1. Introduction
The phenomenon of postmortem dark scleral spots (PDSS), also known as ‘tache noire’, was first documented by Sommers in 1833 [
1]. These spots are morphologically characterized by a discoloration of the scleral surface in cadavers, ranging from brown to black, and typically exhibiting a vaguely triangular shape. They appear in the exposed scleral area during the early postmortem interval. Historically, PDSS has been considered a nonspecific sign, observed in some cadavers where the eyelids were not closed postmortem, allowing the sclera to be exposed to air.
Tache noire forms due to the postmortem drying of the sclera when the eyes are left open after death. This condition causes the exposed area of the sclera to desiccate, leading to the formation of a brownish-black, and sometimes reddish, band-like discoloration. The drying effect is more pronounced when the eyes are open, leading to rapid evaporation of moisture from the corneal surface.
PDSS can develop relatively quickly after death. If the eyes are open, the cornea begins to dry within 10 minutes, losing its brightness and becoming cloudy. This process continues, and within 1-2 hours postmortem, triangular or linear dark discolorations form on both sides of the cornea. Initially, these discolorations are yellowish, then turn yellowish-brown, and eventually black. The evolution of tache noire starts with the formation of yellowish discolorations that progress to yellowish-brown and finally to black as desiccation continues. The process is influenced by environmental factors such as air movement, temperature, and humidity, which can accelerate or decelerate the drying process. The eyeball itself loses tension due to the hypostasis of transcellular liquids, contributing further to the formation and evolution of the discoloration. Tache noire typically appears as triangular or linear discolorations located on both sides of the cornea, where the conjunctiva is exposed to air. The specific shape and extent of the discoloration are determined by the degree of exposure and the environmental conditions. These markings are distinct and localized to the areas not covered by the eyelids.
However, it is the practical experience of every forensic pathologist that mere exposure of the eye to ambient air, when an individual dies with their eyes open and this condition persists postmortem, is not sufficient to cause the formation of PDSS [
2,
3,
4,
5,
6,
7].
Recently, some preliminary studies have focused on PDSS, providing new insights from an epidemiological, morphological, and thanatogenesis perspective.
Regarding epidemiology, a study on a sample of 905 cases revealed the presence of PDSS only in 21 cases (2.3%). The same epidemiological investigation showed that the phenomenon was bilateral in 71% of the cases and unilateral in only 29%. The most significant finding, however, concerned the cause of death: the data indicated that the manifestation of PDSS was essentially linked to three main dynamics. In 33.3% of the cases, it was due to violent mechanical asphyxiation from hanging; in another 33.3%, it was caused by hemorrhagic shock; and in the remaining 33.3%, death resulted from cranial-encephalic trauma [
8].
Further preliminary studies on the pathogenesis of PDSS have highlighted that, in addition to dehydration, choroidal detachment at the site of spot formation may be a contributing factor to the phenomenon [
9,
10].
Preliminary studies suggest that, contrary to previous beliefs, Postmortem Drying Sclera Spots (PDSS) may not be merely a nonspecific sign resulting from the dehydration of the ocular surface. Instead, they could provide valuable information not only on the postmortem interval (PMI) but also on the cause of death. Despite these new findings, very few studies, especially in the modern era, have considered the significance of this sign, its pathogenesis, and its practical utility. This gap in research is primarily due to the rarity of the sign, which occurs in a small percentage of cadavers where death resulted from hemorrhagic shock, violent mechanical asphyxia by hanging, and cranial trauma, with the eyes remaining open postmortem, exposing the sclera to dehydration. Additionally, there is the challenge of studying such a delicate tissue in humans, particularly when cadavers must be returned to their families after forensic activities are completed. However, the emergence of these new insights necessitates further investigation into this sign, the observation of which could have significant implications for forensic practice.
The aim of the current work, considering the potential practical implications of the data from preliminary studies, is to create an experimental animal model to study PDSS.
2. Materials and Methods
Twenty heads of young adult female sheep (Ovis aries), aged 24-48 months and having passed standard food consumption controls, were obtained from a local slaughterhouse (CO.AL.BE. dei F.lli Contu & C. s.n.c. Selargius, Cagliari, Sardinia, Italy). The animals were sacrificed by jugular vein incision following electrical stunning. Since sheep heads are considered waste material, there was no need for a specific animal protocol, approval of an ethical committee or associated costs. Once the ovine specimens were sacrificed, they were decapitated as per the protocol for meat processing. The heads obtained from the slaughterhouse were then transported directly to the morgue for observation. In our experiment, the samples were transported to a room with known temperature and humidity conditions (21.5 ± 0.7 °C, 45% humidity) for observation.
Upon arrival at the morgue, one eye (the right) from each head was kept open by surgically removing all three eyelids using a cold blade scalpel. An incision was made in the periorbital skin approximately two centimeters from the bony margins of the orbit, extending down to the lower edge of the palpebral conjunctiva. To maximize air exposure for each sample, the third eyelid (present in sheep but not in humans) was removed while keeping the eye open.
In contrast, the other eye was kept closed using an adhesive patch, which could be removed to allow for observation
The heads were divided into different groups according to their position for observation. Specifically, 12 heads were placed in a natural position with the cut surface resting on the table. The others were positioned using a special cardboard support following these orientations: 2 heads with the snout facing down, 2 heads with the snout facing up, 2 with the open eye facing up, 2 with the open eye facing down, and 2 with the top of the head resting on the support.
Continuous observations, supplemented with photographs, were conducted by three operators for the first 12 hours to identify the onset of scleral discoloration. Subsequent observations were made at intervals of 2 hour from 14th to 24th hour to identify the onset of the full manifestation of the sign in its typical form (dark brown color).
The confirmation that the observed spots were postmortem scleral discolorations and not due to other phenomena was carried out through a single OCT scan (iVue SD-OCT, Optovue Inc, Fremont, CA) performed during the first observation of the SDPP.
Confirmation using a portable OCT programmed for ocular surface scans was performed in two stages for each sample. The first scan was conducted at the initial manifestation of scleral discoloration, and the second scan was performed when the spot had developed the characteristic black-brown coloration. As the device was an intraoperative OCT, the examination was carried out in situ by moving the instrument within the observation room with minimal movement of the head to facilitate the examination.
3. Results
The protocol employed allowed the detection of scleral spots in all 20 eyes (100%) kept open through eyelid excision. In contrast, the appearance of scleral spots was not detected in any of the eyes kept closed using adhesive support. This evidence is so compelling that it renders a statistical analysis of the data unnecessary. However, some chronological and morphological parameters have been examined and will be reported below.
3.1. Timing
The manifestation of the initial discoloration occurred in all samples with the eye open at an average interval of 223,5 ± 53,75 minutes post-mortem. The full manifestation of the sign was verified at an average of 817,65± 401,74 minutes post-mortem.
Table 1.
The table describes, for each sample, the following details: in the left column, the post-mortem interval in minutes at which discoloration of the sclera began and was macroscopically appreciable; in the right column, the time of observation at which the scleral spot assumed its characteristic dark brown to blackish coloration.
Table 1.
The table describes, for each sample, the following details: in the left column, the post-mortem interval in minutes at which discoloration of the sclera began and was macroscopically appreciable; in the right column, the time of observation at which the scleral spot assumed its characteristic dark brown to blackish coloration.
| Samples |
Time of initial discoloration manifestation in the right open eye (minutes) |
Time of full dark sign manifestation in the right open eye (minutes) |
| Sample 1 |
162 |
529 |
| Sample 2 |
186 |
349 |
| Sample 3 |
202 |
567 |
| Sample 4 |
183 |
1080 |
| Sample 5 |
213 |
568 |
| Sample 6 |
233 |
350 |
| Sample 7 |
161 |
1440 |
| Sample 8 |
187 |
351 |
| Sample 9 |
195 |
570 |
| Sample 10 |
197 |
570 |
| Sample 11 |
178 |
573 |
| Sample 12 |
195 |
575 |
| Sample 13 |
273 |
1440 |
| Sample 14 |
299 |
1440 |
| Sample 15 |
275 |
1320 |
| Sample 16 |
357 |
1200 |
| Sample 17 |
273 |
576 |
| Sample 18 |
236 |
577 |
| Sample 19 |
289 |
1080 |
| Sample 20 |
176 |
1200 |
| Mean |
223,5 |
817,65 |
| Standard deviation |
53,75 |
401,74 |
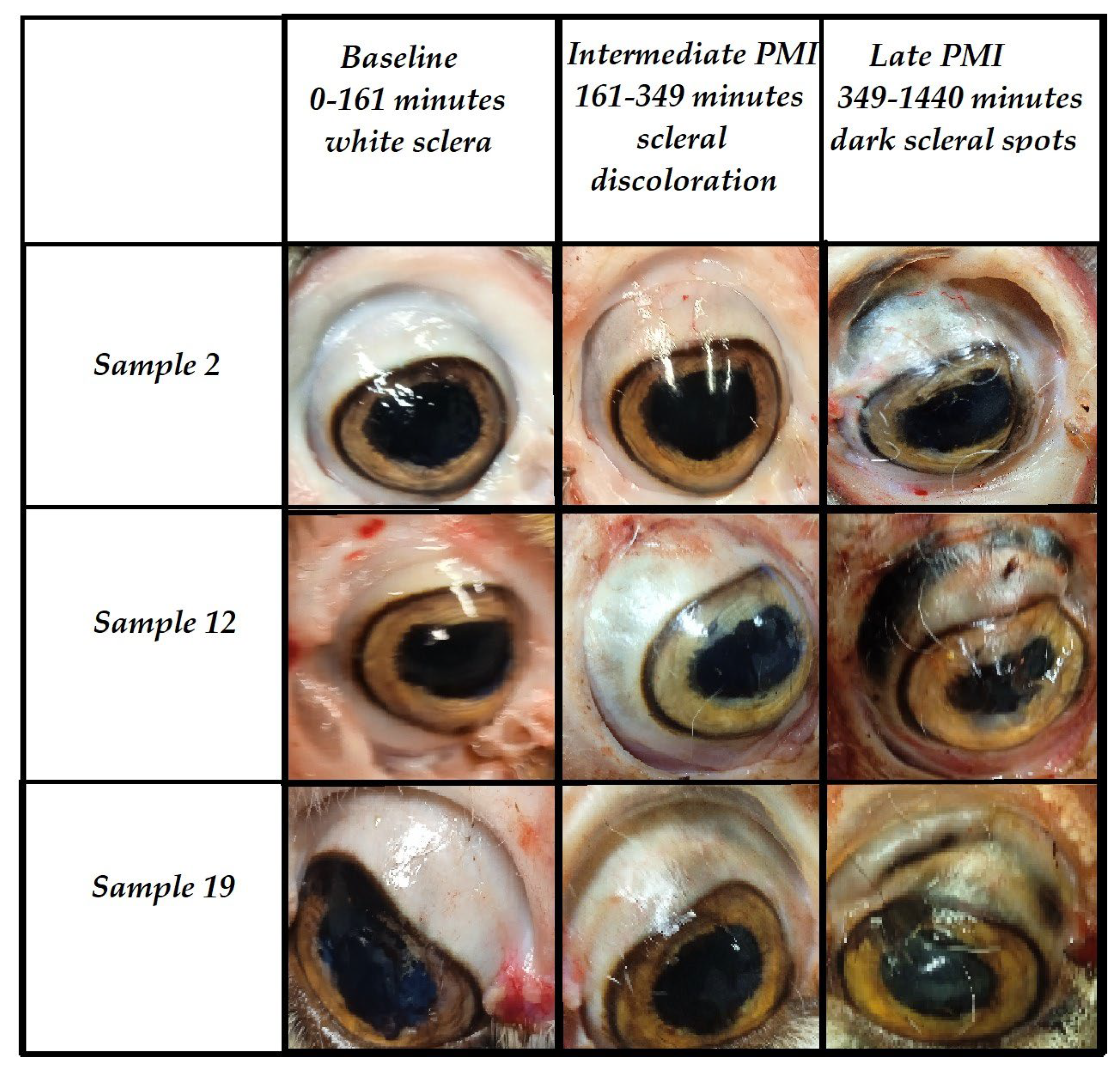
Macroscopically, the evolution of the sign is characterized by an initial period during which the sclera appears completely white upon observation. Starting from the 2nd hour post-mortem, a discoloration begins to manifest, becoming increasingly intense and reaching its peak in 100% of cases by the 24th hour post-mortem.
Figure 1.
The figure shows the macroscopic characteristics of the eye at baseline (0-161 minutes postmortem), in an intermediate PMI (161-349 minutes-scleral discoloration), and in a late PMI (349-1440 minutes dark scleral spot).
Figure 1.
The figure shows the macroscopic characteristics of the eye at baseline (0-161 minutes postmortem), in an intermediate PMI (161-349 minutes-scleral discoloration), and in a late PMI (349-1440 minutes dark scleral spot).
3.2. Morphology
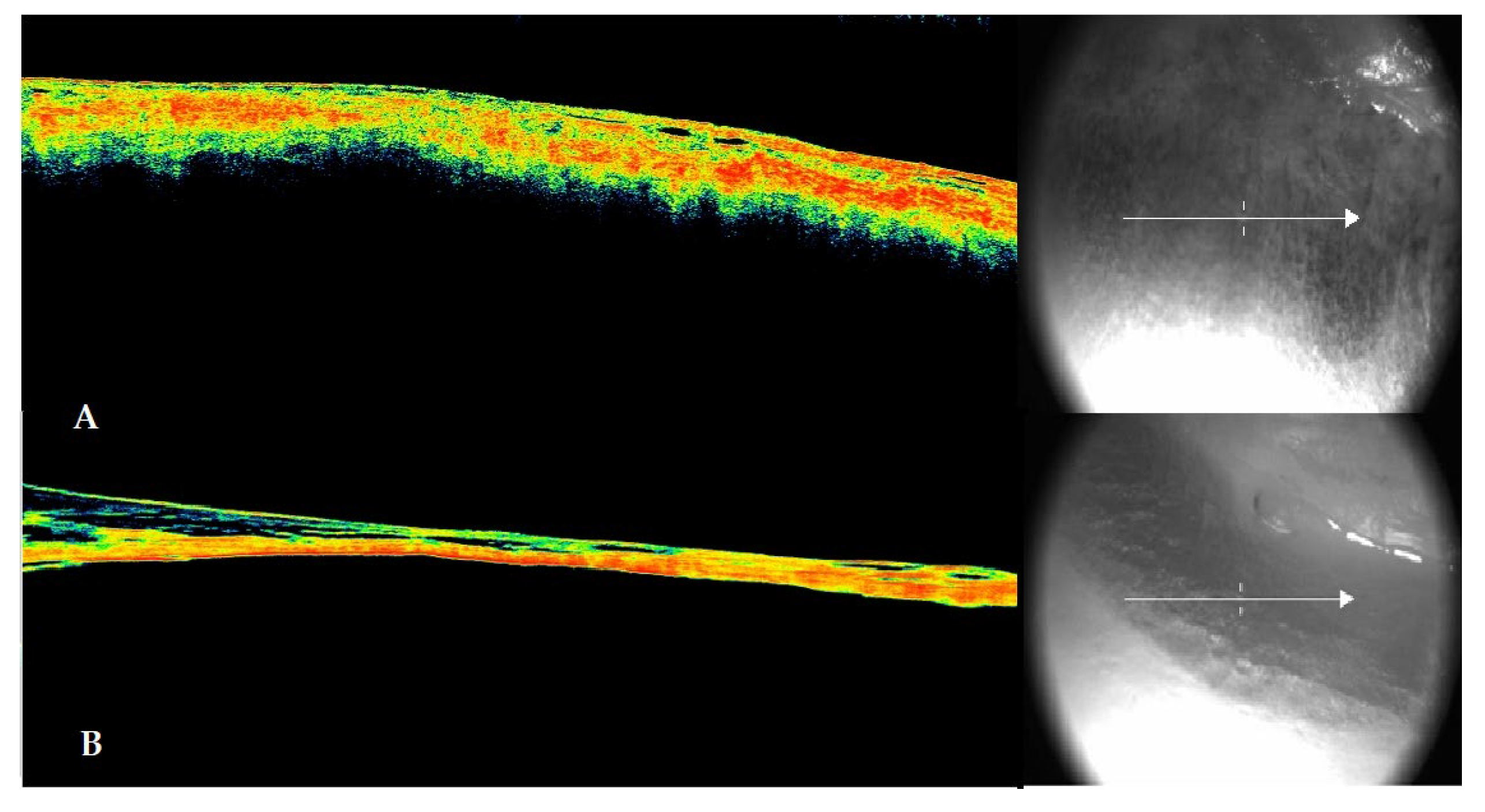
At the first detection of discoloration on the scleral surface, an OCT scan of the structure was performed. The data showed in 100% of early observations, consistent with previous studies, a morphology characterized by the detachment between the scleral layer and the choroid. In the later stages, when the sign was fully manifested, the choroidal detachment was associated with significant dehydration of the sclera, accompanied in all cases by delamination of this structure.
Figure 2.
The figure shows in panel A the morphological configuration of the scleral discoloration highlighted through OCT (left side) and the corresponding section (right side). A detachment between the sclera and choroid and initial dehydration of the ocular surface are evident. In panel B, the OCT scan configuration of the dark brown to blackish scleral spot that appeared in the later post-mortem intervals is shown. Both the advanced dehydration of the ocular surface and the clear detachment between the sclera and vitreous (which appears less dense due to probable liquefaction) are evident. The sclera appears delaminated at several points in the inner layers of the tunic.
Figure 2.
The figure shows in panel A the morphological configuration of the scleral discoloration highlighted through OCT (left side) and the corresponding section (right side). A detachment between the sclera and choroid and initial dehydration of the ocular surface are evident. In panel B, the OCT scan configuration of the dark brown to blackish scleral spot that appeared in the later post-mortem intervals is shown. Both the advanced dehydration of the ocular surface and the clear detachment between the sclera and vitreous (which appears less dense due to probable liquefaction) are evident. The sclera appears delaminated at several points in the inner layers of the tunic.
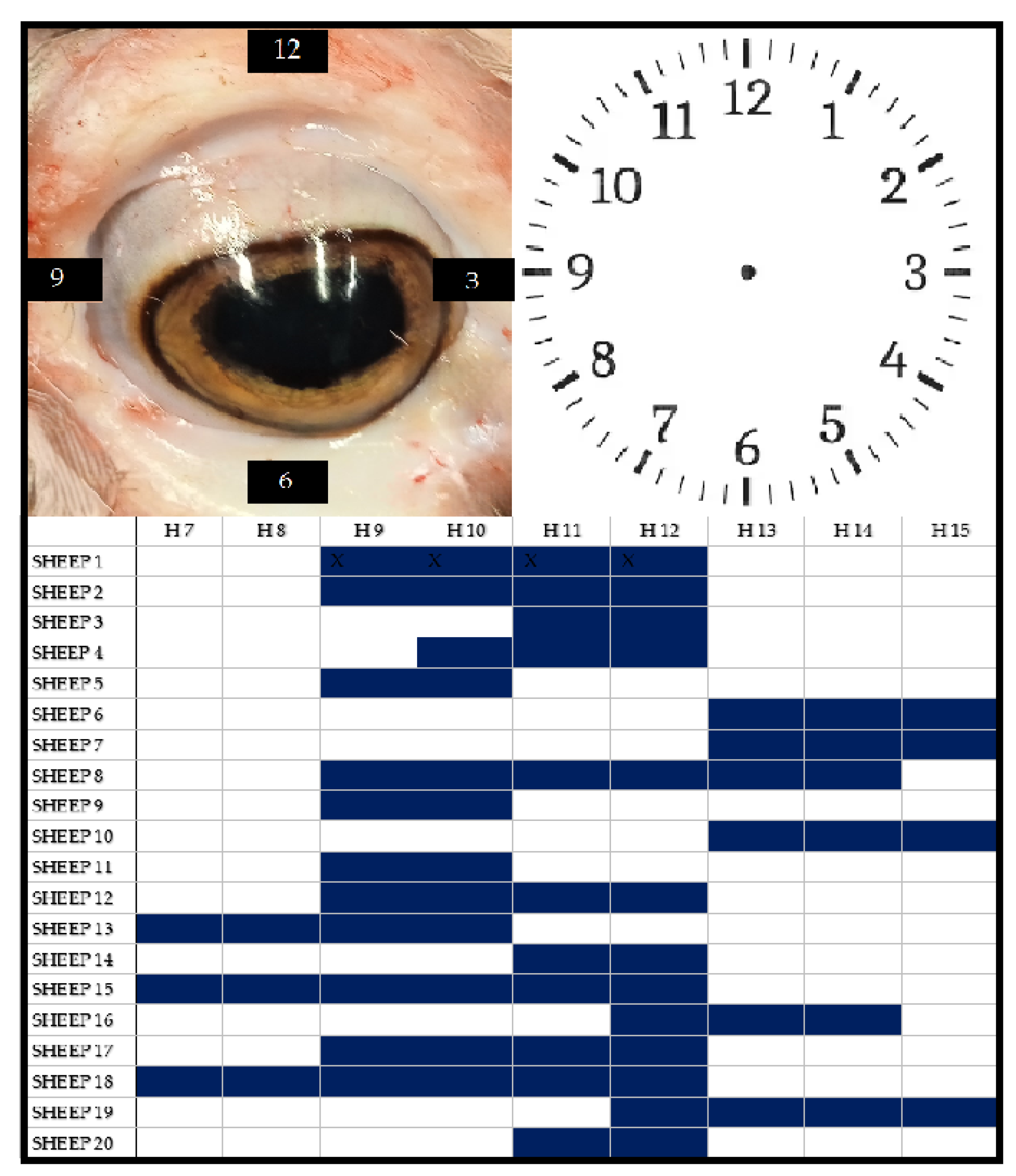
Another notable finding regarding morphology is the location where PDSS manifested in our model. While scleral spots in humans typically appear at the equator of the eyeball (i.e., 3 o’clock and 9 o’clock positions), in the experimental model, the appearance was predominantly observed in the upper quadrant (9 o’clock to 12 o’clock positions). Surprisingly, the localization of the spots was not influenced by the positioning of the head in space. In fact, the spots were predominantly found in the supero-lateral quadrants (9-12). Only in three out of twenty cases (15%), where the head was maintained in a natural position, these portions of the sclera were not involved.
Figure 3.
In the upper part of the figure, on the left, there is a photo of a sheep’s eye, and on the right, the schematic representation of the ocular portions of the sclera based on the classic ‘clock-face’ scheme used in clinical practice. The lower part of the figure shows, in blue, the location of the post-mortem dark scleral spots for each examined sample.
Figure 3.
In the upper part of the figure, on the left, there is a photo of a sheep’s eye, and on the right, the schematic representation of the ocular portions of the sclera based on the classic ‘clock-face’ scheme used in clinical practice. The lower part of the figure shows, in blue, the location of the post-mortem dark scleral spots for each examined sample.
Additionally, the shape of the spot is not linear or triangular as described in human cadavers, but rather appears vaguely ribbon-like and semicircular.
4. Discussion
The aim of the current study was to experimentally reproduce an animal model through which to study PDSS. The experimental results demonstrated that the protocol implemented is capable of reproducing postmortem scleral spots in 100% of cases.
An additional result of the study was the confirmation that postmortem dehydration of the ocular surface exposed to air is a necessary (though not sufficient) element for the manifestation of the sign: PDSS did not appear in any eyes that were kept covered by the eyelid. Postmortem, particularly in the first few hours, if the eye is exposed to air (regardless of temperature), rapid evaporation and drying occur in the superficial layers of the exposed ocular surface [
11,
12,
13,
14].
Dehydration is further promoted by the cessation of homeostatic mechanisms postmortem, which normally maintain ocular surface hydration. Specifically, there is a complete cessation of tear film production, and profound alterations occur in both the aqueous humor and vitreous humor. These changes impact the chemical composition of these biofluids and, consequently, the characteristics of the ocular surface. Numerous studies have demonstrated that alterations in the ocular surface are accompanied by chemical and physical changes within the eye, which become more pronounced with the increase of the postmortem interval. The aqueous humor undergoes a slow and progressive evaporation, resulting in alterations to the shape of the corneal surface. Within the aqueous humor, cellular aggregates can be detected, which are indicative of the desquamation of the corneal endothelium and the structure of the anterior chamber. Conversely, the vitreous humor undergoes progressive liquefaction, leading to a physical state change from gelatinous to liquid. While such alterations are clearly visible in the cornea, which requires a high degree of transparency for its lens function, they also affect the sclera, although to a lesser extent in terms of macroscopic detectability [
14,
15,
16,
17].
The results of the current study, in addition to recreating PDSS in an animal model, have allowed for the confirmation of several elements crucial for a deep understanding of the thanatogenesis of the sign. Of significant importance is the confirmation that, in all samples examined morphologically and through imaging, a choroidal detachment was found corresponding to the scleral spot.
This alteration, when the sclera undergoes extensive dehydration, primarily results in the thinning of the overlying scleral portion. The thinning of this structure, in addition to a change in color due to the superficial dehydration of the tissue and conjunctiva, causes the translucency from the thinning to reveal the underlying layer, the choroid, which is blackish in color. This layer is normally invisible under physiological conditions and, in humans, only becomes apparent due to certain pathological conditions, such as connective tissue disorders, giving the scleral surface a bluish tint. Postmortem, the absence of compensatory homeostatic mechanisms results in this phenomenon manifesting in areas with significant exposure to the environment or physiologically thinner regions, which is inextricably linked to the detachment of the choroid.
An additional confirmation obtained from the study data concerns the possibility that the sign manifests when the cause of death, as in our animal model, is hemorrhagic shock.
Despite the fact that every forensic pathologist has likely encountered PDSS at some point in their career, surprisingly, literature on the phenomenon is scarce. The lack of interest is primarily due to the inconsistency of the sign, which is considered nonspecific because not all cadavers with open eyes postmortem exhibit the tache noire. However, when the analysis is restricted to cases where PDSS is present, some preliminary studies have indicated that the causes of death are primarily attributable to three mechanisms: mechanical asphyxia by hanging, cranial trauma and hemorrhagic shock. From a pathophysiological perspective, although the dynamics leading to these types of death are quite different, they share a common feature: a sudden change in intraocular pressure during the perimortem period (either positive or negative), potentially capable of causing a choroidal detachment [
8,
9]. This finding, which still requires further epidemiological investigation with large-scale data, if confirmed, could become an important tool for forensic pathologists in the differential diagnosis of the cause of death.
In the analysis of the results, it is important to highlight some differences compared to the sign commonly observed in routine forensic pathology practice, as well as from empirical observations in some specialized books, given that very few experimental studies have analyzed PDSS. These differences primarily concern the time of formation of the spots, the morphological evolution of the sign over time, and the location where the spots were found.
Certainly, these differences can be attributed to both the anatomical peculiarities of the ovine eye compared to the human eye (larger size with greater thickness, different orbital shape, presence of a third eyelid) and the extremization of the model, which involved exposing not just a portion of the sclera but the entire ocular globe of the animal.
Regarding the timing of the sign’s appearance, while PDSS is expected to appear within 60-120 minutes in humans, the current study revealed that the sign manifested after approximately 223.5 ± 53.75 minutes in the described model [
3]. This difference in the timing of appearance can be primarily attributed to the anatomical differences between the ovine and human eyes, both in terms of size and ultrastructural characteristics. These differences, although relatively modest, may play a role in the delayed manifestation of the sign in our model [
18,
19,
20,
21,
22].
Similarly, regarding the evolution of the sign over time, our model did not exhibit the phase where the portion of the sclera, which later underwent discoloration and the formation of PDSS, showed a yellowish tint due to dehydration. In the examined samples, as previously mentioned, the progression moved directly from discoloration to the formation of spots without other macroscopic manifestations. This phenomenon can also be attributed to the differences in the ocular structure between humans and sheep.
Finally, concerning the portion of the sclera where the sign appeared, the effect can be attributed to various factors. Besides the anatomical characteristics (particularly the different adherence between the vitreous body and the choroid), the type of model used in the protocol, which aimed to maximize the dehydration effect on the entire ocular globe, certainly played a role. This approach resulted in extensive air exposure to areas of the globe that are usually covered by the eyelids, promoting early dehydration.
However, the study revealed that in the model, the spatial localization of the spots is not influenced by the position of the head. The results showed a predominant involvement of the supero-lateral quadrants (85%), which, in open eyes, were unaffected by the spots in only 3 out of 20 cases (15%).
Despite the observed differences compared to forensic practice, the proposed model successfully reproduced a sign that has a rare incidence in real cases. This sign could hold greater significance than currently reported in the literature, both for estimating the postmortem interval (PMI) and determining the causes of death. The data currently available are limited and fragmented. To gain a better understanding of the sign and apply the findings in practice, further studies are needed, primarily using animal models. These studies should analyze the phenomenon with a greater number of samples using OCT, as well as employing different methodologies compared to those used in the current work (e.g., histology, immunohistochemistry, other imaging techniques). An epidemiological analysis based on a large sample is also essential to statistically highlight the correlation between the presence of the sign and the three different types of death identified.
The current study has some limitations. The first is the difference between the human and sheep eye, which primarily impacts the timing of the sign’s formation. The second is the extremization of the model, which affects the morphology and locations of PDSS appearance. The third limitation is the small sample size. This was partly due to the limited literature available on tache noir, making it impossible to conduct a preliminary sample size calculation. However, this limitation is somewhat mitigated by the robustness and consistency of the results obtained. The fourth limitation is that in all cases, death was due to hemorrhagic shock. However, this limitation also serves to confirm that deaths from hemorrhagic shock represent a type of death where choroidal detachment occurs and PDSS can manifest macroscopically.
5. Conclusions
The experimental data from the study confirmed that the model created, despite some limitations, is capable of reproducing PDSS in all samples where the protocol was applied. The application of this model could lead to a better understanding of the significance of PDSS and provide further insights into the postmortem interval (PMI) and causes of death in cases where there is a macroscopic manifestation of this sign.
Author Contributions
Conceptualization, M.N., P.E.N., M.F. and E.d’A.; methodology, M.N., P.E.N., M.F. and E.d’A.; software, P.E.N., M.N.; formal analysis, M.N., P.E.N., M.F. and E.d’A.; investigation, M.N., P.E.N, A.C, D.N; resources, E.d’A, M.F.; data curation, M.N., P.E.N.,A.C., D.N.; writing—original draft preparation, M.N, P.E.N., D.N.; writing—review and editing, M.F, E.d’A.; visualization, M.N., P.E.N.,D.N., A.C., M.F. E.d’A.; supervision, E.d’A., M.F.; funding acquisition, E.d’A., M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable: this article does not include any studies involving human participants conducted by the authors. Since sheep heads are considered waste material, no specific animal protocol was required, and there were no associated costs
Informed Consent Statement
Not applicable.
Data Availability Statement
the data presented in this study are available on request from the corresponding author (MN).
Acknowledgments
the authors thank Drs. Elia Porru, Luca Natali, and Roberto Caria for their contributions to the preliminary studies that were essential for the creation of the model.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sommer, A.G. Dissertationis de Signis, Mortem Hominis Absolutam ante Putredinis Accessum Indicantibus, Particula Prior [at Posterior]; Forbes, J., Conolly, J., Eds.; n.p.: Copenhagen, Denmark, 1833; p. 277. [Google Scholar]
- Forbes, J.; Conolly, J. Sommer on the signs of death. In British and Foreign Medical Review; Sherwood Gilbert and Piper: Volume IV, London, UK, . 1837. [Google Scholar]
- Madea, B.; Henssge, C.; Reibe, S.; Tsokos, M.; Kernbach-Wighton, G. Postmortem changes and time since death. Handbook of forensic medicine, 2014, 75–133. [CrossRef]
- Madea, B.; Dettmeyer, R.; Schmidt, P. Thanatologie. In Praxis Rechtsmedizin; Springer: Berlin, Heidelberg, 2007. [Google Scholar] [CrossRef]
- Byard, R.W. Pekka Saukko, Bernard Knight: Knight’s forensic pathology 4th ed. Forensic Sci Med Pathol 2018, 14, 147. [Google Scholar] [CrossRef]
- DiMaio, D.; DiMaio, V.J. Time of death. In Forensic pathology, 2nd Ed ed; CRC Press: London, 2001; pp. 35–36. [Google Scholar] [CrossRef]
- DiMaio, V.J.; Molina, D.K. Postmortem Changes, Time of Death and Identification In DiMaio’s forensic pathology. CRC Press. 2021. [CrossRef]
- Nioi, M.; Napoli, P.E.; d’Aloja, E.; Nieddu, D.; Chighine, A.; Fossarello, M.; Demontis, R. Postmortal Dark Scleral Spots: results from a study of 905 cases from a single Institution. IOVS 2023, 64, 4768–4768. [Google Scholar]
- Napoli, P.E.; Fossarello, M.; d’Aloja, E.; Demontis, R.; Galantuomo, M.S.; Tatti, F.; Nioi, M. Morphological analysis of dark scleral spots by OCT: a preliminary study in human and animal model during the early post-mortem period. IOVS 2023, 64, 3395–3395. [Google Scholar]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; d’Aloja, E. Postmortem ocular findings in the optical coherence tomography era: A proof of concept study based on six forensic cases. Diagnostics, 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. Post-mortem corneal thickness measurements with a portable optical coherence tomography system: a reliability study. Sci. Rep. 2016, 6, 30428. [Google Scholar] [CrossRef] [PubMed]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Locci, E.; Fossarello, M.; d’Aloja, E. Morphological analysis of corneal findings modifications after death: A preliminary OCT study on an animal model. Exp. Eye Res. 2018, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Napoli, P.E.; Nioi, M.; Gabiati, L.; Laurenzo, M.; De-Giorgio, F.; Scorcia, V.; Grassi, S.; d’Aloja, E.; Fossarello, M. Repeatability and reproducibility of post-mortem central corneal thickness measurements using a portable optical coherence tomography system in humans: A prospective multicenter study. Sci. Rep. 2020, 10, 14508. [Google Scholar] [CrossRef] [PubMed]
- Nioi, M.; Napoli, P.E.; Demontis, R.; Chighine, A.; De-Giorgio, F.; Grassi, S.; Scorcia, V.; Fossarello, M.; d’Aloja, E. The influence of eyelid position and environmental conditions on the corneal changes in early postmortem interval: a prospective, multicentric OCT study. Diagnostics, 2022, 12, 2169. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Stocchero, M.; Gottardo, R.; Chighine, A.; De-Giorgio, F.; Ferino, G.; Nioi, M.; Demontis, R.; Tagliaro, F.; d’Aloja, E. PMI estimation through metabolomics and potassium analysis on animal vitreous humour. Int. J. LegalMed. 2023, 137, 887–895. [Google Scholar] [CrossRef]
- Rosa, M.F.; Scano, P.; Noto, A.; Nioi, M.; Sanna, R.; Paribello, F.; De-Giorgio, F.; Locci, E.; d’Aloja, E. Monitoring the modifications of the vitreous humor metabolite profile after death: an animal model. BioMed Res. Int. 2015, 627201. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Stocchero, M.; Gottardo, R.; De-Giorgio, F.; Demontis, R.; Nioi, M.; Chighine, A.; Tagliaro, F.; d’Aloja, E. Comparative use of aqueous humour 1H NMR metabolomics and potassium concentration for PMI estimation in an animal model. Int. J.Legal Med. 2021, 135, 845–852. [Google Scholar] [CrossRef]
- Gogola, A.; Jan, N.J.; Lathrop, K.L.; Sigal, I.A. Radial and circumferential collagen fibers are a feature of the peripapillary sclera of human, monkey, pig, cow, goat, and sheep. IOVS 2018, 59, 4763–4774. [Google Scholar] [CrossRef]
- Creveling, C.J.; Colter, J.; Coats, B. Changes in vitreoretinal adhesion with age and region in human and sheep eyes. Front. Bioeng. Biotechnol. 2018, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Reynolds, J.N. The sheep as a large animal model for the investigation and treatment of human disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, G.; Wulc, A.E. Applicability of a sheep model for training in plastic surgery of eyelids and orbit. Ear Nose Throat J. 2022, 101, 43S–49S. [Google Scholar] [CrossRef] [PubMed]
- Caspar, K.R.; Hüttner, L.; Begall, S. Scleral appearance is not a correlate of domestication in mammals. Zoological Lett., 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).