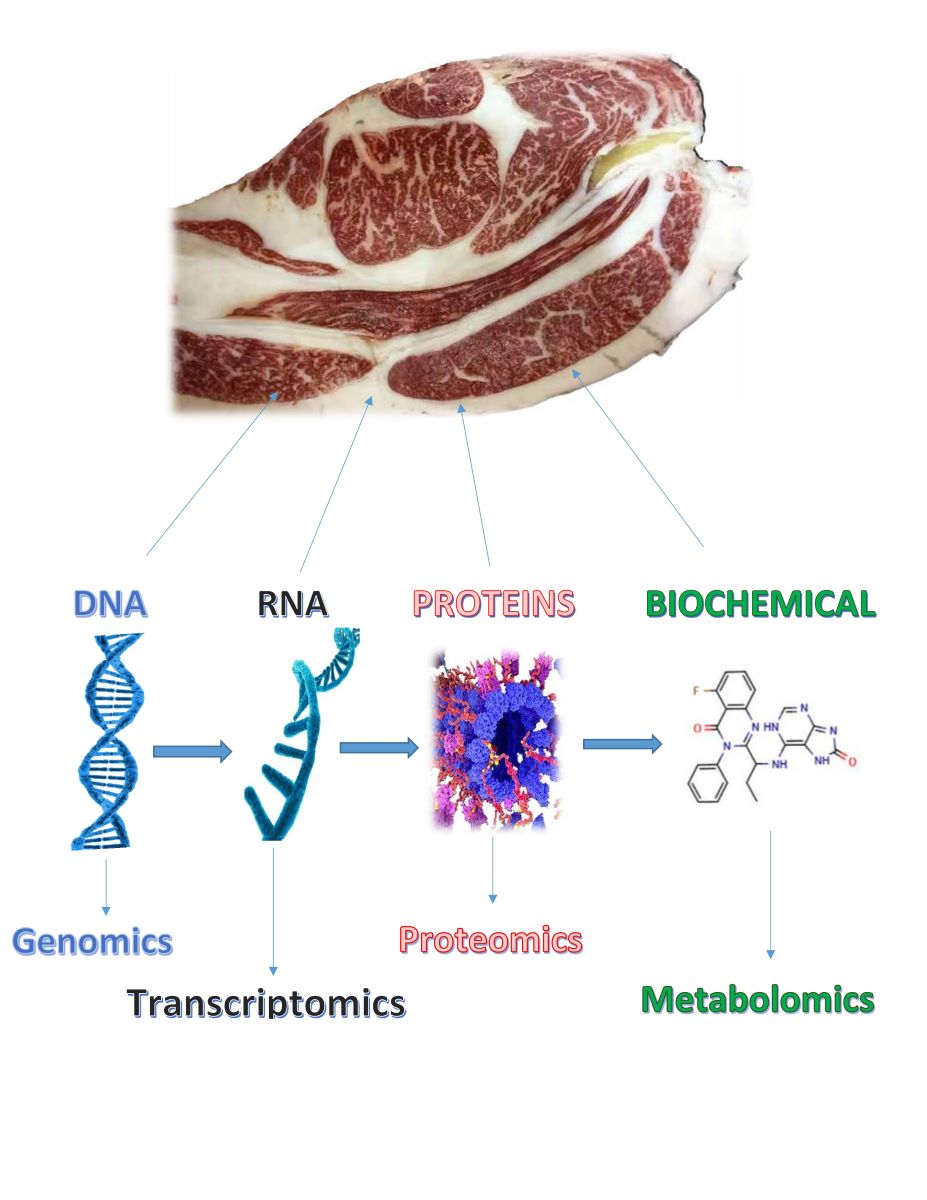
Multi-omics approach has the power to resolve the meat quality research into an image of what is being expressed, translated and produced, which incorporates technologies characterizing various biological products, including DNA (genomics), RNA (transcriptomics), protein (proteomics) and metabolites (metabolomics) in biological samples. Genomic approaches, such as genome-wide association studies (GWAS) and genomic selection (GS), have provided valuable insights into the genetic architecture of beef quality traits. While these methods have identified several genetic markers and regions associated with these traits, their practical application in enhancing beef quality through selective breeding remains limited and requires further research and validation. These techniques enable the identification and utilization of functional variants associated with desirable phenotypes, thereby accelerating genetic improvement, and enhancing the efficiency of breeding programs. Transcriptomics, which examines the expression patterns of genes, provides insights into the molecular pathways and regulatory networks governing muscle development, metabolism, and meat quality attributes. Proteomics, on the other hand, offers a comprehensive view of the functional proteins involved in these processes, elucidating their roles and interactions. Complementing these approaches, metabolomics unveils the complex metabolic landscapes that shape the biochemical composition and sensory properties of beef. This article aims to summarize the latest findings from these advanced scientific approaches in beef quality genetics. By exploring research from GWAS, genomic selection, transcriptomics, proteomics, and metabolomics, we seek to provide a comprehensive understanding of how genetic factors, gene expression, proteins, and metabolic processes influence beef quality. The goal is to offer valuable insights for researchers and industry professionals, potentially improving breeding strategies and production methods to enhance beef quality.
2. Functional Mutations and Commercialized DNA Tests for Beef Quality
At the beginning of the genomics era during the 1980s, the primary application of this technology in livestock breeding revolved around developing standalone genome marker tests, particularly for identifying inherited diseases and parentage testing. However, as the field progressed, the focus shifted towards integrating quantitative and genomic approaches to identify genomic variants with substantial effects on desirable traits of interest. These DNA tests were then leveraged in breeding programs, enabling breeders to make more informed decisions by selecting animals with favorable genetic profiles for specific traits, thereby accelerating genetic improvement in livestock populations.
Kostusiak et al. (2023) provided a comprehensive review of the effects of single nucleotide polymorphisms (SNPs) in four key genes - myostatin (MSTN), thyroglobulin 5 (TG5), μ-calpain (CAPN1), and calpastatin (CAST) - on beef cattle productivity and meat quality traits. MSTN is a negative regulator of muscle growth. Inactivating mutations or suppression of the MSTN gene leads to a "double-muscled" phenotype with increased muscle mass and reduced fat deposition in cattle breeds like Belgian Blue and Piedmontese (Fiems, 2012). Meat from MSTN-null cattle exhibits improved tenderness across all cuts, including typically tougher cuts like chuck and round. This is likely due to increased muscle fiber hyperplasia rather than just hypertrophy (Aiello et al., 2018). While inactivating the MSTN gene can dramatically increase muscle yields and tenderness in beef cattle, it comes at the cost of reduced marbling and juiciness. An optimal approach leverages MSTN alongside other genes to strike a balance between production efficiency, leanness, and eating quality traits like tenderness and flavor. Esmailizadeh et al. (2008) investigated the effects of a specific single nucleotide polymorphism (SNP) in the myostatin (MSTN) gene, resulting in a phenylalanine to leucine substitution at position 94 (F94L), on various beef production and quality traits. The F94L variant of MSTN was found to provide a more desirable intermediate phenotype than the severe double-muscling caused by complete MSTN inactivation, offering improved meat yield while maintaining acceptable meat quality traits like tenderness (Esmailizadeh et al., 2008).
Polymorphisms in the thyroglobulin (TG5) gene can significantly impact beef quality, particularly in terms of intramuscular fat (IMF) content and marbling. The TG5 gene is located on bovine chromosome 14 and encodes the thyroglobulin protein, which plays a role in fat metabolism. Wood et al. (2006), in their meta-analysis, found that there was a positive association between the polymorphic forms of TG5 and the degree of meat marbling. A specific single nucleotide polymorphism (SNP) in the 5' untranslated region of TG5, characterized by a C>T transition at position -422 (X05380.1:g.-422C>T), has been widely studied (Kostusiak et al. (2023). The TG5 C allele has been associated with higher levels of IMF and increased marbling scores in beef cattle across multiple breeds (Wood et al.,2006). Higher IMF and marbling are desirable traits as they enhance beef flavor, juiciness, and tenderness, improving overall eating quality and palatability. However, some consumers, especially those in developed countries, prefer leaner beef with lower fat content for health reasons, creating a conflict with the preference for marbled, flavorful meat in blind taste tests.
CAPN1 and CAST genes encode the calpain and calpastatin enzymes that regulate protein degradation and meat tenderization post-mortem. CAPN1 encodes the enzyme μ-calpain, which is a calcium-dependent cysteine protease that breaks down muscle proteins during the meat tenderization process after slaughter. CAST encodes the protein calpastatin, which is an endogenous inhibitor of μ-calpain and other calpain enzymes, thereby modulating the extent of protein degradation and meat tenderization. Studies across multiple breeds have validated SNP markers in CAPN1 (e.g. 316, 530, 4558, 4684) and CAST (e.g. 282, 589) as useful for marker-assisted selection to improve beef tenderness (Morris et al, 2006; Sun et al., 2018; Lee et al., 2019).
Polymorphisms in
CAPN1 that beneficially associate with beef tenderness are reported to antagonistically associate with calving day in beef heifers (Tait et al., 2018) and post-partum interval to estrus in beef cows (Collis et al., 2012). However, the results of Cushman et al. (2021) indicate that molecular breeding for slice shear force, calculated based on
CAPN1 and calpastatin (
CAST) genotypes, had minimal or no antagonistic association with reproductive performance in heifers.
Table 1 lists some of the commercially available DNA tests for beef quality, although there are more tests in the literature than are being offered to farmers.
The integration of functional mutations in genes such as MSTN, TG5, CAPN1, and CAST has led to the development of commercial DNA tests that enhance beef quality traits like tenderness, marbling, and flavor. However, future research should focus on optimizing these genetic advancements alongside animal welfare and environmental factors to ensure sustainable production. Additionally, exploring the interactions between genetic traits and management practices will be crucial for fully realizing the potential of these genomic tools in the beef industry.
3. Genome-Wide Association Studies for Beef Quality Traits
Initial genome-wide scans to locate quantitative trait loci (QTL) for beef quality traits were based on linkage analysis within families. For example, Esmailizadeh et al. (2011) reported a whole-genome scan to detect QTL for meat quality traits like tenderness (measured as shear force on two muscles), meat color, pH, and cooking loss, as well as metabolic traits in cattle populations from New Zealand and Australia. The study used backcross calves with Jersey and Limousin backgrounds, with the New Zealand cattle reared on pasture and the Australian cattle finished on grain. A total of 18 significant QTL for meat quality traits and 11 significant QTL for metabolic traits were detected across multiple chromosomes. Genome-wide association studies (GWAS), available since 2005 in human genetics, are based on linkage disequilibrium at the level of a population and involve scanning the entire genome for single nucleotide polymorphisms (SNPs) that are statistically associated with a particular phenotype of interest. GWAS have been successful in the identification of numerous genetic variants associated with complex traits for uncovering novel biological pathways and elucidating the genetic architecture of various traits (Visscher, et al., 2017).
Genome association studies provide knowledge about the genetic architecture of beef-related traits that allow linking the target phenotype to genomic information aiding breeding decisions. GWAS in cattle breeds like Hanwoo (Korean native cattle) have identified 107 significant SNPs on chromosome 14 and candidate genes associated with economically important beef quality traits such as marbling, meat color, texture, and fat color (Bedhane et al. 2019). Nearby genes like SFT2 Domain Containing 3 (SFT2D3) and Ectonucleotide Pyrophosphatase/Phosphodiesterase 2 (ENPP2) have been highlighted as potential candidate genes affecting beef traits such as marbling and meat color (Bedhane et al., 2019).
GWAS results from the study of Forutan et al. (2023) implicate some interesting candidate genes (KIF13A and APOB) for eating quality. Kinesin Family 13A (KIF13A) is in a pathway associated with skeletal muscle cells that increase insulin signaling, glucose uptake, and maximal oxygen consumption (Massart et al., 2021). Apolipoprotein B (APOB) is a building block of a type of lipoprotein called a chylomicron. As food is digested, chylomicrons form to carry fat and cholesterol from the intestine into the bloodstream. (Forutan et al., 2023).
A recent study (Arikawa et al., 2024) performed genome-wide association analyses on Nellore cattle to identify genomic regions and candidate genes influencing carcass traits and meat quality traits (shear force, marbling score, intramuscular fat content). The top 10 genomic regions explained 8-22% of the additive genetic variance for these traits, harboring a total of 119-155 positional candidate genes. Relevant genes like CAST, PLAG1, XKR4, PLAGL2, AQP3/AQP7, MYLK2, WWOX, CARTPT, and PLA2G16 are involved in physiological processes affecting muscle growth, lipid metabolism, adipose tissue development, and signaling pathways like the insulin/IGF-1 pathway.
Mateescu et al. (2017) explored the complexity of meat quality, by combining GWAS with gene network analysis to identify genes and pathways associated with meat quality traits like tenderness, juiciness, and flavor in Angus cattle. They revealed several modules of co-expressed genes associated with meat quality traits. Key genes identified included
CAST and
CAPN1 for tenderness,
FASN and
SCD for marbling, and
MYOZ1,
MYOZ3, and
CASQ1 for color score. The study highlights the utility of network analysis for identifying candidate genes from GWAS results in beef cattle. Several beef cattle studies conducted GWAS to identify genomic regions associated with marbling score, intramuscular fat deposition, and fatty acid composition and revealed several significant SNPs and candidate genes on different chromosomes associated with specific fatty acids and fat content (
Table 2).
Genome-wide association and gene enrichment analyses on 672 steers from a multibreed Angus-Brahman beef cattle population have identified membrane anchoring and structural proteins (e.g. ANO2, NTF3, EVC2, ANXA10, PALLD, PKHD1) associated with meat quality traits like tenderness, marbling, cooking loss, and sensory panel ratings for tenderness, juiciness, connective tissue amount, and flavor (Leal-Gutiérrez et al. 2019). A gene network analysis identified EVC2, ANXA10, and PKHD1 as potentially harboring multiple QTL for meat quality. The results of Leal-Gutiérrez et al. (2019) suggest that polymorphisms in structural proteins can modulate muscle fiber organization and postmortem proteolysis, directly impacting meat quality.
Despite their remarkable success, GWAS have faced several challenges, including the need for larger sample sizes to detect variants with smaller effect sizes and the limited representation of diverse ancestral populations (Mills and Rahal, 2019). Additionally, many GWAS are descriptive rather than functionally identifying causal variants. Efforts have been made to increase the diversity of GWAS cohorts and to conduct meta-analyses combining data from multiple studies to enhance statistical power in human genetics (Visscher et al., 2017) and recently in beef cattle (Sanchez et al., 2023). As GWAS continue to evolve, integrating complementary approaches such as functional genomics, epigenomics, and proteomics will be crucial for translating genetic associations into mechanistic insights and understanding the molecular mechanisms underlying beef quality traits.
4. Genomic Prediction and Selection for Beef Quality
Genomic selection (GS) which was first introduced by Lande and Thompson (2000) and popularized by Meuwissen et al. (2001) utilizes genome-wide marker data to predict so-called genome-enhanced or genomic estimated breeding values (GEBV) of the selection candidates. It involves developing prediction models from a training population with both genotypic and phenotypic data and then applying these models to predict the breeding values of individuals in a separate population based solely on their genotypic information. This approach enables more accurate selection of superior individuals at an early stage, accelerating the rate of genetic gain compared to traditional phenotypic selection. GS relies on capturing the effects of all QTL through linkage disequilibrium between markers and QTL, as well as leveraging genetic relationships between the training and prediction populations (Lee et al., 2017). Key factors influencing the accuracy of genomic predictions include the size and genetic diversity of the training population, the heritability of the trait, and the extent of relatedness between the training and prediction sets (Lee et al., 2017; Dekkers et al. 2021). GS holds the promise to be particularly beneficial in selecting for traits such as beef quality traits that are difficult and expensive to measure.
Fernandes Júnior et al. (2022) highlighted the long generation interval of beef cattle and the importance of genomic selection in accelerating genetic gains for meat quality traits. Beef tenderness is a significant challenge in the Zebu beef cattle industry. Reported heritability estimates for meat tenderness ranged from 0.11 to 0.45 (Wheeler et al. 2010; Gordo et al., 2018). However, selection for meat quality has only recently (last 10–15 years) been implemented, and due to the long generation interval of beef cattle, substantial genetic improvement is yet to be realized. Additionally, this trait is costly and difficult to measure, and slaughterhouses do not offer differential payment for tender beef. Furthermore, breeding programs have focused more on improving meat quantity over quality attributes. Considering various methods (Bayesian ridge regression, Bayesian LASSO, Bayes A, Bayes B, and Bayes Cπ) and a training population of 426 Nellore animals, Magnabosco et al. (2016) reported prediction accuracies for beef tenderness ranging from 0.52 to 0.59 . Moderate accuracies for beef tenderness (0.57 to 0.60) have also been reported considering GBLUP, LASSO, and Bayes Cπ in a Nellore training population (n = 4,500 animals) (Fernandes Júnior et al., 2022). Accuracies between 0.23 and 0.73 were also described by the authors for lipid content, marbling, and meat color (
Table 3).
The fatty acid profile is an important indicator of beef quality and studies have revealed the possibility of genetic improvement of fatty acid composition by selection of both major candidate genes and genomic selection strategies in beef cattle (Chiaia et al., 2017; Magalhães et al. 2019).
Forutan et al. (2023) discussed the use of genomic selection to improve meat quality in beef cattle. They highlighted the shift from producer-driven to consumer-driven beef production and the importance of consumer satisfaction in beef quality. Forutan et al. (2023) determined the most accurate method for predicting phenotypes of beef eating quality traits from genotypes and other factors such as carcass weight and days aged. They found that the accuracy of phenotype prediction for beef eating quality traits was sufficiently high that such predictions could be useful in predicting eating quality from samples taken from an animal/carcass as it enters the processing plant, to sort for markets with different quality. Forutan et al. (2023) emphasized that future predictions should be expanded to incorporate all the parameters in the Meat Standards Australia (MSA) models (Watson et al., 2008) as well as genotype information.
It has been challenging to implement genomic selection in multi-breed tropical beef cattle populations. If commercial (often crossbred) animals could be used in the reference population for these genomic evaluations, this could allow for very large reference populations. In tropical beef systems, such animals often have no pedigree information. Hayes et al. (2023) addressed the challenges of implementing genomic selection in multi-breed tropical beef cattle populations, especially when no pedigree information is available. They evaluated potential models using marker heterozygosity and breed composition derived from genetic markers. The study demonstrated that moderately accurate genomic estimated breeding values (GEBV) can be calculated using these models, with BayesR resulting in the highest accuracy.
The limitations, complexity, and loss of information associated with the multiple-step genomic selection approach (Legarra et al., 2009) have led to the development of single-step approaches (Aguilar et al., 2010; Christensen and Lund, 2010). Single-step genomic best linear unbiased prediction (ssGBLUP) is a widely used method that combines the pedigree-based numerator relationship matrix (A) and the genomic relationship matrix (G) to construct a combined relationship matrix (H). This allows information from genotyped and non-genotyped individuals to be used simultaneously in one step. The key advantage of single-step methods is that all available information (phenotypic, pedigree, and genomic) is used optimally, leading to greater accuracy and persistence of genomic predictions across generations. It avoids the need for separate evaluations for genotyped and non-genotyped individuals and accounts for potential pre-selection biases. Adekale et al. (2023) used the ssGBLUP approach and combined pedigree, genomic, and phenotypic data into one evaluation, and genomic evaluations increased the accuracy of estimated breeding values (EBVs) compared to pedigree-based evaluations alone. They demonstrated the successful implementation of single-step genomic evaluations for improving the accuracy of EBVs in German beef cattle breeding programs across multiple breeds (Adekale et al.,2023).
In summary, challenges in obtaining high-quality and adequately detailed phenotype data, along with frequently incomplete pedigree information, hamper traditional genetic evaluations for beef quality traits. The challenges in collecting beef quality data for genetic evaluations can be attributed to several factors, such as the complexity and variability of the traits being measured, the need for specialized equipment or expertise, and the time and resources required to gather data from a large number of individuals. Additionally, the lack of standardized protocols and the potential for human error in data collection can contribute to the challenges in obtaining high-quality phenotypic data for beef quality traits. Therefor, GS has the potential to substantially increase the genetic gain by increased selection accuracy at an early age (Montaldo et al. 2012; Stock and Reents, 2013). However, the heterogeneity of breeds, less developed breeding programs and infrastructures, the predominance of natural service, and the population substructures with frequent crossbreeding in commercial herds have restricted the widespread implementation of GS in beef cattle. Multi-breed genomic evaluation and single-step GS are the most recent developments in implementing GS in beef cattle breeding. Challenges include access to large phenotypic datasets across breeds/environments and low-cost genotyping for widespread adoption (Garrick, 2011). Extension of genomic predictions to beef quality traits influencing consumer satisfaction will further require a focus on the collection of reliable phenotypic information across the broad range of traits. Collecting such information will likely rely on public funding efforts. The novel high-throughput phenotyping technologies that facilitate the collection of phenotypes on large cohorts will also be invaluable (Garrick, 2011).
5. Transcriptomics of Beef Quality
Transcriptomics, one of the most developed fields in the post-genomic era, is the genome-wide study of the complete set of transcribed sequences, including messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), and regulatory noncoding RNA in a tissue or a specific cell type at a given time or under a specific physiological condition. Transcriptomics focuses on RNA expression levels to reveal the molecular mechanisms involved in specific biological processes. High-throughput sequencing technologies like bulk RNA-Seq and single-cell RNA-Seq (scRNA-Seq) have transformed transcriptomics research, including studies related to beef quality. Bulk RNA-Seq characterizes average gene expression profiles across samples, enabling the identification of differentially expressed genes and splicing variants associated with meat traits. scRNA-Seq captures cell-type-specific transcriptomes in muscle tissues, revealing cellular heterogeneity and facilitating the discovery of novel cell populations linked to meat quality traits. Together, these complementary high-throughput approaches provide comprehensive insights into transcriptome landscapes and accelerate the development of transcriptome resources for improving beef quality. In addition, the available transcriptomics datasets in cattle such as the transcriptome atlas (Fang et al., 2020) can serve as a primary source for biological interpretation and functional validation of transcriptomics studies addressing beef quality complexities.
Intramuscular fat (IMF) deposition has been a central focus of numerous transcriptomics investigations aimed at elucidating the molecular determinants of beef quality (e.g., Liu et al., 2020; Yu et al., 2024). A significant proportion of transcriptome research in the realm of beef quality has concentrated on unraveling the genetic and regulatory mechanisms underlying variations in intramuscular fat content, given its pivotal role in influencing meat tenderness, juiciness, and flavor. The study by Yu et al. (2024) employed an integrated transcriptomics and metabolomics approach to elucidate the regulatory mechanisms underlying intramuscular fat deposition in three cattle breeds - Qinchuan, Nanyang, and Japanese Black. The Japanese Black breed had significantly higher IMF content compared to the Chinese indigenous breeds. Transcriptomic analysis revealed genes like ITGB1 were enriched in pathways related to fatty acid metabolism, suggesting their roles in regulating IMF content.
Several key regulatory genes have been identified that influence adipocyte differentiation and intramuscular fat deposition, which are important for beef quality. For example, transcription factors like C/EBPα and PPARγ play crucial roles in promoting adipocyte development and fatty acid biosynthesis in beef cattle (Liu et al., 2020). Krüppel-like factors (KLFs) are a family of transcription factors that regulate adipogenesis in cattle. KLFs can act as positive or negative regulators of adipocyte differentiation through crosstalk with C/EBP and PPARγ (Raza et al., 2022). Adipogenic genes like DGAT1, FABP3, FABP4, and FASN are upregulated during early adipocyte differentiation in cattle (Hausman et al., 2009). In summary, transcription factors like C/EBP, PPARγ and KLFs, fatty acid metabolism genes, and growth-related genes play key regulatory roles in controlling adipocyte differentiation and intramuscular fat deposition, which are crucial determinants of beef quality. Identifying genetic markers in these pathways can help improve meat quality through breeding programs.
A recent study (Zhang et al., 2023) suggests that long non-coding RNAs (lncRNA) may have critical functional roles in intramuscular fat accumulation. Zhang et al. (2023) reported that a lncRNA named long non-coding RNA BNIP3 (lncBNIP3) inhibited the proliferation of bovine intramuscular preadipocytes through the cell cycle pathway, revealing potential new strategies for improving beef quality.
Transcriptomics has been widely exploited to study the effects of diverse feeding systems, production practices, and rearing conditions on beef quality. Researchers have investigated the transcriptomic profiles associated with different dietary regimes, feed restriction and compensatory growth, production systems, and environmental stressors (heat, transportation). These studies aim to elucidate the molecular mechanisms underlying variations in beef quality traits influenced by various production factors. For example, the study by Zhao et al. (2012) investigated the effects of acute stress on beef tenderness and the underlying molecular mechanisms in Angus cattle using a functional genomics approach. They found that acute stress significantly increased beef tenderness, measured by the Warner-Bratzler shear force (WBSF). Microarray analysis identified 147 differentially expressed genes (DEGs) between the stressed and control groups, with the majority of DEGs being downregulated in the stressed group. Functional annotation revealed that these DEGs were enriched in pathways related to muscle structure and integrity, including cytoskeletal organization, muscle contraction, and calcium signaling. Key DEGs included CAPN1, CAPN2, CAST, and CALM, which are involved in the calpain-calpastatin system regulating protein degradation and tenderization. The study also identified potential transcriptional regulators, such as NFKB1, CREB1, and FOXO3, that may mediate the stress response and influence beef tenderness. Overall, this functional genomics study provided insights into the molecular mechanisms by which acute stress improves beef tenderness, highlighting the role of the calpain system and related pathways (Zhao et al. (2012). Sweeney et al. (2016) identified 26 differentially expressed (DE) genes related to lipid metabolism between pasture-fed and concentrate-fed cattle. The expression of ALAD, EIF4EBP1 and NPNT could be used to classify the samples based on the production system with 95-100% accuracy (Sweeney et al., 2016). In addition, Deng et al. (2024) analyzed transcriptomes of cattle under varied restricted feeding conditions to study compensatory growth effects on meat quality. Compensatory growth was observed in the restricted groups, accompanied by alterations in meat quality traits like pH, cooking loss, and fat content compared to the ad libitum group. Transcriptome analysis identified DEGs unique to each feeding group as well as shared DEGs involved in pathways related to muscle growth, lipid metabolism, and nutrient utilization. Gene set enrichment analysis further highlighted pathways associated with compensatory growth, such as protein synthesis, cell cycle regulation, and energy metabolism.
The study by Zhang et al. (2022) employed comparative transcriptomics to characterize region-specific gene expression patterns across five different beef cuts (tenderloin, longissimus lumborum, rump, neck, chuck) from cattle. They identified a total of 80 region-specific genes (RSGs) and 25 transcription factors regulating these RSGs. Through co-expression network analysis, seven region-specific modules were detected, including three positively and four negatively correlated modules. Their analysis revealed 91 candidate genes associated with meat quality traits, enriched in pathways related to muscle fiber structure, fatty acid metabolism, amino acid metabolism, ion channel binding, protein processing, and energy production. Key genes identified included TNNI1, TNNT1 (muscle structure), SCD, LPL (fatty acids metabolism), ALDH2, IVD, ACADS (amino acids metabolism), PHPT1, SNTA1, SUMO1, CNBP (ion binding), CDC37, GAPDH, NRBP1 (protein processing), and ATP8, COX8B, NDUFB6 (energy metabolism) (Zhang et al., 2022). The differential expression of these RSGs and candidate genes across beef cuts suggests they play a key role in determining region-specific differences in nutrient profiles like fatty acid composition and amino acid content, as well as meat quality traits like tenderness and flavor.
Transcriptomics can provide insights into the molecular mechanisms regulating beef quality traits such as water-holding capacity (WHC). In this regard, Du et al. (2021) investigated the molecular mechanisms underlying WHC in Chinese Simmental beef cattle through transcriptome profiling. The longissimus dorsi muscles from 49 cattle were evaluated for meat quality traits, including WHC, water loss, intramuscular fat content, shear force, and pH. Eight individuals with extreme WHC values were selected for RNA-sequencing analysis. A total of 865 DEGs were identified between the high and low WHC groups. These DEGs were involved in pathways related to muscle structure, energy metabolism, and protein folding. The study confirmed seven previously known genes (HSPA12A, HSPA13, PPARγ, MYL2, MYPN, TPI, and ATP2A1) and identified six novel candidate genes (ATP2B4, ACTN1, ITGAV, TGFBR1, THBS1, and TEK) potentially affecting WHC (Du et al., 2021).
In summary, the recent high-throughput transcriptomic studies have identified differentially expressed genes and pathways involved in lipid metabolism, muscle fiber properties, energy production, and other processes that influence beef quality traits like tenderness, fatty acid composition, and nutrient content across different production systems, feeding regimes, and muscle cuts. This knowledge on the region-specific, breed-specific, and production system-specific gene expression patterns that regulate various aspects of beef quality can guide targeted breeding programs and optimized management practices to improve beef quality.
6. Proteomics of Beef Quality
Although transcriptomics tools such as RNA-seq offer a massively parallel approach to genome-wide mRNA expression analysis, there is often no direct relationship between the in vivo concentration of an mRNA and its encoded protein. The association of protein expression levels with biological changes is one of the most fundamental approaches to understanding the functions of individual proteins in complex cellular processes. Proteomics, a large-scale study of proteins, is a biomarker approach for the identification and quantification of all proteins, the proteome, of a given biological system (cell, tissue, organ, biological fluid, or organism) at a specific point in time. Mass spectrometry (Rozanova et al., 2021) coupled with advanced separation techniques like two-dimensional gel electrophoresis and liquid chromatography is the technique most often used for proteomics. In the context of beef quality, proteomics provides insights into the molecular mechanisms influencing meat tenderness, flavor, and other quality attributes. By analyzing the proteome of beef muscles, researchers can identify biomarkers associated with desirable traits, elucidate pathways regulating meat characteristics, and develop strategies to improve beef quality through breeding or processing methods.
Over the last two decades, proteomics has been employed to decipher the underlying factors contributing to variation in beef tenderness.
Table 4 summarizes some of the published proteomic studies on beef quality. Functional proteomic analysis was used to associate electrophoretic bands from the myofibrillar muscle fraction with meat tenderness to understand the mechanisms controlling tenderness (Zapata et al., 2009). Six significant electrophoretic bands were characterized and sequenced, revealing proteins involved in structural, metabolic, chaperone, and developmental functions (Zapata et al., 2009).
An integromics study was performed to review the status of protein biomarker discovery targeting beef tenderness, gathering and proposing a comprehensive list of 124 putative protein biomarkers derived from 28 independent proteomics-based experiments (Gagaoua et al., 2020a). In the study of Gagaoua et al. (2020a) 33 robust candidates were identified as worthy of evaluation using targeted or untargeted data-independent acquisition proteomic methods. The study provides an overview of the interconnection of the main biological pathways impacting tenderness determination, including structural proteins, enzymes, heat shock proteins, and proteins involved in energy metabolism, response to oxidative stress, and apoptosis (Gagaoua et al., 2020a). Gagaoua et al. (2020a) identified MYOZ3 (Myozenin 3), BIN1 (Bridging Integrator-1), and OGN (Mimecan) as the primary proteins, which accounted for 79% of the variability in shear force values.
Functional proteomic and interactome analysis was used to identify protein biomarkers and biological pathways associated with beef tenderness in Angus cattle (Zhao et al., 2014). The study compared the proteome of longissimus thoracis muscle samples from Angus cattle with divergent tenderness phenotypes. Several proteins involved in structural integrity, energy metabolism, stress response, and proteolysis were found to be differentially abundant between tender and tough meat samples. Interactome analysis revealed complex interactions among these proteins, providing insights into the molecular mechanisms underlying beef tenderness variation. The results of Zhao et al. (2014) suggest that a combination of protein biomarkers could be used to predict and improve beef tenderness in Angus cattle. In addition, proteomic techniques have been applied to investigate different degrees of meat tenderness in the Nellore breed, a Bos indicus breed of cattle (Rosa et al, 2018; Malheiros et al., 2021). The results demonstrate that meat tenderness in Nellore cattle depends on the modulation and expression of a set of proteins. For example, the results of Rosa et al. (2018) demonstrated that polymorphisms at UOGCAST and CAPN4751 SNPs (located on CAST and CAPN1, respectively) are associated with the variability in the expression of proteins that are involved in muscle metabolism, and consequently affect meat tenderness. Malheiros et al. (2021) also identified proteins PFN1, LAP3, PRDX1, PRDX2, HSPD1, and ARHGDIA to be associated with beef tenderness.
The study by López-Pedrouso et al. (2021) employed a quantitative proteomic approach using SWATH-MS (Sequential Window Acquisition of all Theoretical Mass Spectra) to investigate the molecular factors influencing beef tenderness in young Piedmontese bulls. They analyzed the proteome of Longissimus thoracis muscle samples from 10 animals, categorized as tough or tender based on Warner-Bratzler shear force measurements. The SWATH-MS analysis identified and quantified over 1,200 proteins, revealing significant differences in the abundance of 43 proteins between the tough and tender groups. Most of these differentially abundant proteins were associated with energy metabolism pathways. Functional analysis suggested that gluconeogenesis, glycolysis, and the citric acid cycle are key pathways influencing tenderness in Piedmontese beef, with proteins like ACO2, MDH1, MDH2, CS, FBP2, PFKL, LDHA, TPI1, and GAPDH/S playing crucial roles (López-Pedrouso et al.. 2021).
Zhu et al. (2023) used label-free proteomics to identify molecular mechanisms and biomarkers related to beef sensory texture and flavor traits in early post-mortem muscle. The authors revealed 34 putative protein biomarkers that discriminated between tender and tough meat groups, belonging to biological pathways associated with muscle structure, heat shock proteins, energy metabolism, response to oxidative stress, and apoptosis. Many of these proteins were previously identified as biomarkers of beef tenderness in an integromics data mining approach (Gagaoua et al., 2021a). Heat shock protein beta-6 (HSPB6) has been identified as being negatively correlated with tenderness and flavor and positively with stringiness (Zhu et al., 2023). It belongs to small heat shock proteins (HSPs) that are widely considered as useful biomarkers of beef tenderness, color, water-holding capacity, and other quality traits (Gagaoua et al, 2020a; 2020b; Ma, and Kim, 2020).
To provide insights into the molecular mechanisms underlying dark-cutting beef and identify potential biomarkers for predicting and managing this meat quality defect, Gagaoua et al. (2021b) conducted an integromics meta-analysis of proteomics data from eight studies on dark-cutting beef. The authors curated a list of 130 proteins that differed between dark-cutting and normal-pH beef and analyzed them using bioinformatics tools. Key pathways involved in dark-cutting beef development included muscle structure, heat shock proteins, energy metabolism, oxidative stress response, and apoptosis. Also, Kiyimba et al. (2022) compared the mitochondrial proteomes of dark-cutting and normal-pH beef using LC-MS/MS proteomics and found that dark-cutting beef has up-regulation of proteins involved in mitochondrial biogenesis, oxidative phosphorylation, intracellular protein transport, and calcium homeostasis. Mitochondria isolated from dark-cutting beef showed greater mitochondrial complex II respiration and uncoupled oxidative phosphorylation, but no differences in membrane integrity or respiration at complexes I and IV. These results indicate that dark-cutting beef has greater mitochondrial biogenesis proteins, increasing mitochondrial content and contributing to the dark color. The study provides insights into the mechanistic basis of dark-cutting beef and identifies potential candidate markers for detecting pre-slaughter events leading to this meat quality defect.
In summary, proteomics has been extensively applied to study the molecular basis of various beef quality traits, including tenderness, marbling, color, water-holding capacity, and dark-cutting beef. These studies have utilized advanced proteomics techniques, such as 2D-PAGE, mass spectrometry, and bioinformatics, to identify differentially expressed proteins and their associated biological pathways. Key proteins and pathways linked to meat quality include those involved in glycolysis, oxidative phosphorylation, the tricarboxylic acid (TCA) cycle, muscle structure, heat shock response, energy metabolism, oxidative stress, and apoptosis. Proteomics has provided valuable insights into post-mortem changes in muscle proteins and their relation to the development of meat quality traits, as well as identified potential biomarkers for predicting and managing beef quality. Future research should focus on integrating proteomic analyses with other omics approaches, such as transcriptomics and metabolomics, to gain a more comprehensive view of the regulatory networks influencing beef quality.
7. Metabolomics of Beef Quality
Metabolomics is a valuable analytical approach for studying the small molecule metabolites present in biological samples including beef and meat products. It utilizes two major platforms: mass spectrometry (MS) and nuclear magnetic resonance (NMR) to comprehensively profile the metabolite composition. The resulting metabolomic data provide insights into the metabolic state of the beef samples, enabling the discovery of biomarkers associated with desirable beef quality traits like tenderness, flavor, and shelf-life. Additionally, metabolomics elucidates the underlying biochemical pathways that produce key metabolites influencing beef quality characteristics. Metabolomic profile data can also be used to explore the genes responsible for specific metabolite-featured phenotypes in genome-wide association studies. Therefore, by associating metabolite profiles with sensory evaluation, production conditions, and postmortem changes, metabolomics offers a powerful tool for monitoring and predicting beef quality, optimizing animal breeding, and feeding strategies, and improving meat processing methods. Some of the published applications of metabolomics in assessing beef quality are summarized in
Table 5.
Muroya et al. (2020) introduced the concept of “MEATabolomics” - the application of metabolomics to study skeletal muscle and meat in domestic animals. Muscle metabolites, as the major phenotypic components, determine the physiological characteristics of muscle and meat quality traits. Since raw and cooked meat are rich in flavor-associated volatile compounds and precursors (Muroya et al., 2020), MEATabolomics studies in combination with sensory evaluation can be used to explore biomarker candidates associated with eating quality of beef.
Jeong et al. (2020) used NMR spectroscopy to investigate the meat metabolite profiles related to differences in beef quality attributes, specifically comparing high-marbled and low-marbled groups. High-marbled meat had higher levels of taste compounds compared to low-marbled meat. Metabolite analysis revealed differences between the two marbling groups based on partial least square discriminant analysis (PLS-DA). Metabolites identified by PLS-DA, such as N, N-dimethylglycine, creatine, lactate, carnosine, carnitine, sn-glycero-3-phosphocholine, betaine, glycine, glucose, alanine, tryptophan, methionine, taurine, and tyrosine, were directly linked to marbling groups. These potential markers were involved in beef taste-related pathways, including carbohydrate and amino acid metabolism. The findings of Jeong et al. (2020) provide an important understanding of the roles of taste-related metabolites in beef quality attributes and suggest that metabolomics analysis of taste compounds and meat quality may be a powerful method for evaluating beef quality.
Zhang et al. (2021) described recent applications of metabolomics in evaluating meat freshness, composition, authenticity, and origin, highlighting its potential as a powerful tool for meat quality assessment. They discussed the challenges faced, such as sample complexity, lack of specialized databases, and the need for harmonized methods. Finally, they outlined future trends, including the development of standardized protocols, meat metabolome databases, and advanced data analysis tools to fully exploit the potential of metabolomics in meat science (Zhang et al., 2021). Moreover, Ramanathan et al. (2023) provided a recent comprehensive overview of the current state of metabolomics research in meat quality and highlighted the immense potential of metabolomics in advancing meat quality research and its practical applications in the meat industry.
Yu et al. (2024) compared the metabolome of two Chinese indigenous cattle breeds (Qinchuan and Nanyang), and Japanese Black cattle. They reported that the Japanese Black breed had significantly higher IMF content compared to the Chinese indigenous breeds. Metabolomic analysis showed higher levels of monounsaturated and polyunsaturated fatty acids, as well as amino acids like creatine, lysine, and glutamine in the Japanese Black breed, contributing to better flavor formation (Yu et al., 2024).
Metabolomics, especially focusing on volatile compounds, has changed our understanding of beef aroma and flavor. Using techniques like gas chromatography-mass spectrometry (GC-MS), researchers can quantify and correlate metabolites with flavor preferences. This analysis identifies key flavor compounds and their precursors, revealing mechanisms like the Maillard reaction, thermal lipid degradation, and oxidation. In beef, metabolomics shows that flavor results from interactions between aromatics and taste components, with meaty and roasted notes from Maillard reactions (Diez-Simon et al. 2019). This knowledge helps food scientists predict and manipulate flavor profiles, enhancing product development and quality control.
Castejón et al. (2015) investigated the potential of using metabolomics analysis of meat exudate to evaluate beef conservation and aging. These researchers analyzed the exudate from beef samples stored at different temperatures and aging times using NMR spectroscopy. They found that the metabolite profile of the exudate changed significantly with storage temperature and aging time, allowing them to discriminate between fresh and aged meat samples. Specific metabolites like creatine, carnosine, and anserine were identified as potential biomarkers for monitoring meat aging and conservation, demonstrating that metabolomics of meat exudate could be a rapid and non-destructive approach to assessing beef quality during storage and aging processes (Castejón et al., 2015).
Tian et al. (2023) performed a comparative metabolomics analysis on subcutaneous fat samples from crossbred cattle with white and yellow fat color. Through liquid chromatography-mass spectrometry, 235 significant metabolites across five categories were identified, with principal component analysis showing distinct clustering of white and yellow fat samples. White fat exhibited greater metabolite variation, with 163 metabolites having higher and 72 having lower relative abundance compared to yellow fat. Notably, 3-hydroxyoctanoic acid, anethofuran, 9,10-DiHODE, furanoeremophilane, pregeijerene, N-glycolylneuraminic acid, and glycocholic acid were identified as potential biomarkers for differentiating fat color. The findings provide insights into the metabolic mechanisms underlying fat color variation and suggest potential biomarkers for selective breeding programs aimed at achieving desired beef fat color traits.
Next-generation phenotyping (NGP) using metabolomics is becoming a fundamental approach to refine trait description and improve the prediction of breeding values aligned with beef industry objectives. For example, non-invasive urinary biomarkers have been identified for beef production efficiency and carcass quality traits (Artegoitia et al. 2022). These biomarkers are indicative of various aspects of beef quality, such as taste, and appearance that can be used to predict and improve beef quality through targeted breeding and nutrition.
In summary, metabolomics has emerged as a powerful tool for profiling meat quality attributes, such as flavor, color, and texture. Recent studies have successfully applied metabolomics to identify biomarkers related to meat quality and taste, using techniques like nuclear magnetic resonance spectroscopy and mass spectrometry. However, challenges remain in correlating metabolites to specific meat quality traits and elucidating the underlying mechanisms (Zhang et al., 2021; Ramanathan et al.,2023). Future research should focus on developing generic validation schemes for metabolomics-based meat quality control, as well as integrating metabolomics with other omics technologies to provide a more holistic understanding of beef quality.
8. Challenges and Future Directions
While a wide range of omics technologies have been applied to study beef quality traits, several challenges remain in fully harnessing their potential:
-Integrating Multi-Omics Data: Combining genomics, transcriptomics, proteomics, and metabolomics data to elucidate the complex biological networks underlying meat quality is a challenging task that requires robust bioinformatic pipelines and systems biology approaches.
-Implementing Integromics: Integromics, which uses advanced computational and statistical methods to integrate diverse data types, offers a promising platform for advancing beef quality research. However, the implementation of an integromics approach is still in its early stages and requires further development and validation.
-Identifying Causal Functional Mutations: The identification and validation of causal functional mutations through gene editing techniques is crucial for precise genomic selection and breeding programs. While gene editing technologies like CRISPR/Cas9 have been developed, their application in beef quality research is still limited.
-Overcoming Challenges through Interdisciplinary Research: Addressing the challenges in applying omics technologies to beef quality research will require interdisciplinary research efforts and public-private partnerships. The lack of collaboration between different disciplines and stakeholders has hindered progress in this field.
-Translating Multi-Omics Findings into Practical Applications: While multi-omics findings have the potential to improve breeding strategies and genomic predictions for beef quality, the translation of these findings into practical applications is still limited. More research is needed to bridge the gap between research and industry.
Despite these challenges, there have been notable successes, such as the identification of specific genetic variants that have been incorporated into breeding strategies, leading to measurable improvements in meat quality. Future research should focus on refining these techniques, improving data integration methods, and addressing the economic feasibility of implementing functional genomics in commercial cattle breeding. By doing so, we can better harness the potential of these advanced technologies to meet the growing demand for high-quality beef.
Author Contributions
Conceptualization, R.T., A.E.; investigation, J.T., M.M., M.S.; resources, M. Z., S.E.K..; writing—original draft preparation, A.E.; writing—review and editing, Y.L., J.T., M.M.; supervision, R.T.; project administration, H.L., X.W.; funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation (grant number 32060731), Inner Mongolia Agriculture and Animal Husbandry Innovation Fund (grant number 2023CXJJM01), 2023 Inner Mongolia grassland talent program, Inner Mongolia Key research and development and achievement transformation plan projects (grant number 2023YFDZ0035),.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this review are included in the article/Tables within the text; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank our colleagues for their help during the research process.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Esmailizadeh, A.K.; Morris, C.A.; Cullen, N.G.; Kruk, Z.A.; Lines, D.S.; Hickey, S.M.; Dobbie, P.M.; Bottema, C.D.; Pitchford, W.S. Genetic mapping of quantitative trait loci for meat quality and muscle metabolic traits in cattle. Anim. Genet. 2011, 42, 592–599. [Google Scholar] [CrossRef]
- Purslow, P.P.; Archile-Contreras, A.C.; Cha, M.C. Meat Science and Muscle Biology Symposium: manipulating meat tenderness by increasing the turnover of intramuscular connective tissue. J. Anim. Sci. 2012, 90, 950–959. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: a review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Killinger, K.M.; Calkins, C.R.; Umberger, W.J.; Feuz, D.M.; Eskridge, K.M. Consumer visual preference and value for beef steaks differing in marbling level and color. J. Anim. Sci. 2004, 82, 3288–3293. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Pethick, D.W.; Ball, A.J.; Banks, R.G.; Hocquette, J.F. Current and future issues facing red meat quality in a competitive market and how to manage continuous improvement. Anim. Prod. Sci. 2011, 51, 13–18. [Google Scholar] [CrossRef]
- Warner, R.D.; Greenwood, P.L.; Pethick, D.W.; Ferguson, D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010, 86, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ellies-Oury, M.P.; Stoyanchev, T.; Hocquette, J.F. Consumer Perception of Beef Quality and How to Control, Improve and Predict It? Focus on Eating Quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef]
- Kostusiak, P.; Slósarz, J.; Gołębiewski, M.; Grodkowski, G.; Puppel, K. Polymorphism of Genes and Their Impact on Beef Quality. Curr. Issues Mol. Biol. 2023, 45, 4749–4762. [Google Scholar] [CrossRef]
- Fiems, L.O. Double Muscling in Cattle: Genes, Husbandry, Carcasses and Meat. Animals (Basel) 2012, 2, 472–506. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Patel, K.; Lasagna, E. The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 2018, 49, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Esmailizadeh, A.K.; Bottema, C.D.; Sellick, G.S.; Verbyla, A.P.; Morris, C.A.; Cullen, N.G.; Pitchford, W.S. Effects of the myostatin F94L substitution on beef traits. J. Anim. Sci. 2008, 86, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.A.; Moser, G.; Burrell, D.L.; Mengersen, K.L.; Hetzel, D.J. A meta-analytic assessment of a thyroglobulin marker for marbling in beef cattle. Genet. Sel. Evol. 2006, 38, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Cullen, N.G.; Hickey, S.M.; Dobbie, P.M.; Veenvliet, B.A.; Manley, T.R.; Pitchford, W.S.; Kruk, Z.A.; Bottema, C.D.; Wilson, T. Genotypic effects of calpain 1 and calpastatin on the tenderness of cooked M. longissimus dorsi steaks from Jersey x Limousin, Angus and Hereford-cross cattle. Anim. Genet. [CrossRef]
- Sun, X.; Wu, X.; Fan, Y.; Mao, Y.; Ji, D.; Huang, B.; Yang, Z. Effects of polymorphisms in CAPN1 and CAST genes on meat tenderness of Chinese Simmental cattle. Arch. Anim. Breed. 2018, 61, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jin, S.; Kim, H.J.; Bhuiyan, M.S.A.; Lee, D.H.; Lee, S.H.; Jang, S.B.; Han, M.H.; Lee, S.H. Validation Study of SNPs in CAPN1-CAST Genes on the Tenderness of Muscles (Longissimus thoracis and Semimembranosus) in Hanwoo (Korean Cattle). Animals (Basel) 2019, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.G. Jr.; Cushman, R.A.; McNeel, A.K.; Casas, E.; Smith, T.P.L.; Freetly, H.C.; Bennett, G.L. μ-Calpain (CAPN1), calpastatin (CAST), and growth hormone receptor (GHR) genetic effects on Angus beef heifer performance traits and reproduction. Theriogenology 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Collis, E.; Fortes, M.R.; Zhang, Y.; Tier, B.; Schutt, K.; Barendse, W.; Hawken, R. Genetic variants affecting meat and milk production traits appear to have effects on reproduction traits in cattle. Anim. Genet. 2012, 43, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Cushman, R.A.; Bennett, G.L.; Tait, R.G. Jr.; McNeel, A.K.; Casas, E.; Smith, T.P.L.; Freetly, H.C. Relationship of molecular breeding value for beef tenderness with heifer traits through weaning of their first calf. Theriogenology 2021, 173, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- Bedhane, M.; van der Werf, J.; Gondro, C.; Duijvesteijn, N.; Lim, D.; Park, B.; Park, M.N.; Hee, R.S.; Clark, S. Genome-wide association study of meat quality traits in Hanwoo beef cattle using imputed whole-genome sequence data. Front. Genet. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Forutan, M.; Lynn, A.; Aliloo, H.; Clark, S.A.; McGilchrist, P.; Polkinghorne, R.; Hayes, B.J. Predicting phenotypes of beef eating quality traits. Front. Genet. 2023, 14, 1089490. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Sjögren, R.J.; Egan, B.; Garde, C.; Lindgren, M.; Gu, W.; et al. Endurance exercise training-responsive miR-19b-3p improves skeletal muscle glucose metabolism. Nat. Commun. 2021, 12, 5948. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, L.M.; Mota, L.F.M.; Schmidt, P.I.; Frezarim, G.B.; Fonseca, L.F.S.; Magalhães, A.F.B.; Silva, D.A.; Carvalheiro, R.; Chardulo, L.A.L.; Albuquerque, L.G. Genome-wide scans identify biological and metabolic pathways regulating carcass and meat quality traits in beef cattle. Meat Sci. 2024, 209, 109402. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, R.G.; Garrick, D.J.; Reecy, J.M. Network analysis reveals putative genes affecting meat quality in Angus cattle. Front. Genet. 2017, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Leal-Gutiérrez, J.D.; Elzo, M.A.; Johnson, D.D.; Hamblen, H.; Mateescu, R.G. Genome wide association and gene enrichment analysis reveal membrane anchoring and structural proteins associated with meat quality in beef. BMC Genomics 2019, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Rahal, C. A scientometric review of genome-wide association studies. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.P.; Tribout, T.; Kadri, N.K.; Boussaha, M.; Esquerré, D.; Barbat, A.; Deloche, M.C.; Fritz, S.; Phocas, F. Sequence-based GWAS meta-analyses for beef production traits. Genet. Sel. Evol. 2023, 55, 70. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Fan, H.; Chang, T.; Xu, L.; Zhang, W.; Song, Y.; Zhu, B.; Zhang, L.; Gao, X.; Chen, Y.; Li, J.; Gao, H. Searching for new loci and candidate genes for economically important traits through gene-based association analysis of Simmental cattle. Sci. Rep. 2017, 7, 42048. [Google Scholar] [CrossRef] [PubMed]
- Leal-Gutiérrez, J.D.; Rezende, F.M.; Reecy, J.M.; Kramer, L.M.; Peñagaricano, F.; Mateescu, R.G. Whole genome sequence data provides novel insights into the genetic architecture of meat quality traits in beef. Front. Genet. 2020, 11, 538640. [Google Scholar] [CrossRef]
- Pegolo, S.; Cecchinato, A.; Savoia, S.; Di Stasio, L.; Pauciullo, A.; Brugiapaglia, A.; Bittante, G.; Albera, A. Genome-wide association and pathway analysis of carcass and meat quality traits in Piedmontese young bulls. Animal 2020, 14, 243–252. [Google Scholar] [CrossRef]
- Hyeonga, K.E.; Lee, Y.M.; Kim, Y.S.; Nam, K.C.; Jo, C.; Lee, K.H.; Lee, J.E.; Kim, J.J. A whole genome association study on meat palatability in Hanwoo. Asian-Australas. J. Anim. Sci. 2014, 27, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, Y.; Abe, T.; Tameoka, N.; Hasebe, H.; Inoue, K.; Nakajima, H.; Shoji, N.; Kobayashi, M.; Kobayashi, E. Whole-genome association study for fatty acid composition of oleic acid in Japanese Black cattle. Anim. Genet. 2011, 42, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Niu, H.; Zhang, W.; Wang, Z.; Liang, Y.; Guan, L.; Guo, Y.; Chen, Y.; Zhang, L.; Gao, X.; Xu, L.; Li, J.; Jia, Y.; Chen, L.; Deng, W.; Zhang, G.; Jiang, Y.; Zeng, J.; Qiu, Q.; Liu, G.E.; Qin, H.; Zhao, S.; Jiang, L. Genome wide association study and genomic prediction for fatty acid composition in Chinese Simmental beef cattle using high density SNP array. BMC Genomics 2017, 18, 464. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sargolzaei, M.; Kelly, M.; Vander Voort, G.; Wang, Z.; Mandell, I.; Moore, S.; Plastow, G. Genome-wide association analyses for carcass quality in crossbred beef cattle. BMC Genet. 2013, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Yamaji, K.; Uemoto, Y.; Sasago, N.; Kobayashi, E.; Kobayashi, N.; Matsuhashi, T.; Maruyama, S.; Matsumoto, H.; Sasazaki, S.; Mannen, H. Genome-wide association study for fatty acid composition in Japanese Black cattle. Anim. Sci. J. 2013, 84, 675–682. [Google Scholar] [CrossRef]
- Saatchi, M.; Garrick, D.J.; Tait Jr., R. G.; Mayes, M.S.; Drewnoski, M.; Schoonmaker, J.; Diaz, C.; Beitz, D.C.; Reecy, J.M. Genome-wide association and prediction of direct genomic breeding values for composition of fatty acids in Angus beef cattle. BMC Genomics 2013, 14, 730. [Google Scholar] [CrossRef] [PubMed]
- Cesar, A.S.; Regitano, L.C.; Mourão, G.B.; Tullio, R.R.; Lanna, D.P.; Nassu, R.T.; Mudado, M.A.; Oliveira, P.S.; do Nascimento, M.L.; Chaves, A.S.; Alencar, M.M.; Sonstegard, T.S.; Garrick, D.J.; Reecy, J.M.; Coutinho, L.L. Genome-wide association study for intramuscular fat deposition and composition in Nellore cattle. BMC Genet. 2014, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.; Kramer, L.M.; Shabbir, M.I.; Reecy, J.M. Genome-wide association study for fatty acid composition in American Angus cattle. Animals (Basel) 2021, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, F.L.B.; Pereira, A.S.C.; Mueller, L.F.; Fonseca, P.A.S.; Braz, C.U.; Amorin, S.; Espigolan, R.; Lemos, M.A.; de Albuquerque, L.G.; Schenkel, F.S.; Brito, L.F.; Stafuzza, N.B.; Baldi, F. Genome-wide association study for beef fatty acid profile using haplotypes in Nellore cattle. Livest. Sci. 2021, 245, 104396. [Google Scholar] [CrossRef]
- Lande, R.; Thompson, R. Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 2000, 124, 743–756. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Lee, S.H.; Clark, S.; van der Werf, J.H.J. Estimation of genomic prediction accuracy from reference populations with varying degrees of relationship. PLoS One 2017, 12, e0189775. [Google Scholar] [CrossRef]
- Dekkers, J.C.M.; Su, H.; Cheng, J. Predicting the accuracy of genomic predictions. Genet. Sel. Evol. 2021, 53, 55. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Júnior, G.A.; Peripolli, E.; Schmidt, P.I.; Campos, G.S.; Mota, L.F.M.; Mercadante, M.E.Z.; Baldi, F.; Carvalheiro, R.; de Albuquerque, L.G. Current applications and perspectives of genomic selection in Bos indicus (Nellore) cattle. Livest. Sci. 2022, 263, 105001. [Google Scholar] [CrossRef]
- Wheeler, T.L.; Cundiff, L.V.; Shackelford, S.D.; Koohmaraie, M. Characterization of biological types of cattle (Cycle VIII): Carcass, yield, and longissimus palatability traits. J. Anim. Sci. 2010, 88, 3070–3083. [Google Scholar] [CrossRef] [PubMed]
- Gordo, D.G.M.; Espigolan, R.; Bresolin, T.; Fernandes Júnior, G.A.; Magalhães, A.F.B.; Braz, C.U.; Fernandes, W.B.; Baldi, F.; Albuquerque, L.G. Genetic analysis of carcass and meat quality traits in Nelore cattle. J. Anim. Sci. 2018, 96, 3558–3564. [Google Scholar] [CrossRef]
- Magnabosco, C.U.; Lopes, F.B.; Fragoso, R.C.; Eifert, E.C.; Valente, B.D.; Rosa, G.J.; Sainz, R.D. Accuracy of genomic breeding values for meat tenderness in Polled Nellore cattle. J. Anim. Sci. 2016, 94, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Chiaia, H.L.J.; Peripoli, E.; Silva, R.M.O.; Aboujaoude, C.; Feitosa, F.L.B.; Lemos, M.V.A.; Berton, M.P.; Olivieri, B.F.; Espigolan, R.; Tonussi, R.L.; Gordo, D.G.M.; Bresolin, T.; Magalhães, A.F.B.; Júnior, G.A.F.; Albuquerque, L.G.; Oliveira, H.N.; Furlan, J.J.M.; Ferrinho, A.M.; Mueller, L.F.; Tonhati, H.; Pereira, A.S.C.; Baldi, F. Genomic prediction for beef fatty acid profile in Nellore cattle. Meat Sci. 2017, 128, 60–67. [Google Scholar] [CrossRef]
- Magalhães, A.F.B.; Schenkel, F.S.; Garcia, D.A.; Gordo, D.G.M.; Tonussi, R.L.; Espigolan, R.; Silva, R.M.O.; Braz, C.U.; Fernandes Júnior, G.A.; Baldi, F.; Carvalheiro, R.; Boligon, A.A.; de Oliveira, H.N.; Chardulo, L.A.L.; de Albuquerque, L.G. Genomic selection for meat quality traits in Nelore cattle. Meat Sci. 2019, 148, 32–37. [Google Scholar] [CrossRef]
- Johnston, D.J.; Tier, B.; Graser, H.-U. Beef cattle breeding in Australia with genomics: Opportunities and needs. Anim. Prod. Sci. 2012, 52, 100–106. [Google Scholar] [CrossRef]
- Bolormaa, S.; Pryce, J.E.; Kemper, K.; Savin, K.; Hayes, B.J.; Barendse, W.; Zhang, Y.; Reich, C.M.; Mason, B.A.; Bunch, R.J.; Harrison, B.E.; Reverter, A.; Herd, R.M.; Tier, B.; Graser, H.U.; Goddard, M.E. Accuracy of prediction of genomic breeding values for residual feed intake and carcass and meat quality traits in Bos taurus, Bos indicus, and composite beef cattle. J. Anim. Sci. 2013, 91, 3088–3104. [Google Scholar] [CrossRef]
- Watson, R.; Polkinghorne, R.; Thompson, J.M. Development of the Meat Standards Australia (MSA) prediction model for beef palatability. Aust. J. Exp. Agric. 2008, 48, 1368–1379. [Google Scholar] [CrossRef]
- Hayes, B.J.; Copley, J.; Dodd, E.; Ross, E.M.; Speight, S.; Fordyce, G. Multi-breed genomic evaluation for tropical beef cattle when no pedigree information is available. Genet. Sel. Evol. 2023, 55, 71. [Google Scholar] [CrossRef]
- Legarra, A.; Aguilar, I.; Misztal, I. A relationship matrix including full pedigree and genomic information. J. Dairy Sci. 2009, 92, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: a unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef]
- Christensen, O.F.; Lund, M.S. Genomic prediction when some animals are not genotyped. Genet. Sel. Evol. 2010, 42, 2. [Google Scholar] [CrossRef]
- Adekale, D.; Alkhoder, H.; Liu, Z.; Segelke, D.; Tetens, J. Single-step SNPBLUP evaluation in six German beef cattle breeds. J. Anim. Breed. Genet. 2023, 140, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, H.H.; Casas, E.; Ferraz, J.B.S.; Vega-Murillo, V.E.; Román-Ponce, S.I. Opportunities and challenges from the use of genomic selection for beef cattle breeding in Latin America. Anim. Front. 2012, 2, 23–29. [Google Scholar] [CrossRef]
- Stock, K.F.; Reents, R. Genomic selection: Status in different species and challenges for breeding. Reprod. Domest. Anim. 2013, 48, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Garrick, D.J. The nature, scope and impact of genomic prediction in beef cattle in the United States. Genet. Sel. Evol. 2011, 43, 17. [Google Scholar] [CrossRef]
- Fang, L.; Cai, W.; Liu, S.; Canela-Xandri, O.; Gao, Y.; Jiang, J.; Rawlik, K.; Li, B.; Schroeder, S.G.; Rosen, B.D.; Li, C.J.; Sonstegard, T.S.; Alexander, L.J.; Van Tassell, C.P.; VanRaden, P.M.; Cole, J.B.; Yu, Y.; Zhang, S.; Tenesa, A.; Ma, L.; Liu, G.E. Comprehensive analyses of 723 transcriptomes enhance genetic and biological interpretations for complex traits in cattle. Genome Res. 2020, 30, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Wang, X.; Ma, Y. Transcription factors regulate adipocyte differentiation in beef cattle. Anim. Genet. 2020, 51, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, S.; Guo, J.; Wang, J.; Mei, C.; Abbas Raza, S.H.; Cheng, G.; Zan, L. Comprehensive analysis of transcriptome and metabolome reveals regulatory mechanism of intramuscular fat content in beef cattle. J. Agric. Food Chem. 2024, 72, 2911–2924. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Pant, S.D.; Wani, A.K.; Mohamed, H.H.; Khalifa, N.E.; Almohaimeed, H.M.; Alshanwani, A.R.; Assiri, R.; Aggad, W.S.; Noreldin, A.E.; Abdelnour, S.A.; Wang, Z.; Zan, L. Krüppel-like factors family regulation of adipogenic markers genes in bovine cattle adipogenesis. Mol. Cell Probes 2022, 65, 101850. [Google Scholar] [CrossRef]
- Hausman, G.J.; Dodson, M.V.; Ajuwon, K.; Azain, M.; Barnes, K.M.; Guan, L.L.; Jiang, Z.; Poulos, S.P.; Sainz, R.D.; Smith, S.; Spurlock, M.; Novakofski, J.; Fernyhough, M.E.; Bergen, W.G. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci. 2009, 87, 1218–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Li, B.; Sun, B.; Yu, S.; Wang, X.; Zan, L. Long non-coding RNA BNIP3 inhibited the proliferation of bovine intramuscular preadipocytes via cell cycle. Int. J. Mol. Sci. 2023, 24, 4234. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, F.; Yu, Y.; Luo, J.; Mitra, A.; Zhan, F.; Hou, Y.; Liu, G.; Zan, L.; Updike, M.S.; Song, J. Functional genomic analysis of variation on beef tenderness induced by acute stress in angus cattle. Comp. Funct. Genomics 2012, 756284. [Google Scholar] [CrossRef]
- Sweeney, T.; Lejeune, A.; Moloney, A.P.; Hamill, R.M.; Cairns, M.T. The application of transcriptomic data in the authentication of beef derived from contrasting production systems. BMC Genomics 2016, 17, 746. [Google Scholar] [CrossRef]
- Deng, T.; Liang, M.; Du, L.; Li, K.; Li, J.; Qian, L.; Xue, Q.; Qiu, S.; Xu, L.; Zhang, L.; Gao, X.; Li, J.; Lan, X.; Gao, H. Transcriptome analysis of compensatory growth and meat quality alteration after varied restricted feeding conditions in beef cattle. Int. J. Mol. Sci. 2024, 25, 2704. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, T.; Niu, Q.; Jiang, Y.; Gao, X.; Li, J.; Gao, H. Comparative transcriptomic analysis reveals region-specific expression patterns in different beef cuts. BMC Genomics 2022, 23, 387. [Google Scholar] [CrossRef]
- Du, L.; Chang, T.; An, B.; Xu, L.; Zhang, L.; Gao, X.; Li, J.; Gao, H. Transcriptome profiling analysis of muscle tissue reveals potential candidate genes affecting water holding capacity in Chinese Simmental beef cattle. Sci. Rep. 2021, 11, 11897. [Google Scholar] [CrossRef]
- Rozanova, S.; Barkovits, K.; Nikolov, M.; Schmidt, C.; Urlaub, H.; Marcus, K. Quantitative Mass Spectrometry-Based Proteomics: An Overview. Methods Mol. Biol. 2021, 2228, 85–116. [Google Scholar] [CrossRef]
- Zapata, I.; Zerby, H.N.; Wick, M. Functional proteomic analysis predicts beef tenderness and the tenderness differential. J. Agric. Food Chem. 2009, 57, 4956–4963. [Google Scholar] [CrossRef]
- Zhu, Y.; Hamill, R.M.; Mullen, A.M.; Kelly, A.L.; Gagaoua, M. Molecular mechanisms contributing to the development of beef sensory texture and flavour traits and related biomarkers: Insights from early post-mortem muscle using label-free proteomics. J. Proteomics 2023, 286, 104953. [Google Scholar] [CrossRef] [PubMed]
- Severino, M.; Gagaoua, M.; Baldassini, W.; Ribeiro, R.; Torrecilhas, J.; Pereira, G.; Curi, R.; Chardulo, L.A.; Padilha, P.; Neto, O.M. Proteomics unveils post-mortem changes in beef muscle proteins and provides insight into variations in meat quality traits of crossbred young steers and heifers raised in feedlot. Int. J. Mol. Sci. 2022, 23, 12259. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.F.; Moncau, C.T.; Poleti, M.D.; Fonseca, L.D.; Balieiro, J.; Silva, S.; Eler, J.P.; Balieiro, J.C.C. Proteome changes of beef in Nellore cattle with different genotypes for tenderness. Meat Sci. 2018, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, J.M.; Enríquez-Valencia, C.E.; Braga, C.P.; Vieira, J.C.S.; Vieira, D.S.; Pereira, G.L.; Curi, R.A.; Neto, O.R.M.; Oliveira, H.N.; Padilha, P.M.; Chardulo, L.A.L. Application of proteomic to investigate the different degrees of meat tenderness in Nellore breed. J. Proteomics 2021, 248, 104331. [Google Scholar] [CrossRef]
- Ma, D.; Kim, Y.H.B. Proteolytic changes of myofibrillar and small heat shock proteins in different bovine muscles during aging: their relevance to tenderness and water-holding capacity. Meat Sci. 2020, 163, 108090. [Google Scholar] [CrossRef]
- Zhao, C.; Zan, L.; Wang, Y.; Updike, M.S.; Liu, G.; Bequette, B.J.; Baldwin, R.L., VI; Song, J. Functional proteomic and interactome analysis of proteins associated with beef tenderness in Angus cattle. Livest. Sci. 2014, 161, 201–209. [Google Scholar] [CrossRef]
- Malheiros, J.M.; Braga, C.P.; Grove, R.A.; Ribeiro, F.A.; Calkins, C.R.; Adamec, J.; Chardulo, L.A.L. Influence of oxidative damage to proteins on meat tenderness using a proteomics approach. Meat Sci. 2019, 148, 64–71. [Google Scholar] [CrossRef]
- Silva, L.H.P.; Rodrigues, R.T.S.; Assis, D.E.F.; Benedeti, P.D.B.; Duarte, M.S.; Chizzotti, M.L. Explaining meat quality of bulls and steers by differential proteome and phosphoproteome analysis of skeletal muscle. J. Proteomics 2019, 199, 51–66. [Google Scholar] [CrossRef]
- Boudon, S.; Ounaissi, D.; Viala, D.; Monteils, V.; Picard, B.; Cassar-Malek, I. Label free shotgun proteomics for the identification of protein biomarkers for beef tenderness in muscle and plasma of heifers. J. Proteomics 2020, 217, 103685. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; Picard, B. Protein array-based approach to evaluate biomarkers of beef tenderness and marbling in cows: understanding of the underlying mechanisms and prediction. Foods 9, 1180. [CrossRef]
- Gagaoua, M.; Hughes, J.; Terlouw, E.M.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic biomarkers of beef colour. Trends Food Sci. Technol. 2020b, S0924224420304660. [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Di Stasio, L.; Brugiapaglia, A.; Franco, D. Quantitative proteomic analysis of beef tenderness of Piedmontese young bulls by SWATH-MS. Food Chem. 2021, 356, 129711. [Google Scholar] [CrossRef]
- Kiyimba, F.; Hartson, S.D.; Rogers, J.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Changes in glycolytic and mitochondrial protein profiles regulates postmortem muscle acidification and oxygen consumption in dark-cutting beef. J. Proteomics 2021, 232, 104016. [Google Scholar] [CrossRef]
- Kiyimba, F.; Hartson, S.D.; Rogers, J.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Dark-cutting beef mitochondrial proteomic signatures reveal increased biogenesis proteins and bioenergetics capabilities. J. Proteomics 2022, 265, 104637. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Warner, R.D.; Purslow, P.; Ramanathan, R.; Mullen, A.M.; López-Pedrouso, M.; Franco, D.; Lorenzo, J.M.; Tomasevic, I.; Picard, B.; Troy, D.; Terlouw, E.M.C. Dark-cutting beef: A brief review and an integromics meta-analysis at the proteome level to decipher the underlying pathways. Meat Sci. 181, 108611. [CrossRef] [PubMed]
- Gagaoua, M.; Terlouw, E.M.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; Picard, B. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 172, 108311. [CrossRef]
- Ueda, S.; Yoshida, Y.; Kebede, B.; Kitamura, C.; Sasaki, R.; Shinohara, M.; Fukuda, I.; Shirai, Y. New implications of metabolites and free fatty acids in quality control of crossbred Wagyu beef during wet aging cold storage. Metabolites 2024, 14, 95. [Google Scholar] [CrossRef]
- Phoemchalard, C.; Uriyapongson, S.; Tathong, T.; Pornanek, P. 1H NMR Metabolic profiling and meat quality in three beef cattle breeds from northeastern Thailand. Foods 2022, 11, 3821. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Kharrati-Koopaee, H.; Asadollahpour Nanaie, H.; Wang, X.; Zhao, M.; Li, H.; Li, Y.; Zhang, H.; Esmailizadeh, A.; Bottema, C.D.K. Comparative metabolomics analysis shows key metabolites as potential biomarkers for selection of beef fat colour. Anim. Prod. Sci. 2023, 63, 1063–1067. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, M.; Ji, S.Y.; Baek, Y.C.; Lee, S.; Oh, Y.K.; Reddy, K.E.; Seo, H.W.; Cho, S.; Lee, H.J. Metabolomics analysis of the beef samples with different meat qualities and tastes. Food Sci. Anim. Resour. 2020, 40, 924–937. [Google Scholar] [CrossRef]
- Artegoitia, V.M.; Newman, J.W.; Foote, A.P.; et al. Non-invasive metabolomics biomarkers of production efficiency and beef carcass quality traits. Sci. Rep. 2022, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef]
- Castejón, D.; García-Segura, J.M.; Escudero, R.; Herrera, A.; Cambero, M.I. Metabolomics of meat exudate: Its potential to evaluate beef meat conservation and aging. Anal. Chim. Acta 2015, 901, 1–11. [Google Scholar] [CrossRef]
- Kodani, Y.; Miyakawa, T.; Komatsu, T.; et al. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci. Rep. 2017, 7, 1297. [Google Scholar] [CrossRef]
- Muroya, S.; Ueda, S.; Komatsu, T.; Miyakawa, T.; Ertbjerg, P. MEATabolomics: Muscle and meat metabolomics in domestic animals. Metabolites 2020, 10, 188. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, C.; Xie, K.; Wang, J.; Pan, Z. Current state of metabolomics research in meat quality analysis and authentication. Foods 2021, 10, 2388. [Google Scholar] [CrossRef]
- Ramanathan, R.; Kiyimba, F.; Suman, S.P.; Mafi, G.G. The potential of metabolomics in meat science: Current applications, trends, and challenges. J. Proteomics 2023, 283–284, 104926. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, S.; Guo, J.; Wang, J.; Mei, C.; Abbas Raza, S.H.; Cheng, G.; Zan, L. Comprehensive analysis of transcriptome and metabolome reveals regulatory mechanism of intramuscular fat content in beef cattle. J. Agric. Food Chem. 2024, 72, 2911–2924. [Google Scholar] [CrossRef] [PubMed]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef] [PubMed]
Table 1.
A list of some of the commercialized DNA tests for beef quality.
Table 1.
A list of some of the commercialized DNA tests for beef quality.
| Gene symbol |
Beef attribute |
Discovered by |
Commercialized by |
| TG |
Marbling |
CSIRO/MLA |
Genetic Solutions Pty Ltd |
| CAST |
Meat tenderness |
CSIRO/MLA/Beef CRC |
Genetic Solutions Pty Ltd |
| CAPN1 |
Meat tenderness |
USDA/AgResearch NZ |
Open |
| GH1 |
Marbling |
NIAS, Japan |
Prescribe Genomics CO |
| LEP |
Marbling/fat traits |
Univ. of Saskatchewan |
Merial |
| Multiple tests |
Marbling |
- |
Genetic Solutions Pty Ltd |
| CAPN3 |
Meat tenderness |
CSIRO/MLA/Beef CRC |
Genetic Solutions Pty Ltd |
| SCD |
Fatty acid composition |
Kobe University |
Prescribe Genomics CO |
Table 2.
Some of the published significant GWAS results for beef quality traits.
Table 2.
Some of the published significant GWAS results for beef quality traits.
| Beef attribute |
Population |
Sample Size |
Genotyping platform |
Significant Genomic Regions/Genes |
Reference |
| Tenderness |
Angus cattle |
1833 |
Illumina BovineSNP50 BeadChip |
CAST and CAPN1 for tenderness |
Mateescu et al., 2017 |
| Marbling score |
Simmental bulls |
785 |
Illumina BovineHD BeadChip |
TUBB1 and RPL27A for marbling score |
Xia et al., 2017 |
| Warner-Bratzler Shear Force (WBSF), marbling, cooking loss, tenderness, juiciness, connective tissue and flavor |
Multibreed Angus-Brahman steers |
672 |
GGP Bovine F-250 chip containing 221,077 SNPs |
LRP5, COL3A1, GRIP1, RECQL5, ANO2, NTF3, CD36, GPR98, MMRN2 and GOSR2. |
Leal-Gutiérrez et al., 2019 |
| Marbling score, meat texture, meat color, and fat color |
Hanwoo steers |
2110 |
Illumina Bovine SNP50 BeadChip imputed to higher density of 15,536,497 SNPs |
SFT2D3 (marbling) located on BTA2, ENPP2 (meat color) on BTA14, CPAMD8 on BTA7 and RHCG on BTA21 for fat color |
Bedhane et al., 2019 |
Tenderness, marbling, and flavor.
marbling, Warner-Bratzler shear force (WBSF), tenderness, and connective tissue |
Angus-sired population of steers, bulls and cows progeny |
2268 |
Bovine SNP50 Infinium II BeadChip imputed to 44.3 million SNPs |
Tenderness: CAST and CAPN1; WBSF: CAPN1, AGAP1, ANXA10, CCDC80, Connective Tissue: UTRN, TMX1, TMEM170B; Marbling: EGR2, RNF130, C1QTNF8, SOX8, SSTR5, TEKT4, SLC20A2
|
Leal-Gutiérrez et al., 2020 |
| Meat color, purge loss, cooking loss, meat Ph, Warner-Bratzler shear force. |
Piedmontese young bulls |
1166 |
GeneSeek Genomic Profiler Bovine LD’ (GGP Bovine LD) array containing 30111 SNPs |
SNPs on BTA4 (at ~112.51 Mb), BTA23 (at ~3.91 and ~7.25 Mb), BTA24 (at ~19.87 Mb) and BTA25 (at ~11.96 Mb) for meat color. Water holding capacity: one SNP located on BTA9 (at ~48.33 Mb) for purge loss, and two SNPs located on BTA6 (at ~29.23 Mb) and on BTA10 (at ~14.57 Mb) for cooking loss, one SNP on BTA8 (at ~28.46 Mb) for meat pH. |
Pegolo et al., 2020 |
| Color, aroma, tenderness, juiciness, palatability |
Hanwoo steers |
250 |
Affymetrix Bovine Axiom Array 640K SNP chip |
Three pleiotropic SNPs (AX-26703353 and AX-26742891 on BTA6, and AX-18624743 on BTA10) influenced multiple traits like tenderness, juiciness, and palatability |
Hyeonga et al., 2014 |
| Oleic acid (C18:1) content in the intramuscular fat |
Japanese Black cattle |
160 |
BovineSNP50 BeadChip |
A total of 32 SNPs, including the FASN gene, had significant effects on C18:1 levels, with 30 SNPs located between 49 and 55 Mbp on chromosome 19 |
Uemoto et al., 2011 |
| Fatty acid composition |
Chinese Simmental beef cattle |
723 |
Illumina BovineHD BeadChip |
SNPs near the FASN gene on BTA19 for C14:0 and C14:1, and the ELOVL5 gene on BTA23 for C14:0. |
Zhu et al., 2017 |
Marbling score, tenderness
|
crossbred beef cattle |
747 |
BovineSNP50 BeadChip |
One SNP (BTA-60019) on BTA25 accounted for 2.67% of the variation in tenderness. |
Lu et al., 2013 |
| Fatty acid composition |
Japanese Black cattle |
461 |
BovineSNP50 BeadChip |
FASN gene on BTA19, one SNP for C18:1 on BTA23, two SNPs for C16:0 on BTA25, and two SNPs for C14:1 near the SCD gene on BTA26. |
Ishii et al., 2013 |
| Fatty acid composition |
Angus beef cattle, |
1713 |
BovineSNP50 BeadChip |
FASN, SCD and THRSP genes |
Saatchi et al., 2013 |
| Intramuscular fat deposition and composition |
Nellore steers |
585 |
Illumina BovineHD BeadChip |
SNPs near the FASN gene on BTA19 for C16:0 and C18:1 fatty acids, and SNPs on BTA7 for intramuscular fat percentage |
Cesar et al., 2014 |
| Fatty acid composition |
American Black Angus calves |
2177 |
574,662 SNPs imputed from BovineSNP50 BeadChip and BovineHD BeadChip |
Candidate genes FABP2, FASN, FADS2, FADS3 and SCD
|
Dawood et al., 2021 |
| Fatty acid composition |
Nellore cattle |
1057 |
Illumina BovineHD BeadChip |
SNPs near the FASN gene on BTA19 for C16:0 and C18:1 and the SCD gene on BTA26 for C14:1 and C16:1., THRSP, ELOVL6 and FADS2
|
Feitosa et al., 2021 |
| Eating quality traits: scores for tenderness, juiciness, flavor overall liking |
Steers, heifers, and bulls from Brahman, Angus, Hereford, Shorthorn, Holstein, Jersey, Belmont Red, Santa Gertrudis composite, crossbred unknown breed. |
1701 |
7,09,068 Imputed SNPs from the Illumina HD array |
Tenderness: CAPN1, CAST genes; juiciness and flavor: MOXD1 APOB, KIF13A
|
Forutan et al., 2023 |
| Shear force, marbling score, intramuscular fat |
Nellore cattle |
6910 young bulls with phenotypic information and 23859 genotyped animals |
435,447 Imputed SNPs from multiple Bead chip assay densities |
Several candidate genes located on chromosomes BTA1, 2, 5, 7, 9, 10, 19, and 25 for Shear force, on BTA4, 7, 10, 11, 12, 13, 15, and 20 for marbling score, and BTA8, 9, 10, 12, 13, and 28 for intramuscular fat |
Arikawa et al., 2024 |
Table 3.
Genomic prediction accuracies for beef quality traits1.
Table 3.
Genomic prediction accuracies for beef quality traits1.
| Trait |
Accuracy |
N |
Reference |
| Meat tenderness |
0.52 to 0.59 |
427 |
Magnabosco et al. (2016) |
| Meat tenderness |
0.57 to 0.60 |
5062 |
Magalhães et al. (2019) |
| Lipids |
0.23 |
3812 |
Magalhães et al. (2019) |
| Marbling |
0.32 |
5039 |
Magalhães et al. (2019) |
| Carcass intramuscular fat % |
0.20 |
1031 |
Johnston et al. (2012) |
| Marbling score |
0.08 to 0.56 |
4228 |
Bolormaa et al. (2013) |
| a* color |
0.40 |
5052 |
Magalhães et al. (2019) |
| b* color |
0.49 to 0.53 |
5046 |
Magalhães et al. (2019) |
| L* color |
0.68 to 0.73 |
5071 |
Magalhães et al. (2019) |
| Sum of SFA |
0.04 to 0.24 |
868 |
Chiaia et al. (2017) |
| Sum of MUFA |
0.05 to 0.13 |
868 |
Chiaia et al. (2017) |
| Sum of PUFA |
0.15 to 0.56 |
868 |
Chiaia et al. (2017) |
Table 4.
Summary of some of the published proteomic studies on beef quality.
Table 4.
Summary of some of the published proteomic studies on beef quality.
| Beef attribute |
Animal and Age at Slaughter |
Sample size |
Protein extracts |
Proteomics platform |
N. of identified proteins |
Reference |
| Sensory Attributes (Tenderness, Chewiness, Stringiness, Flavor) |
Limousin-sired bulls, 16 months |
34 |
Total LD muscle proteins |
LC-MS/MS |
84 |
Zhu et al. (2023) |
| pH, instrumental color, cooking loss, WBSF |
Immunocastrated F1 Montana-Nellore, heifers + steers, 15 months |
16 |
Myofibrillar and sarcoplasmic proteins |
2D-PAGE, MS (ESI-MS/MS)
|
23 |
Severino et al. (2022) |
| Tenderness (WBSF) |
Nellore cattle, steers, and bulls, 27.7 months |
155 |
Whole LD muscle proteins |
2DE and mass spectrometry, MALDI-TOF/TOF MS/MS
|
40 |
Rosa et al. (2018) |
| Tenderness (WBSF) |
Nellore bulls, 27 months |
|
cytoplasmatic proteins |
2D-PAGE, MS (ESI-MS/MS)
|
29 |
Malheiros et al. (2021) |
pH, WBSF, WHC
|
Angus × Simmental beef cattle (USDA Select; A maturity) |
8 |
Whole muscle protein |
Western blots, SDS-PAGE |
14 |
Ma and Kim, (2020) |
| Beef tenderness |
Angus Steers, 18 months
|
6 |
Myofibrillar proteins |
1DE + nano-LC-MS/MS |
19 |
Zapata et al. (2009) |
| Beef tenderness |
Angus Steers, 12 months
|
19 |
High salt and low salt soluble proteins |
1DE + LC-MS/MS |
8 |
Zhao et al. (2014) |
| Beef tenderness |
Angus Steers
|
15 |
Myofibrillar & sarcoplasmic proteins |
2D-DiGE + Linear Ion Trap MS |
28 |
Malheiros et al. (2019) |
| Beef tenderness and intramuscular fat |
Nellore Bulls+Steers
|
12 |
Myofibrillar & sarcoplasmic proteins |
2DE + MALDI-TOF/TOF |
9 |
Silva et al. (2019) |
| Beef tenderness |
Charolais x Aubrac Heifers, 33 ± 3 months
|
10 |
Myofibrillar & sarcoplasmic proteins |
Label free + Nano-LC-MS/MS |
40 |
Boudon et al. (2020) |
| Beef tenderness |
Charolais Bulls, 17 months
|
8 |
Myofibrillar and sarcoplasmic proteins |
2DE + MALDI-TOF/TOF |
23 |
Picard and Gagaoua (2020) |
| Beef Tenderness and Marbling |
PDO Maine Anjou Cows, 67.4 months
|
188 |
Myofibrillar & sarcoplasmic proteins |
RPPA |
10 |
Gagaoua et al. (2020a) |
| Tenderness (shear force) |
Piedmontese bulls, 7 months |
10 |
cytoplasmatic proteins |
SWATH-MS |
43 |
López-Pedrouso et al. (2021) |
| Dark-cutting |
Asturiana de los Valles x Friesian yearling bulls, 14–15 months |
12 |
Liquid isoelectric focusing (OFFGEL) pH range 4–7, and mass spectrometry |
Myofibrillar proteins |
5 |
Fuente-Garcia et al. 2020 |
| Dark-cutting |
6 dark-cutters and 6 normal-pH beef, other information not visible |
12 |
Label-free quantitative proteomics using LC-MS/MS |
Total protein extract |
57 |
Kiyimba et al. (2021) |
Dark-cutting
|
Beef cattle, other information not visible |
22 |
LD muscle mitochondrial proteins |
LC-MS/MS |
12 |
Kiyimba et al. (2022) |
Table 5.
Summary of some of the applications of metabolomics in beef quality analysis.
Table 5.
Summary of some of the applications of metabolomics in beef quality analysis.
| Beef attribute |
Analytical Techniques |
Multivariate analysis Techniques |
Metabolites |
Reference |
| Sensory evaluation of beef taste |
GC/MS |
PCA |
Cold storage led to increased free fatty acids and
Glutamic acid, and decreased creatinine and inosinic acid |
Ueda et al. (2024) |
| Meat color, pH, Water holding capacity, Shear force, Texture |
NMR |
PCA, OPLS-DA |
Beef quality differences related to acetylcholine, valine, adenine, leucine, phosphocreatine, β-hydroxypyruvate, ethanol, adenosine diphosphate, creatine, acetylcholine, and lactate |
Phoemchalard et al. (2022) |
| Beef fat color |
LC-MS |
PCA, PLS-DA |
3-hydroxyoctanoic acid, anethofuran, 9,10-DiHODE, furanoeremophilane, pregeijerene, N-glycolylneuraminic acid, and glycocholic acid were identified as potential biomarkers for differentiating fat color |
Tian et al. (2023) |
| Marbling |
NMR |
PLS-DA |
Carnosine, creatine, glucose, and lactate were associated with higher marbling |
Jeong et al. (2020) |
| Marbling |
Mass spectrometry-based untargeted and targeted metabolomics |
ASCA |
Unconjugated-BA and Glucocorticoids were associated with marbling |
Artegoitia et al. (2022) |
| Aroma of cooked beef |
SPME and GC–MS
|
Linear and logarithmic regression model |
Benzeneacetaldehyde and Heterocyclic compounds |
Watanabe et al. (2015) |
| Meat freshness |
NMR |
PCA, PLS |
60 identified metabolites, metabolomics classified meat samples according to their storage time |
Castejón et al. (2015) |
| Intramuscular fat |
NMR |
PCA, OPLS-DA |
The unsaturation degree of triacylglycerol was estimated by the 1H NMR spectra and was correlated with the content ratio of unsaturated fatty acids and the melting point of IMF. Leucine and creatine were found as biomarkers, positively and negatively correlated with aging duration, respectively. |
Kodani et al. (2017) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).