Introduction
Chronic kidney disease (CKD) is a public health problem with an increasing incidence on the global population and a high mortality rate that is a risk factor for Cardiovascular diseases (CVD) [
1]. Although, Diabetes Mellitus (DM) and Hypertension (HT) are the primary causes of CKD, studies have appeared in recent years that changes in intestinal microbiota and permeability may be related to the disease. As a result of impaired intestinal permeability due to various reasons (tight junction downregulation, viral intestinal infections, environmental toxins, toxic food, etc.) pathogens and toxins originating from the intestine pass into the systemic blood circulation. As a matter of fact, impaired intestinal permeability, together with an increased toxin load in the circulation, paves the way for the formation of DM, HT and eventually CKD [
2,
3].
Zonulin, identified for the first time by Fasano et al., is remarkable among the molecules that are indicators of intestinal permeability [
4]. Dysbiosis in the intestinal system and decreased mucosal permeability pave the way for an increase in plasma zonulin levels. Zonulin, which is associated with many clinical pathologies, especially celiac disease, attracts attention nowadays as a marker of increased intestinal permeability [
5]. Additionally, a few studies conducted in recent years emphasize that serum zonulin level may play a role in the pathogenesis of CKD. Although a few studies in the literature remark that zonulin may be associated with CKD, the pathophysiology of this relationship and the role of the molecule in the disease process are unclear [
6,
7].
Today, we know that in CKD patients, disease prognosis is negatively affected by endothelial dysfunction (ED), impaired metabolic regulation, systemic inflammation (SI), and increased toxic load, specially impaired renal functions. On the other hand, the role of zonulin with pathogenetic processes in CKD patients is fully unknown. In this study, we aimed to examine the relationship between plasma zonulin level and ED, SI, metabolic components, especially renal functions in patient with CKD.
Materials and Methods
The study was conducted as a single-center, cross-sectional study and informed consent was obtained from all participants before the study. The study was designed in accordance with the Declaration of Helsinki and local ethics committee approval was obtained from Local Ethics Committee of Balikesir University Medical School (date: 19.04.2023, approval number: 2023/51).
Study Design and Population
One hundred sixty-three participants were enrolled in this study. All participants were divided into two groups, patient and control groups, according to the presence of CKD. Exclusion criteria were active infection, acute inflammatory diseases (rheumatoid arthritis, inflammatory bowel disease, etc.), malignancies, pregnancy, abnormal thyroid dysfunction, and receiving renal replacement therapy.
Anthropometric Measurements
All participants provided a medical history and underwent a clinical examination. The height and weight of all patients were measured using a standard method, and body mass index (BMI) was evaluated with formula of body weight/height2. Waist circumference (WC) was measured using the lower costal margin and the anterior superior iliac crests as reference. Blood pressure (BP) was measured three times in the outpatient clinic and the average value was registered.
Biochemical Analysis
The complete blood count (CBC) was performed using the DxH 800 (Beckman Coulter Inc., USA) analyzer. Serum fasting plasma glucose (FPG), creatinine, urea, total cholesterol (TC), HDL-C, triglycerides (TGL), sodium, potassium, and albumin levels were measured with the AU680 (Beckman Coulter Inc., USA) biochemical autoanalyzer. The Friedewald formula and the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) formula were used to calculate LDL-C and eGFR, respectively [
8,
9].
Zonulin (Cat No: 201-12-5578, SunRed Biological Technology, Shanghai, China), Claudin-3 (Cat No: 201-12-2303, SunRed), Vascular cell adhesion molecule 1 (VCAM-1) (Cat No: 201-12-0204, SunRed), and Interleukin-6 (IL-6) (Cat No: 201-12-0091, SunRed) levels were analyzed in serum samples using the ELISA (Enzyme-Linked Immunosorbent Assay) method. The measurement ranges for Zonulin, Claudin-3, VCAM-1, and IL-6 were 0.25 - 70 ng/ml, 0.5 - 60 ng/ml, 0.4 - 60 ng/ml, and 3 - 600 ng/L, respectively. Before the analyses, serum samples were diluted with physiological saline solution at ratios of 1/10 for Zonulin analysis and 1/50 for VCAM-1 analysis. No pretreatment was applied to the serum samples for Claudin-3 and IL-6 analyses.
CKD Definition
CKD was defined as the patient group whose eGFR<60 mL/min/1.73m2 for three months. On the other hand, control group was defined as the patient with eGFR>60 mL/min/1.73m2 and without any signs of renal damage (albuminuria, urinary sediment abnormalities, etc.).
Statistical Analysis
All participants divided into two groups and kolmogorov-Smirnov test was applied to determine the suitability of continuous variables for normal distribution among these two groups. Continuous variables are shown as mean ± standard deviation, while categorical variables are expressed as percentage (%). The chi-square test was performed to compare categorical variables. Continuous variables were compared in pairs using the non-parametric Mann-Whitney U test when they were not normally-distributed, and parametric independent student t test in terms of normally-distributed when they were normally-distributed.
The Spearman correlation analysis was performed between zonulin levels and other parameters. The linear regression analysis was performed to show the interaction between eGFR and zonulin levels. The relationship between these variables was examined in an adjusted model that included the covariates identified in the initial univariate analyses. Unadjusted, model 1 and model 2 adjusted β value was calculated. While model 1 was created according to age, model 2 was created according to systolic and diastolic blood pressure in addition to model 1. SPSS package program was performed in the statistical analysis and a p-value of <0.05 was found to be statistically significant.
Results
A total of 104 patients with CKD (mean age 58.9 ±1.4) and 59 control subjects (mean age 59.0 ±1.1) were included. Age (p =0.934) and gender (p =0.196) ratios were similar between the groups and comparisons of the demographic and the laboratory parameters were given in
Table 1.
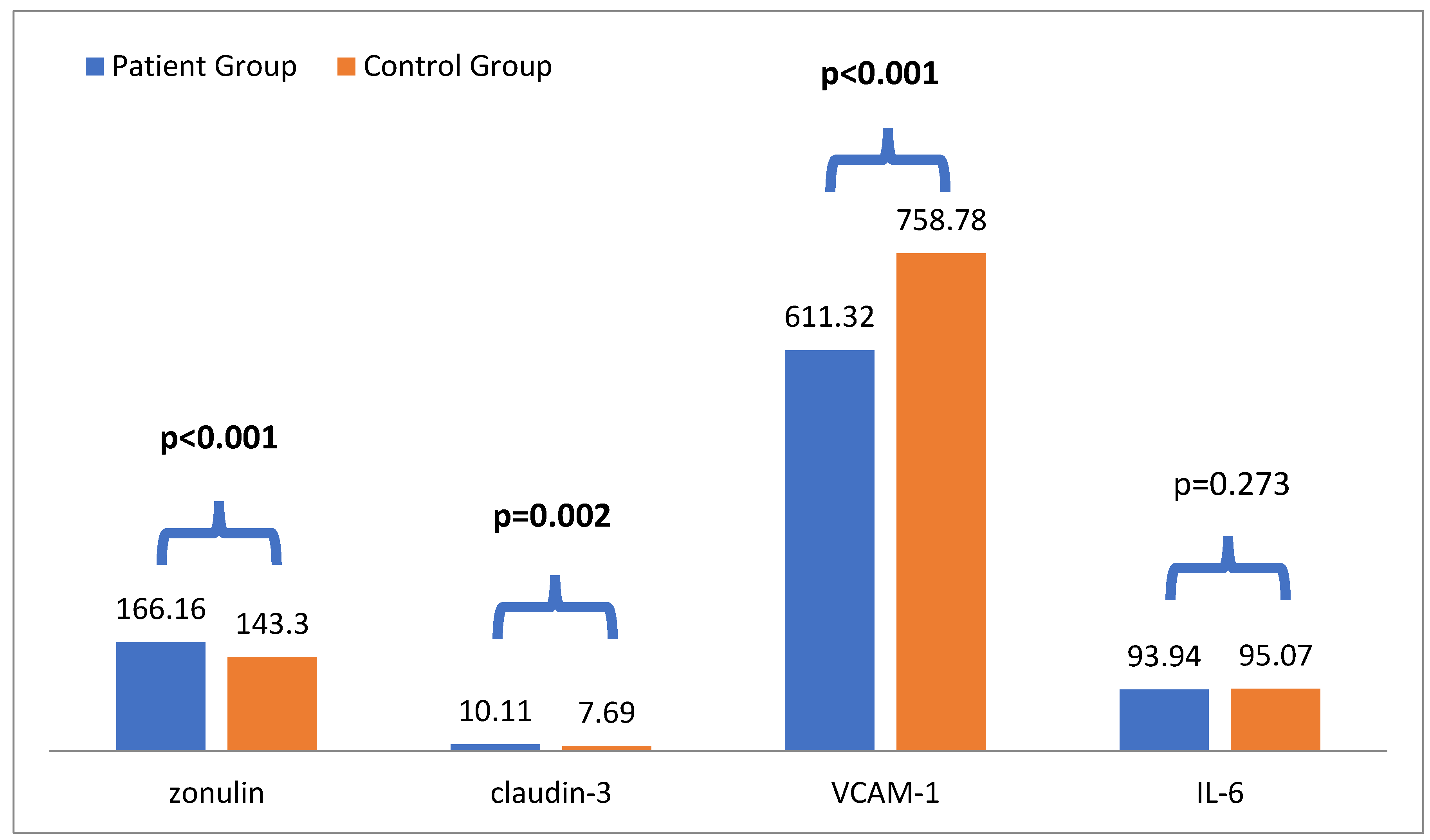
In the comparison analysis, plasma zonulin levels in the CKD group (166.16 ± 53.54) were significantly higher than the controls group (143.30 ± 60.92) (p <0.001). Futhermore, patient with CKD had higher systolic blood pressure (p =0.005), urea, creatinine, potassium (p <0.001 for all) and claudin-3 levels (p =0.002) than the controls. On the other hand, hemoglobin, eGFR and VCAM-1 levels (p <0.001 for all) were lower in patient with CKD compared to control groups (
Table 1 and
Figure 1).
In the correlation analysis, serum zonulin level showed positive correlation with claudin-3 (r =0.612, p <0.001), IL-6 (r =0.307,p <0.00), creatinine (r =0.313, p <0.001) and negative correlation with eGFR (r =-0.320, p <0.001) and TC (r =-0.074, p =0.047) (
Table 2). Moreover, in linear regression analysis, zonulin level was significantly associated to eGFR, after adjustment for age and SBP (β value =-0.918, p =0.012).
Discussion
In the present study, it was found that plasma zonulin levels were statistically significantly higher in patients with CKD compared to the control group. Additionally, plasma zonulin levels were significantly correlated with impaired renal function tests and IL-6, a marker of SI, but this relationship was not detected in terms of ED. In light of these data, current findings suggest that serum zonulin levels are negative correlated with CKD levels and positive related with SI. Therefore, CKD patients may screened for zonulin levels and plasma zonulin levels may used as an SI marker in this patient group.
Zonulin is a haptoglobin protein that can cause an opening in the tight junctions of the intestinal epithelium and plays a role in regulating permeability in the intestinal mucosa [
10,
11]. High zonulin levels increase mucosal permeability and increased mucosal permeability causes endotoxins in the intestinal lumen to pass into the systemic circulation [
12]. In parallel with this data, in the present study, zonulin levels were positively correlated with claudin-3 levels, which have been used as a permeability marker for many years. Moreover, this coundition suggests that elevated zonulin levels may play a role in diseases characterized by SI, especially DM and obesity [
13,
14]. However, the role of plasma zonulin levels is unclear in patients with CKD. In a study by Şirin et al. which compared diabetic renal patients with a control group, no difference was found between the groups in terms of serum zonulin levels [
15]. In another study by Lukaszyk et al. it was showed that zonulin levels were higher in the healthy control group compared to the CKD patient group [
16]. On the other hand, in a study conducted by Carpes LS. et al. on patients with diabetic kidney disease, it was emphasized that zonulin level was correlated positively with eGFR and negatively with creatinine level [
17]. In the light of this data, we found that plasma zonulin levels were higher in patients with CKD compared to the control group. In addition, the fact that zonulin levels were negatively correlated with eGFR and positively with creatinine levels supported our findings. Indeed, in the current modelling, zonulin predicted the change in eGFR independently of age and blood pressure. Our results showed that zonulin, a marker of damaged intestinal mucosal barrier, was elevated in patients with CKD and correlates with impaired renal function tests.
Together with impaired intestinal permeability, increased plasma zonulin levels cause toxic end products from the intestinal mucosa to enter the systemic circulation, which may lead to increased SI over time [
18]. Futhermore, this process may lead to ED in the background of inflammation over time. However, the relationship between plasma zonulin levels with SI and ED has not been fully demonstrated in the current literature. In the study by Al-Obaide et al. plasma zonulin levels were positively correlated with lipopolysaccharide endotoxin levels [
19]. In another study by Ficek et al. found that zonulin levels were correlated with Hs-CRP but not with Il-6 and bacterial lipopolysaccharides [
20]. Additionally, in the study of Lukaszyk et al. shown that plasma zonulin levels were similar in CKD patients with and without inflammation [
16]. In the light of all these data, in the present study, since acute inflammatory conditions were excluded, Il-6 levels were similar between the patient and control groups, as expected. However, plasma zonulin level was positively correlated with Il-6 in the whole group analysis. We believe that this is important in terms of showing that an increase in zonulin levels may lead to inflammatory processes. On the other hand, no significant correlation was observed between zonulin and VCAM levels. To the best of our knowledge, this is the first study to evaluate the relationship between zonulin with ED and SI in patients with CKD. Our findings suggest that zonulin levels may be associated with SI in CKD clinic, but this does not seem to be valid for ED.
The strength of this study includes that this is the first report to investigate the relationship between serum zonulin levels and renal function, SI and ED in patient with CKD. The present study has several limitations: Firstly, the results from this study should be generalized with caution, since it was conducted at a single center. Secondly, since the design of our study was cross-sectional, it does not allow us to make a strong causal inference between zonulin and CKD. Thirdly, the uremic toxin analysis of the patients could not be evaluated. Lastly, ED was evaluated using biomarkers, and imaging methods showing morphological change could not be used.
Conclusion
In conclusion, our results suggest that high zonulin level is independently associated with impaired renal function in patients with CKD. Furthermore, zonulin may also be associated with SI, but this association does not apply to ED. Therefore, checking the serum zonulin levels in patients with CKD seems to be a useful guide to assess the inflammation.
Author Contributions
Conceptualization, A.K., O.T. and S.E.K.; methodology, A.K., O.T., S.E.K. and H.S.; software, A.K., S.E.K. and O.K.B.; validation, A.K., O.T., S.E.K. and S.U.; formal analysis, A.K., S.E.K., S.U., and T.M.; investigation, A.K., O.T. and H.S.; resources; A.K.; data curation A.K., H.S., O.K.B., I.B., E.P., S.U., and T.M.; writing—original draft preparation, A.K., O.T., S.E.K. and S.U.; writing—review and editing, A.K., O.T., S.E.K. H.S., OK.B. and S.U., supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was designed in accordance with the Declaration of Helsinki and local ethics committee approval was obtained from Local Ethics Committee of Balikesir University Medical School (date: 19.04.2023, approval number: 2023/51).
Acknowledgements
We would like to thank Balıkesir University Scientific Research Project unit for their support.
Informed Consent Statement
Patient consent was obtained from all included patients.
Data Availability Statement
Data are available from the correspondent author upon a reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.Ü.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: global dimension and perspectives. Lancet. 2013, 382, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Linh, H.T.; Iwata, Y.; Senda, Y.; Sakai-Takemori, Y.; Nakade, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; et al. Intestinal Bacterial Translocation Contributes to Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000, 355, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef]
- Yu, J.; Shen, Y.; Zhou, N. Advances in the role and mechanism of zonulin pathway in kidney diseases. Int. Urol. Nephrol. 2021, 53, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Dschietzig, T.B.; Boschann, F.; Ruppert, J.; Armbruster, F.P.; Meinitzer, A.; Bankovic, D.; Mitrovic, V.; Melzer, C. Plasma Zonulin and its Association with Kidney Function, Severity of Heart Failure, and Metabolic Inflammation. Clin. Lab. 2016, 62, 2443–2447. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Levey A.S.; Stevens L.A.; Schmid C.H.; Zhang Y.L.; Castro A.F. 3rd; Feldman H.I.; Kusek J.W.; Eggers P.; Van Lente F.; Greene T.; et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009; 150, 604-612. [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, L.; Zheng, Y.; Yue, F.; Russell, R.D.; Zeng, Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res. Clin Pract. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zak-Gołąb, A.; Kocełak, P.; Aptekorz, M.; Zientara, M.; Juszczyk, L.; Martirosian, G.; Chudek, J.; Olszanecka-Glinianowicz, M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int. J. Endocrinol. 2013, 2013, 674106. [Google Scholar] [CrossRef] [PubMed]
- Sirin, F.B.; Korkmaz, H.; Eroglu, I.; Afsar, B.; Kumbul Doguc, D. Serum zonulin levels in type 2 diabetes patients with diabetic kidney disease. Endokrynol. Pol. 2021, 72, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Lukaszyk, E.; Lukaszyk, M.; Koc-Zorawska, E.; Bodzenta-Lukaszyk, A.; Malyszko, J. Zonulin, inflammation and iron status in patients with early stages of chronic kidney disease. Int. Urol. Nephrol. 2018, 50, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Carpes, L.S.; Nicoletto, B.B.; Canani, L.H.; Rheinhemer, J.; Crispim, D.; Souza, G.C. Could serum zonulin be an intestinal permeability marker in diabetes kidney disease? PLoS One. 2021, 16, e0253501. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut Microbiota-Dependent Trimethylamine-N-oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Ficek, J.; Wyskida, K.; Ficek, R.; Wajda, J.; Klein, D.; Witkowicz, J.; Rotkegel, S.; Spiechowicz-Zatoń, U.; Kocemba-Dyczek, J.; Ciepał, J.; et al. Relationship between plasma levels of zonulin, bacterial lipopolysaccharides, D-lactate and markers of inflammation in haemodialysis patients. Int. Urol. Nephrol. 2017, 49, 717–725. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).