Submitted:
14 August 2024
Posted:
15 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- i)
- the actual severity of patients included in trials that explicitly declare to enroll severe subjects, to confirm or refute the anecdotal notion of extreme variability in baseline motor impairment which might be responsible for the hesitating translational success of such interventions [9]
- ii)
- whether some of these technological approaches have been more consistently tested on severe patients than others and eventually speculate on why they have.
2. Methods
2.1. Protocol and Eligibility Criteria
- published between the period of 2008–2024,
- written in English,
- involved human participants in the framework of a Randomised Controlled Trial.
- Papers were excluded if they
- did not fit into the conceptual framework of the study,
- were reviews, study protocols, and meta-analyses.
2.2. Information Sources and Search Strategy
2.3. Selection of Sources of Evidence
2.4. Data Charting Process and Data Items
- First Author Name
- Year of publication
- Source
- Population sample size (participants per group)
- Inclusion Criteria in the RCT related to the upper limb impairment
- Availability of the dataset used (Yes/No)
-
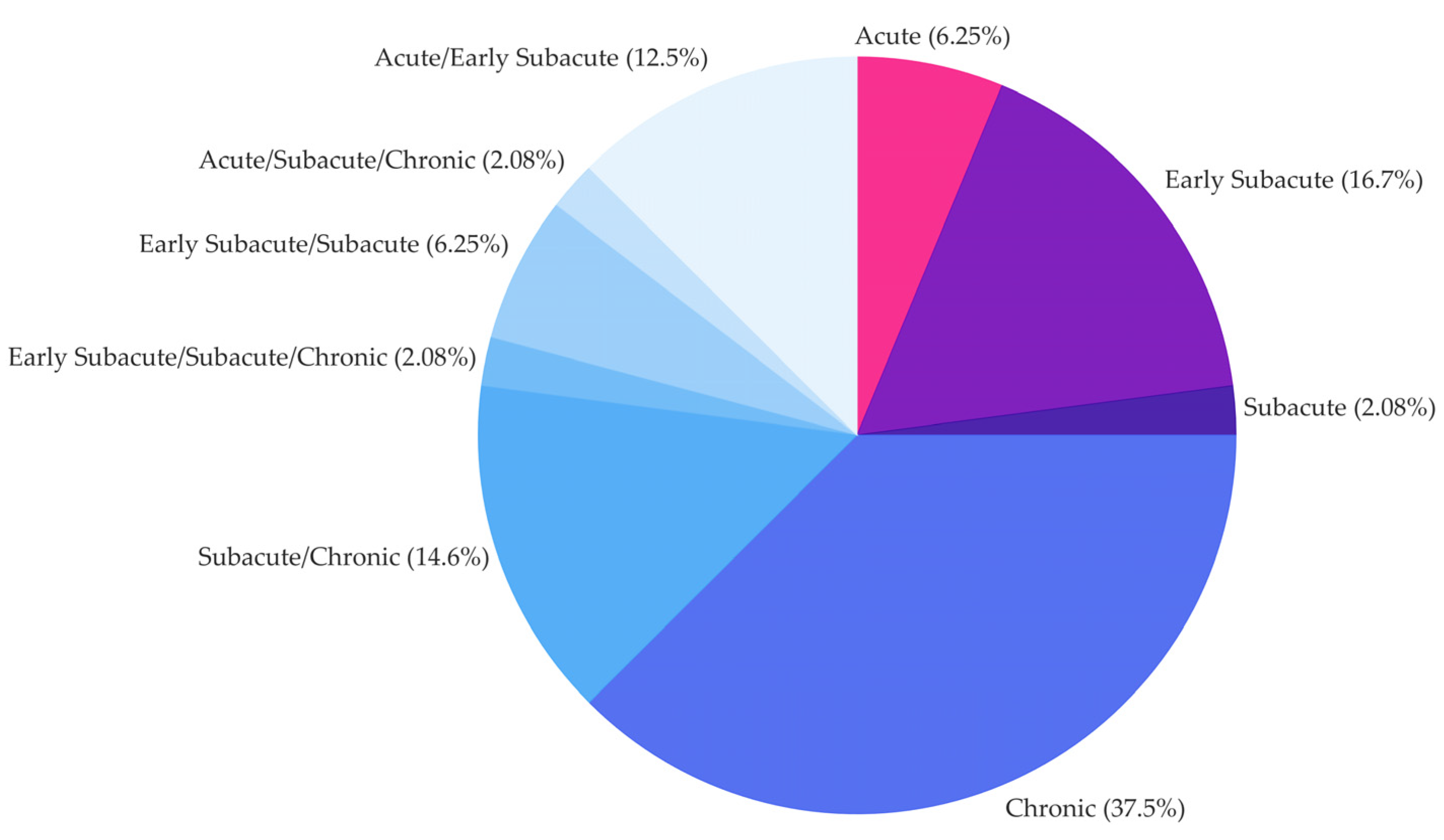
Time since injury (TSI), i.e., stroke event, classified as
- ◦
- ≤ 1 month (acute)
- ◦
- ≤ 3 months (early subacute)
- ◦
- ≤ 6 months (subacute)
- ◦
- > 6 months (chronic)
-
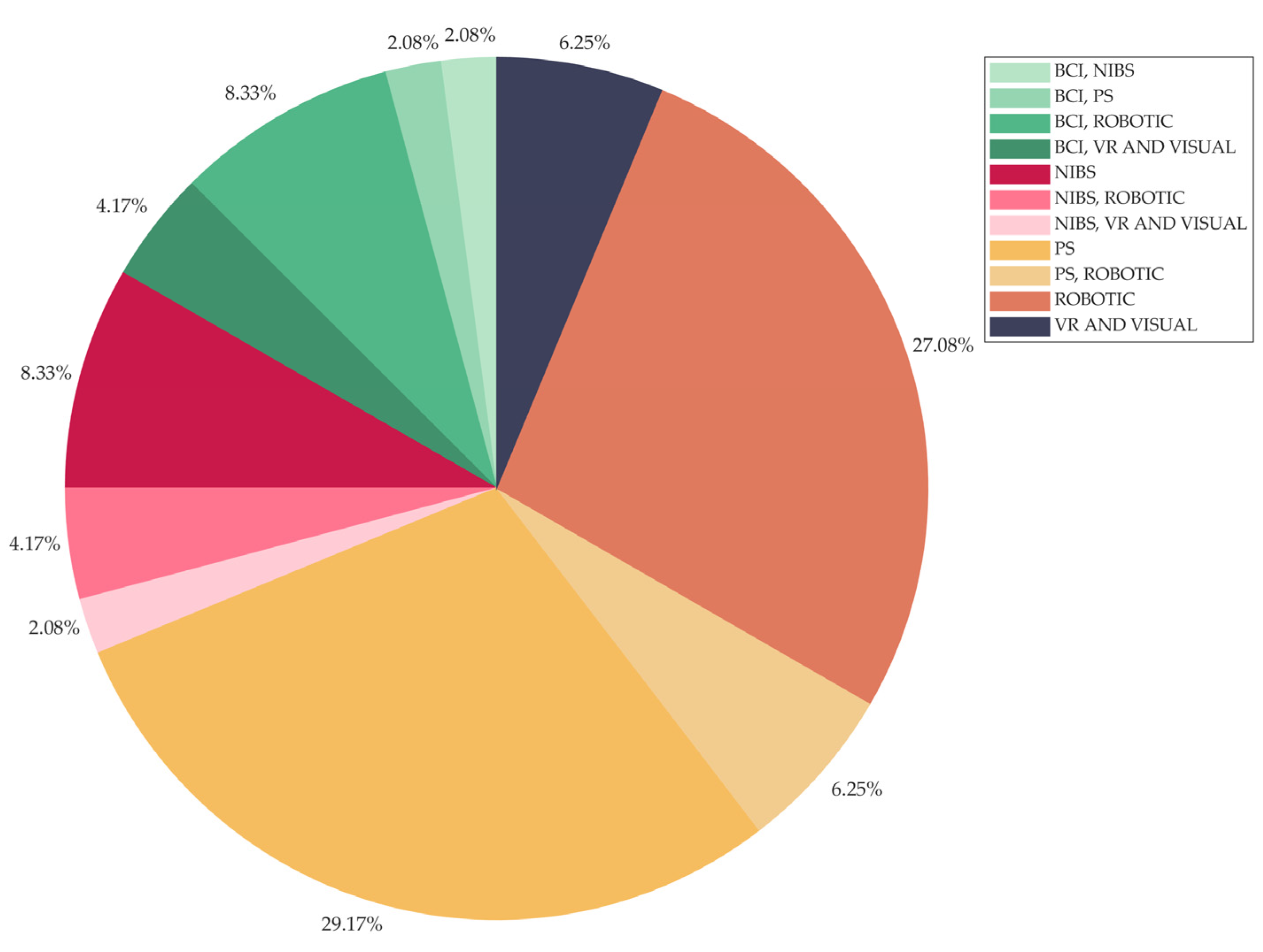
Intervention type, classified as
- ◦
- Brain-Computer Interface (BCI)
- ◦
- Non-Invasive Brain Stimulation (NIBS)
- ◦
- Peripheral Stimulation (PS)
- ◦
- Robotic
- ◦
- Virtual Reality (VR) and Visual
- Comparator, i.e., control interventions and/or comparison conditions
-
Active Motor Action required (Yes/Yes whenever possible/No):
- ◦
- Yes, if the intervention type requires participant’s residual motor ability (active motor exercise from the participant)
- ◦
- Yes whenever possible refers to conditions foreseeing active motor exercise when feasible, with the technology providing assistance as needed (e.g., robotics)
- ◦
- No otherwise
- Combination of technological interventions (Yes/No)
- Dose, expressed as minutes x number of sessions
- Primary and Secondary Outcomes
-
Key Findings, classified as Positive, Positive on secondary analyses, Negative. We define Key Findings as
- ◦
- Positive if between-group statistical analyses evaluated for the primary outcomes statistically confirm the hypothesis investigated in the study.
- ◦
- Positive on secondary analyses if between-group statistical analyses evaluated for sub-items of the primary/secondary outcomes or considering sub-groups of the population under investigation confirm the hypothesis investigated in the study or if within-group statistical analyses evaluated for the primary/secondary outcomes reveal a statistical improvement only for the experimental group.
- ◦
- Negative if between- and within-group analyses do not reveal statistically significant differences among groups.
- Stratification for secondary analyses according to an upper limb impairment criterium
- Follow-up (Yes/No), i.e., if Yes, we reported the number of months after the end of the intervention
- Setting: Inpatient/Outpatient.
2.5. Synthesis of Results
2.5.1. Descriptive and Frequency Analysis
2.5.2. Association Analysis
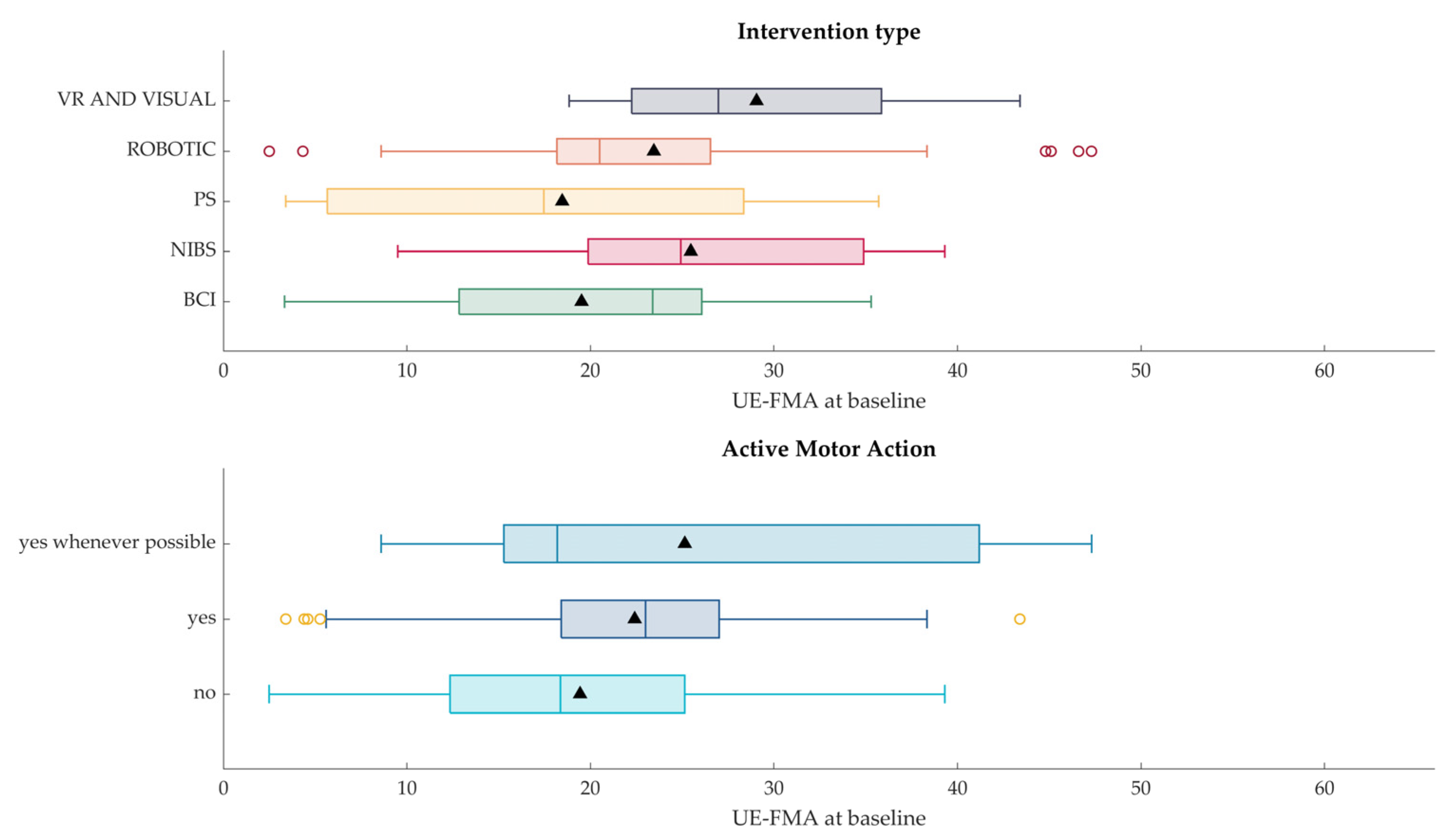
- type of intervention (e.g., Robotic, BCI, PS, …)
- required active upper limb motor actions from the participant by the intervention itself.
3. Results
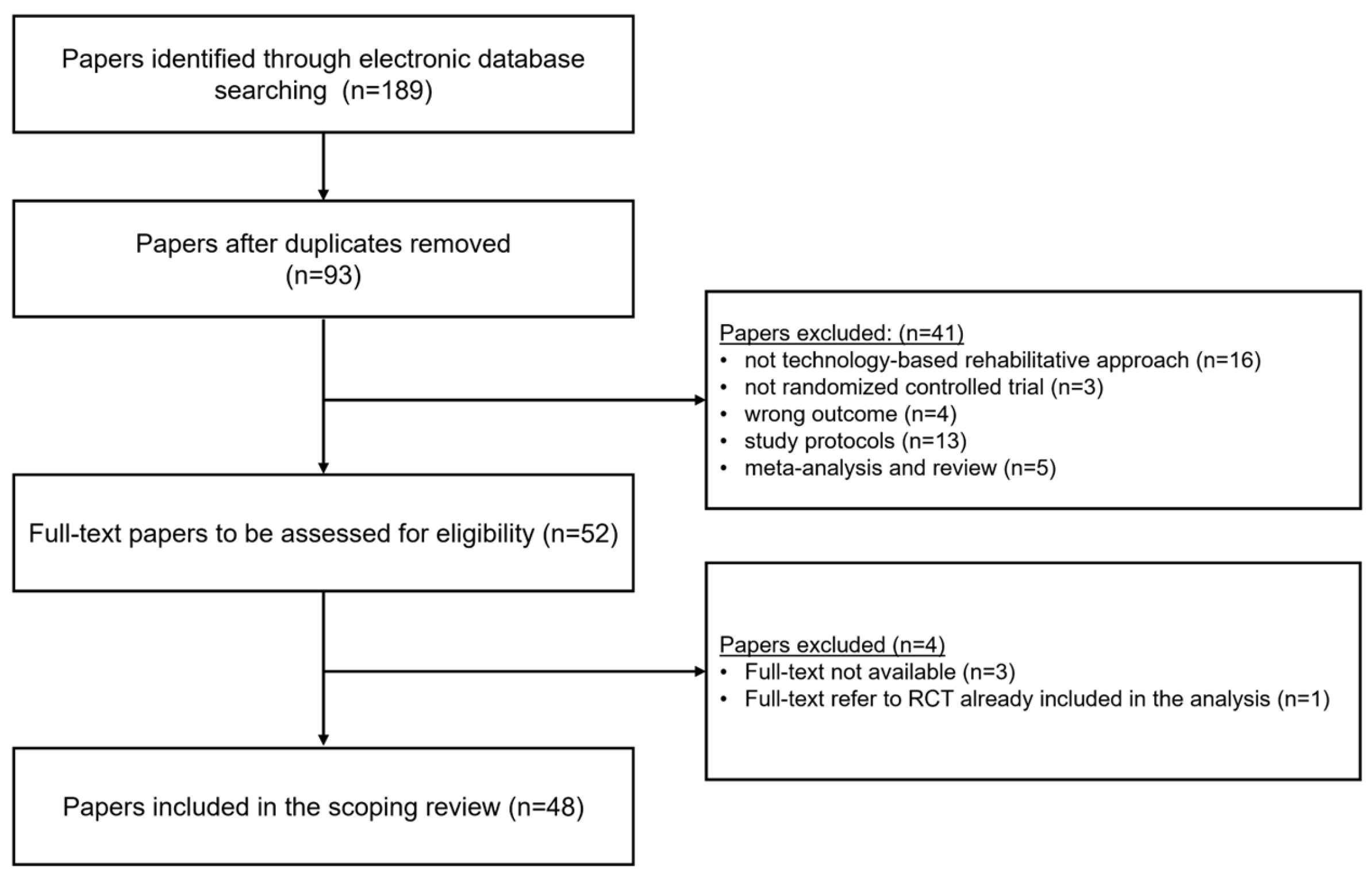
3.1. Selection of Sources of Evidence
- the rehabilitative intervention under investigation does not include a technology-based approach; videos/instruction displayed on screens or other devices of everyday use (personal computers, tablet, smartphones) were not included
- the design of the study does not follow the Randomised Controlled Trial design (wrong study design)
- the effectiveness of the rehabilitative intervention under investigation was not assessed in term of motor function improvement (wrong outcome)
- the paper presents a study protocol, a review or meta-analysis.
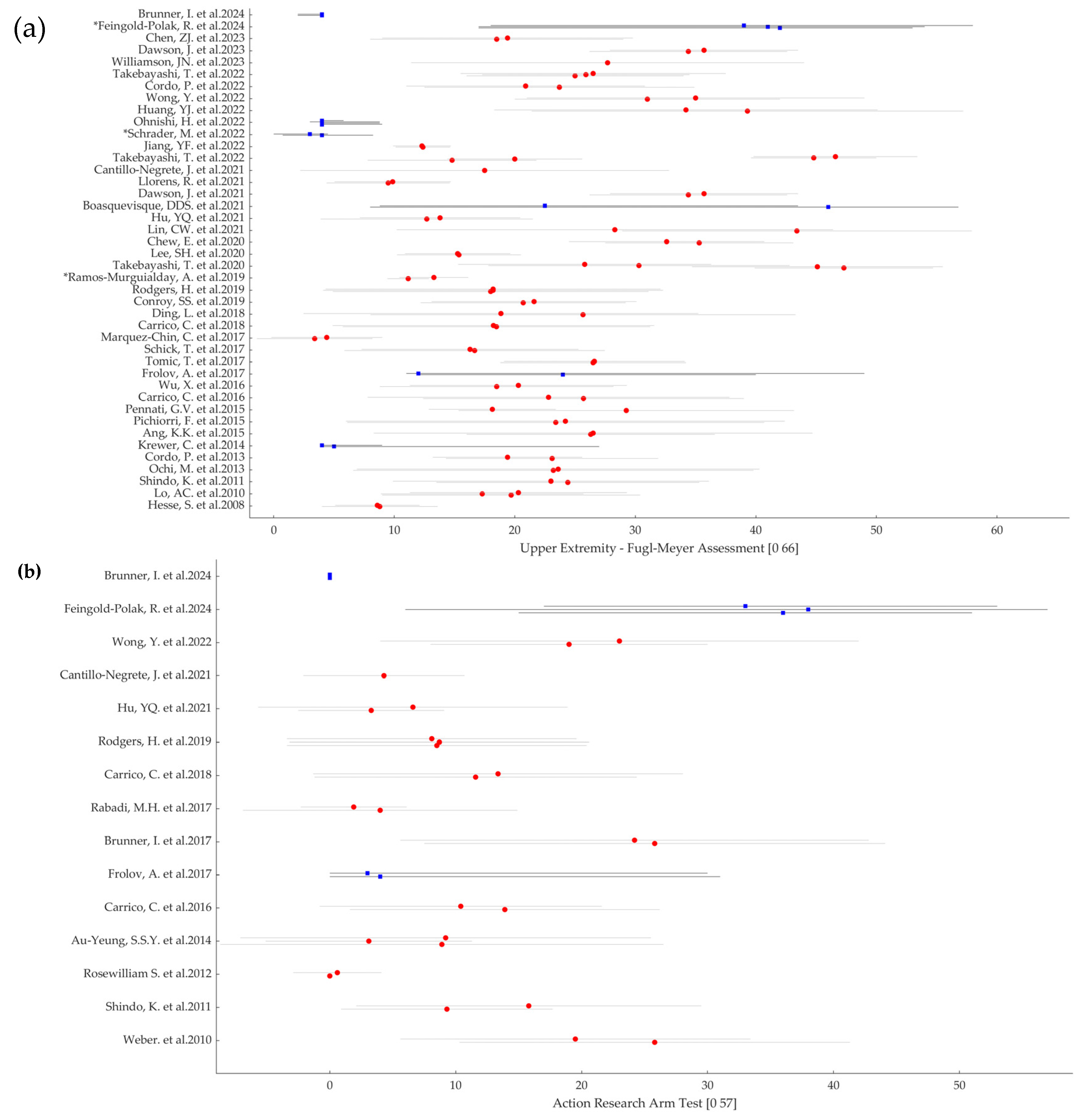
3.2. Results of Individual Sources of Evidence
- First Author Name and Year of Publication
- Population sample size (participants per group)
- Severity of the impairment (FMA and ARAT per group, whenever available)
- Inclusion Criteria for the RCT related to the upper limb impairment
- Time since Injury
- Intervention Type
- Active Motor Action required
- Comparator
- Primary Outcome
- Key Findings
3.3. Synthesis of Results
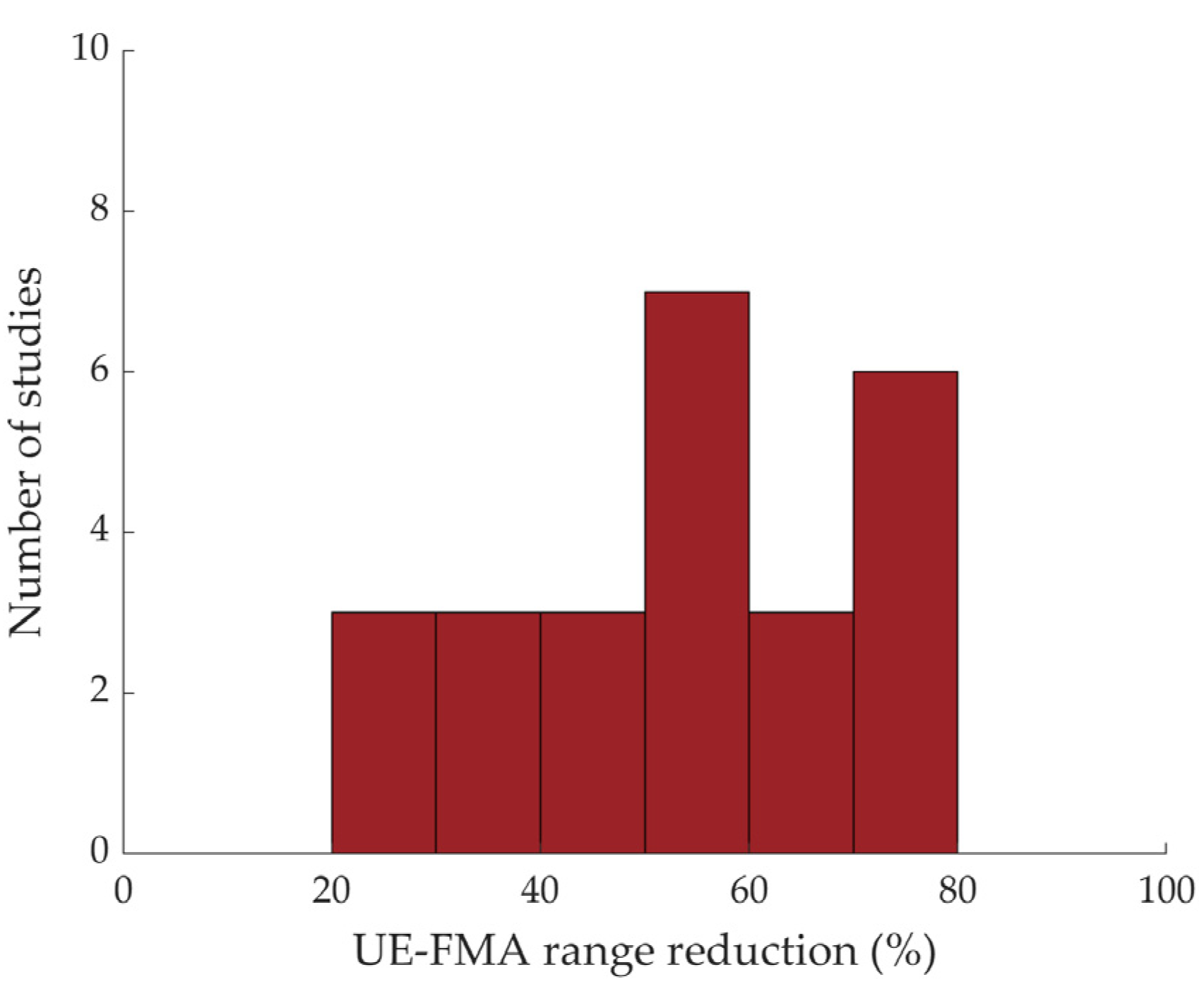
3.3.1. Descriptive and Frequency Results
- Sham Stimulation/Control (applies to NIBS/PS and BCI studies, referring to conditions where the participants are induced to believe they are receiving stimulation or controlling a BCI system while they are not): 28.20%
- Similar intervention “without technology” (e.g., mirror therapy in contrast to VR-based mirror therapy): 17.95%
- Usual care: 17.95%
- Dose equivalent upper limb training (dose equivalent therapy session focused on the upper limb, considered in add-on to usual care): 10.26%
- Different combinations of technology-based approaches: 10.26%
- Different technology: 7.69%
- Different parameters of the same technology (e.g., different robotic assistance, anodal vs cathodal transcranial direct-current stimulation): 7.69%.
3.3.2. Association Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iosa, M.; Morone, G.; Fusco, A.; Bragoni, M.; Coiro, P.; Multari, M.; Venturiero, V.; De Angelis, D.; Pratesi, L.; Paolucci, S. Seven Capital Devices for the Future of Stroke Rehabilitation. Stroke Res Treat 2012, 2012, 187965. [Google Scholar] [CrossRef] [PubMed]
- Coscia, M.; Wessel, M.J.; Chaudary, U.; Millán, J.D.R.; Micera, S.; Guggisberg, A.; Vuadens, P.; Donoghue, J.; Birbaumer, N.; Hummel, F.C. Neurotechnology-Aided Interventions for Upper Limb Motor Rehabilitation in Severe Chronic Stroke. Brain 2019, 142, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Brander, F.; Kelly, K. Intensive Upper Limb Neurorehabilitation in Chronic Stroke: Outcomes from the Queen Square Programme. J Neurol Neurosurg Psychiatry 2019, 90, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Tenberg, S.; Mueller, S.; Vogt, L.; Roth, C.; Happ, K.; Scherer, M.; Behringer, M.; Niederer, D. Comparative Effectiveness of Upper Limb Exercise Interventions in Individuals With Stroke: A Network Meta-Analysis. Stroke 2023, 54, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Dboba, M.M.; Mohd Nordin, N.A.; Manaf, H.; Mohd Rasdi, H.F. Effect of Constraint-Induced Movement Therapy Combined with Neuromuscular Electrical Stimulation on Upper Extremity Function in Stroke Survivors: A Protocol for Systematic Review. Medicine 2023, 102, e34249. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Paolucci, S.; Mattia, D.; Pichiorri, F.; Tramontano, M.; Iosa, M. The 3Ts of the New Millennium Neurorehabilitation Gym: Therapy, Technology, Translationality. Expert Rev Med Devices 2016, 13, 785–787. [Google Scholar] [CrossRef]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of Upper Limb Recovery after Stroke: A Systematic Review and Meta-Analysis. Clin Rehabil 2012, 26, 291–313. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Medical Research Methodology 2018, 18, 143. [Google Scholar] [CrossRef]
- Morone, G.; Pichiorri, F. Post-Stroke Rehabilitation: Challenges and New Perspectives. J Clin Med 2023, 12, 550. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Journal of Clinical Epidemiology 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—a Web and Mobile App for Systematic Reviews. Systematic Reviews 2016, 5, 210. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The Post-Stroke Hemiplegic Patient. 1. a Method for Evaluation of Physical Performance. Scand J Rehabil Med 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; di Bella, P.; Johnson, G. Reliability and Validity of Arm Function Assessment with Standardized Guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A Multicentre Study. Clin Rehabil 2005, 19, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Medical Research Methodology 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Werner, C.; Pohl, M.; Mehrholz, J.; Puzich, U.; Krebs, H.I. Mechanical Arm Trainer for the Treatment of the Severely Affected Arm after a Stroke: A Single-Blinded Randomized Trial in Two Centers. American Journal of Physical Medicine and Rehabilitation 2008, 87, 779–788. [Google Scholar] [CrossRef]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. Robot-Assisted Therapy for Long-Term Upper-Limb Impairment after Stroke. N. Engl. J. Med. 2010, 362, 1772–1783. [Google Scholar] [CrossRef]
- Weber, D.J.; Skidmore, E.R.; Niyonkuru, C.; Chang, C.-L.; Huber, L.M.; Munin, M.C. Cyclic Functional Electrical Stimulation Does Not Enhance Gains in Hand Grasp Function When Used as an Adjunct to onabotulinumtoxinA and Task Practice Therapy: A Single-Blind, Randomized Controlled Pilot Study. Arch Phys Med Rehabil 2010, 91, 679–686. [Google Scholar] [CrossRef]
- Shindo, K.; Fujiwara, T.; Hara, J.; Oba, H.; Hotta, F.; Tsuji, T.; Hase, K.; Liu, M. Effectiveness of Hybrid Assistive Neuromuscular Dynamic Stimulation Therapy in Patients with Subacute Stroke: A Randomized Controlled Pilot Trial. Neurorehabilitation and Neural Repair 2011, 25, 830–837. [Google Scholar] [CrossRef]
- Rosewilliam, S.; Malhotra, S.; Roffe, C.; Jones, P.; Pandyan, A.D. Can Surface Neuromuscular Electrical Stimulation of the Wrist and Hand Combined with Routine Therapy Facilitate Recovery of Arm Function in Patients with Stroke? Archives of Physical Medicine and Rehabilitation 2012, 93, 1715–1721.e1. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Saeki, S.; Oda, T.; Matsushima, Y.; Hachisuka, K. Effects of Anodal and Cathodal Transcranial Direct Current Stimulation Combined with Robotic Therapy on Severely Affected Arms in Chronic Stroke Patients. Journal of Rehabilitation Medicine 2013, 45, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Cordo, P.; Wolf, S.; Lou, J.-S.; Bogey, R.; Stevenson, M.; Hayes, J.; Roth, E. Treatment of Severe Hand Impairment Following Stroke by Combining Assisted Movement, Muscle Vibration, and Biofeedback. Journal of Neurologic Physical Therapy 2013, 37, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Krewer, C.; Hartl, S.; Müller, F.; Koenig, E. Effects of Repetitive Peripheral Magnetic Stimulation on Upper-Limb Spasticity and Impairment in Patients with Spastic Hemiparesis: A Randomized, Double-Blind, Sham-Controlled Study. Archives of Physical Medicine and Rehabilitation 2014, 95, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Au-Yeung, S.S.Y.; Hui-Chan, C.W.Y. Electrical Acupoint Stimulation of the Affected Arm in Acute Stroke: A Placebo-Controlled Randomized Clinical Trial. Clinical Rehabilitation 2014, 28, 149–158. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.G.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.K.; Low, W.; Guan, C. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clinical EEG and Neuroscience 2015, 46, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain-Computer Interface Boosts Motor Imagery Practice during Stroke Recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonzaiuti, D. How Could Robotic Training and Botolinum Toxin Be Combined in Chronic Post Stroke Upper Limb Spasticity? A Pilot Study. European Journal of Physical and Rehabilitation Medicine 2015, 51, 381–387. [Google Scholar]
- Carrico, C.; Chelette, K.C.; Westgate, P.M.; Powell, E.; Nichols, L.; Fleischer, A.; Sawaki, L. Nerve Stimulation Enhances Task-Oriented Training in Chronic, Severe Motor Deficit after Stroke: A Randomized Trial. Stroke 2016, 47, 1879–1884. [Google Scholar] [CrossRef]
- Wu, X.; Guarino, P.; Lo, A.C.; Peduzzi, P.; Wininger, M. Long-Term Effectiveness of Intensive Therapy in Chronic Stroke. Neurorehabilitation and Neural Repair 2016, 30, 583–590. [Google Scholar] [CrossRef]
- Frolov, A.A.; Mokienko, O.; Lyukmanov, R.; Biryukova, E.; Kotov, S.; Turbina, L.; Nadareyshvily, G.; Bushkova, Y. Post-Stroke Rehabilitation Training with a Motor-Imagery-Based Brain-Computer Interface (BCI)-Controlled Hand Exoskeleton: A Randomized Controlled Multicenter Trial. Front Neurosci 2017, 11. [Google Scholar] [CrossRef]
- Tomić, T.J.D.; Savić, A.M.; Vidaković, A.S.; Rodić, S.Z.; Isaković, M.S.; Rodríguez-De-Pablo, C.; Keller, T.; Konstantinović, L.M. ArmAssist Robotic System versus Matched Conventional Therapy for Poststroke Upper Limb Rehabilitation: A Randomized Clinical Trial. BioMed Research International 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Schick, T.; Schlake, H.-P.; Kallusky, J.; Hohlfeld, G.; Steinmetz, M.; Tripp, F.; Krakow, K.; Pinter, M.; Dohle, C. Synergy Effects of Combined Multichannel EMG-Triggered Electrical Stimulation and Mirror Therapy in Subacute Stroke Patients with Severe or Very Severe Arm/Hand Paresis. Restorative Neurology and Neuroscience 2017, 35, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmus, J.; Becker, F.; Sanders, A.-M.; Pallesen, H.; Kristensen, L.Q.; Michielsen, M.; Thijs, L.; et al. Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES): A Multicenter RCT. Neurology 2017, 89, 2413–2421. [Google Scholar] [CrossRef]
- Rabadi, M.H.; Aston, C.E. Effect of Transcranial Direct Current Stimulation on Severely Affected Arm-Hand Motor Function in Patients After an Acute Ischemic Stroke: A Pilot Randomized Control Trial. American journal of physical medicine & rehabilitation 2017, 96, S178–S184. [Google Scholar] [CrossRef]
- Marquez-Chin, C.; Bagher, S.; Zivanovic, V.; Popovic, M.R. Functional Electrical Stimulation Therapy for Severe Hemiplegia: Randomized Control Trial Revisited. Canadian Journal of Occupational Therapy 2017, 84, 87–97. [Google Scholar] [CrossRef]
- Carrico, C.; Westgate, P.M.; Salmon Powell, E.; Chelette, K.C.; Nichols, L.; Pettigrew, L.C.; Sawaki, L. Nerve Stimulation Enhances Task-Oriented Training for Moderate-to-Severe Hemiparesis 3-12 Months After Stroke: A Randomized Trial. Am J Phys Med Rehabil 2018, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, X.; Guo, X.; Chen, S.; Wang, H.; Jiang, N.; Jia, J. Camera-Based Mirror Visual Feedback: Potential to Improve Motor Preparation in Stroke Patients. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2018, 26, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.S.; Wittenberg, G.F.; Krebs, H.I.; Zhan, M.; Bever, C.T.; Whitall, J. Robot-Assisted Arm Training in Chronic Stroke: Addition of Transition-to-Task Practice. Neurorehabilitation and Neural Repair 2019, 33, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): A Multicentre Randomised Controlled Trial. The Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; Curado, M.R.; Broetz, D.; Yilmaz, Ö.; Brasil, F.L.; Liberati, G.; Garcia-Cossio, E.; Cho, W.; Caria, A.; Cohen, L.G.; et al. Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-Up. Neurorehabil Neural Repair 2019, 33, 188–198. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Domen, K.; Hachisuka, K. Impact of Initial Flexor Synergy Pattern Scores on Improving Upper Extremity Function in Stroke Patients Treated with Adjunct Robotic Rehabilitation: A Randomized Clinical Trial. Topics in Stroke Rehabilitation 2020, 27, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, G.; Cho, D.Y.; Kim, H.Y.; Lee, J.-Y.; Kim, S.; Park, S.-B.; Shin, J.-H. Comparisons between End-Effector and Exoskeleton Rehabilitation Robots Regarding Upper Extremity Function among Chronic Stroke Patients with Moderate-to-Severe Upper Limb Impairment. Scientific Reports 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.; Teo, W.-P.; Tang, N.; Ang, K.K.; Ng, Y.S.; Zhou, J.H.; Teh, I.; Phua, K.S.; Zhao, L.; Guan, C. Using Transcranial Direct Current Stimulation to Augment the Effect of Motor Imagery-Assisted Brain-Computer Interface Training in Chronic Stroke Patients—Cortical Reorganization Considerations. Frontiers in Neurology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Kuo, L.-C.; Lin, Y.-C.; Su, F.-C.; Lin, Y.-A.; Hsu, H.-Y. Development and Testing of a Virtual Reality Mirror Therapy System for the Sensorimotor Performance of Upper Extremity: A Pilot Randomized Controlled Trial. IEEE Access 2021, 9, 14725–14734. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Gao, T.-H.; Li, J.; Tao, J.-C.; Bai, Y.-L.; Lu, R.-R. Motor Imagery-Based Brain-Computer Interface Combined with Multimodal Feedback to Promote Upper Limb Motor Function after Stroke: A Preliminary Study. Evidence-based Complementary and Alternative Medicine 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Boasquevisque, D.D.S.; Servinsckins, L.; De Paiva, J.P.Q.; Dos Santos, D.G.; Soares, P.; Pires, D.S.; Meltzer, J.A.; Plow, E.B.; De Freitas, P.F.; Speciali, D.S.; et al. Contralesional Cathodal Transcranial Direct Current Stimulation Does Not Enhance Upper Limb Function in Subacute Stroke: A Pilot Randomized Clinical Trial. Neural Plasticity 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Liu, C.Y.; Francisco, G.E.; Cramer, S.C.; Wolf, S.L.; Dixit, A.; Alexander, J.; Ali, R.; Brown, B.L.; Feng, W.; et al. Vagus Nerve Stimulation Paired with Rehabilitation for Upper Limb Motor Function after Ischaemic Stroke (VNS-REHAB): A Randomised, Blinded, Pivotal, Device Trial. The Lancet 2021, 397, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Llorens, R.; Fuentes, M.A.; Borrego, A.; Latorre, J.; Alcañiz, M.; Colomer, C.; Noé, E. Effectiveness of a Combined Transcranial Direct Current Stimulation and Virtual Reality-Based Intervention on Upper Limb Function in Chronic Individuals Post-Stroke with Persistent Severe Hemiparesis: A Randomized Controlled Trial. Journal of NeuroEngineering and Rehabilitation 2021, 18. [Google Scholar] [CrossRef]
- Cantillo-Negrete, J.; Carino-Escobar, R.I.; Carrillo-Mora, P.; Rodriguez-Barragan, M.A.; Hernandez-Arenas, C.; Quinzaños-Fresnedo, J.; Hernandez-Sanchez, I.R.; Galicia-Alvarado, M.A.; Miguel-Puga, A.; Arias-Carrion, O. Brain-Computer Interface Coupled to a Robotic Hand Orthosis for Stroke Patients’ Neurorehabilitation: A Crossover Feasibility Study. Frontiers in Human Neuroscience 2021, 15. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Okita, Y.; Kubo, H.; Hachisuka, K.; Domen, K. Impact of the Robotic-Assistance Level on Upper Extremity Function in Stroke Patients Receiving Adjunct Robotic Rehabilitation: Sub-Analysis of a Randomized Clinical Trial. Journal of NeuroEngineering and Rehabilitation 2022, 19. [Google Scholar] [CrossRef]
- Jiang, Y.-F.; Zhang, D.; Zhang, J.; Hai, H.; Zhao, Y.-Y.; Ma, Y.-W. A Randomized Controlled Trial of Repetitive Peripheral Magnetic Stimulation Applied in Early Subacute Stroke: Effects on Severe Upper-Limb Impairment. Clinical Rehabilitation 2022, 36, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Sterr, A.; Kettlitz, R.; Wohlmeiner, A.; Buschfort, R.; Dohle, C.; Bamborschke, S. The Effect of Mirror Therapy Can Be Improved by Simultaneous Robotic Assistance. Restor Neurol Neurosci 2022, 40, 185–194. [Google Scholar] [CrossRef]
- Ohnishi, H.; Miyasaka, H.; Shindo, N.; Ito, K.; Tsuji, S.; Sonoda, S. Effectiveness of Repetitive Facilitative Exercise Combined with Electrical Stimulation Therapy to Improve Very Severe Paretic Upper Limbs in with Stroke Patients: A Randomized Controlled Trial. Occup Ther Int 2022, 2022, 4847363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-J.; Wang, S.-M.; Chen, C.; Chen, C.-A.; Wu, C.-W.; Chen, J.-J.; Peng, C.-W.; Lin, C.-W.; Huang, S.-W.; Chen, S.-C. High-Definition Transcranial Direct Current with Electrical Theta Burst on Post-Stroke Motor Rehabilitation: A Pilot Randomized Controlled Trial. Neurorehabilitation and Neural Repair 2022, 36, 645–654. [Google Scholar] [CrossRef]
- Wong, Y.; Li, C.-J.-Z.; Ada, L.; Zhang, T.; Månum, G.; Langhammer, B. UPPER LIMB TRAINING A WITH HAND ORTHOSIS IN EARLY SUBACUTE STROKE: A PILOT RANDOMIZED TRIAL. Journal of Rehabilitation Medicine 2022, 54. [Google Scholar] [CrossRef] [PubMed]
- Cordo, P.; Wolf, S.; Rymer, W.Z.; Byl, N.; Stanek, K.; Hayes, J.R. Assisted Movement With Proprioceptive Stimulation Augments Recovery From Moderate-To-Severe Upper Limb Impairment During Subacute Stroke Period: A Randomized Clinical Trial. Neurorehabilitation and Neural Repair 2022, 36, 239–250. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Amano, S.; Gosho, M.; Sakai, M.; Hashimoto, K.; Hachisuka, K.; Uchiyama, Y.; Domen, K. Robot-Assisted Training as Self-Training for Upper-Limb Hemiplegia in Chronic Stroke: A Randomized Controlled Trial. Stroke 2022, 53, 2182–2191. [Google Scholar] [CrossRef]
- Williamson, J.N.; James, S.A.; He, D.; Li, S.; Sidorov, E.V.; Yang, Y. High-Definition Transcranial Direct Current Stimulation for Upper Extremity Rehabilitation in Moderate-to-Severe Ischemic Stroke: A Pilot Study. Frontiers in Human Neuroscience 2023, 17. [Google Scholar] [CrossRef]
- Dawson, J.; Engineer, N.D.; Cramer, S.C.; Wolf, S.L.; Ali, R.; O’Dell, M.W.; Pierce, D.; Prudente, C.N.; Redgrave, J.; Feng, W.; et al. Vagus Nerve Stimulation Paired With Rehabilitation for Upper Limb Motor Impairment and Function After Chronic Ischemic Stroke: Subgroup Analysis of the Randomized, Blinded, Pivotal, VNS-REHAB Device Trial. Neurorehabilitation and Neural Repair 2023, 37, 367–373. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Gu, J.; Tan, J.; Hu, T. Effectiveness of Soft Robotic Glove versus Repetitive Transcranial Magnetic Stimulation in Post-Stroke Patients with Severe Upper Limb Dysfunction: A Randomised Controlled Trial. Frontiers in Neurology 2023, 13. [Google Scholar] [CrossRef]
- Chen, Z.-J.; He, C.; Xu, J.; Zheng, C.-J.; Wu, J.; Xia, N.; Hua, Q.; Xia, W.-G.; Xiong, C.-H.; Huang, X.-L. Exoskeleton-Assisted Anthropomorphic Movement Training for the Upper Limb After Stroke: The EAMT Randomized Trial. Stroke 2023, 54, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Feingold-Polak, R.; Barzel, O.; Levy-Tzedek, S. Socially Assistive Robot for Stroke Rehabilitation: A Long-Term in-the-Wild Pilot Randomized Controlled Trial. IEEE Trans Neural Syst Rehabil Eng 2024, 32, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Lundquist, C.B.; Pedersen, A.R.; Spaich, E.G.; Dosen, S.; Savic, A. Brain Computer Interface Training with Motor Imagery and Functional Electrical Stimulation for Patients with Severe Upper Limb Paresis after Stroke: A Randomized Controlled Pilot Trial. Journal of NeuroEngineering and Rehabilitation 2024, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Wallace, D.; Lai, S.M.; Johnson, D.; Embretson, S.; Laster, L.J. The Stroke Impact Scale Version 2.0. Evaluation of Reliability, Validity, and Sensitivity to Change. Stroke 1999, 30, 2131–2140. [Google Scholar] [CrossRef]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel Index and Modified Rankin Scale in Acute Stroke Trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook III, E.W.; Taub, E. The Reliability of the Wolf Motor Function Test for Assessing Upper Extremity Function after Stroke. Archives of Physical Medicine and Rehabilitation 2001, 82, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; O’Connor, R.J.; Makower, S.; Levesley, M.; Bhakta, B. Systematic Review of Outcome Measures Used in the Evaluation of Robot-Assisted Upper Limb Exercise in Stroke. J Rehabil Med 2011, 43, 181–189. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W. Rasch Analysis Staging Methodology to Classify Upper Extremity Movement Impairment after Stroke. Arch Phys Med Rehabil 2013, 94, 1527–1533. [Google Scholar] [CrossRef]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; McCombe Waller, S. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch Phys Med Rehabil 2017, 98, 456–462. [Google Scholar] [CrossRef]
- Luft, A.R.; McCombe-Waller, S.; Whitall, J.; Forrester, L.W.; Macko, R.; Sorkin, J.D.; Schulz, J.B.; Goldberg, A.P.; Hanley, D.F. Repetitive Bilateral Arm Training and Motor Cortex Activation in Chronic Stroke: A Randomized Controlled Trial. JAMA 2004, 292, 1853–1861. [Google Scholar] [CrossRef]
- Zeiler, S.R.; Krakauer, J.W. The Interaction between Training and Plasticity in the Poststroke Brain. Curr Opin Neurol 2013, 26, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Langhorne, P.; Kidd, L.; Wu, O.; McConnachie, A.; van Wijck, F.; Dawson, J. SaeboGlove Therapy for Upper Limb Disability and Severe Hand Impairment after Stroke (SUSHI): Study Protocol for a Randomised Controlled Trial. Eur Stroke J 2021, 6, 302–310. [Google Scholar] [CrossRef]
- Dai, W.; Yang, X.; Liu, C.; Ding, H.; Guo, C.; Zhu, Y.; Dong, M.; Qian, Y.; Fang, L.; Wang, T.; et al. Effects of Repetitive Transcranial Magnetic Stimulation over the Contralesional Dorsal Premotor Cortex on Upper Limb Function in Severe Ischaemic Stroke: Study Protocol for a Randomised Controlled Trial. BMJ Open 2023, 13, e074037. [Google Scholar] [CrossRef]
- Gonzalez-Santos, J.; Soto-Camara, R.; Rodriguez-Fernández, P.; Jimenez-Barrios, M.; Gonzalez-Bernal, J.; Collazo-Riobo, C.; Jahouh, M.; Bravo-Anguiano, Y.; Trejo-Gabriel-Galan, J.M. Effects of Home-Based Mirror Therapy and Cognitive Therapeutic Exercise on the Improvement of the Upper Extremity Functions in Patients with Severe Hemiparesis after a Stroke: A Protocol for a Pilot Randomised Clinical Trial. BMJ Open 2020, 10, e035768. [Google Scholar] [CrossRef]
- Kinoshita, S.; Ikeda, K.; Yasuno, S.; Takahashi, S.; Yamada, N.; Okuyama, Y.; Sasaki, N.; Hada, T.; Kuriyama, C.; Suzuki, S.; et al. Dose-Response of rPMS for Upper Limb Hemiparesis after Stroke. Medicine (Baltimore) 2020, 99, e20752. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, W.-S.; Park, J.; Kim, J.; Paik, N.-J. Effects of Anodal Transcranial Direct Current Stimulation over the Contralesional Hemisphere on Motor Recovery in Subacute Stroke Patients with Severe Upper Extremity Hemiparesis: Study Protocol for a Randomized Controlled Trial. Medicine (Baltimore) 2020, 99, e19495. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, S.; Long, L.; Zang, Q.; Jia, G. Efficacy and Safety of Electroacupuncture at Auricular Concha Region in Promoting of Rehabilitation of Ischemic Stroke Patients with Upper Limb Motor Dysfunction: A Study Protocol for a Randomized Pilot Trial. Medicine (Baltimore) 2022, 101, e28047. [Google Scholar] [CrossRef]
- Mizuno, K.; Abe, T.; Ushiba, J.; Kawakami, M.; Ohwa, T.; Hagimura, K.; Ogura, M.; Okuyama, K.; Fujiwara, T.; Liu, M. Evaluating the Effectiveness and Safety of the Electroencephalogram-Based Brain-Machine Interface Rehabilitation System for Patients With Severe Hemiparetic Stroke: Protocol for a Randomized Controlled Trial (BEST-BRAIN Trial). JMIR Res Protoc 2018, 7, e12339. [Google Scholar] [CrossRef]
- Mohan, A.; Knutson, J.S.; Cunningham, D.A.; Widina, M.; O’Laughlin, K.; Arora, T.; Li, X.; Sakaie, K.; Wang, X.; Uchino, K.; et al. Contralaterally Controlled Functional Electrical Stimulation Combined With Brain Stimulation for Severe Upper Limb Hemiplegia-Study Protocol for a Randomized Controlled Trial. Front Neurol 2022, 13, 869733. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H.; Shaw, L.; Bosomworth, H.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; Dawson, J.; Eyre, J.; Finch, T.; et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): Study Protocol for a Randomised Controlled Trial. Trials 2017, 18, 340. [Google Scholar] [CrossRef] [PubMed]
- Stoykov, M.E.; Biller, O.M.; Wax, A.; King, E.; Schauer, J.M.; Fogg, L.F.; Corcos, D.M. Bilateral Upper Extremity Motor Priming (BUMP) plus Task-Specific Training for Severe, Chronic Upper Limb Hemiparesis: Study Protocol for a Randomized Clinical Trial. Trials 2022, 23, 523. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, E.C.C.; Visser-Meily, J.M.A.; Neggers, S.F.W.; van der Worp, H.B.; Dijkhuizen, R.M. Brain Stimulation for Arm Recovery after Stroke (B-STARS): Protocol for a Randomised Controlled Trial in Subacute Stroke Patients. BMJ Open 2017, 7, e016566. [Google Scholar] [CrossRef]
- Wei, D.; Hua, X.-Y.; Zheng, M.-X.; Wu, J.-J.; Xu, J.-G. Effectiveness of Robot-Assisted Virtual Reality Mirror Therapy for Upper Limb Motor Dysfunction after Stroke: Study Protocol for a Single-Center Randomized Controlled Clinical Trial. BMC Neurol 2022, 22, 307. [Google Scholar] [CrossRef]
- Wei, X.; Xia, N.; Li, Y.-A.; Gu, M.; Zhang, T.; Gao, W.; Liu, Y. Immediate and Short-Term Effects of Continuous Theta Burst Transcranial Magnetic Stimulation over Contralesional Premotor Area on Post-Stroke Spasticity in Patients with Severe Hemiplegia: Study Protocol for a Randomized Controlled Trial. Front Neurol 2022, 13, 895580. [Google Scholar] [CrossRef] [PubMed]
| First Author Name, Year of Publication | Population sample size (participants per group) | Severity of the impairment (UE-FMA and/or ARAT per group) | IC | Time since Injury | Intervention Type | Active Motor Action required | Comparator | Primary Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| Hesse, 2008 [16] | EG: 27 CG: 27 |
UE-FMA EG: 8.8 ± 4.5 CG: 8.6 ± 3.5 |
Yes | ES | PS, Robotic | Yes, whenever possible | Different Technology | UE- FMA | Neg |
| Lo, 2010 [17] | EG: 47 CG1: 46 CG2: 27 |
UE-FMA EG: 19.7 ± 10.7 CG1: 17.3 ± 8.4 CG2: 20.3 ± 9.0 |
Yes | C | Robotic | Yes | Dose Equivalent UL training, Usual Care |
UE- FMA | Pos On Sec |
| Weber, 2010 [18] | EG: 10 CG: 13 |
ARAT EG: 19.5 ± 13.9 CG: 25.8 ± 15.5 |
Yes | C | PS | Yes | Without Technology | MAL | Neg |
| Shindo, 2011 [19] | EG: 10 CG: 10 |
UE-FMA EG: 24.4 ± 10.9 CG: 23.0 ± 13.1 ARAT EG: 9.3 ± 8.4 CG: 15.8 ± 13.7 |
Yes | ES | PS | Yes | Without technology | UE-FMA | Pos |
| Rosewilliam, 2012 [20] | EG: 31 CG: 36 |
ARAT EG: 0.0 ± 0.0 CG: 0.6 ± 3.5 |
Yes | A/ES | PS | No | Usual Care | ARAT | Neg |
| Ochi, 2013 [21] | EG: 9 CG: 9 |
UE-FMA EG: 23.2 ± 16.6 CG: 23.6 ± 16.7 |
Yes | C | NIBS, Robotic | No | Different Parameters | UE-FMA | Pos On Sec |
| Cordo, 2013 [22] | EG1: 22 EG2: 21 |
UE-FMA EG1: 23.1 ± 8.8 EG2: 19.4 ± 6.2 |
Yes | C | PS, Robotic | Yes | Different Technology | UE-FMA | Pos On Sec |
| Krewer, 2014 [23] | EG: 31 CG: 32 |
UE-FMA EG: 5 (4-27) CG: 4 (4-9) |
Yes | C | PS | No | Sham Stimulation | MTS UE-FMA |
Pos On Sec |
| Au-Yeung, 2014 [24] | EG: 29 CG1: 21 CG2: 23 |
ARAT EG: 8.9 ± 17.6 CG1: 3.1 ± 8.2 CG2: 9.2 ± 16.3 |
Yes | A | PS | No | Sham Stimulation, Usual Care | Force measures | Pos |
| Ang, 2015 [25] | EG: 11 CG: 14 |
UE-FMA EG: 26.3 ± 10.3 CG: 26.5 ± 18.2 |
Yes | C | BCI, Robotic | Yes | Different Combination | UE-FMA | Neg |
| Pichiorri, 2015 [26] | EG: 14 CG: 14 |
UE-FMA EG: 23.4 ± 17.3 CG: 24.2 ± 18.2 |
No | ES/S | BCI, VR and Visual | No | Without Technology | UE-FMA | Pos |
| Pennati, 2015 [27] | EG1: 8 EG2: 7 |
UE-FMA EG1: 29.25 ± 13.91 EG2: 18.14 ± 5.27 |
No | C | Robotic | Yes | Different Combination | UE-FMA BBT FIM MAS |
Neg |
| Carrico, 2016 [28] | EG: 18 CG: 18 |
UE-FMA EG: 25.7 ± 13.3 CG: 22.8 ± 15.0 ARAT EG: 13.9 ± 12.3 CG: 10.4 ± 11.2 |
Yes | C | PS | Yes | Sham Stimulation | UE-FMA |
Pos |
| Wu, 2016 [29] | EG: 99 CG: 28 |
UE-FMA EG: 18.5 ± 9.7 CG: 20.3 ± 9.0 |
Yes | C | Robotic | Yes | Usual Care | UE-FMA | Pos |
| Frolov, 2017 [30] | EG: 55 CG: 19 |
UE-FMA EG: 24.0 (12.0-40.0) CG: 12.0 (11.0-49.0) ARAT EG: 4.0 (0.0-31.0) CG: 3.0 (0.0-30.0) |
No | S/C | BCI, Robotic | No | Sham Control | UE-FMA ARAT |
Pos On Sec |
| Tomic, 2017 [31] | EG: 13 CG: 13 |
UE-FMA EG: 26.5 ± 7.7 CG: 26.6 ± 7.5 |
Yes | ES | Robotic | Yes | Dose Equivalent UL Training | UE-FMA | Pos |
| Schick, 2017 [32] | EG: 16 CG: 17 |
UE-FMA EG: 16.67 ± 10.80 CG: 16.29 ± 9.00 |
Yes | ES | PS | No | Different Combination | UE-FMA | Pos On Sec |
| Brunner, 2017 [33] | EG: 57 CG: 55 |
ARAT EG: 25.8 ± 18.3 CG: 24.2 ± 18.6 |
Yes | ES | VR and Visual | Yes | Dose Equivalent UL Training | ARAT | Neg |
| Rabadi, 2017 [34] | EG: 8 CG: 8 |
ARAT EG: 4.0 ± 10.9 CG: 1.9 ± 4.2 |
Yes | A | NIBS | No | Sham Stimulation | ARAT | Neg |
| Marquez-Chin, 2017 [35] | EG: 10 CG: 11C |
UE-FMA EG: 3.4 ± 4.8 CG: 4.4 ± 4.6 |
Yes | A/ES | PS | Yes | Usual Care | FIM UE-FMA |
Pos |
| Carrico, 2018 [36] | EG: 33 CG: 22 |
UE-FMA EG: 18.48 ± 12.75 CG: 18.23 ± 13.34 ARAT EG: 11.58 ± 12.80 CG: 13.36 ± 14.68 |
Yes | S/C | PS | No | Sham Stimulation | WMFT | Pos |
| Ding, 2018 [37] | EG: 38 CG: 41 |
UE-FMA EG: 25.66 ± 17.63 CG: 18.85 ± 16.38 |
No | S/C | VR and Visual | Yes | Dose Equivalent UL Training | UE-FMA | Pos |
| Conroy, 2019 [38] | EG: 22 CG: 19 |
UE-FMA EG: 20.7 ± 8.5 CG: 21.6 ± 8.5 |
Yes | C | Robotic | Yes | Different Combination | UE-FMA | Pos On Sec |
| Rodgers, 2019 [39] | EG: 239 CG1: 246 CG2: 223 |
UE-FMA EG: 18.0 ± 13.1 CG1: 18.2 ± 14.1 CG2: 18.2 ± 13.9 ARAT EG: 8.5 ± 11.9 CG1: 8.7 ± 11.9 CG2: 8.1 ± 11.5 |
Yes | S/C | Robotic | Yes, whenever possible | Dose Equivalent UL Training, Usual care | ARAT | Neg |
| Ramos-Murguialday, 2019 [40] | EG: 16 CG: 12 |
UE-FMA EG: 11.16 ± 1.73 CG: 13.29 ± 2.86 |
Yes | C | BCI, Robotic | Yes | Sham Control | UE-FMA (54) | Pos On Sec |
| Takebayashi, 2020 [41] | EG: 30 CG: 26 |
UE-FMA EG: 47.3 ± 7.4 (mild) CG: 45.1 ± 19.4 (mild) EG: 30.3 ± 12.5 (moderate) CG: 25.8 ± 10.5 (moderate) EG: 16.1 ± 10.5 (severe) CG: 14.8 ± 4.7 (severe) |
Yes | ES | Robotic | Yes, whenever possible | Dose Equivalent UL Training | UE-FMA | Pos On Sec |
| Lee, 2020 [42] | EG1: 19 EG2: 19 |
UE-FMA EG1: 15.37 ± 5.14 EG2: 15.26 ± 4.37 |
Yes | S/C | Robotic | Yes, whenever possible | Different Technology | UE-FMA WMFT |
(*) |
| Chew, 2020 [43] | EG: 10 CG: 9 |
UE-FMA EG: 35.3 ± 7.8 CG: 32.6 ± 8.1 |
Yes | C | BCI, NIBS | No | Sham Stimulation | UE-FMA |
Pos On Sec |
| Lin, 2021 [44] | EG: 9 CG: 9 |
UE-FMA EG: 43.4 ± 14.5 CG: 28.3 ± 18.1 |
Yes | C | VR and Visual | Yes | Without technology | UE-FMA | Pos |
| Hu, 2021 [45] | EG: 7 CG: 5 |
UE-FMA EG: 12.70 ± 8.80 CG: 13.80 ± 6.65 ARAT EG: 3.29 ± 5.79 CG: 6.60 ± 12.29 |
Yes | S/C | BCI, VR and Visual | No | Without technology | UE-FMA | Pos On Sec |
| Boasquevisque, 2021 [46] | EG: 15 CG: 15 |
UE-FMA EG: 46 (8-56.8) CG: 22.5 (8.8-43.5) |
No | A/ES | NIBS | No | Sham Stimulation | Safety (**) | Neg |
| Dawson, 2021 [47] | EG: 53 CG: 54 |
UE-FMA EG: 34.4 ± 8.2 CG: 35.7 ± 7.8 |
Yes | C | PS | Yes | Sham Stimulation | UE-FMA | Pos |
| Llorens, 2021 [48] | EG: 14 CG: 15 |
UE-FMA EG: 9.50 ± 5.11 CG: 9.87 ± 4.82 |
Yes | C | NIBS, VR and Visual | Yes | Usual Care | UE-FMA | Pos |
| Cantillo-Negrete, 2021 [49] | 10 cross-over study | UE-FMA 17.5 ± 15.3 ARAT 4.3 ± 6.4 |
Yes | S/C | BCI, Robotic | No | Usual Care | UE-FMA | Neg |
| Takebayashi, 2022 [50] | EG1: 17 EG2: 13 |
UE-FMA EG1: 14.8 ± 7.0 (severe) EG2: 20.0 ± 5.6 (severe) EG1: 44.8 ± 5.2 (moderate) EG2: 46.6 ± 6.8 (moderate) |
Yes | ES | Robotic | Yes, whenever possible | Different parameters | UE-FMA WMFT |
Pos On Sec |
| Jiang, 2022 [51] | EG: 24 CG: 20 |
UE-FMA EG: 12.38 ± 2.26 CG: 12.30 ± 2.39 |
Yes | A | PS | No | Usual Care | UE-FMA | Pos |
| Schrader, 2022 [52] | EG: 14 CG: 10 |
UE-FMA EG: 4.00 (0.75-8.25) CG: 3.00 (0.00-4.50) |
Yes | A/S/C | Robotic | No | Without technology | UE-FMA (60) | Pos |
| Ohnishi, 2022 [53] | EG1: 25 EG2: 22 EG3: 26 CG: 26 |
UE-FMA EG1: 4.0 (4.0-9.0) EG2: 4.0 (4.0-8.8) EG3: 4.0 (3.0-8.8) CG: 4.0 (4.0-5.8) |
Yes | EA | PS | Yes | Different parameters, Usual Care |
SIAS UE-FMA MAS FIM |
Pos |
| Huang, 2022 [54] | EG: 13 CG: 11 |
UE-FMA EG: 39.3 ± 17.9 CG: 34.2 ± 15.9 |
Yes | C | NIBS | No | Sham Stimulation | UE-FMA | Pos On Sec |
| Wong, 2022 [55] | EG: 15 CG: 15 |
UE-FMA EG: 31 ± 11 CG: 35 ± 14 ARAT EG: 19 ± 11 CG: 23 ± 19 |
Yes | A/ES | PS | Yes | Without technology | ARAT | Neg |
| Cordo, 2022 [56] | EG: 44 CG: 39 |
UE-FMA EG: 20.9 ± 9.9 CG: 23.7 ± 11.2 |
Yes | ES/S | PS, Robotic |
Yes | Different parameters | UE-FMA | Pos |
| Takebayashi, 2022 [57] | EG1: 42 EG2: 39 CG: 36 |
UE-FMA EG1: 25.9 ± 8.6 EG2: 26.5 ± 11.0 CG: 25.0 ± 0.9 |
Yes | C | Robotic | Yes | Without technology | UE-FMA | Pos On Sec |
| Williamson, 2023 [58] | 8 cross-over study | UE-FMA 27.7 ± 16.3 |
Yes | ES/S/C | NIBS | No | Different Parameters, Sham Stimulation | UE-FMA | Pos |
| Dawson, 2023 [59] | EG: 53 CG: 55 |
UE-FMA EG: 34.4 ± 8.2 CG: 35.7 ± 7.8 |
Yes | C | PS | Yes | Sham Stimulation | UE-FMA | Pos |
| Wang, 2023 [60] | EG1: 23 EG2: 23 CG: 23 |
UE-FMA EG1: 9 (IQR: 12) EG2: 11 (IQR: 8) CG: 14 (IQR: 16) |
Yes | ES/S | NIBS, Robotic | Yes | Different Technology, Usual care |
UE-FMA BI |
Pos |
| Chen, 2023 [61] | EG: 40 CG: 40 |
UE-FMA EG: 18.5 ± 10.5 CG: 19.4 ± 10.4 |
Yes | A/ES | Robotic | Yes | Usual Care | UE-FMA | Pos |
| Feingold-Polak, 2024 [62] | EG1: 10 EG2: 8 CG: 8 |
UE-FMA (60) EG1: 42 (17-53) EG2: 41 (17-54) CG: 39 (18-58) ARAT EG1: 36 (15-51) EG2: 38 (6-57) CG: 33(17-53) |
Yes | S | Robotic | Yes | Different Technology, Usual Care |
UE-FMA (60) ARAT MAL SIS |
Pos On Sec |
| Brunner, 2024 [63] | EG: 15 CG: 20 |
UE-FMA EG: 4 (2-4) CG: 4 (2-4) ARAT EG: 0 (0-0) CG: 0 (0-0) |
Yes | A/ES | BCI, PS | No | Usual Care | ARAT | Neg |
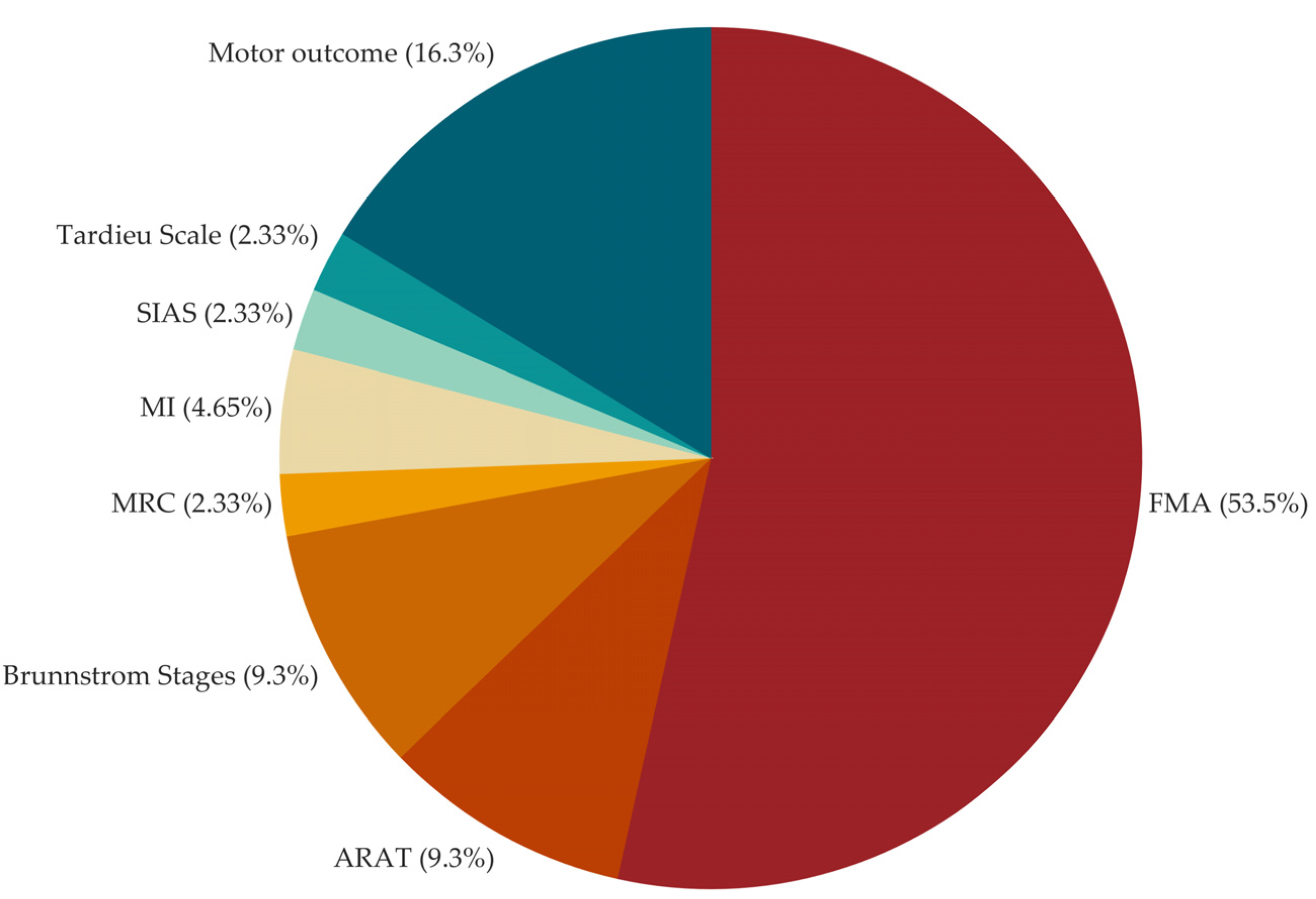
| Outcome | As Primary number of studies |
As Secondary number of studies |
|---|---|---|
| Upper Extremity Fugl-Meyer Assessment | 38 | 5 |
| Action Research Arm Test | 8 | 8 |
| Wolf Motor Function Test | 3 | 9 |
| Functional Independence Measure | 3 | 2 |
| Modified Ashworth Scale | 2 | 9 |
| Motor Activity Log | 2 | 6 |
| Stroke Impact Scale | 1 | 11 |
| Barthel Index | 1 | 11 |
| Box and Block Test | 1 | 4 |
| Stroke Impairment Assessment Set: knee-mouth and finger function test | 1 | |
| Modified Tardieu Scale | 1 | |
| Goal Attainment Scaling | 2 | |
| Motricity Index | 2 | |
| Medical Research Council Scale | 2 | |
| National Institutes of Health Stroke Scale | 2 | |
| Finger-Nose Test | 1 | |
| Jebsen-Taylor Hand Function Test | 1 | |
| Hamilton Depression Scale | 1 | |
| Mental Rotation Task | 1 | |
| Modified Rankin Scale | 1 | |
| Montreal Cognitive Assessment | 1 | |
| Nine-hole peg test | 1 | |
| Nottingham Sensory Assessment | 1 | |
| Numeric Rating Scale Pain | 1 | |
| Rancho Los Amigos Scale | 1 | |
| Rivermead Assessment of Somatosensory Performance | 1 | |
| Stroke specific Quality of Life Scale | 1 | |
| Motor outcome (kinematic, kinetic, electromyographic parameters) | 1 | 10 |
| Brain outcome (transcranial magnetic stimulation and electroencephalographic parameters) | 5 | |
| Safety (adverse events) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





